Graphical abstract
Keywords: Pseudoaneurysm, Myocardial infarction, Multimodality imaging
Highlights
-
•
LV pseudoaneurysm is a rare but fatal complication of myocardial infarction.
-
•
Multimodality imaging is important in the diagnosis.
-
•
Surgical repair is the mainstay of treatment.
-
•
Conservative management may be necessary in patients with excessive surgical risk.
Introduction
Left ventricular (LV) pseudoaneurysm is a rare complication of myocardial infarction with a high risk for mortality. The incidence has decreased in the era of early and aggressive revascularization. Surgical repair remains the cornerstone of treatment. We describe the case of a 72-year-old woman who presented for routine evaluation of atypical chest pain and was noted to have impressive findings of a chronic massive LV pseudoaneurysm with thrombus demonstrated through multimodality imaging. She was ultimately treated conservatively in view of her prohibitive surgical risk.
Case Presentation
A 72-year-old woman was referred by her primary care physician for further evaluation of chronic atypical chest pain and dyspnea. She had a long-standing history of destructive rheumatoid arthritis on disease-modifying therapy with methotrexate 20 mg weekly and hydroxychloroquine 200 mg/d. Her other cardiac risk factors were significant for hypertension, dyslipidemia, and an extensive smoking history. She had no known ischemic heart disease.
The patient reported atypical chest pain several months before presentation and had markedly reduced exercise capacity. Her chest pain was described as brief, sharp, nonexertional, and transient, with episodes lasting up to 2 minutes at most. She denied any prolonged or severe episode of chest pain. She also reported dyspnea, although this was chronic and multifactorial because of her significant functional debilitation and heavy smoking history, with no acute change in symptomatology. Although she denied any clear heart failure symptoms such as peripheral edema and orthopnea, low cardiac output from impaired overall systolic function and coronary artery disease provided a strong suspicion in view of her cardiac risk profile. Her functional impairment was long-standing and had rendered her largely housebound.
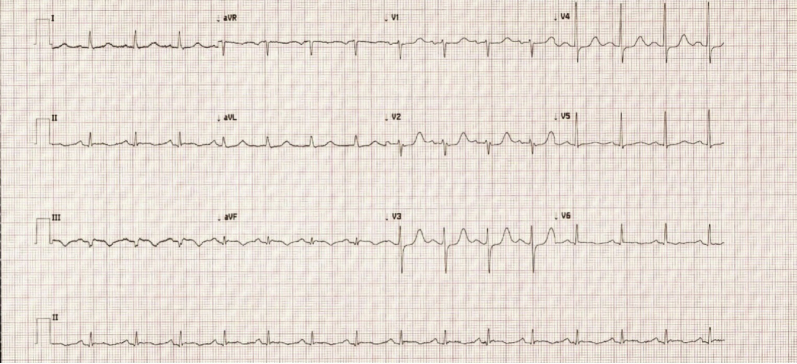
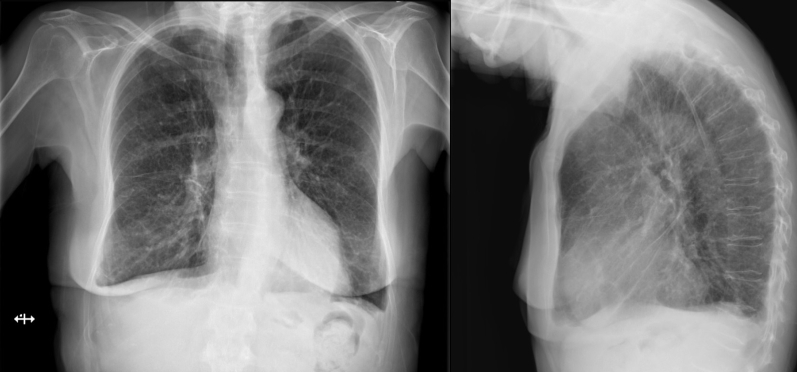
Electrocardiography demonstrated sinus rhythm with a Q wave in lead III and T-wave inversions in leads III and aVF (Figure 1). Chest radiography revealed a normal cardiothoracic ratio with evidence of congestion on the posterior-anterior projection and a protuberant posterior LV contour on the lateral view (Figure 2).
Figure 1.
Electrocardiogram demonstrating sinus rhythm with Q waves in lead III and T-wave inversions in leads III and aVF.
Figure 2.
Posterior-anterior and lateral views of chest radiograph with evidence of congestion and protuberant posterior LV contour (arrow).
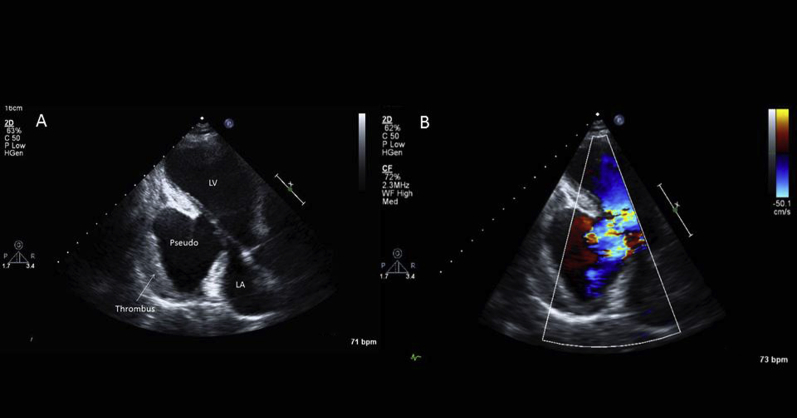
Transthoracic echocardiography was unexpectedly suspicious for a large inferolateral wall pseudoaneurysm with organized thrombus (Figure 3, Video 1). Her overall LV ejection fraction was difficult to estimate, but it was clear that she had a severely reduced ejection fraction in the setting of this large pseudoaneurysm.
Figure 3.
(A) Transthoracic echocardiogram: long-axis view demonstrating large inferolateral wall pseudoaneurysm with evidence of thrombus. (B) Color flow imaging showing clear communication between pseudoaneurysm and left ventricular cavity. LA, Left atrium; LV, left ventricle.
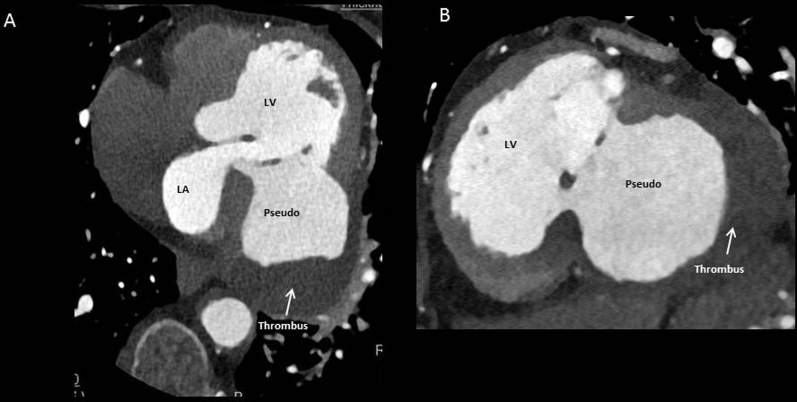
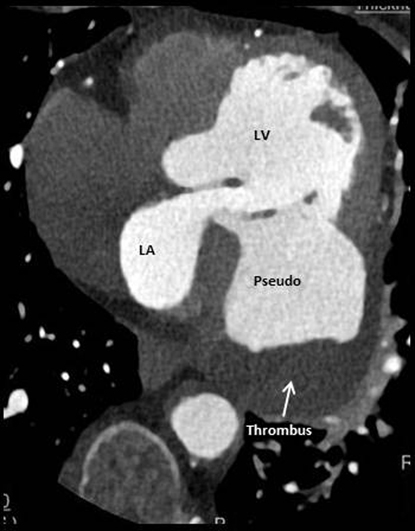
Although transthoracic echocardiography was essentially diagnostic, further imaging was required for better anatomic delineation, surgical planning, and coronary artery status. She underwent retrospective helical cardiac computed tomography (CT), which confirmed a large pseudoaneurysm associated with rupture of the basal to mid-inferolateral/inferior segments measuring 83 × 83 × 72 mm, with a neck up to 36 × 56 mm (Figure 4, Figure 5, Video 2, Video 3). Delayed phase contrast demonstrated a significant filling defect consistent with organized thrombus. Severe right coronary artery disease was also demonstrated, with total occlusion at the mid segment and no significant stenosis in her other coronary arteries.
Figure 4.
(A) Computed tomographic coronary angiogram demonstrating four-chamber axial view of large pseudoaneurysm and filling defect consistent with organized thrombus. (B) Short-axis view. LA, Left atrium; LV, left ventricle.
Figure 5.
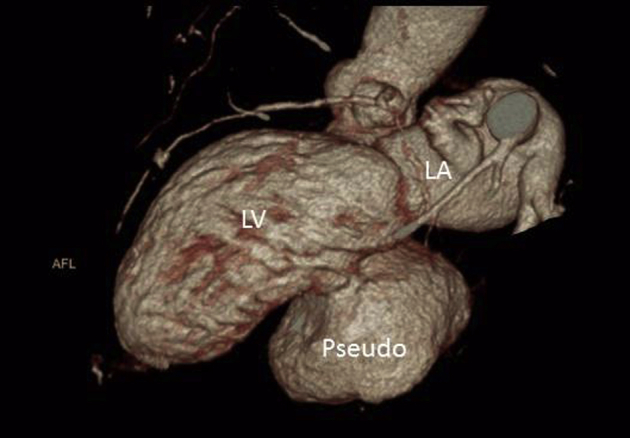
Four-dimensional computed tomographic reconstruction. LA, Left atrium; LV, left ventricle.
She was referred for consideration of surgical management of this large LV pseudoaneurysm. After heart team review and discussion with the patient and her family, she was deemed too high risk in view of her significant frailty and other medical comorbidities.
It was unclear what her prognosis would be at this stage given the chronicity of her pseudoaneurysm as well as the systemic embolization risk. The patient and family were keen to know her prognosis, and as such we performed limited noncontrast cardiac magnetic resonance imaging (MRI) to facilitate tomographic surveillance of possible pseudoaneurysm expansion for prognostication purposes. This further delineated massive pseudoaneurysm and organized thrombus (Figure 6, Video 4), with a severely dilated left ventricle and severely reduced ejection fraction. Accurate estimation of overall ejection fraction was not feasible by any modality, but it was clearly severely reduced (<30%) by visual estimation. Accordingly, conservative management was pursued, and she currently remains stable on optimal heart failure therapy.
Figure 6.
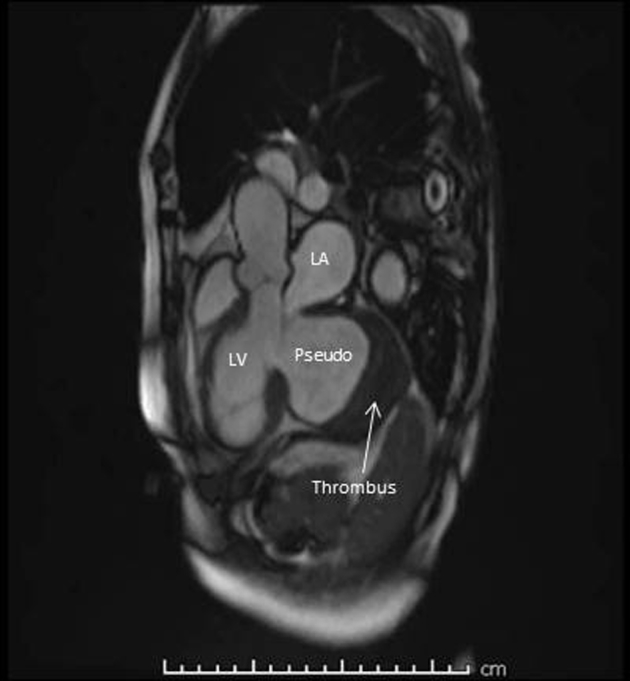
Cardiac MRI showing pseudoaneurysm with thrombus. LA, Left atrium; LV, left ventricle.
Discussion
LV pseudoaneurysm occurs as a result of contained cardiac rupture limited by adherent pericardium.1 The resultant aneurysm often has a thin, akinetic wall composed mainly of pericardium and fibrous tissue and hence poses a significant risk for rupture with high mortality.1, 2 This is in contrast to true aneurysms, which are formed from endocardium, myocardium, and pericardium and have less clinical and surgical urgency.2 Differentiation between the two entities is of paramount importance because of the prognostic and management implications. The presumed risk for rupture is between 30% and 45% in the case of LV pseudoaneurysms.1, 3 Symptoms of LV pseudoaneurysm include congestive heart failure, chest pain, and embolism, but >10% are asymptomatic.1, 2 Pseudoaneurysms typically have a narrow neck with a ratio of the breadth in the wall to maximum diameter of <50%.4 In contrast, a true aneurysm will have a broad neck.4
Typically, the main etiology for this condition is transmural myocardial infarction, but other less common causes include postsurgical, trauma, and infection.1, 5 Early and aggressive revascularization has certainly reduced the incidence of this complication. The cause in our patient's case was presumably a remote silent myocardial infarction. Inferior myocardial infarction seems to account for twice as many cases as anterior infarctions, with subsequent pseudoaneurysmal formation on the inferoposterior surface of the left ventricle being more common.1, 2, 3 One proposed explanation is that anterior rupture is more likely to lead to hemopericardium, shock, and death compared with posterior rupture, which is more likely to be contained by pericardium with inflammatory adhesions.1, 3 In contrast, true aneurysms generally tend to occur in the anterior and apical walls.6
Risk factors for the development of LV pseudoaneurysm include female sex, first occurrence of myocardial infarction, age > 60 years, and severe single-vessel coronary disease.7 Rheumatoid arthritis and indeed chronic inflammation remain potent contributors to cardiovascular risk.8 Through chronic inflammation, rheumatoid arthritis can cause endothelial and vascular dysfunction and resultant coronary artery disease. Chronic immunosuppressive therapy is well known to affect wound healing, but whether this might play a role in the extent and severity of our patient's pseudoaneurysm remains speculative. Indeed animal studies have suggested that corticosteroids may lead to myocardial scar thinning and possibly increased aneurysmal formation following myocardial infarction.9
LV angiography has traditionally been considered the best available test for the diagnosis of LV pseudoaneurysm, often because coronary angiography was necessary before surgery.1 Transthoracic echocardiography remains important as a noninvasive means of diagnosis, and increasing use of multimodality imaging through cardiac CT and cardiac MRI often diminishes the need for upfront invasive angiography for diagnosis.10 Although transthoracic echocardiography was essentially diagnostic in this case, further imaging with cardiac CT was required for improved anatomic delineation, surgical planning, and coronary artery status. However, once a decision of conservative treatment was taken, limited cardiac MRI was performed to facilitate tomographic surveillance of possible pseudoaneurysm expansion for prognostic purposes as per the patient's wishes. Lack of radiation was a clear advantage of cardiac MRI over cardiac CT for serial follow-up. In cases of smaller pseudoaneurysm, the sensitivity of echocardiography is reduced, and in this situation cardiac MRI may be useful in diagnosis.1 Although delayed enhancement imaging is an important technique to define myocardial elements and infarct extent, we believed that this would not add any further information in our clinical scenario. If there was difficulty defining myocardium from thrombus, then certainly delayed enhancement would be imperative for accurate differentiation.
Preemptive surgery remains the cornerstone of treatment because of the high risk for rupture. However, mortality rates are still high even with surgery: 23% to 30%, with a rate even higher at 48% for those patients treated conservatively.1, 11, 12 Improvements in surgical technique may have reduced this perioperative mortality rate to <10%.11 Surgery is urgently recommended when pseudoaneurysm complicates myocardial infarction early (i.e., within 2–3 months), as rupture may be more unpredictable.12 Less is known about incidentally discovered chronic pseudoaneurysms and risk for rupture, with operative intervention being guided by symptoms in these cases.12 Although surgical intervention is preferred, conservative management in those high-risk patients may be reasonable, with no deaths due to further rupture reported in a case series of 52 patients between 1980 and 1996.13 Indeed, in this series, 48% of pseudoaneurysms were diagnosed incidentally, as in the case of our patient.13 Percutaneous closure by occlusion devices in these high-risk patients has been described in case reports and may be feasible in selected patients.14 A series of seven cases demonstrated successful repair, with improved New York Heart Association functional class over a mean follow-up period of 18 ± 12.5 months.14 Preprocedural planning and detailed multimodality imaging were critical to device sizing and delivery. Percutaneous closure may therefore be considered to reduce the risk for fatal rupture, improve heart failure symptoms, and potentially reduce systemic embolism risk. However, this was not considered suitable in our patient because of the massive size of her pseudoaneurysm.
Medical therapy often involves optimizing pharmacotherapy for heart failure as well as emphasis on blood pressure control to minimize wall stress. Certainly static blood flow in the akinetic pouch predisposes to thrombus formation. Oral anticoagulation may be considered in cases of thrombus formation, as in cases of LV aneurysm, although the risk for embolism is relatively unclear.1, 13, 15 In one series of 69 patients not anticoagulated, the incidence of systemic embolization in chronic LV aneurysm was low at 0.35 per 100 patient-years.15 Indeed, the literature in this area is controversial and lacking, and generally refers to LV aneurysm rather than pseudoaneurysm. After discussion with our patient, we elected to treat with aspirin alone, in view of her increased bleeding risk.
Conclusions
LV pseudoaneurysm remains a lethal complication of myocardial infarction that often necessitates urgent surgical repair. Multimodality imaging is important in the diagnosis and management of such complications. Our patient has thus far remained clinically stable nearly 12 months since the diagnosis.
Footnotes
Conflicts of interest: The authors reported no actual or potential conflicts of interest relative to this document.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.case.2017.11.003.
Supplementary Data
Transthoracic echocardiogram: long-axis view demonstrating large inferolateral wall pseudoaneurysm with evidence of thrombus. Color flow imaging shows clear communication between pseudoaneurysm and left ventricular cavity. LA, Left atrium; LV, left ventricle.
Computed tomographic coronary angiographic inflow-outflow cine demonstrating massive pseudoaneurysm with thrombus. LA, Left atrium; LV, left ventricle.
Four-dimensional computed tomographic reconstruction showing relationship of pseudoaneurysm (pseudo) to other chambers. LA, Left atrium; LV, left ventricle.
Cardiac MRI demonstrating massive pseudoaneurysm and thrombus. LA, Left atrium; LV, left ventricle.
References
- 1.Frances C., Romero A., Grady D. Left ventricular pseudoaneurysm. J Am Coll Cardiol. 1998;32:557–561. doi: 10.1016/s0735-1097(98)00290-3. [DOI] [PubMed] [Google Scholar]
- 2.Bisoyi S., Dash A., Nayak D., Sahoo S., Mohapatra R. Left ventricular pseudoaneurysm versus aneurysm a diagnosis dilemma. Ann Card Anaesth. 2016;19:169–172. doi: 10.4103/0971-9784.173042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vlodaver Z., Coe J., Edwards J. True and false left ventricular aneurysms: propensity for the latter to rupture. Circulation. 1975;51:567–572. doi: 10.1161/01.cir.51.3.567. [DOI] [PubMed] [Google Scholar]
- 4.Hulten E., Blankstein R. Pseudoaneurysms of the heart. Circulation. 2012;125:1920–1925. doi: 10.1161/CIRCULATIONAHA.111.043984. [DOI] [PubMed] [Google Scholar]
- 5.Friedman B., Dunn M. Postinfarction ventricular aneurysms. Clin Cardiol. 1995;18:505–511. doi: 10.1002/clc.4960180905. [DOI] [PubMed] [Google Scholar]
- 6.Tuan J., Kaivani F., Fewins H. Left ventricular pseudoaneurysm. Eur J Echocardiogr. 2008;9:107–109. doi: 10.1016/j.euje.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 7.Ludmir K., Kapoor K., George P., Khural J., Barr B. Left ventricular pseudoaneurysm following inferior myocardial infarction: a case for conservative management. Cardiol Res. 2016;7:32–35. doi: 10.14740/cr449w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowson C., Liao K., Davis J., Solomon D., Matteson E., Knutson K. Rheumatoid arthritis and cardiovascular disease. Am Heart J. 2013;166:622–628.e1. doi: 10.1016/j.ahj.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.March K., Sawada S., Tarver R., Kesler K., Armostrong W. Current concepts of left ventricular pseudoaneurysm: pathophysiology, therapy and diagnostic imaging methods. Clin Cardiol. 1989;12:531–540. doi: 10.1002/clc.4960120911. [DOI] [PubMed] [Google Scholar]
- 10.Ho H., Sinaga D., Lee E., Watson T., Hon J. Left ventricular pseudoaneurysm. J Geriatr Cardiol. 2017;14:78–80. doi: 10.11909/j.issn.1671-5411.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Csapo K., Voith L., Szuk T., Edes I., Kereiakes D. Postinfarction left ventricular pseudoaneurysm. Clin Cardiol. 1997;20:898–903. doi: 10.1002/clc.4960201021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prifti E., Bnoacchi M., Baboci A., Giunti G., Veshti A., Demiraj A. Surgical treatment of post-infarction left ventricular pseudoaneurysm: case series highlighting various surgical strategies. Ann Med Surg (Lond) 2017;16:44–51. doi: 10.1016/j.amsu.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeo T., Malouf J., Oh J., Seward J. Clinical profile and outcome in 52 patients with cardiac pseudoaneurysm. Ann Intern Med. 1998;128:299–305. doi: 10.7326/0003-4819-128-4-199802150-00010. [DOI] [PubMed] [Google Scholar]
- 14.Dudiy Y., Jelnin V., Einhorn B., Kronzon I., Cohen H., Carlos R. Percutaneous closure of left ventricular pseudoaneurysm. Circ Cardiovasc Interv. 2011;4:322–326. doi: 10.1161/CIRCINTERVENTIONS.111.962464. [DOI] [PubMed] [Google Scholar]
- 15.Lapeyre A., Steele P., Kazmier F., Chesebro J., Vlietstra R., Fuster V. Systemic embolism in chronic left ventricular aneurysm: incidence and role of anticoagulation. J Am Coll Cardiol. 1985;6:534–538. doi: 10.1016/s0735-1097(85)80109-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transthoracic echocardiogram: long-axis view demonstrating large inferolateral wall pseudoaneurysm with evidence of thrombus. Color flow imaging shows clear communication between pseudoaneurysm and left ventricular cavity. LA, Left atrium; LV, left ventricle.
Computed tomographic coronary angiographic inflow-outflow cine demonstrating massive pseudoaneurysm with thrombus. LA, Left atrium; LV, left ventricle.
Four-dimensional computed tomographic reconstruction showing relationship of pseudoaneurysm (pseudo) to other chambers. LA, Left atrium; LV, left ventricle.
Cardiac MRI demonstrating massive pseudoaneurysm and thrombus. LA, Left atrium; LV, left ventricle.