Abstract
Purpose
To study a novel and fast optical coherence tomography (OCT) device for home-based monitoring in age-related macular degeneration (AMD) in a small sample yielding sparse OCT (spOCT) data and to compare the device to a commercially available reference device.
Methods
In this prospective study, both eyes of 31 participants with AMD were included. The subjects underwent scanning with an OCT prototype and a spectral-domain OCT to compare the accuracy of the central retinal thickness (CRT) measurements.
Results
Sixty-two eyes in 31 participants (21 females and 10 males) were included. The mean age was 79.6 years (age range, 69–92 years). The mean difference in the CRT measurements between the devices was 4.52 μm (SD ± 20.0 μm; range, −65.6 to 41.5 μm). The inter- and intrarater reliability coefficients of the OCT prototype were both >0.95. The laser power delivered was <0.54 mW for spOCT and <1.4 mW for SDOCT. No adverse events were reported, and the visual acuity before and after the measurements was stable.
Conclusion
This study demonstrated the safety and feasibility of this home-based OCT monitoring under real-life conditions, and it provided evidence for the potential clinical benefit of the device.
Translational Relevance
The newly developed spOCT is a valid and readily available retina scanner. It could be applied as a portable self-measuring OCT system. Its use may facilitate the sustainable monitoring of chronic retinal diseases by providing easily accessible and continuous retinal monitoring.
Keywords: age-related macular degeneration, monitoring, optical coherence tomography, retina
Introduction
Optical coherence tomography (OCT) is a noninvasive imaging technique used to capture cross-sectional images of the retina.1,2 The OCT retina-mapping software analyzes both qualitative and quantitative changes, based on eye conditions that may be diagnosed or monitored longitudinally, including age-related macular degeneration (AMD),3–5 retinal vein occlusion,6–9 and diabetic retinopathy.10–12
These three pathologies often cause gradual vision loss and blindness.13,14 Therefore, it is important to detect pathologic changes in the retina early to allow for timely treatment, which results in better outcomes9 and reduction in the significant long-term costs of health care systems.15–17 Because frequent medical visits impose a great burden of treatment on patients and clinics, a novel, more compact prototype of a self-measuring OCT device was developed and designed for OCT scanning in elderly patients. The downscaled proportions of the device allowed for its portability and reduced size. Thus, the sparse OCT (spOCT) pattern enabled very fast scanning, and it is potentially suitable for use at home or outside the clinical setting.18
We propose a novel home-based retina monitoring that could be very useful for seamless monitoring and OCT data communication in a wide range of retinal diseases beyond AMD—such as myopic choroidal neovascularization, diabetic macular edema, retinal vein occlusion, and chronic central serous chorioretinopathy—in short, in every eye disease that is associated with the accumulation of pathologic fluid in the retina or beneath.
In this article we report, we believe for the first time, the performance of this portable spOCT system and compare it with a standard desktop OCT with regard to feasibility, scan quality, and the patient's comfort and safety.
Methods
This study was designed as a prospective, open-label, nonrandomized investigation to assess the prototype of a novel OCT retina scanner intended for the self-measuring and monitoring of retinal pathologies. Approval for the study was obtained from the local ethics committee (KEK-ZH-Nr: 2015-0316) and Swissmedic (ID no. 2015-MD-0015). Written informed consent was obtained in advance from each participant in this study, which adhered to the tenets of the Declaration of Helsinki.
Investigational Device
The investigational medical device (IMD) consisted of a spectral-domain OCT prototype (marked as MIMO_02), which was developed at the ARTORG Center (Bern, Switzerland) and the Institute for Human Centered Engineering OptoLab at the University of Applied Sciences Engineering and Information Technology (Biel/Berne, Switzerland). A superluminescent diode-generated light (SLED) with a 52.5-nm full-width half-maximum spectral bandwidth at a center wavelength of 841 nm was guided into a fiber coupler and divided into sample and reference arms by a 50/50 beam splitter. It was possible to operate the system in different densities on data samples (Fig. 1). To illustrate the high imaging capabilities of the new OCT device, a single line scan pattern with parameters comparable to the reference device was performed on the same healthy eye (Fig. 2). In both OCT systems, the scan length was 5 mm. The IMD had a high sample resolution of 400 pixels with an A-scan rate of 15 kHz. The averaging of maximal 22 B-scans was possible at this time. The OCT (Spectralis; Heidelberg Engineering, Heidelberg, Germany) single line acquisition had a resolution of 768 pixels, with an average automatic real-time tracking of 25 B-scans, excluding the technique of enhanced depth imaging.
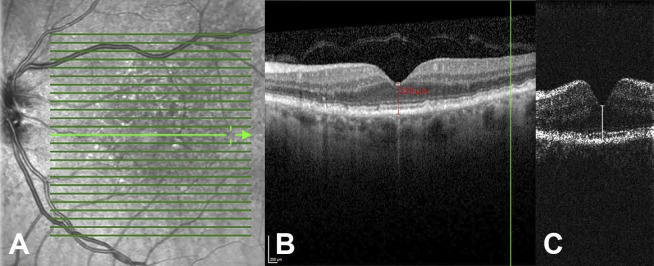
Figure 1.
Comparison of standard Heidelberg Spectralis desktop OCT to novel investigational spOCT. (A) Scanning laser ophthalmoscopy (SLO) of AMD. (B) Corresponding cross-section scan of the same eye as in (A) with illustration of manual central retinal thickness (CRT) measurement of standard Spectralis OCT (B) and spOCT (C). Note that in the spOCT system the scan resolution, thus the redundancy of image information, is reduced to allow rapid and repeated measurement of OCT volumes. However, despite the low sampling, CRT measurement was achieved in spOCT images comparable to the reference device.
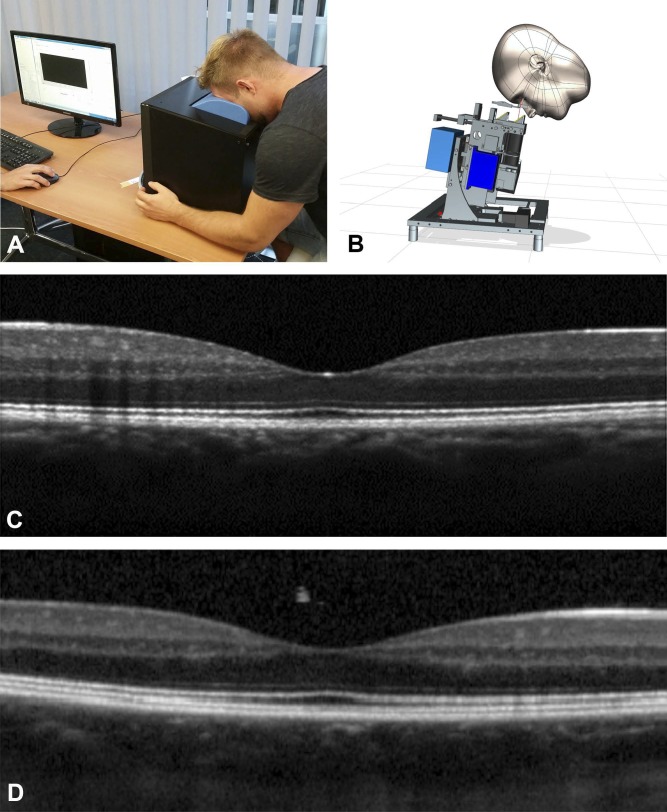
Figure 2.
Illustration and performance of the novel sparseOCT device (spOCT, MIMO_02) compared to standard Heidelberg Spectralis desktop OCT. (A) Illustration of the complete prototype spOCT (black box) shows compact design and novel headrest position to reduce moving artifacts. (B) The direction of the light beam can be adjusted to the position of the subject to provide a comfortable user experience. High sample recording of the single line scan of a healthy macula obtained by spOCT (C) compared to Heidelberg desktop OCT (D) of the same eye with comparable scan settings.
In addition, the IMD was able to operate the small sample acquisition method, spOCT. This novel volume scan protocol was established to investigate extremely fast volume scanning capabilities, generating an optical specimen of a cube 3.8 × 3.8 mm with resolutions of 50 × 50 pixels, 100 × 100 pixels, or 150 × 150 pixels; a scan depth of 4.2 mm; and a depth resolution of 2048 pixels. Because the A-scan rate of the prototype spOCT was tunable, it was adjusted to 15 kHz.
The ergonomics of the spOCT device with minimal operator interaction was enhanced and improved by inclining the subject's head in a novel position (Fig. 2A, 2B). The head was laid on a specific headrest. It was stabilized by the weight of the head itself to provide maximum comfort and reduce moving artifacts. Because the investigational scanner was a prototype, it did not feature all the lay-user operating interfaces. Therefore, during this study a qualified engineer operated the device in some of the examination procedures.
Intended Use
The performance of the newly developed spOCT device was compared with a standard OCT (SDOCT, Spectralis; Heidelberg Engineering, Heidelberg, Germany). The overall objective of this study was to obtain and assess the functionality and specific aspects of the spOCT prototype, such as diagnostic performance and patient experience during acquisition, which are necessary for its improvement. The primary objective of the study was to perform a comparative analysis of the measurements of the central retinal thickness (CRT) captured by the investigational and reference device. The secondary objective of the study was to collect data on the measurement duration and to collect and evaluate patient comfort during the examination with the spOCT.
Study Population
Patients with AMD visiting the Department of Ophthalmology at the University Hospital Zurich in Zurich, Switzerland, were prospectively recruited for the study. The inclusion criteria were as follows: German-speaking adults with and an established diagnosis of AMD. To reflect accurately real-world conditions, all stages of AMD, including geographic atrophy and fibrovascular scarring, were included. To challenge the new device, ocular comorbidities, such as any degree of cataract, dry eye syndrome, and glaucoma, were accepted for inclusion, which facilitated the identification of potential problems that future patients would have in using the device.
The exclusion criteria were as follows: other comorbidities, such as epilepsy, dementia, Parkinson's disease, serious mental health illness, developmental disability or cognitive impairment; any general disability that would preclude the patient's adequate comprehension of the informed consent; subjects who did not sign informed consent; and subjects who used electronic medical devices (e.g., hearing aid, cochlear implant, pacemaker, defibrillator, or infusion pump) in order to ensure the highest possible electromagnetic safety.
Sample Size Calculation
Considering the overall objective of the study (i.e., to collect data on the functionality of the spOCT to improve imaging performance and patient comfort), 30 participants were deemed sufficient to identify the major design deficiencies that would inform the subsequent developmental steps. Therefore, in this pilot study, the number of patients was based on the usability literature.19
Clinical Data Collection
All patients selected for the study were assigned to the same investigation procedure. To test the objectives of the clinical investigation, the performances of the spOCT and the commercial SDOCT scanner were compared. For this reason, the eyes of the study participants were scanned with both OCT systems, and the CRT values were then compared using a pairwise sample analysis. Additionally, the duration of the retinal measurement carried out by both devices was recorded. After the scanning procedure, the subjects were interviewed about their perceptions and comfort during the OCT.
Because the spOCT was not yet equipped with the fully automatic analysis software, the retinal thickness on the spOCT scans was measured manually by one grader using custom software written in MATLAB (MATLAB R 2017a; The MathWorks, Inc., Natick, MA).
The quantitative OCT analysis of the reference device was performed manually by a second, independent grader using the manufacturer's built-in caliber software tool (Spectralis HRA Version 1.9.13.0, Viewing Module 6.5.2.0; Heidelberg Engineering).
To determine the safety performance of the prototype, visual acuity (VA) was measured before and after scanning with the spOCT using charts from the Early Treatment Diabetic Retinopathy Study (ETDRS). Any adverse events (AEs), serious adverse events (SAEs), and/or serious adverse device effects (SADEs) were collected, fully investigated, and reported if and when they occurred in the course of the study.
Clinical Investigation: Primary Objective
To achieve the primary objective, a true/false criterion was applied to determine whether the measurement of the distance between the inner limiting membrane (ILM) of the retina and retinal pigment epithelium (RPE) (i.e., CRT, ILM to RPE distance) was successful. After the CRT measurement was performed, the CRT thickness was measured (in micrometers) by using both the investigative and the reference devices.
The CRT was measured to obtain a relatively easy and comparable landmark in both devices. Indeed, the new OCT device is intended to operate with a specifically designed artificial intelligence software that is capable of automatically analyzing the intra- and subretinal changes in a diseased retina.
The reproducibility of the investigative medical device was assessed by determining the inter- and intrarater reliability coefficients. The differences in CRT measurements between the spOCT and the reference device in the range of CRT values were graphically analyzed by a Bland-Altman plot. Finally, to evaluate further the measurement quality and to contextualize the performance of the prototype spOCT, the results of a study that compared CRT measurements in healthy eyes according to six different commercially available OCT instruments were used as a benchmark.20
Clinical Investigation: Secondary Objective
To achieve the secondary objective, the patients' narrative descriptions of their comfort during the examination with the spOCT were evaluated. The goal was to collect and assess the participants' feedback on how the device should be modified in order to improve patient comfort during the OCT imaging. The descriptions were not quantified.
In addition, the duration of the acquisition with the spOCT was recorded. Documentation of the acquisition time started with the calibration of the device and ended after the first OCT measurement was performed.
Clinical Investigation: Safety Objective
The safety objective was formulated as the change in the logarithmic minimum angle of resolution (logMAR) measured using an ETDRS test chart before and after the nonmydriatic measurements were taken. One letter on the chart represents 0.02 logMAR units. The VA was measured before and after the examination using the spOCT. Potentially, the side effects (e.g., nervousness or dry eyes) of an OCT examination could temporarily reduce the VA if patients do not blink enough. If the VA score differed by more than 0.1 logMAR (i.e., a change of more than five letters) before and after the OCT imaging, a repeated measurement was performed 15 minutes later to exclude the temporary side effects. In cases where the remeasured VA difference was still greater than 0.1 logMAR, the primary investigator (PI) examined the eye for AE and, if necessary, took appropriate measures.
Data Quality Assurance
For each participant enrolled in the study, an anonymized case report form (CRF) was completed and signed by the PI. The participants' identities were coded using a participant identification number (INU). The data analysis and outcome evaluation were performed using anonymized data; no other blinding procedures were applied.
The procedure used to conduct the study was fully documented, and the study data were subsequently verified as required by ISO 14155 and local regulations. Any CRF entries and corrections were performed by staff members on site and authorized by the PI. The entries were checked using the Integrated Scientific Services' study monitor (ISS AG, Biel, Switzerland), and any errors or inconsistencies were then clarified. At the end of the study, the ISS AG collected original, completed, and signed CRFs. Their copies were stored at the study site.
The data were collected using the custom-built software program (OpenClinica Database Version 3.13; ISS AG). Double data entry was applied. The data processing was performed using OpenClinica and its query management. The narrative data and commentaries were controlled visually. All requests for information and clarification were responded to at the investigational site. The data were then exported as indexed lists into a statistics program (JMP 9; SAS Institute Inc., Cary, NC) for the final analysis.
Statistical Analysis
The descriptive statistics were computed for the CRT measurements and for the duration of the OCT examination performed using the spOCT. Before and after this examination, the change in the VA score was calculated for each examined participant. The patients' narrative descriptions of their comfort during the OCT scanning were not analyzed statistically.
The reproducibility of the method used to investigate the medical device was assessed by determining the inter- and intrarater reliability coefficients according to Eliasziw et al.21 Three randomly selected raters performed three CRT measurements of 12 eyes in six randomly selected patients.
A Bland-Altman plot22 was used to show the differences in CRT measurements between the spOCT and the reference device in the range of the obtained CRT values. A paired Student's t-test was performed to determine whether the spOCT values were significantly different from the reference measurements.
The data were analyzed using the statistics software R (https://www.r-project.org/; provided free in the public domain by R Core Team 2017, R Foundation for Statistical Computing, Vienna, Austria). R provides a language and environment for statistical computing using the package irr for the reliability coefficients and the package BlandAltmanLeh for the Bland-Altman plot.
Results
Patient Demographics
A total of 62 eyes in 31 participants (68% females [N = 21] and 32% males [N = 10]) who met the inclusion criteria were recruited for OCT retinal examination by the investigational and reference devices. The overall median average age was 79 years (age range, 68–92 years). The median age of the female patients was 78 years (age range, 70–92 years), and the median age of the male patients was 79.5 years (age range, 68–85 years).
The characteristics of dry eye syndrome were observed in nine patients. Thirty-one eyes had clinical signs of cataract development, and 31 were pseudophakic. One patient was diagnosed with ocular hypertension, one with corneal scarring, and one with posterior capsule opacification. One patient presented with a tremor caused by a previous cortisone inhalation.
From 62 eyes, three eyes (patients 16, 19, 29) were not examined by the spOCT because of technical issues related to the power supply or media opacity secondary to corneal scarring. Two eyes (patients 19, 29) could not be measured by the reference device for the reasons described above. Furthermore, one eye was excluded from the analysis because the off-target measurement produced by the spOCT prototype was too high. To allow the pairwise analysis, two eyes that were examined by the reference scanner were also excluded from the macular thickness analysis.
Quantitative Analysis of Central Retinal Thickness as the Primary Objective
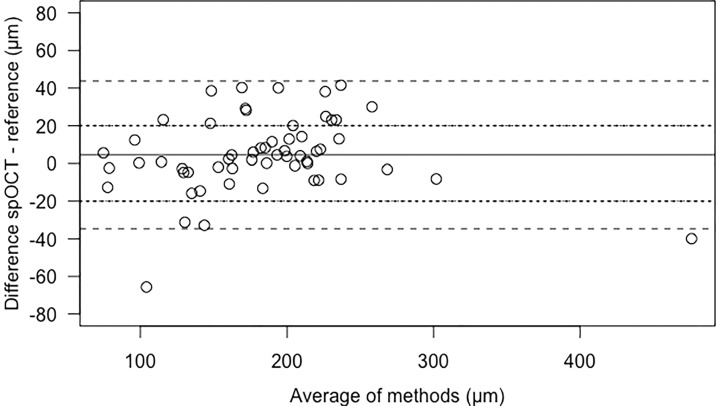
The Bland-Altman plot in Figure 3 shows the differences between the prototype and the commercial scanner according to the range of 58 CRT values measured and included in the analysis. The differences ranged between −65.6 and +41.5 μm with a mean of 4.52 μm (SD = 20.00 μm), which was equivalent to 2.5% of the mean of reference measurements with 181.26 μm. Seventeen spOCT measurements deviated by >20 μm from the reference value.
Figure 3.
Bland-Altman plot of difference against the mean of spOCT scanner and Heidelberg Spectralis (reference) measurements of CRT (μm). The lines represent mean difference (solid) and mean ±1.96 standard deviations (dashed) as the 95% limits of agreement. Sixty-nine percent of the differences are in the range of ±20 μm (dotted lines).
In 38 (65%) of the 58 eyes, the difference in CRT measured with the spOCT was within ±10% of the respective reference value. In 13 eyes, the values differed by more than ±10% and less than ±20%. In six eyes the values were greater than ±20% but less than ±30%. The difference in the CRT measurements obtained using the two devices was not statistically significant (paired t-test, two-sided, t = 1.7198, df = 57, P = 0.091).
The CRT values of 12 eyes in six randomly selected patients were measured by three randomly selected raters. The results showed an intrarater reliability coefficient of 0.968 (lower bound of one-sided 95% confidence interval [CI]: 0.935) and an interrater reliability coefficient of 0.958 (lower bound of one-sided 95% CI: 0.917). The individual intrarater reliabilities for each rater with their lower bound of a one-sided 95% CI were 0.973 (0.940), 0.947 (0.885), and 0.987 (0.971).
Qualitative OCT Image Comparison
The cross-section OCT image information derived from the spOCT using high sample acquisition parameters was shown to be comparable with the SDOCT reference device (Fig. 2C, 2D). In the spOCT image, the retinal zones appeared less blurred and the retinal vessels were slightly better demarcated. The choroid and the vitreous were more easily recognizable in the Spectralis scan, but they were associated with more speckle noise. Because the device was designed to fulfill its intended purpose, a quick and easily repeatable measurement, the small sample OCT acquisition (spOCT), showed a more pixelated and grainy image (Fig. 1C).
Secondary Objective
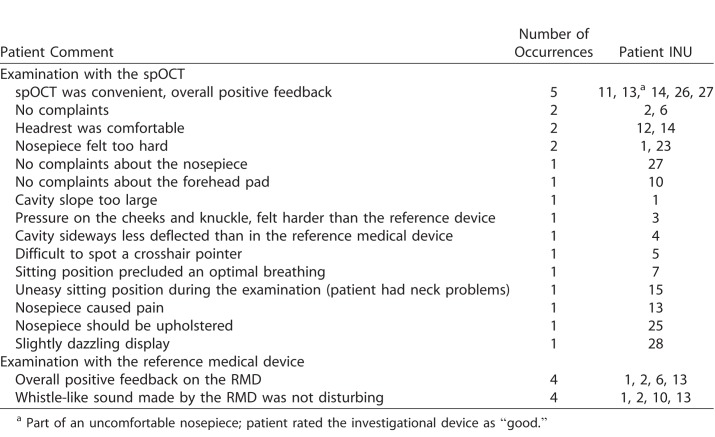
The patients' narrative feedback on their comfort during the OCT imaging procedure is summarized in Table 1. Commentaries were obtained from 19 AMD patients. Only five patients reported mild discomfort related to the nosepiece material of the spOCT.
Table.
Summary of Patient Feedback on the Examination Performed With the Investigational, spOCT Device and Reference OCT System
The length of the retinal assessment procedure included the phase between device calibration and the first OCT measurement, which was on average 2.51 minutes in duration (SD = 0.83; 95% CI: 2.82–2.17) as measured in all the examined patients.
Safety Objective
No safety concerns as defined by VA loss, AEs, SAEs, or SADEs were observed.
Discussion
The U.S. Food and Drug Administration has already approved perimetric devices for home monitoring, and these devices have been successful in previous clinical trial settings.23,24 The home-based structural imaging of the retina is the next logical step. We believe that the described, rapid spOCT examination could be an important development in the longitudinal three-dimensional monitoring of retina structure pathologies. In the present study, we showed that the spOCT was capable of delivering OCT images with an adequate resolution under real-life conditions, thus enabling measurements of retinal thickness in AMD patients with various associated pathologies, such as cataract, dry eye, and tremor. The intra- and interrater reliability coefficients of >0.95 suggest both the high consistency and the reproducibility of the measurements. The very short acquisition time could have positive effects on improving the patient's comfort and reducing potential acquisition artifacts, which is especially important in elderly individuals. Most importantly, patients with a limited fixation could benefit from the short acquisition time. Such brief measurements could further improve clinical efficiency, which was previously slow, regarding new concepts for the use of decentralized OCT imaging.25
The system was specifically designed for handling by patients and not by health care professionals in order to allow automatic image acquisition in patients' homes or in nursing homes. This need is exacerbated by the mounting pressure to monitor a growing number of patients with chronic eye diseases26 in an increasingly resource-constrained environment.27 For example, in the United States, over 100 million people with chronic diseases account for about 75% of health care expenditures.28 The possible benefit of the presented OCT device arises from the potential of telehealth and the delivery of more efficient, convenient, cost-effective, and patient-centered care.29–31
The self-OCT monitoring of patients at home would avoid referrals and save travel time and costs as well as involve patients in their long-term care at a high level. Compared with commercial desktop OCT devices, the cost has been shown to be at least one third that of similar devices because of optimized design. Loans to purchasers (e.g., patients and health care providers) could be made possible, depending on the regulations in each country.
Validated components were also used for their potential to reduce the size and weight of the device.
The rate of positive patient feedback on the ophthalmology examination conducted using the spOCT was comparable to that on the reference desktop OCT device. Some other helpful comments collected during the examination using spOCT will be used to improve some of the device's components, particularly the nosepiece and the interface cavity.
The close examination of the distribution of the retinal thickness data revealed some limitations associated with the precision of the spOCT measurements of the areas of interest. The differences in the CRT measurements between the spOCT and the reference device were not statistically significant, and the mean difference of 4.52 μm was well within the range of the mean differences found in pairwise comparisons of six commercially available OCT devices (2–77 μm).20 However, the Bland-Altman plot (Fig. 3) shows that about one third of the spOCT measurements deviated by more than 20 μm from the reference value, and one outlier was as high as −65.6 μm. This disparity may be attributed to the manual quantification method required in the spOCT.
Moreover, the results of the quantitative OCT analysis differed between various commercial ultra-high-resolution OCT instruments because they employed different postprocessing protocols and relied on inconsistent definitions of retinal margins.32,33 Because of these technical disparities, retinal thickness values are often not uniformly measured by different OCT systems, especially regarding abnormalities in the retinal tissue. Other limitations of this study include the small number of participants and the narrow inclusion criteria. However, these limitations are acceptable in an initial feasibility study.19
The results of this study showed that the new device could also handle different sample resolutions that were appropriate to clinical requirements. The imaging information about the new device using a high sample acquisition protocol was similar to that of the reference OCT device. Because the fully detailed OCT image looks natural to the human eye, it may serve to promote visual discrimination in humans. However, these images are time-consuming to acquire, and future studies should be conducted to demonstrate the level of image redundancy that is needed to achieve the maximum reliability of OCT scans.34 Thus, further studies are warranted to validate the system and to run fully automatic image analyses using methods such as machine learning.
Automatic image analysis would be helpful in the anti-vascular endothelial growth factor (anti-VEGF) therapy. Anti-VEGF is currently the standard for the treatment of neovascular AMD (nAMD), diabetic macular edema, retinal vein occlusion, and other vascular pathologies of the retina. Different treatment protocols have been developed to optimize the benefit-to-risk ratio and the cost-effectiveness of anti-VEGF agents.35–38 Nevertheless, recently published data under real-life conditions showed poor functional results.39,40 In contrast, in a recent study the data showed a positive association among more frequent visits, repeated OCTs, and more injections, with a better visual outcome.41 Furthermore, regarding the detection of impending VA deterioration, evidence showed that central foveal thickness measurement may be a sensitive and early predictor of VA deterioration.42 Despite the sophisticated anti-VEGF protocols, the follow-up decision to treat reflects only the best possible estimation rather than an accurate decision based on timely and true retinal measurements. Thus, it may be speculated that, hypothetically, continuous retinal monitoring with spOCT could close this information gap and lead to a legitimated and individualized treatment regimen. This approach could not only open the best therapeutic window for the optimal effect of a drug but also allow for the detection of early conversion in fellow eyes.43,44 Additionally, the optimization of the number of retreatments, the reduction of in-clinic visits, and the improvement of treatment logistics based on telehealth communication systems would be provided.
In the future, it will be necessary to define new criteria for retreatment (interval) and to determine reliable predictive factors, thus meeting the real-life requirements of AMD and other diseases. In this context, OCT telehealth45 may be a mechanism to facilitate the more efficient and better delivery of medicine despite regulatory, economic, and technological obstacles.24,46–49 The home-based primary care model has received increasing attention worldwide.50–53 In many countries, the population of patients who manage their own health outside professional health care by employing home-use medical devices is on the rise.54 Thus, the further development of the spOCT device software, ergonomics, and telecommunications will be the objective of future research.
This study showed positive results for the performance and safety of the newly developed, robust, self-contained retina OCT scanner MIMO_02 in an elderly population suffering from AMD and could be applied beyond. In this setting, our portable, relatively inexpensive, and patient-operated OCT scanner showed promise as an important aid to disease management and prompt treatment decisions to safeguard patients' health and comfort. This important novel monitoring device, combined with a machine learning software that compiles the OCT data automatically and enhanced with self-acting data communication, may eventually transform the ways in which patients with retinal diseases are followed and treated into a telehealth loop that could be described as “OCT singularity”.
Acknowledgments
The authors thank Wolfgang Wild at the University of Zurich, Switzerland, who performed visual acuity testing and OCT scanning. ISS AG, Biel, Switzerland, notably Michel Weber, PhD, developed the study design and contributed to the manuscript. The Institute for Human Centered Engineering OptoLab at the University of Applied Sciences Engineering and Information Technology in Biel/Berne, Switzerland, designed the MIMO prototype.
This work was supported in part by the Swiss Commission for Technology and Innovation (CTI, no. 14761.1PFLS-LS) and MIMO AG, Bern, Switzerland.
Disclosure: P. Maloca, owns the intellectual properties mentioned in the article; P.W. Hasler, None; D. Barthelmes, None; P. Arnold, None; M. Matthias, None; H.P.N. Scholl, None; H. Gerding, None; J. Garweg, None; T. Heeren, None; K. Balaskas, None; J.E.R. de Carvalho, None; C. Egan, None; A. Tufail, None; S.A. Zweifel, None
References
- 1.Huang D., Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimoto J., Huang D. Foreword: 25 years of optical coherence tomography. Invest Ophthalmol Vis Sci. 2016;57:OCTi–OCTii. doi: 10.1167/iovs.16-20269. [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Erfurth U., Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi: 10.1016/j.ophtha.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V., et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–2548. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Pielen A., Clark WL, Boyer DS, et al. Integrated results from the COPERNICUS and GALILEO studies. Clin Ophthalmol. 2017;11:1533–1540. doi: 10.2147/OPTH.S140665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016;123:330–336. doi: 10.1016/j.ophtha.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155:429–437.e7. doi: 10.1016/j.ajo.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Korobelnik JF, Holz FG, Roider J., et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the phase 3 GALILEO study. Ophthalmology. 2014;121:202–208. doi: 10.1016/j.ophtha.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Kang SW, Park CY, Ham DI. The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol. 2004;137:313–322. doi: 10.1016/j.ajo.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Voo I., Mavrofrides EC, Puliafito CA. Clinical applications of optical coherence tomography for the diagnosis and management of macular diseases. Ophthalmol Clin North Am. 2004;17:21–31. doi: 10.1016/j.ohc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Korobelnik JF, Do DV, Schmidt-Erfurth U., et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. doi: 10.1016/j.ophtha.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Klein R., Klein BE, Linton KL, DeMets DL. The Beaver Dam Eye Study: the relation of age-related maculopathy to smoking. Am J Epidemiol. 1993;137:190–200. doi: 10.1093/oxfordjournals.aje.a116659. [DOI] [PubMed] [Google Scholar]
- 14.Klein R., Wang Q., Klein BE, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Invest Ophthalmol Vis Sci. 1995;36:182–191. [PubMed] [Google Scholar]
- 15.Pezzullo L., Streatfeild J., Simkiss P., Shickle D. The economic impact of sight loss and blindness in the UK adult population. BMC Health Serv Res. 2018;18:63. doi: 10.1186/s12913-018-2836-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunnlaugsdottir E., Halldorsdottir S., Klein R., et al. Retinopathy in old persons with and without diabetes mellitus: the Age, Gene/Environment Susceptibility–Reykjavik Study (AGES-R) Diabetologia. 2012;55(3):671–680. doi: 10.1007/s00125-011-2395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JJ, Rochtchina E., Lee AJ, et al. Ten-year incidence and progression of age-related maculopathy: the blue Mountains Eye Study. Ophthalmology. 2007;114:92–98. doi: 10.1016/j.ophtha.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 18.UAB gets $1.9 million to begin glaucoma screenings in Walmarts. 2012 November 16. Available at: https://www.reviewofoptometry.com/article/glaucoma-screening-at-walmart Accessed July 17, 2017.
- 19.Faulkner L. Beyond the five-user assumption: benefits of increased sample sizes in usability testing. Behav Res Methods Instrum Comput. 2003;35:379–383. doi: 10.3758/bf03195514. [DOI] [PubMed] [Google Scholar]
- 20.Wolf-Schnurrbusch UE, Ceklic L., Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–3437. doi: 10.1167/iovs.08-2970. [DOI] [PubMed] [Google Scholar]
- 21.Eliasziw M., Young SL, Woodbury MG, Fryday-Field K. Statistical methodology for the concurrent assessment of interrater and intrarater reliability: using goniometric measurements as an example. Phys Ther. 1994;74:777–788. doi: 10.1093/ptj/74.8.777. [DOI] [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 23.Chakravarthy U., Goldenberg D., Young G., et al. Automated identification of lesion activity in neovascular age-related macular degeneration. Ophthalmology. 2016;123:1731–1736. doi: 10.1016/j.ophtha.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 24.Wittenborn JS, Clemons T., Regillo C., Rayess N., Liffmann Kruger D., Rein D. Economic evaluation of a home-based age-related macular degeneration monitoring system. JAMA. Ophthalmol. 2017;135:452–459. doi: 10.1001/jamaophthalmol.2017.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callaway NF, Park JH, Maya-Silva J., Leng T. Thinking lean: improving vitreoretinal clinic efficiency by decentralizing optical coherence tomography. Retina. 2016;36:335–341. doi: 10.1097/IAE.0000000000000712. [DOI] [PubMed] [Google Scholar]
- 26.Hashemi H., Khabazkhoob M., Nabovati P., et al. The prevalence of age-related eye disease in an elderly population. Ophthalmic Epidemiol. 2017;24:222–228. doi: 10.1080/09286586.2016.1270335. [DOI] [PubMed] [Google Scholar]
- 27.Rein DB, Zhang P., Wirth KE, et al. The economic burden of major adult visual disorders in the United States. Arch Ophthalmol. 2006;124:1754–1760. doi: 10.1001/archopht.124.12.1754. [DOI] [PubMed] [Google Scholar]
- 28.Gerteis J., Izrael D., Deitz D., LeRoy L., Ricciardi R., Miller T., Basu J. Multiple Chronic Conditions Chartbook. Rockville, MD: Agency for Healthcare and Quality;; 2014. [Google Scholar]
- 29.Raftery J., Clegg A., Jones J., Tan SC, Lotery A. Ranibizumab (Lucentis) versus bevacizumab (Avastin): modelling cost effectiveness. Br J Ophthalmol. 2007;91:1244–1246. doi: 10.1136/bjo.2007.116616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotery A., Xu X., Zlatava G., Loftus J. Burden of illness, visual impairment and health resource utilisation of patients with neovascular age-related macular degeneration: results from the UK cohort of a five-country cross-sectional study. Br J Ophthalmol. 2007;91:1303–1307. doi: 10.1136/bjo.2007.116939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith AF. The growing importance of pharmacoeconomics: the case of age-related macular degeneration. Br J Ophthalmol. 2010;94:1116–1117. doi: 10.1136/bjo.2010.179945. [DOI] [PubMed] [Google Scholar]
- 32.Giani A., Cigada M., Choudhry N., et al. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am J Ophthalmol. 2010;150(6):815–824. doi: 10.1016/j.ajo.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 33.Giani A., Cigada M., Esmaili DD, et al. Artifacts in automatic retinal segmentation using different optical coherence tomography instruments. Retina. 2010;30:607–616. doi: 10.1097/IAE.0b013e3181c2e09d. [DOI] [PubMed] [Google Scholar]
- 34.Gurses-Ozden R., Ishikawa H., Hoh ST, et al. Increasing sampling density improves reproducibility of optical coherence tomography measurements. J Glaucoma. 1999;8:238–241. [PubMed] [Google Scholar]
- 35.Rofagha S., Bhisitkul RB, Boyer DS, Sadda SR, Zhang K. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP) Ophthalmology. 2013;120:2292–2299. doi: 10.1016/j.ophtha.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 36.Lee AY, Lee CS, Egan CA, et al. UK AMD/DR EMR Report IX: comparative effectiveness of predominantly as needed (PRN) ranibizumab versus continuous aflibercept in UK clinical practice. Br J Ophthalmol. 2017;101:1683–1688. doi: 10.1136/bjophthalmol-2016-309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freund KB, Korobelnik Jf, Devenyi R., et al. Treat-and-extend regimens with anti-VEGF agents in retinal diseases: a literature review and consensus recommendations. Retina. 2015;35:1489–1506. doi: 10.1097/IAE.0000000000000627. [DOI] [PubMed] [Google Scholar]
- 38.Rufai SR, Almuhtaseb H., Paul RM, et al. A systematic review to assess the “treat-and-extend” dosing regimen for neovascular age-related macular degeneration using ranibizumab. Eye (Lond) 2017;31:1337–1344. doi: 10.1038/eye.2017.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Writing Committee for the UK Age-Related Macular Degeneration EMR Users Group. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections: report 1: visual acuity. Ophthalmology. 2014;121:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 40.Wolf A., Kampik A. Efficacy of treatment with ranibizumab in patients with wet age-related macular degeneration in routine clinical care: data from the COMPASS health services research. Graefes Arch Clin Exp Ophthalmol. 2014;252:647–655. doi: 10.1007/s00417-013-2562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holz FG, Tadayoni R., Beatty S., et al. Multi-country real-life experience of anti-vascular endothelial growth factor therapy for wet age-related macular degeneration. Br J Ophthalmol. 2015;99:220–226. doi: 10.1136/bjophthalmol-2014-305327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerding H., Loukopoulos V., Riese J., Hefner L., Timmermann M. Results of flexible ranibizumab treatment in age-related macular degeneration and search for parameters with impact on outcome. Graefes Arch Clin Exp Ophthalmol. 2011;249:653–662. doi: 10.1007/s00417-011-1636-6. [DOI] [PubMed] [Google Scholar]
- 43.Amissah-Arthur KN, Panneerselvam S., Narendran N., Yang YC. Optical coherence tomography changes before the development of choroidal neovascularization in second eyes of patients with bilateral wet macular degeneration. Eye (Lond) 2012;26:394–399. doi: 10.1038/eye.2011.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbazetto IA, Saroj N., Shapiro H., Wong P., Ho AC, Freund KB. Incidence of new choroidal neovascularization in fellow eyes of patients treated in the MARINA and ANCHOR trials. Am J Ophthalmol. 2010;149:939–946.e1. doi: 10.1016/j.ajo.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 45.Perilli R., Di Biagio R., Seller R., et al. Teleretinography into diabetes integrated care: an Italian experience. Ann Ist Super Sanita. 2016;52:598–602. doi: 10.4415/ANN_16_04_22. [DOI] [PubMed] [Google Scholar]
- 46.Pare G., Jaana M., Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc. 2007;14:269–277. doi: 10.1197/jamia.M2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the eye (HOME) study. Ophthalmology. 2014;121:535–544. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Halpern MT, Schmier JK, Covert D., Venkataraman K. Resource utilization and costs of age-related macular degeneration. Health Care Financ Rev. 2006;27:37–47. [PMC free article] [PubMed] [Google Scholar]
- 49.Day S., Acquah K., Lee PP, Mruthyunjaya P., Sloan FA. Medicare costs for neovascular age-related macular degeneration, 1994–2007. Am J Ophthalmol. 2011;152:1014–1020. doi: 10.1016/j.ajo.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karunanithi M., Zhang Q. An innovative technology to support independent living: the smarter safer homes platform. Stud Health Technol Inform. 2018;246:102–110. [PubMed] [Google Scholar]
- 51.Sadek I., Mohktari M. Nonintrusive remote monitoring of sleep in home-based situation. J Med Syst. 2018;42:64. doi: 10.1007/s10916-018-0917-6. [DOI] [PubMed] [Google Scholar]
- 52.Ho CYD, Wu Z., Turpin A., et al. A tablet-based retinal function test in neovascular age-related macular degeneration eyes and at-risk fellow eye. Trans Vis Sci Tech. 2018;7(2):2. doi: 10.1167/tvst.7.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ledolter J., Kardon RH. Does testing more frequently shorten the time to detect disease progression? Trans Vis Sci Tech. 2017;6(3):1. doi: 10.1167/tvst.6.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parimbelli E., Bottalico B., Losiouk E., et al. Trusting telemedicine: a discussion on risks, safety, legal implications and liability of involved stakeholders. Int J Med Inform. 2018;112:90–98. doi: 10.1016/j.ijmedinf.2018.01.012. [DOI] [PubMed] [Google Scholar]