Abstract
Most cellular phenomena of interest to mammalian biology occur within the context of living tissues and organisms. However, today’s most advanced tools for observing and manipulating cellular function – based on fluorescent or light-controlled proteins – work best in cultured cells, transparent model species or small, surgically accessed anatomical regions. Their reach into deep tissues and larger animals is limited by photon scattering. To overcome this limitation, we must design biochemical tools that interface with more penetrant forms of energy. For example, sound waves and magnetic fields easily permeate most biological tissues, allowing the formation of images and delivery of energy for actuation. These capabilities are widely used in clinical techniques such as diagnostic ultrasound, magnetic resonance imaging, focused ultrasound ablation and magnetic particle hyperthermia. Each of these modalities offers spatial and temporal precision that could be used to study a multitude of cellular processes in vivo. However, connecting these techniques to cellular functions such as gene expression, proliferation, migration and signaling requires the development of new biochemical tools that can interact with sound waves and magnetic fields as optogenetic tools interact with photons. Here, we discuss the exciting challenges this poses for biomolecular engineering, and provide examples of recent advances pointing the way to greater depth in in vivo cell biology.
Length scales for studying cellular function in vivo
Before discussing technologies for cellular imaging and control, it is useful to think about the length scales on which these techniques must operate. Consider three representative biological systems: the mammalian microbiome, the adaptive immune system and the brain (Figure 1a). A microbe’s life in the mammalian gastrointestinal tract is intricately linked to its location along the length of the tract, its radial position within the lumen, and its place within a microenvironment such as the colonic crypt1. These locations are associated with length scales of centimeters, millimeters and microns, respectively. Likewise, adaptive immunity is a multi-scale phenomenon. Antigen presentation and recognition occur at sub-micron immunological synapses while immune cel recruitment from blood and lymphoid organs, proliferation and regulatory signaling occu on the scale of millimeters to centimeters. Similarly, neura signaling is organized a length scales ranging from sub-micron synapses to millimeter-sized brain region and centimeter-scale axonal projections.
Figure 1 -. Modalities for in vivo imaging and control of cellular function.
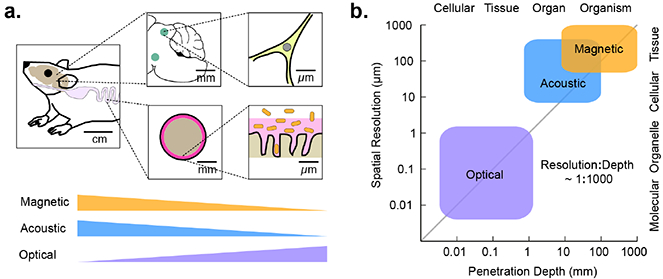
(a) Diagram of the length scales of several biological processes of interest in vivo, and the degree to which these length scales are accessible by imaging technologies. (b) Approximate length scales and maximal tissue penetration depths accessible by optical, acoustic, or magnetic imaging.
In all three systems, key biological questions involve the function of particular cell types within a certain spatially-defined anatomical context. For example, which microbes can successfully colonize the small intestine? Which genes do T-cells express after migrating into a tumor and recognizing a neoantigen? How does the activity of excitatory neurons in a certain part of the hippocampus relate to the development of seizures? Each of these questions involves ensemble cellular behaviors occurring on the millimeter scale, which are difficult to recapitulate in in vitro models. Studying biology at this scale complements the understanding gained by examining cells at the single-cell and sub-cellular level, and requires a dedicated set of experimental tools.
Forms of energy for biological imaging and control
The key elements of any technology for cellular imaging and control are the form of energy applied to or measured from the sample and the molecular mechanisms connecting this energy to a biological process of interest (Figure 1b). Since the work of van Leeuwenhoek, the dominant energy type used to study biological phenomena has been visible light, with modern microscopy taking advantage of an impressive array of molecular tools to optically visualize and perturb cellular processes. Unfortunately, visible light gets scattered within approximately one millimeter in most tissues, limiting its use to in vitro specimens and shallow or surgically accessed anatomical regions.
On the other hand, sound waves and magnetic fields are capable of penetrating deep into tissues. Ultrasound at MHz frequencies permeates through several centimeters, enabling imaging or focused energy deposition with a wavelength-dependent resolution down to approximately 100 μm2. This is further improved to below 10 pm with recently developed super-resolution techniques3. Due to this excellent performance, ultrasound imaging is widely used in the clinic and in pre-clinical research. In addition, ultrasound can be focused at depth to deliver mechanical forces or localized heating4. These capabilities are used clinically for non-invasive ablation of diseased tissues.
Likewise, magnetic fields experience minimal tissue attenuation. They can be used to produce high-contrast images of many organs by exploiting the context-dependent magnetic resonance behavior of nuclear spins, with a spatial resolution on the order of 100 μm. In addition, static or time-varying magnetic fields can produce mechanical forces or heat in tissues containing magnetic nanomaterials5, which can be localized in to the millimeter scale using field-free point scanning techniques6.
Based on their tissue penetration and spatiotemporal resolution, sound waves and magnetic fields are well-suited to imaging and controlling the function of cells in vivo (Figure 1b). All that is needed is a set of biomolecular tools that can link these forms of energy to specific cellular functions such as gene expression and signaling. Developing such tools presents an exciting challenge to biomolecular engineers. Just as the discovery of the green fluorescent protein stimulated the development of hundreds of reporters, sensors and actuators through creative protein engineering, recent developments in acoustically and magnetically active proteins may allow us to engineer a similar variety of biological tools for ultrasound and magnetic resonance. Initial inroads towards this goal are described in the following sections.
Biomolecular tools for ultrasound imaging
Diagnostic ultrasound uses the scattering of sound waves to delineate tissue boundaries, monitor the motion of organs such as the heart and quantify the velocity of blood flow (Figure 2a). Until recently, the prospect of using ultrasound to image the function of specific cells was remote due to the lack of suitable molecular reporters. Conventional ultrasound contrast agents are micron-sized synthetic bubbles that resonantly scatter sound waves. Although these microbubbles can be targeted to specific endovascular targets for molecular imaging in the bloodstream, their size and longterm instability makes it difficult to use them in labeling and monitoring the function of specific cells7. Alternatively, scattering synthetic nanoparticles have been explored as ultrasound contrast agents with the potential for cell labeling and extravascular interrogation8,9.
Figure 2 -. Biomolecular tools for ultrasound imaging.
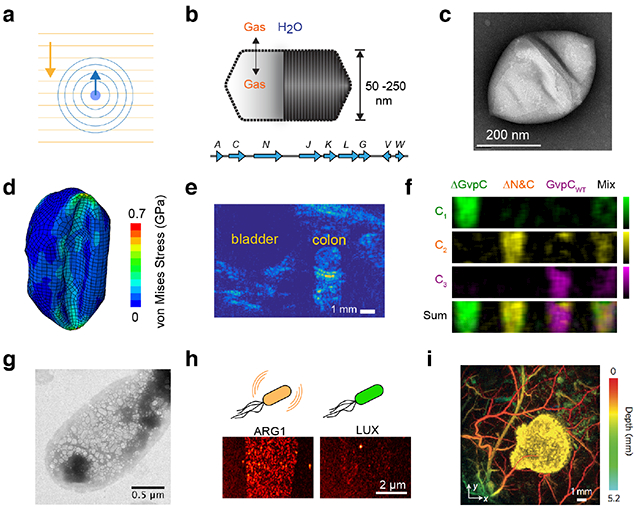
(a) Illustration of sound propagation in the imaging medium and received echo used to form the ultrasound image. (b) GVs are hollow protein nanostructures that freely allow diffusion of dissolved gas through their shell but exclude water11. GVs are encoded by operons consisting of 814 genes. (c) Representative transmission electron micrograph of purified GV from Halobacterium11. (d) Simulation illustrating nanoscale deformation of GVs under ultrasound leading to nonlinear backscattered echo12. (e) Amplitude-modulation pulse sequence reveal GVs in mouse colon13 (Reprinted from Maresca, D et al (2017). Nonlinear ultrasound imaging of nanoscale acoustic biomolecules. Applied Physics Letters, 110(7), 73704, with the permission of AIP Publishing). (f) Multiplexed imaging of genetically engineered GVs14. (g) Heterologous expression of GVs in E. coli using an optimized GV gene cluster15. (h) Ultrasound image of E. coli expressing GVs or the non-echogenic luminescence reporter, luciferase15. (i) Photoacoustic imaging of tumor expressing tyrosinase, and surrounding blood vessels52.
To connect ultrasound more closely with molecular and cellular biology, we recently adapted a unique class of gas-filled proteins, called gas vesicles or GVs, as the first biomolecular acoustic reporters. GVs evolved in aquatic photosynthetic microbes as a means to regulate buoyancy for optimal access to sunlight and other nutrients10. Despite their name, gas vesicles contain no lipids; they comprise a 2 nm-thick protein shell enclosing a hollow interior with dimensions on the order of 250 nm (Figure 2, b-c). Their shell allows gases dissolved in the surrounding media to freely permeate in and out of their interior, while their hydrophobic inner surface prevents the formation of a liquid aqueous phase. GVs are encoded by clusters of 8–14 genes, including two primary structural proteins and several minor constituents, chaperones and regulators.
In 2014, we showed that GVs can produce ultrasound contrast in purified form, inside cells and in vivo, establishing them as the first acoustic biomolecules11. Since this initial discovery, considerable advances have been made in understanding the acoustic properties of GVs and improving the ability of ultrasound to detect them with greater sensitivity and specificity. One key finding was that GVs undergo nanoscale buckling deformations under ultrasound (Figure 2d), resulting in non-linear scattering and allowing amplitude-modulated pulse sequences to detect GVs with greater specificity against background tissues (Figure 2e) in a process analogous to two-photon microscopy12,13. Another key finding was that the acoustic properties of GVs can be engineered at the genetic level. In particular, a key component of the GV shell called GvpC influences the response of GVs to pressure, setting thresholds for buckling and irreversible collapse14. Tuning GvpC at the genetic level enables modulation of GVs’ nonlinear signals, as well as multiplexed imaging of GV variants with differential pressure sensitivity14 (Figure 2f). Additionally, fusions of GvpC with other polypeptides enable the tailoring of GV surface properties such as charge or affinity for molecular imaging targets14.
A major effort is also underway to express GVs heterologously as genetically encoded reporters. As an initial target, we have developed genetic constructs to express GVs in model commensal and pathogenic microbes such as E. coli and S. typhimurium (Figure 2g)15. Imaging these and other microbes in mammalian hosts could enable new studies of the microbiome and the tracking of engineered probiotic therapies. Cells expressing the current generation of acoustic reporter genes can be visualized at densities below 108 cells/ml, corresponding to a volume fraction of 0.005% – a level compatible with imaging microbes in the GI tract or tumors (Figure 2h)15.
An alternative mechanism by which ultrasound can facilitate the visualization of cells in vivo is photoacoustic imaging, a technique wherein optical excitation is absorbed and converted into thermoelastic pressure waves, which are detected by ultrasound transducers16. This enables the use of light to image deeper structures because photons are allowed to scatter en route to their target, with spatial information provided by ultrasound. The major advantage of photoacoustic imaging compared to pure ultrasound is its ability to leverage existing molecular tools developed for optical imaging, including fluorescent proteins or light-absorbing pigments such as melanin (Figure 2i)17,18. However, this technique is still difficult to employ at depths beyond one to two centimeters without causing tissue phototoxicity.
Biomolecular tools for ultrasonic actuation
In addition to imaging, ultrasound can be used to deliver energy to focused regions of tissue, with targeting on the scale of a single millimeter. Depending on beam intensity and pulse duration, this energy can be used to apply mechanical forces, drive resonant cavitation of bubbles, or deposit heat (Figure 3a)4. These capabilities are used clinically for non-invasive surgery19. If they could instead be harnessed, at lower intensities, to modulate the activity of specific cells in vivo, this would facilitate the study of cellular function within relevant anatomical contexts.
Figure 3 -. Biomolecular tools for acoustic control.
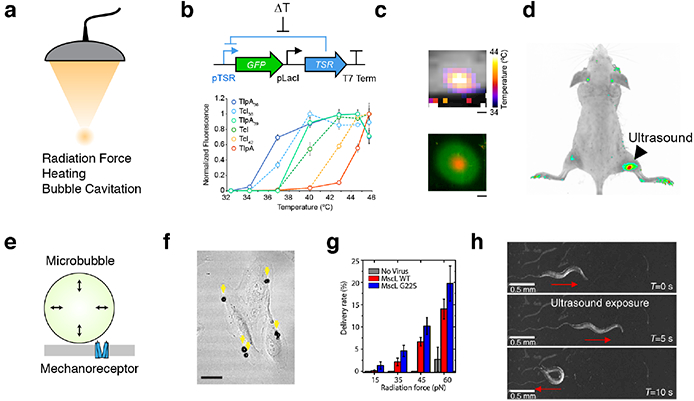
(a) Ultrasound can be focused at depth in tissue and apply several forms of energy to interface with cells. (b) Schematic of the gene circuit utilized to gate a GFP reporter gene with a temperature-sensitive repressor (TSR), and a panel of tuned variants of temperature-sensitive repressors22. (c) MRI thermometry imaging demonstrates a spatial temperature gradient induced by FUS on a plate of bacterial cells, resulting in spatially targeted gene expression22. (d) E. coli were injected into both hindlimbs of a nude mouse; after FUS application to the right hindlimb, reporter gene expression is significantly enriched at the site of heating22. (e) Diagram of mechanism by which microbubble cavitation can result in membrane deformation leading to mechanoreceptor activation. (f) Microbubbles attached to cultured retinal pigment epithelium cells23. (g) Uptake of membrane-impermeable dye into retinal pigment epithelium cells expressing MscL and functionalized with microbubbles23. (h) C. elegans worm motor response to ultrasound in a bath of microbubbles24.
Several nascent approaches have been proposed to enable this possibility. For example, the ability of ultrasound to controllably heat tissue within the well tolerated range of 37–42°C can be coupled to natural or engineered temperature-dependent signaling pathways. This approach has been used to remotely activate transcription driven by the heat shock promoter, pHSP70, in cultured mammalian cells and live mice20. While this approach is highly effective in certain contexts, the thermal set-point and activity level of heat shock promoters varies between cell types, is not easily tunable, and responds to other stimuli in addition to temperature21.
In bacteria, endogenous heat shock promoters have only modest activation in response to this range of temperatures, necessitating the development of engineered thermal bioswitches. To address this need, we recently introduced two families of orthogonal, tunable temperature-dependent transcriptional repressors for remote control of bacterial function22. These bioswitches are based on the TlpA transcriptional repressor from S. typhimurium and a variant of the cI repressor from the Lambda bacteriophage (TcI). Unlike the ~ 10-fold thermal induction of heat shock promoters, the expression of genes downstream of TlpA and TcI operators is turned on by more than 100-fold in response to mild heating. We showed that TlpA and TcI can be engineered through directed evolution to actuate at different desired temperatures, as required by a given application (Figure 3b). In addition, they can be used in combination to build thermal logic circuits, for example to turn on two different functions at two different temperatures. We have demonstrated that these switches can be used to spatially pattern gene expression in plated bacterial cells (Figure 3c) and also in bacteria implanted in vivo (Figure 3d).
Besides heating, ultrasound is also able to apply mechanical forces to tissues. These forces, which are amplified by acoustically active structures such as microbubbles, could be coupled to signaling elements such as mechanosensitive ion channels, allowing non-invasive control of cellular signaling. This concept was recently demonstrated in vitro by combining synthetic microbubbles with mammalian cells heterologously expressing the E. coli mechanosensitive ion channel MscL23. Microbubbles were similarly used to stimulate the endogenous mechanosensor Trp4 in C. elegans24. Unlike thermal stimuli, which are typically associated with timescales on the order of seconds, mechanical effects can be produced on the order of milliseconds, potentially allowing more rapid control of cellular signaling. However, techniques requiring microbubbles are limited in their application to mammals due to the difficulty of delivering bubbles to relevant tissues.
In addition to directly controlling cellular function, ultrasound can be used to spatially target the delivery of genetically encoded tools or treatments. In the brain, such delivery can be targeted non-invasively by opening the blood-brain barrier reversibly at a specific location using focused ultrasound and intravascular microbbubles25. This technique allows the delivery of adeno-associated virus (AAV) vectors to targeted regions with millimeter precision26.
Biomolecular tools for magnetic resonance imaging
Like ultrasound, MRI derives contrast from both, endogenous variation in the properties of tissue and molecular contrast agents. Taking advantage of the rich behavior of nuclear spins under various physical and chemical conditions has enabled the development of several classes of biomolecular MRI reporters27,28. One major class comprises proteins that contain paramagnetic metals, such as iron or manganese, or lead to the accumulation of these ions in tissue. Proteins in this class include ferritin, bacterial cytochromes, the transferrin receptor and other transporters (Figure 4, a-b). Paramagnetic species in these proteins produce T1 contrast through spin exchange of coordinated water protons and T2 contrast by distorting the magnetic field near the protein. Another class of reporters includes proteins with large numbers of exchangeable protons – the nuclear spin most commonly imaged with MRI. These protein-bound protons resonate at a distinct frequency (chemical shift) relative to water-bound protons, and can be selectively saturated with radiofrequency pulses, quenching their MRI signal. By applying such saturation while these protons exchange rapidly with the aqueous pool, the signal of the entire pool can be substantially reduced. This “catalytic” contrast scheme is called chemical exchange saturation transfer, or CEST. Proteins detectable with this method include a synthetic lysine-rich protein29 and human protamine30.
Figure 4 -. Biomolecular tools for magnetic resonance imaging.
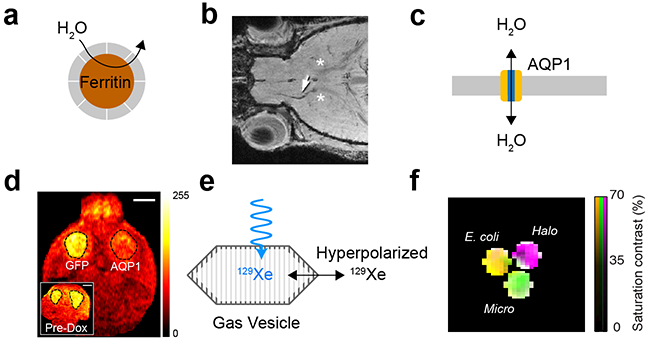
(a) Metalloproteins interact magnetically with aqueous 1H nuclear spins, leading to T1 or T2 MRI contrast. (b) Migrating neuroblasts expressing ferritin produce a hypointense track (arrow) in T2 weighted MRI53. Asterisks denote adenovirus injection sites. (c) Overexpression of aquaporin enhances passive diffusion of water across the cell membrane, resulting in contrast on diffusion weighted MRI. (d) AQPi expression in mouse xenograft shows significant contrast compared to contralateral GFP expressing xenograft after expression is induced with doxycycline31. (e) GVs interact with hyperpolarized xenon dissolved in biological media, producing contrast in 129Xe MRI. (f) Genetically distinct GVs produce different chemical shifts in 129Xe MRI, enabling multiplexed imaging34.
While these pioneering reporter types have been used to demonstrate the imaging of genetically defined cells using MRI, they are generally limited by their low molecular sensitivity (requiring protein concentrations on the order of μΜ) or the requirement of metal cofactors, which may not always be bioavailable. Recent efforts have therefore been focused on developing alternative classes of reporters that are more sensitive and do not require metals. For example, we recently introduced aquaporin 1 as a biomolecular reporter for MRI based on its ability to enhance the diffusion of water across cell membranes (Figure 4c)31. Aquaporins are transmembrane channels that passively conduct water with exquisite selectivity at rates of up to one billion water molecules per channel per second. We showed that the overexpression of this autologous, non-toxic, metal-free molecule produces contrast in diffusion-weighted MRI at concentrations below 500 nM, allowing non-invasive imaging of gene expression in vitro and in vivo (Figure 4d). In addition to aquaporin 1, other water-permeable channels such as the urea transporter can produce diffusion-based contrast, albeit with lower channel selectivity32.
To push the molecular and cellular sensitivity of MRI even further, recent work has focused on directly addressing a fundamental physical limitation of conventional magnetic resonance: the weak magnetic alignment of nuclear spins under thermal equilibrium. This low polarization results in overall MRI signals approximately 105 times weaker than they could be if all the available spins aligned with the applied magnetic field. This limitation can be overcome with hyperpolarization – an advanced technique in which nuclei are prepared via physical methods in a state of non-equilibrium polarization that is up to 10,000-fold stronger than baseline33. Hyperpolarized nuclei such as the noble gas 129Xe can then be delivered to the body by inhalation to be imaged during their polarization half-life of a few seconds. Because each hyperpolarized atom carries a much stronger signal than thermally polarized molecules, MRI reporters acting on these nuclei are detectable at much lower concentrations than their conventional counterparts. The first biomolecular reporters for hyperpolarized xenon MRI were GVs, the aforementioned gas-filled protein nanostructures. GVs allow xenon dissolved in surrounding media to exchange in and out of their gaseous compartment, producing MRI contrast through CEST at picomolar concentrations (Figure 4, e-f)34. Other proteins active as contrast agents for 129Xe MRI and other hyperpolarized nuclei have also been reported35,36.
In addition to reporters connected to gene expression, biomolecules have also been engineered as MRI sensors – allowing dynamic tracking of processes such as neurotransmission and kinase signaling. One class of such sensors, inspired by pioneering synthetic approaches37, comprises iron-containing metalloproteins in which the accessibility of an open iron coordination site to water is modulated by the binding of small molecules, thereby altering T1 contrast38. Directed evolution allows the tuning of this small molecule binding site for selective interactions with neurotransmitters, such as dopamine and serotonin. The resulting reporters have been used to image the dynamics of neurotransmitter release and reuptake in rodent brains39. Other biomolecular sensor constructs, based on T2 and CEST mechanisms, have been developed to image signals such as kinase and protease activity40.
Biomolecular tools for magnetic control
Magnetic fields exert forces on magnetically active materials such as superparamagnetic and ferromagnetic particles5. Depending on the particle type, these forces can be sufficient to guide the movement of materials or cells in the body and actuate receptor signaling (Figure 5a). In addition, rapidly alternating magnetic fields can generate heat in particles whose magnetization oscillates with the applied field, which in turn can be used to control temperature-dependent processes (Figure 5b).
Figure 5 -. Biomolecular tools for magnetic control.
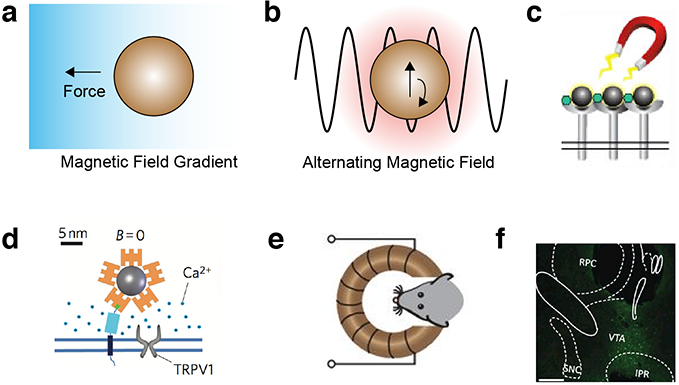
(a) Magnetic field gradients exert a force on magnetic particles. (b) Alternating magnetic fields heat magnetic particles by inducing oscillations in the magnetic moment of the nanoparticle. (c) Magnetic fields can induce the clustering of magnetic particles, and receptors to which they are bound41. (d) Coupling magnetic nanoparticles to the heat-sensitive ion channel TRPV1 enables magnetic control of calcium influx to the cell42. (e) Remote deep brain stimulation using alternating magnetic fields applied to mice with implanted magnetic nanoparticles and virally transduced TRPV143. (f) Expression of the neural activity marker cFos in the ventral tegmental area (VTA) of the mouse brain after injection with TRPV1-encoding virus, magnetic nanoparticles and the application of alternating field stimulation43.
Most strategies for magnetic control of cell function have relied on synthetic magnetic nanoparticles as transducers of the magnetic field. For example, superparamagnetic iron oxide nanoparticles have been used to cluster cell surface receptors or apply directly actuating forces on integrin and notch41 (Figure 5c). In addition, superparamagnetic particles have been used in combination with alternating fields to activate temperature-sensitive ion channels such as TRPV142 (Figure 5d). This approach enabled remote control of neural signaling in vivo in mice surgically implanted with such particles43 (Figure 5, e-f). Additionally, cells containing iron oxide particles have been concentrated at certain locations in vivo44,45.
Translating these approaches into more versatile, fully-genetic constructs is challenging due to the unsolved problem of heterologous biosynthesis of strongly magnetic nanomaterials. Although superparamagnetic and ferromagnetic iron oxide nanocrystals are made by magnetotactic bacteria46, the genes encoding their specialized organelle machinery for such synthesis have so far been transferred only to their close genetic relatives. The magnetic nanostructures formed in commensal microbes and mammalian cells, such as ferritin, tend to be paramagnetic or weakly superparamagnetic. Efforts to increase the magnetic strength of ferritin through genetic engineering have so far come up short of qualitatively altering its magnetic character.
Despite this physical limitation, some groups have reported that fusions of ferritin to temperature- and mechanically-sensitive ion channels allow neurons expressing these channels to be activated remotely using both alternating and static magnetic fields47,48. These reports are somewhat surprising based on classical theoretical estimates of the forces and temperatures that could be produced by ferritin49. However, it is possible that as-yet unknown alternative mechanisms are at play.
Outlook
The development of biomolecular tools for non-invasive cellular imaging and control is in its infancy. Inspired by the history and impact of fluorescent and optogenetic proteins, many opportunities exist to develop acoustic and magnetic technologies connected to a variety of cellular processes. For example, biomolecular ultrasound imaging is a new field animated 3 years ago with the development of GVs as its first biomolecular reporter. Much remains to be learned about the acoustic properties of these molecules and how they can be tuned at the genetic level to increase imaging sensitivity, or engineered to respond dynamically as sensors of cellular signaling. In addition, more work is needed to transfer the machinery encoding GVs to a greater number of species. In particular, expressing GVs in mammalian cells represents a major unsolved challenge in genetic engineering, given the need to functionally transfer a large operon driving the self-assembly of a complex macromolecular structure between two domains of life. This work takes place against a backdrop of other exciting developments in ultrasound, exemplified by super-resolution imaging3 and the recent invention of functional ultrasound (fUS), a technique for imaging neural activity non-invasively with improved spatiotemporal resolution compared to functional MRI (< 100 μm and < 10 ms) using transducers that can be mounted on freely moving animals50.
Biomolecular MRI is only slightly more mature, with an expanding variety of contrast mechanisms but no clear frontrunner to become the go-to molecule for in vivo cellular imaging. Recently developed aquaporin-based reporter genes offer unique advantages in terms of their simplicity and biocompatibility, while GVs have the potential to bring the advantages of hyperpolarization to boosting the in vivo sensitivity of cellular MRI. Engineering both of these molecules and accompanying in vivo imaging methods for maximum sensitivity and potential use as dynamic sensors are major avenues for future research. In addition, an outstanding grand challenge is the engineering of heterologous magnetite biosynthesis, which would provide powerful MRI contrast, as well as opportunities for actuation. In parallel with these molecular efforts, progress is being made on improving the information content of MRI images. For example, we recently used nitrogen vacancy diamond magnetometry, an optical technique for imaging magnetic fields, to map the nanoscale magnetic field in cells containing iron oxide nanoparticles and connect these maps to the T2 contrast seen by MRI51. This study demonstrated experimentally that the spatial arrangement of magnetic materials inside cells strongly influences contrast, guiding the development of magnetic cellular reporters and sensors and imaging parameters for their specific identification in vivo.
Complementing these evolving imaging technologies, much additional work is needed on genetically encodable agents to control cellular responses with acoustic or magnetic energy. For example, there is a lack of mammalian thermal bioswitches that are orthogonal to pleotropic heat shock pathways and tunable to different temperature thresholds analogously to the system we developed for microbial remote control. In addition to regulating gene expression, tools are needed to connect thermal inputs directly to signaling pathways. Similarly, for ultrasound actuation based on mechanical forces, use in mammals will require eliminating the need for synthetic microbubbles to produce constructs that can be fully genetically encoded. Likewise, the synthetic magnetic particles uses in well-accepted magnetic control techniques using thermal, mechanical or clustering mechanisms must be replaced with genetically encodable materials. The potential use of ferritin for this purpose requires further study to reconcile its encouraging empirical performance and predicted lack of efficacy based on previously studied physical properties.
In summary, going deeper into the body to study cellular function within its native in vivo context requires engineering interactions between deeply penetrant forms of energy and biomolecules to enable non-invasive imaging and control. Several recent advances have provided exciting proofs or concept for this approach, and inform the development of new classes of biomolecular tools. Many depths remain to be plumbed.
ACKNOWLEDGEMENTS
We thank members of the Shapiro Laboratory for helpful discussions. Related work in the Shapiro laboratory is also supported by the Heritage Medical Research Institute, the National Institutes of Health, the Defense Advanced Research Projects Agency, the Jacobs Institute for Molecular Engineering in Medicine, the Caltech Center for Environmental Microbial Interactions, the Human Frontiers Science Program, the Burroughs Wellcome Fund, the Pew Scholarship in the Biomedical Sciences, the Sontag Foundation, the Packard Fellowship for Science and Engineering. D.M. is supported by the Human Frontiers Science Program Cross Disciplinary Postdoctoral Fellowship (Award No. LT000637/ 2016). A.F. is supported by the Natural Sciences and Engineering Research Council of Canada PGSD.
Footnotes
AUTHOR CONTRIBUTIONS
All authors wrote the manuscript.
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- (1).Donaldson GP, Lee SM, and Mazmanian SK (2015) Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol 14, 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, and Turnbull DH (2000) Advances in ultrasound biomicroscopy. Ultrasound Med. Biol 26, 1–27. [DOI] [PubMed] [Google Scholar]
- (3).Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, and Tanter M (2015) Ultrafast ultrasound localization microscopy for deep super-resolution vascular imaging. Nature 527, 499–502. [DOI] [PubMed] [Google Scholar]
- (4).Humphrey VF (2007) Ultrasound and matter-Physical interactions. Prog. Biophys. Mol. Biol 93, 195–211. [DOI] [PubMed] [Google Scholar]
- (5).Pankhurst QA, Connolly J, Jones SK, and Dobson J (2003) Applications of magnetic nanoparticles in biomedicine. J. Phys. D. Appl. Phys 36, R167–R181. [Google Scholar]
- (6).Tasci TO, Vargel I, Arat A, Guzel E, Korkusuz P, and Atalar E (2009) Focused RF hyperthermia using magnetic fluids. Med. Phys 36, 1906–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Abou-Elkacem L, Bachawal SV, and Willmann JK (2015) Ultrasound molecular imaging: Moving toward clinical translation. Eur. J. Radiol 84, 1685–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sheeran PS, Luois SH, Mullin LB, Matsunaga TO, and Dayton PA (2012) Design of ultrasonically-activatable nanoparticles using low boiling point perfluorocarbons. Biomaterials 33, 3262–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Chen F, Ma M, Wang J, Wang F, Chern S-X, Zhao ER, Jhunjhunwala A, Darmadi S, Chen H, and Jokerst JV (2017) Exosome-like silica nanoparticles: a novel ultrasound contrast agent for stem cell imaging. Nanoscale 9, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Pfeifer F (2012) Distribution, formation and regulation of gas vesicles. Nat. Rev. Microbiol 10, 705–715. [DOI] [PubMed] [Google Scholar]
- (11).Shapiro MG, Goodwill PW, Neogy A, Yin M, Foster FS, Schaffer DV, and Conolly SM (2014) Biogenic gas nanostructures as ultrasonic molecular reporters. Nat. Nanotechnol 9, 311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Cherin E, Melis JM, Bourdeau RW, Yin M, Kochmann DM, Foster FS, and Shapiro MG (2017) Acoustic behavior of Halobacterium salinarum gas vesicles in the high frequency range: experiments and modeling. Ultrasound Med. Biol 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Maresca D, Lakshmanan A, Lee-Gosselin A, Melis JM, Ni Y-L, Bourdeau RW, Kochmann DM, and Shapiro MG (2017) Nonlinear ultrasound imaging of nanoscale acoustic biomolecules. Appl. Phys. Lett 110, 73704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lakshmanan A, Farhadi A, Nety SP, Lee-Gosselin A, Bourdeau RW, Maresca D, and Shapiro MG (2016) Molecular Engineering of Acoustic Protein Nanostructures. ACS Nano 10, 7314–7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Bourdeau R, Lee-Gosselin A, Lakshmanan A, Kumar S, Farhadi A, and Shapiro M Acoustic reporter genes for non-invasive imaging of microbes in mammalian hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Wang LV, and Yao J (2016) A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 13, 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Jathoul AP, Laufer J, Ogunlade O, Treeby B, Cox B, Zhang E, Johnson P, Pizzey AR, Philip B, Marafioti T, Lythgoe MF, Pedley RB, Pule MA, and Beard P (2015) Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photonics 9, 239–246. [Google Scholar]
- (18).Yao J, Kaberniuk AA, Li L, Shcherbakova DM, Zhang R, Wang L, Li G, Verkhusha VV, and Wang LV (2015) Multiscale photoacoustic tomography using reversibly switchable bacterial phytochrome as a near-infrared photochromic probe. Nat. Methods 13, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Hynynen K (2011) MRIgHIFU: A tool for image-guided therapeutics. J. Magn. Reson. Imaging 34, 482–493. [DOI] [PubMed] [Google Scholar]
- (20).Kruse DE, Mackanos M. a, O’Connell-Rodwell CE, Contag CH, and Ferrara KW (2008) Short-duration-focused ultrasound stimulation of Hsp70 expression in vivo. Phys. Med. Biol 53, 3641–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Aït-Aïssa S, Porcher JM, Arrigo AP, and Lambré C (2000) Activation of the hsp70 promoter by environmental inorganic and organic chemicals: Relationships with cytotoxicity and lipophilicity. Toxicology 145, 147–157. [DOI] [PubMed] [Google Scholar]
- (22).Piraner DI, Abedi MH, Moser BA, Lee-Gosselin A, and Shapiro MG (2016) Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem. Biol 13, 75–80. [DOI] [PubMed] [Google Scholar]
- (23).Heureaux J, Chen D, Murray VL, Deng CX, and Liu AP (2014) Activation of a bacterial mechanosensitive channel in mammalian cells by cytoskeletal stress. Cell. Mol. Bioeng 7, 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Ibsen S, Tong A, Schutt C, Esener S, and Chalasani SH (2015) Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans. Nat. Commun 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Hynynen K, McDannold N, Vykhodtseva N, and Jolesz F. a. (2001) Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology 220, 640–646. [DOI] [PubMed] [Google Scholar]
- (26).Poon C, McMahon D, and Hynynen K (2016) Noninvasive and targeted delivery of therapeutics to the brain using focused ultrasound. Neuropharmacology 4, 519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gilad AA, Ziv K, Mcmahon MT, Van Zijl PCM, Neeman M, Bulte JWM, and Kennedy HM (2008) MRI Reporter Genes. Ɉ Nucl Med 49, 1905–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Mukherjee A, Davis HC, Ramesh P, Lu GJ, and Shapiro MG (2017) Biomolecular MRI Reporters: evolution of new mechanisms. Prog. Nucl. Magn. Reson. Spectrosc [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Farrar CT, Buhrman JS, Liu G, Kleijn A, Lamfers MLM, McMahon MT, Gilad AA, and Fulci G (2015) Establishing the Lysine-rich Protein CEST Reporter Gene as a CEST MR Imaging Detector for Oncolytic Virotherapy. Radiology 275, 746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Oskolkov N, Bar-Shir A, Chan KWY, Song X, Van Zijl PCM, Bulte JWM, Gilad AA, and McMahon MT (2015) Biophysical characterization of human protamine-1 as a responsive CEST MR contrast agent. ACS Macro Lett 4, 34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Mukherjee A, Wu D, Davis HC, and Shapiro MG (2016) Non-invasive imaging using reporter genes altering cellular water permeability. Nat. Commun 7, 13891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Schilling F, Ros S, Hu D-E, D’Santos P, McGuire S, Mair R, Wright AJ, Mannion E, Franklin RJM, Neves AA, and Brindle KM (2016) MRI measurements of reporter-mediated increases in transmembrane water exchange enable detection of a gene reporter. Nat. Biotechnol 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Barskiy DA, Coffey AM, Nikolaou P, Mikhaylov DM, Goodson BM, Branca RT, Lu GJ, Shapiro MG, Telkki V, Zhivonitko VV, Koptyug IV, Salnikov OG, Kovtunov KV, Bukhtiyarov VI, Rosen MS, Barlow MJ, Safavi S, Hall IP, Schröder L, and Chekmenev EY (2016) NMR Hyperpolarization Techniques of Gases. Chem. - A Eur. Ɉ 725–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Shapiro MG, Ramirez RM, Sperling LJ, Sun G, Sun J, Pines A, Schaffer DV, and Bajaj VS (2014) Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nat. Chem 6, 629–34. [DOI] [PubMed] [Google Scholar]
- (35).Wang Y, Roose BW, Palovcak EJ, Carnevale V, and Dmochowski IJ (2016) A Genetically Encoded β-Lactamase Reporter for Ultrasensitive 129Xe NMR in Mammalian Cells. Angew. Chemie - Int. Ed 55, 8984–8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Patrick PS, Kettunen MI, Tee S-S, Rodrigues TB, Serrao E, Timm KN, McGuire S, and Brindle KM (2015) Detection of transgene expression using hyperpolarized 13 C urea and diffusion-weighted magnetic resonance spectroscopy. Magn. Reson. Med 73, 1401–1406. [DOI] [PubMed] [Google Scholar]
- (37).Li WH, Fraser SE, and Meade TJ (1999) A calcium-sensitive magnetic resonance imaging contrast agent. J. Am. Chem. Soc 121, 1413–1414. [Google Scholar]
- (38).Shapiro MG, Westmeyer GG, Romero P. a, Szablowski JO, Kuster B, Shah A, Otey CR, Langer R, Arnold FH, and Jasanoff A (2010) Directed evolution of a magnetic resonance imaging contrast agent for noninvasive imaging of dopamine. Nat. Biotechnol 28, 264–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Lee T, Cai LX, Lelyveld VS, Hai A, and Jasanoff A (2014) Molecular-level functional magnetic resonance imaging of dopaminergic signaling. Science 344, 533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Airan RD, Bar-Shir A, Liu G, Pelled G, McMahon MT, Van Zijl PCM, Bulte JWM, and Gilad AA (2012) MRI biosensor for protein kinase A encoded by a single synthetic gene. Magn. Reson. Med 68, 1919–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, and Ingber DE (2008) Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol 3, 36–40. [DOI] [PubMed] [Google Scholar]
- (42).Huang H, Delikanli S, Zeng H, Ferkey DM, and Pralle A (2010) Remote control of ion channels and neurons through magnetic-field heating of nanoparticles. Nat. Nanotechnol 5, 602–606. [DOI] [PubMed] [Google Scholar]
- (43).Chen R, Romero G, Christiansen MG, Mohr A, and Anikeeva P (2015) Wireless magnetothermal deep brain stimulation. Science 347, 1477–80. [DOI] [PubMed] [Google Scholar]
- (44).Muthana M, Kennerley AJ, Hughes R, Fagnano E, Richardson J, Paul M, Murdoch C, Wright F, Payne C, Lythgoe MF, Farrow N, Dobson J, Conner J, Wild JM, and Lewis C (2015) Directing cell therapy to anatomic target sites in vivo with magnetic resonance targeting. Nat. Commun 6, 8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Felfoul O, Mohammadi M, Taherkhani S, de Lanauze D, Zhong Xu Y, Loghin D, Essa S, Jancik S, Houle D, Lafleur M, Gaboury L, Tabrizian M, Kaou N, Atkin M, Vuong T, Batist G, Beauchemin N, Radzioch D, and Martel S (2016) Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions. Nat. Nanotechnol 11, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Faivre D, and Schuler D (2008) Magnetotactic Bacteria and Magnetosomes. Chem. Rev 108, 4875–4898. [DOI] [PubMed] [Google Scholar]
- (47).Stanley SA, Sauer J, Kane RS, Dordick JS, and Friedman JM (2015) Remote regulation of glucose homeostasis in mice using genetically encoded nanoparticles. Nat Med 21, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Wheeler MA, Smith CJ, Ottolini M, Barker BS, Purohit AM, Grippo RM, Gaykema RP, Spano AJ, Beenhakker MP, Kucenas S, Patel MK, Deppmann CD, and Guler AD (2016) Genetically targeted magnetic control of the nervous system. Nat Neurosci 19, 756–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Meister M (2016) Physical limits to magnetogenetics. Elife 5, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Sieu L-A, Bergel A, Tiran E, Deffieux T, Pernot M, Gennisson J-L, Tanter M, and Cohen I (2015) EEG and functional ultrasound imaging in mobile rats. Nat Methods 12, 831–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Davis HC, Ramesh P, Bhatnagar A, Lee-gosselin A, Barry JF, and David R (2016) Mapping the Microscale Origins of MRI Contrast with Subcellular NV Diamond Magnetometry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Jathoul AP, Laufer J, Ogunlade O, Treeby B, Cox B, Zhang E, Johnson P, Pizzey AR, Philip B, Marafioti T, Lythgoe MF, Pedley RB, Pule MA, and Beard P (2015) Deep in vivo photoacoustic imaging of mammalian tissues using a tyrosinase-based genetic reporter. Nat. Photonics 9, 239–246. [Google Scholar]
- (53).Iordanova B, and Ahrens ET (2012) In vivo magnetic resonance imaging of ferritin-based reporter visualizes native neuroblast migration. Neuroimage 59, 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]


