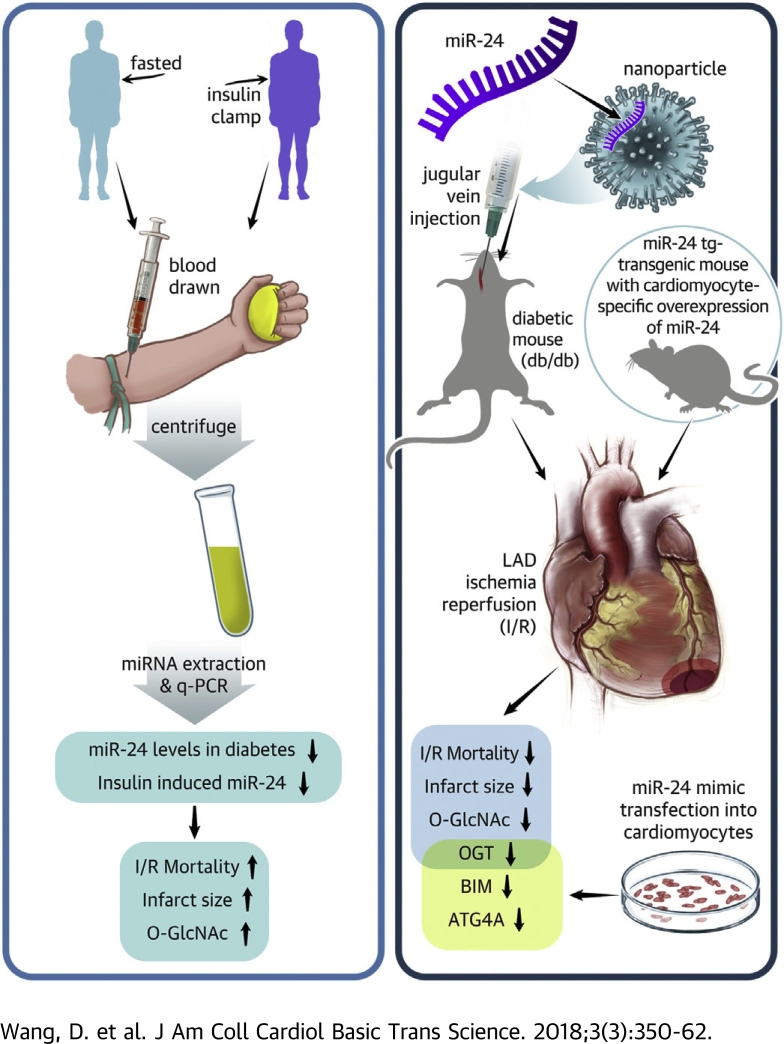
Visual Abstract
Key Words: hyperinsulinemia, infarct size, microRNA, O-GlcNAcylation
Abbreviations and Acronyms: ATG4A, autophagy-related gene 4a; BIM, Bcl-2-like protein 11; CVD, cardiovascular disease; DM, diabetes mellitus; I/R, ischemia/reperfusion; MI, myocardial infarction; OGT, O-GlcNac transferase
Highlights
-
•
Optimal treatment for patients with diabetes and myocardial infarction remains a challenge.
-
•
Hyperglycemia- and hyperinsulinemia-induced miR-24 reduction and O-GlcNAcylation in the diabetic heart contributes to poor survival in diabetic myocardial I/R and increased infarct size post-I/R.
-
•
Overexpression of miR-24 in murine hearts significantly reduces myocardial infarct size.
-
•
miR-24 targets multiple key proteins including O-GlcNac transferase, ATG4A (a key protein in autophagy), and BIM (a pro-apoptosis protein) to protect the myocardium from I/R injury.
-
•
miR-24 is a promising therapeutic candidate for diabetic I/R injury.
Summary
Management for patients with diabetes experiencing myocardial infarction remains a challenge. Here the authors show that hyperglycemia- and hyperinsulinemia-induced microRNA-24 (miR-24) reduction and O-GlcNAcylation in the diabetic heart contribute to poor survival and increased infarct size in diabetic myocardial ischemia/reperfusion (I/R). In a mouse model of myocardial I/R, pharmacological or genetic overexpression of miR-24 in hearts significantly reduced myocardial infarct size. Experimental validation revealed that miR-24 targets multiple key proteins, including O-GlcNac transferase, ATG4A, and BIM, to coordinately protect the myocardium from I/R injury. These results establish miR-24 as a promising therapeutic candidate for diabetic I/R injury.
Diabetes mellitus (DM) is of growing concern, with a prevalence approaching 400 million worldwide and 30 million in the United States (1). Type 2 diabetes mellitus (T2DM), accounting for approximately 95% of DM, is particularly increasing due to insulin resistance from obesity (1). Both type 1 diabetes mellitus (T1DM) and T2DM enhance the risk for cardiovascular disease (CVD) by 2- to 6-fold (2), with mortality arising predominantly from acute thrombotic cardiovascular events (3). Intensive glycemic control appears to reduce the risk of CVD in T1DM (4). In contrast, whether there is a beneficial role for intensive glycemic control on CVD in T2DM remains unclear 5, 6, 7, 8, 9, 10, 11, 12, 13.
Over the past several years it has become clear that alterations in the expression of microribonucleic acid (microRNA or miRNA) contribute to the pathogenesis of diabetes 14, 15, 16, 17, 18. MicroRNAs are small (∼22 nt) regulatory ribonucleic acid (RNA) molecules that functionally modulate the activity of specific messenger RNA targets involved in a wide range of physiological and pathological processes (19). Profiling of microRNA expression in patients with diabetes has identified signatures associated with diagnosis, progression, prognosis, and response to treatment (14). We recently reported that miR-24 is significantly reduced in both T1DM (glycated hemoglobin [HbA1c] >6.5% with absent C-peptide levels) and T2DM (HbA1c >6.5% with normal or higher C-peptide levels) due to hyperglycemia, contributing to endothelial dysfunction (20). In the present study, we have uncovered a possible unifying mechanism that reconciles the disparate glucose control and cardiovascular disease results between T1DM and T2DM. Excessive insulin in T2DM patients, from the use of insulin therapy on a background of increased insulin, leads to dysregulation of a key protective miRNA: miR-24. These results may present a significant therapeutic dilemma in treating patients with both T2DM and MI 21, 22, 23. Our study suggests that therapeutic strategies leading to reduced insulin usage, and thus up-regulating miR-24, potentially protect against acute cardiovascular events in T2DM.
Methods
Human studies
Clamp study
A total of 5 individuals with T1DM participated in the study and underwent hyperinsulinemic (2 mU/kg/min) hypoglycemic (glucose ∼2.8 mmol/l) clamp studies, as previously described (24). Subjects had no medical problems other than T1DM and had a normal physical examination and electrocardiogram. Blood tests confirmed normal liver and renal function, but absent C-peptide levels. Briefly, an intravenous catheter was inserted into an antecubital vein; a primed continuous infusion of insulin (regular human insulin, Novo Nordisk, Bagsvaerd, Denmark) was then initiated and maintained at a constant rate of 2.0 mU/kg/min, and a variable rate of 20% dextrose was infused concomitantly.
Blood miRNA analysis
Blood samples were drawn from consenting volunteers (healthy and DM subjects) with overnight fasting at Yale University School of Medicine (Human Investigation Committee 1005006865). Written informed consent was obtained from all participating individuals. A total of 40 subjects with DM (27 T2DM and 13 T1DM, American Diabetic Association definition) and 10 healthy control subjects (HCs) were consecutively recruited for the studies (Supplemental Table 1). A glucose tolerance test was utilized to exclude participants with pre-diabetes (impaired glucose tolerance). Platelet-rich plasma (PRP) was prepared from blood by venipuncture into 3.8% trisodiumcitrate (weight/volume). PRP was obtained by centrifugation of blood at 250 g (or 1,200 revolutions/min) at 25°C for 15 min. Platelet-poor plasma (PPP) was obtained by centrifugation of the PRP at 1,400 g at 25°C for 10 min (or 1,800 revolutions/min for 5 min). The supernatant PPP was stored at −80°C. PPP was used to determine levels of miR-24 as described in the previous text.
Animals
All mouse studies were approved by Yale Institutional Animal Care and Use Committee. Wild-type (WT) (C57BL/6J background) and diabetic mice (BKS.Cg-Dock7m1/1 Lepr d/b/j) were purchased from The Jackson Laboratory (Farmington, Connecticut). Streptozotocin (STZ)-induced diabetic mice in the C57BL/6J background were purchased from The Jackson Laboratory and were injected with STZ (50 mg/kg) intraperitoneally for 5 consecutive days to induce recurrent episodes of acute hyperglycemia. Four weeks after STZ administration, diabetic mice were injected with insulin (lantus), and the whole hearts were harvested. To generate the Myh6-miR-24 mice, a cDNA construct containing a 400-bp mouse genomic region encompassing the miR-24-2 locus was cloned downstream of the mouse cardiac myosin heavy chain (aMHC/Myh6) promoter. Transgenic mice were produced by microinjection of the Myh6-miR-24 construct into fertilized mouse embryos (C57BL/6 background), as previously described. Transgenic mice were identified by polymerase chain reaction (PCR) analysis of tail genomic DNA.
Plasma analysis
Glucose was measured from the tail-tip with a glucometer. The total RNA, including miRNAs, was extracted and purified using QIAzol lysis reagent and kits according to the manufacturer’s instructions (Qiagen, Hilden, Germany). Plasma insulin levels were measured using an ultrasensitive mouse insulin enzyme-linked immunosorbent assay kit (Crystal Chem, Downers Grove, Illinois).
miRNA mimics delivery in vivo
The mirVanamiR-24 mimic and mimic controls (Life Technologies, Waltham, Massachusetts) were complexed with Invivofectamine 3.0 reagent (Invitrogen, Waltham, Massachusetts) to form nanoparticles for in vivo applications. miRNA oligonucleotides (5 mg/ml in water, 250 μl) were mixed with manufacturer’s complexation buffer (250 μl), and then Invivofectamine 3.0 reagent was added (500 μl). After a 30-min incubation at 50°C, filtration was performed in 15 ml phosphate-buffered saline to remove excessive salts and solvents. The final concentration of miRNA oligonucleotides was 1.25 mg/ml and was intravenously delivered to db/db mice at a concentration of 5 mg/kg body weight. Mice were euthanized 2 weeks after in vivo delivery for detailed assessment.
In vivo myocardial ischemia and reperfusion
WT, db/db, and miR-24-tg mice were anesthetized with pentobarbital (60 mg/kg intraperitoneal injection) and subjected to left coronary artery occlusion for 20 min, followed by 3 h of reperfusion. Hearts were then excised and stained with Evans blue and triphenyl tetrazolium chloride to measure the ischemic area at risk and the area of necrosis (25), respectively. Serum troponin I was measured by enzyme-linked immunosorbent assay (Life Diagnostics, West Chester, Pennsylvania). In separate mice, reperfusion was limited to 10 min, and hearts were then removed to study early reperfusion cell signaling.
Vectors, plasmids, and luciferase assays
The human Bcl-2-like protein 11 (BIM) 3ʹUTR (NM_138621, 4216bp), O-GlcNac transferase (OGT) 3ʹUTR (NM_181672, 2103bp), and autophagy-related gene 4a (ATG4a) 3ʹUTR (NM_052936, 959bp) were amplified from human genomic DNA by PCR, confirmed by sequencing, and cloned into the XhoI and NotI sites of psiCHECK-2 (Promega, Madison, Wisconsin). The sequence GAGC in the predicted seed sequences of BIM/OGT/ATG4A was mutated to ACTA. Luciferase assays were performed in 96-well plates. 293T cells were cotransfected with the 3ʹ UTR reporter or the empty control reporter, and a single miRNA construct or an empty construct in each well. After 2 days, luciferase assays were carried out using the Dual-Glo Luciferase kit (Promega) according to the manufacturer's instructions.
miRNA transfection assays
Transient liposomal transfection of miRNAs was performed according to the manufacturers' instructions. Briefly, cells were split 1 day before transfection to reach 60% to 70% confluence on the day of transfection. miR-24 mimic (Ambion, Thermo Fisher Scientific, Waltham, Massachusetts; Catalog #4464066, mirVana, miRNA mimic) and negative control (Ambion) and Lipofectamine RNAiMAX Transfection Reagent were mixed separately and incubated for 5 min with Opti-MEM I media. Complexes were added together and incubated for 20 min. Media were changed to antibiotic-free media before the addition of liposomal/miRNA complexes (final concentration 100 nmol/l). Cells were incubated for 4 h before the media were changed to fresh media. Silencing of miRNA targets was monitored for 48 h after transfection by quantitative PCR analysis or by Western blot analysis.
Cardiomyocyte culture and treatment
Cardiomyocytes were isolated from male mice (1 to 2 days). In brief, after dissection, hearts were washed and minced in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–buffered saline solution. Tissues were then dispersed in a series of incubations at 37°C in 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid–buffered saline solution containing 1.2 mg/ml pancreatin and 0.14 mg/ml collagenase. Subsequent supernatants were collected and centrifuged at 200 g for 5 min. After centrifugation, cells were resuspended in Dulbecco’s modified Eagle medium/F-12 (GIBCO, Thermo Fisher) containing 5% heat-inactivated horse serum, 0.1 mmol/l ascorbate, insulin-transferring-sodium selenite media supplement, 100 U/ml penicillin, 100 mg/ml streptomycin, and 0.1 mmol/l bromodeoxyuridine. The dissociated cells were pre-plated at 37°C for 1 h. The cells were then diluted to 1 × 106 cells/ml and plated in 10 mg/ml laminin-coated different culture dishes according to the specific experimental requirements. Isolated cardiomyocytes or H9C2 cells were transfected with miR-24 mimic (Ambion, mirVana miRNA mimic) or negative control (Ambion, Catalog #4464066) via Lipofectamine RNAiMAX Transfection Reagent. Western Blot will be applied for ATG4a (Abcam ab108322), OGT (CST #5368), LC3 (Abcam ab51520), O-GlcNAc (CTD110.6 #12938), and BIM (CST #2933). For O-glcNAcylation detection, CTD110.6 antibody was covalently conjugated to anti-IgM as described previously (26).
Statistical analyses
Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, La Jolla, California) and SPSS (IBM, Statistics V22, Armonk, New York). Survival curves were analyzed using a log-rank (Mantel-Cox) test. Statistical differences were assessed with unpaired or paired Student’s t test using GraphPad Prism 6. Otherwise, statistical significance was determined using 1-way analysis of variance followed by Bonferroni’s multiple comparison correction. A 1-way analysis of covariance was conducted to compare differences of 3 or more independent groups while controlling for covariates. Relationships between variables were determined by the Pearson correlation coefficient. All p values <0.05 were considered statistically significant.
Results
Diabetes exacerbates myocardial infarction and ischemia/reperfusion injury
We initially applied 2 diabetic mouse models (STZ alone modeling insulin deficient T1DM and db/db for T2DM) (Figures 1A and 1B, Supplemental Figures 1A to 1D) to perform myocardial I/R surgery (subjected to 20 min ligation of left anterior descending coronary artery) followed by reperfusion for 3 h (for observation of infarct size) or 4 weeks (for observation of survival). The infarct size in db/db mice had over a 3-fold increase compared with WT mice (Figures 1C to 1E), consistent with previous clinical reports of increased morbidity and mortality post-myocardial infarction (MI) in diabetes mellitus (1). STZ-induced diabetic mice had significantly decreased survival post-I/R relative to that of WT mice (survival 31% vs. 91%; p = 0.0023) (Figure 1F, Table 1). However, insulin infusion significantly increased survival post-I/R in STZ-induced diabetic mice compared with noninsulin treated mice (survival 77% vs. 31%; p = 0.0223) (Figure 1F, Table 1, Supplemental Figure 1D). In contrast, there was a 45% decrease in survival post-I/R between insulin- and noninsulin-treated db/db mice (survival 69% vs. 25%; p = 0.0241) (Figure 1F, Table 1, Supplemental Figure 1B). These suggest both T1DM and T2DM contributed to worse outcomes in myocardial ischemia/reperfusion (I/R), and the difference in response to insulin therapy between them was possibly due to their baseline insulin level and/or insulin sensitivity.
Figure 1.
Diabetes Exacerbates Myocardial Infarction and Ischemia/Reperfusion Injury
(A) Comparison of fasting blood glucose levels in diabetic mouse models (n = 10 to 12). (B) Enzyme-linked immunosorbent assay analysis of fasting plasma insulin levels in diabetic mouse models (n = 8 to 10). (C to E) Comparison of infarct size between wild-type (WT) and db/db (leptin receptor knockout) mice subjected to ischemia for 20 min and reperfusion for 3 h. (F) Survival curve (log-rank [Mantel-Cox] test) of WT, streptozotocin (STZ)-induced type 1 diabetes (with or without insulin therapy), and db/db (with or without insulin therapy) subjected to 20 min ligation of left anterior descending coronary artery (LAD) followed by ischemia/reperfusion (I/R) for 4 weeks.
Table 1.
Survival in WT and Diabetic Mice Subjected to MI or I/R
| Mice Type | 1-Week Survival |
4-Week Survival |
||
|---|---|---|---|---|
| MI | MI + Insulin | I/R | I/R + Insulin | |
| WT (C57BL/6J) | 58% | NA | 91% | 100% |
| STZ (T1DM) | 14%∗ | 63%† | 31%‡ | 77%§ |
| Db/db (T2DM) | NA | NA | 69% | 25%‖ |
Survival curves were analyzed using GraphPad Prism 6 (GraphPad Software, La Jolla, California) (a log-rank [Mantel-Cox] test).
I/R = ischemia/reperfusion; MI = myocardial infarction; STZ = streptozotocin; WT = wild-type.
MI, STZ vs. WT, 14% vs. 58%; p = 0.0099.
MI, STZ + insulin vs. STZ, 63% vs. 14%; p < 0.0065.
I/R, STZ vs. WT, 31% vs. 91%; p = 0.0023.
I/R, STZ + insulin vs. STZ, 31% vs. 77%; p = 0.0223.
I/R, db/db + insulin vs. db/db, 25% vs. 69%; p = 0.0241.
Down-regulation of miR-24 by insulin
T1DM is characterized with hyperglycemia plus hypoinsulinemia, whereas T2DM (early stage) characterized with hyperglycemia plus hyperinsulinemia. To assess whether insulin regulates miR-24 levels, we performed in vivo insulin infusion clamp studies in DM human subjects (Yale Institutional Review Board–approved protocol). To confirm that insulin was being infused, plasma insulin levels were measured as well as progression to hypoglycemia (Figures 2A and 2B). Level of miR-24 decreased despite progressing from hyperglycemia to euglycemia (from 0 to 50 min), supporting that the insulin infusion rather than glucose levels is the direct cause for the reduction in miR-24 level (Figure 2C). As further supporting evidence, in patients with T1DM (where patients are on various forms of exogenous insulin therapy for low insulin levels) we observed an inverse correlation between circulating miR-24 and plasma levels of insulin (Figure 2D). In a small pilot study, we observed an intriguing significant reduction in plasma miR-24 levels in T2DM (n = 27) compared with T1DM patients (n = 13) (Figure 2E, Supplemental Table 1), despite no significant differences in fasting glucose or HbA1c. Almost one-half of T2DM patients were on insulin for glucose control, so we separated the T2DM patients who took insulin. The lowest miR-24 levels were observed in T2DM patients on insulin therapy (Figure 2F, Supplemental Table 1). This suggested that insulin itself (particularly on a high background of insulin/insulin resistance) may reduce miR-24 plasma level and possibly further affect cardiac miR-24.
Figure 2.
Down-Regulation of miR-24 in Response to Insulin Infusion in Human Plasma
Human plasma insulin (A), glucose (B), and miR-24 level (C) response to insulin infusion, normalized to spiked-in cel-miR-238. (D) Correlation of plasma miR-24 levels with insulin levels in T1DM patients. (E) Comparison of human plasma miR-24 levels in type 1 diabetes mellitus (T1DM) (n = 13) and type 2 diabetes mellitus (T2DM) (n = 27), normalized to spiked-in cel-miR-238; analysis of covariance was conducted while adjusting for age and sex. (F) Comparison of human plasma miR-24 levels in healthy subjects (HC) (n = 10) and T2DM without insulin therapy (n = 13) or with insulin therapy (n = 13), normalized to spiked-in cel-miR-238; analysis of covariance was conducted while adjusting for age and sex.
Overexpression of miR-24 reduces cardiac damage after myocardial I/R injury
To determine the potential consequences of reduced miR-24 on myocardial function, we first compared the myocardial infarction size in response to I/R between WT and db/db mice (Figure 3A). As mentioned in the previous text, we observed a significantly increased infarct size in diabetic hearts (Figures 1C to 1E). Remarkably, with systemic injection of miR-24 mimic into db/db mice (2 weeks prior to I/R), we observed significant restoration of cardiac miR-24 levels (Figure 3B) (miR-126 served as a control) and a reduction in infarct size (Figures 3C to 3E). The increase in infarct size in DM mice (low miR-24) and its rescue by exogenous miR-24 (restoring levels of miR-24) further support a myocardial protective role for miR-24. Notably, we also observed a significant reduction in plasma insulin level and fasting glucose level with intravenously delivery of miR-24 to db/db mice (Figures 3F and 3G), which could contribute to the decrease in infarct size in DM mice.
Figure 3.
Overexpression of miR-24 Ameliorates Diabetic Ischemia/Reperfusion Injury
(A) Schematic protocol of miR-24 delivery and I/R surgery. (B) Quantitative polymerase chain reaction analysis of miR-24 expression in hearts of db/db mice treated with mimic control (n = 6) or treated with miR-24 mimic (n = 6) for 2 weeks (normalized to U6). (C) Myocardial infarction size assessed following in vivo I/R (20 min LAD ligation and 3 h reperfusion) after treatment with miR-24 mimic (5 mg/kg) or scramble control intravenously, 2 weeks before LAD ligation. Representative images of myocardial tissue slices stained with Evans blue and triphenyl tetrazolium chloride. (D) Quantitation of infarct risk area. (E) Quantitation of infarct area expressed as percentage of the nonperfused risk area during coronary occlusion. (F) Enzyme-linked immunosorbent assay analysis of fasting plasma insulin levels in WT and db/db mice with without miR-24 mimic delivery (n = 8 to 10). (G) Fasting plasma glucose levels in db/db mice with without miR-24 mimic delivery (n = 10 to 12) (paired t test). Abbreviations as in Figure 1.
To demonstrate a cardiac-specific effect, we used a cardiac-specific miR-24 overexpression mouse model. Circulating miR-24 (including nanoparticle-packaged miR-24) is easily endocytosed by cardiomyocytes (27). Interestingly, miR-24 inhibits cardiomyocyte apoptosis 28, 29, 30. To clarify cell-autonomous effects of miR-24 on myocardial function during I/R, we used a Myh6-miR-24 transgenic mouse model where miR-24 is overexpressed in cardiomyocytes (Figure 4A). In response to I/R injury (Figure 4B), overexpression of miR-24 in cardiomyocytes ameliorated the damages caused by myocardial I/R compared with littermate controls (Figures 4C to 4E). The protective effects observed in our transgenic mice under myocardial I/R supports an essential role for miR-24 in reducing myocardial infarct size.
Figure 4.
Cardiomyocyte-Specific Knock-In miR-24 Ameliorates Ischemia/Reperfusion Injury
(A) Quantitative polymerase chain reaction analysis of miR-24 expression in hearts of WT and transgenic mice [miR-24 tg] (normalized to U6). (B) Schematic protocol for myocardial I/R surgery. (C) Representative images of WT and transgenic mice myocardial tissue slices stained with Evans blue and triphenyl tetrazolium chloride. (D) Quantitation of infarct risk area. (E) Quantitation of infarct area expressed as percentage of the nonperfused risk area during coronary occlusion. Abbreviations as in Figure 1.
miR-24 down-regulates multiple targets to coordinately protect myocardium from I/R injury
We then characterized the molecular signaling from insulin to miR-24 and determined its cardiac protection effect in vivo. A time course after insulin injection in mice followed by cardiomyocyte analysis demonstrated a significant increase in canonical signaling through Akt phosphorylation at 30 min (post-insulin infusion), which persisted for approximately 2 h (Supplemental Figure 2A). This was associated with phosphorylation of c-Myc which peaked at 60 min, and a delayed increase in total c-Myc (Supplemental Figure 2B). c-Myc is known to be downstream of Akt (31). We have previously demonstrated that c-Myc negatively regulates miR-24 (20).
The important question remained as to the molecular mechanism by which miR-24 can protect against cardiac damage after myocardial I/R injury. During myocardial reperfusion, the electron transport chain is reactivated, generating reactive oxygen species (ROS). Other sources of ROS include xanthine oxidase from endothelial cells and NADPH oxidase from neutrophils. Such excessive ROS can mediate cardiac myocyte damage, apoptosis, and necrosis. Like other miRNAs, miR-24 has many predicted targets, including key proteins involved in myocardial reperfusion pathophysiology. Using both TargetScan and miRANDA, we selected potential miR-24 targets that intersected myocardial I/R response genes and diabetic response genes (Supplemental Figure 3). Four interesting potential targets were identified: BIM (a pro-apoptosis protein), OGT (a protein involved in O-GlcNAc transfer) (Figure 5A), and ATG4a (a key protein in the autophagy pathway) (Figure 5B). To confirm direct binding, we generated a reporter vector containing a mutant in each 3′UTR of OGT, ATG4a and BIM (the sequence GAGC in the predicted seed sequences of each 3′UTR was mutated to ACTA). Cotransfection of the miR-24 mimic with these constructs in HEK 293T cells markedly decreased the luciferase expression from the WT sequence, but not each mutant OGT/ATG4A/BIM construct (Figure 5C). The addition of miR-24 to cardiomyocytes significantly decreased the expression of BIM, OGT, and ATG4A (Figures 5D and 5E, top), consistent with the bioinformatics analysis and previous findings (29) showing direct targeting of BIM by miR-24. Intriguingly, when cardiomyocytes were overexpressed with miR-24 treated with different concentrations of insulin for 1 h, we observed that miR-24 effectively reversed the insulin-induced up-regulation of OGT in high glucose-cultured cardiomyocytes (Figure 5E, bottom). We further examined the OGT expression and O-GlcNAcylation in mice models we utilized. Both OGT and O-GlcNAcylation in heart lysates were remarkably reduced when overexpression of miR-24 with either genetic (miR-24 tg) or pharmacological approaches (miR-24 mimic delivery in vivo) (Figure 5F). Taken together, these data further support that OGT, ATG4A, and BIM are direct targets of miR-24, all of which contribute to myocardial I/R injury.
Figure 5.
miR-24 Regulates OGT and ATG4A to Play Cardioprotective Effects
Sequence alignment of miR-24 targeting sites on OGT (A) and ATG4A (B) 3ʹUTR among mammals based on the Targetscan database information. (C) miR-24 inhibited luciferase activity of WT 3ʹUTR of OGT, ATG4A, and BIM, but exhibited no effect on luciferase activity of each mutant 3ʹUTR. The assays were performed in triplicate from 3 independent experiments. (D) The change in intracellular miR-24 levels when cardiomyocytes were transfected with miR-24 mimics and performed in triplicate from 3 independent experiments. (E) (Top) Representative Western blot analyses of OGT, ATG4A, and BIM expression in response to miR-24 overexpression in cardiomyocytes, normalized to HSP90 and actin. (Bottom) Cardiomyocytes were transfected with miR-24 mimic (Ambion mirVana miRNA mimic) or negative control via Lipofectamine RNAiMAX Transfection Reagent. A total of 48 h after transfection, cells were treated with 100 nmol/l insulin for another 1 h before harvested. The expression of OGT was analyzed by western blot. (F) OGT expression and O-GlcNAcylation of heart lysates from WT mice or mice with cardiomyocyte-specific overexpression of miR-24, and heart lysates from in db/db mice with control or miR-24 mimic (Life Technologies, 5 mg/kg) delivery. Abbreviations as in Figure 1.
Discussion
Cardioprotection or cardiotoxicity associated with insulin therapy
Our studies help explain some of the longstanding conundrum of why intensive glucose control does not appear to improve cardiovascular morbidity and mortality in patients with T2DM. Our data suggests that insulin therapy on a background of higher insulin levels may lead to reduced myocardial protection through a reduction of miR-24. A significant percentage of T2DM patients receive exogenous insulin injections despite already having high baseline levels in the context of insulin resistance. However, the mechanism underlying intensive insulin therapy–induced cardiac injury in T2DM remained unclear. Nolan et al. (32) proposed insulin resistance as a physiological defense against metabolic stress in T2DM. High doses of exogenous insulin therapy promote excess glucose uptake and glucolipotoxicity, resulting in increased ROS generation and toxic lipid accumulation that contributes to cardiac injury. We previously reported hyperglycemia repression of miR-24 through activation of aldose reductase and ROS production (20). Here, we found that short-term insulin injection decreased miR-24 in diabetic mice hearts, and intensive insulin injection also decreased miR-24 in the plasma of T1DM patients (Figure 2C). Our combined data supports that excessive insulin leads to reduced miR-24 levels, which acts as a key factor in myocardial dysfunction associated with myocardial infarction in DM. Apart from insulin therapy, whether other antidiabetic agents such as DDP4i (33) and GLP1RA (34) also affect miR-24 levels remains largely unknown.
miR-24 targets and cardiovascular disease
miR-24 has multiple targets in cardiomyocytes (29), endothelial cells (28), and macrophages (35), all of which are involved in diabetic cardiac complications. In the heart, miR-24 has been reported to target BIM and junctophilin-2 in cardiomyocytes, and targeting transcription factor GATA2, p21-activated kinase PAK4, and eNOS in endothelial cells (36). In the vascular system, miR-24 was found to target chitinase 3-like 1 to limit vascular inflammation and matrix metalloproteinase-14 in macrophage to retard atherosclerotic plaque progression (35). Apart from the reported targets of miR-24, bioinformatics also predicts other targets, some of which are validated in vitro in the present study, including O-GlcNAc transferase (OGT). In diabetic hearts, O-GlcNAc levels are increased and removal of O-GlcNAcylation restores Ca2+ handling in diabetic hearts 37, 38, 39, suggesting that targeting OGT represents a new possible strategy for treating diabetic cardiomyopathy. Although increased O-GlcNAcylation is directly linked to hyperglycemia-induced glucose toxicity (40) and insulin resistance (26), O-GlcNAcylation has been reported to contribute to cardioprotection against I/R in WT mice (41). In addition, cardiac-specific deletion of OGT exacerbates heart failure, although cardiac-specific ablation of OGT had no effect on infarct size (24 h) and survival (4 weeks) (42). Thus, we suggest that fine-tuning of microRNA-mediated repression of OGT and O-GlcNAcylation is indispensable for cardiac function. Our findings support a novel mechanism for the cardiovascular outcome when chronic hyperglycemia (detrimentally increased O-GlcNAcylation) encounters acute myocardial I/R (beneficially increased O-GlcNAcylation). Interestingly, miR-24 is also highly expressed in beta cells, which requires further exploration.
Conclusions
We have previously demonstrated that high glucose levels can also reduce miR-24 from the endothelium predisposing to thrombosis (20). Together, high glucose and high insulin, as observed most dramatically in T2DM, lead to a severe reduction in miR-24 level and an enlarged infarct through promoting apoptosis and excessive O-GlcNAcylation (Figure 6). Our data suggest that overload insulin is detrimental to myocardial function through reducing the protective miR-24, particularly because the heart is normally one of the highest producers of miR-24. There is strong evidence that patients with an AMI and increasingly high glucose levels have increased mortality at both 30 days and 1 year (43). This can be attributed to both the hyperglycemia and insulin therapies in addition to other factors.
Figure 6.
miR-24 Overexpression Ameliorates Diabetic Ischemia/Reperfusion Injury
We observed an increase in mortality post-myocardial infarction or ischemia/reperfusion (I/R) in type 2 diabetic mice. We also found a reduction of plasma and heart miR-24 levels in diabetic mice. Systemic enrichment of miR-24 in db/db mice or cardiomyocyte-specific overexpression of miR-24 in wild-type (WT) mice significantly reduced myocardial infarct size and alleviated cardiac injury. The possible mechanism underlying miR-24–based therapeutics in diabetic I/R may involve O-GlcNac transferase (OGT)–mediated heart protein O-GlcNAcylation, autophagy-related gene 4a (ATG4A)–mediated autophagy, and Bcl-2-like protein 11 (BIM)–mediated apoptosis.
Clinical Perspectives
Our data presented here pose a therapeutic dilemma as well as its possible solution: if insulin therapy is potentially detrimental to cardiac outcomes, as data from our group and others suggest 32, 44, then how can we approach T2DM patients with hyperglycemia not controlled by standard oral therapy? Although 1 analysis of the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial does not support that insulin dose contributed to cardiovascular mortality (45), our results demonstrate that type of insulin infusion affects survival post-myocardial I/R in type 2 diabetic mice. Although most pharmacological agents used in the treatment of T2DM are geared toward increasing endogenous insulin production, only a small number enhance insulin sensitivity or result in a lowering of circulating insulin levels. Those agents include metformin, thiazolidinediones, and SGLT2 inhibitors. It is intriguing to speculate that miR-24 may play an important role in the hereto unexplained large benefit of empagliflozin on cardiovascular disease reported in T2DM patients (46). However, based on our current and recent findings that decreased miR-24 in diabetes increases the risk of diabetic thrombotic and vascular complications, miR-24 enrichment therapy may be an appealing adjunct therapy for patients with diabetes who are at particular cardiac risk. Although the cell-specific delivery, dosage, and timing for delivery are the important factors for consideration, the use of miR-24 mimics to diabetic cardiomyopathy and thrombosis may offer exciting new therapeutic opportunities.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Insulin infusion decreased miR-24 levels in patients and survival post-myocardial I/R in diabetic mice. Overexpression of miR-24 in murine hearts significantly reduced myocardial infarct size.
TRANSLATIONAL OUTLOOK: Given that miRNA inhibitors (e.g., miR-122 and -34) are already in clinical trials, the present study motivates advanced clinical testing of miR-24 mimic therapeutics in diabetic cardiomyopathy and MI.
Acknowledgments
The authors thank Robert Sherwin and Silvio Inzucchi for their support in recruiting the patients; Dr. Yi-Han Chen for support in clinical studies and animal experiments; and Drs. Shengxian Li and Jijun Cheng for comments on the manuscript.
Footnotes
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants (RO1HL122815, RO1HL115247, and U54 HL117798 to Dr. Hwa), and grants from the National Natural Science Foundation of China (81770256) and the Fundamental Research Funds for the Central Universities (both to Dr. Xiang). Dr. Young has served as a consultant to Portage Pharmaceuticals; and has received grant support through Yale University from Merck Pharmaceuticals. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Li Qian, Email: li_qian@med.unc.edu.
John Hwa, Email: john.hwa@yale.edu.
Yaozu Xiang, Email: yaozu.xiang@tongji.edu.cn.
Appendix
References
- 1.Nathan D.M. Diabetes: advances in diagnosis and treatment. JAMA. 2015;314:1052–1062. doi: 10.1001/jama.2015.9536. [DOI] [PubMed] [Google Scholar]
- 2.Preis S.R., Pencina M.J., Hwang S.J. Trends in cardiovascular disease risk factors in individuals with and without diabetes mellitus in the Framingham Heart Study. Circulation. 2009;120:212–220. doi: 10.1161/CIRCULATIONAHA.108.846519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Di Angelantonio E., Kaptoge S., Wormser D., for the Emerging Risk Factors Collaboration Association of cardiometabolic multimorbidity with mortality. JAMA. 2015;314:52–60. doi: 10.1001/jama.2015.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan D.M., Cleary P.A., Backlund J.Y. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holman R.R., Paul S.K., Bethel M.A., Matthews D.R., Neil H.A. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 6.Hayward R.A., Reaven P.D., Wiitala W.L. Follow-up of glycemic control and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;372:2197–2206. doi: 10.1056/NEJMoa1414266. [DOI] [PubMed] [Google Scholar]
- 7.Group A.C., Patel A., MacMahon S. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein H.C., Miller M.E., Ismail-Beigi F., for the ACCORD Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerstein H.C., Miller M.E., Ismail-Beigi F. Effects of intensive glycaemic control on ischaemic heart disease: analysis of data from the randomised, controlled ACCORD trial. Lancet. 2014;384:1936–1941. doi: 10.1016/S0140-6736(14)60611-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lago R.M., Singh P.P., Nesto R.W. Congestive heart failure and cardiovascular death in patients with prediabetes and type 2 diabetes given thiazolidinediones: a meta-analysis of randomised clinical trials. Lancet. 2007;370:1129–1136. doi: 10.1016/S0140-6736(07)61514-1. [DOI] [PubMed] [Google Scholar]
- 11.McMurray J.J., Gerstein H.C., Holman R.R., Pfeffer M.A. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–851. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 12.Committee ASGW. The ACCORD Study Group Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 2016;39:701–708. doi: 10.2337/dc15-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zoungas S., Chalmers J., Neal B. Follow-up of blood-pressure lowering and glucose control in type 2 diabetes. N Engl J Med. 2014;371:1392–1406. doi: 10.1056/NEJMoa1407963. [DOI] [PubMed] [Google Scholar]
- 14.Guay C., Regazzi R. Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat Rev Endocrinol. 2013;9:513–521. doi: 10.1038/nrendo.2013.86. [DOI] [PubMed] [Google Scholar]
- 15.Poy M.N., Eliasson L., Krutzfeldt J. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 16.Trajkovski M., Hausser J., Soutschek J. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- 17.Kornfeld J.W., Baitzel C., Konner A.C. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;494:111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- 18.Belgardt B.F., Ahmed K., Spranger M. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med. 2015;21:619–627. doi: 10.1038/nm.3862. [DOI] [PubMed] [Google Scholar]
- 19.Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 20.Xiang Y., Cheng J., Wang D. Hyperglycemia repression of miR-24 coordinately upregulates endothelial cell expression and secretion of von Willebrand factor. Blood. 2015;125:3377–3387. doi: 10.1182/blood-2015-01-620278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Group B.D.S., Frye R.L., August P. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holzmann M.J., Rathsman B., Eliasson B. Long-term prognosis in patients with type 1 and 2 diabetes mellitus after coronary artery bypass grafting. J Am Coll Cardiol. 2015;65:1644–1652. doi: 10.1016/j.jacc.2015.02.052. [DOI] [PubMed] [Google Scholar]
- 23.Dangas G.D., Farkouh M.E., Sleeper L.A. Long-term outcome of PCI versus CABG in insulin and non-insulin-treated diabetic patients: results from the FREEDOM trial. J Am Coll Cardiol. 2014;64:1189–1197. doi: 10.1016/j.jacc.2014.06.1182. [DOI] [PubMed] [Google Scholar]
- 24.Page K.A., Williamson A., Yu N. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes. 2009;58:1237–1244. doi: 10.2337/db08-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller E.J., Li J., Leng L. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–582. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 26.Yang X., Ongusaha P.P., Miles P.D. Phosphoinositide signalling links O-GlcNAc transferase to insulin resistance. Nature. 2008;451:964–969. doi: 10.1038/nature06668. [DOI] [PubMed] [Google Scholar]
- 27.Fiedler J., Jazbutyte V., Kirchmaier B.C. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 28.Meloni M., Marchetti M., Garner K. Local inhibition of microRNA-24 improves reparative angiogenesis and left ventricle remodeling and function in mice with myocardial infarction. Mol Ther. 2013;21:1390–1402. doi: 10.1038/mt.2013.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian L., Van Laake L.W., Huang Y., Liu S., Wendland M.F., Srivastava D. miR-24 inhibits apoptosis and represses Bim in mouse cardiomyocytes. J Exp Med. 2011;208:549–560. doi: 10.1084/jem.20101547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo C., Deng Y., Liu J., Qian L. Cardiomyocyte-specific role of miR-24 in promoting cell survival. J Cell Mol Med. 2015;19:103–112. doi: 10.1111/jcmm.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J., Blenis J., Yuan J. Activation of PI3K/Akt and MAPK pathways regulates Myc-mediated transcription by phosphorylating and promoting the degradation of Mad1. Proc Natl Acad Sci U S A. 2008;105:6584–6589. doi: 10.1073/pnas.0802785105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nolan C.J., Ruderman N.B., Kahn S.E., Pedersen O., Prentki M. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes. 2015;64:673–686. doi: 10.2337/db14-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanasaki K., Shi S., Kanasaki M. Linagliptin-mediated DPP-4 inhibition ameliorates kidney fibrosis in streptozotocin-induced diabetic mice by inhibiting endothelial-to-mesenchymal transition in a therapeutic regimen. Diabetes. 2014;63:2120–2131. doi: 10.2337/db13-1029. [DOI] [PubMed] [Google Scholar]
- 34.Shang J., Li J., Keller M.P. Induction of miR-132 and miR-212 expression by glucagon-like peptide 1 (GLP-1) in rodent and human pancreatic beta-cells. Mol Endocrinol. 2015;29:1243–1253. doi: 10.1210/me.2014-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maegdefessel L., Spin J.M., Raaz U. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boon R.A., Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 37.Ramirez-Correa G.A., Ma J., Slawson C. Removal of abnormal myofilament O-GlcNAcylation restores Ca2+ sensitivity in diabetic cardiac muscle. Diabetes. 2015;64:3573–3587. doi: 10.2337/db14-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu Y., Belke D., Suarez J. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 39.Erickson J.R., Pereira L., Wang L. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–376. doi: 10.1038/nature12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K., Paterson A.J., Chin E., Kudlow J.E. Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc Natl Acad Sci U S A. 2000;97:2820–2825. doi: 10.1073/pnas.97.6.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z.V., Deng Y., Gao N. Spliced X-box binding protein 1 couples the unfolded protein response to hexosamine biosynthetic pathway. Cell. 2014;156:1179–1192. doi: 10.1016/j.cell.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watson L.J., Facundo H.T., Ngoh G.A. O-linked beta-N-acetylglucosamine transferase is indispensable in the failing heart. Proc Natl Acad Sci U S A. 2010;107:17797–17802. doi: 10.1073/pnas.1001907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kosiborod M., Rathore S.S., Inzucchi S.E. Admission glucose and mortality in elderly patients hospitalized with acute myocardial infarction: implications for patients with and without recognized diabetes. Circulation. 2005;111:3078–3086. doi: 10.1161/CIRCULATIONAHA.104.517839. [DOI] [PubMed] [Google Scholar]
- 44.Khunti K., Davies M., Majeed A., Thorsted B.L., Wolden M.L., Paul S.K. Hypoglycemia and risk of cardiovascular disease and all-cause mortality in insulin-treated people with type 1 and type 2 diabetes: a cohort study. Diabetes Care. 2015;38:316–322. doi: 10.2337/dc14-0920. [DOI] [PubMed] [Google Scholar]
- 45.Siraj E.S., Rubin D.J., Riddle M.C. Insulin dose and cardiovascular mortality in the ACCORD Trial. Diabetes Care. 2015;38:2000–2008. doi: 10.2337/dc15-0598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zinman B., Wanner C., Lachin J.M., for the EMPA-REG OUTCOME Investigators Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.