Abstract
Glucose and related hexoses play central roles in the biochemistry and metabolism of single cell parasites such as Leishmania, Trypanosoma, and Plasmodium that are the causative agents of leishmaniasis, African sleeping sickness, and malaria. Glucose transporters and the genes that encode them have been identified in each of these parasites and their functional properties have been scrutinized. These transporters are related in sequence and structure to mammalian facilitative glucose transporters of the SLC2 family, but they are nonetheless quite divergent in sequence. Hexose transporters have been shown to be essential for the viability of the infectious stage of each of these parasites and thus may represent targets for development of novel anti-parasitic drugs. The study of these transporters also illuminates many aspects of the basic biology of Leishmania, trypanosomes, and malaria parasites.
Keywords: Glucose transporters, parasitic protozoa, nutrient uptake, inhibitors of glucose transport, drug development
1. Introduction
The objective of this chapter is to provide a brief review of glucose transporters in three parasitic protozoa that cause important diseases in humans: Leishmania mexicana, Trypanosoma brucei, and Plasmodium falciparum. These parasites are unicellular eukaryotes that are the causative agents of cutaneous leishmaniasis, African sleeping sickness, and malaria respectively, diseases that cause major health burdens worldwide (http://www.who.int/mediacentre/factsheets/fs094/en/). The review focuses on these parasites because i) glucose transporters play central roles in the nutrition and metabolism of each organism and appear to be essential for their viability, and ii) substantial work has been accomplished at the molecular level on the glucose transporters from each species. Given the central role of glucose uptake in these parasites, an understanding of this process at the molecular, cellular, and genetic levels will promote a better understanding of the fundamental biochemistry and physiology of each pathogen. In addition, the molecular dissection of parasite glucose transporters may provide a target for development of novel therapeutics that selectively inhibit the essential parasite permeases without altering uptake of glucose in the mammalian host.
2. Leishmania mexicana
The genus Leishmania encompasses a variety of species that are pathogenic to humans and which cause distinct diseases collectively designated as leishmaniases (1). These parasites exhibit two principal stages in their life cycles. Promastigotes are slender, flagellated protozoa (Fig. 1A) that live in the gut of the insect vector, any of several species of hematophagous sand flies, but can be cultured easily in a variety of tissue culture media. When infected female sand flies feed upon a vertebrate host (to obtain protein from the blood meal which is required for egg formation), they inject promastigotes into the skin, where they are taken up by host macrophages. Inside the macrophage, promastigotes transform into amastigotes, smaller, oval, non-flagellated forms (Fig. 1B) that are adapted for survival and growth within the acidified phagolysosomal vesicles of the macrophage. The physiological environments of extracellular promastigotes and intracellular amastigotes are quite distinct, with promastigotes being exposed to high levels of sugars from the plant nectar meals taken by the sand flies for general nutrition (2) and amastigotes probably being exposed to much lower levels of sugars inside the macrophage phagolysosome (3–5).
Fig. 1.
Microscopic images of promastigotes and amastigotes of L. mexicana. Panels represent a phase contrast image of promastigotes (A), a phase contrast image of a murine peritoneal macrophage infected with amastigotes (B), and immunofluorescence (C) of the same image as in (B) stained with an antibody directed against the amastigote-specific surface protein HASPB (62) to visualize intracellular parasites.
L. mexicana, one agent of cutaneous leishmaniasis that induces ulcerating skin lesions, has been employed by my laboratory to examine the function of glucose transporters in these parasites. Initial studies revealed that the genome of L. mexicana encompasses a cluster of three linked glucose transporter genes designated LmGT1, LmGT2, and LmGT3 (6) (Fig. 2). The proteins encoded by these genes are related in sequence and predicted secondary structure to mammalian facilitative glucose transporters (SLC2 family, http://www.bioparadigms.org/slc/intro.asp), which have 12 transmembrane domains (Fig. 3), but were only ~20% identical in sequence to the closest homologue, human GLUT1. The three transporters are isoforms that are similar but not identical to each other over the region encompassing the transmembrane domains and connecting loops, but they differ significantly in sequence in the hydrophilic domains at the NH2 and COOH termini.
Fig. 2.
Diagram of the glucose transporter gene cluster in L. mexicana and the strategy for generation of the Δlmgt null mutant by targeted gene replacement. Open rectangles designate the open reading frames for the GT1, GT2, and GT3 genes. The construct below the gene cluster contains a puromycin acetyl transferase gene (PAC) employed as a marker to confer resistance to puromycin and cloned segments from upstream (US) and downstream (DS) of the GT cluster to target gene replacement by homologous recombination. Integration of this construct (heavy vertical arrow) leads to replacement of the GT cluster with the drug resistance marker. Since Leishmania are diploid, generation of the homozygous null mutant required a subsequent gene replacement with a second drug resistance marker (22). This figure was reproduced from reference (22) with permission.
Fig. 3.
Topology of Leishmania glucose transporters and regions of sequence divergence. They gray horizontal rectangle represents the plasma membrane, the numbered white vertical rectangles are predicted hydrophobic transmembrane segments, and the black lines indicate hydrophilic segments that connect transmembrane domains. The stipled lines at the NH2- and COOH-termini indicate sequences that are highly divergent between the LmGT1, LmGT2, and LmGT3 proteins. The figure is modified from Fig. 2 in reference (63).
Expression of each isoform, initially in Xenopus oocytes (6) but later in a glucose transporter null mutant of L. mexicana (7), revealed that they were hexose transporters with the ability to take up glucose, fructose, mannose, and galactose. The affinity for glucose was high relative to mammalian homologs, with Km values ranging from ~100 μM to ~1 mM.
There has been some uncertainty about whether the Leishmania glucose transporters are facilitative permeases, similar to their homologues in mammals (8) referred to as GLUTs, or concentrative proton symporters (9; 10). However, glucose transport studies carried out on intact parasites reveal that glucose uptake is inhibited by protonophores such as FCCP and respiratory inhibitors such as sodium azide (11–13), suggesting a role for proton symport. In addition, glucose-induced deacidification of the medium was observed when D-glucose, but not L-glucose, was added to de-energized parasites, also suggesting a role for proton symport. Furthermore, studies with sealed plasma membrane ghosts from L. donovani (14) showed that an imposed pH gradient could drive uptake of labeled 2-deoxyglucose into the ghosts, as would be expected for a proton symporter. Addition of Mg+2-ATP to everted membrane vesicles resulted in extrusion of preloaded [14C]D-glucose from the vesicles by activating the proton-ATPase of the plasma membrane to concentrate protons in the interior of the vesicle. This observation that acidification of the everted vesicle lumen promoted extrusion of labeled glucose also supports an active proton-symport mechanism. Although studies with Leishmania glucose transporters expressed in Xenopus oocytes did not reveal substrate-induced currents (15), this may have been due to loss of proton coupling in the heterologous expression system. It is also noteworthy that the structurally related myo-inositol transporter from L. donovani did generate substrate induced currents with a charge to substrate stoichiometry of 1:1 when expressed in oocytes (16), establishing that members of the SLC2 family in Leishmania can function as protons symporters.
Despite the apparent similarity of LmGT1, LmGT2, and LmGT3 to each other regarding biochemical properties, some important biological distinctions emerged. First, LmGT2 mRNA levels were strongly developmentally regulated being ~15-fold more abundant in promastigotes compared to amastigotes (6), consistent with previous biochemical studies demonstrating that promastigotes both imported (13) and metabolized (17; 18) glucose at much higher rates compared to amastigotes. In contrast, LmGT1 and LmGT3 mRNAs were constitutively expressed in both life cycle stages. Localization of functionally active fusion proteins between each permease and green fluorescent protein revealed that LmGT2 and LmGT3 were targeted to the pellicular plasma membrane surrounding the cell body of the amastigote, but they were excluded from the flagellar membrane. In striking contrast, LmGT1 was targeted preferentially to the flagellar membrane (Fig. 4). Studies performed with the GT1 isoform from the related Leishmania species L. enriettii (19) revealed that two distinct regions of the divergent amino terminus of the flagellar isoform are required for flagellar targeting. These distinctions in developmental regulation and subcellular location of each isoform suggest that each permease subserves distinct functions for the parasite. The function of the flagellar isoform is not clear at present, but it is possible that it could serve some role in sensing glucose. This speculation is consistent with the observation that membrane proteins that are selectively localized to flagellar or ciliary membranes in a variety of eukaryotes are often involved in sensing the environment (20) and with the well-established role of glucose transporter-like proteins as nutrient sensors in yeast (21).
Fig. 4.
Fluorescence images of L. mexicana promastigotes expressing LmGT1-GFP (A and C) and LmGT2-GFP (B and D) fusion proteins. The top images (A,B) represent GFP fluorescence and indicate that LmGT1 is localized to the flagellum and LmGT2 to the pellicular plasma membrane surrounding the cell body. The bottom images (C,D) represent the same cells as in (A,B) stained with anti-α-tubulin antibody to localize the flagellar axoneme and the pellicular microtubules that are attached to the cytosolic side of the pellicular plasma membrane. (A,C) are conventional fluorescence micrographs, whereas (B,D) are confocal micrographs representing a 0.5 μ section through the image.
A genetic approach to defining glucose transporter function in L. mexicana was applied by generating a null mutant deficient in all three linked glucose transporter genes. Targeted gene replacement was employed to replace the LmGT1-LmGT2-LmGT3 cluster on both homologous chromosomes with drug resistance markers (22) (Fig. 2). This Δlmgt null mutant was deficient in uptake of glucose, fructose, mannose, and galactose, and complementation of the null mutant with each open reading frame (ORF) on an episomal expression vector restored uptake of all four sugars (7). These results confirmed that each permease is a hexose transporter with broad substrate specificity. The Δlmgt promastigotes, the life cycle stage in which the null mutant was made, grew more slowly and to a lower density than wild type parasites, and complementation with each LmGT ORF was able restore growth to near wild type levels.
Infection of murine primary peritoneal macrophages with the Δlmgt null mutant revealed an unexpected result. While the null mutant was able to enter macrophages with equal efficiency as wild type promastigotes (22) and to transform into intracellular amastigotes (23), the mutant parasites did not replicate but died over the course of 6 days, whereas wild type L. mexicana amastigotes replicated. Complementation with LmGT2 or LmGT3 was able to restore, at least partially, growth of Δlmgt amastigotes in macrophages. These results suggest that glucose transporters are essential for the viability of intracellular amastigotes, despite the fact that glucose transport (13) and metabolism (17; 18) are downregulated in amastigotes compared to promastigotes. Consistent with the above observations, Δlmgt promastigotes did not transform into axenic culture form amastigotes, and wild type axenic amastigotes lost viability when glucose was withdrawn from the medium (22). Finally, while either wild type or hemizygous knockouts in the glucose transporter gene cluster were able to form cutaneous lesions in BALB/c mice, the Δlmgt promastigotes were unable to establish lesions (unpublished observations of Richard Burchmore and Graham Coombs).
All the above observations point to the essential nature of glucose transporters in the infectious stage of the parasite life cycle. These results are unanticipated for several reasons. First, these permeases are required for viability in the life cycle stage in which their expression, as well as glucose metabolism, is downregulated, but they are not essential in promastigotes where the wild type parasites express higher levels of the permease and metabolize glucose robustly. Second, while glucose and fructose reach high levels in the sandfly gut at least temporarily (2), hexoses are thought to be relatively less abundant within the parasitized phagolysosome (3–5), although no direct measurements of metabolites exist for these organelles. Finally, gluconeogenesis is active and essential in amastigotes of L. major, as demonstrated by the non-viability of null mutants in the gluconeogenic enzyme fructose 1,6-bisphosphatase in the amastigote stage of the life cycle (24). Curiously, it appears that both glucose uptake and gluconeogenesis are essential for survival of these intracellular parasites.
Several metabolic and cellular phenotypes of the Δlmgt promastigotes suggest reasons why they may be impaired in viability inside the macrophage (7; 25). First, these null mutants are more susceptible to oxidative killing than wild type promastigotes or null mutants that have been complemented with the LmGT2 ORF. Second, the null mutants have reduced viability at elevated temperatures (e.g. 33°C compared to the usual temperature for cultivation of promastigotes, 27°C) compared to either wild type parasites or null mutants complemented with the LmGT2 gene (23). Third, null mutants are more susceptible to nutrient limitation than are wild type or complemented mutants (23). All three of these ‘environmental insults’ are conditions that amastigotes are exposed to when they reside within macrophage phagolysosomes, and the increased susceptibility of the null mutants to these stresses likely contributes to their non-viability inside the host cell. In addition, the major storage carbohydrate of Leishmania promastigotes and amastigotes, the mannose polymer β-mannan, is reduced ~3-fold in level in Δlmgt promastigotes compared to wild type promastigotes (7), and the reduction of this established virulence factor (26) is likely to impair amastigote viability and possibly contribute to the increased susceptibility to nutrient limitation, increased oxidative stress, and elevated temperature noted above.
Another confirmation of the essential role of glucose uptake in amastigotes emerged from the detection and analysis of spontaneous suppressor mutants that arose when the promastigotes were passaged for several months in glucose replete medium (23). These variants were initially observed to survive within primary murine macrophages significantly better than the original Δlmgt mutants, although they did not replicate as robustly as wild type amastigotes. In addition, the suppressors grew with similar kinetics compared to wild type promastigotes rather than at the lower rate and density characteristic of Δlmgt promastigotes. Furthermore, the suppressors had re-established the ability to transport glucose and fructose, although not to the same degree as wild type promastigotes. Molecular analysis of these suppressors revealed that they had amplified another permease gene, LmGT4, on a circular amplicon and that they overexpressed LmGT4 mRNA and protein compared to either wild type or null mutant parasites. These suppressors had also at least partially restored relative resistance to oxidative stress, nutrient limitation, and elevated temperature, and they had re-established wild type levels of β-mannan. The LmGT4 gene (previously called the D2 gene) had been characterized previously as a single copy hexose transporter gene unlinked to the LmGT1-LmGT2-LmGT3 locus (15). Curiously, Δlmgt mutants do not exhibit any detectable hexose uptake even though the LmGT4 gene is intact, and it is the amplification by ~50–100-fold of the subchromosomal region encompassing this gene that partially restores hexose transport. It is likely that the suppressors arose initially by the rare spontaneous generation of a circular element containing the LmGT4 gene. Generation of circular extrachromosal elements that subsequently undergo amplification upon genetic selection for a gene encompassed within that element is an event that has been well-documented for other loci in Leishmania (27). Since null mutants were passaged in glucose-replete medium, a genetic selection was being applied inadvertently for any variant that amplified an alternative hexose transporter gene such as LmGT4. Such genetic variants would be able to grow more rapidly in glucose-replete medium than the null mutants and hence would overtake the culture, leading to a uniform population of suppressor mutants.
3. Use of the Δlmgt Null Mutant to Express Heterologous Glucose Transporters
In addition to studying the biological roles of glucose transporters in Leishmania parasites, the Δlmgt mutant provides a useful glucose transport-deficient background in which to express heterologous glucose transporters. The THT1 hexose transporter from T. brucei (28), the PfHT1 hexose transporter from the malaria parasite Plasmodium falciparum (29), and the human glucose transporter GLUT1 (30) were all able to restore hexose transport to mutants when expressed in the null mutant using episomal expression vectors (31). These heterologous expressors provide a potential assay system for screening chemical libraries for selective inhibitors of those permeases. Indeed the Δlmgt null mutants require amino acids such as proline as an alternate source of metabolic energy (9; 22), since they cannot take up and metabolize hexoses. Consequently, growth of the Δlmgt null mutant in proline-deficient medium is dependent upon both glucose in the medium and a functional complementing hexose transporter gene (31). Thus compounds that inhibit the heterologous transporter, such as the glucose analogue 3-O-(undec-10-en)yl-D-glucose that is a selective inhibitor of PfHT1 (32; 33), will inhibit growth of null mutants expressing that heterologous transporter. In principal, this growth assay could be employed to identify compounds that inhibit growth of null mutants expressing the THT1, PfHT1, or any of the Leishmania glucose transporters but not inhibiting growth of null mutant expressing the human glucose transporter GLUT1. Such selective inhibitors could be both useful reagents for probing structure and function of each permease and potential leads for development of drugs that inhibit essential parasite transporters.
4. Trypanosoma brucei
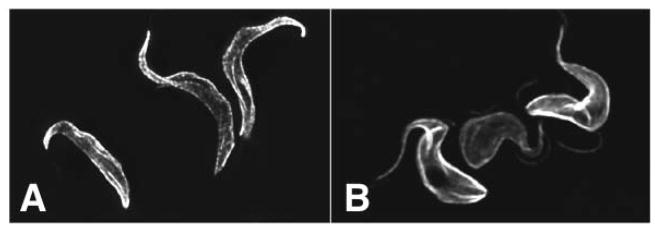
The African trypanosome, T. brucei and its subspecies T. b. gambiense and T. b. rhodesiense, are the causative agents of the disease nagana in cattle and African sleeping sickness in humans (1). Procyclic forms (PFs) are flagellated parasites (Fig. 5A) that live and divide within the midgut of the tsetse fly, whereas bloodstream forms (BFs) are flagellated parasites (Fig, 5B) that live extracellularly within the bloodstream of the mammalian host. Since BF trypanosomes live in a high glucose environment, they are able to generate energy exclusively by glycolysis, and they do not possess a fully functional Krebs cycle or oxidative phosphorylation (34). As a result, BF parasites utilize large amounts of glucose to generate a small yield of ATP, and these life cycle forms are exquisitely dependent upon glucose uptake for viability. As such, trypanosome hexose transporters have been considered as potential targets for development of drugs against these important pathogens (35–38).
Fig. 5.
Immunofluorescence micrographs of procyclic (A) and bloodstream (B) form T. brucei parasites stained with an anti-α-tubulin antibody. This antiserum reacts with the subpellicular microtubules attached to the cytosolic face of the plasma membrane and provides an image of the cell body.
Early studies on transport of glucose or its analogues in BF trypanosomes (34; 39–41) revealed robust substrate saturable uptake activity with Km values in the low mM range. This uptake could be inhibited by other hexoses such as fructose and mannose, suggesting that the transport system was probably a broad specificity hexose permease. Classical inhibitors of the human facilitative glucose transporter GLUT1, such as cytochalasin B and phloretin, could inhibit uptake of glucose by BF trypanosomes, but cytochalasin B inhibition required ~1000-fold higher concentrations compared to the concentrations effective in inhibition of glucose uptake in human red blood cells that express GLUT1. Studies on the uptake of 2-deoxyglucose by PF trypanosomes (42) revealed a distinct, high affinity transport activity (Km for uptake of ~38 μM compared to ~1 mM for BF trypanosomes) that was sensitive to respiratory inhibitors and ionophores, suggesting that it represented proton coupled, active transport. These distinctions between hexose transport in BF and PF trypanosomes would be consistent with the high levels of glucose available in mammalian blood compared to the much lower levels present in the gut of the tsetse fly.
Molecular cloning demonstrated that the T. brucei genome contains a cluster of hexose transporter genes (43). In the EATRO-164 strain first studied, this cluster encompasses six copies of the THT1 gene followed by five copies of the THT2 gene (43). The THT1 and THT2 proteins are 82% identical in sequence. THT1 mRNA is expressed almost exclusively in BF parasites (28), whereas THT2 mRNA is expressed primarily in PF parasites but is present at a lower but detectable level on BFs (44). Kinetic studies performed on BF trypanosomes revealed that THT1 is a low affinity, high capacity transporter that takes advantage of the high concentration of glucose in blood, whereas THT2 is a high affinity, low capacity transporter adapted for life in the relatively low glucose environment of the tsetse midgut. THT1 appears to be a facilitative transporter (44), but it has been suggested that THT2 may function as a proton symporter at low glucose concentration but as a facilitative transporter at high glucose concentration (45), i.e. that it undergoes ‘slippage’ regarding coupling of protons to glucose transport. To date, no subcellular localization studies have been performed for THT1 or THT2.
Two studies have sought to identify high-affinity inhibitors of glucose transport in BF trypanosomes (37; 38). Triazine compounds, especially Cibacron blue, were found to be moderately good inhibitors of glucose transport (37). Examination of glucose and fructose analogues revealed that adding a bromo-acetamide group onto the 2-O position of D-glucose or the 6-O position of D-fructose produced analogues that were competitive inhibitors of D-glucose uptake by BF parasites (38), and it was suggested that the alkylated side chain may react covalently with a nucleophilic group on the trypanosome hexose transporter. These two compounds had trypanocidal effects with LD50 values in the low micromolar range. While these compounds by themselves are unlikely to provide novel trypanocides in their own right, their ability to inhibit the parasite hexose transporter and kill trypanosomes in culture suggests that this permease is a potential drug target.
5. Plasmodium falciparum
Malaria in humans is caused by several species of Plasmodium, with the most severe, life threatening disease resulting from infection by P. falciparum (46). These parasites display a complex life cycle with various developmentally distinct stages occurring in both the mosquito vector and the human host, but the major disease causing form of the parasite invades and replicates within the erythrocyte. These life cycle forms occupy an internal membrane bound parasitophorous vacuole (PV) (Fig. 6). As such, nutrients must pass across three membranes, the red blood cell plasma membrane (RBCPM), the PV membrane (PVM), and the parasite plasma membrane (PPM).
Fig. 6.
Diagram of an erythrocyte infected with the malaria parasite P. falciparum. The gray circle represents the host red blood cell outlined by the red blood cell plasma membrane (RBCPM), the white oval is the parasitophorous vacuole surrounded by the parasitophorous vacuole membrane (PVM), and the inner shaded circle represents the malaria parasite delimited by the parasite plasma membrane (PPM). The red cell glucose transporter GLUT1 and the parasite glucose transporter PfHT1 are indicated by circles with arrows indicating the direction of glucose uptake. In addition channels with broad permeant specificity are depicted by rectangles in the red blood cell membrane (RBC channels or NPPs) and the PVM (PVM channel).
Intraerythrocytic parasites acquire glucose from host blood and are completely dependent upon anaerobic glycolysis for viability (47). Consequently, parasitized red blood cells utilize glucose at ~25–50-fold higher level than non-parasitized erythrocytes (48–50) and export increased levels of lactate, an end product of glycolysis. The host cell glucose transporter GLUT1 is the major mediator of glucose transport across the RBCPM of the parasitized red blood cell (Fig. 6) (51). Various parasite induced channel activities have been proposed to play roles in transport of multiple nutrients across the RBCPM (designated New Permeation Pathways or NPPs) (52; 53), but these NPPs do not play a significant role in glucose uptake compared to GLUT1 (51). There is a high capacity, low specificity channel in the PVM (54) that is likely to be the route for passage of glucose across that intracellular membrane. Early studies on uptake of glucose or its analogues by malaria infected erythrocytes (50; 55–57) suggested that the parasite expressed a facilitative glucose carrier in its plasma membrane that mediated the uptake of this essential nutrient into the parasite cytoplasm. Subsequent studies in which polyadenylated RNA isolated from intraerythrocytic stage parasites was injected into Xenopus oocytes (58) revealed a significant stimulation of uptake of radiolabeled 2-deoxy-D-glucose compared to water injected oocytes, and this uptake was steroespecific and not inhibited by L-glucose. These results implied that the parasite genome encodes endogenous glucose transporters likely to be involved in uptake of this nutrient across the PPM.
A major breakthrough in understanding glucose transport in malaria parasites emerged from identification, among sequences from the then ongoing P. falciparum genome project (http://www.sanger.ac.uk/Projects/P_falciparum/), of an open reading frame (ORF) designated PfHT1 that exhibited significant (20–30%) amino acid sequence identify to human GLUTs (29). Expression of the PfHT1 ORF in Xenopus oocytes induced saturable uptake of 2-deoxyglucose and glucose with a Km for the latter substrate of 0.48 mM. Transport of both substrates was inhibited by fructose, mannose, galactose, and the glucose analogues 6-deoxyglucose and 3-O-methylglucose, indicating that the permease had broad specificity for hexoses. In addition, the classical inhibitors of mammalian GLUTs cytochalasin B, phloretin, and phloridzin exhibited partial inhibition of substrate uptake when applied in the concentration range of 50–500 μM. In synchronized infections of erythrocytes, PfHT1 mRNA levels were strongly developmentally regulated. Transporter mRNA peaked in early ring stage parasites about 8 hr post invasion, fell about 10-fold in level by 20 hr, and then accumulated to intermediate levels in more mature intraerythrocytic parasites between 30–40 hr. In addition, the mRNA was expressed at very low levels in gametocytes, sexual stages of the parasite present in the blood of the infected host. A polyclonal antibody directed against a PfHT1 peptide recognized a single band on Western blots of lysates from infected erythrocytes and stained the plasma membrane of the intracellular parasites, confirming that this permease is localized to the surface membrane of the parasite rather than being inserted into host cell membranes. In summary, the first molecular studies on glucose transport in malaria parasites identified the gene encoding a high affinity hexose transporter that mediates uptake of sugars across the parasite plasma membrane.
Further studies by the same group (59) demonstrated that PfHT1 transports both radiolabeled glucose and fructose, either of which can serve as an energy source for intraerythrocytic parasites. Furthermore mutation of the Q169 residue, located within predicted transmembrane helix 5, to N resulted in a permease that still transported glucose but was deficient in fructose uptake, indicating that this amino acid is involved in determining the substrate specificity of PfHT1.
The likely role of PfHT1 in providing essential nutrients to the intraerythrocytic parasites suggested that it played a vital role in survival of the infecting microorganism and that selective inhibitors of PfHT1 might provide promising leads for development of new antimalarial drugs. To validate PfHT1 as a potential drug target, the Krishna group examined the ability of various glucose analogues to selectively inhibit PfHT1 (32). The observation that 3-O-methylglucose strongly inhibited uptake of glucose by PfHT1 suggested that other 3-O derivatives might provide high affinity inhibitors. Indeed the glucose derivative 3-O-((undec-10-en)-1-yl)-D-glucose, also referred to as compound 3361, inhibited uptake of labeled glucose by PfHT1 with a Ki of ~50 μM but was a very poor inhibitor of human GLUT1 (Ki ~ 3 mM). Notably compound 3361 killed P. falciparum intraerythrocytic parasites in culture with an IC50 value of 16 μM. Furthermore, treatment of mice infected with the murine malaria parasite P. yoelii with 25 mg/kg compound 3361 twice a day for 4 days resulted in significant suppression of parasitemia. This work provides proof of principle that the parasite glucose transporter could serve as a potential target for development of drugs that selectively inhibit its transport activity.
It is noteworthy that the completed genome of P. falciparum encodes two other potential sugar transporters in addition to PfHT1 (60). These other ORFs are more divergent in sequence from known glucose transporters than PfHT1 and thus might represent transporters for other sugar or non-sugar ligands, but they have not been functionally characterized to date. These permeases might be important in the insect stages of the life cycle, where sugars other than glucose are likely to be present from the plant diet of the mosquito. It thus seems likely that PfHT1 is the glucose transporter that plays an essential nutritional role for the intraerythrocytic parasites and that inhibition of this permease by compound 3361 undermines the viability of the parasite within the red blood cell.
6. Summary and Perspectives
The work summarized here underscores the essential role of glucose transporters in the infectious stages of three parasites of importance to global health. For T. brucei and P. falciparum that live in glucose rich blood or in erythrocytes and rely upon glucose metabolism exclusively, the essential role of hexose permeases is a logical expectation. The reason for the essential nature of glucose transporters in the intracellular stage of Leishmania parasites, where hexoses are thought to be relatively non-abundant and where glucose transport and metabolism are downregulated compared to the insect stage of the life cycle, is more obscure. Nonetheless, the essential nature of hexose permeases in each parasite, demonstrated either genetically in L. mexicana or by the inhibition of parasite growth by selective inhibitors for T. brucei and P. falciparum, indicates that these permeases are central to the metabolism of each parasite and present the potential for exploitation as therapeutic targets. The low degree of sequence identity between the microbial transporters and their GLUT homologues in mammals suggests that many inhibitors may be selective for the parasite over the human permeases.
The identification of hexose analogues that inhibit trypanosome or malaria hexose transporters provides an experimental demonstration that selective inhibitors do exist, and their ability to inhibit growth of parasites in vitro and in animal models of infection confirm the therapeutic potential of inhibiting these nutrient carriers. Nonetheless, the inhibitory substrate analogues identified to date are not likely to provide compounds useful as antiparasitic drugs. An alternative approach suggested by multiple workers would be to screen large libraries of small compounds for those that selectively inhibit the parasite transporters and then to examine these compounds for those with the most likely properties as drug leads. For parasites that live in the bloodstream and are exposed to high concentrations of glucose, non-substrate analogues that inhibit noncompetitively at sites distinct from the substrate may offer an advantage. The efficacy of such inhibitors would not be compromised by competition with the natural substrate for access to the permease.
In addition to their potential role in drug development, hexose transporters provide opportunities to study many basic problems central to the biochemistry, physiology, and cell biology of these parasites. Where multiple members of the family exist, it is imperative to understand the distinct functions of each permease. For the THT1 and THT2 permeases of trypanosomes, the preferential expression in BF and PF trypanosomes respectively correlates with transport properties required to support life in high versus low glucose concentrations. However, for the three glucose transporters expressed by Leishmania parasites, the distinct functions of each isoform are less clear. Differential subcellular targeting of distinct glucose transporters provides a model for understanding how membrane proteins are trafficked to specific organelles in parasites. One striking example of discrete organellar targeting is provided by the LmGT1 flagellar glucose transporter in L. mexicana. Since other proteins are targeted to the flagellar membranes of kinetoplastid parasites such as Leishmania and Trypanosoma (20), LmGT1 may provide insights regarding how an entire class of membrane proteins are trafficked to the flagellum. While we know some information about sequences required for flagellar targeting, we do not know what cellular machinery directs such proteins to the flagellar membrane. As for various nutrients in kinetoplastid parasites, growth under conditions of glucose limitation induces higher glucose uptake capacity \, and at least some of this increase may be due to enhanced expression of glucose transporters. In parallel, deletion of the glucose transporter genes or starvation for glucose in Leishmania leads to changes in the levels of many other proteins (unpublished data of Xiuhong Feng, Scott Landfear, and Richard Burchmore), suggesting that the parasites respond to the inability to take up a metabolically important nutrient by altering expression of other proteins in ways that may promote parasite viability or alter metabolism. Thus getting to know the lives of glucose transporters may provide a rich body of information on regulation of gene and protein expression, differential targeting of membrane proteins, and physiological requirements for nutrient uptake and metabolism among parasitic protozoa.
Acknowledgments
The author would like to thank Dr. Richard Burchmore for communicating unpublished data regarding murine infections with L. mexicana glucose transporter null mutants and protein expression in these null mutants and for providing a critical reading of the manuscript. Images for figures included in this chapter were provided by Cosmo Buffalo, Dayana Rodriguez-Contreras, and Marco Sanchez in the author’s laboratory. This work was supported by grant number AI25920 from the National Institutes of Health.
References
- 1.Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, McKerrow J, Reed S, Tarleton R. Kinetoplastids: related protozoan pathogens, different diseases. J Clin Invest. 2008;118:1301–1310. doi: 10.1172/JCI33945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schlein Y. Sandfly diet and Leishmania. Parasitol Today. 1986;2:175–177. doi: 10.1016/0169-4758(86)90150-x. [DOI] [PubMed] [Google Scholar]
- 3.Burchmore RJ, Barrett MP. Life in vacuoles--nutrient acquisition by Leishmania amastigotes. Int J Parasitol. 2001;31:1311–1320. doi: 10.1016/s0020-7519(01)00259-4. [DOI] [PubMed] [Google Scholar]
- 4.McConville MJ, de Souza D, Saunders E, Likic VA, Naderer T. Living in a phagolysosome; metabolism of Leishmania amastigotes. Trends Parasitol. 2007;23:368–375. doi: 10.1016/j.pt.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Naderer T, McConville MJ. The Leishmania-macrophage interaction: a metabolic perspective. Cell Microbiol. 2008;10:301–308. doi: 10.1111/j.1462-5822.2007.01096.x. [DOI] [PubMed] [Google Scholar]
- 6.Burchmore RJS, Landfear SM. Differential regulation of multiple glucose transporter genes in the parasitic protozoan Leishmania mexicana. J Biol Chem. 1998;273:29118–29126. doi: 10.1074/jbc.273.44.29118. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Contreras D, Feng X, Keeney KM, Bouwer HG, Landfear SM. Phenotypic characterization of a glucose transporter null mutant in Leishmania mexicana. Mol Biochem Parasitol. 2007;153:9–18. doi: 10.1016/j.molbiopara.2007.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manolescu AR, Witkowska K, Kinnaird A, Cessford T, Cheeseman C. Facilitated hexose transporters: new perspectives on form and function. Physiology (Bethesda) 2007;22:234–240. doi: 10.1152/physiol.00011.2007. [DOI] [PubMed] [Google Scholar]
- 9.ter Kuile BH. Glucose and proline transport in kinetoplastids. Parasitol Today. 1993;9:206–210. doi: 10.1016/0169-4758(93)90009-5. [DOI] [PubMed] [Google Scholar]
- 10.ter Kuile BH. Membrane-related processes and overall energy metabolism in Trypanosoma brucei and other kinetoplastid species. J Bioenerg Biomembr. 1994;26:167–172. doi: 10.1007/BF00763065. [DOI] [PubMed] [Google Scholar]
- 11.Zilberstein D, Dwyer D. Glucose transport in Leishmania donovani promastigotes. Mol Biochem Parasitol. 1984;12:327–336. doi: 10.1016/0166-6851(84)90089-6. [DOI] [PubMed] [Google Scholar]
- 12.Zilberstein D, Dwyer D. Proton force-driven active transport of D-glucose and L-proline in the protozoan parasite Leishmania donovani. Proc Natl Acad Sci USA. 1985;82:1716–1720. doi: 10.1073/pnas.82.6.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burchmore RJS, Hart DT. Glucose transport in promastigotes and amastigotes of Leishmania mexicana: characterization and comparison with host glucose transporters. Mol Biochem Parasitol. 1995;74:77–86. doi: 10.1016/0166-6851(95)02485-9. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee T, Mandal D, Bhaduri A. Leishmania plasma membrane Mg+2 ATPase is a H+/K+-antiporter involved in glucose symport. J Biol Chem. 2001;276:5563–5569. doi: 10.1074/jbc.M008469200. [DOI] [PubMed] [Google Scholar]
- 15.Langford CK, Kavanaugh MP, Stenberg PE, Drew ME, Zhang W, Landfear SM. Functional expression and subcellular localization of a high-Km hexose transporter from Leishmania donovani. Biochemistry. 1995;34:11814–11821. doi: 10.1021/bi00037a020. [DOI] [PubMed] [Google Scholar]
- 16.Drew ME, Langford CK, Klamo EM, Russell DG, Kavanaugh MP, Landfear SM. Functional expression of a myo-inositol/H+ symporter from Leishmania donovani. Mol Cell Biol. 1995;15:5508–5515. doi: 10.1128/mcb.15.10.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart DT, Coombs GH. Leishmania mexicana: energy metabolism of amastigotes and promastigotes. Exp Parasitol. 1982;54:397–409. doi: 10.1016/0014-4894(82)90049-2. [DOI] [PubMed] [Google Scholar]
- 18.Rainey PM, MacKenzie NE. A carbon-13 nuclear magnetic resonance analysis of the products of glucose metabolism in Leishmania pifanoi amastigotes and promastigotes. Mol Biochem Parasitol. 1991;45:307–316. doi: 10.1016/0166-6851(91)90099-r. [DOI] [PubMed] [Google Scholar]
- 19.Nasser MIA, Landfear SM. Sequences required for the flagellar targeting of an integral membrane protein. Mol Biochem Parasitol. 2004;135:89–100. doi: 10.1016/j.molbiopara.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Landfear SM, Ignatushchenko M. The flagellum and flagellar pocket of trypanosomatids. Mol Biochem Parasitol. 2001;115:1–17. doi: 10.1016/s0166-6851(01)00262-6. [DOI] [PubMed] [Google Scholar]
- 21.Johnston M, Kim JH. Glucose as a hormone: receptor-mediated glucose sensing in the yeast Saccharomyces cerevisiae. Biochem Soc Trans. 2005;33:247–252. doi: 10.1042/BST0330247. [DOI] [PubMed] [Google Scholar]
- 22.Burchmore RJS, Rodriguez-Contreras D, McBride K, Merkel P, Barrett MP, Modi G, Sacks DL, Landfear SM. Genetic characterization of glucose transporter function in Leishmania mexicana. Proc Natl Acad Sci U S A. 2003;100:3901–3906. doi: 10.1073/pnas.0630165100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng X, Rodriguez-Contreras D, Buffalo C, Bouwer A, Kruvand E, Beverley SM, Landfear SM. Amplification of an alternate transporter gene suppresses the avirulent phenotype of glucose transporter null mutants in Leishmania mexicana*. Mol Microbiol. 2008 doi: 10.1111/j.1365-2958.2008.06531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naderer T, Vince JE, McConville MJ. Surface determinants of Leishmania parasites and their role in infectivity of the mammalian host. Curr Mol Med. 2004;4:649–665. doi: 10.2174/1566524043360069. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Contreras D, Landfear SM. Metabolic changes in glucose transporter-deficient Leishmania mexicana and parasite virulence. J Biol Chem. 2006;281:20068–20076. doi: 10.1074/jbc.M603265200. [DOI] [PubMed] [Google Scholar]
- 26.Ralton JE, Naderer T, Piraino HL, Bashtannyk TA, Callaghan JM, McConville MJ. Evidence that intracellular {beta}1-2 mannan Is a virulence vactor in Leishmania parasites. J Biol Chem. 2003;278:40757–40763. doi: 10.1074/jbc.M307660200. [DOI] [PubMed] [Google Scholar]
- 27.Beverley SM. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- 28.Bringaud F, Baltz T. A potential hexose transporter gene expressed predominantly in the bloodstream form of Trypanosoma brucei. Mol Biochem Parasitol. 1992;52:111–122. doi: 10.1016/0166-6851(92)90040-q. [DOI] [PubMed] [Google Scholar]
- 29.Woodrow CJ, Penny JI, Krishna S. Intraerythrocytic Plasmodium falciparum expresses a high affinity facilitative hexose transporter. J Biol Chem. 1999;274:7272–7277. doi: 10.1074/jbc.274.11.7272. [DOI] [PubMed] [Google Scholar]
- 30.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 31.Feistel T, Hodson CA, Peyton DH, Landfear SM. An expression system to screen for inhibitors of parasite glucose transporters. Mol Biochem Parasitol. 2008;162:71–76. doi: 10.1016/j.molbiopara.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joet T, Eckstein-Ludwig U, Morin C, Krishna S. Validation of the hexose transporter of Plasmodium falciparum as a novel drug target. Proc Natl Acad Sci U S A. 2003;100:7476–7479. doi: 10.1073/pnas.1330865100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ionita M, Krishna S, Leo PM, Morin C, Patel AP. Interaction of O-(undec-10-en)-yl-d-glucose derivatives with the Plasmodium falciparum hexose transporter (PfHT) Bioorg Med Chem Lett. 2007;17:4934–4937. doi: 10.1016/j.bmcl.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 34.ter Kuile BH, Opperdoes FR. Glucose uptake by Trypanosoma brucei. J Biol Chem. 1991;266:857–862. [PubMed] [Google Scholar]
- 35.ter Kuile BH. Glucose uptake mechanisms as potential targets for drugs against trypanosomatids. In: Coombs GH, North M, editors. Biochemical Protozoology. Taylor and Francis; London and Washington: 1991. p. 635. [Google Scholar]
- 36.Bakker BM, Westerhoff HV, Opperdoes FR, Michels PA. Metabolic control analysis of glycolysis in trypanosomes as an approach to improve selectivity and effectiveness of drugs. Mol Biochem Parasitol. 2000;106:1–10. doi: 10.1016/s0166-6851(99)00197-8. [DOI] [PubMed] [Google Scholar]
- 37.Bayele HK. Triazinyl derivatives that are potent inhibitors of glucose transport in Trypanosoma brucei. Parasitol Res. 2001;87:911–914. doi: 10.1007/s004360100473. [DOI] [PubMed] [Google Scholar]
- 38.Azema L, Claustre S, Alric I, Blonski C, Willson M, Perie J, Baltz T, Tetaud E, Bringaud F, Cottem D, Opperdoes FR, Barrett MP. Interaction of substituted hexose analogues with the Trypanosoma brucei hexose transporter. Biochem Pharmacol. 2004;67:459–467. doi: 10.1016/j.bcp.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Eisenthal R, Game S, Holman GD. Specificity of hexose transport in Trypanosoma brucei. Biochim Biophys Acta. 1988;985:81–89. doi: 10.1016/0005-2736(89)90107-7. [DOI] [PubMed] [Google Scholar]
- 40.Munoz-Antonia T, Richards FF, Ullu E. Differences in glucose transport between bloodstream and procyclic forms of Trypanosoma brucei rhodesiense. Mol Biochem Parasitol. 1991;47:73–82. doi: 10.1016/0166-6851(91)90149-z. [DOI] [PubMed] [Google Scholar]
- 41.Seyfang A, Duszenko M. Specificity of glucose transport in Trypanosoma brucei: Effective inhibition by phloretin and cytochalasin B. Eur J Biochem. 1991;202:191–196. doi: 10.1111/j.1432-1033.1991.tb16362.x. [DOI] [PubMed] [Google Scholar]
- 42.Parsons M, Nielsen B. Active transport of 2-deoxy-D-glucose in Trypanosoma brucei. Mol Biochem Parasitol. 1990;42:197–204. doi: 10.1016/0166-6851(90)90162-f. [DOI] [PubMed] [Google Scholar]
- 43.Bringaud F, Baltz T. Differential regulation of two distinct families of glucose transporter genes in Trypanosoma brucei. Mol Cell Biol. 1993;13:1146–1154. doi: 10.1128/mcb.13.2.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrett MP, Tetaud E, Seyfang A, Brignaud F, Baltz T. Trypanosome glucose transporters. Mol Biochem Parasitol. 1998;91:195–205. doi: 10.1016/s0166-6851(97)00192-8. [DOI] [PubMed] [Google Scholar]
- 45.Barrett MP, Tetaud E, Seyfang A, Bringaud F, Baltz T. Functional expression and characterization of the Trypanosoma brucei procyclic glucose transporter, THT2. Biochem J. 1995;312:687–691. doi: 10.1042/bj3120687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winzeler EA. Malaria research in the post-genomic era. Nature. 2008;455:751–756. doi: 10.1038/nature07361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherman IW. Biochemistry of Plasmodium (malarial parasites) Microbiol Rev. 1979;43:453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roth EF., Jr Malarial parasite hexokinase and hexokinase-dependent glutathione reduction in the Plasmodium falciparum-infected human erythrocyte. J Biol Chem. 1987;262:15678–15682. [PubMed] [Google Scholar]
- 49.Sherman IW. The Wellcome Trust lecture. Mechanisms of molecular trafficking in malaria. Parasitology. 1988;96(Suppl):S57–81. doi: 10.1017/s003118200008598x. [DOI] [PubMed] [Google Scholar]
- 50.Tanabe K. Glucose transport in malaria infected erythrocytes. Parasitol Today. 1990;6:225–229. doi: 10.1016/0169-4758(90)90199-e. [DOI] [PubMed] [Google Scholar]
- 51.Kirk K, Saliba KJ. Targeting nutrient uptake mechanisms in Plasmodium. Curr Drug Targets. 2007;8:75–88. doi: 10.2174/138945007779315560. [DOI] [PubMed] [Google Scholar]
- 52.Becker K, Kirk K. Of malaria, metabolism and membrane transport. Trends Parasitol. 2004;20:590–596. doi: 10.1016/j.pt.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 53.Staines HM, Alkhalil A, Allen RJ, De Jonge HR, Derbyshire E, Egee S, Ginsburg H, Hill DA, Huber SM, Kirk K, Lang F, Lisk G, Oteng E, Pillai AD, Rayavara K, Rouhani S, Saliba KJ, Shen C, Solomon T, Thomas SL, Verloo P, Desai SA. Electrophysiological studies of malaria parasite-infected erythrocytes: current status. Int J Parasitol. 2007;37:475–482. doi: 10.1016/j.ijpara.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Desai SA, Krogstad DJ, McCleskey EW. A nutrient-permeable channel on the intraerythrocytic malaria parasite. Nature. 1993;362:643–646. doi: 10.1038/362643a0. [DOI] [PubMed] [Google Scholar]
- 55.Izumo A, Tanabe K, Kato M, Doi S, Maekawa K, Takada S. Transport processes of 2-deoxy-D-glucose in erythrocytes infected with Plasmodium yoelii, a rodent malaria parasite. Parasitology 98 Pt. 1989;3:371–379. doi: 10.1017/s0031182000061448. [DOI] [PubMed] [Google Scholar]
- 56.Kirk K, Horner HA, Kirk J. Glucose uptake in Plasmodium falciparum-infected erythrocytes is an equilibrative not an active process. Mol Biochem Parasitol. 1996;82:195–205. doi: 10.1016/0166-6851(96)02734-x. [DOI] [PubMed] [Google Scholar]
- 57.Goodyer ID, Hayes DJ, Eisenthal R. Efflux of 6-deoxy-D-glucose from Plasmodium falciparum-infected erythrocytes via two saturable carriers. Mol Biochem Parasitol. 1997;84:229–239. doi: 10.1016/s0166-6851(96)02802-2. [DOI] [PubMed] [Google Scholar]
- 58.Penny JI, Hall ST, Woodrow CJ, Cowan GM, Gero AM, Krishna S. Expression of substrate-specific transporters encoded by Plasmodium falciparum in Xenopus laevis oocytes. Mol Biochem Parasitol. 1998;93:81–89. doi: 10.1016/s0166-6851(98)00024-3. [DOI] [PubMed] [Google Scholar]
- 59.Woodrow CJ, Burchmore RJ, Krishna S. Hexose permeation pathways in Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci U S A. 2000;97:9931–9936. doi: 10.1073/pnas.170153097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin RE, Henry RI, Abbey JL, Clements JD, Kirk K. The ‘permeome’ of the malaria parasite: an overview of the membrane transport proteins of Plasmodium falciparum. Genome Biol. 2005;6:R26. doi: 10.1186/gb-2005-6-3-r26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seyfang A, Landfear SM. Substrate depletion upregulates uptake of myo-inositol, glucose and adenosine in Leishmania. Mol Biochem Parasitol. 1999;104:121–130. doi: 10.1016/s0166-6851(99)00138-3. [DOI] [PubMed] [Google Scholar]
- 62.Nugent PG, Karsani SA, Wait R, Tempero J, Smith DF. Proteomic analysis of Leishmania mexicana differentiation. Mol Biochem Parasitol. 2004;136:51–62. doi: 10.1016/j.molbiopara.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 63.Landfear SM. Drugs and transporters in kinetoplastid protozoa. Adv Exp Med Biol. 2008;625:22–32. doi: 10.1007/978-0-387-77570-8_3. [DOI] [PubMed] [Google Scholar]