Virtually all cases of chronic lymphocytic leukemia (CLL) are preceded by a pre-malignant condition known as monoclonal B-cell lymphocytosis (MBL).1 MBL is defined as a clonal lymphoproliferative disorder with an absolute B-cell count of <5 × 109/L, in the absence of symptoms, organomegaly, and lymphadenopathy.2–4 MBL is classified into low-count MBL (absolute B-cell count <0.5 × 109/L, identified during population screening studies) and high count MBL (absolute B-cell count ≥0.5 × 109/L, typically identified during the work-up of low-count lymphocytosis).5,6 Retrospective studies of ~20–300 individuals with MBL and relatively short follow up (median 3.5 years) have reported that the risk of progression to CLL requiring therapy ranges from 1.1% to 5% per year.7–13 Data regarding risk factors for progression are limited, with some reports suggesting CD38 expression, unmutated immunoglobulin heavy chain (IGHV) genes and high-risk cytogenetic aberrations, as determined by fluorescence in situ hybridization, are associated with a higher risk of progression.8,9,12 However, these analyses are limited by the small numbers of individuals with MBL, short follow-up period and lack of survival analysis. In this study, we describe the outcomes of a large cohort of individuals with high-count MBL seen at the Mayo Clinic.
The Mayo Clinic CLL Database includes adults with a clonal B-cell population of the CLL immunophenotype seen at Mayo Clinic (Rochester, MN, USA) who permit their records to be used for research purposes.14–17 Using this database, we identified individuals with clinically ascertained high-count MBL who were seen between January 1, 1995 and May 31, 2016. Individuals with atypical-CLL and non-CLL MBL immunophenotype, and those with a concomitant lymphoproliferative neoplasm within three months of MBL ascertainment were excluded. Indications for treatment were classified into: (i) progression to CLL requiring therapy; (ii) immune complications; (iii) high-grade lymphoma; and (iv) non-MBL related conditions. The percent of bone marrow infiltration by monoclonal B cells among individuals who underwent bone marrow biopsy was recorded. The Mayo Clinic Institutional Review Board approved this study.
Time to first therapy was defined as the time interval between the date of MBL diagnosis and the date of first therapy for an MBL-related reason. Time to first therapy was analyzed using cumulative incidence methods accounting for competing risk of death. Overall survival was estimated by the Kaplan–Meier method and defined as the time from the date of MBL diagnosis until death or last follow up. Multivariable Cox proportional hazards regression analysis was used to identify factors that predicted time to first treatment and overall survival. Because of missing data for some prognostic parameters, multiple models were run, introducing one novel prognostic factor at a time to the base model (consisting of age and sex). Overall survival was compared to the age- and sex-matched population of the state of Minnesota.
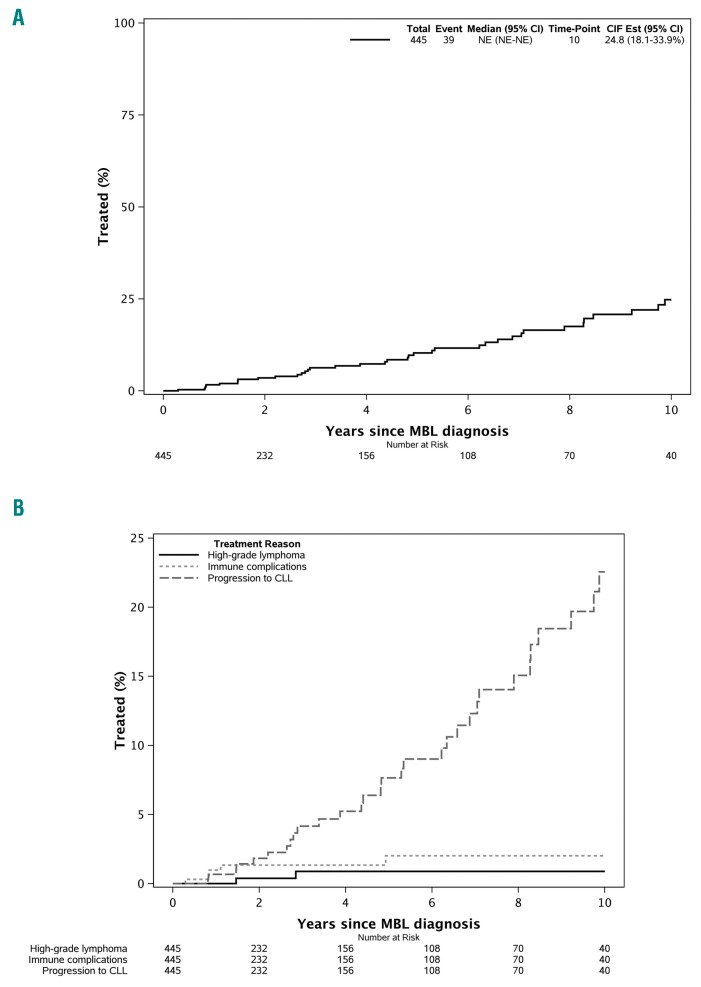
Four hundred and forty-five individuals with clinically ascertained high-count MBL were identified. Their median age at the time of diagnosis of MBL was 69 years (range, 44–95 years). Two hundred and sixty-three (59%) were males, and the median absolute B-cell count was 2.9 × 109/L (range, 0.5–4.9 × 109/L) (Table 1). The median infiltration of bone marrow by the B-cell clone was 15% (range, 0–70%) among 52 individuals who underwent a clinical bone marrow biopsy. After a median follow up of 6.4 years, 45 individuals required therapy. Of these 45 individuals, 39 (87%) received treatment for MBL-related reasons (including 32 for progression to CLL requiring therapy; 5 for immune complications, and 2 for high-grade lymphoma) and 6 (13%) for non-MBL-related conditions (Online Supplementary Table S1). When considering MBL-related reasons only as an indication to start treatment, the rate of progression was estimated to be ~1.4% per year (Figure 1A). The estimated 2-, 5- and 10-year incidences of requiring therapy were, respectively, 1.8%, 7.7%, and 22.6% for progressive CLL, 1.3%, 2.0%, and 2.0% for immune complications, and 0.4%, 0.9%, and 0.9% for high-grade lymphoma (Figure 1B).
Table 1.
Baseline characteristics of individuals with clinically ascertained monoclonal B-cell lymphocytosis.
Figure 1.
Time to first therapy in clinically ascertained high-count monoclonal B-cell lymphocytosis. (A) For the overall cohort. (B) According to reason for therapy (progression to chronic lymphocytic leukemia, immune complications, and high-grade lymphoma).
Of the 32 individuals treated for progression to CLL requiring therapy, 9 (28%) received anti-CD20 monoclonal antibody alone, 8 (25%) received a combination of an alkylator and anti-CD20 monoclonal antibody, 7 (22%) received chemoimmunotherapy, 6 (19%) received an alkylator alone, and 2 (6%) received ibrutinib. Seven individuals received therapy for an indication besides CLL progression: 5 for immune complications (autoimmune hemolytic anemia, n=3; membranoproliferative glomerulonephritis, n=2) and 2 patients with diffuse large B-cell lymphoma. Therapy for autoimmune hemolytic anemia included steroids alone (n=2) and steroids with rituximab (n=1), resulting in complete response in one patient and partial response in 2 patients. Both individuals with glomerulonephritis received rituximab-based therapy and achieved a significant reduction in proteinuria. Two individuals treated for diffuse large B-cell lymphoma, at 17 and 34 months after MBL, received cyclophosphamide, doxorubicin, vincristine, prednisone and rituximab.
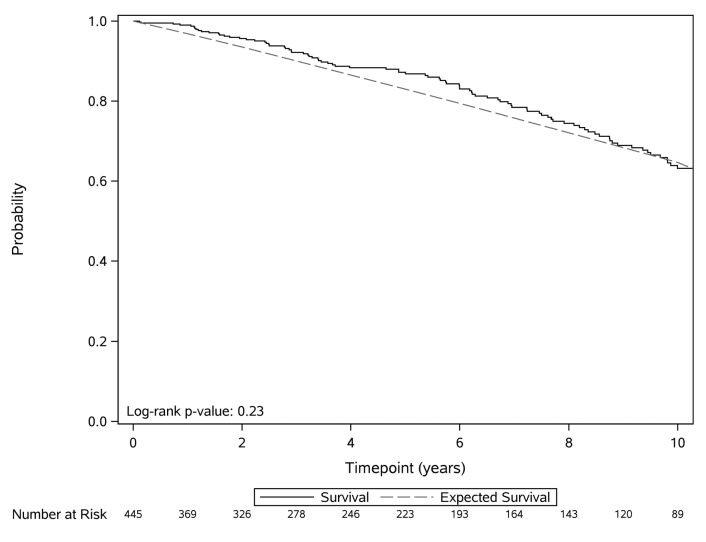
The median overall survival of the entire cohort was 11.7 years. Online Supplementary Table S2 shows factors associated with shorter time to first treatment and overall survival on multivariable analysis. After adjusting for age and sex, serum β2-microglobulin ≥3.5 μg/mL [Hazard Ratio (HR): 6.2, P<0.0001], CD38 ≥30% (HR: 3.5, P<0.0001), unmutated IGHV (HR: 3.1, P=0.003), and CD49d ≥30% (HR: 3.1, P=0.004) were independently associated with shorter time to first treatment. After adjusting for age and sex, serum β2-microglobulin ≥3.5 μg/mL (HR: 2.7, P=0.002), and unmutated IGHV (HR: 2.4, P=0.004), were independently associated with shorter overall survival. The absolute B-cell count and percent bone marrow infiltration by the B-cell clone were not predictive for time to first treatment or overall survival. The overall survival among individuals with MBL was similar to that of the age- and sex-matched general population of Minnesota (P=0.23) (Figure 2).
Figure 2.
Overall survival of individuals with clinically ascertained monoclonal B-cell lymphocytosis compared to the age- and sex-matched general population of Minnesota.
Multiple prior studies evaluating the natural history of MBL have estimated that the risk of progression to CLL requiring therapy is approximately 1–5%.7–13 Consequently, expert guidelines recommend annual visits for individuals with MBL, with particular focus on physical examination (to detect lymphadenopathy and organomegaly), and a complete blood count to detect worsening lymphocytosis or cytopenias.6 Results from our study confirm the previously reported rate of progression with long-term follow up, and extend our understanding of the reasons for treatment. Consistent with current knowledge, the most common reason for therapy in our study of high-count MBL individuals was progression to CLL. Investigators of the UK Haematological Malignancy Research Network reported that 6/353 (1.7%) individuals with MBL were treated with corticosteroids for an autoimmune complication.18 The findings were similar in our current study in which ~1% individuals with MBL were treated for an immune complication with steroids alone or in combination with anti-CD20 monoclonal antibody. We also observed that a small number of individuals were treated for renal manifestations of the malignant B-cell clone. This observation is similar to the recently described entity of monoclonal gammopathy of renal significance in patients with plasma cell disorders,19 indicating that treatment of the underlying B-cell clone may favorably affect organ function. Finally, a small proportion of individuals with MBL required therapy for a high-grade lymphoma diagnosed ~2 years after the ascertainment of MBL. Collectively, these findings reinforce our suggestion above that clinicians need to be cognizant of disease complications other than CLL progression in individuals with high-count MBL.20,21
Although it is not yet clinically indicated to perform prognostic testing in individuals with MBL, results from this study provide important data that biological markers associated with risk of progressive disease in CLL22–24 are also associated with risk of progression in MBL. Given that these markers were not available for ~30% of our patients and the limited number of events, we were unable to perform comprehensive modeling (e.g. calculating the CLL-International Prognostic Index)25 in this group of patients. Results from our study confirm prior findings that, as a group, the overall survival of individuals with MBL is similar to that of the age- and sex-matched general population.10,26 Multivariable analysis of factors associated with shorter overall survival includes unmutated IGHV genes and high serum β2-microglobulin, suggesting that the shorter survival may be related to the presence of the B-cell clone. Recent studies have also shown that individuals with MBL have a higher risk of serious infections and non-hematologic malignancies compared to the general population and similar to that of patients with CLL.20,21 Preliminary studies from our group have also identified specific immune defects in MBL, including increased exhausted T cells and impaired immunological T-cell synapse formation.27 Although cause of death could not be ascertained in this cohort, one can speculate that adverse biological factors related to the B-cell clone and/or a immune deficits could contribute to shorter survival in MBL.
Our study has several limitations. It is a single-center study and additional studies are needed to demonstrate that the results are generalizable. Individuals with MBL were included in this study based on physical examination findings;2 however, it is possible that some individuals with small lymphocytic lymphoma may have been included, potentially overestimating the risk of disease progression.
In summary, data from our cohort of individuals with clinically ascertained MBL demonstrate that the risk of requiring therapy is ~1.4% per year, after a median follow up of ~6.5 years. In addition to the risk of progression to CLL requiring therapy (7% of the overall cohort), immune complications (1% of the overall cohort) and high-grade lymphoma (0.4% of the overall cohort) are additional indications for therapy. These findings have important implications for counselling individuals with clinically ascertained high-count MBL.
Supplementary Material
Footnotes
Funding: SAP and SSK are recipients of the K12 CA090628 grant from the National Cancer Institute (Paul Calabresi Career Development Award for Clinical Oncology).
This work was supported in part by CA 197120 (NEK and TDS).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Landgren O, Albitar M, Ma W, et al. B-cell clones as early markers for chronic lymphocytic leukemia. N Engl J Med. 2009;360(7):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xochelli A, Kalpadakis C, Gardiner A, et al. Clonal B-cell lymphocytosis exhibiting immunophenotypic features consistent with a marginal-zone origin: is this a distinct entity? Blood. 2014;123(8):1199–1206. [DOI] [PubMed] [Google Scholar]
- 4.Ghia P, Prato G, Scielzo C, et al. Monoclonal CD5+ and CD5- B-lymphocyte expansions are frequent in the peripheral blood of the elderly. Blood. 2004;103(6):2337–2342. [DOI] [PubMed] [Google Scholar]
- 5.Parikh SA, Kay NE, Shanafelt TD. Monoclonal B-cell lymphocytosis: update on diagnosis, clinical outcome, and counseling. Clin Adv Hematol Oncol. 2013;11(11):720–729. [PubMed] [Google Scholar]
- 6.Shanafelt TD, Ghia P, Lanasa MC, Landgren O, Rawstron AC. Monoclonal B-cell lymphocytosis (MBL): biology, natural history and clinical management. Leukemia. 2010;24(3):512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rawstron AC, Bennett FL, O’Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med. 2008;359(6):575–583. [DOI] [PubMed] [Google Scholar]
- 8.Shanafelt TD, Kay NE, Rabe KG, et al. Brief report: natural history of individuals with clinically recognized monoclonal B-cell lymphocytosis compared with patients with Rai 0 chronic lymphocytic leukemia. J Clin Oncol. 2009;27(24):3959–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossi D, Sozzi E, Puma A, et al. The prognosis of clinical monoclonal B cell lymphocytosis differs from prognosis of Rai 0 chronic lymphocytic leukaemia and is recapitulated by biological risk factors. Br J Haematol. 2009;146(1):64–75. [DOI] [PubMed] [Google Scholar]
- 10.Molica S, Mauro FR, Giannarelli D, et al. Differentiating chronic lymphocytic leukemia from monoclonal B-lymphocytosis according to clinical outcome: on behalf of the GIMEMA chronic lymphoproliferative diseases working group. Haematologica. 2011;96(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fung SS, Hillier KL, Leger CS, et al. Clinical progression and outcome of patients with monoclonal B-cell lymphocytosis. Leuk Lymphoma. 2007;48(6):1087–1091. [DOI] [PubMed] [Google Scholar]
- 12.Kern W, Bacher U, Haferlach C, et al. Monoclonal B-cell lymphocytosis is closely related to chronic lymphocytic leukaemia and may be better classified as early-stage CLL. Br J Haematol. 2012;157(1):86–96. [DOI] [PubMed] [Google Scholar]
- 13.Xu W, Li JY, Wu YJ, et al. Clinical features and outcome of Chinese patients with monoclonal B-cell lymphocytosis. Leuk Res. 2009;33(12):1619–1622. [DOI] [PubMed] [Google Scholar]
- 14.Parikh SA, Leis JF, Chaffee KG, et al. Hypogammaglobulinemia in newly diagnosed chronic lymphocytic leukemia: natural history, clinical correlates, and outcomes. Cancer. 2015;121(17):2883–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parikh SA, Rabe KG, Call TG, et al. Diffuse large B-cell lymphoma (Richter syndrome) in patients with chronic lymphocytic leukaemia (CLL): a cohort study of newly diagnosed patients. Br J Haematol. 2013;162(6):774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shanafelt TD, Geyer SM, Bone ND, et al. CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: a prognostic parameter with therapeutic potential. Br J Haematol. 2008;140(5):537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanafelt TD, Rabe KG, Kay NE, et al. Age at diagnosis and the utility of prognostic testing in patients with chronic lymphocytic leukemia. Cancer. 2010;116(20):4777–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawstron A, de Tute R, Burton C, Roman E, Hillmen P. Recommendations for monitoring of newly diagnosed patients with CLL and MBL: 10-year experience from the UK Haematological Malignancy Research Network (HMRN). XVI International Workshop on Chronic Lymphocytic Leukemia; 2015; Sydney, Australia: Leukemia and Lymphoma; 2015. p. 1–166. [Google Scholar]
- 19.Leung N, Bridoux F, Hutchison CA, et al. Monoclonal gammopathy of renal significance: when MGUS is no longer undetermined or insignificant. Blood. 2012;120(22):4292–4295. [DOI] [PubMed] [Google Scholar]
- 20.Moreira J, Rabe KG, Cerhan JR, et al. Infectious complications among individuals with clinical monoclonal B-cell lymphocytosis (MBL): a cohort study of newly diagnosed cases compared to controls. Leukemia. 2013;27(1):136–141. [DOI] [PubMed] [Google Scholar]
- 21.Solomon BM, Rabe KG, Moreira J, et al. Risk of cancer in patients with clinical monoclonal B-cell lymphocytosis (MBL): a cohort Study of newly diagnosed patients compared to controls. ASH Annual Meeting Abstracts. 2012;120(21):2893. [Google Scholar]
- 22.Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer. 1981;48(1):198–206. [DOI] [PubMed] [Google Scholar]
- 23.Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood. 1975;46(2):219–234.1139039 [Google Scholar]
- 24.Parikh SA, Shanafelt TD. Prognostic factors and risk stratification in chronic lymphocytic leukemia. Semin Oncol. 2016;43(2):233–240. [DOI] [PubMed] [Google Scholar]
- 25.An international prognostic index for patients with chronic lymphocytic leukaemia (CLL-IPI): a meta-analysis of individual patient data. Lancet Oncol. 2016;(6):779–790. [DOI] [PubMed] [Google Scholar]
- 26.Shanafelt TD, Kay NE, Rabe KG, et al. Survival of patients with clinically identified monoclonal B-cell lymphocytosis (MBL) relative to the age- and sex-matched general population. Leukemia. 2012;26(2):373–376. [DOI] [PubMed] [Google Scholar]
- 27.Parikh SA, Ramsay A, Boysen J, et al. Longitudinal Evaluation of T-cells in Clinical Monoclonal B-cell Lymphocytosis (MBL). 21st Annual Congress of European Haematology Association; 2016; Copenhagen, Denmark: Haematologica; 2016. Abstract P580. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.