Platelets play a critical role in prevention of bleeding at sites of inflammation independent of “classical” hemostasis. For instance, in mouse models of immune complex-mediated skin inflammation and glomerulonephritis, early protective recruitment of platelets to the inflamed vasculature occurs without visual evidence of platelet aggregate formation as assessed by intravital microscopy or immunohistological analysis.1,2 Furthermore, neither GPIb nor integrin αIIbβ3 are required for prevention of bleeding in the inflamed skin.1,3
A role for GPVI and CLEC-2 in prevention of bleeding following cutaneous reverse passive Arthus reaction (rpA) and LPS-induced lung inflammation has been reported following adoptive transfer of receptor-deficient platelets into hIL4R/GPIbα transgenic (hIL4R/GPIbα-tg) mice rendered thrombocytopenic with an antibody to hIL4R.4 Using GPVI-deficient mice, the role of GPVI in limiting bleeding in the cutaneous rpA model has been confirmed in mice with normal platelet count, and was shown to involve repairing of neutrophil-inflicted vascular injury.5 The role of CLEC-2 in limiting bleeding in mice with a normal platelet count has not been investigated.
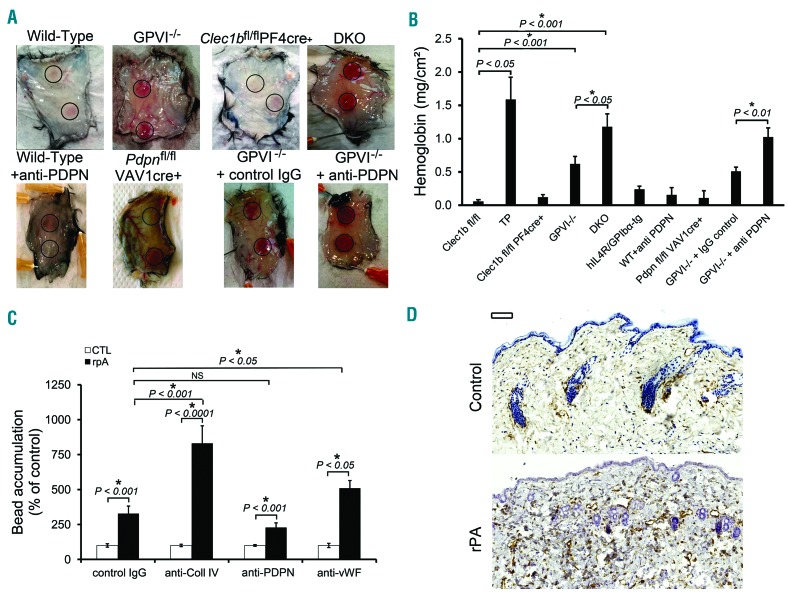
In order to address the contribution of platelet receptors to inflammation-mediated bleeding, we used cutaneous rpA and lung inflammation models. We compared the contribution of GPVI and targeted deletion of CLEC-2 and podoplanin to prevention of bleeding in the cutaneous rpA model. Mice with a deficiency in CLEC-2 on platelets or in its ligand podoplanin on all hematopoietic cells did not bleed (Figure 1A and B). This is in contrast to the petechial bleeding observed in GPVI-deficient mice (Figure 1A) which is mediated by repair of neutrophil-induced vascular injury.4,5 Absence of bleeding in the CLEC-2-deficient mice was not due to altered neutrophil recruitment as this was comparable to that in GPVI-deficient mice (Online Supplementary Figure S1).
Figure 1.
Platelet GPVI supports a first-line repair mechanism of inflammatory hemostasis in the inflamed skin with back up from the CLEC-2/podoplanin axis. Mice were subjected to cutaneous reverse passive Arthus reaction (rpA) and skin biopsies were collected after 4 hours of reaction. (A) Representative images of the rpA-challenged skin of wild-type (WT), GPVI deficient (GPVI−/−), CLEC-2 deficient (Clec1bfl/flPF4cre+) mice, and mice deficient in both receptors (DKO), WT mice treated with a blocking antibody to podoplanin (mAb 8.1.1 100 μg/mouse, intravenous; anti-PDPN), mice with a deficiency of podoplanin in hematopoietic cells (Pdpnfl/flVAV1cre+), or GPVI−/− mice treated with a control or blocking antibody to podoplanin. (B) Hemoglobin content in inflamed and control skin biopsies. Platelet-depletion of WT mice served as a positive control. Induction of the rpA, led to significantly increased hemoglobin levels in GPVI−/− and DKO but not in platelet CLEC2-deficient mice. N=6. (C) Fluorescent microspheres (1 μm-diameter) coated with control IgG or with antibodies to collagen (Coll IV), podoplanin (PDPN), or von Willebrand factor (VWF) were injected intravenously to mice 30 minutes after eliciting rpA. Accumulation at the site of rpA was quantified by flow cytometry analysis of digested skin biopsies. N=10 mice per group. Results are expressed as percentage of bead accumulation measured in unchallenged control skin biopsies. NS: not significant. (D) Representative images of unchallenged control and rpA-challenged skin biopsies from WT mice stained for podoplanin (brown) and hematoxylin (blue). Scale bar=100 μm.
Mice lacking the extracellular domain of GPIbα did not bleed following cutaneous rpA (Figure 1B), confirming previous observations that prevention of bleeding in this model is independent of the GPIbα/von Willebrand factor (VWF) axis.6 Nevertheless, bleeding in GPVI−/− mice was less severe than in thrombocytopenic mice, indicating involvement of one or more additional platelet receptors. Bleeding in mice deficient in GPVI and in platelet CLEC-2 (DKO) approached that of thrombocytopenic mice, and was similar to that in GPVI−/− mice treated with an anti-podoplanin blocking antibody (Figure 1A and B). These results show a potential role for CLEC-2/podoplanin axis in maintaining vascular integrity in the inflamed skin, only in the absence of GPVI.
The functional hierarchy between GPVI and CLEC-2 suggested possible differences in the exposure and/or localization of their respective ligands during cutaneous rpA. To investigate this, we compared the recruitment of microspheres (1 μm-diameter) coated with antibodies to podoplanin, collagen, VWF antibody or an irrelevant IgG to the reaction site (Figure 1C). Microspheres coated with antibodies to collagen or VWF but not to podoplanin accumulated significantly more than control microspheres. These results indicate that basement membrane components but not podoplanin become exposed and accessible to the circulation during cutaneous rpA. Concordantly, immunostaining shows that podoplanin is up-regulated in the extravascular space in the skin on macrophages and other stromal cells following rpA (Figure 1D and Online Supplementary Figure S2A–C). It appears that, in the absence of GPVI, engagement of platelet CLEC-2 by these podoplanin-positive cells limits bleeding in the inflamed skin. Supporting this hypothesis, platelets interacting with podoplanin-expressing cells were observed in the inflamed skin of GPVI−/− mice (Online Supplementary Figure S2D).
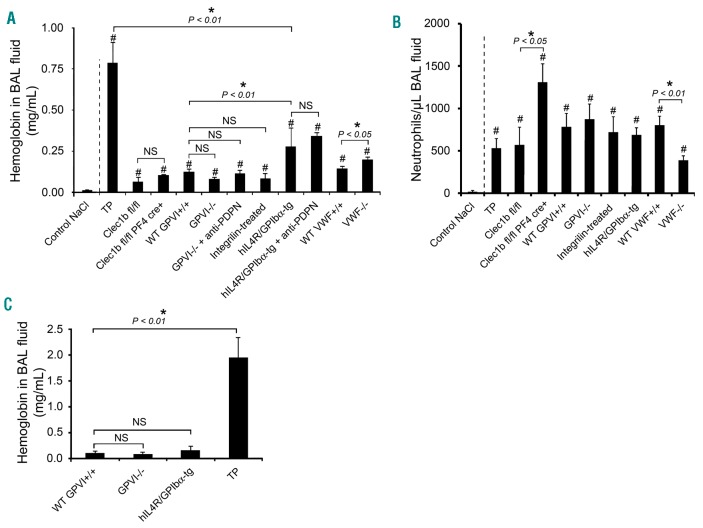
As previously shown,1 immunodepletion of platelets leads to pulmonary bleeding following LPS inhalation-induced lung inflammation (Figure 2A) and this is through neutrophil-mediated bleeding.7 GPVI and platelet CLEC-2 have been shown to play a critical role in this model by adoptive transfer into the hIL4R/GPIbα-tg transgenic thrombocytopenic mouse model.4 In contrast, however, bleeding was not observed following LPS challenge in mice deficient in GPVI or CLEC-2 (Figure 2A) even though neutrophil recruitment developed normally in GPVI−/− mice and was increased in platelet CLEC-2-deficient mice (Figure 2B), as previously shown.8 Bleeding was also absent in mice treated with the blocking anti-podoplanin mAb 8.1.1 or the αIIbβ3 antagonist, integrilin (Figure 2A). These data show that GPVI, CLEC-2 and integrin αIIbβ3 are not required for prevention of bleeding in LPS-inflamed lungs.
Figure 2.
Contribution of GPVI and CLEC-2 to inflammatory hemostasis in the inflamed lungs. (A) Hemoglobin levels in bronchoalveolar lavage (BAL) fluid collected after an overnight incubation following P. aeruginosa lipopolysaccharide (LPS) intranasal instillation in platelet-depleted (TP), wild-type (WT GPVI+/+ and WT VWF+/+), GPVI deficient (GPVI−/−), CLEC-2 deficient (Clec1bfl/flPF4cre+), and mice lacking the extracellular domain of GPIb (hIL4R/GPIbα-tg), Integrilin-treated WT mice, and GPVI−/− and hIL4R/GPIb-tg mice treated with a blocking antibody to podoplanin (mAb 8.1.1 100 μg/mouse, intravenous; anti-PDPN). Control NaCl corresponds to hemoglobin levels measured in BAL fluid from control mice instilled with NaCl. N=8–21 mice per group. #Significant difference (P<0.05) as compared to control NaCl. (B) Neutrophil count in BAL fluid collected after LPS-induced lung inflammation. N=8–21 mice per group. #Significant difference (P<0.05) as compared to control NaCl. (C) Hemoglobin levels measured in BAL collected after lung rpA. N=7–9 mice.
The observation of moderate bleeding in hIL4R/GPIbα-tg mice in the absence of immunodepletion (Figure 2A) indicates a role for GPIb-IX-V in maintenance of vascular integrity in the inflamed lung. Interestingly, bleeding in mice deficient in the GPIb-IX-V ligand, VWF, was even milder than in hIL4R/GPIbα-tg mice (Figure 2A), thus suggesting that the protective function of GPIb-IX-V in LPS-inflamed lungs does not solely depend on its interaction with VWF. A similar observation has been made for comparison of the roles of GPIb-IX-V and VWF in models of arterial thrombosis.9 In contrast to hIL4R/GPIbα-tg mice, however, VWF−/− mice were characterized by a reduction in neutrophil infiltration in LPS-inflamed lungs (Figure 2B) and this could account for the reduction in bleeding. Consistent with this, the role of VWF in mediating neutrophil recruitment in the cutaneous rpA model has been shown to be independent of GPIb-IX-V.6 Interestingly, the limited bleeding of hIL4R/GPIbα-tg mice following LPS inhalation compared to thrombocytopenic mice suggests the involvement of additional platelet receptors. Treatment of hIL4R/GPIbα-tg mice with an antibody to podoplanin, however, did not modify their bleeding phenotype, suggesting that the podoplanin-CLEC-2 axis does not provide a back-up mechanism.
The findings in this study show a difference in platelet receptor hierarchy in inflammatory hemostasis between the cutaneous rpA and LPS-induced lung inflammation models. To address whether this difference is model or organ dependent, we compared hIL4R/GPIbα-tg and GPVI−/− mice challenged with the rpA reaction in lung. Whereas pulmonary bleeding was observed following rpA in lung in thrombocytopenic mice, there was no bleeding in hIL4R/GPIbα-tg or GPVI−/− mice, in contrast to the results for rpA in skin (Figure 2C). This demonstrates that prevention of inflammatory bleeding in the rpA model is organ and stimulus dependent.
In conclusion, our results show that the role of GPVI, CLEC-2 and GPIb-IX-V in maintenance of inflammatory hemostasis is organ and stimulus dependent, demonstrating an unexpected complexity in the regulation of vascular integrity independent of classical hemostasis.
Supplementary Material
Footnotes
Funding: this work was supported by grants from the British Heart Foundation (CH/03/003 and RG/13/18/30563), La Société Française d’Hématologie, Force Hémato, La Fondation Arc (PJA 20151203107), and L’INCA (RPT16002MMA).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Goerge T, Ho-Tin-Noe B, Carbo C, et al. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111(10):4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirahashi J, Hishikawa K, Kaname S, et al. Mac-1 (CD11b/CD18) links inflammation and thrombosis after glomerular injury. Circulation. 2009;120(13):1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deppermann C, Kraft P, Volz J, et al. Platelet secretion is crucial to prevent bleeding in the ischemic brain but not in the inflamed skin or lung in mice. Blood. 2017;129(12):1702–1706. [DOI] [PubMed] [Google Scholar]
- 4.Boulaftali Y, Hess PR, Getz TM, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013; 123(2):908–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gros A, Syvannarath V, Lamrani L, et al. Single platelets seal neutrophil-induced vascular breaches via GPVI during immune-complex-mediated inflammation in mice. Blood. 2015;126(8):1017–1026. [DOI] [PubMed] [Google Scholar]
- 6.Hillgruber C, Steingraber AK, Poppelmann B, et al. Blocking von Willebrand factor for treatment of cutaneous inflammation. J Invest Dermatol. 2014;134(1):77–86. [DOI] [PubMed] [Google Scholar]
- 7.Hillgruber C, Poppelmann B, Weishaupt C, et al. Blocking neutrophil diapedesis prevents hemorrhage during thrombocytopenia. J Exp Med. 2015;212(8):1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lax S, Rayes J, Wichaiyo S, et al. Platelet CLEC-2 protects against lung injury via effects of its ligand podoplanin on inflammatory alveolar macrophages in the mouse. Am J Physiol Lung Cell Mol Physiol. 2017;313(6):1016–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmeier W, Piffath CL, Goerge T, et al. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc Natl Acad Sci USA. 2006;103(45):16900–16905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.