Abstract
Although inhibitors of bromodomain and extra terminal domain (BET) proteins show promising clinical activity in different hematologic malignancies, a systematic analysis of the consequences of pharmacological BET inhibition on healthy hematopoietic (stem) cells is urgently needed. We found that JQ1 treatment decreases the numbers of pre-, immature and mature B cells while numbers of early pro-B cells remain constant. In addition, JQ1 treatment increases apoptosis in T cells, all together leading to reduced cellularity in thymus, bone marrow and spleen. Furthermore, JQ1 induces proliferation of long-term hematopoietic stem cells, thereby increasing stem cell numbers. Due to increased numbers, JQ1-treated hematopoietic stem cells engrafted better after stem cell transplantation and repopulated the hematopoietic system significantly faster after sublethal myeloablation. As quantity and functionality of hematopoietic stem cells determine the duration of life-threatening myelosuppression, BET inhibition might benefit patients in myelosuppressive conditions.
Introduction
The molecular mechanisms that govern hematopoietic stem cell (HSC) activity and lineage specification are increasingly well known and it has been demonstrated that abnormalities in pathways controlling these functions are a major cause of malignant transformation.1 Moreover, investigation of HSC biology has changed the view of cancer, and it is now believed that tumors are sustained by cells with a cancer stem cell phenotype, a highly malignant subpopulation which maintains the uncontrolled production of less malignant progeny.2 It is, therefore, essential to identify pathways that control key stem cell functions in order to better understand genes and mechanisms which are involved in transformation, tumor progression and relapse.
In recent years, increasing evidence has linked epigenetic dysregulation with aberrant gene expression ultimately leading to the development of cancer. It was shown that 50% of human cancers harbor mutations in enzymes that are important for proper chromatin organization.3 Members of the family of bromodomain and extra terminal domain (BET) proteins include BRD2, BRD3, BRD4 and BRDT which function as epigenetic readers and have been shown to facilitate transcription through interaction with acetylated histones.4 Chromatin binding of BET proteins is known to drive MYC expression5 which is an oncogenic driver in several hematologic malignancies such as acute myeloid leukemia (AML),6 lymphoma7 or multiple myeloma (MM).8
Consequently, epigenetic regulators have become attractive therapeutic targets and, despite their toxic and non-specific side effects, drugs interfering with epigenetic pathways have entered clinical practice.9
The BET inhibitor JQ1 binds with highest affinity to bromodomain 1 of BRD4 and prevents it binding to acetylated histones at promoters and linage specific enhancers thereby decreasing transcription of some lineage specific genes.10 At a therapeutic level, it exerts potent anti-cancer effects in a broad range of human AML subtypes,5 acute lymphoblastic leukemia (ALL),11 and MM,12 and the structurally similar BET inhibitor (OTX015) recently showed promising clinical results in these patients.13,14
Although it has recently been shown that JQ1 decreases pluripotency of embryonic stem cells by suppression of Nanog and Lefty1,15 the consequences of BET inhibition for adult HSC have not yet been analyzed in detail.
The fact that BET inhibitors are already used in clinical studies today underlines the need for a systematic approach to understand the impact of pharmacological BET inhibition on healthy hematopoiesis.
We, therefore, addressed this issue and show for the first time that BET inhibition by JQ1 induces expansion of HSC thereby promoting recovery of the hematopoietic system after myelosuppression or stem cell transplantation.
Methods
More detailed information can be found in the Online Supplementary Appendix.
Animals
All experiments were carried out in accordance with the institutional guidelines for the welfare of animals and were approved by the local licensing authority (Behörde für Soziales, Gesundheit, Familie, Verbraucherschutz; Amt für Gesundheit und Verbraucherschutz, Hamburg, Germany, project n. G128/16).
Treatments and mixed bone marrow chimeras
Ly5.1 animals received daily intraperitoneal (i.p.) injections with 50 mg/kg JQ1 or control for 21 days. To generate bone marrow (BM) chimeras, BM of treated Ly5.1 mice was mixed with 2×105 untreated Ly5.2 supporter BM cells and injected intravenously (i.v.) into lethally irradiated Ly5.2 mice (9 Gy). Transplantations were performed with total numbers of BM cells per condition and not corrected for equal numbers of HSC. Detailed information about the normalization in secondary recipients and the calculation of repopulating units can be found in the Online Supplementary Appendix.
Hematopoietic recovery after sublethal myeloablation
Mice received daily i.p. injections with 50 mg/kg JQ1 or control for 21 days. Myeloablation was then induced via sublethal irradiation (5 Gy). Hematopoietic recovery was monitored in peripheral blood (PB) and BM.
Colony formation assays
Colony formation assays for quantification of hematopoietic stem and progenitor cells (GF M3434, H4034 Optimum) or megakaryocytes (MegaCult-C) were performed according to the manufacturer’s instructions.
ELISA and RT-qPCR
Transcript levels were analyzed using Power SYBR Green RT-qPCR and protein concentrations were determined using ELISA according to the manufacturer’s instruction.
Flow cytometry
Different cell populations in thymus, PB, BM and spleen were stained with antibodies and analyzed on a FACS Canto II flow cytometer.
Statistical analysis
Data represent mean±Standard Error of Mean (SEM) of representative experiments, which were carried out at least in duplicates, unless otherwise stated. Statistical significance was calculated by Student’s t-test or ANOVA. Statistical significance in Kaplan-Meier plots was calculated by log-rank test.
Results
JQ1 treatment alters normal hematopoiesis in a cell context-dependent manner
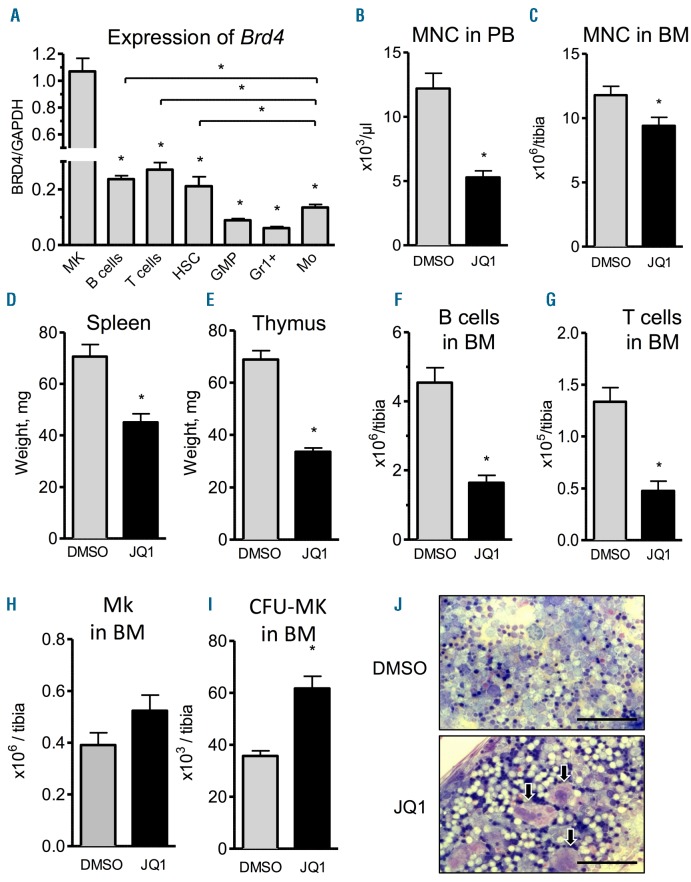
As a first step, we analyzed the mRNA expression patterns of Brd2-4 in all major hematopoietic subpopulations, because BET family members represent the main molecular targets of JQ1.5 Expression of Brd4 mRNA was most abundant in megakaryocytes (MK), followed by B cells, T cells and HSC (Figure 1A). Brd2 expression showed a similar distribution whereas Brd3 expression was highest in MK and T cells (Online Supplementary Figure S1A and B).
Figure 1.
JQ1 treatment affects normal hematopoiesis in cell context-dependent manner. (A) Sorted megakaryocytes (MK), hematopoietic stem cells (HSC), granulocyte-monocyte progenitors (GMP), Gr1+ cells (GR1+) and monocytes (Mo) from bone marrow (BM) of mice were analyzed for baseline RNA expression of Brd4 via qRT-PCR (n=2–3; *P<0.05). (B–J) Animals received daily intraperitoneal (i.p.) injections of 50 mg/kg JQ1 for 21 days after which the different parameters were analyzed. (B) Mononucleated cells in peripheral blood (n=8; *P<0.05). (C) Mononucleated cells in BM (n=8; *P<0.05). (D and E) Spleen (D) and thymus (E) weight (n=7–8; *P<0.05). (F and G) B-cell (F) and T-cell (G) counts in BM (n=8; *P<0.05). (H) Flowcytometric quantification of MK in BM (n=4; *P<0.05). (I) Colony formation assay from BM to quantify megakaryocytic progenitors (n=5–7; *P<0.05). (J) Representative images showing increased numbers of MK (arrows) upon JQ1 treatment in BM. Scale bars represent 100 μm.
To characterize the effect of BET inhibition by JQ1 on hematopoietic development in vivo, we analyzed 7-week old mice which were treated with 50 mg/kg JQ1 for three weeks, a dose level previously used in literature.5,16
As reported previously, JQ1 treatment resulted in delayed growth of mice (Online Supplementary Figure S2A). Moreover, JQ1-treated mice were leukopenic (Figure 1B) and showed reduced numbers of mononucleated cells (MNC) in the BM (Figure 1C). In contrast, the numbers of erythrocytes and platelets were not affected by JQ1 (Online Supplementary Figure S2B and C). Spleen and thymus weight were reduced upon treatment, indicating a JQ1-induced reduction of immune cells in secondary lymphoid organs in agreement with the literature16 (Figure 1D and E). Indeed, flow-cytometric analysis of lymphoid and myeloid cell populations showed that absolute cell numbers of both B220+ B cells and CD3+ T cells were lower after treatment (Figure 1F and G). In contrast, absolute cell numbers of myeloid cells remained constant (Online Supplementary Figure S2D) indicating a more lymphoid-specific and cell context-dependent role of BET inhibition by JQ1.
It has been shown that JQ1-mediated effects occur due to downregulation of c-Myb, which is known to be a potent negative modulator of megakaryopoiesis.17 As Brd4 is also highly expressed in MK (Figure 1A), we examined their prevalence in the BM and found numerically increased absolute numbers of CD41+CD61+ MK with enhanced colony forming unit-megakaryocyte (CFU-MK) upon JQ1 treatment (Figure 1H and I). Wright’s-Giemsa staining of BM and splenocytes confirmed the presence of more MK upon JQ1 treatment (Figure 1J). Thus, JQ1 treatment induces quantitative and phenotypic changes within cell populations showing the highest level of Brd4 expression.
JQ1 blocks B-cell maturation and induces T-cell apoptosis
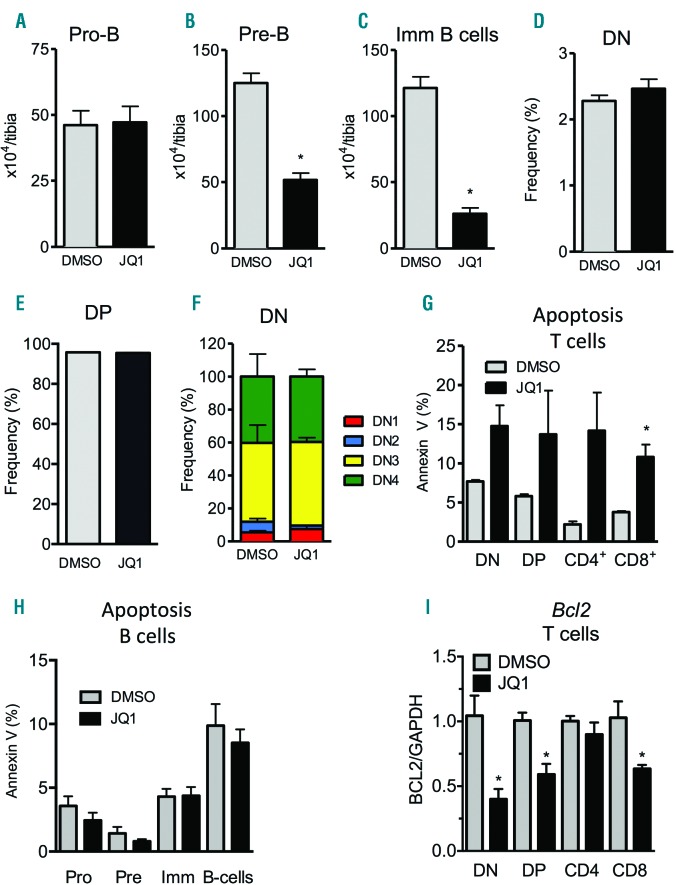
Since BET inhibition by JQ1 reduced the numbers of mature B cells, we next analyzed the different stages of B-cell development in the BM and spleen. The earliest pro-B-cell progenitors (Lin-B220+CD43+IgM−) were present in normal numbers after treatment (Figure 2A). However, numbers of late pre-B (Lin−B220+CD43−IgM−) and immature B cells (Lin−B220+IgM+) were reduced by 2- and 5-fold, respectively (Figure 2B and C).
Figure 2.
JQ1 induces T-cell apoptosis and inhibits B-cell maturation. Animals received daily intraperitoneal (i.p.) injections of 50 mg/kg JQ1 for 21 days after which the different parameters were analyzed. (A–C) Flowcytometric quantification of Pro-B (A), Pre-B (B) and immature B cells to analyze B-cell development in bone marrow (BM) (n=4; *P<0.05). (D–F) Flowcytometric quantification of CD4−CD8− double negative (D), CD4+CD8+ double positive (E) and all differentiation states of CD4−CD8− double negative T cells (DN1-4) in thymus (n=4; *P<0.05). (G) Flowcytometric quantification of apoptosis in CD4−CD8− double negative, CD4+CD8+ double positive and CD4/CD8 single positive T cells using Annexin V (n=3; *P<0.05). (H) Flowcytometric quantification of apoptosis in pro-B, pre-B, immature-B (Imm) and mature B cells using Annexin V (n=4; *P<0.05). (I) FACS-sorted T-cell subpopulations from thymus of placebo or JQ1-treated mice were analyzed for mRNA expression via qRT-PCR (n=5; *P<0.05).
Moreover, JQ1-mediated BET inhibition caused depletion of absolute numbers of T cells (Figure 1G). Analysis of T-cell development in the thymus showed that, despite the reduction of T-cell numbers in the BM (Figure 1G), there were no developmental differences in double negative (DN) or double positive (DP) thymocyte subpopulations (Figure 2D and E). Within the developmental stages of DN thymocytes, JQ1 did not influence the proportions of DN1, DN2, DN3 and DN4 progenitor subpopulations (Figure 2F). Notably, all differentiation stages of T-cell development showed increased apoptosis rates, whereas apoptosis of B-cell subsets was unchanged (Figure 2G and H).
On a transcriptional level, JQ1 treatment decreased the expression of anti-apoptotic mediator Bcl2 in the majority of sorted T-cell subsets (Figure 2I). In contrast, treatment did not affect the expression of pro-apoptotic Bak1 in these cell types (Online Supplementary Figure S1E). Further analyses revealed no changes in differential expression of other BH3 genes important for apoptosis during particular steps of the development of DN (Mcl1, Bcl2a1a, Bcl2l11), DP (Mcl1, Bclxl, Bcl2l11) or single positive CD4+ or CD8+ (Bclxl, Mcl1, Bcl2l11) T cells18–21 (Online Supplementary Figure S1F–I). This suggests that JQ1 induces apoptosis in T cells by reducing Bcl2 expression.
JQ1 treatment results in expansion and mobilization of HSC
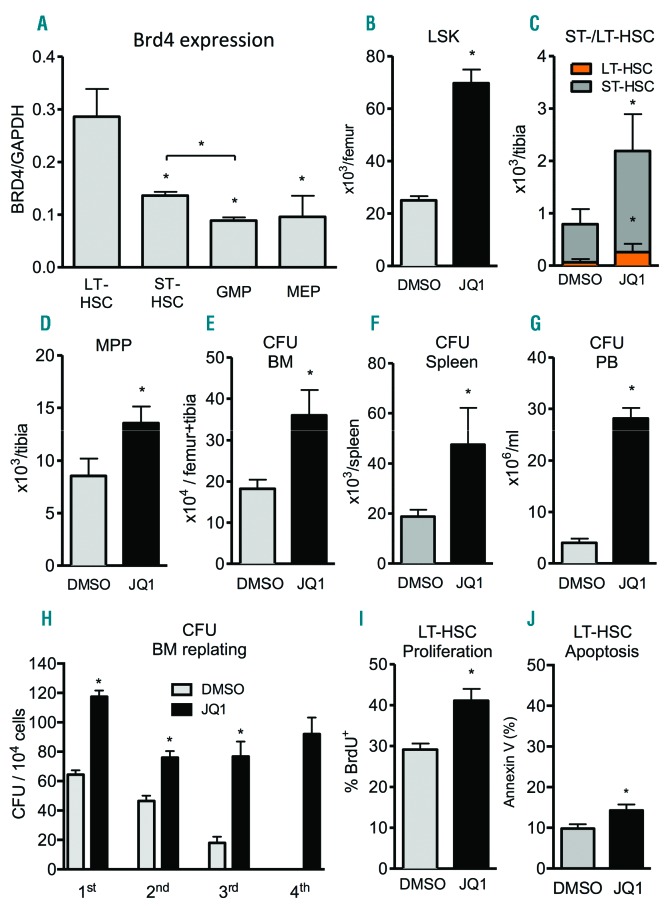
Prompted by the high expression of BET family members in HSCs, we further dissected their expression pattern in different stages during HSC differentiation, such as LT-HSC, ST-HSC, GMP and MEP (Figure 3A). Interestingly, only Brd4 expression was highest in LT-HSC and this decreased with further differentiation via GMP to MEP, whereas no differential expression of Brd2 and 3 was detectable (Online Supplementary Figure S1C and D). Therefore, we examined the impact of JQ1 treatment on hematopoietic stem and progenitor cells (HSPC) in vivo.
Figure 3.
JQ1 treatment results in expansion and mobilization of hematopoietic stem cells (HSC). Animals received daily intraperitoneal (i.p.) injections of 50 mg/kg JQ1 for 21 days after which the different parameters were analyzed. (A) Different FACS-sorted hematopoietic stem and progenitor cells from bone marrow (BM) of mice were analyzed for baseline mRNA expression of Brd4 via qRT-PCR (n=2–3; *P<0.05). (B–D) Flowcytometric quantification of phenotypic lineage-ckit+sca1+ cells (LSK) (B), ST-HSC and LT-HSC (C), as well as MPP (D) in BM (n=5–9; *P<0.05). (E–G) Colony formation assays to quantify hematopoietic progenitors in BM (E), spleen (F), and peripheral blood (G) (n=3–8; *P<0.05). (H) Replating of CFU from BM (n=3; *P<0.05). (I) Flowcytometric quantification of BrdU incorporation in LT-HSC in BM (n=5; *P<0.05). (J) Flowcytometric quantification of apoptosis in LT-HSC in BM using Annexin V (n=5; *P<0.05).
Despite reduced cellularity in BM, the absolute numbers of phenotypic lineage-ckit+sca1+ cells (LSK) were increased 3-fold upon JQ1 treatment (Figure 3B). To rule out the possibility that JQ1 treatment only affects developing hematopoiesis in young mice, we validated the JQ1-induced increase in LSK cells in adult mice (Online Supplementary Figure S2I). Interestingly, an increased cell count was only present in LT-HSC (CD34−CD135−LSK), ST-HSC (CD34+CD135−LSK) and MPP (CD34+CD135+ LSK) (Figure 3C and D), but not in more differentiated lineages such as GMP (lin−sca1−ckit+CD34+FcγRII+) or MEP (lin−sca1−ckit+ CD34−FcγRII−) (Online Supplementary Figure S2E and F).
To assess the functionality of hematopoietic progenitors, we evaluated the colony forming capacity of JQ1-treated MNC from BM and spleen in vitro. We found a 2-to 3-fold increase in total numbers of colony forming units (CFU) in BM and spleen in mice that had received JQ1 treatment (Figure 3E and F). Moreover, JQ1 treatment mobilized early hematopoietic progenitors into the PB, where we could detect a 2.5-fold increase in CFU per mL of blood (Figure 3G).
To further characterize their proliferation and ability to preserve stemness, we serially replated the CFU multiple times (Figure 3H). Intriguingly, and in contrast to control, JQ1-treated BM gave rise to constant CFU numbers after each replating, whereas CFU counts decreased rapidly in the control group. This indicates that, in addition to increasing proliferation, JQ1 treatment helps maintain the regenerative potential of hematopoietic progenitors, indicating a preservation of stemness.
To investigate potential species-to-species variations, we treated purified human CD34+ cells from BM with JQ1 in vitro and assed the colony-forming capacity via colony formation assays. Interestingly, JQ1 induced an increase in CFU numbers when compared to placebo treatment (Online Supplementary Figure S2J), indicating that JQ1 has a similar effect on both murine and human HSC.
To determine whether the accumulation of HSC results from increased proliferation or reduced differentiation, we analyzed BrdU incorporation into HSC in vivo. We found that LT-HSC from JQ1-treated mice incorporated more BrdU than those from control-treated littermates (Figure 3I), suggesting a JQ1-induced increase in HSC-cycling. Interestingly, more differentiated ST-HSC or the pool of LSK cells did not show increased cell cycle activity, indicating specificity of the JQ1-induced effect in LT-HSC (Online Supplementary Figure S2G and H). In addition, HSC proliferation was accompanied by a marginal increase in apoptosis of HSC (Figure 3J). These data strongly suggest that JQ1 promotes HSC proliferation, ultimately leading to an increase in the size of the HSC pool.
JQ1 treatment increases hematopoietic reconstitution after HSC transplantation
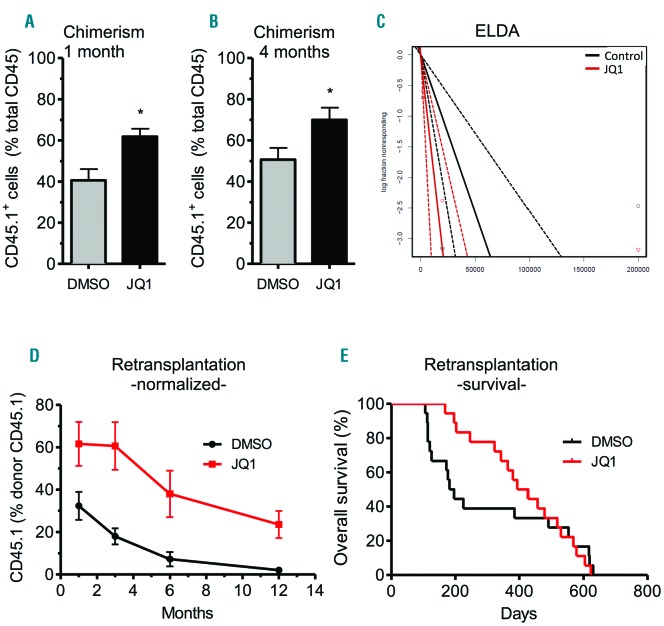
To measure HSC numbers and multipotency at a functional level, we carried out limiting dilution competitive repopulation transplantations. Therefore, we treated CD45.1 mice for three weeks with JQ1 or control before sacrifice. Subsequently, a mix of supporter BM from CD45.2 mice and JQ1- or control-treated BM from CD45.1 mice was transplanted into lethally irradiated CD45.2 recipients. Engraftment of HSC was evaluated by analyzing recipient PB contribution over four months.
Consistent with our in vitro studies, mice transplanted with JQ1-treated BM had higher levels of donor-derived chimerism during the whole observation period, indicating an increased fraction of HSPC in the transplants (Figure 4A and B). Relative calculation of repopulating units (RU) revealed 2.5-fold more HSC in transplants when donors received JQ1 treatment [RUD (DMSO) = 2.15; RUD (JQ1) = 4.9]. In addition, extreme limiting dilution analysis (ELDA) showed a 3-fold increase of HSC frequency in BM upon JQ1 treatment (DMSO: 1/19480 vs. JQ1: 1/6284; n=8–12; *P<0.05) (Figure 4C). Importantly, JQ1 and DMSO-treated HSC reconstituted all major hematopoietic lineages with similar frequencies (Online Supplementary Figure S3A–C). We, therefore, conclude that JQ1 induces HSC pool expansion in donors without induction of lineage priming after transplantation.
Figure 4.
JQ1 treatment increases hematopoietic repopulation after hematopoietic stem cell (HSC) transplantation. Limiting dilution competitive repopulation transplantations were performed by transplanting bone marrow (BM) from CD45.1+ JQ1- or control-treated mice together with supporter BM from CD45.2+ mice into lethally irradiated CD45.2+ recipients. (A and B) Analysis of chimerism after competitive repopulation (n=12; *P<0.05). (C) Extreme limiting dilution analysis (ELDA) for determining the frequency of HSC in transplants (n=8–12; *P<0.05). (D and E) Monitoring of chimerism (D) and survival (E) after retransplantation of the BM of primary recipients into the second generation of mice and over the course of 12 months.
After six months, primary recipients were sacrificed and their BM was transplanted into secondary recipients. As it is well known that HSC engraftment is less effective in secondary recipients, we normalized the measured frequencies of CD45.1+ cells in secondary recipients (Online Supplementary Figure S4) to the known frequencies of their respective donor animals; this allowed us to analyze potential changes of HSC contribution to hematopoiesis independent of their numbers (Figure 4D). In line with our observations following primary transplantation, JQ1-treated HSC also contributed more to hematopoiesis in secondary recipients, indicating a sustained long-term effect of JQ1 on HSC. The contribution of DMSO-treated HSC to hematopoiesis decreased in secondary recipients over the course of 12 months in agreement with literature.22 Interestingly, the decline was slower upon transplantation of JQ1-treated HSC, and after 12 months they still significantly contributed to hematopoiesis while any contribution of DMSO-treated HSC to the host hematopoiesis was no longer detectable (Figure 4D). Therefore, the relative contribution of JQ1 over DMSO-treated HSC to hematopoiesis increased over time, indicating a sustained long-term effect of JQ1 on HSC (1 month: 1.9-fold, 3 months: 3.4-fold, 6 months: 5.2-fold, 12 months: 11.8-fold). Furthermore, the JQ1-induced increase in HSC proliferation did not translate into enhanced exhaustion of HSC, as there was no significant difference in long-term survival of secondary recipients from both groups (Figure 4E).
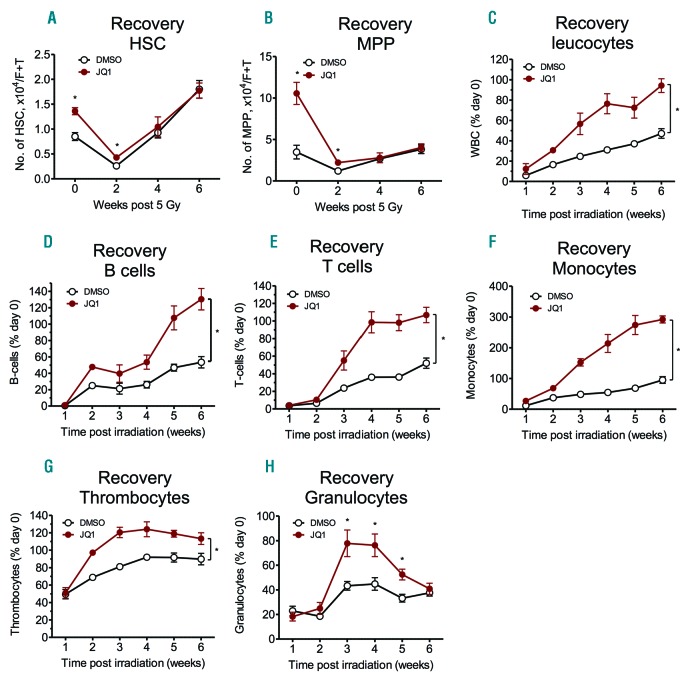
JQ1 treatment accelerates blood recovery after sublethal irradiation
As JQ1 treatment increases HSC numbers by inducing proliferation, we asked whether the pro-stem-cell phenotype elicited by JQ1 might translate into increased hematopoietic recovery after sublethal irradiation, or if this beneficial effect is compromised by increased radiosensitivity of HSC due to increased cell cycle activity.
To verify this, mice were treated with JQ1 for three weeks before they received sublethal irradiation and hematopoietic recovery was monitored. As we found that JQ1 also mediates HSC expansion in 11-week old mice that have been treated with JQ1 for three weeks (Online Supplementary Figure S2I), we used adult mice for these experiments.
Analysis of the BM of irradiated mice revealed a strong decrease in hematopoietic stem and progenitor cells within two weeks after irradiation (Figure 5A and B).
Figure 5.
JQ1 treatment accelerates blood recovery after myeloablation. Animals were injected daily with 50 mg/kg JQ1 intraperitoneal (i.p.) for 21 days before receiving sublethal whole body irradiation with 5 Gy. (A and B) Flowcytometric quantification of hematopoietic stem cells (HSC) (A) and MPP (B) over the course of six weeks after myelosuppression (n=3–5; *P<0.05). (C–H) Analysis of the recovery of leukocytes (C), B cells (D), T cells (E), monocytes (F), thrombocytes (G) and granulocytes (H) after myelosuppression over the course of six weeks (n=3–5; *P<0.05).
Before irradiation, BM of JQ1-treated mice contained 1.6-fold higher numbers of HSC when compared to placebo-treated mice. Interestingly, irradiation led to a similar reduction in HSC in both groups two weeks after treatment, indicated by an unchanged HSC ratio of 1.6-fold (Figure 5A). Similar observations were made for MPP (Figure 5B). This indicates that, despite increasing cell cycle activity, JQ1 does not sensitize HSC to irradiation at a dose level of 5 Gy.
In response to irradiation, cell counts of all major hematopoietic lineages decreased by 75–99% in both groups, with B and T cells being most affected one week after irradiation (Online Supplementary Figure S5A). Interestingly, and in line with the increased numbers of HSC and MPP, JQ1-treated mice showed a significantly faster recovery of all lineages when compared to animals before irradiation.
Within the whole post-irradiation period, the relative recovery of leukocytes was significantly faster in JQ1-treated mice than in the control cohort (Figure 5C). Six weeks after irradiation, total leukocyte counts of JQ1-treated mice fully recovered to levels before irradiation (94±7%; n=5; *P<0.05), whereas recovery was slower in the control group (47±5%; n=5; *P<0.05). Strikingly, recovery of most hematopoietic lineages such as B cells, T cells, monocytes, thrombocytes or granulocytes was accelerated when animals received JQ1 treatment before irradiation (Figure 5D–H). In contrast, the recovery of erythrocytes was delayed upon JQ1 treatment; however, levels never dropped to critical values and fully recovered after irradiation (Online Supplementary Figure S5B).
Although JQ1 induces cycling of HSC, this mild increase does not seem to be sufficient to render HSC more susceptible to irradiation with 5 Gy. Still harboring increased numbers of HSC two weeks after irradiation, JQ1-pre-treated animals recover faster from sublethal myelosuppression than their placebo-treated littermates.
Discussion
Despite the emerging clinical importance of BET inhibitors, a systematic study examining their effects in the adult hematopoietic compartment is urgently needed. We have been able to demonstrate high expression of Brd4 in MK, B cells, T cells and HSC, while it was lower in GMP, monocytes and granulocytes. The expression of Brd2 showed a similar distribution, whereas Brd3 expression was highest in MK and T cells.
In contrast to data with BET inhibitors analyzed in clinical studies, JQ1 treatment of mice did not lead to thrombocytopenia, which might be indicative of species-to-species variations. Interestingly, this comparison is impaired by the fact that JQ1 has never been used in clinical studies due to its short half-life in plasma.23 On the other hand, the different effects on thrombopoiesis seen with JQ1 and other BET inhibitors could also be due to minor structural differences between JQ1 and clinical compounds or the drug administration methods.
Further experiments are warranted in order to determine the reasons for the different effects on thrombopoiesis exerted by JQ1 in mice and by other BET inhibitors in humans.
In agreement with previous literature, JQ1 treatment led to reduced cellularity in peripheral blood and BM due to reduced numbers of B and T cells.16 We could refine these analyzes showing that JQ1 treatment only led to a reduction of pre-, immature and mature B-cell numbers; however, no decrease in pro-B cells was observed. This might indicate a differentiation block occurring at the pro-B to pre-B transition, which would be in line with the high expression of BET proteins starting at the pro-B-cell level. Despite this hypothesis, we cannot rule out the possibility that JQ1 specifically targets all B-cell subsets except for pro-B cells, thereby reducing their numbers.
Furthermore, JQ1 treatment increased apoptosis in all analyzed T- but not B-cell subsets. We have been able to show that JQ1 decreases mRNA expression levels of the anti-apoptotic BH3 protein BCL2 in the majority of T-cell subsets, whereas no changes in the expression of proapoptotic genes could be detected. However, future studies are needed to understand whether this differential regulation of BCL2 mRNA is directly mediated by the effect of JQ1 on known targets including MYC and MYB or by other mechanisms. The finding of reduced numbers of B cells at the pre-B-cell level while pro-B cells are similar upon JQ1 treatment suggests there is a differentiation block, but this needs further clarification.
Although JQ1 induced numerical expansion of megakaryocytes in the BM, other myeloid cells, platelets and RBC remained constant upon treatment. Thus, JQ1 treatment induces profound cell type-specific changes in hematopoietic cell populations displaying high mRNA expression of BET proteins. As JQ1 targets multiple proteins of the BET family, the individual contribution of specific molecular targets to the observed phenotype has to be identified in future studies.
JQ1 induces LT-HSC proliferation, thereby leading to an expansion of LT-HSC, ST-HSC and MPP in BM and spleen. Our data further indicate that JQ1 induces HSC mobilization. At the functional level, JQ1 treatment increased the quantity of HSC leading to better engraftment and faster recovery of the hematopoietic system without inducing HSC exhaustion. It is worthy of note that we did not challenge the secondary recipients of JQ1-or control-treated BM with myelosuppressive stimuli, but only measured their lifespan as a read-out for HSC exhaustion. It might, therefore, be conceivable that transient myeloablation of secondary recipients would reveal potential differences in hematopoietic recovery between both groups. Such experiments are especially important as we found marginal induction of apoptosis in HSC upon JQ1 treatment. Therefore, this aspect should be analyzed in future studies.
Interestingly, recent reports have shown that MYB plays an important role in hematopoiesis by recruiting BRD4, which is functionally suppressed by BET inhibitors such as JQ1.24 In line with this, the MYB polymorphism M303V disrupts the interaction of MYB with its transcriptional coactivator p300, thereby inducing megakaryocytosis, lymphopenia, as well as a pronounced increase in HSC.25 The striking phenotypic similarities of reduced MYB transactivation and JQ1 treatment suggest that the results reported in this study could result from JQ1-mediated suppression of MYB. Furthermore, and in line with our observations, impaired function of MYB was reported to increase cycling of LT-HSC.25 In any case, additional studies are warranted to verify whether JQ1 induces its effects on hematopoiesis via MYB.
With respect to BRD4 inhibition, a previous study demonstrated that short-term reconstitution of LSK cells after lethal irradiation is impaired when the transplanted hematopoietic stem and progenitor cells harbor an shRNA-mediated knockdown of Brd4 (Brd4 KD).26 The reduced repopulative capacity of Brd4 KD HSCP at the LSK level initially seems to contradict our findings. One obvious reason for this discrepancy might be that the shRNA strategy only focuses on the inhibition of Brd4, whereas JQ1 targets the entire BET family. In addition, BRD4 contains other protein domains such as an extra-terminal domain, the function of which is not influenced by JQ1.27
Although the effect of JQ1 described in our study most likely results from the increased number of HSC, other data link it to increased HSC fitness. In colony formation assays, the increased CFU numbers arise from the expanded pool of stem and progenitor cells. However, their increased regenerative potential seen in replating assays may also result from increased HSC fitness. Similarly, the increased contribution of JQ1-treated HSC to hematopoiesis in secondary recipients results from the increased HSC numbers in the graft. However, the decline of the relative contribution to hematopoiesis was slower in the animals that received JQ1-treated HSC, which might indicate enhanced fitness. As in our study it is difficult to discriminate between the effects of JQ1 on HSC quantity and quality, future experiments transplanting equal numbers of JQ1- or control-treated HSC are warranted.
It is worthy of note that the supporter BM cells from the first transplantation outcompeted the DMSO-treated HSC in secondary recipients starting at 12 months after transplantation. Although it is well known that the fitness of HSC dramatically decreases in secondary recipients,22 we also observed an unusually reduced median survival, particularly in the DMSO arm, although this effect was not statistically significant; this has to be considered when interpreting the data. Therefore, future studies are needed to identify the underlying factors for this effect.
Although allogeneic or autologous HSCT is an important option for the treatment of leukemia, severe autoimmunity or myelosuppression,28 due to different limitations, not all patients benefit. It has been shown that the quantity and quality of HSCs are critical factors determining patient survival and time required for engraftment after HSCT.29
Between 2–5% of stem cell donors do not adequately respond to G-CSF treatment and the number of CD34+ HSC mobilized is not sufficient; these donors are called ‘poor mobilizers’.30–33 As a donor’s response to G-CSF varies,34 and HSCT programs have to, therefore, deal with these obvious uncertainties, alternative approaches are needed to obtain sufficient numbers of HSC for transplantation. We were able to show that, besides HSC proliferation, BET inhibition by JQ1 also increases the mobilization of HSC into the PB. Our data, therefore, indicate that BET inhibition might represent an alternative approach to yield increased numbers of HSC. However, future studies are necessary to verify whether this approach is safe or applicable for the G-CSF-independent mobilization of stem cells in humans.
In this context, it should be considered that JQ1 treatment has opposing effects on some mature hematopoietic cell populations and HSC. This leads to the question as to whether the reduction of B and T cells might compromise the beneficial effects on HSC during repopulation of the hematopoietic system. In cases in which the detrimental effect on B and T cells persists even after discontinuation of BET inhibitor treatment in a transplantation setting, this would make HSCT recipients more prone to infections, thereby counteracting the beneficial effects of increased HSC numbers in the grafts. Therefore, further experiments are warranted to clarify the consequences of (transient) BET inhibition on B and T cells and the outcome of HSCT.
Supplementary Material
Acknowledgments
The authors would like to thank the FACS Core Facility (UKE, Hamburg, Germany) for helping with flow cytometry.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/103/6/939
Funding
SL was supported by the German Research Council (DFG LO1863/3-1), by the Margarethe Clemens Stiftung, by a Starting Grant of the European Research Council and is the recipient of a Heisenberg professorhip (DFG, LO1863/4-1); MWr was supported by the Medical Faculty of the University of Hamburg (FFM program); RB is an Erwin-Schrödinger fellow of the Austrian Science Fund (FWF); SK is grateful for support by the Structural Genomics Consortium (SGC); MWa is supported by the Research Training Group Translational Research Innovation - Pharma (TRIP), supported by the Else Kröner-Fresenius Foundation (EKFS). SAJ is supported by the German Research Council (DFG, JO 815/3-2) and the German Cancer Aid-funded PiPAC consortium (70112505). JQ and LW are supported by the NIH/NCI P01 CA066996.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: an evolving concept. Nat Rev Cancer. 2012;12(2):133–143. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Issa JP, Baylin S. Targeting the cancer epigenome for therapy. Nat Rev Genet. 2016;17(10):630–641. [DOI] [PubMed] [Google Scholar]
- 4.Wu SY, Chiang CM. The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J Biol Chem. 2007;282(18):13141–13145. [DOI] [PubMed] [Google Scholar]
- 5.Zuber J, Shi J, Wang E, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukaemia. Nature. 2011;478(7370):524–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvatori B, Iosue I, Djodji Damas N, et al. Critical Role of c-Myc in Acute Myeloid Leukemia Involving Direct Regulation of miR-26a and Histone Methyltransferase EZH2. Genes Cancer. 2011;2(5):585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Hematology Am Soc Hematol Educ Program. 2013;2013:575–583. [DOI] [PubMed] [Google Scholar]
- 8.Holien T, Vatsveen TK, Hella H, Waage A, Sundan A. Addiction to c-MYC in multiple myeloma. Blood. 2012;120(12):2450–2453. [DOI] [PubMed] [Google Scholar]
- 9.Wagner JM, Hackanson B, Lubbert M, Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics. 2010;1(3–4):117–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippakopoulos P, Qi J, Picaud S, et al. Selective inhibition of BET bromodomains. Nature. 2010;468(7327):1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Da Costa D, Agathanggelou A, Perry T, et al. BET inhibition as a single or combined therapeutic approach in primary paediatric B-precursor acute lymphoblastic leukaemia. Blood Cancer J. 2013;3:e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delmore JE, Issa GC, Lemieux ME, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146(6):904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amorim S, Stathis A, Gleeson M, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3(4):e196–204. [DOI] [PubMed] [Google Scholar]
- 14.Berthon C, Raffoux E, Thomas X, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016;3(4):e186–195. [DOI] [PubMed] [Google Scholar]
- 15.Horne GA, Stewart HJ, Dickson J, et al. Nanog requires BRD4 to maintain murine embryonic stem cell pluripotency and is suppressed by bromodomain inhibitor JQ1 together with Lefty1. Stem Cells Dev. 2015;24(7):879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DU, Katavolos P, Palanisamy G, et al. Nonselective inhibition of the epigenetic transcriptional regulator BET induces marked lymphoid and hematopoietic toxicity in mice. Toxicol Appl Pharmacol. 2016;300:47–54. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi E, Bulgarelli J, Ruberti S, et al. MYB controls erythroid versus megakaryocyte lineage fate decision through the miR-486-3p-mediated downregulation of MAF. Cell Death Differ. 2015;22(12):1906–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opferman JT, Letai A, Beard C, et al. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426(6967):671–676. [DOI] [PubMed] [Google Scholar]
- 19.Tripathi P, Koss B, Opferman JT, Hildeman DA. Mcl-1 antagonizes Bax/Bak to promote effector CD4(+) and CD8(+) T-cell responses. Cell Death Differ. 2013; 20(8):998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma A, Pena JC, Chang B, et al. Bclx regulates the survival of double-positive thymocytes. Proc Natl Acad Sci USA. 1995; 92(11):4763–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Q, Sader A, Parkman JC, Baldwin TA. Bim-mediated apoptosis is not necessary for thymic negative selection to ubiquitous self-antigens. J Immunol. 2009; 183(12):7761–7767. [DOI] [PubMed] [Google Scholar]
- 22.Carnevalli LS, Scognamiglio R, Cabezas-Wallscheid N, et al. Improved HSC reconstitution and protection from inflammatory stress and chemotherapy in mice lacking granzyme B. J Exp Med. 2014;211(5):769–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trabucco SE, Gerstein RM, Evens AM, et al. Inhibition of bromodomain proteins for the treatment of human diffuse large B-cell lymphoma. Clin Cancer Res. 2015; 21(1):113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roe JS, Mercan F, Rivera K, Pappin DJ, Vakoc CR. BET Bromodomain Inhibition Suppresses the Function of Hematopoietic Transcription Factors in Acute Myeloid Leukemia. Mol Cell. 2015;58(6):1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandberg ML, Sutton SE, Pletcher MT, et al. c-Myb and p300 regulate hematopoietic stem cell proliferation and differentiation. Dev Cell. 2005;8(2):153–166. [DOI] [PubMed] [Google Scholar]
- 26.Bolden JE, Tasdemir N, Dow LE, et al. Inducible in vivo silencing of Brd4 identifies potential toxicities of sustained BET protein inhibition. Cell Rep. 2014;8(6):1919–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahman S, Sowa ME, Ottinger M, et al. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol Cell Biol. 2011;31(13):2641–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamadani M, Craig M, Awan FT, Devine SM. How we approach patient evaluation for hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45(8):1259–1268. [DOI] [PubMed] [Google Scholar]
- 29.Ogonek J, Kralj Juric M, Ghimire S, et al. Immune Reconstitution after Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2016;7:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ings SJ, Balsa C, Leverett D, et al. Peripheral blood stem cell yield in 400 normal donors mobilised with granulocyte colony-stimulating factor (G-CSF): impact of age, sex, donor weight and type of G-CSF used. Br J Haematol. 2006;134(5):517–525. [DOI] [PubMed] [Google Scholar]
- 31.Anderlini P, Rizzo JD, Nugent ML, et al. Peripheral blood stem cell donation: an analysis from the International Bone Marrow Transplant Registry (IBMTR) and European Group for Blood and Marrow Transplant (EBMT) databases. Bone Marrow Transplant. 2001;27(7):689–692. [DOI] [PubMed] [Google Scholar]
- 32.Anderlini P, Champlin RE. Biologic and molecular effects of granulocyte colony-stimulating factor in healthy individuals: recent findings and current challenges. Blood. 2008;111(4):1767–1772. [DOI] [PubMed] [Google Scholar]
- 33.Anderlini P, Donato M, Chan KW, et al. Allogeneic blood progenitor cell collection in normal donors after mobilization with filgrastim: the M.D. Anderson Cancer Center experience. Transfusion. 1999; 39(6):555–560. [DOI] [PubMed] [Google Scholar]
- 34.Holig K. G-CSF in Healthy Allogeneic Stem Cell Donors. Transfus Med Hemother. 2013;40(4):225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.