Telomeres are nucleoprotein structures composed of long Deoxyribonucleic Acid (DNA) sequence repeats (TTAGGG) located at the ends of chromosomes which function, in conjunction with telomere-associated proteins, to prevent inappropriate activation of DNA damage signaling pathways and hence to maintain genomic stability.1 Telomeres are therefore unique markers of cellular replicative capacity, cellular senescence, and ageing. Moreover, telomere erosion may be accelerated with chronic inflammation and hyperproliferative pressure within the bone marrow niche. Previous reports from several groups have highlighted the presence of Telomere Length (TL) shortening in Myeloproliferative Neoplasms (MPNs) and, in one study, up regulated telomerase activity in isolated CD34+ cells, when compared to healthy donors.2–5
Shortened TL in Essential thrombocythemia (ET) has previously been reported to be associated with an increased incidence of thrombotic or hemorrhagic events, treatment failure and disease progression to Myelofibrosis (Post ET-MF), Myelodysplastic syndromes (MDS) or increased mortality; however, robust clinicopathological and outcome correlations in studies have been heterogeneous.2,4,5 Moreover, the effect of MPN-directed therapies on TL has been unclear. We therefore sought to determine the presence or absence of TL shortening in a large, well-defined cohort of patients with ET (n=100), and correlate TL with mutational status, current and previous treatment modalities, white blood cell counts and platelet count and occurrence of thromboembolic events. We demonstrate that TL is significantly shorter in individuals with ET compared to age and gender matched controls (AGMC) and occurred irrespective of driver mutation status (JAK2V617F, CALR, MPL and the so-called ‘Triple Negative’ (TN)). Moreover, current or prior exposure to the cytoreductive agent hydroxycarbamide (HC) was associated with significant TL shortening compared to patients with no prior exposure HC. There was no significant correlation between either the total leucocyte or platelet count and TL.
The demographic characteristics of enrolled subjects are shown in Table 1 and TL analyses were age-adjusted and compared with AGMC. Patient TL was measured using a monochrome multiplex quantitative Polymerase Chain Reaction (PCR) method.6 This approach compares the amount of PCR product generated from the telomere (T) to a single-copy gene (S) to obtain a T/S ratio, proportional to the TL. In brief, 900nM of telomere-specific primers and 900nM of primers specific to human albumin were used. Per sample, 5–10ng of genomic DNA was utilised (extracted from peripheral blood mononuclear cells) and all samples were analysed in triplicate. Reactions were performed on a Corbett Light Cycler 480 real-time thermal cycler and data analyzed using Rotorgene 6000 software. Standard curves for telomere and human albumin amplification were established by titration of a reference DNA sample. T/S ratios of samples were determined from these curves. Patient samples were then compared to 357 AGMC. Data analysis was performed using Prism GraphPad™ software.
Table 1.
Patient demographics, disease characteristics and treatment.
There were a total of 100 patients with a median age of 49 years (range: 20–86 years) and included 30 males and 70 females. Regarding mutational status, 34% had JAK2V617F, 27% CALR, 2% MPL and 37% were deemed “TN”. The median duration of disease was 9 years (<1 to 26 years). As regards therapy, treatments are summarised in Table 1. Of note, over half of this cohort had been previously exposed to or were receiving therapy with HC.
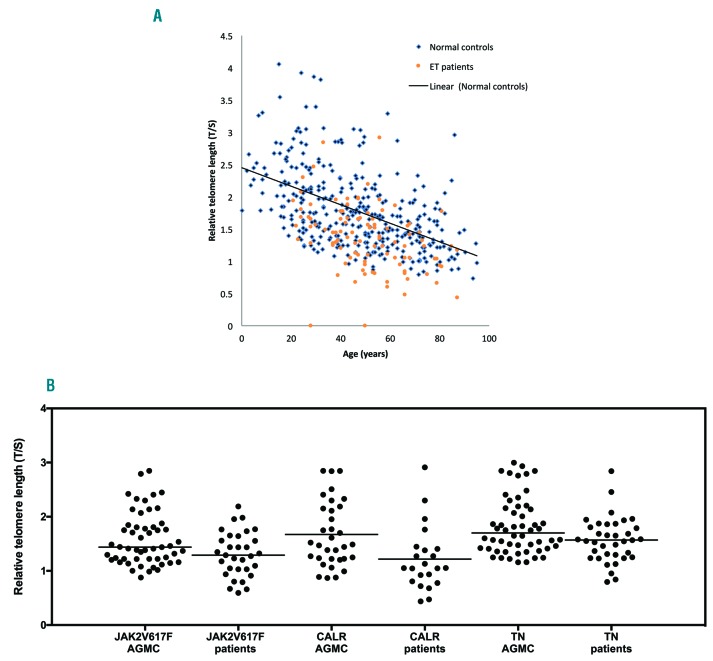
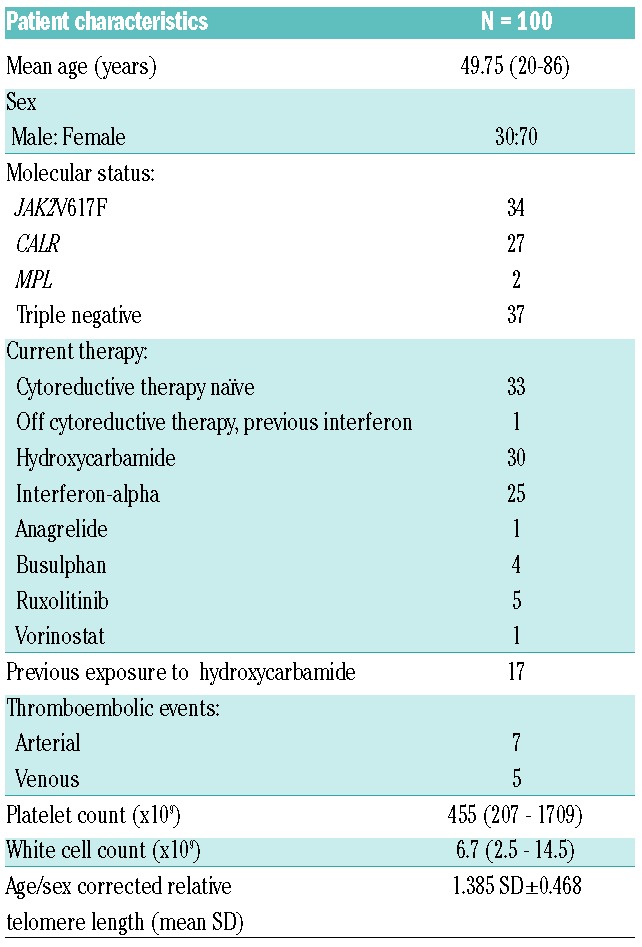
Telomere lengths were significantly shorter (33/100 (33%), P=<0.0001) in patients with ET compared AGMC (Figure 1a). Moreover, shortened TL were present regardless of mutational status when compared to AGMC: CALR (17/33) P=0.0009; JAK2V617F (11/33) P=0.007 and (5/33) P=0.012 in TN patients (Figure 1b). We note with interest that of the 18/100 patients whose TL was below the first centile, indicative of considerable telomere attrition, 55% (10/18) were CALR positive vs. 28% (5/18) JAK2V617F positive vs. only 17% (3/18) of those patients who were TN. We next evaluated the impact of cytoreductive therapy on TL and identified that independent of mutational status, there was TL shortening in treated ET patients 82% (27/33) vs. 18% (6/33) untreated patients (P=0.05) overall, indicating that introduction of therapy may well drive further the propensity for TL shortening. In particular, those ET patients with either current, or prior HC treatment had significantly shortened TL, P=0.0015 (48%, 13/27) and P=<0.0001, respectively (52%, 14/27). Interestingly, treatment with interferon-alpha was more commonly associated with less significant TL shortening except in those patients who had been previously exposed to HC (83% (10/12) normal TL vs. 17% (2/12) shortened TL) (P=<0.0001).
Figure 1.
Distribution of telomere length in patients with essential thrombocythemia and the impact of mutational status. (A) Telomere length is shorter in patients with essential thrombocythaemia when compared with age and gender matched controls (P≤0.0001). B: Telomere length is significantly shorter in patients with essential thrombolecythaemia regardless of mutational status. CALR: calreticulin; TN: triple negative; AGMC: age and gender matched controls.
In our cohort, gender did not have a significant correlation with TL, which is in contrast to previous studies that have described lower TL in male patients compared to their female counterparts.3,4 Furthermore, we could not demonstrate statistically significant difference in the rates of either venous or arterial thrombosis in those with shortened TL (58% (7/12)) versus those ET patients with normal TL (42% (5/12)). On risk stratification, TL was significantly shortened in both low and high-risk ET, P =<0.0005 and P = <0.0001, respectively. Disease duration did not impact on TL, as as it was significantly shortened in patients who have had the disease < or > 10 years (P =0.0029 and P =0.0005, respectively). On further analysis, we interestingly note shortened TLs in 83% (20/24) and 73% (22/30) of patients with disease duration of less than 10 years or greater than 10 years, respectively, who were exposed to HC therapy.
Two patients in the cohort transformed to post ET-myelofibrosis: patient A (27-year-old female with TN ET, disease duration <10 years), whose TL was 1.686, greater than the 10th centile, and patient B (73-year-old female with JAK2V617F positive ET, disease duration >10 years), whose TL was 0.792, below the first centile. Additionally, although both patients were on interferon at the time of analysis, patient B had been exposed to HC previously.
Heterogeneous results have arisen from previous studies of TL and telomerase activity across the MPN spectrum. A key strength of our study is the large number of well-defined ET patients included, but a limitation is that as a cross sectional study, akin to all previously published studies of TL evaluation in MPN, it permits inferences of association only. We have demonstrated significant shortening of TL in ET when compared to appropriately AGMC irrespective of mutational status. This is in keeping with the work of Spanoudakis et al.2 who suggested that TL shortening was a global phenomenon in MPN patients and occurred irrespective of JAK2V617F mutational status, however we additionally demonstrated therapeutic mediated effects on TL. Our data is in contrast to that of Elena et al., whereby in a cohort of MPN patients, no significant correlation between the use or duration of cytoreductive therapy and TL shortening was demonstrated.4 Hence, for the first time, we demonstrate that current or previous HC exposure is independently associated with significant TL shortening in ET when compared to either no therapy or interferon-alpha treatment. Similar findings have been identified in sickle cell disease cohorts who have been exposed to HC.7 Moreover, in the 18 patients with the shortest telomeres, defined as a TL <1st centile, there appeared to be an enrichment of CALR-mutated cases. Although this needs to be evaluated in a larger cohort of CALR-mutated ET patients, it suggests that perhaps the proliferative drive of the MPN clone may differ between JAK2 and CALR mutated and ‘TN’ ET cases. Dahlstrom et al. have previously demonstrated dysregulation of shelterin complex factors in MPN with elevated levels of the shelterin components (Protection of telomeres-1(POT1)) in ET, MF and Polycythemia Vera (PV) and Telomere Regulatory Factor 1-interacting protein 2 (PV and MF), both of which have a negative regulatory role on TL, although, of note, this cohort only comprised 24 ET patients.5 In this study, TL was inversely proportional to JAK2V617F allelic burden and POT1 expression tended to correlate with JAK2V617F allele burden. The TL shortening seen in MPN may at least in part be due to these regulatory components. It will be important to comprehensively evaluate the effect of JAK inhibitor therapy on TL in ET and other MPN, as this group previously demonstrated that v inhibitors suppressed POT1 expression in JAK2V617F harboring cells. It is also of current interest that Baerlocher8 and colleagues reported the use of telomerase inhibitor imetelstat in patients with ET, a 13-mer-thio-phosphoramidate oligonucleotide complimentary to hTR, inhibiting proliferation of megakaryocytic colonies with improvement in hematological parameters8 but no consistent effect on TL. Further updates on the use of telomerase inhibitors in MPN are awaited.
In conclusion, we have demonstrated that, when compared to AGMC, ET patients have shortened TL. This was markedly pronounced in those with CALR and JAK2V617F mutations but also present in TN disease. Consistent with previously published data, patients exposed to HC had significantly shortened TL, we demonstrate that interferon-alpha treatment was associated with more normal TL. Limitations notably include the small sample size and absence of telomerase activity quantification. However, in the era of targeted therapy and with the availability of telomerase inhibitors, there is increasing interest in TL as a marker of disease and response; furthermore, we suggest this may be an additional diagnostic tool in the TN population.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Smogorzewska A, de Lange T. Regulation of telomerase by telomeric proteins. Annu Rev Biochem. 2004;73:177–208. [DOI] [PubMed] [Google Scholar]
- 2.Spanoudakis E, Bazdiara I, Pantelidou D, et al. Dynamics of telomere’s length and telomerase activity in Philadelphia chromosome negative myeloproliferative neoplasms. Leuk Res. 2011;35(4):459–464. [DOI] [PubMed] [Google Scholar]
- 3.Gardner M, Bann D, Wiley L, et al. Gender and telomere length: systematic review and meta-analysis. Exp Gerontol. 2014;51:15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elena C, Rumi E, Portolan M, Della Porta MG, Pascutto C, Passamonti F. Flow-FISH evaluation of telomere length in Philadelphia-negative myeloproliferative neoplasms. Haematologica. 2011;96(8):1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlstrom J, Zhang X, Ghaderi M, Hultcrantz M, Bjorkholm M, Xu D. Dysregulation of shelterin factors coupled with telomere shortening in Philadelphia chromosome negative myeloproliferative neoplasms. Haematologica. 2015;100(10):e402–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37(3):e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drasar ER, Jiang J, Gardner K, Howard J, et al. Leucocyte telomere length in patients with sickle cell disease. Br J Haematol. 2014;165(5):725–727. [DOI] [PubMed] [Google Scholar]
- 8.Baerlocher GM, Burington B, Snyder DS. Telomerase inhibitor imetelstat in essential thrombocythemia and myelofibrosis. N Engl J Med. 2015;373(26):2580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.