Graphical abstract
Keywords: Intracardiac thrombus, Cardiac magnetic resonance imaging, Hypereosinophilic syndrome, Eosinophilic myocarditis
Highlights
-
•
EM involves acute necrotic, intermediate thrombotic, and late fibrotic stages.
-
•
Echocardiography is the initial modality for the diagnosis of EM.
-
•
EMB could miss the diagnosis if the area involved is not biopsied.
-
•
CMR reliably detects all three stages of EM.
Introduction
Hypereosinophilic syndrome (HES) is defined as an absolute eosinophil count > 1.5 × 109/L on two separate examinations separated by at least 1 month and/or pathologic tissue confirmation with evidence of end organ damage in the absence of any known cause of hypereosinophilia. HES is a rare condition with unknown prevalence and is subclassified into primary, secondary, or idiopathic. Primary HES occurs in the setting of stem cell, myeloid, or eosinophilic neoplasm. Secondary HES is a reactive process due to parasitic infections, certain solid tumors, and T-cell lymphoma. Patients are usually diagnosed between 20 and 50 years of age, but HES can also be seen in children.1, 2, 3 In all classes, eosinophils infiltrate and damage common target organs that can include the pulmonary system (Loeffler's syndrome) and/or the cardiovascular system, resulting in eosinophilic myocarditis (EM).4
Case Presentation
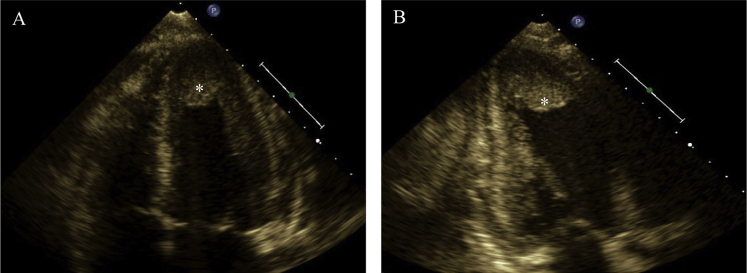
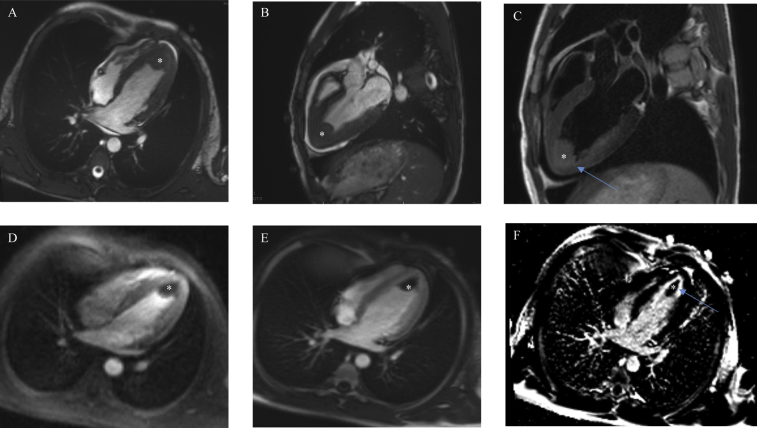
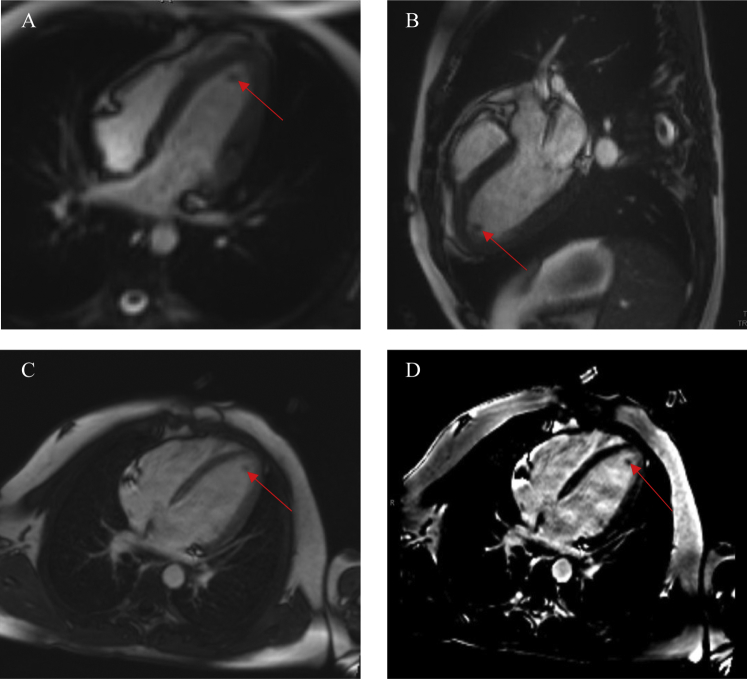
A 15-year-old African American male patient with a medical history significant for asthma as a toddler originally presented to his pediatrician's office because of intermittent palpitations and fatigue. Four days prior, he endorsed palpitations that woke him from sleep and resolved spontaneously within 20 min. The following day he attended football practice, at which he had several presyncopal episodes but no overt syncope. He continued to have intermittent palpitations over the next few days. He otherwise denied fever, weight loss, recent travel, drug exposures, and any known food or drug allergies. He denied any aggravating or alleviating factors. He was diagnosed with a probable viral syndrome and sent home. He continued to have palpitations and fatigue the following day and thus presented to the emergency department. Physical examination was pertinent for tachycardia with a heart rate of 117 beats/min, fever of 38.1°C (311.2 K), and no skin rashes. Laboratory results revealed a white blood cell count of 6.4 × 109/L with 64% eosinophils. Cardiac markers revealed a troponin level of 5.11 μg/L. Electrocardiography showed ST-segment changes concerning for myopericarditis. Subsequent workup with peripheral blood smear and bone marrow biopsy resulted in the diagnosis of B-cell acute lymphoblastic leukemia with marked eosinophilia. Transthoracic echocardiography revealed preserved left ventricular function, a small pericardial effusion, and an apical mass (Figure 1, Video 1, Video 2). Cardiac magnetic resonance imaging (CMR) revealed a large 2.7-cm apical mass in the setting of normal wall motion and overall preserved left ventricular function (Figure 2A and B, Video 3, Video 4). Tissue characterization of the mass with T2-weighted turbo spin echo, short tau inversion recovery, first-pass perfusion, and inversion recovery late gadolinium-contrast sequences were performed. Precontrast T2-weighted turbo spin echo sequences revealed hyperintense signal in the left ventricular myocardium in the apical and inferior wall regions suggestive of myocardial inflammation (Figure 2C). Resting first-pass perfusion images did not reveal any gadolinium uptake in the mass, suggesting absence of vascularity (Figure 2D). Post–gadolinium contrast inversion recovery sequences with high inversion time set at 600 msec revealed homogeneous black appearance of the apical mass consistent with thrombus (Figure 2E). Post–gadolinium late enhancement images set to null the myocardium revealed abnormal enhancement of the apical myocardium, suggestive of adjacent myocardial inflammation (Figure 2F). Diagnosis of eosinophilic heart disease (thrombotic stage) in the setting of acute lymphoblastic leukemia was made, and the patient was started on chemotherapy, glucocorticoids, and anticoagulation. Repeat CMR 3 months later showed reduced thrombus size of 0.2 × 0.7 cm, normal ejection fraction, and no evidence of myocardial fibrosis (Figure 3, Video 5, Video 6).
Figure 1.
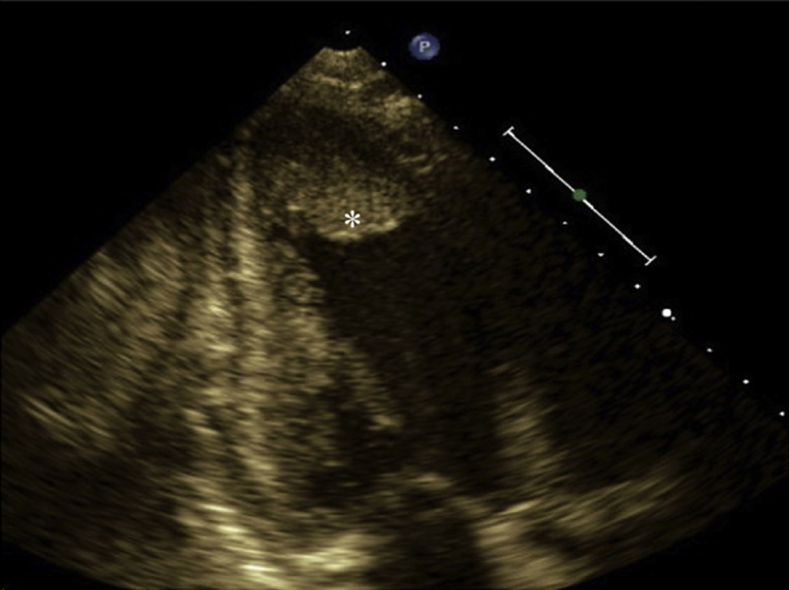
Transthoracic echocardiography, (A) apical four-chamber view and (B) apical two-chamber view, showing apical isoechoic density (asterisk) suspicious for left ventricular mural thrombus.
Figure 2.
Cardiac magnetic resonance, (A) cine steady-state free precession (SSFP) four-chamber view and (B) cine SSFP three-chamber view, showing a large 2.7-cm apical mass (asterisk) in the setting of normal wall motion and overall preserved left ventricular function and small pericardial effusion. (C) T2-weighted fast spin echo sequences with evidence of hyperintense signal in the apical and inferior myocardial wall adjacent to the thrombus suggestive of myocardial inflammation (blue arrow). (D) Resting first-pass perfusion images did not reveal any gadolinium uptake in the mass, suggesting absence of vascularity. Postgadolinium inversion recovery sequences performed with high inversion times (600 ms) in (E) four-chamber view and (F) two-chamber view revealed lack of delayed enhancement and homogeneously black appearance consistent with thrombus and adjacent apical myocardial inflammation.
Figure 3.
Cardiac magnetic resonance, (A) cine steady-state free precession (SSFP) four-chamber view and (B) cine SSFP three-chamber view, showing significant reduction in the size of the apical thrombus (red arrow). Postgadolinium inversion recovery sequences performed in four-chamber view (C,D) revealed no evidence of myocardial fibrosis.
Discussion
EM is present in 60% of patients with HES and remains a major cause of morbidity and mortality, but cardiac symptoms at onset of presentation represent only 5% of patients. Women are less frequently affected than men and in turn have better outcomes.5 The spectrum of presentation ranges from chest pain, dyspnea, and palpitations to cardiogenic shock, with the most common presentation being dyspnea and palpitations occurring in only 8% of patients.6, 7 Elevated troponin concentrations are evidence of eosinophil-mediated cardiac cell damage that involves three stages: an acute necrotic stage, an intermediate stage involving damaged endocardium and thrombus formation, and finally a fibrotic stage with altered cardiac function. The acute necrotic stage is typically of short duration, about 5 to 6 weeks, while the thrombotic and fibrotic stages are usually 10 and 24 months, respectively. The acute necrotic stage entails eosinophilic myocardial infiltration with subsequent necrosis due to degranulating eosinophilic release of toxic cationic proteins.7 Infiltration can range from a mild localized focus to a multifocal or widespread area that can be perivascular or interstitial and involve the epicardium and endocardium.6 It is difficult to diagnose the disease at this stage, as patients often do not have cardiac symptoms, and electrocardiography and echocardiography are unrevealing. This stage is followed by the thrombotic stage that often involves both ventricles because of vascular endocardial damage. Blood flow is relatively static at the endomyocardial surface, particularly at the apices, and this promotes accumulation of clotting factors with subsequent clot formation.7 Blood hypercoagulability also contributes to thrombus formation through release of von Willebrand factor, tissue factor, and factor XII activation by eosinophilic granule proteins. Echocardiography often shows obliteration of the apex by the thrombus, which is later replaced by fibrosis of the apex.8 Patients may not be diagnosed until the fibrotic stage, when they present with cardiac symptoms due to restrictive or dilated cardiomyopathy and/or chordae tendineae entrapment causing mitral or tricuspid regurgitation.9 Classic findings include biventricular apical thrombi in the setting of normal wall motion, posterior mitral leaflet involvement, and endomyocardial thickening, which is often seen in the left ventricular free wall.9 There can also be signs of restrictive cardiomyopathy with valvular regurgitation due to subvalvular damage.7, 9
Although echocardiography is the initial modality performed on patient presentation, findings can be normal during the acute stage. Gadolinium contrast-enhanced CMR, however, reliably detects all stages, including early myocardial eosinophilic inflammation, thrombus formation, and fibrosis. CMR is more sensitive and specific than either transthoracic or transesophageal echocardiography for detection of ventricular thrombi.5, 7 T2-weighted sequences detect myocardial inflammation during the acute phase noted by myocardial hyperintensity, while postgadolinium inversion recovery delayed enhancement sequences can detect thrombotic and fibrotic stages.10 Cine CMR can detect the functional impact of the thrombus and eosinophilic infiltrate, while late enhancement detects myocardial fibrosis and inflammation.11 A study by Tani et al.10 found the extent of the lesion identified on CMR to be inversely proportional to the left ventricular ejection fraction, with another study by Miszalski-Jamka et al.5 finding the maximum measured blood eosinophil count to correlate with the extent of myocardial damage seen on endomyocardial biopsy (EMB). Although EMB is the gold standard and may provide definitive diagnosis, this is typically reserved for those patients in whom there is uncertainty of eosinophilic-induced cardiac disease. EMB has an estimated sensitivity of only 50% and can miss the diagnosis if the area biopsied is not involved. EMB is an invasive procedure and carries risk for iatrogenic complications such as thromboembolism.6, 11 CMR, however, can reliably detect all stages of the disease process and all areas of myocardium involved, with serial CMR allowing disease monitoring and response to treatment, as was the case in our patient.12
Treatment involves glucocorticoids to reduce tissue and peripheral eosinophil levels to <600/μL and subsequent end organ damage. In steroid-resistant cases, imatinib has been shown to be effective.8, 11 For patients who develop congestive heart failure or thrombus, standard heart failure medical therapy is used, as well as anticoagulation for thrombus formation. Up to 25% of patients develop thromboembolic complications, with 5% to 10% resultant mortality.7 Valve replacement or cardiac transplantation is reserved for those with severe valve or myocardial involvement, with bioprosthetic valve replacement being preferred over mechanical because of thrombotic complications.9
Conclusions
Cardiac involvement in HES remains a major cause of morbidity and mortality. Initial patient presentation can be variable, and delayed presentation is common, as patients do not have symptoms until significant disease progression. Echocardiography is the preferred initial modality but can be unrevealing during the acute phase of the disease. EMB is the gold standard for definitive diagnosis, but it is an invasive procedure with potential complications and could still miss the diagnosis if the focal myocardial area is not biopsied. CMR reliably detects all stages of the disease and thus may be the most practical if not best modality for initial diagnosis and follow-up for progression to fibrotic stage or resolution after treatment.
Footnotes
Conflict of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.case.2017.12.003.
Supplementary Data
Transthoracic echocardiography, apical four-chamber view, showing an apical isoechoic density suspicious for a left ventricular mural thrombus.
Transthoracic echocardiography, apical two-chamber view, showing an apical isoechoic density suspicious for a left ventricular mural thrombus.
Cardiac magnetic resonance, cine steady-state free precession four-chamber view, showing a large 2.7-cm apical mass in the setting of normal wall motion and overall preserved left ventricular function and a small pericardial effusion.
Cardiac magnetic resonance, cine steady-state free precession three-chamber view, showing a large 2.7-cm apical mass in the setting of normal wall motion and overall preserved left ventricular function and a small pericardial effusion.
Cardiac magnetic resonance, cine steady-state free precession three-chamber view, showing significant reduction in the size of the apical thrombus.
Postgadolinium inversion recovery sequences performed in four-chamber view revealed no evidence of myocardial fibrosis.
References
- 1.Farruggia P., D’Angelo P., Acquaviva A., Trizzino A., Tucci F., Cilloni D. Hypereosinophilic syndrome in childhood: clinical and molecular features of two cases. Pediatr Hematol Oncol. 2009;26:129–135. doi: 10.1080/08880010902773024. [DOI] [PubMed] [Google Scholar]
- 2.Rapanotti M., Caruso R., Ammatuna E., Zaza S., Trotta L., Divona M. Molecular characterization of paediatric idiopathic hypereosinophilia. Br J Haematol. 2010;151:440–446. doi: 10.1111/j.1365-2141.2010.08394.x. [DOI] [PubMed] [Google Scholar]
- 3.Weller P., Bubley G. The idiopathic hypereosinophilic syndrome. Blood. 1994;83:2759–2779. [PubMed] [Google Scholar]
- 4.Ekin S., Sertogullarindan B., Gunbatar H., Arisoy A., Yildiz H. Loeffler’s syndrome: an interesting case report. Clin Respir J. 2016;10:112–114. doi: 10.1111/crj.12173. [DOI] [PubMed] [Google Scholar]
- 5.Miszalski-Jamka T., Szczeklik W., Karwat K., Sokolowska B., Gasior J., Rucinkska M. MRI-based evidence for myocardial involvement in women with hypereosinophilic syndrome. Magn Reson Med Sci. 2015;14:107–114. doi: 10.2463/mrms.2013-0133. [DOI] [PubMed] [Google Scholar]
- 6.Al Ali A., Straatman L., Allard M., Ignaszewski A. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol. 2006;22:1233–1237. doi: 10.1016/s0828-282x(06)70965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogbogu P., Rosing D., Horne M. Cardiovascular manifestations of hypereosinophilic syndromes. Immunol Allergy Clin North Am. 2007;27:457–475. doi: 10.1016/j.iac.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K., Chuah M., Tang H., Chua T. Hypereosinophilic syndrome with large intracardiac thrombus. Singapore Med J. 2014;55:e129–e131. doi: 10.11622/smedj.2014109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim N., Kim C.Y., Kim J., Jang S., Bae M., Lee J. A hypereosinophilic syndrome with cardiac involvement from thrombotic stage to fibrotic stage. J Cardiovasc Ultrasound. 2015;23:100–102. doi: 10.4250/jcu.2015.23.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tani H., Amano Y., Tachi M., Machida T., Mizuno K., Kumita S. T2-weighted and delayed enhancement MRI of eosinophilic myocarditis: relationship with clinical phases and global cardiac function. Jpn J Radiol. 2012;30:824–831. doi: 10.1007/s11604-012-0130-3. [DOI] [PubMed] [Google Scholar]
- 11.Kleinfeldt T., Nienaber C.A., Kische S., Akin I., Turan R.G., Korber T. Cardiac manifestation of the hypereosinophilic syndrome: new insights. Clin Res Cardiol. 2010;99:419–427. doi: 10.1007/s00392-010-0144-8. [DOI] [PubMed] [Google Scholar]
- 12.Looi J.L., Ruygrok P., Royle G., Raos Z., Hood C., Kerr A.J. Acute eosinophilic endomyocarditis: early diagnosis and localisation of the lesion by cardiac magnetic resonance imaging. Int J Cardiovasc Imaging. 2010;26:151–154. doi: 10.1007/s10554-009-9580-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiography, apical four-chamber view, showing an apical isoechoic density suspicious for a left ventricular mural thrombus.
Transthoracic echocardiography, apical two-chamber view, showing an apical isoechoic density suspicious for a left ventricular mural thrombus.
Cardiac magnetic resonance, cine steady-state free precession four-chamber view, showing a large 2.7-cm apical mass in the setting of normal wall motion and overall preserved left ventricular function and a small pericardial effusion.
Cardiac magnetic resonance, cine steady-state free precession three-chamber view, showing a large 2.7-cm apical mass in the setting of normal wall motion and overall preserved left ventricular function and a small pericardial effusion.
Cardiac magnetic resonance, cine steady-state free precession three-chamber view, showing significant reduction in the size of the apical thrombus.
Postgadolinium inversion recovery sequences performed in four-chamber view revealed no evidence of myocardial fibrosis.