Abstract
Purpose
Safe navigation requires avoiding objects. Visual field loss may affect how one visually samples the environment, and may thus contribute to bumping into objects and falls. We tested the hypothesis that gaze strategies and the number of collisions differ between people with glaucoma and normally sighted controls when navigating around obstacles, particularly under multitasking situations.
Methods
Twenty persons with moderate–severe glaucoma and 20 normally sighted controls walked around a series of irregularly spaced vertical obstacles under the following three conditions: walking with obstacles only, walking and counting backward to simulate a conversation, and walking while performing a concurrent visual search task to simulate locating a landmark. We quantified gaze patterns and the number of obstacle contacts.
Results
Compared with controls, people with glaucoma directed gaze closer to their current position (P < 0.05). They also directed a larger proportion of fixations (in terms of number and duration) to obstacles (P < 0.05). Despite this finding, considerably more people with glaucoma contacted an obstacle (P < 0.05). Multitasking led to changes in gaze behavior in both groups, and this was accompanied by a large increase in obstacle contacts among those with glaucoma (P < 0.05).
Conclusions
Glaucoma alters gaze patterns when negotiating a series of obstacles and increases the likelihood of collisions. Multitasking in this situation exacerbates these changes.
Translational Relevance
Understanding glaucoma-related changes in gaze behavior during walking in cluttered environments may provide critical insight for orientation and mobility specialists and guide the design of gaze training interventions to improve mobility.
Keywords: glaucoma, locomotion, mobility, gaze, obstacle avoidance
Introduction
Eye and/or head movements are used to change the line of sight to visually sample the cluttered environment in which we must navigate. This gaze behavior is necessary to acquire both advanced planning information about the general layout of our surroundings, such as potential hazards and safe locations to step, and information about the relative position of obstacles or other individuals as we move.1–4 When walking around stationary obstacles, individuals tend to look ahead toward the end goal and at features bordering their path,5 which allows for proper steering of the body along a set trajectory. In an environment with moving obstacles, other pedestrians for instance, individuals look more frequently at those people exhibiting a higher probability of collision.6 However, continual fixation of obstacles is not required to properly avoid them, as both children and adults can successfully navigate cluttered environments using only intermittent fixations on obstacles in their path.5,7,8 This highlights the significance of peripheral vision for navigation.
Glaucoma leads to progressive and irreversible loss of vision, typically beginning in the midperiphery.9 It is projected to affect more than 110 million people worldwide by 2040.10 Mobility problems are a major concern in this population. For example, bumping into objects is frequently reported, both during experimental mobility courses11,12 and with subjective quality of life questionnaires.13,14 Perhaps more importantly, glaucoma is associated with a high rate of falls.15–18 Together, these mobility problems may explain why people with glaucoma are less likely to leave their house for extended periods of time.19
Visual field loss may affect how one visually samples the environment, and may thus contribute to bumping into objects and falls. Indeed, people with glaucoma exhibit changes in the frequency, latency, and size of saccades.20–22 For instance, when viewing a virtual driving scene, glaucoma patients make significantly more saccades overall but often miss hazards due to their visual field deficit.23 However, there is limited evidence in the literature regarding how the gaze strategies of people with glaucoma relate to natural motor behavior. Existing work shows that, in a sandwich-making task, people with glaucoma fixate longer and make more frequent saccades than normally sighted controls, albeit saccade amplitude is similar.24 Saccade amplitude is also similar when having to identify safe traffic gaps at intersections, though in this case the fixation area is decreased.25 In addition, people with glaucoma that can search for and collect a series of items in a supermarket within a prescribed time relative to control subjects exhibit a greater frequency of glances toward their visual field defect.26 Recently, we also found the timing of gaze shifts to and from stepping targets with respect to foot placement is significantly altered in this population, particularly when performing a concurrent secondary task.27 These differences in gaze timing are accompanied by reduced foot-placement accuracy.
Understanding the gaze strategies employed during walking in people with glaucoma may provide critical insight for orientation and mobility specialists, and facilitate the refinement or development of interventions aimed at improving mobility in this population. In this study, subjects walked through an array of vertical obstacles to an end goal while we tracked their movement and gaze behavior. We chose this mobility task because avoiding obstacles is a necessity when navigating in our cluttered world, and bumping into objects is a frequent occurrence in the glaucoma population. Because people often engage in conversation or attempt to identify landmarks when walking, subjects performed the obstacle negotiation task in isolation and while performing a concurrent task that simulated these everyday situations. Preliminary research in glaucoma suggests that multitasking affects gaze behavior in other walking tasks27 and general walking function.28 Here, we tested the hypothesis that gaze strategies and the number of collisions differ between people with glaucoma and normally sighted controls when navigating around obstacles, particularly under multitasking situations. The spatial-temporal pattern of gaze to gather necessary environmental information as well as the frequency and duration of gaze fixations to obstacles and route-planning features served as measures of gaze strategies.
Methods
Subjects
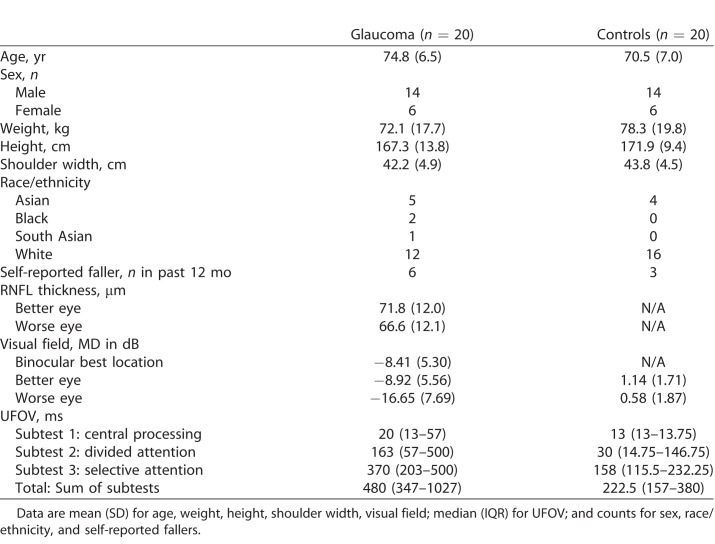
We used G*Power (version 3.1.9; http://www.gpower.hhu.de/) to calculate the required sample size to achieve over 80% power with an alpha of 0.05 for a repeated-measures ANOVA. Unfortunately, there are no relevant studies to help determine an appropriate effect size. Because of the known importance of visual information for negotiating obstacles, and visual field loss associated with glaucoma, we based our sample size calculation on a large effect size (Cohen's f = 0.4). This yielded 18 subjects per group, which we increased to 20 in case of potential equipment problems. Thus, we recruited 20 persons with glaucoma through a collaborating ophthalmologist and 20 normally sighted control subjects through eye clinics, doctor's offices, and community centers. An optometrist screened the control subjects' vision as needed. Potential subjects that met our inclusion criteria (see below) were asked if they would like to participate; we did not recruit based on glaucoma severity. All subjects provided informed written consent prior to participating in this study. The Office of Research Ethics at Simon Fraser University approved the study procedures, which adhered to the tenets of the Declaration of Helsinki.
An ophthalmologist (DRN) had previously diagnosed all persons with glaucoma based on visual field loss on repeated testing, including a Glaucoma Hemifield Test outside of normal limits, and retinal nerve fiber layer (RNFL) loss. Inclusion criteria specific to glaucoma patients included: a Humphrey visual field mean deviation worse than −2 dB in both eyes, habitual binocular visual acuity better than 0.4 logMAR (20/50 Snellen equivalent), and absence of another visual disease that could affect the visual field (e.g., clinically significant cataracts, macular degeneration). Inclusion criteria specific to control subjects included: Humphrey visual field mean deviation better than −2 dB in both eyes, and absence of an eye disease. Additional inclusion criteria for both groups included: aged 60 years and over, free of neurologic (e.g., Parkinson's disease, stroke) or musculoskeletal (e.g., arthritis) disorders that impair mobility, ability to walk without assistance (or mobility aid) for more than 5 minutes, and more than 26 on the Mini-Mental State Exam.
In addition to the above screening, we quantified how often subjects had fallen in the past 12 months. We defined a fall as an unexpected event in which the person landed or came to a rest on the ground, floor, or lower level.29
Visual Assessment
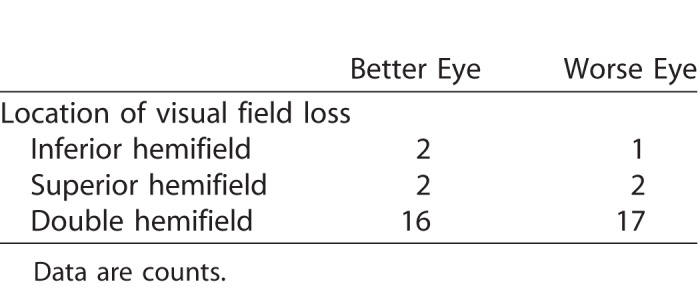
We tested monocular visual fields of the glaucoma subjects using the SITA Fast central 30-2 threshold test procedure (size III Goldmann white target and background luminance of 10.03 cd/m2) with a Humphrey Field Analyzer (model HFA-II 750; Carl Zeiss Meditec, Inc., Dublin, CA). To determine binocular mean deviation (MD) for further analyses, we used the best location model.30 This involved taking the highest sensitivity between the right and left eye at each visual field location, and then averaging these sensitivities across all points. The MD served as a proxy for glaucoma severity. We also determined the location of visual field loss for each eye; loss in a hemifield (inferior, superior) required having a cluster of three or more points depressed below the 5% level on the pattern deviation plot.
For controls, we tested monocular visual fields using frequency-doubling technology (Humphrey 710 FDT Visual Field Instrument; Carl Zeiss Meditec, Inc.). We used this different piece of equipment for these subjects because they were recruited in a different manner than the glaucoma group. However, we did not use these visual field scores in any analyses; they only served to ensure that controls did not have significant visual field loss that could confound our results. This required controls to have a MD score calculated by the device of better than −2 dB in both eyes.
We measured RNFL thickness to confirm the diagnosis of glaucoma using spectral-domain optical coherence tomography (OCT) after pupil dilation with a CIRRUS HD-OCT (model 4000, software version 6.0.2.81; Carl Zeiss Meditec, Inc.). We used the device software and Optic Disc Cube 200 × 200 protocol, which acquires a 6 × 6 × 2-mm data cube in the peripapillary region, to calculate the average RNFL thickness taken from a 3.46-mm circle centered on the optic disc. Controls did not undergo OCT testing.
All subjects also performed the Useful Field of View (UFOV) test (17-inch touch monitor with 75-Hz refresh rate; version 7.0.2; Visual Awareness Research Group, Inc., Punta Gorda, FL) under binocular conditions, which included three subtests of increasing difficulty. Subjects sat 50 cm from the screen. Subtest 1 (central processing) required subjects to identify a central target (car or truck). Subtest 2 (divided attention) required subjects to identify the central target plus a peripheral target (car) simultaneously at one of eight radial locations. Subtest 3 (selective attention) is similar to the previous one except the peripheral target is embedded among 47 triangle distractors. The software decreases the duration of the stimulus presentation until a subject is able to produce a 75% correct response rate.
Obstacle Task
Subjects performed the obstacle negotiation task under single- and dual-task conditions. There were 12 walking trials per condition (i.e., 36 trials total), with the order of conditions randomized. In each condition, subjects walked along a 4.5-m long and 1.25-m wide path to an “end gate” consisting of two blue vertical poles (height = 25 cm; diameter = 6 cm) after navigating four dark gray vertical poles (height = 165 cm; diameter = 3.5 cm; Fig. 1). We positioned the poles 60 cm away from each other in the anterior-posterior direction (i.e., the plane of progression) and varied the pole and end gate positions in the medial-lateral direction on a trial-to-trial basis using one of four predetermined arrangements. This meant that subjects experienced each arrangement for a total of three walking trials. We randomized the order of arrangements on a trial-to-trial basis. An opaque wooden board occluded subjects' vision of the walkway before each trial. The visual occlusion and the use of four, randomized arrangements served to reduce the likelihood of subjects using spatial memory to navigate; thus, ensuring they had to use visual information to complete the task. We instructed subjects to walk at a self-selected speed, to navigate the course without stopping, to take the simplest path through the poles without any part of their body going outside the path's borders, and to avoid contact with the poles. An experimenter demonstrated the task to make sure subjects understood the instructions. Subjects started walking immediately once cued.
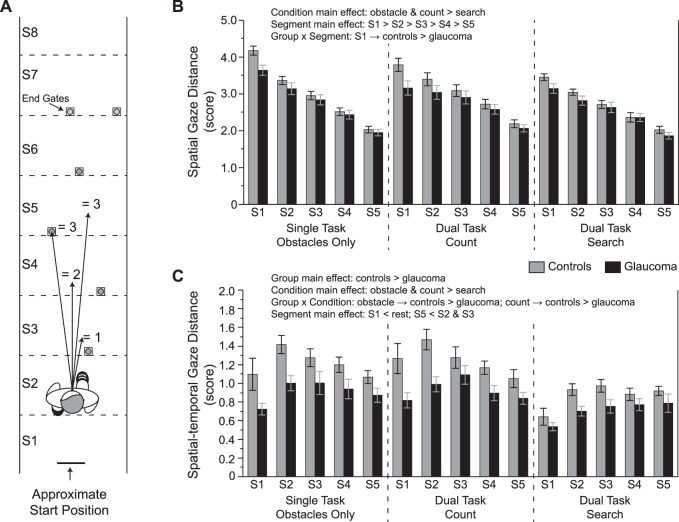
Figure 1.
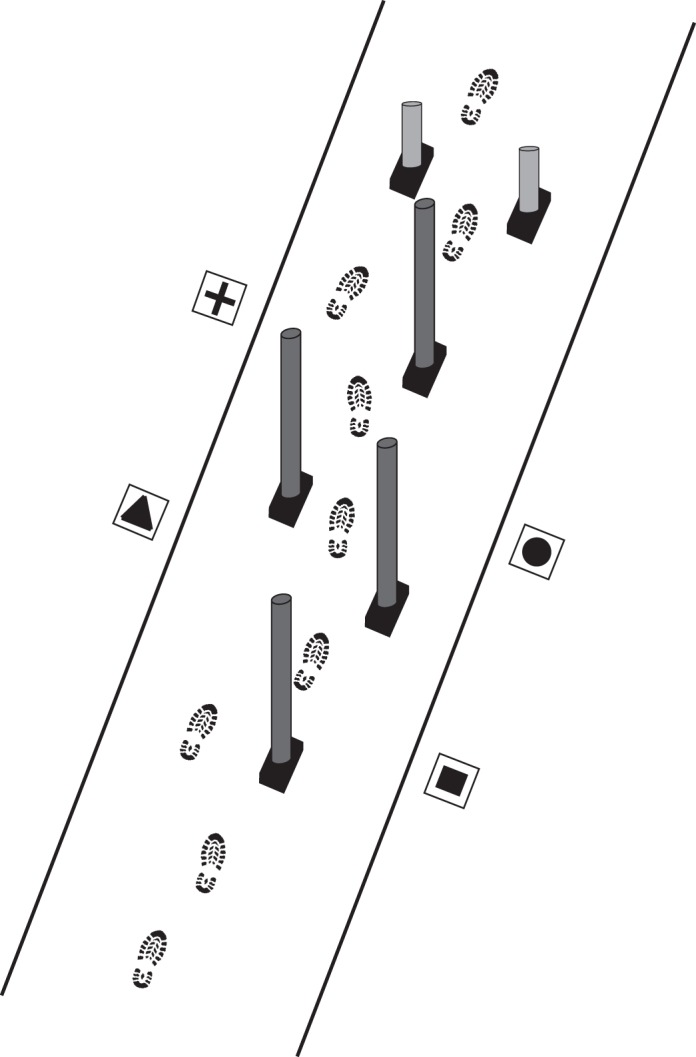
Experimental set-up. An illustration (not drawn to scale) of the obstacle course is shown. The shapes were only present in the visual search dual task condition.
In one (count) dual-task condition, subjects walked while counting backward by threes, a common secondary task that increases cognitive load31 and simulates having a detailed conversation with someone. In the other (visual search) dual-task condition, subjects had to identify the location of a shape at the end of each trial after being cued by an experimenter. In this condition, we positioned four tiles (20 × 15 cm) on the ground on both sides of the path containing the obstacles, each with a different black shape on a white background (see Fig. 1). We randomly varied the sequence of shapes (plus sign, triangle, circle, and square) on a trial-to-trial basis. This dual task condition purposely forced subjects to temporarily direct their gaze away from the path and poles, simulating real-life situations in which we have to both monitor our walking direction and identify landmarks, a task identified by people with eye disease as challenging.32
We also had subjects count and perform visual search trials while not walking. These represented count and visual search baseline (or single-task) conditions. For the count single task, subjects counted by threes for a total of 10 seconds in each of three trials. We calculated the number of correct responses in this task and during the dual-task situation, then divided these values by their respective trial durations. For the visual search single task, subjects viewed the shapes for 5 seconds before having their vision blocked in each of 12 trials. We divided the proportion of correct responses in the single- and dual-task situations by their respective average trial durations. To determine how dual tasking affected counting and visual search performance, we calculated a dual task cost (DTC) value33 for each using the following formula: (dual task – single task) / single task. A negative value represents worse performance in the dual-task situation.
A high-speed, head-mounted, mobile eye-tracker (model H6-HS; Applied Science Laboratories, Bedford, MA) recorded gaze position during the task at 120 Hz. A video camera mounted on the eye tracker recorded the subjects' view of the path at 30 Hz. We calibrated the eye tracker using the system's standard 9-point calibration method. Subjects wore their habitual spectacles, if applicable, during testing in the obstacle task. Two Optotrak Certus (Northern Digital, Inc., Waterloo, Canada) cameras, synchronized with the eye tracker, recorded (at 120 Hz) the time-varying positions of infrared-emitting diodes placed on the obstacles and subject's body. We fixed these markers to the subject's head (using a rigid block at the back of the head and mounted on the eye tracker), chest (midway between the sternal notch and xiphoid process), right shoulder (on the acromion), and bilaterally on the heels, mid-feet (dorsal surface at the approximate level of the metatarsal joint), and above the toes on their shoes.
Gaze and Kinematic Measures
We low-pass filtered kinematic data (with a Butterworth algorithm) at 6 Hz. We calculated gait speed between the first and last obstacle using the marker on the chest, and determined obstacle-crossing events by calculating the time at which the chest marker crossed the anterior-posterior position of each obstacle. Given the reported mobility problems of people with glaucoma and the nature of our mobility task, we also quantified the number of obstacle contacts throughout the experiment. We defined an obstacle contact as a noticeable sway of the obstacle or it falling to the ground after being bumped into with any part of the subject's body. After each walking trial, two experimenters came to a consensus as to whether any contacts occurred and then one of them recorded the event.
For each walking trial, we filtered the horizontal and vertical components of the gaze data (4th order, low-pass, Butterworth algorithm) at 12 Hz and then calculated the vector gaze position at each point in time. The time at which the local angular gaze velocity crossed above or below a threshold of 100°/s for a minimum of 16 ms defined the onset and offset of gaze shifts.27,34 We defined gaze fixations as instances with stable gaze on a location for a minimum of 66 ms.27,35 To determine which aspects of the visual scene subjects fixated, we used the 30-Hz video of the path with crosshairs of gaze position superimposed. Fixation locations included route-planning features (gap between obstacles, ground regions, and end goal region), obstacles (or end gates) and, in the case of the visual search dual-task condition, shapes. Subjects rarely, if ever, fixated outside of these locations.
To determine how subjects allocate gaze to plan their route through the obstacles, we developed the following two measures: spatial gaze distance and spatial-temporal gaze distance. For both measures, we used the positions of the obstacle and the end gates to divide the path into eight segments (S1–S8; see Fig. 2A). Each segment is the same length, expect for the first one (S1) because of the subject's start position and the last one (S8) that represents the end region of the path; these unequal segment lengths are accounted for in the calculation of each of the two gaze distance measures. The anterior-posterior position of the subject's chest marker determines which segment they are located in. For both measures, we determined which segment(s) subjects fixated relative to their location for the first five segments they walked through. We excluded the last three segments because the subjects have walked past the fourth obstacle by this point and gaze begins to deviate from the walking path and becomes erratic as the subjects approach the end of the lab. Each fixation is then assigned a spatial gaze distance score based on how far it is from the subject. This is illustrated in Figure 2A. If a subject fixated the segment within which they were currently located, we scored that fixation a 0.5; this rarely occurred. We scored a fixation to the next segment as a 1, and so on. For example, if the subject is located within segment #2 and is fixating segment #4, that gaze fixation is scored a two. If the subject is located within segment #3 and is fixating segment #4, that gaze fixation is scored a one. Note that subjects had a very strong tendency to direct gaze toward the ground when looking between obstacles (and even fixated obstacles near the bottom). Thus, we are able to assign these fixations to specific ground locations based on where the point of gaze appears on the video image coming from the camera mounted on the eye tracker.
Figure 2.
Gaze distance measures. (A) An illustration of how the gaze distance scores were assigned. In this example, the subject is walking through segment 2 (S2). Gaze is directed to different locations and each fixation is then given a score based on how far ahead the fixation was with respect to the subject. Scores are averaged for each segment. For the spatial-temporal gaze distance measure, scores are scaled according to fixation duration and how long the subject walks within that particular segment. See text for additional details. (B) Spatial gaze distance scores and (C) spatial-temporal gaze distance scores for each condition and group across the five segments included in the analysis. Data are represented as mean ± SE.
For the spatial gaze distance measure, we then averaged the score for each segment. Large values indicate that the subject fixates, on average, a greater distance ahead when negotiating the obstacles. For the spatial-temporal gaze distance measure, the duration of each fixation is divided by the total time the subject spends walking in the segment they are currently located. The spatial gaze distance score of that fixation then multiplies this temporal value. In this way, the spatial gaze distance is scaled based on how long a subject fixates at that distance. These spatial-temporal gaze distance scores are then averaged for each segment. Larger values indicate that the subject allocates gaze, on average, farther ahead for a greater amount of time.
We next quantified (1) the proportion of the number of fixations and (2) the proportion of gaze fixation time to route-planning features, obstacles, and shapes (if applicable) until the subject walked past the fourth obstacle. We excluded data beyond this point because the stability of gaze decreases, as described earlier. We also quantified the time interval (in seconds) between a gaze shift away from an obstacle and anterior-posterior chest crossing time (i.e., the gaze obstacle-crossing interval). Because some subjects fixated a given obstacle more than once, we used the last gaze shift to the obstacle before crossing in this calculation. A negative interval indicates gaze transfer away before crossing the obstacle.
Statistical Analyses
For all ANOVAs, we included subject as a random factor and used Tukey post hoc tests with significant main effects and/or interactions. We also included age as a covariate in all analyses described in this section because the difference in age between groups almost reached significance (see below). To determine differences in the spatial gaze distance and spatial-temporal gaze distance scores between groups, conditions, and segments, we used separate three-way (group × condition × segment) ANOVAs. To determine differences in the proportion of the number of fixations to obstacles and to route-planning features between groups (glaucoma and controls) and across conditions (single task, count dual task, visual search dual task), we used separate two-way (group × condition) ANOVAs. We used identical analyses for the proportion of gaze fixation time to obstacles and to route-planning features. One-way (group) ANOVAs determined differences between groups for both the proportion of the number of fixations to shapes and the proportion of gaze fixation time to shapes as well as the count and visual search DTC measures. Separate two-way (group × condition) ANOVAs assessed differences in gait speed and gaze obstacle-crossing intervals. We used separate generalized estimating equation (GEE) models (negative binomial with log link or binomial logit link, respectively) to compare differences in the number of obstacle contacts and the number of subjects that contacted obstacles between the different conditions and groups. To determine the relationship between obstacle contacts and visual field loss in the glaucoma group, we performed a Poisson regression. We used a similar analysis to determine the relationship between the count (or visual search) dual task cost and obstacle contacts. For all statistical analyses, we used an alpha level of 0.05 and JMP 13 software (SAS Institute, Cary, NC), expect for the GEE model in which we used SPSS 24 software (IBM SPSS Statistics, Armonk, NY).
Results
Characteristics of the glaucoma subjects and normally sighted controls are shown in Tables 1 and 2. The type of glaucoma ranged from primary open-angle (N = 17) to normal- (or low-) tension (N = 2) to pseudoexfoliative (N = 1). Two-sample t-tests showed that age (t38 = 1.99, P = 0.054), height (t38 = −1.22, P = 0.228), weight (t38 = −1.04, P = 0.305), and shoulder width (t38 = −1.08, P = 0.289) did not differ between the groups. Although a greater number of glaucoma subjects fell in the past year compared with controls, this difference did not reach significance (Fisher's Exact test: P = 0.451). All three UFOV subtests and the UFOV total score differed between groups (Wilcoxon rank-sum tests, P < 0.007).
Table 1.
Subject Characteristics
Table 2.
Hemifield Location of Visual Field Loss
The Effects of Glaucoma and Dual Tasking on Gait Speed When Navigating Around Obstacles
Gait speed differed between groups (group main effect: F1,39 = 7.5, P = 0.009), such that control subjects walked faster in the obstacle only condition (controls: 0.89 ± 0.14 m/s; glaucoma: 0.74 ± 0.19 m/s), count dual-task condition (controls: 0.80 ± 0.17 m/s; glaucoma: 0.65 ± 0.2 m/s), and the search dual-task condition (controls: 0.83 ± 0.14 m/s; glaucoma: 0.68 ± 0.19 m/s). Condition also had an effect on gait speed (F2,75 = 35.0, P < 0.0001), as both groups walked faster in the obstacle only condition compared with the count dual task and the visual search dual-task conditions. Despite these differences, our gaze measures are largely independent of gait speed because of the way each measure is calculated (i.e., scaled to segment duration, quantified as proportions).
Gaze Strategies When Navigating Around Obstacles
To determine how subjects allocate gaze to plan their route through the obstacle course, we first quantified how far ahead, on average, they fixated. Figure 2B illustrates these spatial gaze distance scores. Subjects fixated further ahead in the obstacle only and count dual-task conditions compared with the search dual-task condition (F2,532 = 26.8, P < 0.0001). Furthermore, gaze distance scores progressively decreased as subjects walked across each segment (F4,532 = 252.7, P < 0.0001), fixating approximately four segments ahead early in the path and approximately two segments ahead near the end. Spatial gaze distance between groups differed depending on the segment (F4,532 = 5.2, P = 0.0004). Specifically, the glaucoma group demonstrated smaller gaze distance scores versus controls for the first segment, suggesting that they did not shift gaze as far to initially plan their route. However, we found no other group differences in spatial gaze distance for the other segments.
Although knowing the particular distances of fixations provides information on how far ahead subjects may plan their walking trajectory, it is important to recognize that the amount of time spent fixating these distances is also relevant. Our spatial-temporal gaze distance measure captures this aspect of gaze behavior (Fig. 2C). Controls exhibited greater spatial-temporal gaze distance scores compared with the glaucoma group in the obstacle only and count dual-task conditions but not in the search dual task condition (F2,532 = 3.2, P = 0.041). We also found main effects of group (F1,37 = 10.8, P = 0.002), condition (F2,532 = 45.0, P < 0.0001), and segment (F4,532 = 9.7, P < 0.0001) for this measure.
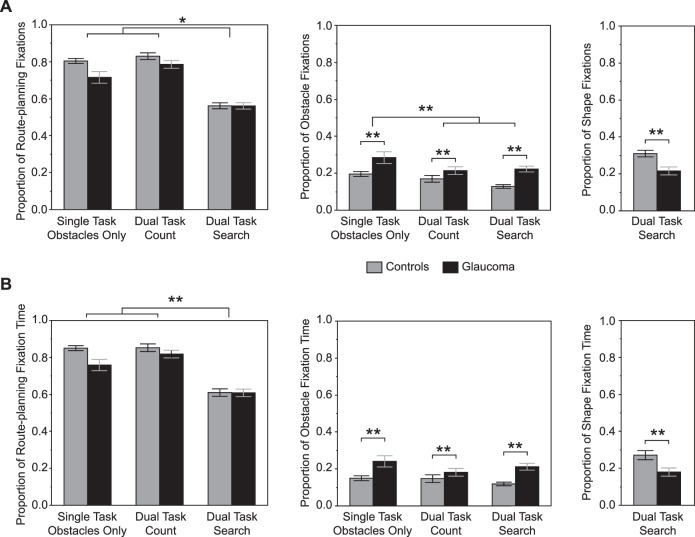
The majority of fixations were directed to route-planning features, ranging from approximately 55% in the search dual-task condition to approximately 80% in the count dual-task condition (Fig. 3A). The proportion of fixations to route-planning features differed depending on the group and condition (F2,76 = 3.1, P = 0.049). However, post hoc tests indicated no group differences, which was also supported by a nonsignificant group main effect (F1,40 = 3.7, P = 0.063). Of note, if age is not included as a covariate, we find that controls make a greater proportion of fixations to these features than the glaucoma group in the obstacle only condition. Regardless of group, the proportion of fixations was significantly reduced in the search dual task relative to the other conditions. This occurred because of the presence of the shapes. In fact, controls made a greater proportion of fixations to the shapes compared with the glaucoma group (F1,37 = 10.0, P = 0.003) as shown in Fig. 3A (right panel). Less than 25% of fixations were directed to the obstacles across all conditions. A greater proportion of obstacle fixations occurred in the obstacle only condition compared with the other conditions (condition main effect: F2,76 = 10.6, P < 0.0001). Furthermore, the glaucoma group exhibited a greater proportion of obstacle fixations versus controls (group main effect: F1,40 = 9.9, P = 0.003). We did not find a statistically significant interaction (F2,76 = 1.8, P = 0.174).
Figure 3.
Gaze fixation locations and times. (A) Proportion of route-planning, obstacle, and shape fixations between groups and conditions. (B) Proportion of route-planning, obstacle, and shape fixation times between groups and conditions. Data are represented as mean ± SE. *Statistically significant post hoc test based on a group × condition interaction, P < 0.05. **Statistically significant main effect of condition or group, P < 0.05.
The proportion of fixation time on route-planning features, obstacles, and shapes closely matched the proportion of fixations to these regions of interest (see left, middle, and right panel, respectively, of Fig. 3B). Although we did not find a statistically significant interaction (F2,76 = 2.8, P = 0.069) or effect of group (F1,39 = 3.2, P = 0.079) for route-planning features, we found an effect of condition (F2,76 = 80.2, P < 0.0001). Post hoc tests indicated that the proportion of fixation time decreased in the search dual-task condition compared with the other conditions. In the search dual task, controls spent a greater proportion of fixation time on the shapes compared with the glaucoma group (27% vs. 18%; F1,37 = 6.1, P = 0.018). In contrast, the glaucoma group spent a greater proportion of time fixating the obstacles compared with controls independent of the condition (F1,40 = 9.5, P = 0.004). However, we did not find a statistically significant effect of condition (F2,76 = 2.5, P = 0.088) or an interaction (F2,76 = 2.3, P = 0.111).
Figure 4 illustrates the gaze obstacle-crossing interval. On average, subjects shifted gaze away from a given obstacle approximately 2 seconds before crossing past it. We found no main effect of group (F1,37 = 1.8, P = 0.185) or group × condition interaction (F2,76 = 1.7, P = 0.191). However, subjects shifted gaze away from obstacles closer to when they crossed past them in the search dual task compared with the count dual task (F2,76 = 3.2, P = 0.045).
Figure 4.
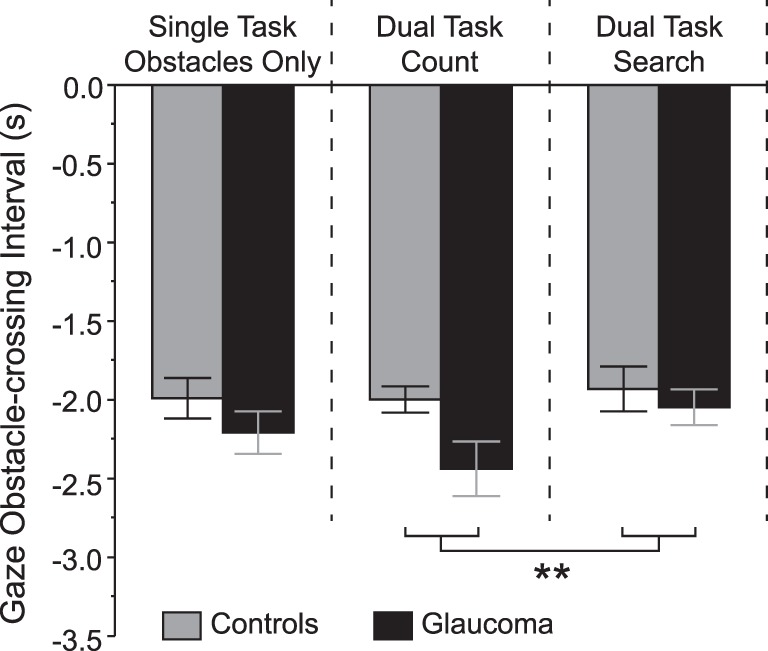
Gaze obstacle-crossing interval for each condition and group. Negative intervals indicate gaze transfer away before crossing the obstacle. Data are represented as mean ± SE. **Statistically significant post hoc test based on a condition main effect, P < 0.05.
Obstacle Contacts
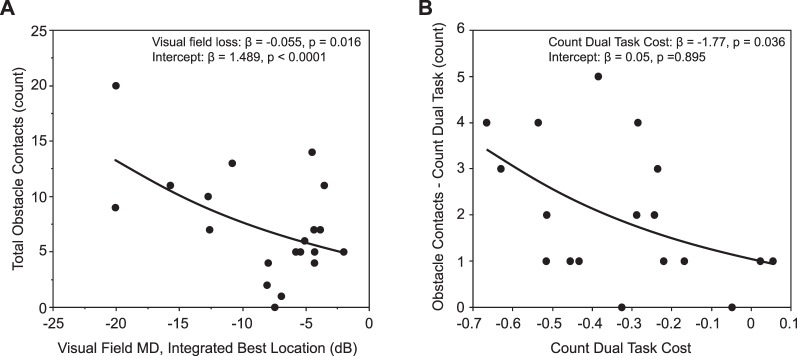
Table 3 summarizes the results of the obstacle contacts. In total, the glaucoma group contacted obstacles 146 times compared with only 35 contacts among the controls. Accordingly, we found an effect of group in the GEE model (χ2 = 25.6; P < 0.0001). We also found an effect of condition (χ2 = 9.7; P = 0.008) in which post hoc tests indicated a greater number of contacts in the count and search dual task conditions versus the obstacle only condition. The group × condition interaction did not reach significance (χ2 = 5.0; P = 0.083). A significantly greater number of glaucoma patients contacted the obstacles than controls (group main effect: χ2 = 9.3, P = 0.002). Furthermore, more subjects contacted an obstacle at least once in the count and search dual-task conditions compared with the obstacle only condition (condition main effect: χ2 = 14.1, P = 0.001). Finally, in the glaucoma group, we determined the relationship between integrated visual field loss and obstacle contacts using a Poisson regression, corrected for over dispersion (over dispersion = 2.45). The results are shown in Figure 5A. Greater visual field loss associated with a greater number of obstacle contacts (P = 0.016).
Table 3.
Obstacle Contacts
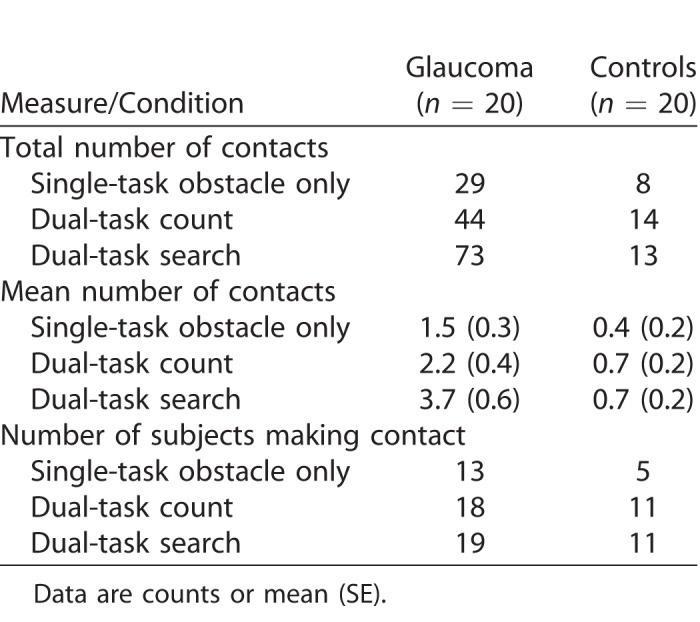
Figure 5.
Poisson regressions for obstacle contacts in the glaucoma group. (A) Relationship between visual field loss and total obstacle contacts. (B) Relationship between count dual task cost and obstacle contacts in that condition.
Dual-Task Costs
The findings above show that gaze measures and the likelihood of contacting an obstacle are affected by having to perform a secondary task. We found a greater cost associated with counting (i.e., worse performance) for the glaucoma group compared with the controls as quantified by our DTC measure (glaucoma count DTC: −0.33 ± 0.21; control count DTC: −0.12 ± 0.19; F1,35 = 9.5, P = 0.004). Note that one subject in the glaucoma group refused to perform the counting task due to its difficulty. In addition, due to technical difficulties we were unable to normalize count performance to trial duration for one glaucoma subject. Therefore, these subjects were excluded from this analysis. Interestingly, greater count dual task cost associated with a greater number of obstacle contacts in this condition (Fig. 5B; P = 0.036). We also found greater cost in terms of visual search performance for the glaucoma group (glaucoma search DTC: −0.20 ± 0.32; control search DTC: 0.09 ± 0.28; F1,37 = 5.5, P = 0.024). However, we did not find a relationship between the visual search dual-task cost and obstacle contacts in this condition (P = 0.315).
Discussion
Safe navigation requires avoiding stationary and moving objects in the environment. Appropriate gaze behavior plays a key role in this success. Unfortunately, people with glaucoma may visually sample the environment differently due to their visual field loss and are more likely to bump into objects than normally sighted individuals. Here, we show that, compared with controls, people with glaucoma direct gaze closer to their current position, and direct a greater proportion of fixations (in terms of number and duration) to obstacles. Despite the latter behavior, considerably more people with glaucoma contacted an obstacle. We also show that multitasking leads to changes in gaze behavior in both groups, and this is accompanied by a large increase in obstacle contacts in those with glaucoma. Greater glaucoma-related visual field loss and difficulty counting as a dual task both associate with a greater number of obstacle contacts. Taken together, we suggest that altered (and arguably inappropriate) gaze strategies as well as deficits in multitasking ability contribute to the mobility problems seen with glaucoma.
As evident from the spatial and spatial-temporal gaze distance measures, the glaucoma subjects directed gaze closer to their current position compared with controls when negotiating the obstacles. This suggests that they prioritize more immediate locations rather than features important for planning a route to the goal area. Interestingly, this is opposite to the gaze strategy observed when walking and stepping onto targets. When precise foot placement is required, people with glaucoma make saccades away from a target sooner in relation to stepping on it.27 This is accompanied by reduced foot-placement accuracy, similar to normally sighted older adults at a high risk of falling.36 In this precision-walking situation, glaucoma subjects prioritize planning future accurate steps at the expense of the current step. Directing gaze closer to their current position may allow glaucoma subjects to better locate obstacles but can also lead to poor path choices that result in the need to make sharper, more frequent turns and/or greater trunk rotations, thus increasing the risk of collision. Orientation and mobility specialists often teach people with low vision to use a gridline scan, which involves scanning in a grid pattern, from in front of the person outward to the destination or farthest point visible, then left-to-right and up-and-down back toward the person to locate hazards.37,38 As ones moves forward, it is important to periodically re-establish lines of direction or the goal. Our results suggest that this may represent an appropriate training strategy for this population.
We found that people with glaucoma allocated a greater proportion of fixations (number and duration) to obstacles but still contacted them more frequently. A reduced visual field may cause subjects to rely more on central rather than peripheral vision to detect obstacles, and thus explain, in part, these results. People with peripheral visual field loss due to retinitis pigmentosa fixate the edges of a doorframe when having to pass through it more than controls; the latter direct fixations more to the door aperture.39 At first glance, a strategy to increase fixations to an obstacle might make sense. Indeed, fixating an obstacle that one must step over is important for determining its location and height.40,41 Recent work shows that people with retinitis pigmentosa spend an equal amount of time fixating an obstacle in this situation, but they spend less time looking past the obstacle to the path ahead, and a greater amount of time fixating the ground before the obstacle compared with normally sighted controls.42 When stepping over an obstacle, normally sighted older adults shift gaze to the obstacle earlier and for longer than young adults.43,44 However, spending more time fixating an obstacle when having to avoid it, rather than when having to step over it, is not necessarily an ideal strategy. This is because there is a natural tendency to veer in the direction you look; that is, you look where you want to go.45–47
The number of obstacle collisions as well as the number of subjects colliding was considerably greater in the glaucoma group. This occurred despite the fact that all subjects were specifically instructed to avoid contact with the obstacles as they traversed the walking path. This suggests that the altered gaze patterns in this group, namely that they direct gaze closer to their current position and fixate obstacles more frequently and for a greater duration, are maladaptive. Although these changes are likely a compensatory strategy due to their eye disease, and they may ultimately let these individuals navigate in the environment, there is a clear need for better training on how to use any remaining vision more effectively.
Counting or searching for a shape concurrently with obstacle negotiation led to changes in gaze patterns and a greater number of obstacle contacts in the glaucoma group. Interestingly, the cost of having to count contributed to obstacle contacts in that condition, as shown in Figure 5B. Difficulty dividing or selecting attention may also partially explain these results, given that the glaucoma group scored considerably worse on subtests one and two of the UFOV. Dual tasking is known to alter gait in older adults. For instance, when circumventing an obstacle and having to remember auditory messages presented during the task, normally sighted older adults walk slower,48 similar to both groups in our experiment. However, there is less research in those with eye disease. Greater visual field loss due to glaucoma does associate with a wider base of support and stride-to-stride variability of step length, stride length, and stride velocity when walking and carrying a cup or tray.28 Furthermore, in a recent study from our lab in people with glaucoma, we found that the count and visual search dual tasks led to a reduction in foot-placement accuracy when walking and stepping onto targets.27 We also found these dual-task conditions affected gaze behavior, such that transferring gaze to and from stepping targets differed compared with the single-task situation. Similarly, Yamada et al.49 found that normally sighted older adults with a history of falls transfer gaze away from an obstacle to step over sooner during a counting dual task condition compared with those without a history of falling. Taken together, it is clear that multitasking can influence gaze and mobility function. This indicates that interventions aimed at improving mobility should consider incorporating dual-task training as a component.
Our study has at least two limitations. First, given the characteristics of our sample, we were unable to stratify glaucoma subjects based on the location and severity of visual field loss. Future research should explore whether gaze strategies differ depending on these factors. Second, although our obstacle negotiation task was designed to mimic real-life scenarios, it may not generalize to all variations and/or environments (e.g., outdoor versus indoor lighting, uneven terrain, moving objects). However, our results provide necessary insight to design future experiments and to guide potential gaze training interventions.
In conclusion, people with glaucoma exhibit altered (and arguably inappropriate) gaze behavior compared with normally sighted controls when negotiating an array of stationary obstacles. Furthermore, they experience a greater number of obstacle contacts. These results suggest that interventions aimed at refining gaze strategies for those with visual field loss might be beneficial. For instance, the use of a gridline scan may serve to increase awareness of hazards in the environment and facilitate the planning of safer routes. In addition, teaching people with glaucoma how to determine safe gaps between obstacles and then look where you want to go may improve mobility. Future research should determine whether these ideas are effective.
Acknowledgments
The authors wish to thank Javier Domínguez-Zamora for help with data collection, and Shaila Gunn for help with data collection and a portion of the data analysis.
Supported by a Glaucoma Research Society of Canada grant and a Simon Fraser University VPR Bridge grant.
Disclosure: K. Lajoie, None; A.B. Miller, None; R.A. Strath, None; D.R. Neima, None; D.S. Marigold, None
References
- 1.Hamid SN, Stankiewicz B., Hayhoe M. Gaze patterns in navigation: encoding information in large-scale environments. J Vis. 2010;10(28):1–11. doi: 10.1167/10.12.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marigold DS. Role of peripheral visual cues in online visual guidance of locomotion. Exerc Sport Sci Rev. 2008;36:145–151. doi: 10.1097/JES.0b013e31817bff72. [DOI] [PubMed] [Google Scholar]
- 3.Marigold DS, Patla AE. Gaze fixation patterns for negotiating complex ground terrain. Neuroscience. 2007;144:302–313. doi: 10.1016/j.neuroscience.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Patla AE, Tomescu SS, Ishac MGA. What visual information is used for navigation around obstacles in a cluttered environment? Can J Physiol Pharmacol. 2004;82:682–692. doi: 10.1139/y04-058. [DOI] [PubMed] [Google Scholar]
- 5.Patla AE, Tomescu SS, Greig M., Novak A. Gaze fixation patterns during goal-directed locomotion while navigating around obstacles and a new route-selection model. In: Van Gompel RPG, MH Fischer, WS Murray, Hill RL, editors. Eye Movements: A Window on Mind and Brain. Elsevier Ltd: Amsterdam, the Netherlands;; 2007. pp. 677–696. [Google Scholar]
- 6.Jovancevic-Misic J., Hayhoe M. Adaptive gaze control in natural environments. J Neurosci. 2009;29:6234–6238. doi: 10.1523/JNEUROSCI.5570-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franchak JM, Adolph KE. Visually guided navigation: head-mounted eye-tracking of natural locomotion in children and adults. Vis Res. 2010;50:2766–2774. doi: 10.1016/j.visres.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marigold DS, Weerdesteyn V., Patla AE, Duysens J. Keep looking ahead? Re-direction of visual fixation does not always occur during unpredictable obstacle avoidance task. Exp Brain Res. 2007;176:32–42. doi: 10.1007/s00221-006-0598-0. [DOI] [PubMed] [Google Scholar]
- 9.Weinreb RN, Aung T., Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014;311:1901–1911. doi: 10.1001/jama.2014.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tham Y-C, Li X., Wong TY, Quigley HA, Aung T., Cheng C-Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Friedman DS, Freeman E., Munoz B., Jampel HD, West SK. Glaucoma and mobility performance: the Salisbury eye evaluation project. Ophthalmology. 2007;114:2232–2237. doi: 10.1016/j.ophtha.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Turano KA, Rubin GS, Quigley HA. Mobility performance in glaucoma. Invest Ophthalmol Vis Sci. 1999;40:2803–2809. [PubMed] [Google Scholar]
- 13.Iester M., Zingirian M. Quality of life in patients with early, moderate and advanced glaucoma. Eye. 2002;16:44–49. doi: 10.1038/sj.eye.6700036. [DOI] [PubMed] [Google Scholar]
- 14.Nelson P., Aspinall P., Papasouliotis O., Worton B., O'Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma. 2003;12:139–150. doi: 10.1097/00061198-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Black AA, Wood JM, Lovie-Kitchin JE. Inferior field loss increases rate of falls in older adults with glaucoma. Optom Vis Sci. 2011;88:1275–1282. doi: 10.1097/OPX.0b013e31822f4d6a. [DOI] [PubMed] [Google Scholar]
- 16.Haymes SA, LeBlanc RP, Nicolela MT, Chaisson LA, Chauhan BC. Risk of falls and motor vehicle collisions in glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1149–1155. doi: 10.1167/iovs.06-0886. [DOI] [PubMed] [Google Scholar]
- 17.Lamoureux EL, Chong E., Wang JJ, et al. Visual impairment, causes of vision loss, and falls: the Singapore Malay eye study. Invest Ophthalmol Vis Sci. 2008;49:528–533. doi: 10.1167/iovs.07-1036. [DOI] [PubMed] [Google Scholar]
- 18.Tanabe S., Yuki K., Ozeki N., Shiba D., Tsubota K. The association between primary open-angle glaucoma and fall: an observational study. Clin Ophthalmol. 2012;6:327–331. doi: 10.2147/OPTH.S28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramulu PY, Hochberg C., Maul EA, Chan ES, Ferrucci L., Friedman DS. Glaucomatous visual field loss associated with less travel from home. Optom Vis Sci. 2014;91:187–193. doi: 10.1097/OPX.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasneci E., Black AA, Wood JM. Eye-tracking as a tool to evaluate functional ability in everyday tasks in glaucoma. J Ophthalmol. 2017;2017:6425913. doi: 10.1155/2017/6425913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lamirel C., Milea D., Cochereau I., Duong M-H, Lorenceau J. Impaired saccadic eye movement in primary open-angle glaucoma. J Glaucoma. 2014;23:23–32. doi: 10.1097/IJG.0b013e31825c10dc. [DOI] [PubMed] [Google Scholar]
- 22.Smith ND, Glen FC, Crabb DP. Eye movements during visual search in patients with glaucoma. BMC Ophthalmol. 2012;12:45. doi: 10.1186/1471-2415-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabb DP, Smith ND, Rauscher FG, et al. Exploring eye movements in patients with glaucoma when viewing a driving scene. PLoS One. 2010;5:e9710. doi: 10.1371/journal.pone.0009710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dive S., Rouland JF, Lenoble Q., Szaffarczyk S., McKendrick AM, Boucart M. Impact of peripheral field loss on the execution of natural actions: a study with glaucomatous patients and normally sighted people. J Glaucoma. 2016;25:e889–e896. doi: 10.1097/IJG.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 25.Cheong AMY, Geruschat DR, Congdon N. Traffic gap judgment in people with significant peripheral field loss. Optom Vis Sci. 2008;85:26–36. doi: 10.1097/OPX.0b013e31815ed6fd. [DOI] [PubMed] [Google Scholar]
- 26.Sippel K., Kasneci E., Aehling K., et al. Binocular glaucomatous visual field loss and its impact on visual exploration – a supermarket study. PLoS One. 2014;9:e106089. doi: 10.1371/journal.pone.0106089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AB, Lajoie K., Strath RA, Neima DR, Marigold DS. Coordination of gaze behavior and foot placement during walking in persons with glaucoma. J Glaucoma. 2018;27:55–63. doi: 10.1097/IJG.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 28.Mihailovic A., Swenor BK, Friedman DS, West SK, Gitlin LN, Ramulu PY. Gait implications of visual field damage from glaucoma. Trans Vis Sci Tech. 2017;6(3):23. doi: 10.1167/tvst.6.3.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lamb SE, Jørstad-Stein EC, Hauer K., Becker C. Development of a common outcome data set for fall injury prevention trials: the prevention of falls network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622. doi: 10.1111/j.1532-5415.2005.53455.x. [DOI] [PubMed] [Google Scholar]
- 30.Nelson-Quigg JM, Cello K., Johnson CA. Predicting binocular visual field sensitivity from monocular visual field results. Invest Ophthalmol Vis Sci. 2000;41:2212–2221. [PubMed] [Google Scholar]
- 31.Beauchet O., Annweiler C., Dubost V., et al. Stops walking when talking: a predictor of falls in older adults? Eur J Neurol. 2009;16:786–795. doi: 10.1111/j.1468-1331.2009.02612.x. [DOI] [PubMed] [Google Scholar]
- 32.Kosnik W., Winslow L., Kline D., et al. Visual changes in daily life throughout adulthood. J Gerontol. 1988;43:P63–P70. doi: 10.1093/geronj/43.3.p63. [DOI] [PubMed] [Google Scholar]
- 33.Beurskens R., Bock O. Age-related deficits of dual-task walking: a review. Neural Plast. 2012;2012:131608. doi: 10.1155/2012/131608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young WR, Hollands MA. Can telling older adults where to look reduce falls? Evidence for a causal link between inappropriate visual sampling and suboptimal stepping performance. Exp Brain Res. 2010;204:103–113. doi: 10.1007/s00221-010-2300-9. [DOI] [PubMed] [Google Scholar]
- 35.Turano KA, Geruschat DR, Baker FH, Stahl JW, Shapiro MD. Direction of gaze while walking a simple route: persons with normal vision and persons with retinitis pigmentosa. Optom Vis Sci. 2001;78:667–675. doi: 10.1097/00006324-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 36.Chapman GJ, Hollands MA. Evidence that older adult fallers prioritise the planning of future stepping actions over the accurate execution of ongoing steps during complex locomotor tasks. Gait Posture. 2007;26:59–67. doi: 10.1016/j.gaitpost.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 37.Geruschat DR, Smith AJ. Improving the use of low vision for orientation and mobility. In: Wiener WR, RL Welsh, Blasch BB, editors. Foundations of Orientation and Mobility, 3rd Edition, Volume 2: Instructional Strategies and Practical Applications. AFB press: American Foundation for the Blind; New York, New York: 2010. pp. 54–90. [Google Scholar]
- 38.Ludt R., Goodrich GL. Change in visual perceptual detection distances for low vision travelers as a result of dynamic visual assessment and training. J Vis Impair Blind. 2002;96:7–15. [Google Scholar]
- 39.Authié CN, Berthoz A., Sahel J-A, Safran AB. Adaptive gaze strategies for locomotion with constricted visual field. Front Hum Neurosci. 2017;11:387. doi: 10.3389/fnhum.2017.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture. 1997;5:54–69. [Google Scholar]
- 41.Patla AE, Vickers JN. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport. 1997;8:3661–3665. doi: 10.1097/00001756-199712010-00002. [DOI] [PubMed] [Google Scholar]
- 42.Timmis MA, Allsop J., Baranian M., et al. Visual search behavior in individuals with retinitis pigmentosa during level walking and obstacle crossing. Invest Ophthalmol Vis Sci. 2017;58:4737–4746. doi: 10.1167/iovs.17-21573. [DOI] [PubMed] [Google Scholar]
- 43.Di Fabio RP, Greany JF, Zampieri C. Saccade-stepping interactions revise the motor plan for obstacle avoidance. J Mot Behav. 2003;35:383–397. doi: 10.1080/00222890309603158. [DOI] [PubMed] [Google Scholar]
- 44.Keller Chandra S., Bockisch CJ, Dietz V., Hegemann SCA, Straumann D., van Hedel HJA. Gaze strategies for avoiding obstacles: differences between young and elderly subjects. Gait Posture. 2011;34:340–346. doi: 10.1016/j.gaitpost.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 45.Hollands MA, Patla AE, Vickers JN. “Look where you're going!”: gaze behaviour associated with maintaining and changing the direction of locomotion. Exp Brain Res. 2002;143:221–230. doi: 10.1007/s00221-001-0983-7. [DOI] [PubMed] [Google Scholar]
- 46.Nummenmaa L., Hyönä J., Hietanen JK. I'll walk this way: eyes reveal the direction of locomotion and make passersby look and go the other way. Psych Sci. 2009;20:1454–1458. doi: 10.1111/j.1467-9280.2009.02464.x. [DOI] [PubMed] [Google Scholar]
- 47.Wann JP, Swapp DK. Why you should look where you are going. Nat Neurosci. 2000;3:647–648. doi: 10.1038/76602. [DOI] [PubMed] [Google Scholar]
- 48.Gerin-Lajoie M., Richards CL, McFadyen BJ. The circumvention of obstacles during walking in different environmental contexts: a comparison between older and younger adults. Gait Posture. 2006;24:364–369. doi: 10.1016/j.gaitpost.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Yamada M., Tanaka H., Mori S., et al. Fallers choose an early transfer gaze strategy during obstacle avoidance in dual-task condition. Aging Clin Exp Res. 2011;23:316–319. doi: 10.1007/BF03337757. [DOI] [PubMed] [Google Scholar]