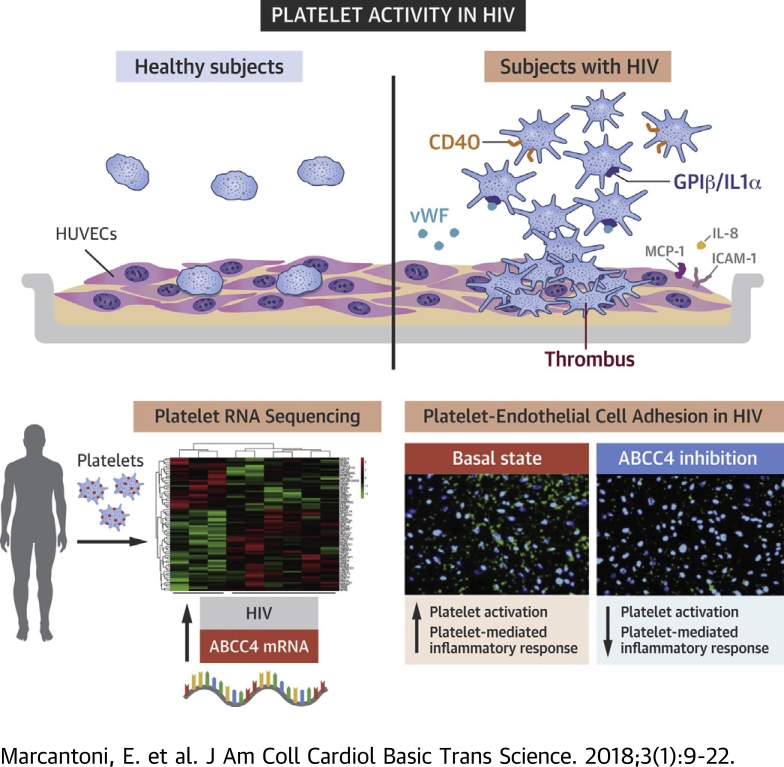
Visual Abstract
Key Words: ABCC4, cardiovascular disease, HIV, platelet activity
Abbreviations and Acronyms: ABCC4, ATP binding cassette subfamily C member 4; ART, antiretroviral therapy; BSA, bovine serum albumin; cAMP, cyclic adenosine monophosphate; CVD, cardiovascular disease; HIV, human immunodeficiency virus; HUVEC, human umbilical vein endothelial cell(s); IL, interleukin; NSAID, nonsteroidal anti-inflammatory drug; PAH, pulmonary artery hypertension; PBS, phosphate-buffered saline; qPCR, quantitative polymerase chain reaction; RNA-Seq, RNA sequencing; RT, room temperature; S1P, sphingosine-1-phosphate; VASP, vasodilator-stimulated phosphoprotein
Highlights
-
•
Platelet activity and its effector cell properties are increased in persons with virologically suppressed HIV on antiretroviral therapy.
-
•
The platelet transcriptome is differentially expressed in participants with HIV compared with healthy individuals.
-
•
ABCC4 expression and translation was enhanced in HIV-infected subjects compared with healthy individuals.
-
•
ABCC4 is a membrane transporter that plays an important role in regulating several cardiovascular processes, including platelet activation and aggregation.
-
•
Platelet ABCC4 inhibition in HIV attenuated platelet activation and platelet effector cell function by regulating cyclic nucleotide homeostasis and the extrusion of platelet proinflammatory mediators.
Summary
An unbiased platelet transcriptome profile identified ATP binding cassette subfamily C member 4 (ABCC4) as a novel mediator of platelet activity in virologically suppressed human immunodeficiency virus (HIV)-infected subjects on antiretroviral therapy. Using ex vivo and in vitro cellular and molecular assays we demonstrated that ABCC4 regulated platelet activation by altering granule release and cyclic nucleotide homeostasis through a cAMP-protein kinase A (PKA)–mediated mechanism. Platelet ABCC4 inhibition attenuated platelet activation and effector cell function by reducing the release of inflammatory mediators, such as sphingosine-1-phosphate. ABCC4 inhibition may represent a novel antithrombotic strategy in HIV-infected subjects on antiretroviral therapy.
Human immunodeficiency virus (HIV)-infected subjects are at significant risk for myocardial infarction and other forms of cardiovascular disease (CVD), even after controlling for traditional risk factors 1, 2. Due to antiretroviral therapy (ART), HIV-infected individuals are living longer, but CVD has become a leading cause of death 3, 4, 5. The mechanism by which HIV infection increases the risk of CVD is not fully known. Possible mechanisms increasing the risk of CVD involve chronic inflammation 6, 7, immune dysregulation (8), metabolic changes (9), increased coagulation (10), dyslipidemia (11), and endothelial dysfunction (12). Data from our group and others have demonstrated that platelets in persons with HIV reveal a basally activated state, which suggests that pathological platelet activation may contribute to HIV-mediated CVD 13, 14, 15, 16.
Platelets play a major role in hemostasis, with increased platelet activity contributing to the pathogenesis of atherothrombosis (17). In addition to their well-known functions in hemostasis and thrombosis, platelets play an important role in inflammation and immune activation (18). Activated platelets synthesize and release a host of pleiotropic inflammatory mediators, including interleukin-1β, CD40L, microparticles, and tissue factor, which interact with leukocytes and endothelial cells 19, 20, 21, 22. Platelets are discoid, anucleate cells generated from bone marrow megakaryocytes that retain megakaryocyte-derived mRNAs and translational machinery for protein biosynthesis 23, 24, 25. Platelet RNA expression profiling has been used in subjects with sickle cell disease, obesity, systemic lupus erythematosus, and CVD 26, 27, 28, 29, 30. Transcriptional profiling may yield novel mechanistic insights, unbiased by pre-existing disease hypotheses. We recently found the platelet transcriptome can: 1) distinguish healthy subjects with hyperreactive versus hyporeactive platelets; and 2) support a mechanistic link between platelet activity and CVD (31).
In the present study, we performed unbiased transcriptome profiling in platelets from subjects with HIV under ART and healthy controls. The platelet transcriptome was analyzed and single-transcript models constructed to identify candidate mRNAs with differential expression. We then sought to validate and study the mechanism of our top candidate transcript using ex vivo and in vitro cellular and molecular assays.
Methods
Study persons
The study was conducted in accordance with policies of the New York University Langone Medical Center Institutional Review Board, Bellevue Hospital Center, and the central office of the New York City Health and Hospital Corporation. Peripheral blood was drawn (3.8% sodium citrate tubes) with written consent, from healthy controls and HIV-infected subjects with HIV RNA viral load <200 copies/ml for ≥3 months on ART. Exclusion criteria included age <18 and >80 years, nonsteroidal anti-inflammatory drug (NSAID) use in the past week (including aspirin), antiplatelet or antithrombotic drug use, CVD, chronic kidney disease, steroids or immunosuppressive agents, active drug or alcohol use, known anemia (hemoglobin <8 mg/dl), or thrombocytopenia (<100 × 103/μl) or thrombocytosis (>500 × 103/μl).
Platelet preparation, lysates and supernatants collection
Platelet-rich plasma was added to 1:10 acid-citrate-dextrose solution, centrifuged (1,000 g, 10 min) and platelet pellet resuspended in Tyrode’s buffer and 1 μmol/l PGE1 (Sigma-Aldrich, St. Louis, Missouri). Platelets were counted on a Coulter AC·T diff2 Hematology Analyzer (Beckman Coulter, Brea, California) and adjusted to the desired concentration by addition of Tyrode’s buffer or endothelial or monocyte starvation medium. Cells were rested 30 min before thrombin activation. Resting or activated platelets were pelleted (14,000 g, 3 min) and lysed in 1% Triton X-100 (Thermo Fisher Scientific, Waltham, Massachusetts) in Tyrode’s buffer containing protease inhibitor cocktail.
Cell culture
Human umbilical vein endothelial cells (HUVECs) (Lonza, Basel, Switzerland) were cultured in endothelial growth medium (Lonza) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, California). For all experiments, HUVECs were used between passages 3 to 5. THP-1 cells (ATCC, Manassas, Virginia) were grown in RPMI 1640 medium (Corning, Corning, New York) supplemented with 10% fetal bovine serum.
Platelet-HUVEC coincubation
HUVECs, serum-starved overnight (0.5% bovine serum albumin [BSA] in basal medium), were incubated with either untreated or stimulated (0.05 U/ml thrombin, 5 min) platelets (1:100 ratio, 2 h, 37°C). Unbound platelets were washed away and HUVECs lysed in QIAzol (Qiagen, Hilden, Germany). Where indicated, platelets were pre-treated (30 min, 37°C) with the highly selective ATP binding cassette subfamily C member 4 (ABCC4) inhibitor Ceefourin 2 (3-chloro-5-4-methylphenyl)-7-(trifluoromethyl)pyrazolo[1,5-a]pyrimidine-2-carboxylic acid; Abcam, Cambridge, United Kingdom) (32) before coincubation.
Platelet-monocyte coincubation
Platelets, resuspended in serum-free RPMI 1640 containing 10 μg/ml polymyxin B sulfate (Sigma-Aldrich) were either left untreated or stimulated (0.25 U/ml thrombin, 5 min) before coincubation with THP-1 (1:100 ratio) in polystyrene round-bottom tubes (2 h, 37°C). THP-1 were pelleted (120 g, 5 min) and stored in QIAzol. Where indicated, platelets were pre-treated with Ceefourin (30 min, 37°C) before coculture.
Platelet purification
As previously described (33), platelets were subjected to negative selection based on magnetic cell sorting using human CD45+ and GLY A+ depletion kit (EasySep, STEMCELL Technologies, Vancouver, British Columbia, Canada). All purified platelets were lysed in QIAzol for RNA isolation. A relative purity (platelet/leukocyte ratio, 1 × 107) of platelet cell populations by flow cytometry and gene expression was obtained (31), consistent with other groups measuring platelet RNA expression (27).
RNA Sequencing
RNA sequencing (RNA-Seq) was performed in leukocyte-depleted platelet RNA from 6 subjects with HIV and 3 controls. Raw sequencing data were received in FASTQ format. Read mapping was performed using TopHat version 2.0.9 (Center for Computational Biology at Johns Hopkins University, Baltimore, Maryland) against the hg19 human reference genome. The resulting BAM alignment files were processed using the HTSeq version 0.6.1 Python framework (Python Software Foundation, Beaverton, Oregon) and respective hg19 GTF (gene transfer format) gene annotation, obtained from the UCSC Genome Browser database. The Bioconductor package DESeq2 (release version 3.2) was used to identify differentially expressed genes. This package provides statistics for determination of differentially expressed genes using a model based on the negative binomial distribution. The resulting values were then adjusted using the Benjamini and Hochberg’s method for controlling false discovery rate. Genes with a nominal p value ≤0.01 were determined differentially expressed. RNA sequencing data from platelet samples and subjects’ clinical characteristics have been submitted to GEO (accession number GSE99737). Gene Set Enrichment Analysis (GO) of transcripts differentially modulated between HIV and controls was performed.
Fluorescence microscopy
Adhesion of platelets to HUVECs was performed as described earlier in the text, with an additional step. Briefly, freshly isolated platelets were stained with 3 μmol/l CellTracker Green CMFDA Dye (Life Technologies, Carlsbad, California) and left untreated or treated with 0.05 U/ml thrombin for 5 min. After incubation with HUVECs, cells were fixed with 3.7% paraformaldehyde (10 min, room temperature [RT]) and permeabilized with 0.5% Triton X-100 in phosphate-buffered saline [PBS] (10 min, RT). Coverslips were mounted with VECTASHIELD Mounting Medium with DAPI (Vector Laboratories, Burlingame, California) and examined on an EVOS FL Imaging System microscope (Thermo Fisher Scientific). In some experiments, platelets were treated with Ceefourin after staining with CellTracker.
For ABCC4 and f-actin double staining, coverslips were coated with 40 μl of human collagen type I (1 mg/ml, Sigma-Aldrich), incubated (1.5 h, 37°C), washed twice with PBS, and blocked by BSA (2 mg/ml, 1 h, 37°C). After another wash with PBS, coverslips were incubated with 20 μl of platelet suspension (106/ml, 30 min, RT), washed with PBS 3×, and fixed by formaldehyde in PBS (1%, 30 min, RT). Platelets were then permeabilized with 0.5% Triton X-100 in PBS (15 min, RT) and blocked by 1% BSA in PBS (30 min, RT). ABCC4 staining was carried out using the ABCC4 antibody (1:500, overnight, 4°C; Abcam) and secondary fluorescein isothiocyanate antibody (1:200, 1 h, RT, Santa Cruz Biotechnology, Austin, Texas). Phalloidin staining, was added with ABCC4 secondary antibody (1:40, Thermo Fisher Scientific). Imaging was performed on a Zeiss AxioObserver with 63× N.A. 1.40 lens, Axiocam 503 mono, and narrow pass fluorescent filter blocks (Carl Zeiss, Oberkochen, Germany). For ABCC4 quantification, platelets from controls and HIV-infected subjects were compared. Fifty cells of each condition were imaged. Fields were imaged randomly, and all cells in each field were manually counted for the number of green dots per cell.
Mepacrine assay
Washed platelets (108/ml) were diluted 1:40 in Hanks' balanced salt solution. Ceefourin or vehicle was added to platelets to a final concentration of 10 μmol/l (30 min, 37°C). Platelets were then stimulated with thrombin (0, 0.05 or 0.25 U/ml, 5 min, 37°C). Mepacrine (10 μmol/l, Sigma-Aldrich) was added (30 min, 37°C) to stain dense granules. Samples, diluted 1:2 in Hanks' balanced salt solution were run on the flow cytometer. In these experiments, 10,000 platelets were collected off of forward and side scatter properties.
Sphingosine-1-phosphate measurement
Sphingosine-1-phosphate (S1P) was analyzed, with minor modifications, as described (34). Twenty-five or 50 μl of sample (sera and supernatants, respectively) were extracted by vortexing in a 1:30 v/v solution of diethylamide 10%/dichloromethanol: methanol 1:1 containing sphingomyelin C12 (d18:1/12:0, 120 μmol/l) as internal standard. External standards were quantified and processed identical to samples. The analyses were carried out in organic acid-resistant deep 96-well plates (Agilent Square 96-well, 2 ml # 51333009, Agilent, Santa Clara, California).
Statistics
Data were analyzed using standard descriptive and multivariable methods. Data were expressed as mean ± SEM or median (25th, 75th percentile), as appropriate. The statistical significance between 2 groups was determined by parametric (Student t test) or nonparametric (Mann Whitney U test) testing, as appropriate. Unadjusted and multivariable linear regression analysis was used to determine the impact of HIV status on platelet mRNA ABCC4 expression, controlling for potentially influential demographic and biological covariables, including duration of HIV, CD4 count, and HIV therapy type. Probability values <0.05 were considered statistically significant. Analyses were performed using SAS (version 9.3, SAS Institute, Cary, North Carolina) and GraphPad Prism (version 7.00 for Windows, GraphPad Software, La Jolla, California).
Methods for quantitative polymerase chain reaction (qPCR), Western blot, flow cytometry, and cyclic adenosine monophosphate (cAMP) measurement available in the Supplemental Methods.
Results
Subjects demographics
Median age of HIV-infected subjects was 53.5 (range 29 to 68) years. Nearly 82% of the population was black, and 57% were men. Fifty percent were current smokers. Mean CD4+ T-cell count was 665.6 (range 214 to 1,727). Mean years of HIV-1 diagnosis was 19.7 (range 5 to 32) years, and mean years on effective ART therapy with a suppressed HIV-1 RNA viral load was 14.4 (range 1 to 26) years. An overview of clinical characteristics and treatment at the time of blood sampling is presented in Table 1 and Supplemental Table 1.
Table 1.
Study Population Characteristics
| Controls (n = 7) | HIV∗ (n = 55) | |
|---|---|---|
| Age, yrs | 42.1 ± 8.5 | 53.5 ± 7.8 |
| Female | 57.1 | 42.6 |
| BMI, kg/m2 | 25.6 ± 1.8 | 27.0 ± 5.5 |
| Race | ||
| White | 71.4 | 18.5 |
| Black | 28.6 | 81.5 |
| Asian | 0 | 0 |
| Other | 0 | 1.9 |
| Ethnicity | ||
| Hispanic | 28.6 | 11.1 |
| Smoking status | ||
| Current | 0 | 50.0 |
| Former | 14.3 | 40.0 |
| Never | 85.7 | 10.0 |
| CD4+ T-cell count (c/mm3) | 0 | 665.6 ± 353.2 |
| Years of HIV duration | 0 | 19.7 ± 7.0 |
| Years on antiretroviral therapy | 0 | 14.4 ± 6.3 |
Values are mean ± SD or %.
BMI = body mass index; HIV = human immunodeficiency virus.
All variables were significantly different between HIV and controls (p < 0.05) except for BMI (0.51).
Endothelial cell and monocyte activation by platelets from HIV-infected subjects
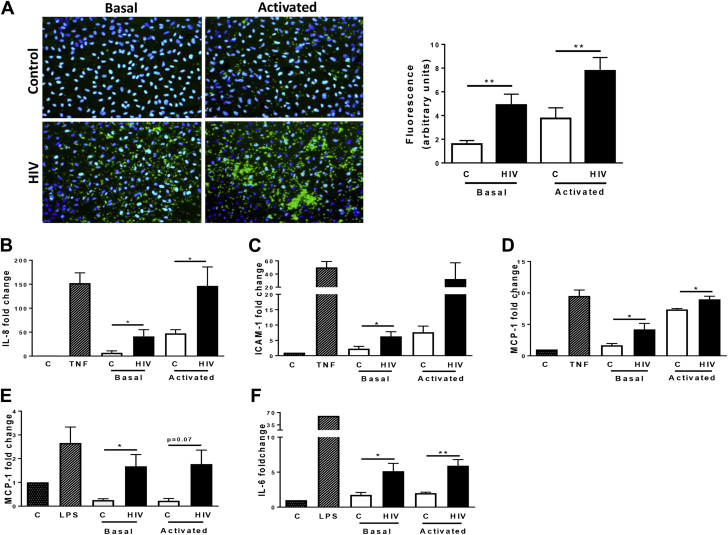
We and others have previously shown that platelet activity is increased in the setting of HIV 13, 14, 15, 16. We now sought to determine the effects of HIV-related platelet activation on endothelial cells and monocytic cell line because these cell types play important roles in CVD pathogenesis 35, 36. Labeled platelets isolated from HIV and healthy individuals were cocultured with HUVECs for 2 h in unstimulated (basal) conditions and following incubation with thrombin (0.05 U/ml). In healthy subjects, few unstimulated platelets adhered to HUVECs (Figure 1A). By contrast, platelets in their basal state isolated from subjects with HIV had increased adhesion to endothelial cells (Figure 1A). Thrombin stimulation further increased platelet adhesion in both groups, with a more pronounced effect in the HIV group (Figure 1A). Moreover, we observed that thrombin-activated platelets from subjects with HIV surrounded HUVECs forming rosette-like aggregates (Figure 1A).
Figure 1.
Endothelial and Monocytic Activation by Platelets Is Enhanced in HIV Subjects
(A) Immunofluorescence microscopy of platelet adhesion to HUVECs in healthy and HIV subjects. Freshly isolated platelets (green) from controls (C) and HIV subjects (HIV) were stained with CellTracker dye and left untreated (Basal, left panels) or treated with thrombin (0.05 U/ml for 5 min; Activated, right panels) before incubating with HUVECs. HUVEC nuclei are stained with DAPI (blue). The images are representative of 3 subjects for each group. Magnification, 20×. **p < 0.01. (B to D) HUVECs were coincubated with resting (Basal) or activated platelets from HIV (n = 6 to 7) and healthy controls (n = 6 to 7) for 2 h. Interleukin (IL)-8 (B), intercellular adhesion molecule (ICAM)-1 (C), and monocyte chemotactic protein (MCP)-1 (D) expression was assessed by quantitative polymerase chain reaction (qPCR). Values, normalized on 18S5 RNA represent fold change versus respective control. TNF (10 ng/ml) represents the positive control. *p < 0.05. (E and F) THP-1 were cultured with HIV platelets (HIV, n = 7) or control platelets (C, n = 4) at basal (Basal) or after stimulation (thrombin 0.25 U/ml, 5 min) for 2 h. MCP-1 (E) and IL-6 (F) expression was assessed by qPCR and normalized on 18S5 RNA. Lipopolysaccharide (100 ng/ml) was used as positive control. Values represent fold increase versus respective control. *p < 0.01, **p < 0.05, and p = 0.07. HIV = human immunodeficiency virus; HUVEC = human umbilical vein endothelial cell.
We next evaluated whether adherent platelets induced mRNA expression of endothelial cells and monocytic cells activation markers. Consistent with prior reports 37, 38, 39, 40, 41, we found that incubating endothelial cells or monocytic cells with healthy donor platelets that were activated by thrombin resulted in up-regulation of inflammatory gene expression (Figures 1B to 1F). Platelets isolated from subjects with HIV (vs. controls) induced the expression of interleukin (IL)-8, intercellular adhesion molecule (ICAM)-1, and monocyte chemotactic protein (MCP)-1 in HUVECs. These differences were observed from platelets in their basal state and after treatment with thrombin (Figures 1B to 1D). Platelets isolated from subjects with HIV were also able to induce expression of IL-6 and MCP-1 in THP-1 cells (Figures 1E and 1F). Altogether, these data demonstrate that platelets from HIV-infected subjects cause endothelial cell and monocyte activation.
Differentially expressed platelet mRNA in HIV
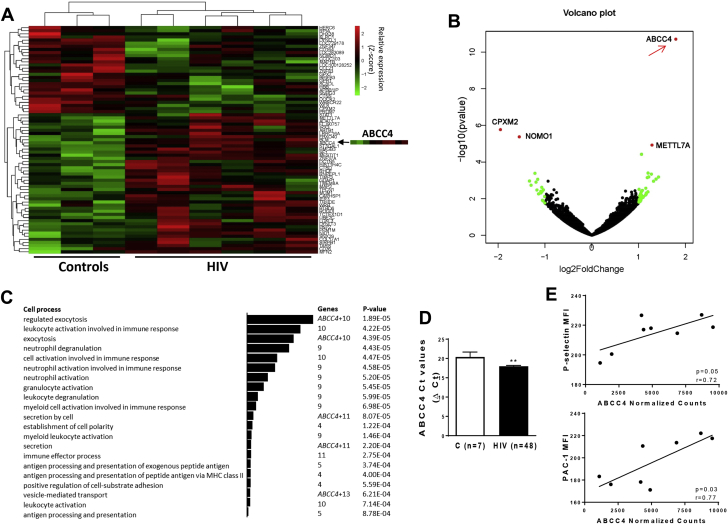
To characterize transcriptional changes associated with the hyperreactive platelet phenotype observed in persons with HIV, we conducted an unbiased RNA-Seq analysis of platelet mRNA from 6 HIV and 3 control subjects. Across the 9 platelet samples, there was an average of 23 million mapped reads per sample with an average unique mapping rate of 78.6%. Using a cutoff of normalized counts ≥1 averaged across the 9 samples, we found 11,988 expressed transcripts (Supplemental Figure 1). We identified candidate transcripts differentially expressed in platelets between HIV and controls (Figure 2A, Supplemental Table 2). The volcano plot of log2 (fold change) versus log10 (adjusted p values) from differentially expressed genes between the 2 cohorts is reported in Figure 2B. Using filtering criteria of a p value <0.01, GO analysis was performed on 73 differentially expressed transcripts (Supplemental Table 3).
Figure 2.
Platelet Transcriptome Profiling in HIV
(A) Heat map from hierarchical clustering of differentially expressed genes between controls and HIV platelets. Genes with p < 0.01 were considered for the analysis. The ABCC4 heat map is highlighted. (B) The data for all genes are plotted as log2 fold change versus the log10 of the p value. The top genes that are significantly different (sorted by p value) are indicated. (C) GO enrichment analysis (2 unranked list) of differentially expressed genes (p < 0.01). Bars indicate significantly enriched GO terms associated with cellular process and cell function. (D) ABCC4 mRNA expression in platelets from healthy volunteers (n = 7) and HIV (n = 48) subjects. qPCR analysis was reported expressing the ABCC4 Ct values after normalization with 18S5 RNA. **p < 0.01. (E) ABCC4 expression (RNA-Seq normalized counts) correlates with surface P-selectin and PAC-1 (activated Integrin αIIbβ3) staining in persons with HIV and controls, measured by flow cytometry (r = 0.72; p = 0.05 and r = 0.77; p = 0.03, respectively). Ct = concentration-time product; MFI = median fluorescence intensity; MHC = major histocompatibility complex; PAC-1 = activated glycoprotein IIb/IIIa receptor; other abbreviations as in Figure 1.
Platelets from persons with HIV had increased expression of genes involved in secretion and exocytosis and leukocyte activation in immune responses (Figure 2C, Supplemental Table 3). The gene encoding ABCC4 (ATP binding cassette subfamily C member 4, also called ABCC4) was the most up-regulated gene in the platelet transcriptome of HIV-infected subjects versus controls (3.5-fold change, p < 0.0001). Consistent with pathways analysis, ABCC4 is a protein-coding gene known to play an important role in platelet degranulation and activation (Figure 2C). Therefore, we selected ABCC4 as our candidate transcript for further analyses.
We validated ABCC4 up-regulation in HIV platelets by qPCR; ABCC4 expression was increased 2.3-fold in HIV versus controls (Supplemental Figure 2). To determine the robustness of our findings, ABCC4 platelet mRNA expression was assessed in a larger cohort of HIV-infected persons (n = 48) and healthy controls. Consistently, ABCC4 was significantly up-regulated in the HIV population compared with the control group, supporting our platelet RNA-Seq data (Figure 2D). In unadjusted analyses, HIV status was significantly associated with higher platelet mRNA ABCC4 expression. Additional variables associated with higher ABCC4 platelet expression were male sex and Hispanic ethnicity (Supplemental Table 4). No significant difference in ABCC4 expression was noted for hepatitis B, hepatitis C, CD4 count, smoking status, HIV duration, or type of ART (Supplemental Table 3). After adjustment for age, sex, race/ethnicity, and body mass index, persons with HIV had significantly higher ABCC4 than controls (β = −2.9; p = 0.001) (Supplemental Table 5).
Because platelet activity is increased in persons with HIV, we examined whether expression of platelet ABCC4 mRNA was associated with platelet activity. Platelet surface expression of P-selectin (r = 0.72; p = 0.046) and activated Integrin αIIbβ3(r = 0.77; p = 0.025) significantly correlated with ABCC4 platelet mRNA expression (Figure 2E).
ABCC4 protein levels in platelets of HIV subjects
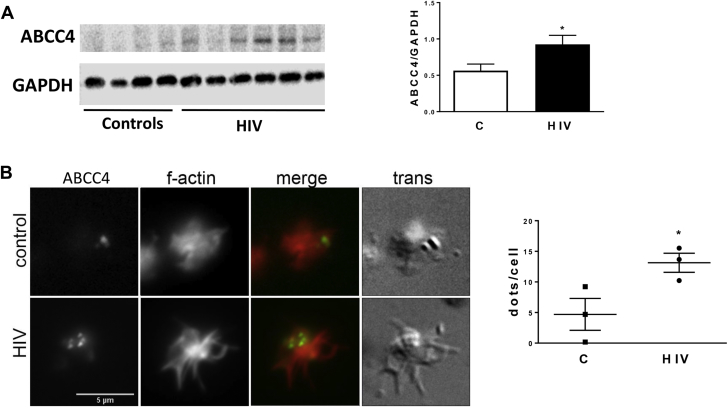
ABCC4 transcriptional up-regulation in subjects with HIV was assessed and confirmed at the protein level. Western blot analysis of platelet lysates showed a significant increase of ABCC4 protein expression in HIV-infected subjects versus controls (p < 0.05) (Figure 3A). Immunofluorescence microscopy of collagen-spread platelets further confirmed increased ABCC4 expression in platelets isolated from subjects with HIV (Figure 3B).
Figure 3.
Enhanced ABCC4 Protein Expression in HIV
(A) Immunoblots of ABCC4 in platelet lysates from controls (C) (n = 8) and HIV (n = 14) subjects. Thirty micrograms of protein were loaded, and GAPDH was used as a loading control. Quantification after normalization is reported. *p < 0.05. (B) Fluorescence microscopy showing ABCC4 expression (green) and f-actin (red) in HIV (n = 3) and controls (n = 3). Image is representative of a single platelet for each group. The quantification reports ABCC4 green dots/cell counted in 3 subjects per group (>50 cells for each sample). *p<0.05. Scale bar: 5 μm. GAPDH = glyceraldehyde 3-phoshate dehydrogenase; other abbreviations as in Figure 1.
ABCC4 inhibition reduces platelet granule release and impairs cyclic nucleotide homeostasis in HIV
Because ABCC4 is involved in the transport of diverse endogenous compounds, including dense granule content, we sought to investigate dense granule release in controls and HIV-infected subjects. Accumulation of the fluorescent mepacrine is used as a dense granule marker 42, 43.
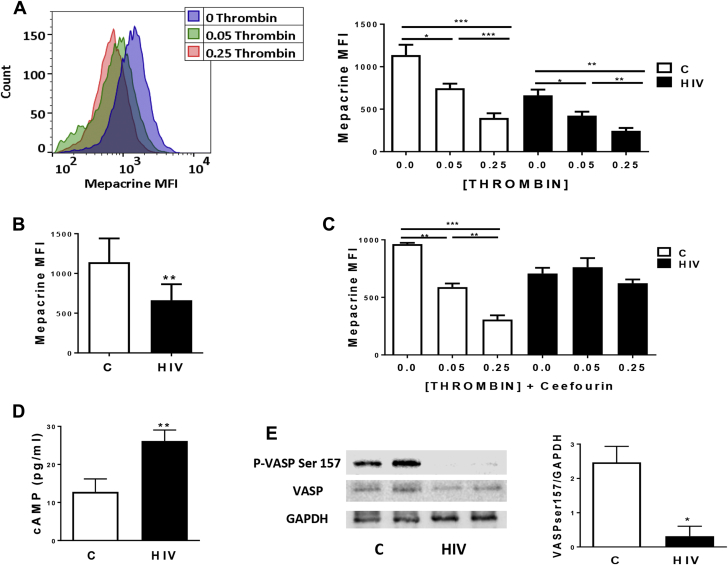
Consistent with the role of thrombin in inducing dense granule release (44), thrombin stimulation decreased mepacrine staining (e.g., increase in dense granule release) in both groups in a concentration-dependent manner (Figure 4A). Notably, in the basal state, subjects with HIV showed decreased mepacrine staining compared with controls (Figure 4B), supporting our previous data of enhanced basal platelet activation in HIV (16).
Figure 4.
ABCC4 Affects Dense Granule Release and Platelet cAMP Homeostasis in HIV
(A) Mepacrine staining was assessed via flow cytometry in platelets from controls (C) and HIV before and after activation with 0.05 or 0.25 U/ml thrombin. A representative histogram of 1 healthy control is shown. Quantitative analysis of 8 controls and 9 HIV is shown. *p < 0.05 and ***p < 0.0001. (B) Mepacrine staining via flow cytometry of platelets from controls (n = 9) and HIV (n = 8) at basal state. **p< 0.01. (C) Platelets from controls (n = 5) and HIV subjects (n = 4) were pre-treated with Ceefourin 2 (Ceefourin, 10 μmol/l), activated with 0, 0.05, or 0.25 U/ml thrombin and assessed for mepacrine staining. **p < 0.01 and ***p < 0.0001. (D) cAMP was quantified in the supernatants of activated (0.05 U/ml thrombin, 5 min) platelets from controls (n = 9) and subjects with HIV (n = 13). Levels are reported as pg/ml. **p < 0.01. (E) Platelets from HIV (n = 3) and controls (n = 3) were harvested for Western blot analysis. Blots were probed with anti P-VASP Ser 157 and anti-VASP, and loading was controlled with anti-GAPDH. Quantitative analysis was obtained by densitometry using ImageJ 1.51n software (NIH, Bethesda, Maryland). *p < 0.05. cAMP = cyclic adenosine monophosphate; GAPDH = glyceraldehyde 3-phoshate dehydrogenase; MFI = mean fluorescence intensity; VASP = vasodilator-stimulated phosphoprotein; other abbreviations as in Figure 1.
Delta granule release was further characterized by the highly selective ABCC4 inhibitor, Ceefourin 2. Delta granule release was measured after incubation with Ceefourin in both HIV and control subjects. Pre-treatment of HIV platelets with Ceefourin prevented granule release after thrombin stimulation. By contrast, Ceefourin had no effect on granule release in platelets from controls (Figure 4C). Our data suggest that ABCC4 inhibition would impair delta granule release in HIV-infected persons, for example, those with increased expression and function of ABCC4.
Upon platelet activation and certain pathophysiological conditions, ABCC4 translocates to the plasma membrane and alters platelet function by increasing transport of several substrates 45, 46. In platelets, a rise in cyclic nucleotides prevents activation of signaling pathways. Thus, any change in the distribution or availability of cyclic nucleotides may interfere with platelet reactivity. We therefore analyzed whether ABCC4 overexpression in HIV was associated with platelet levels of intracellular cAMP and downstream signaling. A significant increase in the amount of secreted cAMP was observed in persons with HIV versus controls (p < 0.01) (Figure 4D). To determine whether these differences in cAMP secretion affected downstream signaling, we analyzed vasodilator-stimulated phosphoprotein (VASP) phosphorylation on Ser157, a preferential cAMP-dependent protein kinase phosphorylation site. Western blot analysis of platelet lysates in HIV-infected individuals and healthy subjects revealed that VASP phosphorylation was significantly reduced in HIV (Figure 4E), suggesting a decrease in cytosolic cAMP levels in HIV platelets. Altogether, these data suggest that ABCC4 overexpression observed in HIV contributes to decreased cytosolic cAMP levels in platelets, resulting in a decreased VASP phosphorylation, thus leading to enhanced platelet activation.
ABCC4 inhibition decreases platelet effector cell function and platelet S1P release in HIV
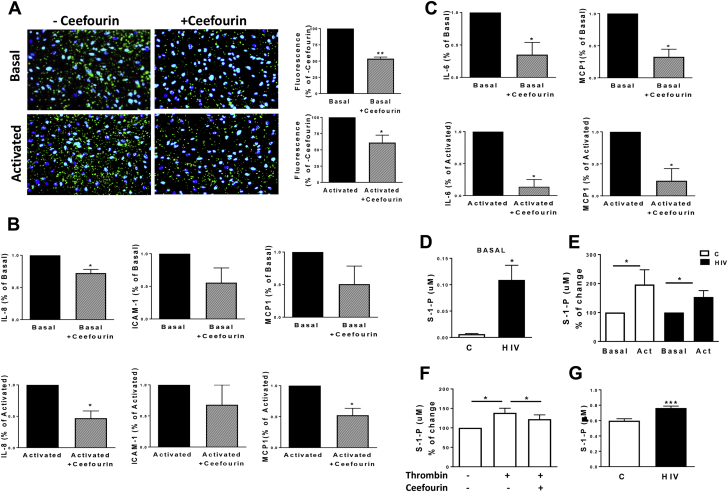
To investigate the potential role of ABCC4 in platelets as effector cells, we tested platelet adhesion to endothelial cells in the presence of Ceefourin. Pre-treatment with Ceefourin of platelets isolated from HIV-infected persons significantly reduced platelet adhesion to HUVECs in the basal state or following thrombin stimulation (47% and 39% reduction compared with controls, respectively [Figure 5A]), whereas no differences were observed in healthy subjects (Supplemental Figure 3). Consistent with reduced platelet adhesion to endothelial cells, ABCC4 inhibition in platelets resulted in the reduction of HUVEC activation markers IL-8, ICAM-1, and MCP-1 and THP-1 markers IL-6 and MCP-1 only in the HIV cohort (Figures 5B and 5C, Supplemental Figure 4). Altogether, these data suggest that ABCC4 inhibition in HIV may represent a useful mechanism to reduce platelet-induced endothelial cell and monocyte activation, by blocking the release of granule contents from platelets.
Figure 5.
ABCC4 Contributes to Endothelial and Monocyte Activation and Increased S1P Release From Activated Platelets in HIV
(A) Immunofluorescence microscopy of platelet adhesion to HUVECs in subjects with HIV. Stained platelets (green) were left untreated (Untreated) or treated with Ceefourin2 (Ceefourin, 10 μmol/l) for 30 min. Thrombin (0.05 U/ml, 5 min) was then added (Activated). HUVEC nuclei are stained with DAPI (blue). The images are representative of 3 subjects for each group. Values are represented as percentage of respective controls. **p < 0.01 and *p < 0.05. Magnification = 20×. (B and C) Gene expression analysis of HUVEC inflammatory genes IL-8, ICAM-1, and MCP1, and THP-1 markers IL-6 and MCP-1. HUVECs and THP-1 were cocultured with untreated or pre-treated (Ceefourin, 10 μmol/l) platelets from HIV-infected subjects at basal (upper graphs) and after stimulation with thrombin (0.05 U/ml, bottom graphs). Results were normalized on 18S5 RNA. Values represent fold change after normalization to basal or activated condition in 3 different subjects. **p < 0.01 versus Basal and *p < 0.05 versus Activated. (D) Sphingosine-1-phosphate (S1P) in platelet supernatants of HIV (n = 16) and controls (C) (n = 8) at the basal state was analyzed by high-performance liquid chromatography-mass spectrometry (HPLCMS/MS) and normalized for protein content. *p < 0.05. (E) S1P quantification in platelet supernatants of subjects with HIV (n = 17) and healthy controls (n = 7) before and after stimulation with thrombin (0.05 U/ml, 5 min). Levels are reported after normalization to respective basal levels. *p < 0.05. (F) Platelets from healthy subjects (n = 5) were left untreated or stimulated with thrombin in presence or absence of Ceefourin (10 μmol/l). S1P levels were quantified in platelet supernatants, and values expressed as percentage of resting platelets. *p < 0.05. (G) S1P quantified in plasma of subjects with HIV (n = 40) and controls (n = 16) by HPLCMS/MS. Abbreviations as in Figure 1.
S1P, an immune modulating lipid mediator, has been reported to induce proinflammatory signaling pathways in the immune and vascular system 47, 48, 49. Platelets release S1P upon activation, and ABCC4 has been reported to mediate its release from platelets (50). We therefore sought to investigate whether platelets from HIV-infected subjects with ABCC4 overexpression released greater amount of S1P compared with controls. In the basal state, S1P levels were markedly increased in HIV platelet supernatants (Figure 5D). After platelet activation, S1P increased in both groups (Figure 5E). To confirm whether ABCC4 inhibition affects S1P levels, we measured S1P release in supernatants of Ceefourin–pre-treated platelets. ABCC4 inhibition significantly reduced S1P levels following platelet activation (Figure 5F). Finally, S1P was measured in plasma of HIV and controls. S1P levels were significantly increased in plasma of subjects with HIV (p < 0.0001) (Figure 5G).
Discussion
Prior studies have demonstrated increased cardiovascular risk in persons with virologically controlled HIV on ART 12, 51. Nonetheless, the reason(s) for this heightened risk have yet to be fully clarified. Platelet activation and immune activation leading to a prothrombotic state have been proposed as significant contributors to CVD 6, 16. Platelets isolated from persons with HIV have increased surface expression of P-selectin, activated glycoprotein IIb/IIIa, and increased aggregation in response to submaximal agonist simulation 16, 52. In the current study, we expand on these findings and demonstrate an enhanced platelet effector role in subjects with HIV. Both in the basal state and following agonist stimulation, platelets from subjects with HIV were more adherent to endothelial cells and monocytic cells and were able to induce a proinflammatory effect on these cell types. These findings support the role of platelets as inflammatory mediators in persons with HIV infection.
In pathological conditions in response to extracellular signaling, a transcriptional modulation during megakaryopoiesis may occur (53). Although anucleate, platelets retain megakaryocyte-derived cytoplasmic RNA and may translate small amounts of mRNAs as well as process miRNAs 54, 55, 56. Therefore, platelet transcript content may play a biological role beyond being remnant RNA derived from the megakaryocyte. Herein, we present an unbiased characterization of the transcriptome of platelets isolated from HIV subjects under ART using RNA-Seq, to identify mRNAs associated with increased platelet activity in HIV. Our platelet transcriptome analysis identified pathways differentially expressed between HIV and healthy individuals, including exocytosis and secretion, inflammatory response, and immune cell trafficking.
Notably, ABCC4 mRNA emerged as the most upregulated transcript in HIV compared with controls. ABCC4 has been shown to play an important role in conveying several molecules that control multiple cardiovascular processes, including smooth muscle cell proliferation, cardiomyocyte contractility, and platelet activation 57, 58, 59. Recent findings indicate that hemostasis and thrombosis are affected in ABCC4-deficient mice, with ABCC4 promoting platelet aggregation by modulating the cAMP-protein kinase A (PKA) signaling (44). These results are in line with studies showing that the absence or inhibition of ABCC4 affects platelet activation and aggregation 60, 61. Therefore, ABCC4 was chosen as a candidate for further analyses and was validated in a larger cohort of persons with HIV and controls. In this second larger cohort, increased ABCC4 gene expression was also demonstrated, and remained significant after multivariable adjustment. Although HIV status was associated with platelet expression of ABCC4 mRNA, no association was noted for different HIV ART regimens, length of HIV diagnosis, smoking status, and CD4 count.
We and others have previously noted that platelet activity is in a heightened state in persons with HIV on ART 16, 52, 62, 63. It was therefore not surprising that ABCC4 overexpression in HIV significantly correlated with platelet activation markers P-selectin and PAC-1, supporting a link between ABCC4 and platelet activity in these persons.
Localization of ABCC4 in platelets has been debated and remains uncertain. ABCC4 was demonstrated to be highly expressed on the membrane of dense granules, which facilitates ADP accumulation and export 42, 46. It was also proposed that ABCC4 localization in platelets can be shifted from granules to the plasma membrane in certain high-risk conditions, including platelet activation 45, 64. Other groups found that ABCC4 is localized primarily to the plasma membrane in platelets 65, 66. We investigated dense granule ADP accumulation and release in HIV-infected subjects and healthy controls. We confirmed enhanced platelet activity in HIV-infected individuals, compared with controls in the basal state. The export of ADP and granule mediators was evaluated in both groups using the highly selective ABCC4 inhibitor, Ceefourin 2 (32). In previous reports, ABCC4 platelet inhibition used the MK571 antagonist 65, 67, 68. However, MK571 has been noted to be a non-specific ABCC4 inhibitor as well as a potent leukotriene D4 receptor and MRP1 antagonist (69). In persons with HIV infection, Ceefourin inhibited dense granule release after thrombin activation, suggesting that ABCC4 impairs dense-granule release in HIV.
ABCC4 has been reported to be an endogenous regulator of intracellular cAMP and cAMP-mediated signaling pathway in platelets 44, 65, 68. ABCC4 inhibition was found to protect from hypoxemia-induced pulmonary hypertension in a murine model by increasing intracellular cAMP levels and preventing activation of cAMP-mediated pathways (68). In murine platelets, absence of ABCC4 attenuated collagen-mediated aggregation and impaired thrombus formation by producing an elevation in cAMP level (65). Therefore, we sought to investigate whether ABCC4 modulated platelet activation in HIV through a cAMP-mediated mechanism. We measured platelet cAMP secretion and cAMP cytosolic levels through VASP phosphorylation on Ser157. Our data demonstrated that platelet ABCC4 is an important contributor to platelet activity in HIV, by impairing cAMP homeostasis.
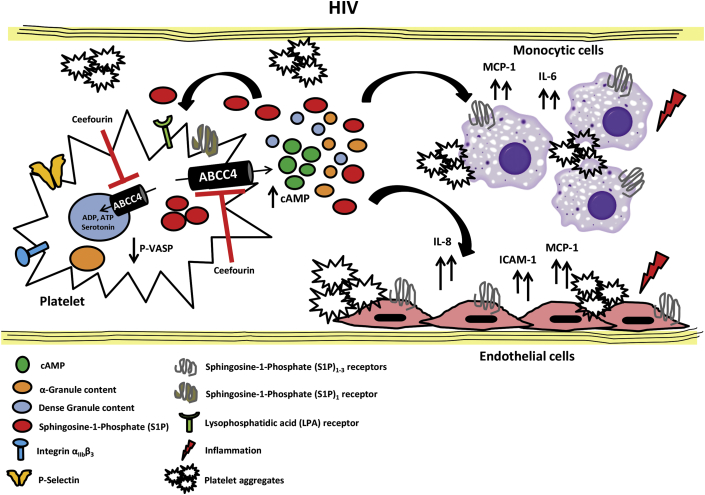
We then demonstrated that ABCC4 inhibition in platelets attenuated endothelial cell and monocyte activation, both in the basal state and after activation. These data support a new role for platelet ABCC4 in mediating, not only platelet function, but also the platelet-mediated effector cell function in HIV. The mechanism linking platelet ABCC4 expression and monocyte/endothelial cell activation is unknown, but signaling lipid S1P is a likely intermediary. Platelets are known to release high levels of a multifunctional S1P upon direct activation of protein kinase C signaling (e.g., thrombin) or during blood clotting 70, 71. Cellular S1P secretion requires active transport across the membrane by ATP-dependent carriers, such as ABCC4 (50). A link between platelet S1P in inflammatory processes and immune response has already been suggested 48, 72, 73. In our study, we demonstrated an enhanced basal secretion of S1P from platelets in HIV, consistent with a hyperreactive platelet phenotype in the basal state. Moreover, ABCC4 inhibition was able to reduce S1P levels following thrombin-induced platelet activation, suggesting a role of ABCC4 as a mediator of S1P release in subjects with HIV who showed a basally active platelet phenotype. A model depicting the role of ABCC4 on platelet function and regulation of inflammatory response of endothelial cells and monocytic cell line in HIV disease is reported in Figure 6.
Figure 6.
Schematic Model of Platelet ABCC4 Role in HIV
In HIV, circulating platelets have increased expression of ABCC4 as consequence of enhanced platelet activation. ABCC4 overexpression on platelet plasma membrane contributes to increased excretion of platelet mediators in the extracellular space, including cAMP, which plays a crucial role in maintaining platelets in the inactive state. In turn, platelet inflammatory mediators activate endothelial cells and monocytic cells, and also platelets, promoting the formation of circulating platelet aggregates. The large amount of S1P released by activated platelets likely contributes to amplify platelet response and the inflammatory process in HIV. Platelet ABCC4 inhibition by Ceefourin in HIV prevents platelet activation by maintaining cAMP homeostasis. Moreover, the inhibition of ABCC4 has an important role in mediating platelet–cell interaction and the inflammatory response in HIV, by decreasing platelet-mediator release and activation of target cells in circulation. Abbreviations as in Figure 1.
As previously mentioned, ABCC4 is involved in the development and progression of pulmonary artery hypertension (PAH) (68). Many clinical studies have demonstrated an association between PAH and HIV infection (74), both before and after ART 75, 76. The impact of ABCC4 on PAH incidence and severity in persons with HIV is unknown and deserves further investigation.
Finally, NSAIDs, including aspirin, increase ABCC4 expression 45, 77. ABCC4-mediated aspirin extrusion from the platelet cytosol causes an incomplete COX-1 inhibition in persons after coronary artery bypass graft surgery (45). In the current study, NSAID use was an exclusion criterion for participation. A recent randomized trial from our group demonstrated no significant benefit on immune activity or vascular health from low-dose aspirin in persons with HIV (78). Whether this neutral effect was mediated, in part, by ABCC4 overexpression in HIV-infected persons is unknown. A pilot trial of a different antiplatelet therapy, clopidogrel, in persons with HIV is ongoing.
Conclusions
Our study is the first to identify increased levels of ABCC4 mRNA as a novel mediator regulating platelet function in persons with HIV. Moreover, we provide insights into the molecular mechanisms by which ABCC4 mediates a hyperreactive platelet phenotype and platelet effector cell function in the setting of HIV. These findings may have important clinical implications in HIV-infected persons. In fact, by acting on the extrusion of cyclic nucleotides and inflammatory mediators, ABCC4 inhibition might represent a novel antithrombotic strategy for virologically suppressed HIV-infected subjects on ART.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: HIV infection significantly increases the risk of myocardial infarction and other forms of CVD. Multiple factors including pathological platelet activation contribute to this enhanced risk. However, the mechanism by which HIV infection increases platelet activity is unknown.
TRANSLATIONAL OUTLOOK: The characterization of the platelet transcriptome profile in persons with virologically controlled HIV on antiretroviral therapy revealed ABCC4 as a central mediator of platelet activity and platelet-mediated proinflammatory response in endothelial cells and monocytic cell line. Targeting ABCC4 in HIV-infected subjects may represent a novel antithrombotic strategy in persons with HIV.
Acknowledgments
The authors thank the Genome Technology Center at the NYU Langone Medical Center for expert RNA-Seq library preparation and sequencing, and the Applied Bioinformatics Laboratories (ABL) at the NYU School of Medicine for providing bioinformatics support and helping with the analysis and interpretation of the data. This work has used computing resources at the NYU High Performance Computing Facility (HPCF).
Footnotes
This work was supported, in part, by the National Heart and Lung Blood Institute of the National Institutes of Health (R01HL114978 to Dr. Berger; and 1R24OD018340) and a Hirschl-Weill-Caulier Career Scientist Award (to Dr. Berger). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors' institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Freiberg M.S., Chang C.C., Kuller L.H. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos A.G., Hulzebosch A., Grobbee D.E., Barth R.E., Klipstein-Grobusch K. Association between immune markers and surrogate markers of cardiovascular disease in HIV positive patients: a systematic review. PLoS One. 2017;12 doi: 10.1371/journal.pone.0169986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Moller N., Thiebaut R., Reiss P. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491–501. doi: 10.1097/HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 4.Triant V.A. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205 Suppl 3:S355–S361. doi: 10.1093/infdis/jis195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nou E., Lo J., Hadigan C., Grinspoon S.K. Pathophysiology and management of cardiovascular disease in patients with HIV. Lancet Diabetes Endocrinol. 2016;4:598–610. doi: 10.1016/S2213-8587(15)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nou E., Lo J., Grinspoon S.K. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS. 2016;30:1495–1509. doi: 10.1097/QAD.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Triant V.A., Meigs J.B., Grinspoon S.K. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lichtenstein K.A., Armon C., Buchacz K. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 9.Hadigan C., Meigs J.B., Corcoran C. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001;32:130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 10.Kuller L.H., Tracy R., Belloso W. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5 doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riddler S.A., Smit E., Cole S.R. Impact of HIV infection and HAART on serum lipids in men. JAMA. 2003;289:2978–2982. doi: 10.1001/jama.289.22.2978. [DOI] [PubMed] [Google Scholar]
- 12.Torriani F.J., Komarow L., Parker R.A. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: the ACTG (AIDS Clinical Trials Group) study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allam O., Samarani S., Jenabian M.A. Differential synthesis and release of IL-18 and IL-18 binding protein from human platelets and their implications for HIV infection. Cytokine. 2017;90:144–154. doi: 10.1016/j.cyto.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 14.DAD Study Group. Friis-Moller N., Reiss P. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 15.Hauguel-Moreau M., Boccara F., Boyd A. Platelet reactivity in human immunodeficiency virus infected patients on dual antiplatelet therapy for an acute coronary syndrome: the EVERE2ST-HIV study. Eur Heart J. 2017;38:1676–1686. doi: 10.1093/eurheartj/ehw583. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien M., Montenont E., Hu L. Aspirin attenuates platelet activation and immune activation in HIV-1-infected subjects on antiretroviral therapy: a pilot study. J Acquir Immune Defic Syndr. 2013;63:280–288. doi: 10.1097/QAI.0b013e31828a292c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davi G., Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 18.Rondina M.T., Weyrich A.S., Zimmerman G.A. Platelets as cellular effectors of inflammation in vascular diseases. Circ Res. 2013;112:1506–1519. doi: 10.1161/CIRCRESAHA.113.300512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gawaz M., Langer H., May A.E. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klinger M.H., Jelkmann W. Role of blood platelets in infection and inflammation. J Interferon Cytokine Res. 2002;22:913–922. doi: 10.1089/10799900260286623. [DOI] [PubMed] [Google Scholar]
- 21.Linden M.D., Jackson D.E. Platelets: pleiotropic roles in atherogenesis and atherothrombosis. Int J Biochem Cell Biol. 2010;42:1762–1766. doi: 10.1016/j.biocel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Dovizio M., Alberti S., Guillem-Llobat P., Patrignani P. Role of platelets in inflammation and cancer: novel therapeutic strategies. Basic Clin Pharmacol Toxicol. 2014;114:118–127. doi: 10.1111/bcpt.12156. [DOI] [PubMed] [Google Scholar]
- 23.Denis M.M., Tolley N.D., Bunting M. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122:379–391. doi: 10.1016/j.cell.2005.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weyrich A.S., Lindemann S., Tolley N.D. Change in protein phenotype without a nucleus: translational control in platelets. Semin Thromb Hemost. 2004;30:491–498. doi: 10.1055/s-2004-833484. [DOI] [PubMed] [Google Scholar]
- 25.Bray P.F., McKenzie S.E., Edelstein L.C. The complex transcriptional landscape of the anucleate human platelet. BMC Genomics. 2013;14:1. doi: 10.1186/1471-2164-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eicher J.D., Chen M.H., Pitsillides A.N. Whole exome sequencing in the Framingham Heart Study identifies rare variation in HYAL2 that influences platelet aggregation. Thromb Haemost. 2017;117:1083–1092. doi: 10.1160/TH16-09-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Freedman J.E., Larson M.G., Tanriverdi K. Relation of platelet and leukocyte inflammatory transcripts to body mass index in the Framingham heart study. Circulation. 2010;122:119–129. doi: 10.1161/CIRCULATIONAHA.109.928192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kondkar A.A., Bray M.S., Leal S.M. VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA. J Thromb Haemost. 2010;8:369–378. doi: 10.1111/j.1538-7836.2009.03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lood C., Amisten S., Gullstrand B. Platelet transcriptional profile and protein expression in patients with systemic lupus erythematosus: up-regulation of the type I interferon system is strongly associated with vascular disease. Blood. 2010;116:1951–1957. doi: 10.1182/blood-2010-03-274605. [DOI] [PubMed] [Google Scholar]
- 30.Raghavachari N., Xu X., Harris A. Amplified expression profiling of platelet transcriptome reveals changes in arginine metabolic pathways in patients with sickle cell disease. Circulation. 2007;115:1551–1562. doi: 10.1161/CIRCULATIONAHA.106.658641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montenont E., Echagarruga C., Allen N., Araldi E., Suarez Y., Berger J.S. Platelet WDR1 suppresses platelet activity and is associated with cardiovascular disease. Blood. 2016;128:2033–2042. doi: 10.1182/blood-2016-03-703157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung L., Flemming C.L., Watt F. High-throughput screening identifies Ceefourin 1 and Ceefourin 2 as highly selective inhibitors of multidrug resistance protein 4 (MRP4) Biochem Pharmacol. 2014;91:97–108. doi: 10.1016/j.bcp.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 33.Plé H., Landry P., Benham A., Coarfa C., Gunaratne P.H., Provost P. The repertoire and features of human platelet microRNAs. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bui H.H., Leohr J.K., Kuo M.S. Analysis of sphingolipids in extracted human plasma using liquid chromatography electrospray ionization tandem mass spectrometry. Anal Biochem. 2012;423:187–194. doi: 10.1016/j.ab.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 35.Jackson S.P. Arterial thrombosis–insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. doi: 10.1038/nm.2515. [DOI] [PubMed] [Google Scholar]
- 36.von Hundelshausen P., Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 37.Weyrich A.S., Elstad M.R., McEver R.P. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passacquale G., Vamadevan P., Pereira L., Hamid C., Corrigall V., Ferro A. Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nhek S., Clancy R., Lee K.A. Activated platelets induce endothelial cell activation via an interleukin-1beta pathway in systemic lupus erythematosus. Arterioscler Thromb Vasc Biol. 2017;37:707–716. doi: 10.1161/ATVBAHA.116.308126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kojima H., Kanada H., Shimizu S. CD226 mediates platelet and megakaryocytic cell adhesion to vascular endothelial cells. J Biol Chem. 2003;278:36748–36753. doi: 10.1074/jbc.M300702200. [DOI] [PubMed] [Google Scholar]
- 41.Bombeli T., Schwartz B.R., Harlan J.M. Adhesion of activated platelets to endothelial cells: evidence for a GPIIbIIIa-dependent bridging mechanism and novel roles for endothelial intercellular adhesion molecule 1 (ICAM-1), alphavbeta3 integrin, and GPIbalpha. J Exp Med. 1998;187:329–339. doi: 10.1084/jem.187.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jedlitschky G., Tirschmann K., Lubenow L.E. The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood. 2004;104:3603–3610. doi: 10.1182/blood-2003-12-4330. [DOI] [PubMed] [Google Scholar]
- 43.Gordon N., Thom J., Cole C., Baker R. Rapid detection of hereditary and acquired platelet storage pool deficiency by flow cytometry. Br J Haematol. 1995;89:117–123. doi: 10.1111/j.1365-2141.1995.tb08901.x. [DOI] [PubMed] [Google Scholar]
- 44.Decouture B., Dreano E., Belleville-Rolland T. Impaired platelet activation and cAMP homeostasis in MRP4-deficient mice. Blood. 2015;126:1823–1830. doi: 10.1182/blood-2015-02-631044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattiello T., Guerriero R., Lotti L.V. Aspirin extrusion from human platelets through multidrug resistance protein-4-mediated transport: evidence of a reduced drug action in patients after coronary artery bypass grafting. J Am Coll Cardiol. 2011;58:752–761. doi: 10.1016/j.jacc.2011.03.049. [DOI] [PubMed] [Google Scholar]
- 46.Jedlitschky G., Cattaneo M., Lubenow L.E. Role of MRP4 (ABCC4) in platelet adenine nucleotide-storage: evidence from patients with delta-storage pool deficiencies. Am J Pathol. 2010;176:1097–1103. doi: 10.2353/ajpath.2010.090425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang F., Xia Y., Yan W. Sphingosine 1-phosphate signaling contributes to cardiac inflammation, dysfunction, and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2016;310:H250–H261. doi: 10.1152/ajpheart.00372.2015. [DOI] [PubMed] [Google Scholar]
- 48.Rauch B.H. Sphingosine 1-phosphate as a link between blood coagulation and inflammation. Cell Physiol Biochem. 2014;34:185–196. doi: 10.1159/000362994. [DOI] [PubMed] [Google Scholar]
- 49.Aoki M., Aoki H., Ramanathan R., Hait N.C., Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediators Inflamm. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ulrych T., Bohm A., Polzin A. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J Thromb Haemost. 2011;9:790–798. doi: 10.1111/j.1538-7836.2011.04194.x. [DOI] [PubMed] [Google Scholar]
- 51.Liao J.K. Linking endothelial dysfunction with endothelial cell activation. J Clin Invest. 2013;123:540–541. doi: 10.1172/JCI66843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holme P.A., Muller F., Solum N.O., Brosstad F., Froland S.S., Aukrust P. Enhanced activation of platelets with abnormal release of RANTES in human immunodeficiency virus type 1 infection. FASEB J. 1998;12:79–89. doi: 10.1096/fasebj.12.1.79. [DOI] [PubMed] [Google Scholar]
- 53.Tijssen M.R., Ghevaert C. Transcription factors in late megakaryopoiesis and related platelet disorders. J Thromb Haemost. 2013;11:593–604. doi: 10.1111/jth.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rowley J.W., Oler A.J., Tolley N.D. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118:e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagalla S., Shaw C., Kong X. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117:5189–5197. doi: 10.1182/blood-2010-09-299719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edelstein L.C., Bray P.F. MicroRNAs in platelet production and activation. Blood. 2011;117:5289–5296. doi: 10.1182/blood-2011-01-292011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sassi Y., Lipskaia L., Vandecasteele G. Multidrug resistance-associated protein 4 regulates cAMP-dependent signaling pathways and controls human and rat SMC proliferation. J Clin Invest. 2008;118:2747–2757. doi: 10.1172/JCI35067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sassi Y., Abi-Gerges A., Fauconnier J. Regulation of cAMP homeostasis by the efflux protein MRP4 in cardiac myocytes. FASEB J. 2012;26:1009–1017. doi: 10.1096/fj.11-194027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belleville-Rolland T., Sassi Y., Decouture B. MRP4 (ABCC4) as a potential pharmacologic target for cardiovascular disease. Pharmacol Res. 2016;107:381–389. doi: 10.1016/j.phrs.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Jedlitschky G., Vogelgesang S., Kroemer H.K. MDR1-P-glycoprotein (ABCB1)-mediated disposition of amyloid-beta peptides: implications for the pathogenesis and therapy of Alzheimer's disease. Clin Pharmacol Ther. 2010;88:441–443. doi: 10.1038/clpt.2010.126. [DOI] [PubMed] [Google Scholar]
- 61.Borgognone A., Pulcinelli F.M. Reduction of cAMP and cGMP inhibitory effects in human platelets by MRP4-mediated transport. Thromb Haemost. 2012;108:955–962. doi: 10.1160/TH12-04-0232. [DOI] [PubMed] [Google Scholar]
- 62.Satchell C.S., Cotter A.G., O'Connor E.F. Platelet function and HIV: a case-control study. AIDS. 2010;24:649–657. doi: 10.1097/QAD.0b013e328336098c. [DOI] [PubMed] [Google Scholar]
- 63.Corrales-Medina V.F., Simkins J., Chirinos J.A. Increased levels of platelet microparticles in HIV-infected patients with good response to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;54:217–218. doi: 10.1097/QAI.0b013e3181c8f4c9. [DOI] [PubMed] [Google Scholar]
- 64.Jedlitschky G., Greinacher A., Kroemer H.K. Transporters in human platelets: physiologic function and impact for pharmacotherapy. Blood. 2012;119:3394–3402. doi: 10.1182/blood-2011-09-336933. [DOI] [PubMed] [Google Scholar]
- 65.Cheepala S.B., Pitre A., Fukuda Y. The ABCC4 membrane transporter modulates platelet aggregation. Blood. 2015;126:2307–2319. doi: 10.1182/blood-2014-08-595942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bai J., Lai L., Yeo H.C., Goh B.C., Tan T.M. Multidrug resistance protein 4 (MRP4/ABCC4) mediates efflux of bimane-glutathione. Int J Biochem Cell Biol. 2004;36:247–257. doi: 10.1016/s1357-2725(03)00236-x. [DOI] [PubMed] [Google Scholar]
- 67.Lien L.M., Chen Z.C., Chung C.L. Multidrug resistance protein 4 (MRP4/ABCC4) regulates thrombus formation in vitro and in vivo. Eur J Pharmacol. 2014;737:159–167. doi: 10.1016/j.ejphar.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Hara Y., Sassi Y., Guibert C. Inhibition of MRP4 prevents and reverses pulmonary hypertension in mice. J Clin Invest. 2011;121:2888–2897. doi: 10.1172/JCI45023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reid G., Wielinga P., Zelcer N. The human multidrug resistance protein MRP4 functions as a prostaglandin efflux transporter and is inhibited by nonsteroidal antiinflammatory drugs. Proc Natl Acad Sci U S A. 2003;100:9244–9249. doi: 10.1073/pnas.1033060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tantry U.S., Bonello L., Aradi D. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–2273. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 71.Yatomi Y., Ohmori T., Rile G. Sphingosine 1-phosphate as a major bioactive lysophospholipid that is released from platelets and interacts with endothelial cells. Blood. 2000;96:3431–3438. [PubMed] [Google Scholar]
- 72.English D., Welch Z., Kovala A.T. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- 73.Mahajan-Thakur S., Bohm A., Jedlitschky G., Schror K., Rauch B.H. Sphingosine-1-phosphate and its receptors: a mutual link between blood coagulation and inflammation. Mediators Inflamm. 2015;2015:831059. doi: 10.1155/2015/831059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morris A., Gingo M.R., George M.P. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS. 2012;26:731–740. doi: 10.1097/QAD.0b013e32835099ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Speich R., Jenni R., Opravil M., Pfab M., Russi E.W. Primary pulmonary hypertension in HIV infection. Chest. 1991;100:1268–1271. doi: 10.1378/chest.100.5.1268. [DOI] [PubMed] [Google Scholar]
- 76.Sitbon O., Lascoux-Combe C., Delfraissy J.F. Prevalence of HIV-related pulmonary arterial hypertension in the current antiretroviral therapy era. Am J Respir Crit Care Med. 2008;177:108–113. doi: 10.1164/rccm.200704-541OC. [DOI] [PubMed] [Google Scholar]
- 77.Temperilli F., Di Franco M., Massimi I. Nonsteroidal anti-inflammatory drugs in-vitro and in-vivo treatment and Multidrug Resistance Protein 4 expression in human platelets. Vascul Pharmacol. 2016;76:11–17. doi: 10.1016/j.vph.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 78.O'Brien M.P., Hunt P.W., Kitch D.W. A randomized placebo controlled trial of aspirin effects on immune activation in chronically human immunodeficiency virus-infected adults on virologically suppressive antiretroviral therapy. Open Forum Infect Dis. 2017;4:ofw278. doi: 10.1093/ofid/ofw278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.