Summary
Eroom’s law (Moore’s law spelled backwards), describes adverse trends towards declining innovation and rising costs of drug development over the last several decades. Therapeutics for cardiovascular diseases (CVD) appear to have been particularly sensitive to these trends. Thirty-three percent fewer CVD therapeutics were approved between 2000 and 2009 compared to the previous decade, and the number of CVD drugs starting all clinical trial stages declined in both absolute and relative numbers between 1990 and 2012. In the last 5 years, drugs to treat CVD disease comprised just 6% of all new drug launches. This review discusses the decline in CVD therapeutics, the reasons behind it, and ways in which this trend is being or might be addressed.
Key Words: Eroom’s law, innovation, drug approval
Abbreviations and Acronyms: CVD, cardiovascular disease; FDA, Food and Drug Administration; NIH, National Institutes of Health; OD, orphan drug; PPMD, parent project muscular dystrophy; RCT, randomized controlled trials; R&D, research and development; ROI, return on investment; SDLT, severely debilitating or life-threatening; TB, tuberculosis
In 1965, Intel co-founder Gordon Moore noted that the number of transistors per square inch on integrated circuits had roughly doubled every year since the time of their invention, and formulated “Moore’s Law” predicting that this trend would continue into the foreseeable future. Moore’s law is used more generally to describe technologies that improve exponentially over time. In contrast, many indicators dating as far back as the 1950s suggest that rate of new drug discovery is decelerating, and the cost of drug development is increasing despite breathtaking improvements in new drug technologies, such as high throughput screening, combinatorial chemistry, and computational drug design. 1, 2. Jack Scannel et al. 2, 3 coined the term “Eroom’s Law” (“Moore’s Law” spelled backwards) to describe the observation that the number of new drugs developed per 1 billion dollars of research and development (R&D) spending is halved every 9 years.
Cardiovascular disease (CVD) accounts for nearly 1 in 3 deaths globally, or over 17 million deaths annually 4, 5. That number is expected to reach over 24 million by 2030 as developing countries conquer diseases that impede longevity, and shift their focus toward CVD and other chronic diseases affecting their aging populations 6, 7. Despite this, few drugs that truly improve patient outcomes over existing therapies are reaching clinicians and patients, to meet the anticipated growth in CVD (8). Medical innovation faces increasing challenges, including formulation of new ideas, R&D barriers, regulatory uncertainty, growing payer pressures and skyrocketing costs of bringing a therapy to market 9, 10. This review discusses evidence that medical innovation is in fact slowing for CVD therapeutics, possible reasons for that phenomenon, and ways in which challenges to innovation might be addressed.
The CVD Therapeutics Pipeline
Overall, investment in biomedical research and adaptation of regulatory requirements for treatments that meet unmet medical needs has actually been successful in improving productivity for some drug pipelines. Annual new “molecular entity” filings with the U.S. Food and Drug Administration (FDA) were 23 and 41 in 2011 and 2016 respectively, an increase of 78%, and biological licensing approvals in 2011 and 2015 were 23 and 35 respectively, an increase of 67% (11). However, the CVD drug pipeline was a glaring exception to those trends. Most new therapeutics approved by the FDA from 2014 to 2016 were for oncology, infectious disease, and orphan diseases (33, 19 and 19 out of 108 drugs, respectively). FDA approvals for CVD therapies declined 33% between 2000 and 2009 compared to the previous decade (7). Just a handful of CVD drugs (11 of 108) were approved between 2014 and 2016 (11).
Industry has responded to the challenges of drug development by refocusing on therapeutic areas that optimize probability of market success, reduce development costs, are more likely to reach rapid regulatory approval and are relatively resistant to pricing pressure (9), thus improving their return on investment (ROI). Those adjustments are negatively impacting CVD therapeutics out of proportion to other clinical areas. Pfizer has 94 clinical product pathways, over one-half of which are devoted to oncology and rare diseases, and only 7 for CVD products (12). Merck has 17 oncology programs versus 2 CVD programs, and Allergan has no CVD programs (12). Based on FDA new drug applications, a “tipping point” in therapeutics occurred around 2008, away from CVD and toward oncology and central nervous system disease (9).
The number of new CVD drugs starting trials of all stages between 1990 and 2012 declined in both absolute and relative numbers (Figure 1) 2, 13. The percentages of phase I, II, and III clinical trials initiated in 2012 involving CVD drugs were 3%, 3%, and 7%, respectively, compared to 13%, 12%, and 21% respectively of trials initiated in 1990. Around the same period there was a shift from “follow-on” compounds to drugs targeting novel therapeutic pathways (defined as drugs targeting a biological pathway for which the FDA had not yet approved a drug). In 2012, drugs targeting novel pathways constituted 57% of all new phase III trials, up from 27% in 1990. In the last 5 years, drugs to treat CVD, novel or not, comprised just 6% of new drug launches, down from 13% in the mid 1990s (14).
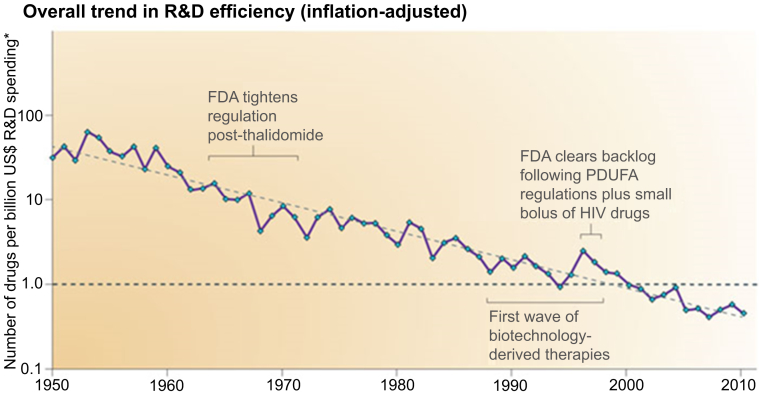
Figure 1.
Inflation-Adjusted Trends in R&D Efficiency 1950 to 2010
*Adjusted for inflation and PDUFA. Used with permission from Scannell et al. (2). FDA = Food and Drug Administration; HIV = human immunodeficiency virus; PDUFA = Prescription Drug User Fee Act; R&D = research and development.
Only about one-third of CVD drugs approved since 2000 have a novel mechanism of action (8). Recent regulatory measures favor drugs with novel mechanisms, although novelty does not assure that a drug will meet an unmet therapeutic need or represent a major therapeutic advance, either of which can allow a company to pursue expedited pathways for approval (8). Ward et al. (15) determined that drugs representing true therapeutic advances accounted for just 26% of new drugs entering the British National Formulary between 2001 and 2012. In 2012, Congress approved the Breakthrough Therapy designation program (16), to expedite development of drugs with preliminary evidence of substantial improvement over available therapies. But as of the end of 2016, about 45% of all breakthrough designations were for oncology drugs, and only 2% for CVD therapeutics. Between 2007 and 2015, only 6% of the FDA’s fast track designations were for CVD therapeutics, compared to 21% each for cancer and antiviral therapies (Figure 2) 8, 17.
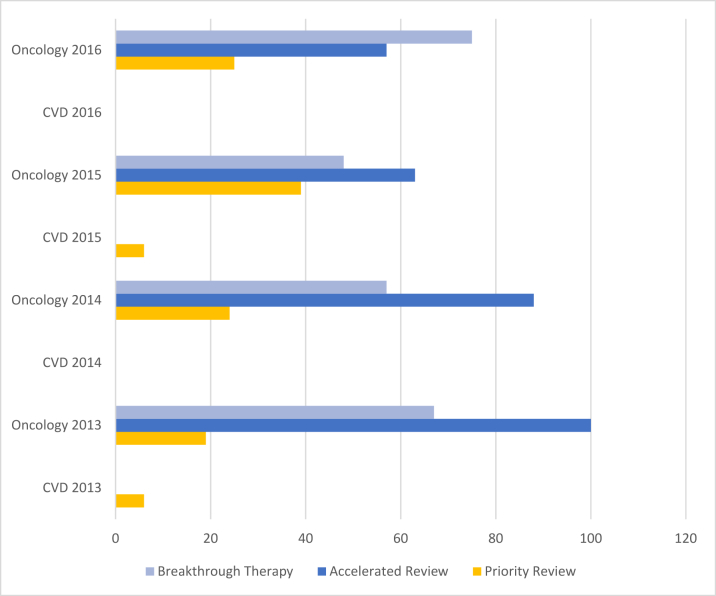
Figure 2.
Percentages of FDA Approved Priority Reviews, Accelerated Reviews, and Breakthrough Therapies for CVD vs. Oncology Therapeutics (2013 to 2016)
CVD therapeutics received approvals (priority reviews) in only 2 of the 4 years, and in none of the 4 years did CVD therapeutics receive accelerated review or breakthrough therapy designations.
Challenges in Developing Cardiovascular Therapies
Why has the rate of development of new drugs for CVD declined more than therapies for other classes of disease? Some reasons may include: 1) disproportionately low funding of CVD basic research; 2) declining biological targets for CVD therapies compared to other diseases; 3) focus by pharmaceutical companies on target-based research; 4) higher costs of CVD clinical trials compared to other diseases; 5) failures of CVD therapies in late stage clinical trials; 6) lack of strong public advocacy for CVD therapeutics; and 7) failure of CVD researchers and commercial entities to exploit the same regulatory changes that thus far have favored other disease entities over CVD.
Funding barriers
U.S. public funding of CVD therapies is disproportionately low compared to the burden of disease. National Institutes of Health (NIH) funding of basic science research for CVD in 2015 comprised only 10% of appropriations, compared to oncology (16%) and allergy and infectious disease (15%) (14). Ringel et al. (14) estimate that there is an approximate 3-fold mismatch in CVD between the burden of disease and the level of U.S. federal funding, R&D pipeline volumes, and the number of new drug launches that would be needed to generate enough medications to meet this burden. Declining basic science research for CVD therapeutics today will be felt for years to come, because the average timeline for bench-to-market drug development is 12 years (18).
Negative impact of target-based research
In 1990, James D. Watson, director of the National Center for Human Genomic Research, predicted that the ability to cost-effectively sequence DNA would “provide the technological bases for a new era in drug development” (19). Sequencing human DNA was supposed to usher in an era of cures, based on the resulting understanding of malfunctioning genes and their progeny, malfunctioning proteins (20). Those proteins, were to become the specific targets of putative drugs.
While there have certainly been important discoveries along these lines (e.g., Gleevec [Novartis, East Hanover, New Jersey] for chronic myelogenous leukemia) the majority of diseases that affect large numbers of people, such as CVD, are not based on single genetic variants amenable to target-based research. Jack Scannell postulates that, contrary to speeding up drug development, genomics, with its false promise of rapid access to widespread cures, may have actually slowed it down by diverting R&D resources to treatments aimed at single-mutation diseases that only affect very small populations (19). The majority of new drugs approved by the FDA in 2013 were specialty treatments used by only around 1% of the population 19, 21—e.g., Kalydeco (Vertex Pharmaceuticals, Boston Massachusetts), which treats a genetic variant of cystic fibrosis that affects only 1,200 people in the United States. Target-based genomics has offered little benefit for widespread diseases, and yet, according to David Swinney, CEO of the Institute of Rare and Neglected Diseases Drug Discovery, 80% of commercial R&D remains devoted to target-based research (19).
More traditional, “phenotypic” research (in which a specific molecular target or drug action isn’t known prior to studies) has historically been far more successful overall, and less costly than target-based research. Sweeney et al. (22) analyzed 259 agents approved by the FDA between 1999 and 2008, and reported that phenotypic screening led to the successful discovery of first-in-class small molecule drugs 1.6 times more often than target-based screening, despite the fact that the major focus of pharmaceutical companies at the time was target-based research.
Target exhaustion
In theory, as underlying targets of diseases such as CVD are identified and exploited, the number of remaining therapeutic targets declines (2). In the past decade, there have been only 2 first-in-class therapeutics developed for hypertension (aliskiren and valsartan/sacubitril) and few additional “targets” in the renin-angiotensin system are known. The 10 top-revenue producing CVD drugs since 1990 address only 4 unique targets, compared to 9 unique targets for the top 10 oncology drugs (14). Such target “exhaustion” may be restricting the flow of novel agents into the CVD pipeline. According to the Pharmacogenomics Knowledge Base, which publishes a compilation of known biological pathways for drug development (23), as of 2017 over twice as many biological pathways are known for oncology (n = 35) than for CVD (n = 17), affording greater opportunity for target-based therapeutic research in oncology compared to CVD. This underscores the need for increased early-stage research to identify new pathways and targets, and the importance of closing the NIH funding gap in CVD basic science.
“Better Than the Beatles” and “Low Hanging Fruit”
New drugs targeting steps in known biological pathways that already have successful therapeutics can usually only modestly improve on previous, successful ones. In other words, incremental successes usually have progressively smaller effects. As the catalogue of successful therapies targeting similar biologic pathways grows, the development processes for each successive therapy increases in complexity and cost; when differences between successive drugs become smaller, the size and duration of clinical trials must increase in order to demonstrate statistically significant improvements in efficacy. Furthermore, when a new drug is developed for an already exploited target, its success must be sufficiently greater than the last to compete in the market with the older, generally much cheaper, therapy. Scannell et al. (2) describe this as the “Better Than the Beatles Effect”; i.e., it would become increasingly difficult to come up with successful pop songs if every song had to be substantially better than the Beatles, and/or if the public didn’t also become rapidly bored with songs that already exist.
A second, potentially related, effect is that of “low hanging fruit”. Easiest drug targets are exploited first, because of lower costs and complexity. Thus, later drugs therapies are incrementally more complex and costlier to produce. For CVD therapeutics, both phenomena are magnified by the fact that CVD clinical trials are already significantly larger at baseline than those for many other therapeutic areas.
Limitations of randomized controlled trials
Although randomized controlled trials (RCTs) are favored in drug approval studies, they are nevertheless plagued with uncertainty, such as gross experimental error and bias, and random type I (false-positive) and type II (false-negative) errors, that can be minimized but not entirely eliminated in the study design process (24). Further, even a perfect RCT does not test drug performance in a “real-world” environment, where the same controls on medication administration, patient adherence, and restrictions with regard to all potential other drugs a patient might be concomitantly taking do not exist. An RCT may therefore not be borne out in clinical practice, and adverse effects that occur later in real life use may go undisclosed by an RCT.
Risk acceptance and threshold
Ultimately, drug approvals occur within an acknowledged benefit/risk balance, and the acceptable threshold for this balance above which a drug license is approved and below which it is denied cannot usually be described by a single metric—and varies highly from drug to drug. Regulators and the public are both more willing to accept a higher level of benefit/risk uncertainty for life-threatening or severe conditions with an unmet medical need compared to less severe conditions. For example, the FDA approved bedaquiline, for drug-resistant tuberculosis (TB) on the basis of only 2 studies involving a total of about 200 patients. Studies showed that bedaquiline improved clearance of TB from patient sputum, but they also demonstrated that patients receiving the drug were about 2.5 times more likely to die of TB than controls (25). The drug was nevertheless approved, because multidrug-resistant TB is a life-threatening condition with few available treatment options (26). Similar acceptance of higher risk occurs with many oncology drugs, which are often approved based on smaller numbers of patients, single-arm trials, and/or surrogate endpoint information, compared to the large RCTs generally required for CVD drugs (27). Regulatory acceptance of greater benefit/risk uncertainty for drugs of some classes is built into federal drug approval pathways, such as the orphan drug (28), fast track, priority review, accelerated review and breakthrough therapy designations (16), some of which require continued post-market clinical trials for safety and efficacy.
CVD clinical trial size and late trial failures
A trial failure in late stage for a CVD drug carries a heavier proportional impact on commercial ROI, because CVD clinical trials are both larger and generally more expensive than those for other therapeutic areas. According to a 2015 report by Battelle (29), late phase CVD trials enroll an average of 503 patient per study, versus 162 for oncology and 204 for central nervous system diseases. The average cost per patient in late clinical trials was $20,500, $59,500, and $36,000, respectively. This translates into average late phase trial costs of $10,865,00, $9,639,000, and $7,344,000 respectively. Moreover, the sheer size of CVD trials leads to longer trial durations: a median of 24 weeks for CVD, versus 18.5 weeks for oncology and 5 weeks for infectious disease (30). Longer trial duration not only increases direct trial expense, but runs down the clock on a drug’s patent, reducing the subsequent time of market exclusivity following approval, and consequently reducing ROI.
Enthusiasm for expedited drug approvals is attenuated by the occurrences of late trial failures and post-market findings that have uncovered unexpected or unacceptable levels of patient harm and necessitated drug withdrawals, restrictions or “black box” warnings by the FDA. Late trial failures are common across all drug development classes, affecting over 54% of investigational drugs that enter late-stage clinical development (31). The rate of failure of drugs for CVD is similar to other drug classes, with about 44% of failures of CVD drugs due to inadequate efficacy, and 24% due to safety issues (13). Drug failures often follow promising preclinical trials that use surrogate markers of success, as in the VISTA-16 (Vascular Inflammation Suppression to Treat Acute coronary syndrome for 16 weeks) trial of a secretory phospholipase A2 inhibitor, which was terminated early when, despite achieving the targeted levels of low-density lipoprotein cholesterol and C-reactive protein, the drug was associated with a significantly higher risk of mortality (32). This demonstrates the potential shortcomings of biomarkers and other surrogate endpoints, and the importance of well-designed clinical trials of patient-centered outcomes to assure drug safety and efficacy.
Regulatory uncertainty
Pathways for drug approval are becoming more complex, and there have been well-publicized instances of drugs receiving regulatory approval only to result in patient harm revealed in post market analysis—e.g., Vioxx (Merck, Kenilworth, New Jersey), which was withdrawn from the market after it was associated in post-market studies with increased cardiovascular morbidity and mortality (24). Therefore, there is intense U.S. government and public scrutiny focused on expedited approval pathways. Regulatory agencies face the challenge of balancing demands for rapid access to new and effective drugs with the need for comprehensive safety and efficacy data prior to patient use.
Regulatory gaps for CVD drug approvals
The FDA defines “life-threatening conditions” as: 1) diseases in which the risk of death is high unless the course of disease is interrupted; and 2) disease or conditions with potentially fatal outcomes, where the endpoint of a clinical trial analysis is survival 33, 34. Severely-debilitating or life-threatening (SDLT) diseases are conditions in which life expectancy is short or quality of life is greatly diminished despite available therapies. There is no doubt that many CVD diseases, such as advanced heart failure, should be considered SDLTs, and it stands to reason that approval of SDLT disease therapeutics should parallel that of advanced oncologic drugs. Yet although the FDA presents regulatory guidelines for advanced cancer therapies (35) and for rare diseases (36), there is minimal FDA guidance for early development and availability of the drugs for non-oncologic SDLT diseases for which there are few available therapeutic options.
Low regulatory priority for CVD
Of 13 treatment areas investigated by Ringel et al. (14), CVD was 11th on the list for frequency of any type of FDA expedited reviews which are intended to move high-priority treatments to market for unmet needs. From 1987 through 2014, comparing CVD drugs approved for expedited review by the FDA to those for oncology and infectious disease, 10% were approved as orphan drugs (vs. 61% and 13%, respectively), 1% received accelerated approval (vs. 48% and 36%, respectively), 0% for fast track (vs. 30% and 20%, respectively) and 16% as priority review (vs. 76% and 61%, respectively). Under the FDA’s new “breakthrough therapy” designation, of the 28 approvals granted since inception, 54% were for oncology, 21% for infectious disease, and none for CVD (14).
Commercialization Challenges for Cardiovascular Disease Therpeutics
Industry perception of commercialization risk
Higher average patient enrollment and higher costs of CVD late clinical trials present significant barriers to CVD drug development, although, interestingly, overall costs may not be significantly increased. Phase I and II CVD trials are actually less costly than oncology trials 14, 37, and median time from early phase to market launch is significantly less for CVD drugs than for oncology drugs 14, 38. Success rates are similar, or sometimes even higher for CVD drugs compared to those for oncology therapy (14). Reluctance to pursue commercialization of CVD drugs may be due to a misperception in industry that they are more costly than other therapeutics to bring to market, boosted by some spectacular late-phase failures, such as that of torcetrapib, an anticholesterol drug, at a cost of $800 million (39).
Patient advocacy
Public and private entities involved in medical therapeutics—including researchers, funding agencies, regulators, and commercial entities—are motivated by public advocacy as well as potential market share. Public advocacy for therapeutics that have large benefits for small numbers of patients can bias all phases of medical development away from treatments that have somewhat lower individual patient benefit, but affect much higher population numbers.
The public does not appear to advocate for CVD treatments in the same way that they do for other diseases. One reason may be that other diseases inspire more dread. In 1 study of patients over age 50 who were asked which of 8 diseases they most fear, 30% reported fear of Alzheimer’s, 30% reported cancer 30%, and only 2% reported CVD (40), similar to findings by others (41). Alzheimer’s disease and cancer funding proportionally outstrip funding for CVD diseases.
Adults in the United States also significantly overestimate deaths from cancer and HIV/AIDS, and a majority do not recognize the higher mortality of CVD (42); over 50% of Americans surveyed indicated that finding a cure for cancer was the 1 thing they would like to see within 10 years (43). Furthermore, in repeated surveys conducted since 1992, CVD is named <10% of the time as the most important health issue in the U.S. today (44).
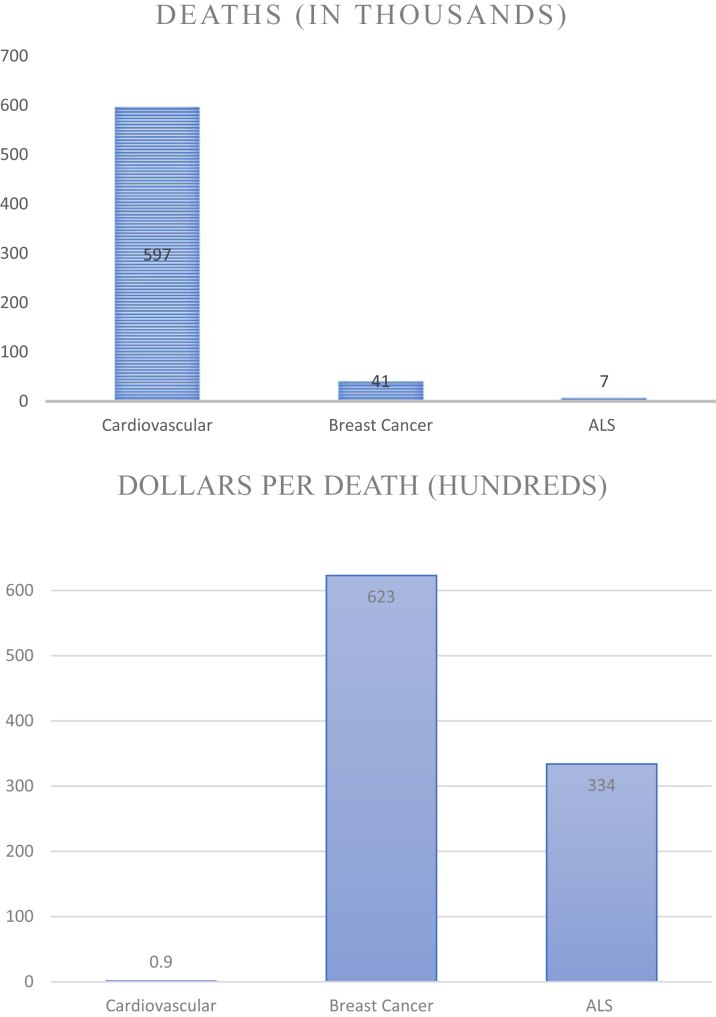
This bias is also reflected in donations to public health charities that fund research. In 2011, donations to several major charities, relative to the respective mortality of those diseases, strongly favored neuromuscular disease and cancer causes over CVD: e.g., heart disease (Jump Rope for Heart) $91 per death, versus motor neuron disease (ALS Ice Bucket Challenge) $3,344 per death, breast cancer (Komen Race for the Cure) $6,232 per death and prostate cancer (Movember) $6,942 per death (Figure 3) (45). Many factors drive disparities in public health advocacy, including perceptions of individual risk and susceptibility, and individual control over those factors. Chronic diseases that develop slowly and are perceived to result from factors that individuals can control elicit less concern than diseases in which control is limited (46). Accordingly, CVD, a chronic, usually slowly developing disease with “controllable” risk factors such as diet, exercise, and smoking cessation elicits much less concern in the public eye than cancer.
Figure 3.
Relative Disproportion of Charitable Donations* per Death Versus Annual U.S. Mortality From CVD, Breast Cancer, and ALS
*Charities surveyed: Jump Rope for Heart, Koman Race for the Cure, and ALS Ice Bucket Challenge (45).
Public advocacy also strongly affects payer reimbursements, particularly in cases where the disease makes up a small percentage of the overall patient/cost base 47, 48 or when a political or advocacy response is expected if there are limitations to drug access 48, 49. Strong patient advocacy may push a payer to accept higher pricing, especially if the patient group being advocated for is small, and expensive pricing will have limited impact on the payer’s bottom line.
Promoting Innovation in Drug Research and Development
Changing the way drug R&D is carried out can facilitate faster conclusions regarding clinical efficacy and safety, lead to findings that are more likely to be borne out in “real world” clinical scenarios, reduce the number of research subjects needed for clinical trials (reducing trial duration and costs), or in cases where large trials simply can’t be avoided or modified, facilitate recruitment of sufficiently sized patient populations through global research outreach. In December 2016, the U.S. Congress passed the 21st Century Cures Act 11, 50 allotting $6.3 billion for NIH funding to speed regulatory approval of medical therapies, in part by loosening clinical data requirements to include observational data, insurance data, patient input and anecdotal data rather than full clinical trials. These changes can have a strong positive impact development of CVD therapeutics. In addition, innovative clinical trial designs, such as those being employed in oncology trials, may also decrease the cost of drug development, and allow newer drugs to gain regulatory approval sooner (51).
Patient and physician advocacy-initiated FDA guidance documents and policies
The potential power of patient advocacy in shaping the future of CVD therapeutics should not be overlooked, and there are lessons to be learned from the accomplishments of patient advocacy groups, such as the Parent Project Muscular Dystrophy (PPMD). Despite over a decade of work, the PPMD felt that the FDA had not adequately addressed the needs of their patient community, so they sought opportunities to shift FDA policies and influence FDA guidance documents regarding muscular dystrophy research.
The FDA Good Guidance Practices regulation (52) specifically provides that stakeholders, including patients and patient-advocacy groups can: 1) suggest guidance topics; and 2) submit drafts of proposed guidance. Such guidance documents “do not create or confer rights for any persons or bind the FDA to any particular actions” (53), but are extremely important expressions of current FDA thinking, and the FDA tends to rely on them as a source for informal policy making. The FDA is often constrained by limited resources from developing guidance in response to stakeholder suggestions, and several FDA Centers and Offices have therefore been encouraging stakeholders to submit their own proposed guidances (52). If the topic involves new or novel ideas, such drafts can expedite the process of issuing official guidances, which in turn can expedite research and drug approval.
The PPMD accordingly drafted a guidance for the FDA (53), which in 2015 became the first ever patient-initiated draft guidance published by the FDA 53, 54. Creation of draft guidance documents is a potential way in which CVD patients and physicians can call attention to their unmet medical need, educate FDA offices about the ways in which a disease may parallel others that have achieved approval for expedited review, and advocate for drug development policies and expedited reviews for drugs that target treatment for CVD.
Prescott et al. (34) suggest that, since CVD is a SDLT condition, guidance documents for development of CVD therapeutics should be modeled on those for advanced cancer. They point out that such guidance could defer or eliminate activities or nonclinical studies that are not essential to support safety in light of the unmet medical need. Early clinical development guidance would be aimed at proof of concept in the target patient population, and allow patients access early to potential benefits of experimental therapies. Guidance could allow continued experimental therapy following trial completion but before formal drug approval—something that is routinely allowed in early-stage studies for advanced cancer—so long as the disease appears to be responding and the rate and nature of adverse events is acceptable. Prescott’s proposed approach allows greater autonomy of patients who suffer from severe CVD with inadequate therapeutic options to undertake less known and possibly greater risks for management of their disease during drug development itself. Using advance-stage heart failure as an example of a disease that might benefit from this approach, Prescott et al. estimate that the time to filing for drug approval could be accelerated by 2 to 3 years over current, standard clinical studies, and save almost $42 million over current development costs (34).
Incentivizing the Commercialization of Therapies for CVD
Several FDA drug approval processes have been created to shorten time from drug conception to market approval for therapies that meet certain requirements, and these all warrant renewed scrutiny with regard to their relevance to CVD therapeutics. These include fast track, breakthrough therapy and priority review designations, and the FDA accelerated review pathway (Table 1) 17, 55. In addition, the orphan drug (OD) designation 26, 28, 55 qualifies a drug for expedited FDA processes. All of these processes can speed up drug approval, saving money in development, and preserving a longer time on the drug’s patent for market exclusivity once approved, both of which improve ROI. Each of these pathways and processes can potentially be exploited to improve commercial interest in developing certain CVD therapeutics.
Table 1.
Comparison of FDA-Expedited Drug Approval Programs
| Fast Track | Breakthrough Therapy | Accelerated Approval | Priority Review | |
|---|---|---|---|---|
| Type of program | Designation | Designation | Approval Pathway | Designation |
| Criteria relevant to CVD drug approval |
|
|
|
|
| Features |
|
|
|
|
| Additional factors |
|
|
|
|
CVD = cardiovascular disease; FDA = Food and Drug Administration.
Adapted from Prescott et al. (34).
Orphan drug designation
Therapeutics for diseases that affect small numbers of patients suffered from a lack of interest in commercialization until relatively recently. Such patient populations are often so small, that it can be nearly impossible to generate the traditional RCTs needed to show both efficacy and safety, even if nearly every single patient is enrolled in a clinical trial. Once approved, ROI was limited, due to the limited marketing potential for the drug’s primary use, and the limited time for market exclusivity left on the drug’s patent “clock”. Patient advocacy groups, such as NORD (National Organization for Rare Disorders) (56) arose in the 1970s and 1980s, consolidating the efforts of multiple, smaller patient advocacy groups to push for federal support to develop treatments for these heretofore neglected diseases. Their efforts culminated in the passage of the Orphan Drug Act of 1983 (28). The act designates ODs as those that treat diseases affecting fewer than 200,000 patients residing in the United States, or for which there is proof that if more than 200,000 patients are affected, there is “no reasonable expectation that the sales of the drug will be sufficient to offset the costs of developing and making the drug available in the U.S. market” (57). OD designation results in grants for drug development; fast track approvals and access to the Investigational New Drug Approval program at the FDA that cut the time to market and decrease direct development costs; tax credits; a waiver of user fees that can result in a savings of millions of dollars of development costs; and 7-year market exclusivity after development independent of the drug’s current patent status.
ROI for certain drug classes has significantly improved under OD designations. In 1 review, 86 companies holding OD approvals in Europe and/or the United States were found to have 9.6% higher ROIs than 258 controls. For every OD a company sold, ROI increased by 11% (58). A direct result is that ODs accounted for nearly one-half (21 out of 45) of all new innovative drugs approved by the FDA in 2015. The OD market is expected to reach $176 billion by 2020, and account for 19% of all branded drug sales (58). The new and somewhat paradoxical profitability of ODs is boosted by shorter development times, incentives for R&D, reduced marketing costs, and premium pricing. Some of the largest pharmaceutical companies now have dedicated rare disease units, or have acquired smaller biotech companies in the rare disease sector in an effort to boost their bottom line 59, 60, 61.
A review of current drugs in the pipeline that have received OD designation shows that of the 14 ODs approved in 2016 (62), none were for conditions related to CVD. Of 316 entities (the “pipeline” drugs) that received OD designation between January 1 and August 16, 2017, none were primarily for any adult cardiac disease, although 2 (0.6%) were for treatment of pulmonary hypertension (63). While the OD designation is unlikely to be widely applicable for the general categories of common CVD, it nevertheless could prove useful for certain CV diseases that affect small numbers of patients—for example, genetically-mediated cardiac arrhythmic disorders.
Fast track designation
Fast track designation is for drugs that treat serious illnesses plus fill an unmet medical need. The definition of a “serious illness” is subjective, but generally based on whether the drug will impact disease survival or day-to-day functioning, and the likelihood that if the disease is untreated it will progress to a more serious condition. Heart failure is 1 example of a disease that carries the “serious” designation. “Fulfilling an unmet medical need” is defined as providing therapy where none exists, or else providing a therapy that may substantially better than available therapy (16). Fast track designation can be based on theoretical rationale and mechanistic rationale (nonclinical data) and prioritizes the drug for more frequent meetings with the FDA during the drug development plan, more frequent written communication from the FDA, eligibility for “accelerated approval” and “priority review” in some cases, and “rolling reviews” in which the FDA accepts sections of the biological license application and new drug application as they are completed, rather than waiting for all sections to be completed before beginning review.
Breakthrough therapy
The breakthrough designation can be sought for drugs that are intended to treat a serious condition that have preliminary clinical evidence of substantial improvement on a clinically significant endpoint over other available therapies. This designation can be applied based on preliminary data before trials for full drug approval are complete (16). Unlike fast track designation, the breakthrough therapy designation requires preliminary clinical evidence, meaning evidence that is sufficient to indicate a potential, substantial improvement in safety or efficacy—but that evidence need not be as stringent as that required for the final approval. Nonclinical evidence is acceptable in support of the clinical evidence. Breakthrough therapy designation earns the developer intensive FDA guidance on an efficient drug development program, starting as early as Phase 1. Breakthrough therapies may also be allowed rolling reviews, and may be eligible for priority review.
Early data regarding breakthrough drug designation is promising, but can be over-interpreted: many drugs approved had already been waiting in the in pipeline for companies to get extra guidance from the FDA. Of 10 breakthrough products approved in 2015, 6 had already been in the pipeline for 4 years before the designation was introduced. However, several of the remaining drugs clearly benefitted from significantly reduced approval times: idanucizumab (a reversal agent for the blood thinner dabigatan etexilate), and osmertinib, and alectinib, both lung cancer treatment agents (64). Time saving translates into lower development costs and increases the period of market exclusivity left on patent during which the company will face little or no market competition.
Accelerated approval can be obtained for drugs that treat a serious condition, plus provide a meaningful advantage over existing therapies, plus demonstrates an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that is likely to predict irreversible morbidity and mortality (16). Accelerated approval is conditional on the performance of confirmatory trials, and is subject to expedited withdrawal from the market if such trials do not confirm the clinical effects.
Priority review designation is for drugs that treat a serious condition plus provide a significant improvement in efficacy or safety over previous treatments (16). Priority review designation requires the FDA to take action earlier (6 months vs. 10 months standard review time) on the new drug marketing application.
There are concerns about whether priority review is “safe”—not because it affects requirements for drug efficacy and safety studies (it does not), but because the obligation for shorter review times for new drug applications at the FDA may increase errors in the FDA review process itself. At least 1 study supports this concern: drugs that were approved by the FDA within 2 months of their deadline (i.e., presumably under pressure) were significantly more likely to be withdrawn later or require black box warnings for safety problems (65). Similar problems have been shown with the other accelerated pathways (66). Priority reviews also indirectly increase insurer costs. If a drug is approved 4 months earlier in its patent, insurers will be required to pay for 4 months of drug with exclusive marketing status and thus higher pricing. Such increases are likely to be passed on to patients in the form of higher insurance premiums.
Pricing Policies and Payer Reimbursements
Pricing practices and payer reimbursement policies significantly affect both the willingness of commercial developers to pursue drug development and the quality of their innovations. An in-depth discussion of pricing, reimbursements, and innovation is beyond the scope of this review, but because pricing in turn affects all drug development, including CVD drugs, it warrants at least a short discussion.
For drug development, “cost-effectiveness” refers to the question of whether a drug is “good value for the money” and whether its effectiveness warrants both the cost of development and the price. In general, commercial entities want to avoid investing R&D costs into drugs that either end up not delivering on their promise of efficacy (and therefore do not achieve a hoped-for market share), and/or are note safe, and producing drugs whose pricing will not ultimately provide substantial, or even exorbitant, ROI.
Even older drugs, that have presumably long ago returned the cost of development through profits, have been subject recently to significant price increases, as drug companies claim that they need to expand profits on older therapies to cover R&D costs for new drug development. For example, in 2015, Valeant Pharmaceuticals (Bridgewater, New Jersey) raised the price of 2 newly acquired drugs, Isuprel and Nitropress, by 525% and 212%, respectively, and Turing Pharmaceuticals (New York, New York) justified a 5,000% increase of the toxoplasmosis drug Daraprim, a 62-year old drug, as necessary to support future R&D (67).
But are these claims actually true? It remains to be seen whether, if the costs of R&D fell, company stakeholders would support lowering the drug prices, if it meant that their stock holdings would lose value. Stakeholders in drug company profits and share prices include the senior executives, investors, general investment funds, and even private and public retirement accounts. In fact, a recent study demonstrates that between 2006 and 2015, the 18 pharmaceutical companies of the Standard and Poor’s 500 index, spent billions of dollars more on dividends and buy backs, than on new R&D (68). The authors postulate that a major factor suppressing drug innovation was the pay packages of their senior executives, which “reward stock-price speculation and manipulation rather than the company’s success at innovation”. William Lazonick, an author of the study, commented in an interview that “there really is very little drug development going on in companies showing the highest profits and capturing much of the gains” (69). The authors of the study further declared that “the key cause of high drug prices, restricted access to medicines and stifled innovation [italics added], we submit, is a social disease called ‘maximizing shareholder value’” (69).
Lazonick et al. concluded that eradicating the maximizing shareholder value ideology of corporate governance should be the primary objective of all government agencies, civil-society organization and businesses that seek innovative and affordable drugs. They proposed the following regulatory steps: 1) ban pharmaceutical companies from doing stock repurchases and excessive shareholder distributions to encourage a refocus on access to medicines in the industry; 2) structure executive compensation to reward executives for generating innovative drugs at affordable prices, rather than for increases in stock value; 3) place stakeholders representing households as taxpayers, workers and consumers on boards of directors; 4) regulate the price of any drug that has benefited from government funding, subsidies and protections; 5) increase the returns to taxpayers for their investments in life-sciences research through their federal funding of research; and 6) use government funding in collaboration with innovative businesses to ensure “collective and cumulative careers” of life-sciences researchers, the lowest paid PhDs in the natural sciences (69).
In addition to company practices, the impact of global political forces on drug innovation and pricing can no longer be ignored. In most cases, the majority of a drug’s price will be paid by a health insurer—often a public entity—and that insurer decides at what price the drug will be reimbursed. Factors that have significant effects on optimum pricing are national laws, the nature of the insurer (single public payer, mixed public and private payers, or private payer), the regulatory authority and its policies, and market size 70, 71. While there are rules that help determine such reimbursements, they vary from country to country, and among insurers. Countries, such as Germany for example, that adhere to free market pricing schemes have higher drug prices than countries with price controls, such as Spain (70). U.S. drug prices run 74% to 181% higher than in the United Kingdom, and are the highest in the world (69).
The exit of the United Kingdom from the European Union is likely to impact drug approval, regulation and pricing throughout Europe, and indirectly in the United States, although it is as of yet unclear exactly how much and in what ways. Pan-European regulation of medicine development will certainly change, and at a time when the taxonomy of disease is shifting toward molecular mechanisms rather than target tissues, and therefore toward smaller patient target groups. If the fragmentation of the European Medicine Agency leads to more regulatory hurdles, price volatility, and other changes in ROI, this may further complicate new drug development for all drug therapies, including CVD drugs (72).
Moving Beyond EROOM’s Law
Moore’s law held for more than half a century, but ultimately become outdated because the very technology for which it was coined approached its limits. On the other hand, the validity of Eroom’s law appears to be going strong, and to be particularly germane with regard to CVD therapeutics. Currently, the potential new targets for CVD therapeutics appear to be less obvious, and many CVD diseases may be less amenable to target-based research, because CVD “phenotypes” may have multiple, different pathophysiologic sources. CVD controlled trials are larger and more expensive than for other therapeutic areas, and the results of incremental drug development make future trials more costly and complex to complete, and therefore less attractive to commercialize. Moreover, insurance companies appear to be increasingly less willing to pay for expensive new life saving CVD drugs, which creates further headwinds for developing new CVD therapies. Future rejuvenation of the CVD drug “pipeline” will likely require efforts on multiple fronts, including more basic science research funding for CVD to identify future therapeutic targets; more innovative trial designs outside of traditional RCTs; aggressive use of current FDA accelerated pathways to shorten time for drug approval; outreach for heightened patient advocacy for CVD therapeutics; and ultimately, significant changes, in the business models of the U.S. pharmaceutical industry.
Footnotes
Dr. Van Norman has received financial support from the Journal of the American College of Cardiology.
The author attests he is in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Lowe D. Eroom’s Law. Science Translational Medicine: In the Pipeline. March 8, 2012. Available at: http://blogs.sciencemag.org/pipeline/archives/2012/03/08/erooms_law. Accessed August 24, 2017.
- 2.Scannell J.W., Blanckley A., Boldon H., Warrington B. Diagnosing the decline in pharmaceutical R&D efficiency. Nature Rev Drug Discov. 2012;11:191–200. doi: 10.1038/nrd3681. [DOI] [PubMed] [Google Scholar]
- 3.Harper M. See editor’s note to: Scannel J. Four reasons why drugs are expensive, of which two are false. Forbes Oct 13, 2015. Available at: https://www.forbes.com/sites/matthewherper/2015/10/13/four-reasons-drugs-are-expensive-of-which-two-are-false/#3e235ab14c3b. Accessed August 8, 2017.
- 4.Terzic A., Waldman S. Chronic diseases: the emerging pandemic. Clin Transl Sci. 2011;4:225–226. doi: 10.1111/j.1752-8062.2011.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benjamis E.J. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2017 update. A report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuster V., Frazer J., Snair M., Vendanthan R., Dzau V. Global health and the future role of the United States. J Am Coll Cardiol. 2017 In press. [Google Scholar]
- 7.Fordyce C.B., Roe M.T., Ahmad T. Cardiovascular drug development. Is it dead or just hibernating? J Am Coll Cardiol. 2015;15:1567–1582. doi: 10.1016/j.jacc.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Hwang T.J., Kesselheim A.S. Challenges in the development of novel cardiovascular therapies. Clin Pharmacol Ther. 2017;102:194–196. doi: 10.1002/cpt.703. [DOI] [PubMed] [Google Scholar]
- 9.Honig P., Huang S.M. Intelligent pharmaceuticals: beyond the tipping point. Clin Pharm Ther. 2014;95:455–459. doi: 10.1038/clpt.2014.32. [DOI] [PubMed] [Google Scholar]
- 10.Lauer M., Gordon D., Wei G., Pearson G. Efficient design of clinical trials and epidemiological research: is it possible? Nat Rev Cardiol. 2017;14:493–501. doi: 10.1038/nrcardio.2017.60. [DOI] [PubMed] [Google Scholar]
- 11.Honig P., Terzic A. Affairs of the Heart: innovation in cardiovascular research and development. Clin Pharm Ther. 2017;102:162–168. doi: 10.1002/cpt.737. [DOI] [PubMed] [Google Scholar]
- 12.LaMattina J. Death of Pfizer heart drug shows the challenges of CV R&D and why companies are bowing out. Forbes. November 2, 2016 Available at: https://www.forbes.com/sites/johnlamattina/2016/11/02/death-of-pfizer-heart-drug-shows-the-challenges-of-cv-rd-and-why-companies-are-bowing-out/#204a443e46f3. Accessed July 30, 2017.
- 13.Hwang T.J., Lauffenburger J.L., Franklin J.M., Kesselheim A.S. Temporal trends and factors associated with cardiovascular drug development. J Am Coll Cardiol Basic Trans Sci. 2016;1:301–308. doi: 10.1016/j.jacbts.2016.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ringel M.S., Shah N.A., Baedeker M., Lim C.T., Lamichhane A., Schultze U. Occlusion of the flow of new drugs for cardiovascular disease. Clin Pharm Ther. 2017;102:246–253. doi: 10.1002/cpt.691. [DOI] [PubMed] [Google Scholar]
- 15.Ward D.J., Angharad S., Genus T., Martino O.I., Stevens A.J. How innovative are new drugs launched in the UK? A retrospective study of new drugs listed in the British National Formulary (BNF) 2001-2012. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-006235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.S. Food and Drug Administration. Fast track, breakthrough therapy, accelerated approval, priority review. Available at: https://www.fda.gov/forpatients/approvals/fast/ucm20041766.htm. Accessed August 25, 2017.
- 17.U.S. Food and Drug Administration. Drug and biological approval and IND activity reports. Available at: https://wayback.archive-it.org/7993/20170403225619/https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/default.htm. Accessed August 26, 2017.
- 18.Van Norman G.A. Drugs, devices and the FDA: Part 1. JACC Basic Transl Sci. 2016;1:170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurley D. Why are so few blockbuster drugs invented today? The New York Times Magazine. Nov 13, 2014. Available at: https://www.nytimes.com/2014/11/16/magazine/why-are-there-so-few-new-drugs-invented-today.html. Accessed August 8, 2017.
- 20.U.S. Food and Drug Administration. Targeted drug development: why are so many diseases lagging behind? U.S. FDA, July 2015. Available at: https://www.fda.gov/forpatients/approvals/fast/ucm20041766.htm. Accessed August 25, 2017.
- 21.Mullard A. 2013 FDA drug approvals. Nature Rev Drug Discov. 2014;13:85–89. doi: 10.1038/nrd4239. [DOI] [PubMed] [Google Scholar]
- 22.Swinney D.C., Anthony J. How were medicines discovered? Nature Rev Drug Discov. 2011;10:507–519. doi: 10.1038/nrd3480. [DOI] [PubMed] [Google Scholar]
- 23.PharmGKB, Stanford California Available at: https://www.pharmgkb.org/view/pathways.do, Accessed August 2, 2017.
- 24.Eichler Hans-Georg, Pignatti F., Flamion B., Leuflkens H., Breckenridge A. Balancing early market access to new drugs with the need for benefit/risk data: a mounting dilemma. Nat Rev Drug Discov. 2008;7:818–826. doi: 10.1038/nrd2664. [DOI] [PubMed] [Google Scholar]
- 25.Diacon A.H., Pym A., Grobusch M.P. Multidrug-resistant tuberculosis and culture conversion with bedaquiline. N Engl J Med. 2014;271:723–732. doi: 10.1056/NEJMoa1313865. [DOI] [PubMed] [Google Scholar]
- 26.Kesselheim A.S., Darrow J.J. FDA designations for therapeutics and their impact on drug development and regulatory review outcomes. Clin Pharm Ther. 2015:29–36. doi: 10.1002/cpt.1. [DOI] [PubMed] [Google Scholar]
- 27.Garattini S., Bertele V. New approach to clinical trials and drug registration. Author’s suggestions for drug approval are questionable. BMJ. 2001;323:341. doi: 10.1136/bmj.323.7308.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orphan Drug Act, Pub.L. No.97-414, 96 Stat. 2049, §1 (Jan 4, 1983).
- 29.Biopharmaceutical Industry-Sponsored Clinical Trials: Impact on State Economies. Battelle Technology Partnership Practice; a report for the Pharmaceutical Research and Manufacturers of America (PhRMA), March 2015. Available at: http://phrma-docs.phrma.org/sites/default/files/pdf/biopharmaceutical-industry-sponsored-clinical-trials-impact-on-state-economies.pdf. Accessed August 2, 2017.
- 30.Downing N.S., Aminawung J.S., Shah N.D., Krumholz H.M., Ross J.S. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311:368–377. doi: 10.1001/jama.2013.282034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMurray J.J., Packer M., Desai A.S. Angiotensin-nepriysin inhibition versus enalapril in heart failure. N Eng J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 32.Nicholls S.J., Kastelein J.J., Schwartz G.G. Varespladib and cardiovascular events in patients with an acute coronary syndrome: the VISTA-16 randomized clinical trial. JAMA. 2014;311:252–262. doi: 10.1001/jama.2013.282836. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Food and Drug Administration. Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry: information program on clinical trials for serious or life-threatening diseases and conditions. March 2002. Available at: https://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126838.pdf. Accessed August 25, 2017.
- 34.Prescott J.S., Andrews P.A., Backer R.W. Evaluation of therapeutics for advanced-stage heart failure and other severely-debilitating or life-threatening diseases. Clin Pharm Ther. 2017;102:219–227. doi: 10.1002/cpt.730. [DOI] [PubMed] [Google Scholar]
- 35.U.S Food and Drug Administration Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Guidance for industry. Cancer drug and biological products—clinical data in marketing applications. October, 2001. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071323.pdf. Accessed August 25, 2017.
- 36.US Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER). Draft guidance for industry. Rare diseases: common issues in drug development. August 2015. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM458485.pdf. Accessed August 25, 2017.
- 37.Sertkaya A., Wong H.H., Jessup A., Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials. 2016;13:117–126. doi: 10.1177/1740774515625964. [DOI] [PubMed] [Google Scholar]
- 38.Kaitin K.I., Dimasi J.A. Pharmaceutical innovation in the 21st century: new drug approvals in the first decade, 2000-2009. Clin Pharmacol Ther. 2011;89:183–188. doi: 10.1038/clpt.2010.286. [DOI] [PubMed] [Google Scholar]
- 39.Adams C.P., Branter V.V. Estimating the cost of new drug development: is it really 802 million dollars? Health Aff. 2006;25:420–428. doi: 10.1377/hlthaff.25.2.420. [DOI] [PubMed] [Google Scholar]
- 40.Bystad M, GrØnil O, Lilleeggen C, Aslaksen PM. Fear of diseases among people over 50 years of age: a survey. Scand Psychol 2016;3:e19.
- 41.What America thinks: MetLife Foundation Alzheimer’s survey, February 2011. Available at: https://www.metlife.com/assets/cao/foundation/alzheimers-2011.pdf. Accessed August 25, 2017.
- 42.Attitudes. Global Health Research Survey. Research! America. 2006. Available at: http://www.researchamerica.org/sites/default/files/uploads/poll2006ghr.pdf. Accessed August 25, 2017.
- 43.Americans Expect Medical Breakthroughs. Research!America. Pharma. Nov 2007 Available at: http://www.researchamerica.org/sites/default/files/uploads/poll.report.2007.transforminghealth.pdf. Accessed on August 3, 2017.
- 44.Research!America Research Enterprise Survey 2010. Available at http://www.researchamerica.org/sites/default/files/uploads/ResearchEnterprisePoll.pdf. Accessed August 3, 2017.
- 45.Belluz J. The truth about the Ice Bucket Challenge: viral memes shouldn’t dictate our charitable giving. Vox. August 20, 2014. Available at: https://www.vox.com/2014/8/20/6040435/als-ice-bucket-challenge-and-why-we-give-to-charity-donate. Accessed August 3, 2017.
- 46.Weinstein N.D. Why it won’t happen to me: perceptions of risk factors and susceptibility. Health Psychol. 1984:431–457. doi: 10.1037//0278-6133.3.5.431. [DOI] [PubMed] [Google Scholar]
- 47.Morel T., Popa C., Simoens S. Market watch: are orphan drug companies the pick of the pharmaceutical industry? Nat Rev Drug Discov. 2014;10:13. doi: 10.1038/nrd4205. [DOI] [PubMed] [Google Scholar]
- 48.Nahuis R., Boon W.P. The impact of patient advocacy: the case of innovative breast cancer drug development and approval programs, 1987-2014: cohort study. BMJ. 2015;351:h4633. doi: 10.1136/bmj.h4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Epstein S. Patient groups and health movements. In: The Handbook of Science and Technology Studies, Hackett EJ, Amsterdamska O, Lynch M, Bacjman J, Eds. MIT Press, Cambridge MA, 2007. Available at:. http://www.faculty.umb.edu/pjt/epi/epstein05.pdf. Accessed August 25, 2017.
- 50.H.R.34 – 21st Century Cures Act. (114th Congress 2015-2016). Available at: https://www.congress.gov/bill/114th-congress/house-bill/34/. Accessed August 25, 2017.
- 51.Shah S.J. Innovative clinical trial designs for precision medicine in heart failure with preserved ejection fraction. J Cardiovasc Transl Res. 2017;10:322–336. doi: 10.1007/s12265-017-9759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.U.S. Food and Drug Administration. U.S. Food and Drug Administration Report on Good Guidance Practices: Improving Efficiency and Transparency. Dec 2011, U.S. Department of Health and Human Services Food and Drug Administration. Available at: https://www.fda.gov/downloads/AboutFDA/Transparency/TransparencyInitiative/UCM285124.pdf. Accessed August 6, 2017.
- 53.Furlong P., Bridges J.F., Charnas l. How a patient advocacy group developed the first proposed draft guidance document for industry for submission to the U.S. Food and Drug Administration. Orphanet J Rare Dis. 2015;10:82. doi: 10.1186/s13023-015-0281-2. https://ojrd.biomedcentral.com/articles/10.1186/s13023-015-0281-2 Available at: Accessed August 6, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.U.S. Food and Drug Administration. Guidance (Drugs) Available at: https://www.fda.gov/Drugs/GuidancecomplianceRegulatoryInformation/Guidances/default.htm. Accessed August 6, 2017.
- 55.U.S. Food and Drug Administration. Designating an orphan product: drugs and biological products. Available at: https://www.fda.gov/ForIndustry/DevelopingProductsforRareDiseasesConditions/HowtoapplyforOrphanProductDesignation/default.htm. Accessed August 25, 2017.
- 56.NORD. National Organization for Rare Disorders. Available at https://rarediseases.org/about/what-we-do/history-leadership/. Accessed August 16, 2017.
- 57.U.S. Government Publishing Office. Electronic Code of Federal Regulations. Title 21 Part 316. Orphan Drugs. Available at: https://www.ecfr.gov/cgi-bin/retrieveECFR?gp=&SID=718f6fcbc20f2755bd1f5a980eb5eecd&mc=true&n=sp21.5.316.c&r=SUBPART&ty=HTML#se21.5.316_121. Accessed August 16, 2017.
- 58.Hughes D.A., Poletti-Hughes J. Profitability and market value of orphan drug companies: a retrospective, propensity-matched case-control study. PLOS one. 2016;11:e0164681. doi: 10.1371/journal.pone.0164681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDuling J. Forget viagra: why rare diseases are big pharma’s latest obsession. Quarz Media LLC. Available at: https://qz.com/145941/why-rare-diseases-are-big-pharmas-latest-obsession/. Accessed August 6, 2017.
- 60.L.E.K. Consulting. Raising orphans: how pharma can capture value while treating rare diseases. Executive Insights 2014; volume XVI issue 9. Available at: https://www.lek.com/sites/default/files/LEK_1609_Orphan_Web_2.pdf. Accessed August 6, 2017.
- 61.Thomas K. Making ‘every patient counts’ a business imperative. The New York Times. Jan 30, 2013 http://www.nytimes.com/2013/01/31/business/orphan-drugs-for-rare-diseases-gain-popularity-with-pharmaceutical-companies.html Available at: Accessed August 6, 2017. [Google Scholar]
- 62.U.S. Food and Drug Administration. CDER Rare Disease and Orphan Drug Designated Approvals. CY 2016. Available at: https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/NDAandBLAApprovalReports/UCM544019.pdf. Accessed August 16, 2017.
- 63.U.S. Food and Drug Administration. Orphan Drug Designations and Approvals. Available at: https://www.accessdata.fda.gov/scripts/opdlisting/oopd/listResult.cfm?StartRow=51&EndRow=75. Accessed August 16, 2017.
- 64.Jarvis L.M. The year in new drugs: speedier development and regulatory process contributed to a peak in product approvals in 2015. The Year in New Drugs. 2016;94:12–17. [Google Scholar]
- 65.Carpenter D., Zucker E.J., Avorn J. Drug-review deadlines and safety problems. New Engl J Med. 2008;358:1354–1361. doi: 10.1056/NEJMsa0706341. [DOI] [PubMed] [Google Scholar]
- 66.Frank C., Himmelstein D.U., Wollhandler S. Era of faster FDA approval has also seen increased black-box warnings and market withdrawals. Health Aff. 2014;33:1453–1459. doi: 10.1377/hlthaff.2014.0122. [DOI] [PubMed] [Google Scholar]
- 67.Pollack A. Drug goes from $13.50 a tablet to $750, overnight. The New York Times; September 20, 2015.
- 68.Morgenson G. Fair game. Big pharma spends on share buybacks, but R&D? Not so much. The New York Times; July 14, 2017.
- 69.Lazonick W, Hopkins M, Jacobson K, Kakinc ME, Tulum O. US pharma’s financialized business model. Working paper No. 60, July 13, 2017. Institute for New Economic Thinking. New York, New York. Available at: https://www.ineteconomics.org/uploads/papers/WP_60-Lazonick-et-al-US-Pharma-Business-Model.pdf. Accessed August 23, 2017.
- 70.Simoens S. Pricing and reimbursement of orphan drugs: the need for more transparency. Orphanet J Rare Dis. 2011;6:42. doi: 10.1186/1750-1172-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jobjornsson S., Forster M., Pertile P., Burman C.F. Late-stage pharmaceutical R&D and pricing policies under two-stage regulation. J Health Econ. 2016;50:298–311. doi: 10.1016/j.jhealeco.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 72.Jackson E., Feldschreiber P., Breckenridge A. Regulatory consequences of “Brexit” for the development of medicinal products. Heart Failure. 2017;102:183–184. doi: 10.1002/cpt.706. [DOI] [PubMed] [Google Scholar]