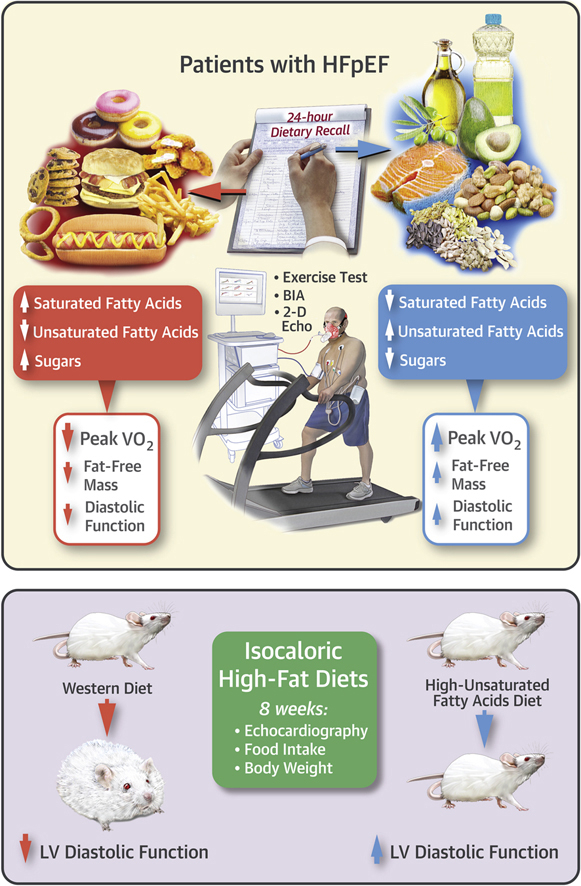
Visual Abstract
Key Words: body composition, diet, heart failure with preserved ejection fraction, obesity, unsaturated fatty acids
Abbreviations and Acronyms: CPX, cardiopulmonary exercise testing; CRF, cardiorespiratory fitness; CV, cardiovascular; DT, deceleration time; FFM, fat-free mass; FM, fat mass; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; IQR, interquartile range; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; UFA, unsaturated fatty acid; Vo2, oxygen consumption
Highlights
-
•
The effects of UFA on CRF in patients with HFpEF are unknown.
-
•
In obese HFpEF patients, UFA consumption analyzed with a validated 24-h dietary recall was positively associated with improved body composition, cardiac diastolic function and greater CRF, measured as peak VO2 at maximal cardiopulmonary exercise testing. Conversely, sugars consumption was associated with worse CRF.
-
•
In mice, an isocaloric high-fat diet, high in UFA and low in saturated fat prevented cardiac diastolic dysfunction measured with echocardiography and body weight gain in a model of cardiac dysfunction and obesity induced by Western diet, despite similar total caloric intake.
-
•
A high-UFA diet is associated with preservation of CRF in patients with HFpEF and cardiac function in the mouse.
Summary
Heart failure with preserved ejection fraction (HFpEF) is associated with obesity and, indirectly, with unhealthy diet. The role of dietary components in HFpEF is, however, largely unknown. In this study, the authors showed that in obese HFpEF patients, consumption of unsaturated fatty acids (UFA), was associated with better cardiorespiratory fitness, and UFA consumption correlated with better diastolic function and with greater fat-free mass. Similarly, mice fed with a high-fat diet rich in UFA and low in sugars had preserved myocardial function and reduced weight gain. Randomized clinical trials increasing dietary UFA consumption and reducing sugar consumption are warranted to confirm and expand our findings.
Heart failure (HF) affects more than 38 million people worldwide, almost 6 million in the United States alone, with an extremely high mortality and hospital admission rates (1). In one-half of patients diagnosed with HF, the ejection fraction is preserved (2), and in a large proportion of those patients, shortness of breath, exercise intolerance, and reduced cardiorespiratory fitness (CRF) cannot be attributed to coronary, valvular, or pericardial disease but rather to an impairment in diastolic function and/or functional and structural abnormalities of body composition compartments, defining the syndrome of heart failure with preserved ejection fraction (HFpEF) (3).
Peak oxygen consumption (Vo2) assessed by cardiopulmonary exercise testing (CPX) (4) is a measure of exercise capacity in patients with HF and an independent predictor of mortality in both HF with reduced ejection fraction and HFpEF.
HFpEF is a multifactorial disease in which comorbidities such as obesity and diabetes are highly prevalent and likely involved in its pathophysiology 3, 5, 6, 7, 8.
Obesity contributes to exercise intolerance, independent of abnormalities in cardiac function (9). Obesity results from a sustained positive caloric balance, therefore caloric restriction is recommended (10). Caloric restriction leading to 7 kg of weight loss was associated with a +1.3 ml · kg−1 · min−1 improvement in peak Vo2 in patients with HFpEF, without changes in cardiac function (11). Preclinical studies, however, suggest a direct effect of dietary components, namely, saturated fat and sugars, on diastolic function, which appears to be at least in part independent of weight gain 12, 13. These changes are also partially reversible with a low–saturated fat and low-sugar diet, thus offering a promise for intervention (12). Conversely, unsaturated fatty acids (UFAs) exert beneficial effects, potentially by reducing the synthesis of pro-inflammatory cytokines 14, 15, 16, 17, 18, 19. The effects of a UFA-enriched diet on cardiac diastolic function and on body composition are largely unknown, especially in patients with HFpEF.
In clinical studies, the consumption and/or supplementation of foods rich in UFAs such as monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), found in high proportions in olive oil and nuts, but also in canola oil and avocado in non-Mediterranean countries, have been associated with lower major cardiovascular (CV) event and overall mortality rates in subjects at high CV risk, independent of the overall dietary caloric intake or body weight 20, 21. Specific dietary supplement has also been associated with reduced mortality in HF, suggesting a major impact of nutrition in HF (22).
The objective of this study was to determine whether nutrient consumption, particularly UFAs, was associated with better cardiac function, body composition, and CRF in patients with HFpEF and to test whether an isocaloric diet enriched in UFAs could prevent cardiac diastolic dysfunction in a mouse model of diet-induced cardiac dysfunction.
Methods
Clinical study
Subjects
Subjects with symptomatic HFpEF and impaired CRF assessed by maximal CPX were enrolled. Diagnosis of HFpEF was based on clinical, laboratory, recent imaging study, and/or invasive hemodynamic data, according to the European Society of Cardiology consensus statement (23).
Major exclusion criteria included moderate to severe valvular heart disease, pericardial disease, restrictive cardiomyopathy, pulmonary artery hypertension (group I), persistent or permanent atrial fibrillation, active cancer or prior diagnosis within the past 10 years (excluding carcinoma in situ), chronic autoimmune or autoinflammatory disease, stage IV or V renal impairment, severe obstructive pulmonary disease, anemia, mechanical limitation to exertion due to musculoskeletal or neurological disease, and other conditions that may affect the completion or interpretation of maximal (i.e., respiratory exchange ratio >1.0) CPX, such as myocardial ischemia, uncontrolled hypertension, and chronotropic incompetence (24). The local Institutional Review Board approved the study, and all patients provided written informed consent.
Dietary assessment
Subjects underwent standardized 24-h dietary recall. They were asked by the nutritionist to recall foods and beverages consumed in the prior 24 h in a structured fashion. Dietary intake data gathered by interview was governed by a standardized 5-pass interview approach in which items are listed, reviewed, then described in detail (including preparation), quantified, probed for frequently forgotten foods, and eventually reviewed again 25, 26. Data were analyzed using the Nutrition Data System for Research, a computer-based software application developed at the University of Minnesota Nutrition Coordinating Center. The Nutrition Coordinating Center’s Food and Nutrient Database served as the source of food composition information in the Nutrition Data System for Research. Values for calories and nutrients consumption (i.e., MUFAs, PUFAs, total fat, total carbohydrates) expressed in total grams per day and percentage of total calories were calculated from the structured dietary recall interview.
CPX
Subjects underwent symptom-limited CPX, following the American College of Cardiology and American Heart Association guidelines for exercise testing (27). CPX was performed using a metabolic cart interfaced with a treadmill and a conservative incremental ramping protocol whereby speed and grade were increased by about 0.6 estimated METs every 60 s, as previously described (28).
The following parameters were measured and calculated: peak Vo2, minute ventilation, carbon dioxide production, and total exercise time according to standard procedures (27). Peak Vo2 was determined as the highest 10-s interval average obtained from breath-by-breath measurements of Vo2 during the last 30 s of exercise. The peak respiratory exchange ratio (carbon dioxide production/Vo2) was used to determine maximal effort. A respiratory exchange ratio >1.0 was the minimally acceptable threshold for enrollment (4). Of note, the exercise physiologist performing CPX was blinded to echocardiographic and body composition parameters.
Body composition
We measured body composition using a single-frequency bioelectric impedance analysis (Quantum IV, RJL System, Clinton Township, Michigan) to measure fat mass (FM) and fat-free mass (FFM), calculated FM index and FFM index by dividing the amount of FM or FFM by the square of the height expressed in meters, and FFM/FM index by dividing FFM by FM.
Echocardiography
We used transthoracic Doppler echocardiography, as recommended by the American Society of Echocardiography and the European Association of Cardiovascular Imaging 29, 30, to measure left ventricular end-diastolic and end-systolic volumes, ejection fraction, and early transmitral velocity (E) on pulsed-wave Doppler spectra and early mitral annular velocities by tissue Doppler averaged between lateral and septal (E′), and we calculated the E/E′ ratio and the E′ velocity indexed by deceleration time (DT) (E′DT).
Animal study
Eight-week-old CD-1 male mice (n = 5 to 8) were fed for 8 weeks with 1 of the following diets: 1) standard diet; 2) a diet rich in saturated fat (25.9%) and sugars (30%) and low in UFAs (14.7%) (Western diet; TD.88137, Envigo, Huntingdon, United Kingdom); 3) a diet rich in saturated fat (25.9%) and low in sugars (0%) (high–saturated fatty acid [SFA] diet; TD.150507, Envigo); or 4) a diet low in saturated fat (6.5%) and sugars (0%) and high in UFAs (36.7%) (high-fat UFA diet; TD.160017, Envigo) (Table 1). An additional group received a high-sugar (30%), low–saturated fat (6.5%) diet (high-sugar diet; TD.160017, Envigo) (Supplemental Table 1).
Table 1.
Nutritional Characteristics of Experimental Diets in the Mouse
| Standard Diet | Western Diet | High-SFA Diet | High-UFA Diet | |
|---|---|---|---|---|
| Proteins | 25.0 | 15.2 | 15.6 | 15.6 |
| Total fat | 17.0 | 42.0 | 43.2 | 43.2 |
| SFAs (% of total fatty acids) | 14 | 65 | 65 | 15 |
| UFAs (% of total fatty acids) | 86 | 35 | 35 | 85 |
| Total carbohydrates | 58 | 42.7 | 41.2 | 41.2 |
| Sugars | 0 | 30 | 0 | 0 |
| Cholesterol (% of weight) | 0 | 0.2 | 0.2 | 0.2 |
| Energy density (kcal/g) | 3.1 | 4.5 | 4.4 | 4.4 |
SFA = saturated fatty acid; UFA = unsaturated fatty acid.
Values are % of total calories.
We measured food intake daily, body weight weekly, and cardiac function every 4 weeks using transthoracic Doppler echocardiography (Vevo770 Imaging System, VisualSonic, Toronto, Ontario, Canada) and a 30-MHz probe, to measure cardiac systolic and diastolic function, as previously described (31). Experiments were performed following National Institutes of Health guidelines and approved by the Institutional Animal Care and Use Committee.
Statistical analysis
For the clinical study, data are reported as median (interquartile ranges [IQRs]) for potential deviation from the Gaussian distribution. Discrete variables are reported as numbers and percentages. We assessed for correlations between CRF parameters and dietary variables using the Spearman rank test. Correlations between dietary variables and body composition parameters were also performed. A correlation was considered significant at p < 0.05. To further assess the separate contribution of UFAs and SFAs, we performed a multivariate analysis using a linear regression method after determination of nonsignificant deviation from the Gaussian distribution of the 2 variables using the Kolmogorov-Smirnov test (p = 0.20 and p = 0.12, respectively).
For the animal study, because of the low expected variance within the groups, values are expressed as mean and SEM. The differences between groups were assessed using analysis of variance followed by the Student t test for unpaired data to compare the individual groups. SPSS version 23.0 (IBM, Armonk, New York) was used for statistical analyses.
Results
Clinical characteristics
Major demographics and characteristics are summarized in Table 2. Fourteen patients (61%) were female, 13 (57%) African American and the remaining Caucasian, and the median age was 53 years (IQR: 48 to 57 years). The majority of patients had hypertension (n = 21 [91%]) and type 2 diabetes (n = 16 [70%]). All subjects were obese (body mass index ≥30 kg/m2), with 16 subjects (70%) being morbidly obese (body mass index ≥40 kg/m2).
Table 2.
Baseline Characteristics
| Age, yrs | 53 (48–57) |
| Male/female | 9 (39.1%)/14 (60.9%) |
| Race, Caucasian/African American | 10 (43.5%)/13 (56.5%) |
| Major comorbidities | |
| Diabetes mellitus | 16 (69.6%) |
| Coronary artery diseases | 8 (34.8%) |
| Hypertension | 21 (91.3%) |
| Dyslipidemia | 14 (60.9%) |
| Obesity grading | |
| Grade I obesity, BMI ≥30 and <35 kg/m2 | 1 (4.4%) |
| Grade II obesity, BMI ≥35 and <40 kg/m2 | 6 (26.1%) |
| Grade III or severe obesity, BMI ≥40 kg/m2 | 16 (69.6%) |
| Major dietary characteristics | |
| Total daily energy, kcal | 2,066 (1,897–2,642) |
| Fat, % of total energy | 38.3 (29.7–45.6) |
| Monounsaturated fatty acids, % of total energy | 13.8 (10.9–15.3) |
| Polyunsaturated fatty acids, % of total energy | 8.6 (7.4–11.9) |
| Saturated fatty acids, % of total energy | 12.7 (9.4–15.5) |
| Carbohydrates, % of total energy | 47.7 (38.4–56.8) |
| Sugars, % of total energy | 20.3 (14.4–29.8) |
| Protein, % of total energy | 14.1 (12.7–16.1) |
| Sodium, mg | 3,252 (2,578–3946) |
| Cholesterol, mg | 248.1 (200.9–476.3) |
| Body mass and composition | |
| BMI, kg/m2 | 42.4 (35.2–44.4) |
| Fat mass, kg | 54.8 (49.7–59.6) |
| Fat mass index, kg/m2 | 18.8 (16.8–21.3) |
| Fat-free mass, kg | 70.4 (61.0–83.4) |
| Fat-free mass index, kg/m2 | 24.0 (21.6–27.7) |
| Functional capacity | |
| Exercise time, min | 8.5 (7.5–9.8) |
| Respiratory exchange ratio | 1.13 (1.04–1.17) |
| Peak oxygen consumption, ml · kg−1 · min−1 | 14.4 (11.8–18.5) |
| Oxygen uptake efficiency (slope) | 2.2 (1.9–2.5) |
| Peak oxygen pulse, ml · min−1 | 14.8 (13.2–17.1) |
| Doppler echocardiographic parameters | |
| Left ventricular ejection fraction, % | 60.4 (57.1–63.0) |
| Stroke volume, ml | 67.2 (57.9–75.6) |
| E′ velocity, cm/s | 8.0 (6.2–8.4) |
| E/E′ ratio | 12.1 (10.1–15.3) |
| E/A ratio | 1.1 (1.0–1.3) |
| DT, ms | 241.0 (195.8–278.1) |
| E′/DT ratio | 0.33 (0.26–0.41) |
| Biomarkers | |
| NT-proBNP, pg/ml | 90 (47–242) |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 68 (52–85) |
Values are median (interquartile range) or n (%).
BMI = body mass index; DT = deceleration time; NT-proBNP = N-terminal pro–brain natriuretic peptide.
Dietary assessment
Median daily total calories consumption was 2,066 kcal (IQR: 1,897 to 2,642 kcal), and percentages of calories from total fat, total carbohydrates, and total proteins were 38.3% (IQR: 29.7% to 45.6%), 47.7% (IQR: 38.4% to 56.8%), and 14.1% (IQR: 12.7% to 16.1%), respectively (Table 2).
When the type of fat was taken into consideration, median grams and percentage of calories from SFA were 25.6 g (IQR: 23.5 to 35.3 g) and 12.7% (IQR: 9.4% to 15.5%), respectively. Consumption of MUFAs and PUFAs was 29.4 g (IQR: 24.3 to 34.7 g) and 19.0 g (IQR: 15.6 to 26.8 g), respectively, while percentage of calories from MUFAs was 13.8% (IQR: 10.9% to 15.3%) and 8.6% (IQR: 7.4% to 11.9%) from PUFA. Median total sugars was 97.3 g (IQR: 75.4 to 153.8 g), representing 20.3% (IQR: 14.4% to 29.8%) of total calories.
Median daily sodium consumption was 3,252 mg (IQR: 2,578 to 3,946 mg), and cholesterol intake was 248.1 mg (IQR: 200.9 to 476.3 mg).
Body composition
The median FM index was 18.8 kg/m2 (IQR: 16.8 to 21.3 kg/m2), and the median FFM index was 24.0 kg/m2 (IQR: 21.6 to 27.7 kg/m2) (Table 2).
CPX
Median peak Vo2 was 14.4 ml · kg−1 · min−1 (IQR: 11.8 to 18.5ml · kg−1 · min−1), or 52% (IQR: 45% to 61%) of predicted according to age, sex, and ideal body weight. Exercise time was 8.5 min (IQR: 7.5 to 9.8 min). The median respiratory exchange ratio was 1.13 (IQR: 1.04 to 1.17) (Table 2).
Echocardiography
The median left ventricular ejection fraction was 60% (IQR: 57% to 63%), the median E/E′ ratio was 12.1 (IQR: 10.1 to 15.3), median E′ was 8.0 cm/s (IQR: 6.2 to 8.4 cm/s), median DT was 241 ms (IQR: 196 to 278 ms), and median E′/DT ratio was 0.33 (IQR: 0.26 to 0.41) (Table 2).
Correlations between nutrient consumption and CRF
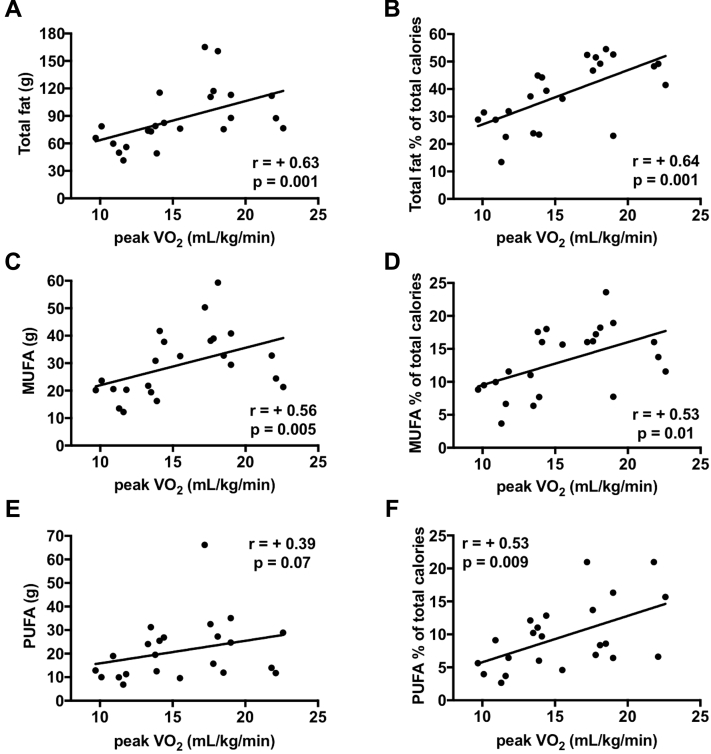
Correlations are shown in Figure 1. Total fat in grams or percentage of calories was associated with greater peak Vo2 (R = +0.63 [p = 0.001] and R = +0.64 [p = 0.001], respectively). MUFAs measured in grams or percentage of calories correlated with peak Vo2 (R = +0.56 [p = 0.005] and R = +0.53 [p = 0.010], respectively). Similarly, PUFAs in grams or percentage of calories correlated with peak Vo2 (R = +0.39 [p = 0.07], R = +0.53 [p = 0.009], respectively).
Figure 1.
Fatty Acid Consumption and Cardiorespiratory Fitness in Patients With Heart Failure With Preserved Ejection Fraction
Total fat consumption was associated with improved peak oxygen consumption (Vo2) (A, B). Monounsaturated fat (MUFA) and polyunsaturated fat (PUFA) consumption in grams (C, E) and in percentage of total calories (D, F) was associated with greater peak Vo2. Unsaturated fat (UFA) consumption (cumulative MUFA and PUFA) (G, H) and saturated fat (SFA) consumption presented a significant positive correlation with peak Vo2(I, J).
When MUFA and PUFA consumption in grams or as percentage of total calories were combined, the correlations with peak Vo2 became stronger (R = +0.45 [p = 0.03] and R = +0.62 [p = 0.001], respectively) (Figure 1). SFAs in grams and percentage of total calories also correlated significantly, although less strongly with peak Vo2 (R = +0.46 [p = 0.03], R = +0.49 [p = 0.02], respectively) (Figure 1); in fact, a multivariate analysis correcting for both SFAs and UFAs showed that UFAs remained significantly related with peak Vo2 (p = 0.017), whereas SFAs no longer did (p = 0.22).
Total carbohydrates in grams and percentage of total calories were inversely correlated with peak Vo2 (R = −0.54 [p = 0.008] and R = −0.59 [p = 0.003], respectively). When sugars (monosaccharides and disaccharides) were subtracted from total carbohydrate consumption, nonsugar carbohydrates showed a weaker correlation with peak Vo2 (R = −0.41, p = 0.053). In contrast, total grams or percentage of calories derived from sugars showed an inverse correlation with peak Vo2 (R < −0.43, p < 0.05 for all) (Supplemental Figure 1), reflecting worse CRF with higher sugar consumption. None of the other analyzed nutrients (i.e., sodium consumption) showed a statistically significant correlation with CPX-derived parameters (all values −0.35 < R < +0.35, p > 0.05).
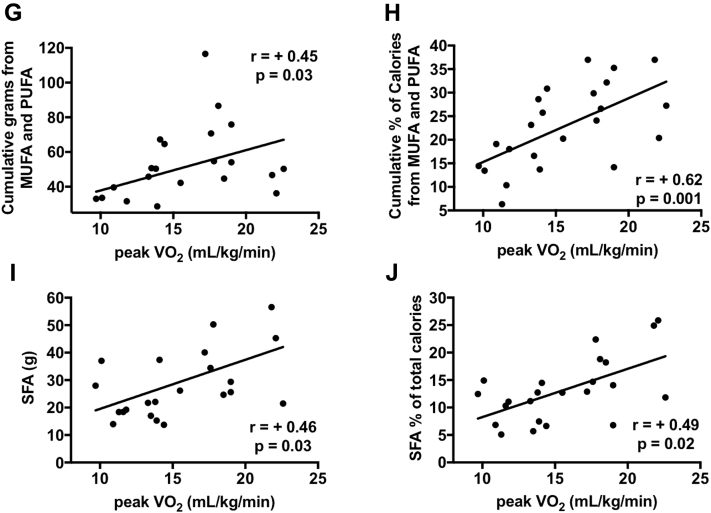
Correlations between nutrient consumption and diastolic function
Significant correlations were found (Figure 2). UFA consumption was associated with measures of more effective diastolic function such as higher E′ (R = +0.38, p = 0.07), lower DT (R = −0.41, p = 0.04), and higher E′/DT ratio (R = +0.48, p = 0.02). SFAs were associated with E′ (R = +0.36, p = 0.09) and E′/DT ratio (R = +0.54, p = 0.006) but not with DT (R = −0.33, p = 0.12).
Figure 2.
Fatty Acid Consumption and Myocardial Relaxation in Patients With Heart Failure With Preserved Ejection Fraction
Unsaturated fat (UFA) consumption (cumulative monounsaturated fat [MUFA] and polyunsaturated fat [PUFA]) were associated with higher E′ (A), lower deceleration time (DT) (C), and higher E′/DT ratio (E). Saturated fatty acids (SFAs) were associated with higher E′ (B) and higher E′/DT (F), but not with DT (D).
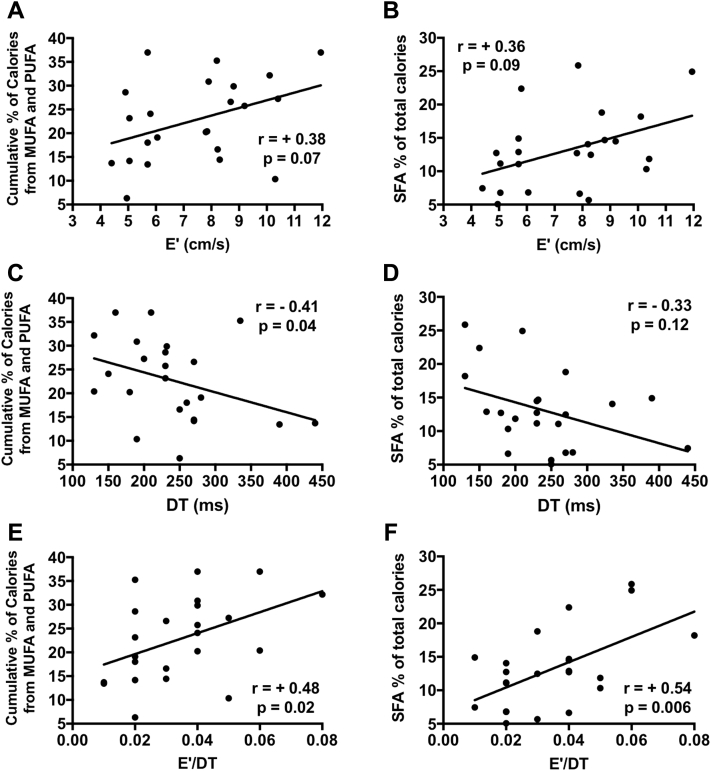
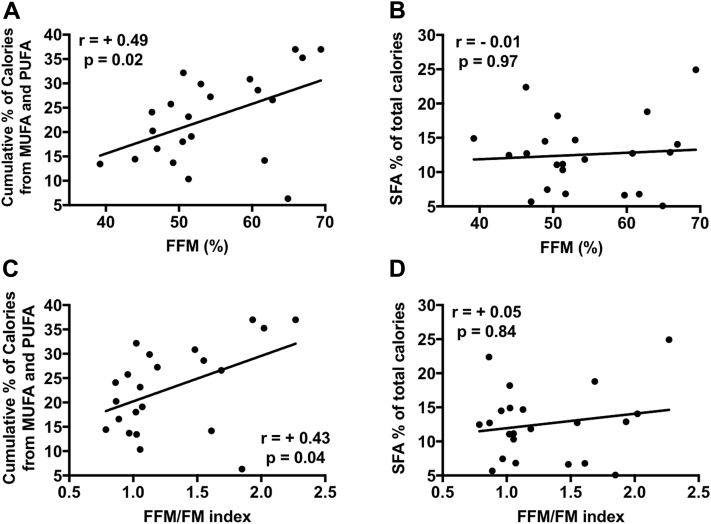
Correlations between nutrient consumption and body composition
UFA consumption was associated with a higher percentage of FFM (R = +0.49, p = 0.02) and with a more favorable FFM/FM ratio (R = +0.43, p = 0.04) (Figure 3). SFA consumption did not correlate with body composition parameters (R < +0.1, p > 0.05) (Figure 3).
Figure 3.
Fatty Acid Consumption and Body Composition in Patients With Heart Failure With Preserved Ejection Fraction
Unsaturated fatty acid (UFA) consumption is associated with higher fat-free mass (FFM) (A) and with higher FFM/fat mass (FM) index (C). Saturated fatty acids (SFA) did not correlate with body composition compartments (B, D).
Experimental study in mice
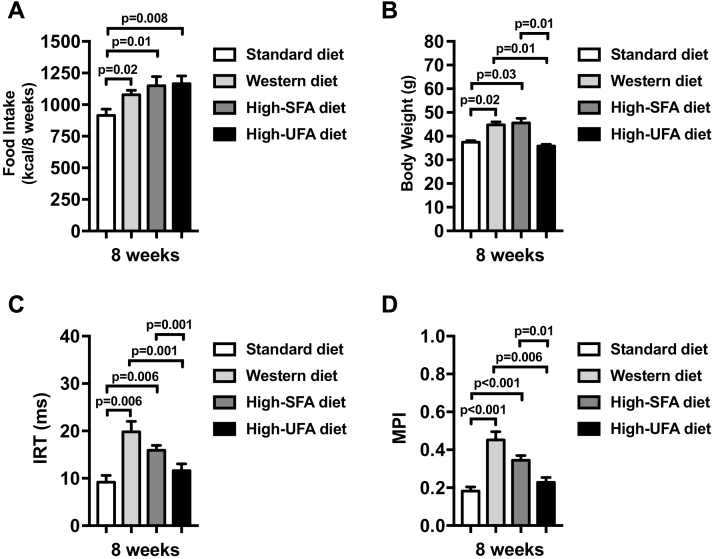
Cumulative 8-week caloric intake was significantly higher in all 3 high-fat diets compared with the standard diet (Figure 4). Mice fed a high–saturated fat and high-sugar diet (Western diet) or a high–saturated fat and low-sugar diet (high-SFA diet) presented significantly higher body weight at 8 weeks compared with those fed standard and high-UFA diets (Figure 4). Both diets rich in saturated fats (with and without high sugar content) induced an impairment in diastolic function, measured as an increase in isovolumetric relaxation time and myocardial performance index at 8 weeks, whereas mice fed the high-UFA diet had preserved diastolic function (Figure 4).
Figure 4.
Diet Modulates Cardiac Function in Mice
Mice fed a Western diet and a high–saturated fatty acids (SFAs) diet presented higher body weight (B), despite similar caloric intake (A) compared with standard diet and compared with high–unsaturated fatty acids (UFAs) diet. Western diet and high-SFA diet-fed mice presented diastolic dysfunction (C, D) after 8 weeks. High-UFA-fed mice presented preserved cardiac diastolic function, which was not different compared with standard diet.
We evaluated also a group of mice fed a high-sugar, low–saturated fat diet (high-sugar diet) and found significant worsening of diastolic function compared with those fed the standard diet (Supplemental Figure 2). Of note, the degree of impairment in diastolic dysfunction was numerically less than what was seen with the Western diet (p < 0.05 for both) but reflected lower food intake, and the effects on weight and cardiac function corrected for food intake were not statistically different from the Western diet (Supplemental Figure 2), suggesting a similar toxic effect, but possibly mice found the high-sugar, low–saturated fat food less appealing.
Discussion
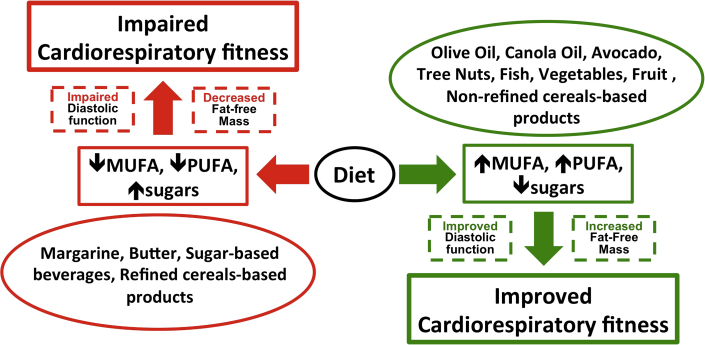
Our data provide a link between dietary UFAs with CRF in obese patients with HFpEF, independent of calorie consumption, potentially through a protective role of MUFAs and PUFAs on FFM and on cardiac diastolic function (Figure 5). Total carbohydrates, in contrast, were associated with worse exercise capacity, which was driven by consumption of sugars.
Figure 5.
Potential Role of Diet on Cardiorespiratory Fitness in Heart Failure With Preserved Ejection Fraction
Diet can have detrimental (red arrows) or beneficial (green arrows) effects on cardiorespiratory fitness (CRF). A diet low in monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) and high sugars may contribute to worsening CRF (red arrows). Conversely, a diet rich in MUFAs and PUFAs and low in sugars may exert beneficial effects in patients with heart failure with preserved ejection fraction by increasing CRF through improvements in myocardial relaxation and body composition (green arrows).
We also performed an experimental study in which mice were fed different forms of high-calorie and high-fat diets, 1 rich in both saturated fat and sugars (Western diet), 1 rich in saturated fat but low in sugars, 1 rich in sugars but low in saturated fats, and another rich in UFAs but low in saturated fat and sugars; despite no differences in caloric intake, the increased consumption of saturated fat and of sugars appears to have an additive deleterious effect on cardiac diastolic function, whereas a diet enriched in UFA and low in saturated fat and sugars did not result in weight gain or in cardiac dysfunction.
Mediterranean diet and CV outcomes
A diet rich in UFAs and low in refined carbohydrates and sugars, typically seen in Mediterranean dietary patterns, has been associated with favorable CV outcomes 32, 33, lower incidence of HF (34), and reduced overall mortality (21).
In the PREDIMED (Primary Prevention of Cardiovascular Disease With a Mediterranean Diet) study (20), an unrestricted-calorie supplementation for almost 5 years with extra-virgin olive oil or nuts, foods rich in UFAs, in a population at high CV risk reduced the incidence of major adverse CV events, the level of N-terminal pro–brain natriuretic peptide (35), body weight, and waist circumference (36).
Effects of UFA on cardiac function and body composition
Guideline recommendations on specific dietary patterns in HFpEF are lacking (37). A weight-loss intervention through caloric restriction improved CRF in obese patients with HFpEF (11). Here we identified putative mechanisms through which diet can adversely affect CRF. On the basis of the knowledge that obesity, particularly the amount and quality of FM and FFM 9, 11, 38, and diastolic function contribute to exercise intolerance, and that UFA consumption is associated with better body composition and better cardiac diastolic function, an intervention aimed at increasing UFA and reducing sugar consumption may be beneficial in patients with HFpEF (Figure 5).
It is unclear, however, whether MUFAs or PUFAs play a more prominent role. Foods rich in MUFA are often also rich in PUFAs, making it difficult to completely differentiate the effects of the 2 in the clinical practice. Foods rich in MUFAs and PUFAs sometimes also contain SFAs, which may explain the correlation seen also between SFA consumption, CRF, and diastolic function. This is supported by the experimental data in mice showing that excess SFA intake results in cardiac dysfunction, whereas high UFA intake does not.
Accordingly, advocating for a generalized low-fat diet may be neutral on CV outcomes (39), if not indirectly detrimental (40). A low-fat diet may result in lower consumption of UFAs, precluding the potential beneficial effects these nutrients exert. Moreover, advocating for a low-fat diet may increase the consumption of sugars as substitutes for fat.
The data presented herein showed indeed that sugar consumption is linked to impaired CRF in patients with HFpEF, and the experimental data in mice confirm the toxic effects of a high-sugar diet. In preclinical studies, a Western-like diet induced cardiac dysfunction, mainly diastolic, which was reversed by switching back to a “healthy” diet (12), and another study in rats showed beneficial effects of oleic acid–enriched canola oil on cardiac function (41). These data highlight the ability of diet to modulate cardiac function in experimental animal settings, showing that the quality of nutrients rather than calories affects cardiac function, with a high-UFA, low-sugar content opposing the detrimental effects of saturated fat and sugars.
A diet rich in UFAs may also play a protective role on body composition by increasing the amount of FFM 42, 43 but also improving FFM quality and strength (42). These findings are particularly relevant because body composition plays a crucial role in affecting exercise capacity (44), to the extent that has been proposed to be the major cause of exercise intolerance in HFpEF (38).
Study limitations
Because of the observational nature of the clinical study, causality between improved CRF, cardiac function, body composition, and UFA consumption should not be assumed. These findings may also not be replicable in a normal-weight population with HFpEF.
Additionally, the 24-h dietary recall, although validated in a number of studies, has its own limitations (26). In this study, a single trained nutritionist performed all assessments following a standardized protocol, yet the 24-h recall only allows the collection of a snapshot of subjects’ dietary patterns and is a memory-based, indirect measure of dietary intake.
Although bioelectric impedance analysis is not the gold-standard technique for body composition assessment, it has been validated in a number of studies against gold-standard techniques, and we have recently shown a highly significant correlation with dual-energy x-ray absorptiometric parameters in HFpEF (9). Finally, the small sample size of the study should be considered, as it may have negatively affected the power of other analyses.
In the preclinical study, we included only male mice. The improved cardiac diastolic function in mice fed a UFA-enriched and low-sugar diet does not necessarily translate to improved exercise capacity in patients with HFpEF. Finally, an assessment of body composition in mice fed with different diets is lacking.
Conclusions
In the present study we found an association between a fat-rich diet, specifically UFAs (MUFAs and PUFAs), and CRF, diastolic function, and body composition in obese patients with HFpEF. The mouse experiments confirmed the protective effect of a high-UFA, low-sugar diet. These results call for targeted dietary interventional randomized trials in patients with HFpEF aimed at increasing UFA consumption, with or without caloric and saturated fat restriction and reduction in sugar consumption. Moreover, the related molecular mechanisms involved in these potential beneficial effects require further investigation.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: A diet rich in UFAs may improve CRF, supporting the concept that life-style intervention plays a central role in the treatment of obese patients with HFpEF.
TRANSLATIONAL OUTLOOK: Future well-designed randomized controlled trials are needed to confirm our findings, and we highlight that a multidisciplinary approach is needed to develop therapeutics for obese patients with HFpEF.
Footnotes
This study was supported by the National Institutes of Health (grants R34HL118348 and UL1TR000058). Dr. Carbone is supported by a Mentored Clinical and Population Research Award from the American Heart Association (16MCPRP31100003). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Abbate A., Arena R., Abouzaki N. Heart failure with preserved ejection fraction: refocusing on diastole. Int J Cardiol. 2015;179:430–440. doi: 10.1016/j.ijcard.2014.11.106. [DOI] [PubMed] [Google Scholar]
- 4.Malhotra R., Bakken K., D’Elia E., Lewis G.D. Cardiopulmonary exercise testing in heart failure. J Am Coll Cardiol HF. 2016;4:607–616. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman D.W., Shah S.J. The HFpEF obesity phenotype: the elephant in the room. J Am Coll Cardiol. 2016;68:200–203. doi: 10.1016/j.jacc.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Carbone S., Lavie C.J., Arena R. Obesity and heart failure: focus on the obesity paradox. Mayo Clin Proc. 2017;92:266–279. doi: 10.1016/j.mayocp.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Lavie C.J., Sharma A., Alpert M.A. Update on obesity and obesity paradox in heart failure. Prog Cardiovasc Dis. 2016;58:393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Eaton C.B., Pettinger M., Rossouw J. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women: clinical perspective. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carbone S., Canada J.M., Buckley L. Obesity contributes to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68:2487–2488. doi: 10.1016/j.jacc.2016.08.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen M.D., Ryan D.H., Apovian C.M. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Obesity Society. J Am Coll Cardiol. 2014;63:2985–3023. doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Kitzman D.W., Brubaker P., Morgan T. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;1045:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone S., Mauro A.G., Mezzaroma E. A high-sugar and high-fat diet impairs cardiac systolic and diastolic function in mice. Int J Cardiol. 2015;198:66–69. doi: 10.1016/j.ijcard.2015.06.136. [DOI] [PubMed] [Google Scholar]
- 13.Gonçalves N., Silva A.F., Rodrigues P.G. Early cardiac changes induced by a hypercaloric Western-type diet in “subclinical” obesity. Am J Physiol Heart Circ Physiol. 2016;310:H655–H666. doi: 10.1152/ajpheart.00684.2015. [DOI] [PubMed] [Google Scholar]
- 14.Wen H., Gris D., Lei Y. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat Immunol. 2011;12:408–415. doi: 10.1038/ni.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X., Du N., Zhang Q. MicroRNA-30d regulates cardiomyocyte pyroptosis by directly targeting foxo3a in diabetic cardiomyopathy. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan Y., Jiang W., Spinetti T. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity. 2013;38:1154–1163. doi: 10.1016/j.immuni.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Marty-Roix R., Lien E. (De-)oiling inflammasomes. Immunity. 2013;38:1088–1090. doi: 10.1016/j.immuni.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finucane O.M., Lyons C.L., Murphy A.M. Monounsaturated fatty acid-enriched high-fat diets impede adipose NLRP3 inflammasome-mediated IL-1β secretion and insulin resistance despite obesity. Diabetes. 2015;64:2116–2128. doi: 10.2337/db14-1098. [DOI] [PubMed] [Google Scholar]
- 19.Reynolds C.M., McGillicuddy F.C., Harford K.A., Finucane O.M., Mills K.H.G., Roche H.M. Dietary saturated fatty acids prime the NLRP3 inflammasome via TLR4 in dendritic cells-implications for diet-induced insulin resistance. Mol Nutr Food Res. 2012;56:1212–1222. doi: 10.1002/mnfr.201200058. [DOI] [PubMed] [Google Scholar]
- 20.Estruch R., Ros E., Salas-Salvadó J. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 21.Wang D.D., Li Y., Chiuve S.E. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176:1134–1145. doi: 10.1001/jamainternmed.2016.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mortensen S.A., Rosenfeldt F., Kumar A. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure. J Am Coll Cardiol HF. 2014;2:641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Paulus W.J., Tschöpe C., Sanderson J.E. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher G.F., Ades P.A., Kligfield P. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation. 2013;128:873–934. doi: 10.1161/CIR.0b013e31829b5b44. [DOI] [PubMed] [Google Scholar]
- 25.Thompson F.E., Byers T. Dietary assessment resource manual. J Nutr. 1994;124(11 Suppl):2245S–2317S. doi: 10.1093/jn/124.suppl_11.2245s. [DOI] [PubMed] [Google Scholar]
- 26.Moshfegh A.J., Rhodes D.G., Baer D.J. The US Department of Agriculture automated multiple-pass method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88:324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 27.Balady G.J., Arena R., Sietsema K. Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:191–225. doi: 10.1161/CIR.0b013e3181e52e69. [DOI] [PubMed] [Google Scholar]
- 28.Canada J.M., Fronk D.T., Cei L.F. Usefulness of C-reactive protein plasma levels to predict exercise intolerance in patients with chronic systolic heart failure. Am J Cardiol. 2016;117:116–120. doi: 10.1016/j.amjcard.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Hear J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 30.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 31.Abbate A., Salloum F.N., Vecile E. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
- 32.Widmer R.J., Flammer A.J., Lerman L.O., Lerman A. The Mediterranean diet, its components, and cardiovascular disease. Am J Med. 2015;128:229–238. doi: 10.1016/j.amjmed.2014.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao Y., Han J., Hu F.B. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369:2001–2011. doi: 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tektonidis T.G., Åkesson A., Gigante B., Wolk A., Larsson S.C. Adherence to a Mediterranean diet is associated with reduced risk of heart failure in men. Eur J Heart Fail. 2016;18:253–259. doi: 10.1002/ejhf.481. [DOI] [PubMed] [Google Scholar]
- 35.Fitó M., Estruch R., Salas-Salvadó J. Effect of the Mediterranean diet on heart failure biomarkers: a randomized sample from the PREDIMED trial. Eur J Heart Fail. 2014;16:543–550. doi: 10.1002/ejhf.61. [DOI] [PubMed] [Google Scholar]
- 36.Estruch R., Martínez-González M.A., Corella D. Effect of a high-fat Mediterranean diet on bodyweight and waist circumference: a prespecified secondary outcomes analysis of the PREDIMED randomised controlled trial. Lancet Diabetes Endocrinol. 2016;4:666–676. doi: 10.1016/S2213-8587(16)30085-7. [DOI] [PubMed] [Google Scholar]
- 37.Yancy C.W., Jessup M., Bozkurt B. ACCF/AHA practice guideline 2013 ACCF/AHA guideline for the management of heart failure a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Upadhya B., Haykowsky M.J., Eggebeen J., Kitzman D.W. Exercise intolerance in heart failure with preserved ejection fraction: more than a heart problem. J Geriatr Cardiol. 2015;12:294–304. doi: 10.11909/j.issn.1671-5411.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Howard B.V., Van Horn L., Hsia J. Low-fat dietary pattern and risk of cardiovascular disease. JAMA. 2006;295:655. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 40.Ludwig D.S. Lowering the bar on the low-fat diet. JAMA. 2016;316:2087–2088. doi: 10.1001/jama.2016.15473. [DOI] [PubMed] [Google Scholar]
- 41.Thandapilly S.J., Raj P., Louis X.L. Canola oil rich in oleic acid improves diastolic heart function in diet-induced obese rats. J Physiol Sci. 2017;67:425–430. doi: 10.1007/s12576-016-0504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelaiditi E., Jennings A., Steves C.J. Measurements of skeletal muscle mass and power are positively related to a Mediterranean dietary pattern in women. Osteoporos Int. 2016;27:3251–3260. doi: 10.1007/s00198-016-3665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Welch A.A., MacGregor A.J., Minihane A.-M. Dietary fat and fatty acid profile are associated with indices of skeletal muscle mass in women aged 18–79 years. J Nutr. 2014;144:327–334. doi: 10.3945/jn.113.185256. [DOI] [PubMed] [Google Scholar]
- 44.Carbone S., Popovic D., Lavie C.J., Arena R. Obesity, body composition and cardiorespiratory fitness in heart failure with preserved ejection fraction. Future Cardiol. 2017 Aug 10 doi: 10.2217/fca-2017-0023. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.