Abstract
Nutrient sensing is a mechanism for organisms to sense their environment. In larger animals, including humans, the intestinal tract is a major site of nutrient sensing for the body, not surprisingly, as this is the central location where nutrients are absorbed. In the gut, bacterial fermentation results in generation of short chain fatty acids (SCFAs), a class of nutrients, which are sensed by specific membrane bound receptors, FFA2, FFA3, GPR109a, and Olfr78. These receptors are expressed uniquely throughout the gut and signal through distinct mechanisms. To date, the emerging data suggests a role of these receptors in normal and pathological conditions. The overall function of these receptors is to regulate aspects of intestinal motility, hormone secretion, maintenance of the epithelial barrier, and immune cell function. Besides in intestinal health, a prominent role of these receptors has emerged in modulation of inflammatory and immune responses during pathological conditions. Moreover, these receptors are being revealed to interact with the gut microbiota. This review article updates the current body of knowledge on SCFA sensing receptors in the gut and their roles in intestinal health and disease as well as in whole body energy homeostasis.
Introduction
“Man is what he eats”
—(Ludwig Feuerbach, 1826)
As an interpretation of Feuerbach’s words, a profound association of diet with health of the body exists. The intestine with its specialized architecture functions to digest and absorb nutrients (211). It also sends messages to the body to relay information on the metabolic processes occurring in the gut. This role of the intestine is facilitated by an assortment of molecular sensors that provide feedback about the environment to the host (202). Undoubtedly, some of these molecular sensors have been shown to be specific to nutrients (202). Nutrients, including carbohydrates, amino acids, and fatty acids, which are essential not only for growth and sustenance, also interact with metabolic and immune systems regulating multiple aspects of health of the host (80). The nutrients interact with these systems through trans-membrane or intracellular molecules, which then transduce “the detection event” to specific cellular responses (152).
While sensing the environment is an important survival technique for single cell organisms, which allows the cell to process cues for self-protection and nutrient availability, this mechanism has continued into more complex organisms including mammals. Most mammalian cells are now known to employ multiple nutrient sensing mechanisms for proper cellular function (106). One type of nutrient sensors, the nutrient sensing G-protein coupled receptor(s) (GPCR), are a versatile class with regards to ligand diversity, ability to act via varied signaling pathways, and widespread tissue expression (152). Over the past 20 years, receptors for almost every nutrient have been revealed (Table 1) (25). The discovery of these GPCRs has dramatically changed our understanding of how the body responds to nutrients, and has heralded great efforts, as these receptors are excellent drug targets (25). An understanding of their signaling mechanisms through G-proteins restricted to cell membranes, and more recently described, their intracellular endosomal signaling has revealed insights into not only how cells sense nutrients (or other molecules like hormones) but also into the extensive roles these receptors play in regulating cell physiology.
Table 1.
Classification of Nutrient Sensing GPCRs by Nutrient Type
| Nutrient | Cognate GPCR |
|---|---|
| Fatty acids (long) | GPR120, GPR40 |
| Fatty acids (medium) | GPR120, GPR109b, GPR84, GPR40 |
| Fatty acids (short) | FFA2, FFA3, GPR109a, OLFR78 |
| Amino acids | GCN2, T1R1 and T1R3, GRM5 GPRC6A, GPR109b |
| Carbohydrates | T1R2 and T1R3 |
While these receptors are expressed broadly, as expected, many of these nutrient-sensing GPCRs are present in intestinal cells (217), as this is the main site for processing and absorption of nutrients. Additionally, these gut nutrient sensing GPCRs have been shown to contribute to gut hormone secretion (129). Among the various nutrient-sensing GPCRs of the intestine, one class responding to free fatty acids (FFA) is the FFA sensing receptor (FFAR) class. FFA ligands for these receptors range in size, from short, medium to long carbon chain length, ranging from C = 2 to 6, C = 6 to 12, and C > 12, respectively (Table 1). This FFAR class is currently comprised of four members, receptors binding long chain fatty acids, free fatty acid receptor 1, FFA1 (previously GPR40) and FFA4 (previously GPR120) and receptors binding short chain fatty acids, FFA2 (previously GPR43) and FFA3 (previously GPR41) (Table 1) (3). Another GPCR, GPR84 reported to bind medium chain fatty acids (carbon chain length ranging from C = 6 to C = 12) lacks sufficient data to be considered as FFA5 and is still classified as orphan (213). The long chain fatty acids like palmitoleic and linoleic acids, which activate FFA1 and FFA4, are either diet derived or produced endogenously during fatty acid metabolism (205). While sensing long chain fatty acids, both of these long chain fatty acid receptors, can enhance insulin secretion by activity on pancreatic beta (β) cell (FFA1) and influence incretin secretion from enteroendocrine cells (FFA1 and FFA4) (61, 88, 113), and thus, modulate whole body energy utilization.
The short chain fatty acids (SCFAs), the ligands of FFA2 and FFA3, are distinct given the nature of their origin. SCFAs are largely thought to be produced by the fermentation of ingested complex carbohydrates by intestinal bacteria (98, 110). The SCFAs are sensed by these receptors, and through this interaction, modulate a variety of physiological and hormonal processes that contribute to whole body energy sensing (33, 98, 152). Consequently, SCFA-cognate receptor signaling presents a novel intestinal (gut) relationship to the intestinal function and to the rest of the body, primarily through hormonal secretion. In addition to FFA2 and FFA3, other GPCRs that can be modulated by SCFAs have been discovered namely, Olfr78 and GPR109a. While not classified as part of the FFAR family of GPCRs, these GPCRs are regulated by SCFAs and/or closely related SCFA intermediates, as we describe below. In this article, we present the current knowledge on the role of SCFA receptors with an emphasis on their function in intestinal physiology and pathophysiology, and will also enumerate on their interrelationship with gut bacteria and the therapeutic relevance in intestinal metabolic and inflammatory disorders.
Diverse Signaling Mechanisms of GPCRs
GPCRs are seven transmembrane spanning proteins, and are the largest receptor class in the human genome. GPCRs mediate their individual signaling characteristics through multiple mechanisms, where the most well characterized mode is via Guanine nucleotide binding proteins (G-proteins) (99). G-proteins are heterotrimeric proteins, containing alpha, beta, and gamma subunits (162). In humans, four primary G-protein alpha subunit classes (Gαs, Gαi/o, Gαq/11, and Gα12/13) are predominant, where each alpha subclass influences important and distinct intracellular pathways (Fig. 1) (162). Gβγ proteins can also mediate their own signaling pathways, and this subunit remains a complex, and does not dissociate. Overall, there are 5 distinct beta and 12 distinct gamma subunits that are expressed in humans (90).
Figure 1.
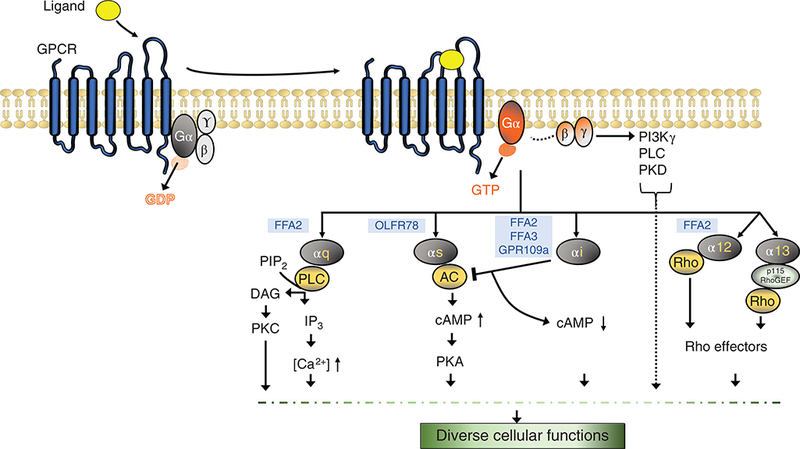
Different G-protein signaling pathways. Ligand (agonist) occupation of the binding pocket in the GPCR leads to conformational changes in the receptor and an altered interaction with heterotrimeric G-proteins. The activated receptor acts as a guanine nucleotide exchange factor that catalyzes the exchange of GDP for GTP on the Gα subunit and induced dissociation of active Gα subunit and Gβγ dimer (activated subunits represented with orange shade). There are four main Gα subunit protein classes, Gαq, Gαs, Gαi, Gα12/13. Each GPCR can couple with one or more Gα subunits. The activated Gα subunits can bind to and regulate the activity of several downstream effector molecules generating a cascade of signaling responses that culminate into specific cellular responses. Gαq/11 family members bind to and activate phospholipase C (PLC) which hydrolyses phosphotidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol triphosphate (IP3) which can mobilize calcium from intracellular stores or activate downstream protein kinases and modulate cell response. Gαs subunit activates adenylyl cyclase (AC) which increases intracellular cyclic adenosine monophosphate (cAMP) levels leading to activation of protein kinase A (PKA). Gαi subunit inhibits AC and reduces intracellular cAMP concentration. Gα12/13 can activate Rho kinases. The Gβγ subunit can activate phosphoinositide 3 kinase γ (PI3Kγ), protein kinases (PKD), and phospholipases (PLC). Various short chain fatty acid receptors (highlighted in blue color) are listed based on their G-protein-coupling preference. FFA2/3, free fatty acid receptor 2/3; Olfr78, olfactory receptor 78; GPR109a, G-protein-coupled receptor 109a (32,135,145,147,152).
Upon GPCR activation, by their specific endogenous ligand or pharmacologic agonist, a conformational change occurs across the GPCR, leading to internal GPCR domain changes in the GPCR and the G-protein complex structural interface (138). This process allows the G-protein complex to be activated, where the GDP on Gα is replaced by GTP, resulting in Gα and Gβγ free to signal the external “ligand” message to the internal mileu (138). While this classical GPCR signaling mechanism has been well validated and characterized, a multitude of mechanisms have been shown to affect each step of this process. A few of these mechanisms are depicted in Figure 2. Along with the availability of a ligand to activate the GPCR, the amount of the plasma membrane receptor available for ligand-induced activation is regulated through desensitization and resensitization processes (14), which are essential to prevent overstimulation of the GPCR and for the recovery of a functional receptor, respectively. Next, the step where GPCR activity is regulated is the GTP hydrolysis rate of the Gα proteins (Fig. 2A). Within the cell, proteins that modulate the intrinsic time of Gα activation influence duration of ligand effect. These special proteins called “regulators of G-protein signaling” (RGS proteins) aid Gα proteins in this function and enhance their GTP hydrolysis potential. GPCR activity is also regulated through the G-protein receptor kinases or GRKs (Fig. 2B). GRKs can phosphorylate specific residues in the cytoplasmic domains of ligand-activated GPCRs allowing dissociation of G-protein subunits from the receptor (112). β-arrestin is another multifunctional protein that acts in concert with GRK regulating desensitization of most receptors (Fig. 2C). Following GRK mediated phosphorylation, β-arrestin binds ligand-activated GPCRs and inhibits G-protein coupling (144). β-arrestin additionally can function as a scaffold for various adaptor proteins like clathrin and promote receptor internalization (endocytosis). The GPCR-arrestin endosome can also have multiple fates. First, GPCR-arrestin complex can direct assembly of a “signalosome” with recruitment of different kinases e.g. mitogen activated kinases like ERK1/2, and c-Jun N terminal kinases (JNK) allowing G-protein-independent signaling by the receptor (Fig. 2D) (81, 112, 179). Second, internalized receptor can either be recycled back to the plasma membrane or third, it can be degraded (Fig. 2E-F). Moreover, the amount of ligand can modulate the response of the GPCRs where higher concentration of the ligand often leads to a greater response (24). Ligands can also show selectivity for β-arrestin-dependent or G-protein-dependent signaling and this mechanism is called as biased agonism (144) (Fig. 2G). Additionally, transcriptional regulation of receptor expression is another mechanism for regulation of GPCR levels (Fig. 2H). And lastly, GPCR dimerization processes occur between the similar and dissimilar GPCRs (homo or hetero-dimers) to modulate the activation of the GPCR and its signaling capabilities (50) (Fig. 2I).
Figure 2.
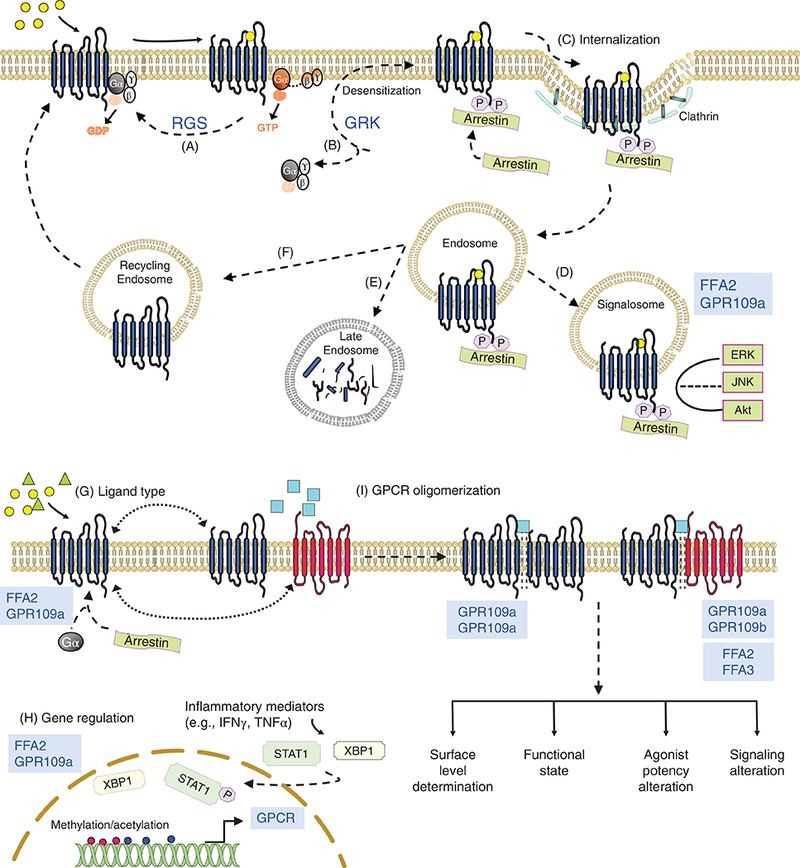
Regulation of GPCR signaling. Binding of agonist to the GPCR initiates a series of events: exchange of GDP for GTP on Gα subunit, dissociation of activated GTP-Gα and Gβγ dimer, stimulation of downstream effectors (depicted in Fig. 1). To prevent overstimulation and assure receptor resensitization, GPCR signaling is regulated by multiple mechanisms (few are enumerated here; see Further Reading list for detailed description). (A) Regulator of G-protein signaling (RGS) proteins function as GTPase-activating proteins, enhance the intrinsic GTP hydrolysis activity of the Gα subunit resulting in generation of inactive GDP-Gα, and promoting the reassembly G-protein heterotrimer and its reassociation with the receptor. (B) GPCR kinases (GRK) phosphorylate specific residues on the intracellular domain of agonist-occupied receptor. Phosphorylated receptor recruits β arrestin that sterically hinders interaction of the receptor with G-protein, thus uncoupling the G-protein complex from the GPCR (termed as desensitization). (C) Binding to the phosphorylated GPCR activates arrestin enabling it to perform ligand regulated scaffolding functions. Activated β arrestin interacts with the endocytic machinery (clathrin and adaptor proteins) and initiates receptor internalization. (D-F) Internalized GPCR in GPCR-β arrestin endosome can have multiple fates. GPCR-β arrestin complex can (D) direct the assembly of signalosome by allowing coupling of mitogen-activated protein kinases propagating further signaling pathways, (E) be targeted for lysosomal degradation, or (F) GPCRs can be trafficked to recycling endosomes and recycled back to the plasma membrane for resensitization. (G) Ligand type can also determine course of GPCR signaling where biased agonists can specifically activate either G-protein or β arrestin-mediated signaling pathway. (H) Gene transcription can also affect the total membrane levels of the GPCR. Transcription can be regulated by various inflammatory mediators, which can activate transcription factors that bind directly like XBP-1 on FFA2 promoter or indirectly affect chromatin accessibility by altering acetylation/methylation of the promoter (IFNγ for GPR109a). (I) GPCRs can form oligomers (homodimers or heterodimers) that can regulate (1) cell surface delivery following receptor maturation or cellular trafficking following agonist activation, and (2) receptor pharmacology and signaling. Short chain fatty acid receptors (highlighted in blue color font) are noted if data exist on their regulatory mechanisms. FFA2/3, free fatty acid receptor 2/3; GPR109a, G-protein-coupled receptor 109a (6, 8, 14, 15, 102, 112, 114, 120, 144, 179, 212).
Among the SCFA receptors, data exists beyond the classic G-protein signaling for FFA2 and GPR109a where β-arrestin-dependent mechanisms have been described. Human FFA2 has been shown to employ β-arrestin-dependent signaling for transcriptional regulation of proinflammatory cytokine expression in vitro (102). GPR109a when activated by nicotinic acid (and not SCFAs, an example of biased agonism) has been shown to recruit ß-arrestin 1 and mediate cutaneous flushing effect associated with nicotinic acid consumption (212). For FFA2, ligand bias extends to selective coupling of Gα proteins. Synthetic ligands at FFA2 can elicit either Gαi/o or Gαq/11 signaling (27) and also physiological conditions like high-fat diet consumption can influence G-protein coupling bias of the receptor itself in favor of Gαq/11 over Gαi/o (120). Furthermore, physiological ligand concentrations possibly affect the SCFA receptors activity (and probably expression) in yet another way. Highest concentration of their ligands (SCFAs) is present in the intestine where butyrate is consumed as energy source and remaining SCFA pool is drained into the portal vein (40). Liver metabolizes propionate, leaving acetate as the SCFA with highest systemic concentration (40). Accordingly, prominent physiological effects of these receptors are noted in the intestine, as discussed in the following text.
In summary, it is clear that GPCR signaling is complex and new modes of signaling are being discovered (for greater detail on GPCR signaling mechanisms and regulation, see Further Reading list). In this overview article, we focus on how SCFA receptors (see Table 2) signal via Gα (and through other pathways, if known) and influence different normal and pathological cellular processes in the gut.
Table 2.
Short Chain Fatty Acid Receptors: Official and Alternative Names
| GPCR (official IUPHAR symbol) |
Alternative symbol | Gene | Name |
|---|---|---|---|
| FFA2 | GPR43, FFAR2 | Ffar2 | Free fatty acid receptor 2 |
| FFA3 | GPR41, FFAR2 | Ffar3 | Free fatty acid receptor 3 |
| GPR109a | HCA2, NIACR1, HM74A, PUMA-G |
Hcar2 | Hydroxycarboxylic acid receptor 2 |
| Olfr78 | PSGR; RA1c; MOL2.3; Or51e2; MOR18–2 |
Olfr78 | Olfactory receptor 78 |
The Short Chain Fatty Acid Receptors
FFA2 and FFA3
Discovery
The discovery of the first short chain fatty acid receptors (SCFA-Rs) and their ligands occurred serendipitously. During the search for human galanin receptor subtypes, a cluster of four intron-less genes, at the time named as GPR40, GPR41, GPR42, and GPR43, was identified downstream of CD22 gene on chromosome 19q13.1 (166). Based on their resemblance with members of a GPCR class (in particular, the class A receptors), these were classified as orphan GPCRs, where this term orphan refers to the lack of known ligand responsible for the particular GPCR [for greater detail on GPCR classes see (145)]. Gene cluster of these FFARs in mice exists on chromosome 7 but devoid of GPR42 which is a feature observed only in the primate FFAR gene cluster (155). A few years later, the first rodent orthologs of FFA2 were cloned from leukocytes and FFA3 from lungs (29, 171). Since then, orthologs from various species have been cloned and characterized (121). Because these receptors (FFA2 and FFA3) are genetically close and were discovered at the same time, we discuss them here together.
Following the discovery of these genes, screening of ligands active at these receptors using recombinant cell systems, reporter gene assays, and Ca2+mobilization/cAMP accumulation assays was done (97, 130). In 2003, multiple groups reported SCFAs to be ligands for FFA2 and FFA3 (32, 100, 130). It was a chance observation that only the compounds with acetate as a counter ion could elicit FFA2 activity. Later acetate alone, salts of acetate and other carboxylate ions up to six carbons in length were revealed to activate both receptors, albeit with different potencies (32, 100, 130). GPR42, which shared 98% homology with FFA3 (166), however, was not activated by any of the SCFAs and was classified as a pseudogene (32). Potentially of importance, it was recently suggested that GPR42 expressed heterologously in rat sympathetic neurons is functional exhibiting lower potency but similar efficacy as FFA3 for propionate (155). The discrepant observations of functional activity of GPR42 in studies of Brown et al. (32) and Puhl et al. (155) may have arisen from use of different assays monitoring the activity. However, if GPR42 truly represents a functionally important gene remains to be confirmed.
Pharmacology
Owing to 43% amino acid sequence identity, it is not surprising that FFA2 and FFA3 also share overlapping ligand selectivity profiles. The consensus of all reports is that the shorter the carbon chain length of the SCFA, the higher the potency at FFA2 and vice versa for FFA3. Accordingly, acetate (C2) and propionate (C3) show highest and equal potency at FFA2 followed by butyrate (C4) (Table 3) (32, 76, 130, 183). Formate (C1) and pentanoate (C5) can also activate FFA2 but to a lesser extent while hexanoate (C6) shows no activity with FFA2 (28, 130). At FFA3, C3, C4, and C5 show highest and equal potency, C2 and C6 can also activate the receptor but to a lesser extent (Table 3) (124). SCFAs with substitution of branched chain alkyl groups are more potent at FFA3 (168). Modifications of SCFA alkyl chain with unsaturated double or triple bond favor FFA2 over FFA3 (168).
Table 3.
Potency (EC50) of Short Chain Fatty Acids at Cognate Receptors
| GPCR | Acetate | Propionate | Butyrate |
|---|---|---|---|
| FFA2 | 35–431 | 14–290 | 28–371 |
| FFA3 | >1000 | 6–127 | 42–158 |
| GPR109a | Does not respond | Does not respond | 700–1600 |
| Olfr78 | 2350 | 920 | NA |
With regards to SCFA potency, an important distinction between human and mouse orthologs of the receptors has been revealed. First, C2 is 20-fold more selective at human FFA2 than at human FFA3 while C2 is equipotent at both the mouse orthologs (168). Next, C3 is 12-fold more selective at mouse FFA3 while exhibiting similar potency at human orthologs (168). Ionic interactions between positively charged amino acid residues and carboxylate group of SCFAs are essential to ligand binding and receptor function (183), and it has been shown that the molecular basis of specific ligand selectivity described above lies in the differences in core amino acids constituting the binding pockets of these receptors (183). These observations will be important as pharmacologic molecules have been developed and some of which are being tested in preclinical studies.
Expression
Both FFA2 and FFA3 are highly expressed in the intestine (Fig. 3). This high expression at the natural site of their ligand production may have evolved as a part of mutualistic association between the gut microbiota and the host. Dass et al. (42) and Karaki et al. (84) were the first reports describing expression of these GPCRs throughout the gastrointestinal (GI) tract. First, the highest expression for FFA2 was observed in the rat colon (42), and its expression was also reported in enteroen-docrine cells expressing (peptide YY) PYY and in mucosal mast cells that express serotonin (84), an observation supported by the same group in human large intestine (85). Of note, enteroendocrine cells are specialized cells of the GI system that release gastrointestinal hormones into blood stream producing an array of systemic metabolic and immunologic effects. There are several types of intestinal endocrine cells including I cells, which secrete cholecystokinin (CCK), K cells that secrete gastric inhibitory peptide (GIP), L cells that secrete glucagon-like peptide 1 (GLP-1), GLP-2 and PYY, and enterochromaffin cells that secrete serotonin. FFA3 has also been observed to be expressed in intestinal tissue, where it was first reported that FFA3 was expressed in the human colon(enterocytes and enteroendocrine cells) but to a lesser extent than FFA2 (197). Immunohistochemistry studies have shown that FFA3 is expressed in the apical cytoplasm of enterocytes, enteroendocrine cells, and several lamina propria cells but not in 5-HT (5-hydroxytryptamine) positive enterochromaf-fin cells of the human colon. All FFA3 positive enteroen-docrine cells were reported to be immunoreactive for PYY producing cells but not vice versa. Interestingly FFA3 was not co-localized in FFA2 immunoreactive enteroendocrine cells (197). In mice, Samuel et al. (165) showed that FFA3 is highly expressed in small intestine especially in ileum. Further studies from Sykaras et al. in mice reported the expression of FFA3 and FFA2 transcripts in duodenal I-cells (188).
Figure 3.
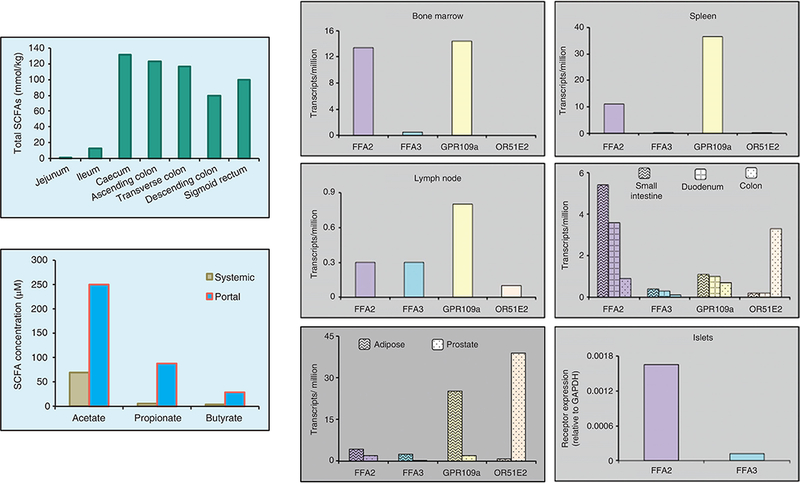
Short chain fatty acid levels and expression of cognate receptors in select tissues. Short chain fatty acid concentrations in contents along the intestine (left upper panel) and systemic and portal blood in human subjects (left lower panel) [adapted, from Cummings et al. (40)]. Right-hand panel, human short chain fatty acid receptor expression in select tissues presented as plot of mean transcripts per million from HPA (human protein atlas) except for expression in human islets that was obtained by qRT-PCR and presented relative to GAPDH (Brian T Layden, unpublished data).
Other than the gut, FFA2 expression is localized to many immune cell types including regulatory T cells (6, 37, 84, 100, 181), bone marrow, and adipose tissue (56, 70, 105, 116). Immune cell expression of FFA2 has been observed to change in response to cell maturation and inflammation (6, 116, 171, 174). For example, in human monocytes, treatment with lipopolysaccharide, tumor necrosis factor (TNF) or granulocyte macrophage colony stimulating factor (GM-CSF) can upregulate FFA2 expression via actions of transcription factors like nuclear factor kB (NFkB) and x-box binding protein-1 (XBP-1) on FFA2 promoter (Fig. 2H) (6, 171, 174). Modulation of FFA2 expression in immune cells consequent to inflammation may indicate the crucial role FFA2 plays in regulating number and function of these cell types in mitigating adverse inflammatory reactions (59, 115).
FFA2 has also been observed to be expressed in gastric ghrelin cells (49) and both FFA2 and FFA3 in gastric brush cells, islet α and β cells, renal cortical epithelial cells and spleen (13, 47, 56, 89, 95, 100, 120, 152, 192, 207). The expression of FFA3 in β cells is regulated in a unique fashion. Its expression is mediated via internal ribosome entry site-dependent translation of bicistronic mRNA encoding FFA3 and GPR40 (13). Additionally, FFA3 expression has been noted in sympathetic and enteric neurons and autonomic ganglia (43, 131, 132), certain immune cells like dendritic cells and monocytes (100), and spleen (Fig. 3) (56, 95, 100). Hence, FFA3 seems to be more widely expressed as compared to FFA2. Its expression in adipose tissue, however, is debated. Initial reports (32, 100, 223) suggesting expression of FFA3 in adipose tissue or cell lines were later refuted when no FFA3 was detected in different adipose depots or confluent and differentiated adipocytes (68, 228). More recent reports have again reinforced its presence in adipose tissue. Using 3T3-L1 adipocyte cell line, these reports have projected a role of adipose expressed FFA3 in insulin signaling and lipolysis (64, 136). While these reports await validation of FFA3 protein level in adipose tissue, it has been identified that its expression in this tissue can be induced under certain conditions such as the lack of FFA2 (in FFA2 knockout mice), dietary SCFA supplementation, or the stage of adipose tissue development in some mammals (23, 105, 108).
Majority of the data on expression of FFA2 and FFA3 (and other nutrient sensing GPCRs in general) has been generated through transcriptomic analyses and there may be uncertainty with detection of false positives (use of primers spanning introns and inappropriate controls, low transcript levels in certain tissues). Moreover, in absence of any reliable antibodies (191, 207, 209) transcript level analysis is the only option currently available for determination of expression of these receptors except for the use of reporter proteins expressed under control of their promoters. An elegant method performed by Nohr et al. (131, 132) used transgenic reporter mice expressing monomeric red fluorescent protein (mRFP) under the control of FFA2 and FFA3 promoters to evaluate their expression patterns in GI tract. Their findings indicated that in the gastric mucosa, FFA3-mRFP is expressed in gastrin and ghrelin-positive cells and in the small intestine, FFA3-mRFP is expressed mainly in the CCK-secretin-GIP-GLP-1-PYY-neurotensin lineage of enteroendocrine cells. Although earlier studies showed expression of FFA2 in GLP-1 positive enteroendocrine cells, utilizing Q-PCR (quantitative polymerase chain reaction), FACS (fluorescence activated cell sorting) analysis and immunohistochemistry, Nohr et al. (131, 132) demonstrated that FFA2 is strongly expressed in enteric leukocytes and rather weakly expressed and only in a fraction of the enteroendocrine cells. Use of similar model for assessment of FFA2 and FFA3 expression in other tissues like adipose will be important. Such models may also be helpful in elucidating mechanisms governing membrane expression and non-G-protein signaling of these receptors.
Signaling
Unique G-protein alpha signaling characteristics of FFA2 and FFA3 lead to distinct downstream effects (Fig. 4). FFA2 is reported to be capable of dual signaling via Gαq/11 and Gαi/o pathways; whereas, FFA3 signals exclusively by Gαi/o. Because of these G-protein-coupling patterns, FFA3 activation predominantly has an inhibitory tone which is associated with inhibition of adenylyl cyclase activity and reduction in intracellular cyclic adenosine monophosphate (cAMP) levels (Fig. 1) (32, 76, 100, 152, 183). Propionate induced activation of FFA3 in islets, for example, inhibits insulin secretion in pertussis toxin (a GPCR-Gαi/o interaction inhibitor) sensitive manner (150). Interestingly though, Gαi/o coupled FFA3 can also induce excitatory responses like sympathetic activation; however, this is via Gβγ-PLC-MAPK pathway (Figs. 1 & 4) (93). As of yet, there are no findings demonstrating the recruitment of GRK-arrestin by ligand activated FFA3 (75, 93) except for the latest report, where ligand activation of heterologously expressed human FFA3 recruited ß-arrestin 2(8).
Figure 4.
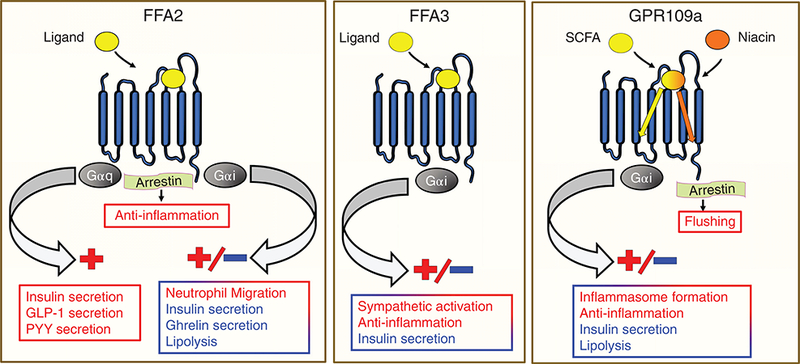
Signaling pathways downstream of FFA2, FFA3, and GPR109a activation and corresponding effects. Apart from G-proteins, FFA2 can signal via arrestin exerting anti-inflammatory effects. Niacin at GPR109a shows biased agonism, arrestin signaling preferentially over G-protein signaling causing flushing.
FFA2 is more versatile than FFA3, as it signals by multiple G-protein signaling pathways. More exactly, it can signal via Gαq/11, Gαi/o, and Gα12/13, and can, therefore, inhibit or activate cellular responses (32, 76, 100, 130, 183) (Fig. 4). For instance, FFA2 activation can enhance insulin secretion via Gαq/11 (120, 151) and inhibit ghrelin secretion through Gαi/o pathway (49). Similar to FFA3, FFA2 can also signal through Gαi/o. In adipocytes, acetate and agonists at FFA2 inhibit insulin signaling in pertussis toxin and Gallein (which is a Gßγ blocker) sensitive manner (94). Activated FFA2 can also recruit arrestin (73, 74, 102, 226). Ligand-activated FFA2 has been shown to recruit ß-arrestin 2, leading to internalized FFA2, and signal dephosphorylation (inactivation) of NFkB (102). This effect has been associated with reduced expression of proinflammatory cytokines in in vitro studies (102).
Ability to evoke different signaling pathways by ligands (referred to as ligand bias) provides another level of dexterity in FFA2 signaling. More precisely, ligand bias refers to the unique ability of a compound to favor one signaling pathway over the other. Several synthetic ligands at FFA2 have been described to selectively elicit Gαq/11, Gαi/o, or β-arrestin pathway (28). One of the first potent activators of FFA2, 4-chloro-α-(1-methylethyl)-N-2-thiazolylbenzeneacetamide (4-CMTB) was originally reported to activate Gαq/11, Gαi/o as well as β-arrestin pathways (103, 180, 216). In our laboratory, glucose-stimulated insulin secretion (GSIS) in studies utilizing islets from FFA2 knockout (KO) and wild-type (WT) mice suggested that this compound showed bias for Gαi/o over Gαq/11 (151). Moreover, in in vitro Ca2+ mobilization assays (a substitute technique for studying Gαq/11 response) in mouse β cell line, we did not observe any activity of this compound at FFA2 (151). Though these data suggest biased agonism by 4-CMTB, this observation, however, requires further investigation.
Previously unknown, FFA2-FFA3 heteromerization shown by Ang et al. recently in both in vivo and in vitro settings has further added complexity to signaling mechanisms of these receptors (8). Heteromers of human FFA2-FFA3 exhibit distinct signaling with: (1) significant elevation in Ca2+mobilization above what was observed for FFA2 alone; (2) marked enhancement in β-arrestin 2 recruitment and; (3) loss of Gαi/o signaling (Fig. 2I) (8). A limitation of these observations is the narrow tissue range screened for this heteromer where only macrophages and monocytes exhibited heteromers but not colonic epithelial cells. Physiological significance of FFA2-FFA3 heteromer and its presence in other tissues still needs clarification.
SCFAs, the natural ligands of these receptors, show species bias at these receptors owing to differences in their ligand binding pockets and their constitutive signaling properties. For example, human FFA2 exhibits high affinity for acetate and also has a high level of constitutive activity, whereas human FFA3 does not exhibit these properties. Moreover, differences in these characteristics exist between species (human vs. mouse isoforms) for the same receptor (73). How this relates to their biological function requires more investigation. For example, acetate enhances GSIS in mouse islets via Gαq/11 pathway but has no net effect on GSIS in the human islets where it evokes both Gαq/11 and Gαi/o pathways (120, 151). Therefore, use of SCFAs and synthetic ligands at these receptors in in vivo settings is challenging, as a means to understand receptor biology. Concomitant use of receptor knockout and overexpression mouse/cell models along with the models expressing the wild-type receptors has allowed partial segregation of tissue specific effects of these receptors (56, 94, 151, 191, 192, 207). As discussed in subsequent sections, FFA2 and FFA3 appear to have roles in numerous health conditions. Hence, synthesis and characterization of specific and selective agonists for these receptors is important for their use in understanding each of their biological effects.
Role of FFA2 and FFA3 in the gut physiology
SCFAs are generated in the colon as end products of bacterial fermentation where the primary ones are acetate, propionate, and butyrate (98). In colon, SCFAs play critical roles as predominant source of metabolic energy, maintenance of epithelial integrity, fluid absorption and have important anti-inflammatory and anti-cancer effects (66, 87, 156, 178, 187, 193, 194). These SCFAs specifically also affect important cellular processes such as proliferation, differentiation, apoptosis, hormonal secretion, and immune responses in the gut, and in part via activation of FFA2 and FFA3 (5, 31, 52, 92, 111, 116, 154, 165, 181, 196), as outlined later.
Role of FFA2 and FFA3 in epithelial barrier function and intestinal inflammation
SCFAs have long been known to exert beneficial effects against intestinal inflammation and protecting intestinal epithelial integrity (66, 87, 187). Recent studies have shown that these protective effects of SCFAs are, in part, through their receptors, FFA2 and FFA3. Loss of these receptors in various experimental models of intestinal inflammatory disorders like inflammatory bowel disease (IBD) has been associated with dysregulated inflammatory responses to different immunologic challenges (92, 111).
The physical barrier of gut epithelium offers first line of defense against injury or infection and optimal gut permeability is associated with development of protective immune response (204). In fact, modest disruption of the epithelial barrier is important to mount proper inflammatory response against pathogenic microbes and antigens (26). Because of their expression patterns, a role for FFA2 and FFA3 in regulation of epithelial barrier function has been investigated. For example, challenging the gut with 2,4,6-trinitrobenzene sulfonic acid (TNBS) increases gut permeability (214) and colonic neutrophil migration only in WT mice but not in mice deficient in FFA2 and FFA3 (92). Also, subsequent to TNBS administration, interleukin-6 (IL-6), IL-12, interferon γ (IFN-γ), and other inflammation-associated genes are induced in the colon of WT mice, but not in FFA2 and FFA3 KO mice (92).
In these above studies, the TNBS treatment led to early proinflammatory changes and set the stage for ousting the immunologic challenge over the long course. These effects were almost absent in FFA2 and FFA3 KO mice (26, 91). Owing to lack of such early inflammatory response, FFA2 KO and FFA3 KO mice show higher susceptibility to C rodentium infection (monitored for 4 weeks post insult), decreased pathogen clearance during the course of infection and reduced Th1 and Th17 responses (92), which were suggested to indicate that FFA2 and FFA3 are crucial regulators of gut permeability (92). FFA2 on dendritic cells can also promote intestinal IgA production, providing additional route of intestinal epithelium protection against pathogenic microbes (190, 220). Similarly, in acute as well as chronic DSS (dextran sodium sulfate) colitis model, which is independent of adaptive immune system, genetic knockout of FFA2 exacerbated the disease, through effects on neutrophil recruitment, cytokine release and gut integrity (111, 116, 117). Mediation of above effects by FFA2 and FFA3 signaling is possible through several mechanisms. SCFAs can induce anti-microbial peptide (AMP) production in gastrointestinal cell lines (167) while in islets this effect of SCFAs probably involves FFA2/3 (185). Islet AMP has immunomodulatory role and induces regulatory dendritic cells, T-cells, and macrophages (185). A similar role of intestinal FFA2 and FFA3 is yet to be determined (79, 185). However, stimulated FFA2 on both gut epithelial cells and immune cells can affect production of immunomodulatory cytokines and regulatory T cells (Treg) and activation of inflammasome (92, 111, 116, 117, 133, 181).
Intestinal epithelial cells (IECs) secrete mucin, antibacterial peptides, and immune mediators that recruit or activate immune cells (143). Moreover, FFA2 and FFA3 expressed in IECs, may mediate some of the above effects. Bone marrow chimera studies have demonstrated a role of IEC-FFA2 in regulation of inflammation (92,111). For example, in DSS, TNBS, and infectious colitis models, FFA2 in nonhematopoietic cell population has emerged as a main player in promotion of gut homeostasis. Transfer of bone marrow from FFA2-KO (donor) to WT (recipient, ensuring absence of the receptor in myeloid cells) was protective against inflammation as compared to transfer of bone marrow from WT→KO. FFA2 in gut epithelia has also been shown to stimulate potassium ion efflux, hyperpolarization of membrane and activate NLRP3 inflammasome pathway via Gαi/o (111). All these processes attenuate colitis by stimulating the expression of IL-18 (111), a critical regulator of gut epithelial integrity (133). FFA3, unlike FFA2, seems to have a more restricted function in gut barrier preservation. FFA3 in the nonimmune cell population of the gut has been shown to confer protection in infectious colitis model mainly by enhancing expression of cytokines and chemokines through MEK-ERK (mitogen-activated protein/extracellular signal regulated kinase kinase) pathway (92). Protective effects of dietary manipulation on mucosal barrier in swine model of weaning associated diarrhea were conjectured to be mediated by enhanced levels of FFA3 in the gut (65). Similarly, protection observed with dietary manipulation in type 1 diabetic FFA2 KO mice is thought to be FFA3 driven (115). Though promising, these data need to be corroborated with loss of function studies in FFA3 knockout models.
Additionally, there is growing evidence that FFA2 modulates immune responses by regulating neutrophil chemotaxis, recruitment of inflammatory mediators and modulation of Treg development (92, 116, 117, 181,210). In both acute (2.5% DSS, 7 days) and chronic (0–6 days 4% DSS, 20–24 days 2%, and 35–39 days 4% DSS) colitis models, whole body FFA2 deficiency results in unabated inflammatory response (116), which includes increased neutrophil infiltration, concomitant increase in myeloperoxidase (MPO) activity with physical features of colitis, for example, reduced colon length (116). Acetate administration could reverse disease features in acute DSS model but only in the WT mice (116). Concordant with a “neutrophil-driven response,” WT mice with bone marrow reconstituted from FFA2 KO mice were not protected from DSS colitis suggesting that immune cell FFA2 is responsible for the phenotype observed in the KO mice (116). Kamp et al. also recently demonstrated that acetate-FFA2 ligand-receptor pair affects neutrophil migration following inflammatory stimulus (82). Of note, FFA2 is highly expressed in neutrophils (6, 32, 130). In light of these above studies, FFA2 appears to be crucial for regulating migratory behavior of neutrophils, and essential to mount anti-inflammatory response in acute as well as chronic inflammation conditions. Similar exacerbation of inflammatory response along with increased colonic levels of IL17 producing T helper cells (particularly CD44+IL17+ T cells) and cytokines has also been shown in TNBS colitis in FFA2 KO mice (116).
Another mechanism by which FFA2 can affect immune homeostasis is through the regulation of the number and function of Tregs, which are specialized T cells noted for immune-suppressive effects against autoantigens (self-antigens) (164). Interestingly, their expansion in the intestine is regulated by SCFAs (140, 181) and involves FFA2 signaling (181, 190). For example, in a study using T cell transfer colitis model, it was shown that genetic deficiency of the receptor could lead to insufficient number of colonic Tregs and reduced antiinflammatory IL-10 levels in intestinal inflammation (181), an effect not reverted by SCFA treatment. Thus, Treg deficiency in part could arise from a role of FFA2 in regulating colonic migration of extraintestinal Tregs (181). Influence of FFA2 signaling on Treg differentiation is, however, debated. Other studies have shown that SCFAs promote differentiation of gut CD4+ T cells to FOXP3+ Treg cells independent of the receptor possibly through histone deacetylase inhibition (9, 20, 57, 140). FFA2 may also affect Treg differentiation indirectly by regulating CD103+ dendritic cells that are chief regulators of inducible mucosal Treg cells (190). Another possibility is that direct regulation of Tregs via FFA2 is tissue specific. For example, FFA2-dependent increase in Treg pool in type 1 diabetes model has been noted but only in peripheral tissues (115). FFA2, thus, has emerged as a key determinant of inflammatory response in intestine, affecting gut integrity, MPO activity, CD44+IL17+ T cells, FOXP3+ Treg cells, and levels of proinflammatory cytokines in the colon tissue (92, 115, 116, 181).
Gut immune homeostasis as achieved by FFA2 and FFA3 signaling can have far-reaching effects (92, 204, 220). Not only SCFA-FFA2 primed gut immune cells can translocate to distant organs but also disrupted intestinal epithelial barrier results in leakage of pathogenic bacteria or their byproducts into systemic circulation (20, 204). For example, FFA2 KO mice were reported to have exacerbated immune response in asthma and arthritis (116) and WT mice showed protection against lung inflammation by FFA3-dependent regulation of production of macrophages and dendritic cells (203). The significance of these defensive roles of FFA2 and FFA3, however, has not been consistently observed (5). Sina et al. (175) and Kim et al. (92) reported contradictory findings as they observed opposing roles for protection against various immunologic insults in FFA2 KO compared to WT mice. The basis for these controversies is not entirely clear and may involve different experimental design/protocols in different studies. While Maslowski et al. suggested that bone marrow-derived cells contribute to the colitis phenotype (116), Kim et al. reported opposite findings by implicating suppression of FFA2-dependent activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling in colonic epithelial cells accountable for the exacerbation of colitis (92). In contrast, Sina et al. (175) demonstrated that activation of p38 mitogen-activated protein kinase by FFA2 in polymorphonuclear leukocytes plays a key role in the pathogenesis of colitis. Interestingly, FFA2-mediated activation of the inflammasome is shown to be protective for gut homeostasis (111) but contributes to development of gouty arthritis (208). Nevertheless, immune-modulatory role of these receptors is likely a balance of pro- or anti-inflammation driven by multiple factors like disease course, gut microbes, and nutritional status (Fig. 5). Furthermore, differences in precise determination of expression of FFA2 in immune cells (191) and possible transcriptional regulation by SCFAs (108), physiological conditions (56, 116, 120, 151), and stress-induced inflammatory mediators like TNFα (6, 86) may be contributing to these divergent effects. Further detailed studies in intestine-specific or cell-specific deletion of these receptors are needed to pinpoint the exact role of these receptors in IBD.
Figure 5.
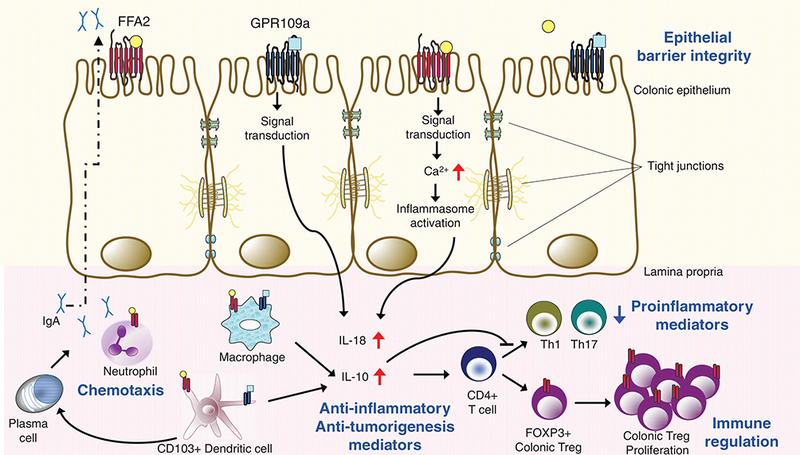
Short chain fatty acid receptors in gut immune homeostasis. Short chain fatty acids produced by fermentative activity of gut microbiota bind and activate receptors on intestinal epithelial cells and immune cells like macrophages, neutrophils, and dendritic cells. Production of cytoprotective IL-18 (from FFA2/GPR109a-dependent activation of inflammasome), anti-inflammatory IgA (from FFA2-dependent activation of B cells), anti-inflammatory IL-10 (from FFA2 activation and GPR109a-dependent activation of macrophages and dendritic cells), and (FFA2-and GPR109a-dependent) differentiation and proliferation of Tregs protect against conditions leading to colitis and colitis associated cancer. Taken together, these processes influence epithelial barrier integrity, adequate neutrophil migration, balanced proinflammatory (Th1 and Th17), and immunosuppressive (Treg) responses under conditions of inflammatory insult. CD, cluster of differentiation; IgA, immunoglobulin A; Th, T helper cells; Treg, regulatory T cells; short chain fatty acids are represented byo, □; FFA2 and GPR109a, respectively, on immune cells are represented by I, I; red upward arrow signifies increase; blue downward arrow signifies decrease (92, 111, 116, 117, 176, 177, 181, 190, 191, 220).
Role of FFA2 and FFA3 in colon cancer
Tumorigenesis and inflammation have interrelated features (71, 186). As such, a role of FFA2 in intestinal cancer may exist, similar to its reported role in mediation of inflammatory responses (111, 177). First of all, FFA2 is consistently down-regulated in cancer tissues and cell lines. For example, its expression was markedly reduced or completely absent in colorectal adenocarcinoma tissues compared to normal colon tissue in humans (177, 195). Going beyond expression changes, various reports have shown that FFA2 activity has antiproliferative effects on cancer cells (21, 22). Tang et al., (195) showed that restoration of FFA2 expression in colon cancer cell lines induced apoptosis and inhibited cell proliferation by arresting cells in G0/G1 phase, upregulating p21, and decreasing the levels of cyclin D3- and cyclin-dependent kinases (CDKs) 1 and 2. Indeed, FFA2 deficiency results in increased number and size of polyps and tumors in the colon of different cancer models, like ApcMin/+ mice and mice cotreated with azoxymethane and DSS (111, 139, 177). Dysregulated inflammatory response with enhanced neutrophil migration and increased expression of proinflammatory markers such as IL-1ß and IL-17α are characteristic feature of rapid carcinogenesis in these models (111, 139, 177). Moreover, anti-cancer effects of FFA2 are not limited to colon. BaF3 leukemic cell proliferation in liver of mice could be countered by activation of FFA2 expressed profusely on BaF3 cells (22, 116). However, these studies are contended by a report demonstrating oncogenic properties of FFA2 (67). Using in vitro focus formation assay and in vivo tumorigenicity assay in nude mice, this report showed that expression of FFA2 transcript and protein is increased in digestive tract tumors (67). Thus, further investigations are warranted to understand whether FFA2 is a tumor suppressor or oncogenic in nature. Unlike FFA2, a role of FFA3 in cancer has not been reported probably because of its low expression compared to FFA2 in cancer cell lines (227) or alternatively due to its pro-cancer effects in vitro (219).
Role of FFA2 and FFA3 in metabolic regulation via actions in the gut
Both these receptors (FFA2 and FFA3) also affect metabolic functions related to their expression in the intestine. Aspects of this role for the receptors, like their role in immune modulation, are also debated. An interaction of SCFAs and these receptors on enteroendocrine cells or enteric neurons results in metabolic regulation through neural and/or gut derived hormonal mechanisms. Initially, FFA2 and FFA3 both were reported to mediate GLP-1 release from L cells with a greater role surmised for FFA2 (201). Low basal serum and colonic levels of active GLP-1 protein corresponded with FFA2 deficiency in mice, and this was suggested to be reflected in glucose intolerance in an oral glucose tolerance test and reduced GLP-1 secretion upon intra-colonic SCFA infusion or with primary colonic cultures from FFA2 KO mice (154, 201). Through this role of secreting GLP-1, FFA2, and possibly FFA3 are suggested to contribute to glucose homeostasis by improving/preserving insulin secretion and limiting insulin resistance (209). However, the role of FFA2, in particular, is debated by some studies with no difference in GLP-1 levels, basal or glucose stimulated observed between FFA2 KO and WT mice (56, 151, 192).
Another means by which these receptors may affect overall energy homeostasis is through modulation of PYY secretion. PYY lowers the intestinal transit rate and enhances satiety (17). By means of receptor KO mouse models and ex vivo colonic cultures, both FFA2 and FFA3 were documented to enhance PYY secretion and reduce gut motility (31, 52, 154, 165, 196). Another variable, for FFA3, is that it is also expressed in mouse and human sympathetic nervous system (93, 132) and it can, in part, mediate gut-brain axis communication (63). For example, a study showed that through gut-brain circuitry, FFA3 could enhance intestinal gluconeogenesis in mice by increasing expression of gluconeogenic enzymes. This effect was blocked by FFA3 antagonism (43). In sum, cumulative role of FFA2 and FFA3 on intestinal epithelial barrier integrity, immune cells, and gut hormone secretion suggests an important novel link between gut inflammation, diabetes, obesity, and SCFAs through these receptors. And how this link holds potential for new therapies against obesity and diabetes still remains to be investigated.
Role of FFA2 and FFA3 in extraintestinal tissues
Insulin secretion is a response to nutrient absorption. Nutrients, primarily glucose and free fatty acids, are well recognized to enhance insulin secretion (149). SCFAs have been suggested for multiple decades to modulate insulin release from β cells (141, 173, 200, 222). More recently, various groups have assessed the role of FFA2 and FFA3 in SCFAs mediation of insulin release (56, 120, 150, 151, 192, 207). Through these studies, FFA3 has emerged as a negative modulator of insulin secretion owing to its Gαi/o coupling (Fig. 4) (150, 192, 207). Consequently, FFA3 KO mice (192) [Priydarshini et al., unpublished data] and FFA3 overexpressing transgenic mice (207) exhibit complementary changes in glucose tolerance upon high-fat diet feeding without effects on insulin sensitivity suggesting that this phenotype is primarily a β cell secretory phenotype. Studies exploring the role of FFA2 on GSIS, however, have resulted in divergent outcomes [reviewed in (152)]. Two studies concluded that FFA2 activation enhances insulin secretion ex vivo as well as in vivo (120, 151) through Gαq/11 pathway. Contrary to this, another study showed that acetate, which these authors suggested is produced in the islets, acts in an autocrine manner inhibiting GSIS through activation and Gαi/o coupling of FFA2 and FFA3 (192). While in human islets, acetate and synthetic ligands of FFA2 evoked Gαi/o or Gαq/11 pathway either by increasing (151), decreasing (192) or no net effect on GSIS (120, 151). Groups supporting FFA2-Gαq/11 coupling in islets showed further that FFA2 signaling maintains its insulinotropic effect and promotes compensatory β cell mass expansion under diet-induced obesity and pregnancy (56, 120, 151). In fact, FFA2 signaling is necessary for establishment of β cell mass. Results from our laboratory show that FFA2 KO mice have reduced β cell mass at birth and this is maintained during adulthood (both in male and female mice) (56, 209). As suggested by our experimental data, akin to its role in regulation of immune cell number, β cell FFA2 regulates aspects of β cell function and survival (209). However, prior to any clinical translational studies, more work is required to clarify these different outcomes between studies.
Besides insulin secretion, FFA2 has been reported to also affect insulin action (94). Using tissue specific overexpression model for this receptor, Kimura et al., showed that FFA2 in the white adipose tissue inhibits insulin mediated fat accumulation, promotes energy homeostasis by enhancing energy expenditure and systemic insulin sensitivity in mice on high fat diet (94). These effects of adipose tissue-FFA2 overexpression were taken to suggest that overstimulation of the receptor is preventive against spontaneous or diet-induced obesity observed in FFA2 KO mice (94). While results from our laboratory and others have shown that FFA2 KO does not lead to higher body weight gain on high-fat diet (23,120,151), each of these studies show divergent outcomes for FFA2-KO effects on systemic insulin sensitivity, reporting either increase (23), decrease (94), or no change (120, 151). These former studies (23, 120, 151) support a role for FFA2 in white (44,68) as well as brown adipogenesis (70). Differing effects of FFA3 on body fat mass are also reported with FFA3 KO exhibiting reduced (165), enhanced (18) or similar (201) body fat mass. Thus, effects of these receptors on adiposity are far from clear but there is strong likelihood of these receptors playing a significant role through modulation of hormones affecting energy balance [leptin and ghrelin secretion by FFA2 (49,228)], satiety [PYY secretion by FFA3 (165), FFA2 [(31, 52, 154)], yet unidentified effects on CCK [(131) (39)] and sympathetic outflow [FFA3 (78, 93)]. Taken together, these receptors modulate secretion of insulin from the β cells directly or indirectly through GLP-1 secretion from L cells (described in the previous section) and mediate its effects on insulin-sensitive tissues like adipose (Fig. 4) (152). How all these peripheral actions of SCFAs work together is beginning to be understood, and it is increasingly apparent that FFA2 and FFA3 are participating in interorgan cross talk mediating the actions of SCFAs (33).
Implications for therapeutic use of FFA2 (and FFA3)
Based on findings reported earlier, gut FFA2 may be a befitting target for promotion of intestinal health and immune homeostasis. Several possibilities exist here and could be leveraged. First, acetate has highest concentration in the gut as well as in systemic circulation (Fig. 3) (96) suggesting that immune homeostasis possibly in the gut could be regulated naturally through consumption of fiber rich diet (111, 115, 181). Next, because functional redundancy of SCFA receptors does not allow partitioning of their physiological effects when using natural ligands, synthetic agonists could be of potential use to circumvent this issue. Third, FFA2 can exert effects both at intestinal epithelial and immune cells, where specific targeting of epithelial cells over immune cells or vice versa through synthetic agonists can be useful in different physiological settings.
Despite the compelling observations from in vivo (rodent) and in vitro (human and rodent cell lines) models, the first phase 2 clinical trial conducted in 2013 using FFA2 synthetic antagonist -[[(R)-1-(benzo[b]thiophene-3-carbonyl)-2-methyl-azetidine-2-carbonyl]-(3-chloro-benzyl)-amino]-butyric acid (GLPG0974, reported by Galapagos NV, Mechelen, Belgium; (28, 127)) failed to provide benefit to ulcerative colitis (UC) patients. This compound had been previously shown in vitro in human cell models to reduce FFA2 induced Ca2+ mobilization and neutrophil migration (28). Premise for using FFA2 antagonist in this 4-week study was that excessive neutrophil migration to the gut drives IBD and FFA2 is the neutrophil-SCFA receptor promoting neutrophil infiltration of the affected tissue (Figs. 4 and 5) (146,191). While GLPG0974 did reduce neutrophil activation and migration in UC patients, this decline did not produce any clinical benefits.
Failure of this trial signifies that our understanding of the complex interaction between FFA2 signaling and immune system may still be insufficient. First, most of our knowledge on the role of FFA2 in intestinal immunity is from mouse studies (92, 111, 116, 117, 181) and there may be differences in the type of inflammatory response elicited at FFA2 between species (7). Further, it has been shown that human FFA2 has constitutive activity (76), and how this constitutive activity is important in normal state and is altered in diseased states is yet unexplored. Also, for this trial, in vivo concentration of SCFAs, bacterial populations, and diet of the study population could also have influenced the results. Moreover, whether neutrophil activation improves or deteriorates the course of IBD is unclear (53). Additionally, FFA2 is expressed on different immune cell types; hence, consequence of its activity on different immune cell populations may vary along with differing effects based on existing (patho)physiological conditions (126). Lastly, as noted before, different ligands behave differently at FFA2 between the species [e.g., GLPG0974 is inactive at the mouse ortholog of the receptor (172) and CPTB stimulates Gαi/o at mouse FFA2 but Gαq/11 at human FFA2 (151)] and therefore, our understanding of this FFA2 antagonist pharmacology may be incomplete [reviewed in (184)].
As apparent, the role of these receptors needs to be definitively described, to resolve the doubts associated with their function (Fig. 6). Novel synthetic ligands active at these receptors are being generated [reviewed in (122)]. However, caution should be exercised with their use and interpretation in biological models until an understanding of downstream effectors is known. Also, understanding if these are allosteric or orthosteric agonists needs to be detailed in interpreting studies (26, 62, 72, 120, 122, 151). Thus, the effect of novel ligands as they are generated should be assessed in different biological models in vitro, ex vivo, and in vivo. Results should be deduced relative to a standard control model. For example, in in vitro systems and primary cells, the stage of cell differentiation and time of harvest of tissue from the animal can cause variation in receptor expression and activity (44, 94, 120). To draw a corollary for human receptors, parallel experiments should be conducted in human cell lines, miniature organs derived from human biopsy tissue (4), or related tissues (151). Awareness of the in vivo models specifically the genetic backgrounds, diet composition, and gut microbiota of mice also needs to be considered (123, 191). To reduce variable outcomes, comparisons should also be made to gnotobiotic, same and nonidentical strains of mice fed diets with similar compositions (as we enumerate in detail in the section on “Interplay of Gut Microbiota, Diet, and Short Chain Fatty Acid Receptor Signaling”).
Figure 6.
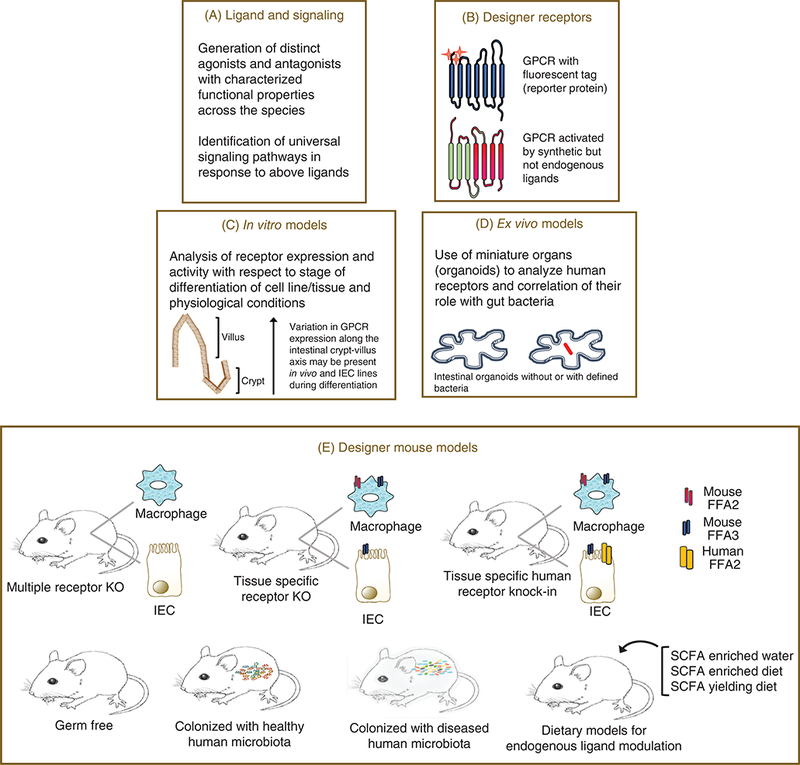
Customizing our approach for resolution of the roles of short chain fatty acid receptors. Evidence connects short chain fatty acid receptors to gut inflammatory responses. For reasons like shared endogenous ligands, differences in ligand efficacy for species orthologs (and others, enumerated in the text) there are hurdles in assigning selective physiological roles to these receptors. Their actual therapeutic potential thus remains unappreciated. These blocks can be overcome by customized approaches like, development of selective and potent agonists and antagonists for these receptors and their use in in vitro and ex vivo models (A and D); use of designer receptors with modified ligand binging sites, allowing activation of a particular member from the family which also contains a fluorescent tag (if attached to the receptor) (B); minimizing discrepancies by determining receptor expression along the course of cell/tissue differentiation (C); several different mouse models can be used to ascertain specific physiological effects of these receptors (E), including use of mice lacking two or more members of the family (multiple receptor KO), tissue specific KO or mice with gene of a human receptor replacing the mouse ortholog (tissue specific human receptor knock-in). These mouse models can then be used in combination with gut microbiota knockdown approaches (germ free), colonized with human microbiota, or fed SCFAs alone or in combination to determine the interrelationship of these receptors with gut microbiota (44,73,94,120,122,123,132,206,218).
Most of the studies described in sections above have utilized global KO models. Considering their overlapping expression and ligand profiles, its imperative to develop double receptor KOs, and tissue specific receptor KOs. For example, for elucidation of their role specifically in intestinal epithelial and immune cells, gut epithelial specific Cre and myeloid specific Cre expressing mice may prove useful to generate mutant mice lacking these receptors specifically in IECs and leukocytes. Problems associated with analysis of human receptor pharmacology and function in physiological settings (which may differ greatly in comparison to the mouse ortholog) requires “humanized mice” expressing human receptor globally or under specific tissue promoter to be developed. The human receptor in such models can also be tagged with reporter protein (e.g., mCherry) or have its active site mutated (73, 131, 206). These tagged receptor models could be useful to explain receptor dynamics and track expression with respect to physiological conditions. On the other hand, active site mutated human receptor is a thoughtful way to distinguish the effects of endogenous as well as synthetic ligand activity at the native and mutated receptor, respectively (73, 206). Such humanized mice when germ free, colonized with specific bacterial strains and normal or diseased human gut microbiota may also prove useful to study association of normal/altered gut microbiota with receptor activity and expression. In addition, studies with human enteroids could also increase our understanding of the role of these receptors. As apparent, unraveling the therapeutic potential of FFA2 (and FFA3) entails further in depth research.
GPR109a
Discovery
In one of the first studies, Benyo et al. (19) reported the expression of GPR109a in macrophages, other immune cells, and in adipocytes. Interestingly, these early studies uncovered that this GPCR was regulated by niacin, a well-known lipid-lowering agent (19). Moreover, the well-described side effect of flushing (which has historically limited its clinical usefulness) was indicated to be mediated through this GPCR. Another receptor GPR109b though closely related by sequence homology to GPR109a was also described; however, it showed much lower affinity to niacin (230). Moreover, this receptor, GPR109b, has thus far been described to be activated by aromatic D-amino acids and also molecules more similar to medium length fatty acids (135).
GPR109a is unique from FFA2 and FFA3, in that it is activated by the longer SCFAs, in particular butyrate, pen-tanoate and hexanoate, but it is also shown to be activated by beta-hydroxybutyrate (BHB) (135, 189). Due to the discovery of this GPCR as a niacin receptor and its well-defined side effects, multiple agonists have been developed with the goal of limiting this challenging side effect of flushing. These agonists largely appear to address the limitation of clinical use of niacin. For example, Richman et al. (159) described a number of pyrazole GPR109a agonists that seem to limit the flushing response to nicotinic acid, by preventing internalization of the receptor or activation of ERK 1/2 mitogen-activated protein kinase phosphorylation.
Expression
In addition to immune cells and adipose tissue (Fig. 3), GPR109a has also been shown to be expressed in pancreatic β cells (215). Though expressed poorly in pancreatic β cell culture lines, it is expressed in both mouse and human β cells within islets. GPR109a is also expressed in human microvas-cular endothelial cells (77) and microglial cells (54, 55). In retinal epithelial cells, it may have an anti-inflammatory role in diabetic retinopathy (58). GPR109a is also expressed in the lumen-facing apical membrane of colonic and intestinal epithelial cells, and is activated by butyrate (198). Interestingly, GPR109a has also been implicated in blood pressure regulation due to its expression in the brain, specifically in the rostral ventrolateral medulla (158). Additionally, GPR109a is expressed in mammary tissue (48), and may play a role in breast tumorigenesis, as noted in the following text. Compared to mammary tissue, its expression in breast cancer samples and cell lines is silenced, and ectopic expression of the receptor in these cell lines leads to cellular apoptosis. Consistent with this, in mice with GPR109a deletion, increased tumori-genesis has been observed (48). Taken together, GPR109a is expressed in several organs, and appears to contribute to a host of metabolic responses.
Pharmacology/signaling
Signaling by GPR109a has been predominantly shown to occur via Gαi/o pathway (215) (Figs. 1 and 4), which leads to lower intracellular cAMP levels. In adipose tissue, this results in reduced lipase activity, plasma triglycerides, and free fatty acid levels. In islets, GPR109a activation reduces insulin secretion and interestingly, its expression is significantly decreased in islets from diabetic patients and murine diabetic models (db/db mice) (215). However, the significant role of GPR109a in the prevention of diabetes needs further investigation. Cell surface levels of GPR109a have been shown to be tightly regulated. In depth analysis of GPR109a internalization process (104) has revealed that it is readily down regulated through clathrin-coated pathways (Fig. 2D). Further, its agonist-dependent internalization is regulated by arrestin-3 and G-protein coupled receptor kinase-2 (GRK2). In an intriguing study, Mandrika et al. (114) showed that GPR109a forms homodimers with itself and also heterodimers with GPR109b, and the dimerization process is not influenced by ligand binding. In this study, the authors described that GPR109a dimerization process occurred prior to it reaching the plasma membrane, possibly during receptor synthesis and might have a role in receptor trafficking (Fig. 2I). As both GPR109a and GPR109b are expressed in adipose tissue and regulate lipolysis, it remains unclear whether homo and/or heterodimer formation of these receptor subtypes plays an important role in their function in adipose tissue.
Role of GPR109a in the gut physiology
Role in nutrient sensing
The nutrient sensing machinery of the intestine includes the trans-membrane solute carrier transporters (SLC) in addition to the GPCRs being described here. While we do not know much about the relationship between SLC and GPCRs, one study examined this interrelationship. In colonic epithelial cells, SCFA uptake is well described to be mediated by luminal membrane MCT-1 (monocarboxylate transporter-1), where butyrate is known to stimulate MCT-1 expression. Borthakur et al. (30) recently demonstrated that GPR109a functions as a novel nutrient sensor, where short-term butyrate treatment (30–120 min) enhanced MCT-1 function and surface membrane expression through GPR109a signaling. Importantly, this increase in MCT-1 function and expression occurred at least in part through cAMP inhibition via GPR109a signaling by Gαi/o pathway (30).
Role of GPR109a in intestinal inflammation
Butyrate-activated GPR109a signaling has been reported to have an essential role in anti-inflammatory effects in the intestine. More specifically, GPR109a KO mice were shown to be more susceptible to DSS-induced colitis by showing increased mortality compared to control (181). Moreover, GPR109a KO mice also had increased susceptibility to colitis when subjected to Azoxymethane (AOM) plus DSS-induced colonic inflammation and carcinogenesis (176). Pro-inflammatory markers such as IL-6, CXCL1, CCL2, IL-17, and IL-1ß were each elevated and anti-inflammatory IL-18 was decreased in colons of GPR109a KO mice compared to WT mice (176). Using GPR109a KO mice and bone marrow chimera models, Macia et al. demonstrated the importance of these receptors in gut homeostasis. Additionally, it was observed that GPR109a-activated NLRP3 inflammasome, which promoted gut epithelial integrity (111). The role of GPR109a in the suppression of colitis thus appears partially to be due to the activation of NLRP3 inflammasome, decreased pro-inflammatory markers and increased IL-18 production in colonic tissue (Fig. 5).
Anti-inflammatory action of GPR109a in colon mainly involves increased production of anti-inflammatory Treg and IL-10 producing CD4+T-cells. CD4+ Treg cells suppress the deleterious activities of T-helper cells and thus play a critical role in immune mechanisms (163). Butyrate is considered to have a strong impact on the induction of Tregs as mice fed with butyrate displayed increased number of Treg cells in the colon compared to other SCFAs such as acetate and propionate. Germ free mice have lower Tregs than conventionally raised mice and high-fiber diet induces greater number of Treg cells than low-fiber diet (57). GPR109a is activated by butyrate and niacin and this signaling has been observed to regulate the production of Treg cells, and IL-18, and IL-10 producing T cells in colon. Colonic lamina propria of GPR109a KO mice also showed reduced frequency of Treg cells, IL-18, and elevated levels of IL-10 and IL-6 compared to WT littermates. However, no difference was observed in the spleens of WT and GPR109a KO mice, signifying that the action of GPR109a may be specific to the colon (111,176). The authors of this study further provided evidence that GPR109a-dependent differentiation of Tregs played an essential role in colonic inflammation as colonic dendritic cells and macrophages from GPR109a KO mice were defective in inducing differentiation of native T cells into Tregs compared to their WT counterparts. In other related studies, Chang et al. reported some of these aforementioned properties to be related to the inhibition of HDACs as a potential mechanism (36). However, whether SCFA-mediated GPCR activation promotes Treg frequency in intestinal lamina propria or impacts T-cell differentiation in lymph nodes remains unclear and further studies with GPR109a KO mice are needed to clarify this issue. Overall, data from multiple independent laboratories indicated that GPR109a-mediated induction of Tregs in colonic dendritic cells and macrophages to be a potential mechanism by which GPR109a regulates anti-inflammatory actions in colon.
While it is clear GPR109a senses SCFAs, it is intriguing that BHB also activates this receptor. As well known, BHB increases during fasting, and other related conditions, such as low calorie or ketogenic diet consumption, where these conditions are known to have metabolic benefits, including diminished inflammation. In fact, it has been suggested that BHB under conditions of fasting inhibits lipolysis through GPR109a and BHB-induced GPR109a activation on monocytes and macrophages reduces neuroinflammation (55,135). Whether the anti-inflammatory effects of BHB-GPR109a interaction are relevant in intestinal inflammation or underlie ketogenic diet induced anti-inflammation needs verification.
Role of GPR109a in colon cancer
In one of the first reports describing this receptor in 2009 (198), GPR109a was observed to be expressed on the luminal plasma membrane of the epithelial cells in colon and small intestine. Interestingly, in this report, GPR109a was found to be downregulated in human colon cancer cell lines (along with mouse models of colon cancer). Moreover, expression of GPR109a in these cells led to apoptosis in the presence of its ligands. These expression patterns were further confirmed in mice, and were observed to be downregulated in germ free mice (38). Using two different models of carcinogenesis (AOM plus DSS-induced carcinogenesis and ApcMin/+-driven intestinal and colon carcinogenesis), Singh et al. demonstrated that GPR109a KO mice were more prone to development of colon cancer than WT mice as GPRKO;ApcMm/+ mice had strikingly more polyps in the colon and small intestine than did ApcMm/+ mice (176). GPR109a levels have also been observed to be downregulated in human and mouse models of colon cancer and in cancer cell lines possibly due to DNA methylation (198). Restoration of GPR109a in cancer cell lines induced apoptosis only in the presence of ligands, for example, niacin and butyrate (198). Apoptosis induced by overexpression of GPR109a in cancer cell lines was not due to inhibition of HDACs but involved inhibition of Bcl-2, Bcl-xL, and cyclin D1, NFκb activity, and upregulation of death receptor pathway (198). Moreover, Bardhan et al. showed that IFN-γ-activated transcription of GPR109a promoter in colon carcinoma cells independent of DNA methylation, leads to its increased transcription, concluding that GPR109a acted as a tumor suppressor in colon cancer by IFN-γ regulation of transcription (15) (Fig. 2H). In summary, it appears that GPR109a plays important roles in gut inflammation and nutrient sensing in the gut, and may have a role in carcinogenesis.
Role of GPR109a in extraintestinal tissues
Role in diabetes
Suggesting a role in diabetes, GPR109a has been observed to be down regulated in islets of diabetic db/db mice as well as in type 2 diabetes (T2D) patients compared to islets of normal control subjects (215). Additionally, in Min6 cells (a mouse pancreatic β cell line), high glucose, plus nicotinic acid or 3 hydroxy butyrate treatment inhibited GPR109a expression (225). Administration of selective GPR109a agonist, GSK256073, in diabetic human subjects significantly reduced serum glucose and nonesterified fatty acids (NEFA) without any complications of flushing and GI disturbances (45). Taken together these studies indicate a potential role of GPR109a in T2D and promising therapeutic value of GPR109a agonist, GSK256073 in the treatment of T2D.
Role in atherosclerosis
Niacin reduces the progression of atherosclerosis by lipid modifying effects. Niacin treatment also significantly reduced the expression of inflammatory markers such as TNFα, IL-6, IL1β, and IL12p40 in LPS-stimulated macrophages from WT mice but not GPR109a KO mice (229). Also, the expression of cholesterol transporter ABCG1 and cholesterol efflux was reduced by niacin in macrophages from GPR109a KO mice compared to WT mice. In addition, activation of GPR109a by niacin reduced macrophage recruitment to atherosclerotic plaques. These reports provide compelling evidence that nicotinic acid reduces the progression of atherosclerosis via activation of GPR109a on immune cells and regulation of inflammation (109).
Therapeutic potential of GPR109a agonists
As described earlier, the antiatherogenic and antiinflammatory effects of GPR109a are not only via antilipolytic effects but also through the role of GPR109a in immune cells. The reduction in the progression of atherosclerosis was attributed to the reduced infiltration of vessel wall by GPR109a expressing macrophages and neutrophils. There is also evidence that dimethyl fumarate (DMF) and dietary metabolites such as butyrate mediate antineuroinflammatory effects in Parkinson’s disease and multiple sclerosis, respectively, via activation of GPR109a (83). DMF has also been shown to have a beneficial effect in the treatment of psoriasis (12, 125). In intestine, butyrate-mediated activation of GPR109a leads to improvement in colitis and limits progression of colon cancer. In summary, many studies have implicated GPR109a agonists as potential therapeutic targets in the treatment of atherosclerosis, neuroinflammatory disorders, colitis, and psoriasis; however, more detailed mechanistic studies are warranted to further establish the direct role of GPR109a in these processes.
Olfr78
Discovery
Olfactory Receptor 78 (Olfr78) also known as OR51E2 in humans (Olfactory receptor family 51, subfamily E, member 2) or PSGR, (prostate-specific G-protein-coupled receptor) is expressed, in multiple tissues (148, 160, 221) (Fig. 3). As compared to FFA2, FFA3, and GPR109a, Olfr78 is overall less well characterized regarding its expression, function, and pharmacology. Although primarily expressed in olfactory tissues, recent reports indicate its expression in other organs. Initial reports observed Olfr78 as a gene upregulated in prostate cancer (224), as such, it was initially called prostate-specific G-protein-coupled receptor. While it was initially observed to be expressed in human prostate tissue, expression in the human brain was also found, specifically in the medulla oblongata and olfactory region. In mice, it was also observed to be expressed in the colon and in rats, expression was observed in the liver (51, 118).
Pharmacology/signaling
Acetate and propionate have both been shown to activate Olfr78, with EC50 values for acetate around 2.4 mmol/L and for propionate ~0.9 mmol/L (Table 3) (148). Regarding its signaling pathway, the early reports suggest it is likely that Olfr78 is a Gαs-coupled receptor, as cAMP levels increase when it is activated (148). In melanocytes and prostate cells, coupling through Gαq has also been reported. However, to the best of our knowledge, the G-protein signaling preferences of Olfr78 have not been extensively explored. Moreover, other characteristics of its signaling mechanisms have not been examined to date.
Role of Olfr78 in the gut physiology/ pathophysiology
As compared to other SCFA receptors, the role of Olfr78 in GI physiology is largely unknown. Thus far, it has been reported that elevated levels of OR51E2/Olfr78 are observed in colon cancers (2, 101, 107). It is also identified as one of the candidate driver genes for microsatellite-unstable colorectal cancer with a somatic mutation frequency of >20% (2). Olfr78 is expressed in colonic epithelial cells, and specifically a class of enteroendocrine cells that coexpress PYY (51). However, its role in these cells is not known and needs further investigation.
Role of Olfr78 in extraintestinal tissues
Recent evidence suggests that Olfr78/OR51E2 plays an important role in nonolfactory organs. For example, this receptor is implicated in blood pressure regulation (147) and a variety of other cellular functions. Pluznick et al. showed that Olfr78 activation increased whereas FFA3 activation was able to reduce blood pressure (148). In another study, Chang et al. reported an activation of Olfr78 by lactate in carotid body under hypoxic conditions, which resulted in elevated blood pressure thereby preventing hypotension and restoring normal blood pressure (35). In prostate cells, overexpression of OR51E2 induced chronic inflammatory response leading to prostate intraepithelial neoplasia (160,161). Ligand-induced activation of OR51E2 has also been shown to affect melanocyte proliferation, differentiation, and melanogenesis (60). Overall, these studies indicate a pathophysiological role of this receptor in blood pressure regulation, colon cancer, and prostate neoplasia. As there are only limited studies thus far, further studies are needed for a thorough analysis of its functional significance in different organs.
Interplay of Gut microbiota, Diet, and Short Chain Fatty Acid Receptor Signaling
So far, the interaction between the receptors and the SCFAs has been described. However, gut microbiota, the major site of SCFA production, can also exert control on the receptors by modulating the concentration of their ligands (69, 142). But this relationship between the gut microbiota and receptors through SCFAs is far from simple. Gut microbial structural components and other metabolites also affect host physiology with potential bearings on SCFA levels and these receptors [reviewed in (169, 199)]. Gut microbiota can also interact with the host genotype, and it can be influenced by hygiene habits (191, 199, 218). Furthermore, it is well documented that mammalian diet influences the compositional properties of the gut bacteria (182). Both these processes along with dietary fiber content govern SCFA production in the gut (96, 110). Of note, intestinal inflammatory diseases like IBD involve complex interaction of all of the above (16, 153). With this obligatory relationship between diet, gut bacteria and SCFAs, involvement of SCFA receptors in dietary intervention induced protection against gut barrier breach and inflammation is possible, and is slowly being experimentally tested.
In WT or gnotobiotic mice, SCFA supplemented drinking water and/or fiber/SCFA enriched diets have been employed to understand the interplay of gut microbiota, diet, and short chain fatty acid receptor signaling in promotion of intestinal health and its discontinuity in intestinal inflammation. From these studies, FFA2 and GPR109a have emerged as links between host immune responses and gut microbiota. These receptors are considered to act as sensors of these gut microbial metabolites, the SCFAs, and maintain intestinal immune homeostasis. A loss of the signals or of the receptors then would be causal for intrinsic immune defects and deregulated immune response to exogenous insults. In fact, SCFA deficit arising from the absence of gut microbiota (as in germ free mice, antibiotic-treated specific pathogen free mice, and antibiotic-treated WT mice) or lack of fiber in the diet reduces colonic Treg cell population and exacerbates DSS colitis in mice (1, 11, 111, 116,157, 176, 181). SCFAs, niacin, or pharmacologic agonist treatment could rescue this phenotype but only in the germ free and gut microbiota-modified mice (1, 116, 176, 181). The FFA2 KO and GPR109a KO mice lacked such protection (116, 176). As the level of SCFAs in gastrointestinal tract is to a large extent determined by fiber content in the diet, a high proportion of fiber should produce similar effects (69, 142). Consumption of fiber-free diet in WT mice worsened and of high fiber diet in FFA2 KO and GPR109a KO mice did not combat DSS colitis (111, 176). Dietary fiber also conferred protection against colitis-associated cancer in FFA2 and GPR109a-dependent manner (111, 176, 177). FFA2 and GPR109a, thus, emerge as functional links between gut microbiota via SCFAs to intestinal immune effects (Fig. 7).
Figure 7.
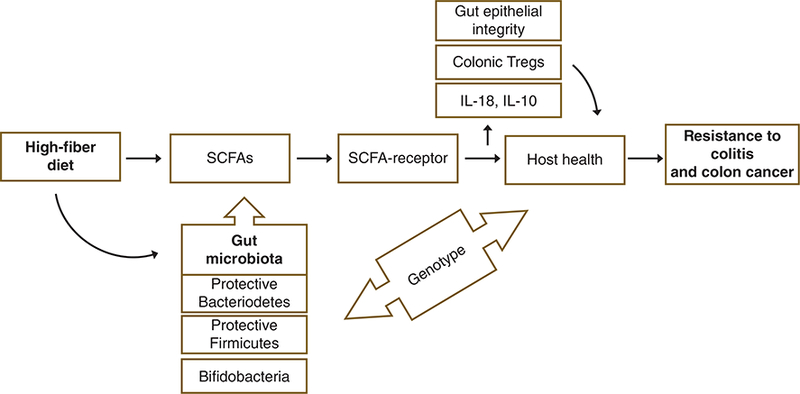
Interplay of diet, gut microbiota, and short chain fatty acid receptors. Gut microbiota converts fiber in the diet to SCFAs. Activity of SCFAs at their receptors on gut cells promotes epithelial integrity and immune homeostasis. High-fiber diet also shapes and selects for beneficial gut microbiota, and this selection is dependent on the genotype of the host. The combinatorial effect of these processes is resistance against inflammatory states like colitis and colitis associated cancer (111,116,181,190,191).
The mechanistic underpinnings of diet induced immune protection extend beyond SCFA-cognate receptor mediated upregulation of protective cytokines (IL-10, IL-18), inflam-masome activation, and increase in colonic Tregs (effects discussed earlier) (111, 116, 176). Diet also can significantly modify gut microbial composition and function (41,119,182). Fiber-deficient diet has been observed to select for members of Bacteroidetes and Proteobacteria families which was associated with severe DSS colitis and reduced IL-18 levels (1, 111). Whereas, high-fiber diet, selected for specific protective members of Bacteroidetes (Porphyromonadaceae and Rikenellaceae) and Firmicutes (Lachnospiraceae) (111) and was found to be associated with reduced severity of DSS colitis and enhanced IL-18 levels. Colonization of germ free mice with high-fiber diet-induced gut microbiota could resist DSS colitis (111). An interesting observation in these studies was the expansion of colitogenic and cancer-associated bacteria (like Proteobacteria, Prevotellaceae, Helicobacter hepaticus) in FFA2 KO and GPR109a KO mice (111, 176–178). In the same line, presence of FFA2 was shown to be necessary for selection of Bifidobacterium species in high fiber diet fed mice (177). Of note, decrease in Bifidobacterium species and increase in Proteobacteria (represented mainly by Escherichia coli) was reported to be associated with intestinal inflammation and colon cancer (1, 10, 46, 134). Consistent with this, these receptor KO mice, which were susceptible to colitis, associated cancer (111,139,176,177), and could be rendered cancer resistant with use of antibiotics (177), suggests that gut microbiota alone could also confer beneficial effects. Indeed, transfer of “protective bacteria” to the deficient mice could confer protection against colitis regardless of receptor or dietary status (111, 177). Moreover, suppression of colon inflammation and carcinogenesis in FFA2 KO mice could be achieved by oral gavage of Bifidobacterium infantis (177, 178) or upon co-housing with WT littermates allowing transfer of protective bacteria from WT to KO mice (111). These results point to the fact that “beneficial bacteria” may confer protection against intestinal inflammatory diseases (137), while SCFAs and cognate receptors, thus, are not the entire story but only part of it. Their contributions are likely additive, to enhance the effects of beneficial microbes, and this needs further investigation.
The recent work of Macia et al. (111), Sivaprakasma et al. (177), and Singh et al. (176) alludes to the role of SCFA receptors in preventing disruption of commensal gut bacteria. Selection of specific gut bacterial profiles associated with absence of receptors (and their signaling) is intriguing. It suggests that receptor signaling is required for basal immune homeostasis and to balance the activities of harmful and beneficial bacteria that cohabit in the gut (170). This also suggests that receptor absence can influence tolerance with contribution from various factors like lack of IgA response (220). In one of our reports, we did not specifically observe any discrimination against cancer/colitis promoting bacteria in FFA2 KO as compared to WT mice (56) while McNelis et al. (120) observed selection of butyrate producers in WT and acetate producers in FFA2 KO mice which could be a compensatory response for lack of acetate receptor (FFA2) signaling. Though high-fat diet is associated with dysbiosis and mild inflammation (34), no inference was drawn regarding increase of intestinal inflammation promoting bacteria in the KO mice (120). While these differences may arise from the distinct focus of these separate studies, future studies are required to understand the complex association of specific gut microbial species in these different KO models.
Gut microbiota dependence of SCFA receptor signaling has also been shown to influence metabolic roles of these receptors. For example, FFA3 plays a crucial role in gut microbiota-dependent host energy balance (165). Reduced adiposity in FFA3 KO mice compared to the WT mice was abrogated in germ free conditions. Using conventionally raised or germ-free mice co-colonized with two human gut microbes the Saccharolytic bacterium, Bacteroides thetaiotaomicron, and the methanogenic archaeon, Methanobrevibacter smithii, the authors demonstrated that co-colonization of germ free mice with these microbes significantly increased the levels of PYY but not in FFA3 KO mice (165). Additionally, intestinal transit rates were faster in FFA3 KO mice compared to WT littermates and this effect was abolished in germ free conditions. Thus, gut microbiota-dependent SCFA production and their signaling at FFA3 are necessary for PYY secretion (165). Similarly, spontaneous obese phenotype of FFA2 KO mice could be rescued under germ free conditions or upon antibiotic treatment (94).
Functional implications of these observations to humans are needed. For example, proof of concept experiments using “humanized mice” colonized with gut microbiota of healthy and IBD patients are needed. In such study, colonization of such humanized mice with healthy bacteria like Firmicutes [anti-inflammatory bacteria expanded on high fiber feeding (111)] was recently shown to be beneficial against colitis but the contribution of the SCFA receptors was not studied (128). To harness the potential of SCFA receptors as therapeutic targets, more detailed and focused studies are required.
Conclusion
SCFAs have been long known to be key nutrients generated in the gut, contributing to GI tract physiology and at times, pathophysiology. During the last 20 years, significant evidence has accumulated that cognate GPCRs, FFA2, FFA3, GPR109a, and Olfr78, serve as sensors for SCFAs. We are just beginning to understand the role of these receptors in health and disease, and this article outlines the observations so far in modulation of gastrointestinal health.
While, the role of FFA3 is still less appreciated and the studies on Olfr78 are in their infancy, we have a bit better understanding of the contributions of FFA2 and GPR109a. Leveraging genetic mouse models (receptor knockout and overexpression), synthetic ligands, latest technologies of gut microbiome profiling, recent studies have corroborated a role for FFA2 and GPR109a in diseases like IBD and colon cancer underscoring their role as potential therapeutic targets. However, there are a number of questions yet unanswered. With single receptor whole body knockouts, the function of these receptors can be compensated. In other words, in absence of the use of selective synthetic agonists in vivo, it is difficult to assign a specific role to just one of these receptors. SCFAs also have receptor-independent effects. Thus, future studies aiming to answer these questions will benefit from the use of more sophisticated mouse models like with double, multiple or tissue specific receptor knockouts, and enteroids prepared from these mouse models and from human intestinal biopsies from healthy subjects and patients with IBD, colon cancer, or metabolic disorders.
In addition, FFA2 and GPR109a can signal through multiple pathways. Activation of one pathway may have beneficial or harmful effects that may also depend on time of activation during the course of disease (e.g., FFA2-dependent inflamma-some activation is beneficial in the gut but may be detrimental in gout (208)). Pharmacologic tools for targeted tissue specific activation of these receptors are thus required. These receptors lie at the crossroad of inflammation and metabolism. How these pathways intersect and influence the course of inflammatory and metabolic disorders through these receptors is intriguing. Furthermore, interdependence of gut microbiota, diet, and short chain fatty acid receptor signaling (with additional effects from host genetics, health status, and environment) may have distinct functional consequences on the host. Thus, several caveats exist to the pharmacologic and therapeutic translation of these receptors to humans. Nonetheless, this interplay of gut microbiota, SCFAs and cognate receptor signaling is the Pandora’s promise of combating serious inflammatory diseases like IBD, colon cancer, and promoting gut health (Fig. 7).
Didactic Synopsis.
Short chain fatty acids (SCFAs) produced by gut microbial fermentation serve both as an energy source and signaling molecules.
Molecular sensors for these SCFAs are G-protein coupled receptors. These GPCRs, namely, FFA2 and −3 and GPR109a have immune as well as metabolic roles.
These receptors, including Olfr78, have overlapping ligand profiles and some functional redundancy, but their restricted expression pattern in various cell types in the gut (and other tissues) and distinct G-protein coupling profiles allows for specific physiologic effects.
FFA2 and GPR109a modify the gut immune cell repertoire and inflammatory responses regulating immune homeostasis, guard intestinal epithelial barrier against pathogenic intrusion and tumor growth.
FFA2 and FFA3 appear to regulate GLP-1, GIP, and PYY secretion exerting effects on glucose homeostasis.
These roles signify a link between gut microbiota and host physiology through SCFA receptors.
Targeted modulation of these SCFA receptors may promote intestinal health.
Acknowledgements
This work was supported by Department of Veterans Affairs Merit Award #BX002011, Veterans Affairs Senior Research Career Scientist Award, and the National Institutes of Health award #R01DK54016 (to P. K. Dudeja), and Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Career Development Grant #1IK2BX001587–01, the National Institutes of Health award #R01DK104927–01A1 and the University of Chicago DR&TC Grant #P30DK020595 (to B. T. Layden).
References
- 1.Agus A, Denizot J, Thevenot J, Martinez-Medina M, Massier S, Sauvanet P, Bernalier-Donadille A, Denis S, Hofman P, Bonnet R, Billard E, Barnich N. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci Rep 6: 19032, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alhopuro P, Sammalkorpi H, Niittymaki I, Bistrom M, Raitila A, Saharinen J, Nousiainen K, Lehtonen HJ, Heliovaara E, Puhakka J, Tuupanen S, Sousa S, Seruca R, Ferreira AM, Hofstra RM, Mecklin JP, Jarvinen H, Ristimaki A, Orntoft TF, Hautaniemi S, Arango D, Karhu A, Aaltonen LA. Candidate driver genes in microsatellite-unstable colorectal cancer. Int J Cancer 130: 1558–1566, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Curto E, Milligan G. Metabolism meets immunity: The role of free fatty acid receptors in the immune system. Biochem Pharmacol 114:3–13, 2016. [DOI] [PubMed] [Google Scholar]
- 4.Anabazhagan AN, Chatterjee I, Priyamvada S, Kumar A, Tyagi S, Saksena S, Alrefai WA, Dudeja PK, Gill RK. Methods to study epithelial transport protein function and expression in native intestine and Caco-2 cells grown in 3D. J Vis Exp 2017(121): e55304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang Z, Ding JL. GPR41 and GPR43 in obesity and inflammation— protective or causative? Front Immunol 7: 28, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ang Z, Er JZ, Ding JL. The short-chain fatty acid receptor GPR43 is transcriptionally regulated by XBP1 in human monocytes. Sci Rep 5: 8134, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang Z, Er JZ, Tan NS, Lu J, Liou YC, Grosse J, Ding JL. Human and mouse monocytes display distinct signalling and cytokine profiles upon stimulation with FFAR2/FFAR3 short-chain fatty acid receptor agonists. Sci Rep 6: 34145, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ang Z, Xiong D, Wu M, Ding JL. FFAR2-FFAR3 receptor heteromerization modulates short-chain fatty acid sensing. FASEB J 32: 289–303, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338: 120–123, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov, II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331:337–341, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atwan A, Ingram JR, Abbott R, Kelson MJ, Pickles T, Bauer A, Piguet V. Oral fumaric acid esters for psoriasis. Cochrane Database Syst Rev. 10(8):CD010497, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahar Halpern K, Veprik A, Rubins N, Naaman O, Walker MD. GPR41 gene expression is mediated by internal ribosome entry site (IRES)-dependent translation of bicistronic mRNA encoding GPR40 and GPR41 proteins. J Biol Chem 287: 20154–20163, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bahouth SW, Nooh MM. Barcoding of GPCR trafficking and signaling through the various trafficking roadmaps by compartmentalized signaling networks. Cell Signal 36: 42–55, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardhan K, Paschall AV, Yang D, Chen MR, Simon PS, Bhutia YD, Martin PM, Thangaraju M, Browning DD, Ganapathy V, Heaton CM, Gu K, Lee JR, Liu K. IFNgamma induces DNA methylation-silenced GPR109A expression via pSTAT1/p300 and H3K18 acetylation in colon cancer. Cancer Immunol Res 3: 795–805, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basson A, Trotter A, Rodriguez-Palacios A, Cominelli F. Mucosal interactions between genetics, diet, and microbiome in inflammatory bowel disease. Front Immunol 7: 290, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beglinger C, Degen L. Gastrointestinal satiety signals in humans— physiologic roles for GLP-1 and PYY? Physiol Behav 89: 460–464, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Bellahcene M, O’Dowd JF, Wargent ET, Zaibi MS, Hislop DC, Ngala RA, Smith DM, Cawthorne MA, Stocker CJ, Arch JR. Male mice that lack the G-protein-coupled receptor GPR41 have low energy expenditure and increased body fat content. Br J Nutr 109: 1755–1764, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Benyo Z, Gille A, Kero J, Csiky M, Suchankova MC, Nusing RM, Moers A, Pfeffer K, Offermanns S. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J Clin Invest 115: 3634–3640, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhutia YD, Ganapathy V. Short, but smart: SCFAs train T cells in the gut to fight autoimmunity in the brain. Immunity 43: 629–631, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bindels LB, Dewulf EM, Delzenne NM. GPR43/FFA2: Physiopathological relevance and therapeutic prospects. Trends Pharmacol Sci 34: 226–232, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Bindels LB, Porporato P, Dewulf EM, Verrax J, Neyrinck AM, Martin JC, Scott kP , Buc Calderon P, Feron O, Muccioli GG, Sonveaux P, Cani PD, Delzenne NM Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br J Cancer 107: 1337–1344, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjursell M, Admyre T, Goransson M, Marley AE, Smith DM, Oscarsson J, Bohlooly YM. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab 300: E211–E220, 2011. [DOI] [PubMed] [Google Scholar]
- 24.Black JB, Premont RT, Daaka Y. Feedback regulation of G protein-coupled receptor signaling by GRKs and arrestins. Semin Cell Dev Biol 50: 95–104, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov 11: 603–619, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Boirivant M, Amendola A, Butera A, Sanchez M, Xu L, Marinaro M, Kitani A, Di Giacinto C, Strober W, Fuss IJ. A transient breach in the epithelial barrier leads to regulatory T-cell generation and resistance to experimental colitis. Gastroenterology 135: 1612–1623 e1615, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Bolognini D, Moss CE, Nilsson K, Petersson AU, Donnelly I, Sergeev E, Konig GM, Kostenis E, Kurowska-Stolarska M, Miller A, Dekker N, Tobin AB, Milligan G. A novel allosteric activator of free fatty acid 2 receptor displays unique Gi-functional bias. J Biol Chem 291: 18915–18931, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bolognini D, Tobin AB, Milligan G, Moss CE. The pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol 89: 388–398, 2016. [DOI] [PubMed] [Google Scholar]
- 29.Bonini JA, Anderson SM, Steiner DF. Molecular cloning and tissue expression of a novel orphan G protein-coupled receptor from rat lung. Biochem Biophys Res Commun 234: 190–193, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Borthakur A, Priyamvada S, Kumar A, Natarajan AA, Gill RK, Alrefai WA, Dudeja PK. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. Am J Physiol Gastrointest Liver Physiol 303: G1126–G1133, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brooks L, Viardot A, Tsakmaki A, Stolarczyk E, Howard JK, Cani PD, Everard A, Sleeth ML, Psichas A, Anastasovskaj J, Bell JD, Bell-Anderson K, Mackay CR, Ghatei MA, Bloom SR, Frost G, Bewick GA. Fermentable carbohydrate stimulates FFAR2-dependent colonic PYY cell expansion to increase satiety. Mol Metab 6: 48–60, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar Dm , Foord SM, Wise A, Dowell SJ The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol 11: 577–591, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Chang AJ, Ortega FE, Riegler J, Madison DV, Krasnow MA. Oxygen regulation of breathing through an olfactory receptor activated by lactate. Nature 527: 240–244, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang PV, Hao L, Offermanns S, Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111: 2247–2252, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox MA, Jackson J, Stanton M, Rojas-Triana A, Bober L, Laverty M, Yang X, Zhu F, Liu J, Wang S, Monsma F, Vassileva G, Maguire M, Gustafson E, Bayne M, Chou cC, Lundell D, Jenh CH Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol 15: 5549–5557, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg 14: 449–461, 2010. [DOI] [PubMed] [Google Scholar]
- 39.Cummings DE, Overduin J. Gastrointestinal regulation of food intake. J Clin Invest 117: 13–23, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28: 1221–1227, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daien CI, Pinget GV, Tan JK, Macia L. Detrimental impact of microbiota-accessible carbohydrate-deprived diet on gut and immune homeostasis: An overview. Front Immunol 8: 548, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dass NB, John AK, Bassil AK, Crumbley CW, Shehee WR, Maurio FP, Moore GB, Taylor CM, Sanger GJ. The relationship between the effects of short-chain fatty acids on intestinal motility in vitro and GPR43 receptor activation. Neurogastroenterol Motil 19: 66–74, 2007. [DOI] [PubMed] [Google Scholar]
- 43.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156: 84–96, 2014. [DOI] [PubMed] [Google Scholar]
- 44.Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, Deldicque L, Bindels LB, Pachikian BD, Sohet FM, Mignolet E, Francaux M, Larondelle Y, Delzenne NM. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem 22: 712–722, 2011. [DOI] [PubMed] [Google Scholar]
- 45.Dobbins RL, Shearn SP, Byerly RL, Gao FF, Mahar KM, Napolitano A, Nachbaur GJ, Le Monnier de Gouville AC GSK256073, a selective agonist of G-protein coupled receptor 109A (GPR109A) reduces serum glucose in subjects with type 2 diabetes mellitus. Diabetes Obes Metab 15: 1013–1021, 2013. [DOI] [PubMed] [Google Scholar]
- 46.Duranti S, Gaiani F, Mancabelli L, Milani C, Grandi A, Bolchi A, Santoni A, Lugli GA, Ferrario C, Mangifesta M, Viappiani A, Bertoni S, Vivo V, Serafini F, Barbaro MR, Fugazza A, Barbara G, Gioiosa L, Palanza P, Cantoni AM, de’Angelis GL, Barocelli E, de’Angelis N, van Sinderen D, Ventura M, Turroni F. Elucidating the gut microbiome of ulcerative colitis: Bifidobacteria as novel microbial biomarkers. FEMS Microbiol Ecol 92: fiw191, 2016. [DOI] [PubMed] [Google Scholar]
- 47.Eberle JA, Widmayer P, Breer H. Receptors for short-chain fatty acids in brush cells at the “gastric groove.” Front Physiol 5: 152, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elangovan S, Pathania R, Ramachandran S, Ananth S, Padia RN, Lan L, Singh N, Martin PM, Hawthorn L, Prasad PD, Ganapathy V, Thangaraju M. The niacin/butyrate receptor GPR109A suppresses mammary tumorigenesis by inhibiting cell survival. Cancer Res 74: 1166–1178, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engelstoft MS, Park WM, Sakata I, Kristensen LV, Husted AS, Osborne-Lawrence S, Piper PK, Walker AK, Pedersen MH, Nohr MK, Pan J, Sinz CJ, Carrington PE, Akiyama TE, Jones RM, Tang C, Ahmed K, Offermanns S, Egerod KL, Zigman JM, Schwartz TW. Seven transmembrane G protein-coupled receptor repertoire of gastric ghrelin cells. Mol Metab 2: 376–392, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Farran B An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol Res 117: 303–327, 2017. [DOI] [PubMed] [Google Scholar]
- 51.Fleischer J, Bumbalo R, Bautze V, Strotmann J, Breer H. Expression of odorant receptor Olfr78 in enteroendocrine cells of the colon. Cell Tissue Res 361: 697–710, 2015. [DOI] [PubMed] [Google Scholar]
- 52.Forbes S, Stafford S, Coope G, Heffron H, Real K, Newman R, Davenport R, Barnes M, Grosse J, Cox H. Selective FFA2 agonism appears to act via intestinal PYY to reduce transit and food intake but does not improve glucose tolerance in mouse models. Diabetes 64: 3763–3771, 2015. [DOI] [PubMed] [Google Scholar]
- 53.Fournier BM, Parkos CA. The role of neutrophils during intestinal inflammation. Mucosal Immunol 5: 354–366, 2012. [DOI] [PubMed] [Google Scholar]
- 54.Fu SP, Liu BR, Wang JF, Xue WJ, Liu HM, Zeng YL, Huang BX, Li SN, Lv QK, Wang W, Liu JX. beta-Hydroxybutyric acid inhibits growth hormone-releasing hormone synthesis and secretion through the GPR109A/extracellular signal-regulated 1/2 signalling pathway in the hypothalamus. J Neuroendocrinol 27: 212–222, 2015. [DOI] [PubMed] [Google Scholar]
- 55.Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL, Li SN, Huang BX, Lv QK, Wang W, Liu JX. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation 12: 9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fuller M, Priyadarshini M, Gibbons SM, Angueira AR, Brodsky M, Hayes MG, Kovatcheva-Datchary P, Backhed F, Gilbert JA, Lowe WL Jr., Layden BT The short-chain fatty acid receptor, FFA2, contributes to gestational glucose homeostasis. Am J Physiol Endocrinol Metab 309: E840–E851, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504: 446–450, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Gambhir D, Ananth S, Veeranan-Karmegam R, Elangovan S, Hester S, Jennings E, Offermanns S, Nussbaum JJ, Smith SB, Thangaraju M, Ganapathy V, Martin PM. GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest Ophthalmol Vis Sci 53: 2208–2217, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garrett WS, Smith PM. Modulation of regulatory t cells via g-coupled protein receptor 43. Google Patents, US Patent 1, (2017 11 30): 59 Pages, 2014.
- 60.Gelis L, Jovancevic N, Veitinger S, Mandal B, Arndt HD, Neuhaus EM, Hatt H. Functional characterization of the odorant receptor 51E2 in human melanocytes. J Biol Chem 291: 17772–17786, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gribble FM, Diakogiannaki E, Reimann F. Gut hormone regulation and secretion via FFA1 and FFA4. Handb Exp Pharmacol 236: 181–203, 2017. [DOI] [PubMed] [Google Scholar]
- 62.Grundmann M, Tikhonova IG, Hudson BD, Smith NJ, Mohr K, Ulven T, Milligan G, Kenakin T, Kostenis E. A molecular mechanism for sequential activation of a G protein-coupled receptor. Cell Chem Biol 23: 392–403, 2016. [DOI] [PubMed] [Google Scholar]
- 63.Haghikia A, Jorg S, Duscha A, Berg J, Manzel A, Waschbisch A, Hammer A, Lee DH, May C, Wilck N, Balogh A, Ostermann AI, Schebb NH, Akkad Da, Grohme DA, Kleinewietfeld M, Kempa S, Thone J, Demir S, Muller DN, Gold R, Linker RA Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 43: 817–829, 2015. [DOI] [PubMed] [Google Scholar]
- 64.Han JH, Kim IS, Jung SH, Lee SG, Son HY, Myung CS. The effects of propionate and valerate on insulin responsiveness for glucose uptake in 3T3-L1 adipocytes and C2C12 myotubes via G protein-coupled receptor 41 PLoS One 9: e95268, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han M, Song P, Huang C, Rezaei A, Farrar S, Brown MA, Ma X. Dietary grape seed proanthocyanidins (GSPs) improve weaned intestinal microbiota and mucosal barrier using a piglet model. Oncotarget 7: 80313–80326, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harig JM, Soergel KH, Komorowski RA, Wood CM. Treatment of diversion colitis with short-chain-fatty acid irrigation. N Engl J Med 320: 23–28, 1989. [DOI] [PubMed] [Google Scholar]
- 67.Hatanaka H, Tsukui M, Takada S, Kurashina K, Choi YL, Soda M, Yamashita Y, Haruta H, Hamada T, Ueno T, Tamada K, Hosoya Y, Sata N, Yasuda Y, Nagai H, Sugano K, Mano H. Identification of transforming activity of free fatty acid receptor 2 by retroviral expression screening. Cancer Sci 101: 54–59, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146: 5092–5099, 2005. [DOI] [PubMed] [Google Scholar]
- 69.Hoverstad T, Midtvedt T. Short-chain fatty acids in germfree mice and rats. J Nutr 116: 1772–1776, 1986. [DOI] [PubMed] [Google Scholar]
- 70.Hu J, Kyrou I, Tan BK, Dimitriadis GK, Ramanjaneya M, Tripathi G, Patel V, James S, Kawan M, Chen J, Randeva HS. Short-chain fatty acid acetate stimulates adipogenesis and mitochondrial biogenesis via GPR43 in brown adipocytes. Endocrinology 157: 1881–1894, 2016. [DOI] [PubMed] [Google Scholar]
- 71.Hu Y, Le Leu RK, Christophersen CT, Somashekar R, Conlon MA, Meng XQ, Winter JM, Woodman RJ, McKinnon R, Young GP. Manipulation of the gut microbiota using resistant starch is associated with protection against colitis-associated colorectal cancer in rats. Carcinogenesis 37: 366–375, 2016. [DOI] [PubMed] [Google Scholar]
- 72.Hudson BD, Christiansen E, Murdoch H, Jenkins L, Hansen AH, Madsen O, Ulven T, Milligan G. Complex pharmacology of novel allosteric free fatty acid 3 receptor ligands.Mol Pharmacol 86:200–210, 2014. [DOI] [PubMed] [Google Scholar]
- 73.Hudson BD, Christiansen E, Tikhonova IG, Grundmann M, Kostenis E, Adams DR, Ulven T, Milligan G. Chemically engineering ligand selectivity at the free fatty acid receptor 2 based on pharmacological variation between species orthologs. FASEB J 26: 4951–4965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hudson BD, Due-Hansen ME, Christiansen E, Hansen AM, Mackenzie AE, Murdoch H, Pandey SK, Ward RJ, Marquez R, Tikhonova IG, Ulven T, Milligan G. Defining the molecular basis for the first potent and selective orthosteric agonists of the FFA2 free fatty acid receptor. J Biol Chem 288: 17296–17312, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hudson BD, Smith NJ, Milligan G. Experimental challenges to targeting poorly characterized GPCRs: Uncovering the therapeutic potential for free fatty acid receptors. Adv Pharmacol 62: 175–218, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Hudson BD, Tikhonova IG, Pandey SK, Ulven T, Milligan G. Extracellular ionic locks determine variation in constitutive activity and ligand potency between species orthologs of the free fatty acid receptors FFA2 and FFA3. J Biol Chem 287: 41195–41209, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hughes-Large JM, Pang DK, Robson DL, Chan P, Toma J, Borradaile NM. Niacin receptor activation improves human microvascular endothelial cell angiogenic function during lipotoxicity. Atherosclerosis 237: 696–704, 2014. [DOI] [PubMed] [Google Scholar]
- 78.Inoue D, Kimura I, Wakabayashi M, Tsumoto H, Ozawa K, Hara T, Takei Y, Hirasawa A, Ishihama Y, Tsujimoto G. Short-chain fatty acid receptor GPR41-mediated activation of sympathetic neurons involves synapsin 2b phosphorylation. FEBS Lett 586: 1547–1554, 2012. [DOI] [PubMed] [Google Scholar]
- 79.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med 7: 180–185, 2001. [DOI] [PubMed] [Google Scholar]
- 80.Iyer A, Brown L, Whitehead JP, Prins JB, Fairlie DP. Nutrient and immune sensing are obligate pathways in metabolism, immunity, and disease. FASEB J 29: 3612–3625, 2015. [DOI] [PubMed] [Google Scholar]
- 81.Jean-Charles PY, Kaur S, Shenoy SK. G protein-coupled receptor signaling through beta-arrestin-dependent mechanisms. J Cardiovasc Pharmacol 70: 142–158, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamp ME, Shim R, Nicholls AJ, Oliveira AC, Mason LJ, Binge L, Mackay CR, Wong CH. G protein-coupled receptor 43 modulates neutrophil recruitment during acute inflammation. PLoS One 11: e0163750, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kappos L, Gold R, Miller DH, Macmanus DG, Havrdova E, Limmroth V, Polman CH, Schmierer K, Yousry TA, Yang M, Eraksoy M, Meluzinova E, Rektor I, Dawson KT, Sandrock AW, O’Neill GN, Investigators BGPIS. Efficacy and safety of oral fumarate in patients with relapsing-remitting multiple sclerosis: A multicentre, randomised, double-blind, placebo-controlled phase IIb study. Lancet 372: 1463–1472, 2008. [DOI] [PubMed] [Google Scholar]
- 84.Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res 324: 353–360, 2006. [DOI] [PubMed] [Google Scholar]
- 85.Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol 39: 135–142, 2008. [DOI] [PubMed] [Google Scholar]
- 86.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134: 743–756, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kataoka K, Ogasa S, Kuwahara T, Bando Y, Hagiwara M, Arimochi H, Nakanishi S, Iwasaki T, Ohnishi Y. Inhibitory effects of fermented brown rice on induction of acute colitis by dextran sulfate sodium in rats. Dig Dis Sci 53: 1601–1608, 2008. [DOI] [PubMed] [Google Scholar]
- 88.Kebede M, Alquier T, Latour MG, Semache M, Tremblay C, Poitout V. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57: 2432–2437, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: Therapeutic implications? Diabetes Obes Metab 11(Suppl 4): 10–20, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khan SM, Sleno R, Gora S, Zylbergold P, Laverdure JP, Labbe JC, Miller GJ, Hebert TE. The expanding roles of Gbetagamma subunits in G protein-coupled receptor signaling and drug action. Pharmacol Rev 65: 545–577, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Kiesler P, Fuss IJ, Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 1: 154–170, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145: 396–406 e391–310, 2013. [DOI] [PubMed] [Google Scholar]
- 93.Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 108: 8030–8035., 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 4: 1829, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kobayashi M, Mikami D, Kimura H, Kamiyama K, Morikawa Y, Yokoi S, Kasuno K, Takahashi N, Taniguchi T, Iwano M. Short-chain fatty acids, GPR41 and GPR43 ligands, inhibit TNF-alpha-induced MCP-1 expression by modulating p38 and JNK signaling pathways in human renal cortical epithelial cells. Biochem Biophys Res Commun 486: 499–505, 2017. [DOI] [PubMed] [Google Scholar]
- 96.Koh A, De Vadder F, Kovatcheva-Datchary P, Backhed F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 165: 1332–1345, 2016. [DOI] [PubMed] [Google Scholar]
- 97.Kotarsky K, Nilsson NE, Olde B, Owman C. Progress in methodology. Improved reporter gene assays used to identify ligands acting on orphan seven-transmembrane receptors. Pharmacol Toxicol 93:249–258, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. Short chain fatty acids and their receptors: new metabolic targets. Transl Res 161:131–140, 2013. [DOI] [PubMed] [Google Scholar]
- 99.Layden BT, Durai V, Lowe WL Jr. G-protein-coupled receptors, pancreatic islets, and diabetes. Nat Educat 3: 13, 2010. [Google Scholar]
- 100.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003. [DOI] [PubMed] [Google Scholar]
- 101.Lee H, Flaherty P, Ji HP. Systematic genomic identification of colorectal cancer genes delineating advanced from early clinical stage and metastasis. BMC Med Genomics 6: 54, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee SU, In HJ, Kwon MS, Park BO, Jo M, Kim MO, Cho S, Lee S, Lee HJ, Kwak YS, Kim S. beta-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-kappaB. Biol Pharm Bull 36:1754–1759, 2013. [DOI] [PubMed] [Google Scholar]
- 103.Lee T, Schwandner R, Swaminath G, Weiszmann J, Cardozo M, Greenberg J, Jaeckel P, Ge H, Wang Y, Jiao X, Liu J, Kayser F, Tian H, Li Y. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol Pharmacol 74: 1599–1609, 2008. [DOI] [PubMed] [Google Scholar]
- 104.Li G, Shi Y, Huang H, Zhang Y, Wu K, Luo J, Sun Y, Lu J, Benovic JL, Zhou N. Internalization of the human nicotinic acid receptor GPR109A is regulated by G(i), GRK2, and arrestin3. J Biol Chem 285: 22605–22618, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li G, Su H, Zhou Z, Yao W. Identification of the porcine G protein-coupled receptor 41 and 43 genes and their expression pattern in different tissues and development stages. PLoS One 9: e97342, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lindsley JE, Rutter J. Nutrient sensing and metabolic decisions. Comp Biochem Physiol B Biochem Mol Biol 139: 543–559, 2004. [DOI] [PubMed] [Google Scholar]
- 107.Link A, Balaguer F, Shen Y, Lozano JJ, Leung HC, Boland CR, Goel A. Curcumin modulates DNA methylation in colorectal cancer cells. PLoS One 8: e57709, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K. Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep 6: 37589, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest 121: 1163–1173, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc 62: 67–72, 2003. [DOI] [PubMed] [Google Scholar]
- 111.Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6: 6734, 2015. [DOI] [PubMed] [Google Scholar]
- 112.Magalhaes AC, Dunn H, Ferguson SS. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br J Pharmacol 165: 1717–1736, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mancini AD, Poitout V. The fatty acid receptor FFA1/GPR40 a decade later: How much do we know? Trends Endocrinol Metab 24: 398–407, 2013. [DOI] [PubMed] [Google Scholar]
- 114.Mandrika I, Petrovska R, Klovins J. Evidence for constitutive dimerization of niacin receptor subtypes. Biochem Biophys Res Commun 395: 281–287, 2010. [DOI] [PubMed] [Google Scholar]
- 115.Marino E, Richards JL, McLeod KH, Stanley D, Yap YA, Knight J, McKenzie C, Kranich J, Oliveira AC, Rossello FJ, Krishnamurthy B, Nefzger CM, Macia L, Thorburn A, Baxter AG, Morahan G, Wong LH, Polo JM, Moore RJ, Lockett TJ, Clarke JM, Topping DL, Harrison LC, Mackay CR. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat Immunol 18: 552–562, 2017. [DOI] [PubMed] [Google Scholar]
- 116.Km Maslowski , Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masui R, Sasaki M, Funaki Y, Ogasawara N, Mizuno M, Iida A, Izawa S, Kondo Y, Ito Y, Tamura Y, Yanamoto K, Noda H, Tanabe A, Okaniwa N, Yamaguchi Y, Iwamoto T, Kasugai K G protein-coupled receptor 43 moderates gut inflammation through cytokine regulation from mononuclear cells. Inflamm Bowel Dis 19: 2848–2856, 2013. [DOI] [PubMed] [Google Scholar]
- 118.Matsubara K, Nakamura N, Sanoh S, Ohta S, Kitamura S, Uramaru N, Miyagawa S, Iguchi T, Fujimoto N. Altered expression of the Olr59, Ethe1, and Slc10a2 genes in the liver of F344 rats by neonatal thyroid hormone disruption. J Appl Toxicol 37: 1030–1035, 2017. [DOI] [PubMed] [Google Scholar]
- 119.McKenzie C, Tan J, Macia L, Mackay CR. The nutrition-gut microbiome-physiology axis and allergic diseases. Immunol Rev 278: 277–295, 2017. [DOI] [PubMed] [Google Scholar]
- 120.McNelis JC, Lee YS, Mayoral R, van der Kant R, Johnson AM, Wollam J, Olefsky JM. GPR43 potentiates beta-cell function in obesity. Diabetes 64: 3203–3217, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mielenz M. Invited review: Nutrient-sensing receptors for free fatty acids and hydroxycarboxylic acids in farm animals. Animal 11: 1008–1016., 2017. [DOI] [PubMed] [Google Scholar]
- 122.Milligan G, Bolognini D, Sergeev E. Ligands at the free fatty acid receptors 2/3 (GPR43/GPR41). Handb Exp Pharmacol 236: 17–32, 2017. [DOI] [PubMed] [Google Scholar]
- 123.Milligan G, Shimpukade B, Ulven T, Hudson BD. Complex pharmacology of free fatty acid receptors. Chem Rev 117: 67–110, 2017. [DOI] [PubMed] [Google Scholar]
- 124.Milligan G, Stoddart LA, Smith NJ. Agonism and allosterism: The pharmacology of the free fatty acid receptors FFA2 and FFA3. Br J Pharmacol 158: 146–153, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mrowietz U, Asadullah K. Dimethylfumarate for psoriasis: More than a dietary curiosity. Trends Mol Med 11: 43–48, 2005. [DOI] [PubMed] [Google Scholar]
- 126.Nakajima A, Nakatani A, Hasegawa S, Irie J, Ozawa K, Tsujimoto G, Suganami T, Itoh H, Kimura I. The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PLoS One 12: e0179696, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Namour F, Galien R, Van Kaem T, Van der Aa A, Vanhoutte F, Beetens J, Van’t Klooster G. Safety, pharmacokinetics and pharmacodynamics of GLPG0974, a potent and selective FFA2 antagonist, in healthy male subjects. Br J Clin Pharmacol 82: 139–148, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Natividad JM, Pinto-Sanchez MI, Galipeau HJ, Jury J, Jordana M, Reinisch W, Collins SM, Bercik P, Surette MG, Allen-Vercoe E, Verdu EF. Ecobiotherapy rich in firmicutes decreases susceptibility to colitis in a humanized gnotobiotic mouse model. Inflamm Bowel Dis 21: 1883–1893, 2015. [DOI] [PubMed] [Google Scholar]
- 129.Nguyen CA, Akiba Y, Kaunitz JD. Recent advances in gut nutrient chemosensing. Curr Med Chem 19: 28–34, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Nilsson NE, Kotarsky K, Owman C, Olde B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem Biophys Res Commun 303: 1047–1052, 2003. [DOI] [PubMed] [Google Scholar]
- 131.Nohr MK, Egerod KL, Christiansen SH, Gille A, Offermanns S, Schwartz TW, Moller M. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience 290: 126–137, 2015. [DOI] [PubMed] [Google Scholar]
- 132.Nohr MK, Pedersen MH, Gille A, Egerod KL, Engelstoft MS, Husted AS, Sichlau RM, Grunddal KV, Poulsen SS, Han S, Jones RM, Offermanns S, Schwartz TW. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short-chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology 154: 3552–3564, 2013. [DOI] [PubMed] [Google Scholar]
- 133.Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, LowJS, Harman CC, Graham M, Elinav E, Flavell RA. Epithelial IL-18 equilibrium controls barrier function in colitis. Cell 163: 1444–1456, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.O’Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol 13: 691–706, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Offermanns S Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol Metab 28: 227–236, 2017. [DOI] [PubMed] [Google Scholar]
- 136.Ohira H, Tsutsui W, Mamoto R, Yamaguchi S, Nishida M, Ito M, Fujioka Y. Butyrate attenuates lipolysis in adipocytes cocultured with macrophages through non-prostaglandin E2-mediated and prostaglandin E2-mediated pathways. Lipids Health Dis 15: 213, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ohland CL, Macnaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 298: G807–G819, 2010. [DOI] [PubMed] [Google Scholar]
- 138.Oldham WM, Hamm HE. Heterotrimeric G protein activation by G protein-coupled receptors. Nat Rev Mol Cell Biol 9: 60–71, 2008. [DOI] [PubMed] [Google Scholar]
- 139.Pan P, C WS, Wang HT, Oshima K, Huang YW, Yu J, Zhang J, M MY, K AA, W RD, Chen X, Wang lS. Loss of free fatty acid receptor 2 enhances colonic adenoma development and reduces the chemopreventive effects of black raspberries in ApcMin/+ mice. Carcinogenesis 38: 86–93, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol 8: 80–93, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Patel DG, Singh SP. Effect of ethanol and its metabolites on glucose mediated insulin release from isolated islets of rats. Metabolism 28: 85–89, 1979. [DOI] [PubMed] [Google Scholar]
- 142.Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, Petersen KF, Kibbey RG, Goodman AL, Shulman GI. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature 534:213–217, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Peterson LW, Artis D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat Rev Immunol 14: 141–153, 2014. [DOI] [PubMed] [Google Scholar]
- 144.Peterson YK, Luttrell LM. The diverse roles of arrestin scaffolds in G protein-coupled receptor signaling. Pharmacol Rev 69: 256–297, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pierce KL, Premont RT, Lefkowitz RJ. Seven-transmembrane receptors. Nat Rev Mol Cell Biol 3: 639–650, 2002. [DOI] [PubMed] [Google Scholar]
- 146.Pizzonero M, Dupont S, Babel M, Beaumont S, Bienvenu N, Blanque R, Cherel L, Christophe T, Crescenzi B, De Lemos E, Delerive P, Deprez P, De Vos S, Djata F, Fletcher S, Kopiejewski S, L’Ebraly C, Lefrancois JM, Lavazais S, Manioc M, Nelles L, Oste L, Polancec D, Quenehen V, Soulas F, Triballeau N, van der Aar EM, Vandeghinste N, Wakselman E, Brys R, Saniere L. Discovery and optimization of an azetidine chemical series as a free fatty acid receptor 2 (FFA2) antagonist: From hit to clinic. J Med Chem 57: 10044–10057, 2014. [DOI] [PubMed] [Google Scholar]
- 147.Pluznick J A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes 5: 202–207, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 110: 4410–4415, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prentki M, Matschinsky FM, Madiraju SR. Metabolic signaling in fuel-induced insulin secretion. Cell Metab 18: 162–185, 2013. [DOI] [PubMed] [Google Scholar]
- 150.Priyadarshini M, Layden BT. FFAR3 modulates insulin secretion and global gene expression in mouse islets. Islets 7: e1045182, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Priyadarshini M, Villa SR, Fuller M, Wicksteed B, Mackay CR, Alquier T, Poitout V, Mancebo H, Mirmira RG, Gilchrist A, Layden BT. An acetate-specific GPCR, FFAR2, regulates insulin secretion. Mol Endocrinol 29: 1055–1066, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Priyadarshini M, Wicksteed B, Schiltz GE, Gilchrist A, Layden BT. SCFA receptors in pancreatic beta cells: Novel diabetes targets? Trends Endocrinol Metab 27: 653–664, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Priyamvada S, Gomes R, Gill RK, Saksena S, Alrefai WA, Dudeja PK. Mechanisms underlying dysregulation of electrolyte absorption in inflammatory bowel disease-associated diarrhea. Inflamm Bowel Dis 21: 2926–2935, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Psichas A, Sleeth ML, Murphy KG, Brooks L, Bewick GA, Hanyaloglu AC, Ghatei MA, Bloom SR, Frost G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int J Obes (Lond) 39: 424–429, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Puhl HL III, Won YJ, Lu VB, Ikeda SR Human GPR42 is a transcribed multisite variant that exhibits copy number polymorphism and is functional when heterologously expressed. Sci Rep 5: 12880, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rajendran VM, Nanda Kumar NS,Tse CM, Binder HJ.Na-H exchanger isoform-2 (NHE2) mediates butyrate-dependent Na+ absorption in dextran sulfate sodium (DSS)-induced colitis. J Biol Chem 290: 25487–25496, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 118: 229–241, 2004. [DOI] [PubMed] [Google Scholar]
- 158.Rezq S, Abdel-Rahman AA. Central GPR109A activation mediates glutamate-dependent pressor response in conscious rats. J Pharmacol Exp Ther 356: 456–465, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Richman JG, Kanemitsu-Parks M, Gaidarov I, Cameron JS, Griffin P, Zheng H, Guerra NC, Cham L, Maciejewski-Lenoir D, Behan DP, Boatman D, Chen R, Skinner P, Ornelas P, Waters MG, Wright SD, Semple G, Connolly DT. Nicotinic acid receptor agonists differentially activate downstream effectors. J Biol Chem 282: 18028–18036, 2007. [DOI] [PubMed] [Google Scholar]
- 160.Rodriguez M, Luo W, Weng J, Zeng L, Yi Z, Siwko S, Liu M. PSGRpromotes prostatic intraepithelial neoplasia and prostate cancer xenograft growth through NF-kappaB. Oncogenesis 3: e114, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rodriguez M, Siwko S, Zeng L, Li J, Yi Z, Liu M. Prostate-specific G-protein-coupled receptor collaborates with loss of PTEN to promote prostate cancer progression. Oncogene 35: 1153–1162, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature 459: 356–363, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10: 490–500, 2010. [DOI] [PubMed] [Google Scholar]
- 164.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 133: 775–787, 2008. [DOI] [PubMed] [Google Scholar]
- 165.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chainfatty-acidbinding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A 105: 16767–16772, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sawzdargo M, George SR, Nguyen T, Xu S, Kolakowski LF, O’Dowd BF. A cluster of four novel human G protein-coupled receptor genes occurring in close proximity to CD22 gene on chromosome 19q13.1. Biochem Biophys Res Commun 239: 543–547, 1997. [DOI] [PubMed] [Google Scholar]
- 167.Schauber J, Svanholm C, Termen S, Iffland K, Menzel T, Scheppach W, Melcher R, Agerberth B, Luhrs H, Gudmundsson GH. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut 52: 735–741, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schmidt J, Smith NJ, Christiansen E, Tikhonova IG, Grundmann M, Hudson BD, Ward RJ, Drewke C, Milligan G, Kostenis E, Ulven T. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: Identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J Biol Chem 286: 10628–10640, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med 22: 1079–1089, 2016. [DOI] [PubMed] [Google Scholar]
- 170.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 13: 800–812, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Senga T, Iwamoto S, Yoshida T, Yokota T, Adachi K, Azuma E, Hamaguchi M, Iwamoto T. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood 101: 1185–1187, 2003. [DOI] [PubMed] [Google Scholar]
- 172.Sergeev E, Hansen AH, Pandey SK, MacKenzie AE, Hudson BD, Ulven T, Milligan G. Non-equivalence of key positively charged residues of the free fatty acid 2 receptor in the recognition and function of agonist versus antagonist ligands. J Biol Chem 291: 303–317, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Shah JH, Wongsurawat N, Aran PP. Effect of ethanol on stimulus-induced insulin secretion and glucose tolerance. A study of mechanisms. Diabetes 26: 271–277, 1977. [DOI] [PubMed] [Google Scholar]
- 174.Shi G, Sun C, Gu W, Yang M, Zhang X, Zhai N, Lu Y, Zhang Z, Shou P, Zhang Z, Ning G. Free fatty acid receptor 2, a candidate target for type 1 diabetes, induces cell apoptosis through ERK signaling. J Mol Endocrinol 53: 367–380, 2014. [DOI] [PubMed] [Google Scholar]
- 175.Sina C, Gavrilova O, Forster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, Franke A, Ott S, Hasler R, Nikolaus S, Folsch UR, Rose-John S, Jiang HP, Li J, Schreiber S, Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J Immunol 183:7514–7522, 2009. [DOI] [PubMed] [Google Scholar]
- 176.Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity 40: 128–139, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sivaprakasam S, Gurav A, Paschall AV, Coe GL, Chaudhary K, Cai Y, Kolhe R, Martin P, Browning D, Huang L, Shi H, Sifuentes H, Vijay-Kumar M, Thompson SA, Munn DH, Mellor A, McGaha TL, Shiao P, Cutler CW, Liu K, Ganapathy V, Li H, Singh N. An essential role of Ffar2 (Gpr43) in dietary fibre-mediated promotion of healthy composition of gut microbiota and suppression of intestinal carcinogenesis. Oncogenesis 5: e238, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sivaprakasam S, Prasad PD, Singh N. Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol Ther 164: 144–151, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Smith JS, Rajagopal S. The beta-arrestins: Multifunctional regulators of G protein-coupled receptors. J Biol Chem 291: 8969–8977, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Smith NJ, Ward RJ, Stoddart LA, Hudson BD, Kostenis E, Ulven T, Morris JC, Trankle C, Tikhonova IG, Adams DR, Milligan G. Extracellular loop 2 of the free fatty acid receptor 2 mediates allosterism of a phenylacetamide ago-allosteric modulator. Mol Pharmacol 80: 163–173, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341: 569–573, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sonnenburg JL, Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 535: 56–64, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J Biol Chem 283: 32913–32924, 2008. [DOI] [PubMed] [Google Scholar]
- 184.Suckow AT, Briscoe CP. Key questions for translation of FFA receptors: From pharmacology to medicines. Handb Exp Pharmacol 236: 101–131, 2017. [DOI] [PubMed] [Google Scholar]
- 185.Sun J, Furio L, Mecheri R, van der Does AM, Lundeberg E, Saveanu L, Chen Y, van Endert P, Agerberth B, Diana J. Pancreatic beta-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 43: 304–317, 2015. [DOI] [PubMed] [Google Scholar]
- 186.Sun M, Wu W, Liu Z, Cong Y. Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J Gastroenterol 52: 1–8, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr 100: 297–305, 2008. [DOI] [PubMed] [Google Scholar]
- 188.Sykaras AG, Demenis C, Case RM, McLaughlin JT, Smith CP. Duodenal enteroendocrine I-cells contain mRNA transcripts encoding key endocannabinoid and fatty acid receptors. PLoS One 7: e42373, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem 280: 26649–26652, 2005. [DOI] [PubMed] [Google Scholar]
- 190.Tan J, McKenzie C, Vuillermin PJ, Goverse G, Vinuesa CG, Mebius RE, Macia L, Mackay CR. Dietary fiber and bacterial SCFA enhance oral tolerance and protect against food allergy through diverse cellular pathways. Cell Rep 15: 2809–2824, 2016. [DOI] [PubMed] [Google Scholar]
- 191.Tan JK, McKenzie C, Marino E, Macia L, Mackay CR. Metabolitesensing G protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol 35: 371–402, 2017. [DOI] [PubMed] [Google Scholar]
- 192.Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nat Med 21: 173–177, 2015. [DOI] [PubMed] [Google Scholar]
- 193.Tang Y, Chen Y, Jiang H, Nie D. The role of short-chain fatty acids in orchestrating two types of programmed cell death in colon cancer. Autophagy 7: 235–237, 2011. [DOI] [PubMed] [Google Scholar]
- 194.Tang Y, Chen Y, Jiang H, Nie D. Short-chain fatty acids induced autophagy serves as an adaptive strategy for retarding mitochondria-mediated apoptotic cell death. Cell Death Differ 18: 602–618, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer 128: 847–856, 2011. [DOI] [PubMed] [Google Scholar]
- 196.Tazoe H, Otomo Y, Kaji I, Tanaka R, Karaki SI, Kuwahara A. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J Physiol Pharmacol 59(Suppl 2): 251–262, 2008. [PubMed] [Google Scholar]
- 197.Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res 30: 149–156, 2009. [DOI] [PubMed] [Google Scholar]
- 198.Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ , Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res 69: 2826–2832, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity 40: 833–842, 2014. [DOI] [PubMed] [Google Scholar]
- 200.Tiengo A, Valerio A, Molinari M, Meneghel A, Lapolla A. Effect of ethanol, acetaldehyde, and acetate on insulin and glucagon secretion in the perfused rat pancreas. Diabetes 30: 705–709, 1981. [DOI] [PubMed] [Google Scholar]
- 201.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61: 364–371, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tolhurst G, Reimann F, Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol 309–335, 2012. [DOI] [PubMed] [Google Scholar]
- 203.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 20: 159–166, 2014. [DOI] [PubMed] [Google Scholar]
- 204.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9: 799–809, 2009. [DOI] [PubMed] [Google Scholar]
- 205.Tvrzicka E, Kremmyda LS, Stankova B, Zak A. Fatty acids as biocompounds: Their role in human metabolism, health and disease—a review. Part 1: Classification, dietary sources and biological functions. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 155:117–130, 2011. [DOI] [PubMed] [Google Scholar]
- 206.Urban DJ, Roth BL. DREADDs (designer receptors exclusively activated by designer drugs): Chemogenetic tools with therapeutic utility. Annu Rev Pharmacol Toxicol 55: 399–417, 2015. [DOI] [PubMed] [Google Scholar]
- 207.Veprik A, Laufer D, Weiss S, Rubins N, Walker MD. GPR41 modulates insulin secretion and gene expression in pancreatic beta-cells and modifies metabolic homeostasis in fed and fasting states. FASEB J 30: 3860–3869, 2016. [DOI] [PubMed] [Google Scholar]
- 208.Vieira AT, Macia L, Galvao I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, Nicoli JR, Harper JL, Teixeira MM, Mackay CR. A role for gut microbiota and the metabolite-sensing receptor GPR43 in a murine model of gout. Arthritis Rheumatol 67: 1646–1656, 2015. [DOI] [PubMed] [Google Scholar]
- 209.Villa SR, Priyadarshini M, Fuller MH, Bhardwaj T, Brodsky MR, Angueira AR, Mosser RE, Carboneau BA, Tersey SA, Mancebo H, Gilchrist A, Mirmira RG, Gannon M, Layden BT. Loss of free fatty acid receptor 2 leads to impaired islet mass and beta cell survival. Sci Rep 6: 28159, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vinolo MA, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly YM, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One 6: e21205, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Volk N, Lacy B. Anatomy and physiology of the small bowel. Gastrointest Endosc Clin N Am 27: 1–13, 2017. [DOI] [PubMed] [Google Scholar]
- 212.Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J Clin Invest 119: 1312–1321, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wang J, Wu X, Simonavicius N, Tian H, Ling L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J Biol Chem 281: 34457–34464, 2006. [DOI] [PubMed] [Google Scholar]
- 214.Wang L, Llorente C, Hartmann P, Yang AM, Chen P, Schnabl B. Methods to determine intestinal permeability and bacterial translocation during liver disease. J Immunol Methods 421: 44–53, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wang N, Guo DY, Tian X, Lin HP, Li YP, Chen SJ, Fu YC, Xu WC, Wei CJ. Niacin receptor GPR109A inhibits insulin secretion and is down-regulated in type 2 diabetic islet beta-cells. Gen Comp Endocrinol 237: 98–108, 2016. [DOI] [PubMed] [Google Scholar]
- 216.Wang Y, Jiao X, Kayser F, Liu J, Wang Z, Wanska M, Greenberg J, Weiszmann J, Ge H, Tian H, Wong S, Schwandner R, Lee T, Li Y. The first synthetic agonists of FFA2: Discovery and SAR of phenylacetamides as allosteric modulators. Bioorg Med Chem Lett 20: 493–498, 2010. [DOI] [PubMed] [Google Scholar]
- 217.Wellendorph P, Johansen LD, Brauner-Osborne H. The emerging role of promiscuous 7TM receptors as chemosensors for food intake. Vitam Horm 84: 151–184, 2010. [DOI] [PubMed] [Google Scholar]
- 218.Wu H, Tremaroli V, Backhed F. Linking microbiota to human diseases: A systems biology perspective. Trends Endocrinol Metab 26: 758–770, 2015. [DOI] [PubMed] [Google Scholar]
- 219.Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics 39: 375–384, 2012. [DOI] [PubMed] [Google Scholar]
- 220.Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y, Huang X, Xiao Y, Yao S, Zhao Q, Liu Z, Cong Y. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 10: 946–956, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Xia C, Ma W, Wang F, Hua S, Liu M. Identification of a prostate-specific G-protein coupled receptor in prostate cancer. Oncogene 20: 5903–5907, 2001. [DOI] [PubMed] [Google Scholar]
- 222.Ximenes HM, Hirata AE, Rocha MS, Curi R, Carpinelli AR. Propionate inhibits glucose-induced insulin secretion in isolated rat pancreatic islets. Cell Biochem Funct 25: 173–178, 2007. [DOI] [PubMed] [Google Scholar]
- 223.Xiong Y, Miyamoto N, Shibata K, Valasek Ma, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci U S A 101: 1045–1050, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Xu LL, Stackhouse BG, Florence K, Zhang W, Shanmugam N, Sesterhenn IA, Zou Z, Srikantan V, Augustus M, Roschke V, Carter K, McLeod DG, Moul JW, Soppett D, Srivastava S. PSGR, a novel prostate-specific gene with homology to a G protein-coupled receptor, is overexpressed in prostate cancer. Cancer Res 60: 6568–6572, 2000. [PubMed] [Google Scholar]
- 225.Yang S, Li X, Wang N, Yin G, Ma S, Fu Y, Wei C, Chen Y, Xu W. GPR109A expression in the murine Min6 pancreatic beta cell line, and its relation with glucose metabolism and inflammation. Ann Clin Lab Sci 45:315–322, 2015. [PubMed] [Google Scholar]
- 226.Yin H, Chu A, Li W, Wang B, Shelton F, Otero F, Nguyen DG, Caldwell JS, Chen YA. Lipid G protein-coupled receptor ligand identification using beta-arrestin PathHunter assay. J Biol Chem 284: 12328–12338, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Yonezawa T, Kobayashi Y, Obara Y. Short-chain fatty acids induce acute phosphorylation of the p38 mitogen-activated protein kinase/heat shock protein 27 pathway via GPR43 in the MCF-7 human breast cancer cell line. Cell Signal 19: 185–193, 2007. [DOI] [PubMed] [Google Scholar]
- 228.Zaibi MS, Stocker CJ, O’Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584: 2381–2386, 2010. [DOI] [PubMed] [Google Scholar]
- 229.Zandi-Nejad K, Takakura A, Jurewicz M, Chandraker AK, Offermanns S, Mount D, Abdi R. The role of HCA2 (GPR109A) in regulating macrophage function. FASEB J 27: 4366–4374, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zellner C, Pullinger CR, Aouizerat BE, Frost PH, Kwok PY, Malloy MJ, Kane JP. Variations in human HM74 (GPR109B) and HM74A (GPR109A) niacin receptors. Hum Mutat 25: 18–21, 2005. [DOI] [PubMed] [Google Scholar]


