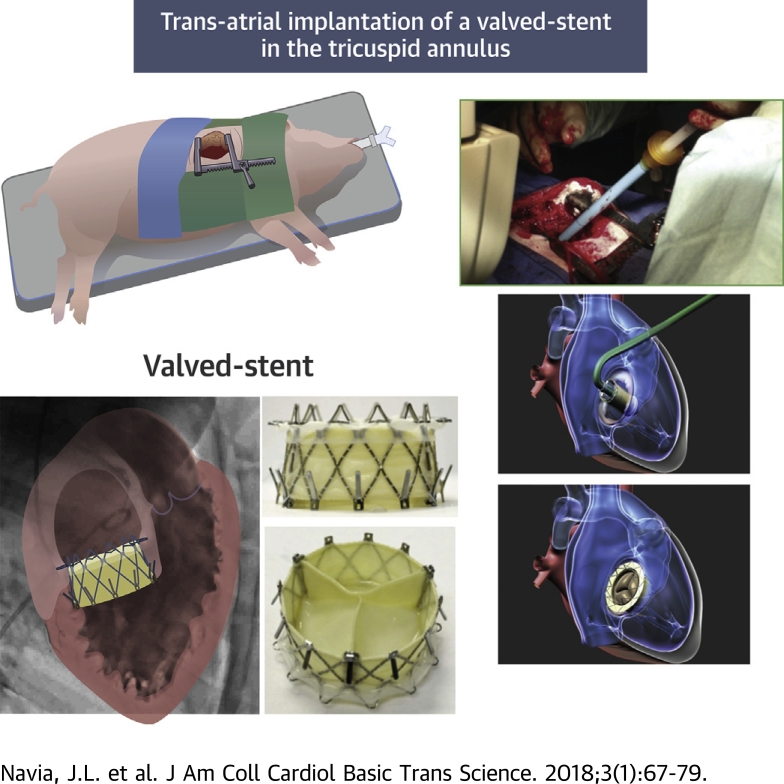
Visual Abstract
Key Words: NaviGate bioprosthesis, preclinical model, transcatheter replacement, tricuspid valve
Abbreviations and Acronyms: ICE, intracardiac echocardiography; PVL, paravalvular leak; RA, right atrium; RHF, right heart failure; RV, right ventricle/ventricular; RVOT, right ventricle outflow; TA, tricuspid annulus; TR, tricuspid regurgitation; TV, tricuspid valve
Highlights
-
•
Surgery for isolated tricuspid regurgitation carries a high mortality risk, especially in the setting of right ventricular dysfunction and reoperation.
-
•
Transcatheter valve therapy is as promising alternative for treatment of isolated tricuspid valve disease associated with right heart failure.
-
•
The NaviGate bioprosthesis is a novel self-expanding valved stent designed to treat functional tricuspid regurgitation.
-
•
The preclinical evaluation shows that transcatheter tricuspid valve implantation using the NaviGate device is safe, is feasible through 2 different approaches, and results in a secure and stable engagement of the native annulus, with excellent hemodynamic and valve performance.
Summary
Patients with isolated functional or recurrent tricuspid regurgitation are often denied surgery because they are considered to be at high risk. Transcatheter valve therapy provides a less invasive alternative for tricuspid regurgitation associated with right heart failure. We have evaluated the feasibility of transcatheter tricuspid valve implantation of the NaviGate valved stent in a long-term swine model. The valved stent was successfully implanted through transjugular and transatrial approaches on the beating heart with excellent hemodynamic and valve performance. No conduction disturbance or coronary obstruction was observed. This technology could provide an alternative treatment for patients who are at high surgical risk with severe tricuspid regurgitation and compromised right ventricular function.
Transcatheter valve technologies have been emerging over the past decade as a new paradigm of heart valve treatment, especially in selected high-risk patients whose care becomes increasingly challenging; these technologies include percutaneous replacement of aortic and pulmonic valves, as well as multiple techniques regarding repairing and replacing the mitral valve for functional or degenerative mitral regurgitation. Secondary tricuspid valve regurgitation (TR) is frequent in patients with long-standing left-sided valve disease, particularly in the setting of severe pulmonary hypertension and atrial fibrillation 1, 2. Uncorrected, moderate, or severe TR is associated with progressive heart failure and premature death (3); even if corrected, it is a marker of lower long-term survival. However, the optimal timing and surgical technique to eliminate TR remains undefined, showing that tricuspid valve (TV) disease is not only underdiagnosed, but is also often surgically ignored. For many years it was believed that secondary TR would improve after mitral valve surgery alone, and it was consequently undertreated (4). Thus, for example, in 1967, Braunwald et al. (5) recommended conservative nonsurgical management of “functional” TR. In the 1980s, it was observed that patients who had undergone successful mitral surgery sometimes returned years later with severe symptomatic TR and right heart failure. When these patients underwent reoperation, mortality was high (3). More recently, compelling data have shown that surgically untreated secondary TR can persist or even worsen despite correction of the associated left-sided lesion (6), suggesting that due to the severity and progressive course of the disease, an aggressive approach toward secondary TR is warranted 7, 8. Unfortunately, this approach is rarely used, so patients usually do not undergo TV reoperations for secondary or recurrent TR, leading to high mortality rates due to worsening right heart failure (9).
In general, most of these patients present with renal impairment, severe pulmonary hypertension, and cardiac cirrhosis late in the disease state; given their coagulopathic state and low likelihood of improvement of right ventricular (RV) function in the perioperative period, they become high-risk surgical candidates. Thereby, transcatheter approaches to TV disease could become an attractive alternative solution for these patients who present a burgeoning clinical unmet need for therapeutic advances. In addition, less invasive strategies may allow for earlier surgical treatment of TR, which may improve functional capacity and reduce end-organ dysfunction.
The NaviGate bioprosthesis (NaviGate Cardiac Structures Inc., Irvine, California) was designed exclusively to overcome the technical challenges associated with transcatheter valve implantation for these high-risk patients. Herein, we describe the preclinical feasibility study of a new transcatheter self-expanding bioprosthetic TV, implanted in a chronic porcine model using transcatheter and minimally invasive approaches on a beating heart with a sutureless valve implantation technique.
Methods
NaviGate bioprosthesis
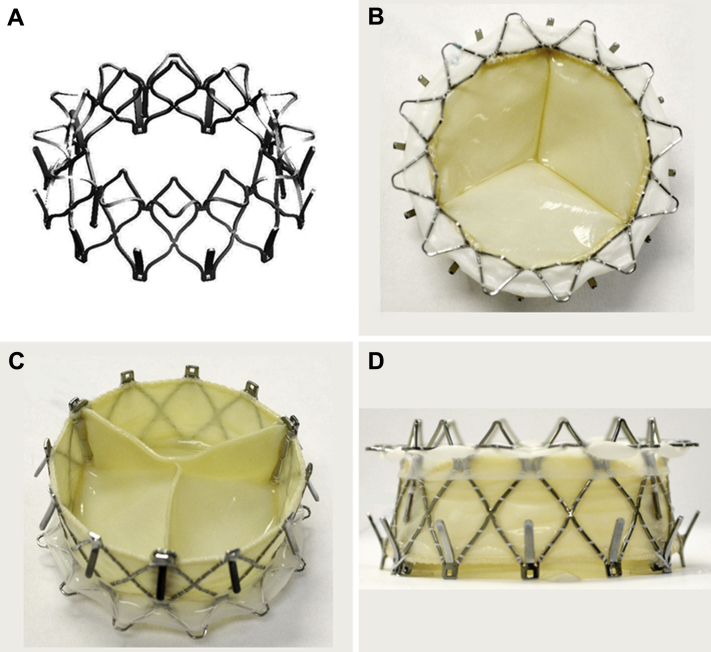
The NaviGate atrioventricular valve is a biological valved stent; it consists of a specifically configured Nitinol alloy stent into which is mounted a trileaflet valvular mechanism fabricated from equine pericardium that has been fixed in low concentrations of buffered glutaraldehyde (Figures 1A to 1D). The configuration of the stent is specifically designed in a geometry that engages the tricuspid annulus (TA) and TV leaflets from both inferior and superior aspects and maintains a minimal extension into both the atrium and ventricle to avoid flow dynamics alterations. Thus, the inferior aspect or ventricular diameter is designed to match the dilated tricuspid annulus typical of secondary TR, which has a mean diametric dimension of 40 ± 5 mm for most patients. For this experimental study, the superior or atrial size of the valve stent is maintained at 30 mm in diameter, and the inferior or ventricular diameter at 40 mm, ensuring that transvalvular gradients will be normal for a TV (ΔPmean ≤5 mm Hg) at normal flows. This configuration results in a truncated conical shape that helps to reduce the height of the valved stent. This results in low intrusion into the right atrium (RA) and RV, giving rise to a divergent nozzle that, by slowing the flow entering the RV, reduces energy loss and flow separation to optimize the transvalvular gradient. From the surface of the truncoconal structures, specially configured structures (winglets and graspers) are incorporated into the proximal (n = 12) and distal (n = 12) ends of the stent. They are deployed sequentially at defined angles to radially fix the bioprosthesis to the TA, minimizing dislocation toward the adjacent chambers. The atrial winglets of the NaviGate device are designed to be relatively short and are covered by woven microfiber polyester fabric lining to avoid compression of the conduction fibers (Figure 1).
Figure 1.
The NaviGate Valved-Stent
(A) Stent. (B) Inflow (left atrial) view. (C) Outflow (left ventricle) view. (D) Side view.
The preserved xenogeneic pericardial membrane is used to fashion both leaflets and inner walls of the valved stent. The lining of woven microfiber polyester fabric supports the pericardial membrane wall to protect its integrity and to prevent paravalvular leakage (PVL). When totally assembled, the bioprosthesis proceeds through a proprietary dehydration process in which all glutaraldehyde and most of its water are removed; it is then sterilized by ethylene oxide exposure and stored in its primary container in the dehydrated form until use.
Delivery system and implantation procedures
Transatrial approach
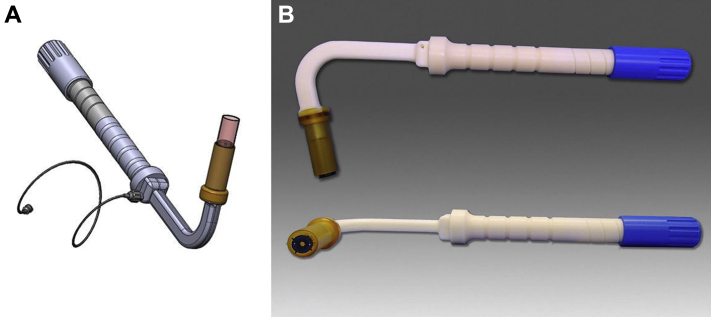
The atrioventricular valved stent is delivered transatrially through a minimally invasive right thoracotomy directly through the free wall of the RA into the TV, using a sutureless technique without cardiopulmonary bypass or rapid ventricular pacing. The delivery device is depicted in Figures 2A and 2B. It evokes the configuration of a mitral commissurotomy device of decades past; when introduced into the left atrium through a small incision, the curved shape and angulation of the shaft leads the distal end into a central alignment position in the TV. The valved stent is loaded and crimped inside the distal capsule, and it can be retracted by simple controlled turning of the proximal handle knob. Retraction causes exposure of the ventricular or distal aspect of the valved stent, causing the ventricular winglets or graspers to deploy that may capture the leaflets above the tricuspid chordae tendineae. The operator can readjust the depth and angle of the valve/delivery system using fluoroscopy and intracardiac echocardiography (ICE) to achieve a good relationship between the RV, RA, and the plane of the TV annulus for final deployment.
Figure 2.
Transatrial Tricuspid Valved Stent Delivery System
(A) Illustrated design of the transatrial delivery system. (B) Lateral view of the transatrial delivery system.
Details of the delivery systems and surgical transatrial implantation technique of the valve are presented in Supplemental Appendix 1.
Transjugular approach
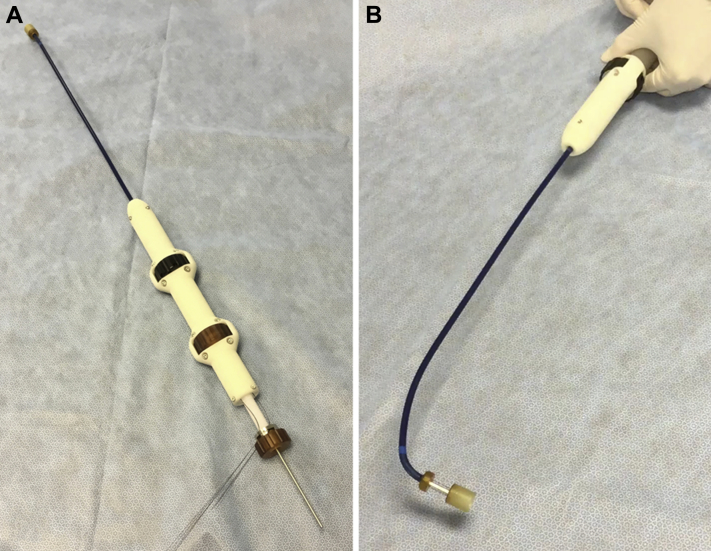
The transjugular delivery system consists of an 80-cm usable catheter with a central conduit accommodating a 0.038-inch guidewire and a 24-F shaft reinforced with interior metallic coiling, for the most part, to optimize its tracking through the vasculature and imaging visibility. The distal section is flexible, as that includes the 30-F outer diameter stent-enclosing capsule and a mechanism that allows steering in 2 directions, including depth control by forward or reverse motion of the distal capsule when in the immediate vicinity of the TV annular plane. The introducer sheath (∼45 cm and 45-F outer diameter) has a hydrophilic coating for atraumatic insertion into the vessel. The catheter is pictured in Figures 3A and 3B. After gaining access into the RA through the right jugular with a modified Seldinger technique (viewed both by fluoroscopy and ICE), the distal end of the delivery system was positioned coaxially to the center of the swine TV, and the tricuspid valved stent was aligned with the plane of the TA. The middle annular region of the tricuspid valved stent was visible by fluoroscopy, with the distal anchors or grasper tips in the contracted or crimped state within the distal capsule. Details of the percutaneously implanted valve are presented in Supplemental Appendix 1.
Figure 3.
Transjugular Tricuspid Valved Stent Delivery System
(A) The delivery system distal capsule extended. (B) The delivery system distal capsule articulated 90°.
Experimental design
In vivo assessment of safety
These studies were designed both to assess the safety of the NaviGate device and to obtain a partial assessment of its effectiveness in performing the designed function; the latter assessment aimed to provide sufficient safety data for the first-in-human evaluation.
Long-term in vivo study
A bioprosthesis valve replacement is intended to remain in situ for its life or the life of the patient. Thus, long-term implantation in the in vivo model was studied to preliminarily assess the continuous function and durability of the device. In the absence of a natural, practical, and cost-effective animal model of tricuspid regurgitation (TR), the healthy swine was chosen to test the safety of the device. Safety includes the proper delivery and deployment of the device with the delivery system. The effectiveness focused on assessment of the performance of the valved stent and its durability. Accordingly, complete percutaneous, transjugular access, catheter-guided implantation of a NaviGate self-expanding bioprosthesis into the native TV of healthy swine was successfully performed (n = 6; 85 to 90 kg) (Table 1).
Table 1.
Model Information for Transjugular Implant in a Long-Term Animal Study
| Animal model | Swine, 85–90 kg |
| Access site for transjugular | Percutaneous right jugular vein access—sutureless implantation technique without cardiopulmonary bypass |
| Delivery system | Size 30-F outer diameter |
| Devices | NaviGate valved-stent, 30-mm inflow and 40-mm outflow, 12 tines in inflow and outflow |
| Implantation interval | Chronic long term (≥25 days) |
| Number of animals | 6 transjugular |
Previously a series of 6 NaviGate tricuspid valved stents were implanted in 6 healthy swine (n = 6; 95 to 105 kg) in the TV orthotopic position for a long-term preclinical study using a minimally invasive right thoracotomy approach on a beating heart with a sutureless implantation technique (Table 2). All valve prostheses used in this study have equivalent design and fabrication methods, and they were evaluated in a pulsatile mock circulatory loop for hemodynamic performance and quality control. The long-term study also assesses the replicability of both implantation approaches, the valved stent’s suitability to maintain valve function and valve hemodynamic function, durability, right ventricular outflow tract (RVOT) obstruction and coronary arteries obstruction if any, the formation of interfering pannus, procedural success and blood loss, possible calcification of biological tissue, and absence of thrombosis.
Table 2.
Model Information for Transatrial Implant in a Long-Term Animal Study
| Animal model | Swine, 95–105 kg |
| Access site for transatrial | Through a mini-thoracotomy RA access—sutureless implantation technique without cardiopulmonary bypass |
| Delivery system | Size 30-F outer diameter |
| Devices | NaviGate valved-stent, 30-mm inflow and 4-mm outflow, 12 tines in inflow and outflow |
| Implantation interval | Chronic long term (≥25 days) |
| Number of animals | 6 transatrial |
Implantation procedure
Pre-operative management
All animals received humane care in compliance with the Animal Welfare Act and Public Health Services policies, and the study protocol was approved by the Institutional Animal Care and Use Committee at the Gateway Innovation Laboratory, Shanghai, China.
The details for each of the modes of approach and anesthesia management are given in Supplemental Appendix 1. In brief, after gaining access into the RA (viewed both by fluoroscopy and ICE) through the antegrade transatrial and transjugular approach, the distal end of the delivery system was positioned coaxially with the center of the swine TV, and the tricuspid valved stent aligned with the plane of the TA. The middle annular region of the tricuspid valved stent is visible by fluoroscopy, with the distal anchors or grasper tips in the contracted or crimped state within the distal capsule. The tricuspid annular plane was defined using fluoroscopic imaging of guidewires in both the right coronary artery and left circumflex artery. Coaxiality of the stented valve to the tricuspid annulus was confirmed prior to deployment using multimodality imaging; fluoroscopic identification of the tricuspid annulus was defined by the guidewire, and positioning in the center of the annulus plane was guided by ICE.
When the operator, guided by imaging, was confident that the valved stent was coaxial in the right landing zone and ready to deploy, further turning of the proximal knob slowly retracted the capsule, allowing the expansion of the valved stent’s distal perimeter to reach close to 80% of the nominal diameter. Then, the ventricular winglets or distal graspers engaged the TV leaflets. The final release was achieved with a turn of the proximal knob, that allowed the atrial anchors to deploy, and the tricuspid valved stent was delivered into its proper locus, anchored from the ventricular as well as from the atrial aspects of the native TV (Figure 4). No rapid pacing was required during this operation as the device functions during a slow controlled release when it reaches 60% of its opening diameter. Thus, there was no obstruction of the blood flow or hemodynamic compromise. Details of the percutaneously and surgically implanted valve are presented in Supplemental Appendix 1.
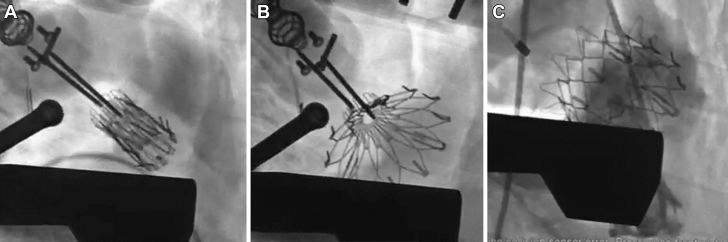
Figure 4.
Fluoroscopy: Transatrial Tricuspid Valved Stent Implant
(A) Positioning of the valved stent in the tricuspid annulus plane. (B) Slow release of the valved stent. (C) Full implantation in the tricuspid annulus.
Post-deployment measurements and management
RV angiography was performed to confirm proper placement of the valve and to rule out RVOT obstruction and/or TR (central to paravalvular regurgitation based on echocardiography guidelines [10]), and a coronary artery angiogram was performed to rule out coronary artery obstruction or compression. Both transesophageal echocardiogram and ICE were used to confirm placement and proper valve function. Echocardiography, hemodynamic interrogations, and valve function assessment were repeated after 20 min when hemodynamic stability was achieved. When hemostasis was ensured, chest, neck, and femoral incisions were closed in layers in the usual fashion. The animals recovered in the acute care unit after the procedure. All animals were followed clinically for manifestation of heart failure and with serial echocardiogram assessment. All animals underwent post-mortem examination of the device and cardiac tissues. Echocardiographic assessment was performed in all animals before euthanasia as well as at 3 and 6 months for live animals. Details of the post-implant measurements and management are presented in Supplemental Appendix 2.
Data analysis
Hemodynamic measurements after NaviGate stented valve implantation were summarized using standard descriptive statistics and are reported as mean ± SD. Analysis of data was performed using a paired Student t test with 2-sided comparisons. A p value of 0.05 was considered to indicate statistical significance. Data analysis was performed using a statistical software package (SPSS for Windows, version 15.0, IBM, Armonk, New York).
Results
Long-term study: transatrial access
Long-term transcatheter RA access of an orthotopic tricuspid valve implantation was performed in the swine model (n = 6; 95 to 105 kg) (≥25 post-operative days). All valves were successfully implanted with no technical difficulties during prostheses deployment, providing a 100% procedural success. Although the deployment was feasible for the novel valved stent, the use of a well-known minimally invasive RA access under direct vision with a sutureless implantation technique provided an advantage. The follow-up varied from 1 to ≥25 days per protocol. All animals but 1 survived the intervention: 1 died during the procedure after developing acute severe TR secondary to annulus-prosthesis mismatch that resulted in prosthesis RV migration. Of the remaining 5 animals, 1 animal survived 30 days and was sacrificed per protocol; 2 animals survived 150 days and were sacrificed per protocol; and 2 animals are still alive. No animals that survived the procedure died secondary to a cardiac or noncardiac complication. Echocardiographic and fluoroscopic assessment confirmed excellent function of the new TV in all animals, with accurate alignment and secure anchoring of the valved stent onto the TA (Figures 4 and 5). Conformance to the tricuspid annulus with no PVL or central TR was observed in 4 valves, with 1 mild PVL and 1 central severe TR/valve dislodgment. In all cases of successful implantation (n = 5) of the new valve, there was a clear unobstructed RVOT, no pericardial effusion, normal coronary artery anatomy, and no arrhythmias, with a preservation of the subvalvular apparatus. Valve function evidenced normal hemodynamic performance and normal pressure gradient across the implanted valve (≤3 mm Hg), as well as RVOT gradients (Table 3). All animals had normal RV function before the procedure and preserved RV function during follow-up period and at the time of euthanasia.
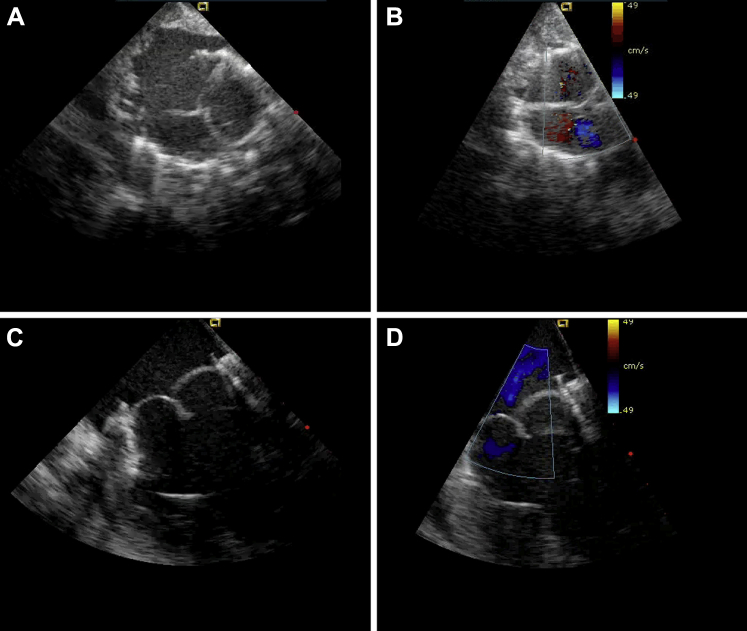
Figure 5.
Echocardiography: Transatrial Tricuspid Valved Stent Implantation
(A and B) Echocardiographic short-axis view. (C and D) Echocardiographic long-axis view.
Table 3.
Chronic Long-Term Tricuspid Valve Study
| Sex, Body Weight (kg) | Model # | Ice C-C Dimension (cm) | Blood Loss (ml) | Time for Implant (min) | ΔPp mm Hg (peak) | ΔPm mm Hg (mean) | RVOT Velocity (m/s) | RVOT Gradient (mean/mm Hg) | Leakage Central or Paravalvular |
Duration (days) |
|---|---|---|---|---|---|---|---|---|---|---|
| F, 105 TA | X27 | 3.59 | 250 | 6.0 | 6.2 | 4.0 | 0.49 | 1.1 | Mild central | 30 |
| F, 104 TA | X27 | 3.93 | 400 | 12 | 6.0 | 2.5 | 0.47 | 1.2 | No | 150 |
| M, 98 TA | X27 | 3.93 | 300 | 5.0 | 4.0 | 2.0 | 0.45 | 1.8 | No | 150 |
| F, 100 TA | X27 | 3.92 | 350 | 7.0 | 5.4 | 1.7 | 0.48 | 0.9 | No | ≥150/alive |
| F, 96 TA | X27 | 4.55 | 1,000 | EUTH | EUTH | — | — | — | TVD | 0.3 |
| F, 95 TA | X27 | 4.42 | 150 | 6.0 | 5.0 | 3.1 | 0.35 | 1.5 | No | ≥150/alive |
| M, 85 TJ | X27 | 4.00 | 50 | 15 | 6.0 | 2.8 | 0.50 | 2.0 | Mild PVL | 210 |
| M, 88 TJ | X27 | 3.70 | 100 | 12 | 5.4 | 2.0 | 0.41 | 1.8 | No | 210 |
| F, 90 TJ | X27 | 4.12 | 50 | 17 | 6.4 | 3.2 | 0.52 | 1.6 | No | ≥120/alive |
| F, 85 TJ | X27 | 3.93 | 75 | 18 | 4.8 | 2.5 | 0.44 | 2.1 | Mod PVL | ≥120/alive |
| M, 89 TJ | X27 | 4.10 | 85 | 15 | 5.0 | 2.7 | 0.53 | 2.2 | No | ≥90/alive |
| M, 90 TJ | X27 | 3.88 | 90 | 13 | 4.7 | 2.2 | 0.49 | 1.9 | No | ≥90/alive |
C-C = commissure-to-commissure; EUTH = euthanasia; ICE = intra-cardiac echocardiography; Pm mmHg = mean pressure gradient across the valved stent; Pp mmHg = peak pressure gradient across the valved stent; PVL = paravalvular leak; RVOT = right ventricular outflow tract; TA = transatrial; TJ = transjugular; TVD = total valve dislodgement.
The entire procedure time in all study animals ranged from 40 to 55 min, the time from the minimally invasive right thoracotomy to position the delivery system at the TV annulus after surgical access ranged from 6 to 9 min, and the NaviGate bioprosthesis deployment time ranged from 1 to 3 min.
Long-term study: transjugular access
Long-term transcatheter right jugular access of an orthotopic tricuspid valve implantation was performed in a swine model (n = 6; 85 to 90 kg) (≥25 post-operative days). All valves were successfully implanted with a complete percutaneous approach, with no technical difficulties during prostheses deployment, providing a 100% procedural success. The delivery system’s articulation feature up to 90° certainly provides an elegant mechanism to align the new valve in the center of the TA and, when combined with the guidewires’ position inside of the right and circumflex coronary arteries that provides a fluoroscopic image of a horizontal plane of the TA, helps the operator secure a safe and accurate landing zone to deploy the valve on a beating heart with a sutureless implantation technique.
All 6 animals survived the intervention: 2 animals survived >210 days, 2 animals survived >120 days and are still alive, and another 2 animals survived >90 days and are both still alive. No animals that survived the procedure died secondary to cardiac or noncardiac complication. Echocardiographic and fluoroscopic assessment confirmed excellent function of the new TV in all animals, with accurate alignment and secure anchoring of the valved stent onto the TA (Figures 6 and 7). Conformance to the tricuspid annulus with no PVL or central TR was observed in 4 valves, whereas 1 animal has mild PVL and 1 animal has moderate PVL. In all 6 animals, the new NaviGate valve left a clear, unobstructed RVOT, no pericardial effusion, normal coronary artery anatomy, and no arrhythmias or atrial ventricular conduction disturbances, with a preservation of the subvalvular apparatus.
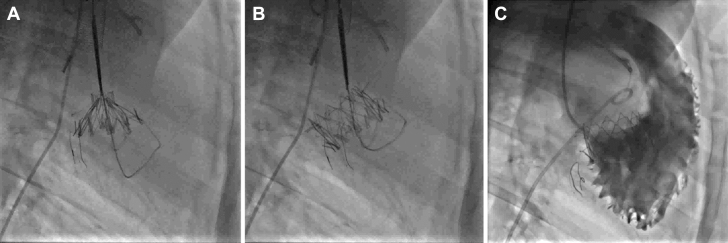
Figure 6.
Fluoroscopy: Transjagular Tricuspid Valved Stent Implantation
(A and B) Fluoroscopy: transjugular tricuspid valved stent. (C) Right ventricle angiogram.
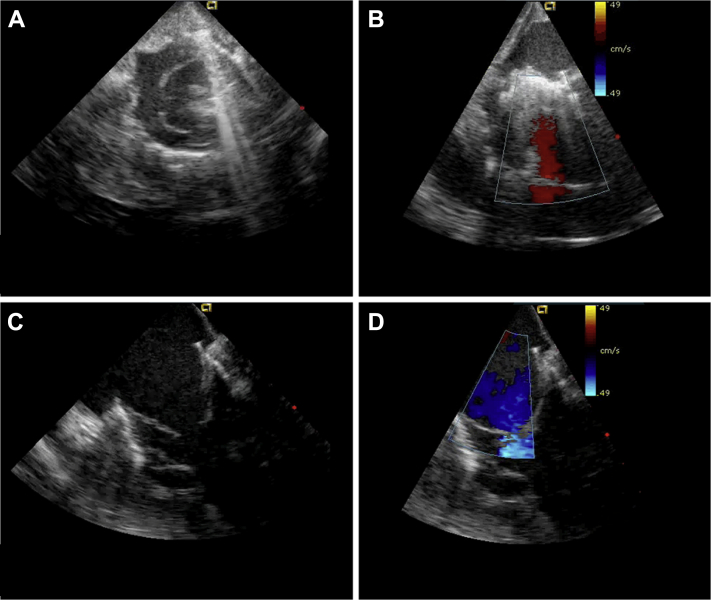
Figure 7.
Echocardiography: Transjagular Tricuspid Valved Stent Implantation
(A and B) Echocardiographic short-axis view. (C and D) Echocardiographic long-axis view.
Valve function evidenced normal hemodynamic performance and normal pressure gradient across the implanted valve (≤4 mm Hg), as well as RVOT gradients (Table 3). All animals had normal RV function before the procedure and preserved RV function during the follow-up period and at the time of euthanasia.
The entire implantation procedure time in the study ranged from 45 to 60 min, the time from the percutaneous right jugular access to position the delivery system at the TV annulus ranged from 12 to 15 min, and the NaviGate bioprosthesis deployment time ranged from 1 to 3 min.
There was no significant difference between the peak gradient across the valved stent in both approaches, transatrial versus transjugular (p = 0.9), indicating feasibility for device implantation using the 2 approaches.
Euthanasia, gross necropsy, and pathology
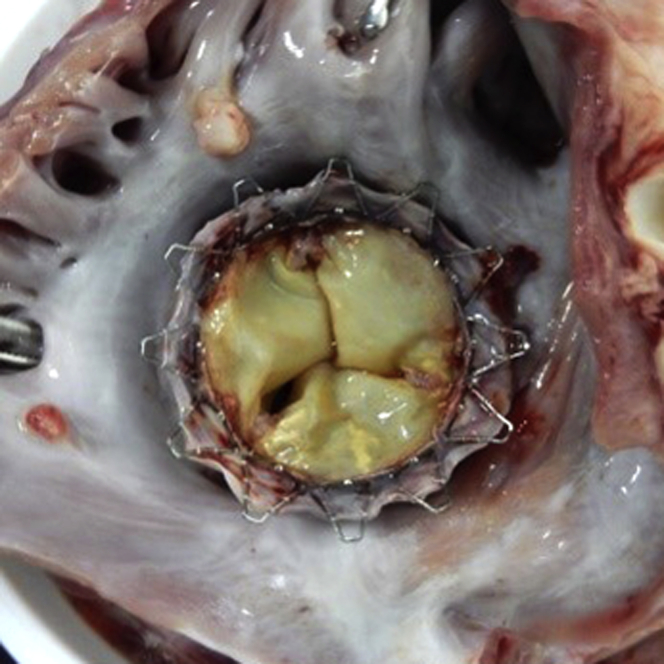
After sacrificing the 3 animals per protocol and 1 animal that died in the operating room, a thorough autopsy was performed according to the Gateway’s detailed protocols for autopsy and pathology, focusing on the biocompatibility of the devices. The heart was excised and weighed. A cursory view of the morphology at necropsy focused on reviewing the placement of the valved stent and its structure and assessment of signs of device-related trauma. A gross examination showed a proper anchoring and alignment of the valve in all animals except 1, which had a total valve dislodgment due to extreme tricuspid annulus to prosthesis mismatch. There were no signs of wear or damage to the anchoring winglets. Assessment of the chronic tissue response to the valve and anchoring mechanism valve struts and leaflets was also of particular interest; there was a normal response and normal evidence of tissue ingrowth, without thrombus formation. There was a complete circumferential seal in both sides of the stented valve without evidence of device-related trauma (Figure 8). Both the stent and valve leaflets were intact in all animals with no signs of damage.
Figure 8.
Pathology
A Faxitron x-ray cell was used to visualize the position of the valved stent within the tricuspid apparatus in the resected rinsed heart, which identified no distortion or fracture of any of the segments or stent elements in the long-term study.
Discussion
Tricuspid valve disease continues to present a challenging problem to cardiovascular specialists, especially in patients who develop TR associated with RV dysfunction and right heart failure (RHF) 11, 12. Recently, a more aggressive approach has been promoted for treatment of functional TR during left heart valve surgery to avoid high-risk reintervention thereafter 13, 14, 15. Patients who present for isolated TV surgery are at high risk of mortality, especially with RV dysfunction and reoperation 16, 17, 18. This could be attributed to RHF phenomena in the setting of long-standing TR that includes systemic venous congestion, volume overload, renal dysfunction, liver congestion, hypersplenism, and severe coagulopathy. Hence, many of these patients have been denied traditional open-heart surgical therapy, although the operation per se is technically less demanding than other valve surgeries.
In light of the former clinical challenges, and along with recent advances in percutaneous valve implantation techniques, the transcatheter approach stands out as a promising alternative for treatment of isolated TV disease associated with RHF. Several transcatheter techniques have been experimentally developed and tested in animal models, but few have been clinically applied in humans for treatment of TR (19): 1) transcatheter valve implantation at the vena cava level to prevent backflow of TR and decrease systemic congestion (Sapien [Edwards Lifesciences, Irvine, California] and Tric Valve [P & F Products & Features Vertriebs GmbH, Weßling, Germany]); 2) devices applied to reduce tricuspid annulus diameter (Mitralign [Mitralgin Inc., Tewksbury, Massachusetts] and TriCinch [4Tech Cardio, Galway, Ireland] devices); 3) devices applied to decrease TR volume and improve leaflet coaptation (FORMA device [Edwards Lifesciences]); 4) the Mitraclip device (Abbott Vascular, Santa Clara, California) used for edge-to-edge repair of TR; and 5) the transatrial intrapericardial tricuspid annuloplasty (TRAIPTA, National Institute of Health, Bethesda, Maryland) device (19). Regarding malfunctioning TV bioprosthesis, few groups have reported successful transcatheter valve-in-valve implantation 20, 21.
All new transcatheter techniques were limited to a small number of patients who were at prohibitive risk for surgical intervention in a trial to improve their clinical symptoms and to test feasibility. Particular concerns were raised for transcatheter caval devices that include persistent right heart volume overload, RV failure, and atrial arrhythmia; however, these concerns were not evidenced on early results 22, 23. The overall early and midterm outcomes showed transcatheter treatment of isolated TV disease to be feasible and promising, yet long-term follow-up as well as prospective trials are required to better evaluate the efficiency and durability of these new techniques. However, there are no reports of transcatheter valve implantation at the native tricuspid annulus level in humans.
In comparison to the evolving transcatheter aortic valve implantation, the TV anatomic features render transcatheter therapy more challenging. Obstacles for percutaneous repair approaches to the TV include the lack of stable adjacent structures for device placement, such as dilated annulus with its complex morphology of the TV; the dynamic geometry of the tricuspid annulus; and the absence of calcification. Furthermore, the close proximity of the coronary sinus ostium, atrioventricular node, and inferior vena cava to the TV result in many more technical difficulties for successfully deploying percutaneous repair devices. However, technology continues to advance, and newer techniques are being developed and tested in the preclinical setting to overcome these challenges before application in humans.
Bai et al. (24) described transcatheter implantation of a porcine pericardial valve in the tricuspid position in 10 healthy sheep under fluoroscopic guidance. Two deaths occurred periprocedurally related to cardiac arrhythmias. No significant TR developed in any animals during the follow-up period (24). Boudjemline et al. (25) have reported successful insertion of a novel percutaneous TV. Complications that were reported in this study include entrapment of the device in tricuspid chordae, leading to its incomplete valve opening, and a significant PVL in a separate animal. This device has not yet advanced to human studies (25). We developed a novel bioprosthesis that fulfilled all criteria required for successful transcatheter TV replacement without cardiopulmonary bypass using 2 different access approaches. Feasibility of the NaviGate prosthesis was tested in long-term preclinical models with overall satisfactory results. Echocardiography showed no significant post-implantation TR or high transvalvular gradients. There was no obstruction of RVOT, coronary arteries, or subvalvular apparatus. The valve anchoring was facilitated by a combination of 3 principles: a gentle radial force of the stent over the TA, a truncated cone or frustum configuration design that uses the RV pressure in systole to maintain the stented valve in proper position, and ventricular winglets that engage the tricuspid annulus, together creating a strong valvular seal.
Small atrial winglets covered with a lining of woven microfiber polyester fabric allow for a low intrusion of the valved stent into the RA; protect the integrity of anatomic structures, such as coronary sinus and conduction bundle; and also prevent PVL, minimizing dislocation toward the adjacent chambers. At this time, the NaviGate device has been implanted in 3 patients in the tricuspid position with no conduction disturbance complications (26).
Study limitations
An important limitation is the use of healthy animal models, because the TV is not enlarged with a very small atrial cavity that precludes substantial extension of the bioprosthesis and easy navigation inside the RA. The healthy pig differs widely from the patient presenting with moderate or severe tricuspid incompetence. Specifically, in humans with long-standing TR resulting from annular dilatation, the tricuspid annulus is >40 mm in diameter in many cases, which is very different from the model used in our study.
Achieving a good measurement of the TA as well as good visualization during valve deployment is critical to avoid mismatch of the TV prosthesis to the native TA; we experienced a total valve migration because of inappropriate TA measurements with intracardiac and transesophageal echocardiogram. That is why imaging technologies such as computerized tomography and 3-dimensional echocardiography are extremely important to provide more accurate guidance when performing transcatheter valve implantation in humans. However, using these imaging technologies would be challenging in our study, as they are not found in animal laboratories as a rule.
The valved stent could not be completely retrieved once released, so to avoid paravalvular leak in the presence of a larger tricuspid annulus, the size of the valved stent should be larger for pathological TR.
Conclusions
Tricuspid valve disease continues to present a challenging problem to cardiovascular specialists, especially in patients who develop tricuspid insufficiency associated with left heart valve disease or severe RV dysfunction. Because reoperation for recurrent TR carries high mortality rates and recurrence of TR after surgical repair is common, few patients are offered reoperation. Percutaneous approaches to TV disease could be an attractive alternative solution. In addition, the less invasive strategies may allow for earlier TR intervention than is currently practiced. There are many challenges and obstacles to emerging percutaneous approaches; however, with the advancement of cardiac imaging and percutaneous devices, development in this area is likely to continue.
This preclinical evaluation shows that transcatheter NaviGate TV orthotopic implantation in a chronic porcine model is feasible and safe and results in a secure and stable engagement of the native annulus with excellent hemodynamics and valve performance. This new device offers both percutaneous and minimally invasive implantation technique options without the need of cardiopulmonary bypass, which may be a potential effective alternative treatment for high-risk patients who are unsuitable for traditional cardiac surgery with severe TR and compromised RV function.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Transcatheter valve therapy has been progressively evolving to provide alternative options for high-risk surgical patients. In this work, we have tested the feasibility of the NaviGate valved stent in an animal model in preparation for clinical application. Two delivery approaches are of particular value for high-risk patients with recurrent TR after prior cardiac surgery. Different sizes of the NaviGate valved stent are available to treat functional TR with dilated annulus and for implantation in a previously placed annuloplasty ring.
TRANSLATIONAL OUTLOOK: At this time, the NaviGate valved stent has been successfully implanted in 2 patients who are at prohibitive surgical risk with severe TR. The short-term results suggest that the procedure is safe and feasible. Further development of the current device and delivery system is ongoing to achieve optimal clinical results.
Footnotes
The Cleveland Clinic has an ownership position on patents licensed to the company; and holds stock in NaviGate Cardiac Structures, Inc. Dr. Navia is the inventor on patents related to this device; and has served as a consultant to and holds stock in NaviGate Cardiac Structures, Inc. Dr. Kapadia has served on the advisory board of Navigate; and has stock options in NaviGate Cardiac Structures, Inc. Dr. Quijano is an inventor on patents related to this device; and holds stock in NaviGate Cardiac Structures, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Singh J.P., Vans J.C., Levy D. Prevalence and clinical determinants of mitral, tricuspid and aortic regurgitation (the Framingham Study) Am J Cardiol. 1999;83:897–902. doi: 10.1016/s0002-9149(98)01064-9. [DOI] [PubMed] [Google Scholar]
- 2.Stuge O., Liddicoat J. Emerging opportunities for cardiac surgeons within structural heart disease. J Thorac Cardiovasc Surg. 2006;132:1258–1261. doi: 10.1016/j.jtcvs.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 3.Groves P.H., Ellis F.H. Late tricuspid regurgitation following mitral valve surgery. J Heart Valve Dis. 1992;1:80–86. [PubMed] [Google Scholar]
- 4.Porter A., Shapira Y., Wurzel M. Tricuspid regurgitation late after mitral valve replacement: clinical and echocardiographic evaluation. J Heart Valve Dis. 1999;8:57–62. [PubMed] [Google Scholar]
- 5.Braunwald N.S., Ross J., Jr., Morrow A.G. Conservative management of tricuspid regurgitation in patients undergoing mitral valve replacement. Circulation. 1967;35:I6–I9. doi: 10.1161/01.cir.35.4s1.i-63. [DOI] [PubMed] [Google Scholar]
- 6.Matsuyama K., Matsumoto M., Sugita T. Predictors of residual tricuspid regurgitation after mitral valve surgery. Ann Thorac Surg. 2003;75:1826–1828. doi: 10.1016/s0003-4975(03)00028-6. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy P.M., Bhudia S.K., Rajeswaran J. Tricuspid valve repair: durability and risk factors for failure. J Thorac Cardiovasc Surg. 2004;127:674–685. doi: 10.1016/j.jtcvs.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Navia J.L., Nowicki E.R., Blackstone E.H. Surgical management of secondary tricuspid valve regurgitation: annulus, commissure, or leaflet procedure? J Thorac Cardiovasc Surg. 2010;139:1473–1482. doi: 10.1016/j.jtcvs.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 9.Dreyfus G.D., Corbi P.J., Chan K.M.J. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–132. doi: 10.1016/j.athoracsur.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 10.Zoghbi W.A., Chambers J.B., Dumesnil J.G. Recommendations for evaluation of prosthetic valves with echocardiography and Doppler ultrasound: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2009;22:975–1014. doi: 10.1016/j.echo.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Desai R.R., Vargas Abello L.M., Klein A.L. Tricuspid regurgitation and right ventricular function after mitral valve surgery with or without concomitant tricuspid valve procedure. J Thorac Cardiovasc Surg. 2013;146:1126–1132.e10. doi: 10.1016/j.jtcvs.2012.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye Y., Desai R., Vargas Abello L.M. Effects of right ventricular morphology and function on outcomes of patients with degenerative mitral valve disease. J Thorac Cardiovasc Surg. 2014;148:2012–2020.e8. doi: 10.1016/j.jtcvs.2014.02.082. [DOI] [PubMed] [Google Scholar]
- 13.Dreyfus G.D., Martin R.P., Chan K.M., Dulguerov F., Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. 2015;65:2331–2336. doi: 10.1016/j.jacc.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura R.A., Otto C.M., Bonow R.O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:e57–e185. doi: 10.1016/j.jacc.2014.02.536. [DOI] [PubMed] [Google Scholar]
- 15.Benedetto U., Melina G., Angeloni E. Prophylactic tricuspid annuloplasty in patients with dilated tricuspid annulus undergoing mitral valve surgery. J Thorac Cardiovasc Surg. 2012;143:632–638. doi: 10.1016/j.jtcvs.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Topilsky Y., Khanna A.D., Oh J.K. Preoperative factors associated with adverse outcome after tricuspid valve replacement. Circulation. 2011;123:1929–1939. doi: 10.1161/CIRCULATIONAHA.110.991018. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y.J., Kwon D.A., Kim H.K. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. 2009;120:1672–1678. doi: 10.1161/CIRCULATIONAHA.109.849448. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.B., Jung S.H., Choo S.J., Chung C.H., Lee J.W. Clinical and echocardiographic outcomes after surgery for severe isolated tricuspid regurgitation. J Thorac Cardiovasc Surg. 2013;146:278–284. doi: 10.1016/j.jtcvs.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Rodés-Cabau J., Hahn R.T., Latib A. Transcatheter therapies for treating tricuspid regurgitation. J Am Coll Cardiol. 2016;67:1829–1845. doi: 10.1016/j.jacc.2016.01.063. [DOI] [PubMed] [Google Scholar]
- 20.Godart F., Baruteau A.E., Petit J. Transcatheter tricuspid valve implantation: a multicentre French study. Arch Cardiovasc Dis. 2014;107:583–591. doi: 10.1016/j.acvd.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 21.Cullen M.W., Cabalka A.K., Alli O.O. Transvenous, antegrade melody valve-in-valve implantation for bioprosthetic mitral and tricuspid valve dysfunction. J Am Coll Cardiol Intv. 2013;6:598–605. doi: 10.1016/j.jcin.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Roberts P.A., Boudjemline Y., Cheatham J.P. Percutaneous tricuspid valve replacement in congenital and acquired heart disease. J Am Coll Cardiol. 2011;58:117–122. doi: 10.1016/j.jacc.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 23.Rogers J.H., Bolling S.F. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation. 2009;119:2718–2725. doi: 10.1161/CIRCULATIONAHA.108.842773. [DOI] [PubMed] [Google Scholar]
- 24.Bai Y., Zong G.-J., Wang H.-R. An integrated pericardial valved stent special for percutaneous tricuspid implantation: an animal feasibility study. J Surg Res. 2010;160:215–221. doi: 10.1016/j.jss.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Boudjemline Y., Agnoletti G., Bonnet D. Steps toward the percutaneous replacement of atrioventricular valves. An experimental study. J Am Coll Cardiol. 2005;46:360–365. doi: 10.1016/j.jacc.2005.01.063. [DOI] [PubMed] [Google Scholar]
- 26.Navia J.L., Kapadia S., Elgharably H. First-in-human implantations of the NaviGate bioprosthesis in a severely dilated tricuspid annulus and in a failed tricuspid annuloplasty ring. Circ Cardiovasc Interv. 2017 Dec 15 doi: 10.1161/CIRCINTERVENTIONS.117.005840. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.