The Critical Care Medicine Department of the National Institutes of Health Clinical Center and the Pulmonary Hypertension Association held a joint symposium to discuss “Challenges in Pulmonary Hypertension: Beating, Breathing, and Beyond.” Communities of interest including patient advocates, clinicians, and academic investigators gathered together to raise awareness and highlight the challenges in diagnosing and treating pulmonary hypertension (PH). While agreeing there is a need for treatments that can cross World Health Organization (WHO) PH classifications, the symposium clearly pointed out that improved phenotyping is still needed to better determine who will benefit from these therapies.
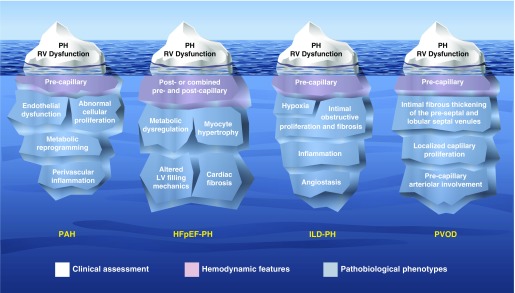
PH, defined hemodynamically as a mean pulmonary artery pressure (mPAP) of 25 mm Hg or greater (1), can arise from pulmonary vascular disease, left heart disease, lung disease, chronic thromboembolic disease, and other causes, forming the basis of WHO classifications (2). The symposium focused on whether or not pulmonary arterial hypertension (PAH) (WHO group 1)-specific treatments are warranted as therapies for heart failure with preserved ejection fraction and PH (HFpEF-PH; WHO group 2.2), interstitial lung disease with PH (ILD-PH; WHO group 3.2), and pulmonary venoocclusive disease (PVOD)/pulmonary capillary hemangiomatosis (PCH) (referred to as PVOD for simplicity; WHO group 1′). Like icebergs in the water, the tips of these specific WHO subgroups can seem similarly menacing, showing elevated pulmonary arterial pressure and right ventricular (RV) dysfunction (Figure 1). Importantly, apart from addressing the underlying substrate in patients with HFpEF-PH and ILD-PH or lung transplant in patients with ILD-PH and PVOD, there are currently no approved evidence-based therapies that specifically address pulmonary vascular disease or a failing RV in these patients (3). Practitioners, in their haste to steer clear of right heart failure, can be conflicted as they navigate a path between the need to treat and the desire above all, to do no harm (primum non nocere [4]).
Figure 1.
Iceberg models illustrating the substrates underlying pulmonary hypertension (PH) due to pulmonary arterial hypertension (PAH), heart failure with preserved ejection fraction (HFpEF), interstitial lung disease (ILD), and pulmonary veno-occlusive disease (PVOD). LV = left ventricular; RV = right ventricular. Illustration by Jacqueline Schaffer.
We need only look to early inotropic strategies using oral milrinone, vesnarinone, and xamoterol in heart failure with reduced ejection fraction to remind ourselves that treating hemodynamics without targeting the underlying pathophysiology does not necessarily improve survival. In fact, these treatments, although occasionally providing symptom relief, uniformly reduced survival (5–7). To avoid therapeutic misadventures, we need to dive deeper to see the phenotypes beneath the surface (Figure 1). PAH is characterized by endothelial dysfunction, abnormal cellular proliferation, metabolic reprogramming, and perivascular inflammation of the precapillary pulmonary arterioles (8–11); HFpEF-PH by metabolic dysregulation, myocyte hypertrophy, cardiac fibrosis, and ultimately altered left ventricular filling mechanics with adverse vascular remodeling involving the postcapillary pulmonary venules and, to varying degrees, the pulmonary capillaries and precapillary pulmonary arterioles (12, 13); ILD-PH by hypoxia, intimal obstructive proliferation and fibrosis, inflammation, and angiostasis (14); and PVOD by extensive occlusion of the postcapillary pulmonary veins by fibrous tissue, intimal thickening of venules in the lobular septa, localized capillary proliferation, and varying degrees of involvement of the precapillary arterioles without plexiform lesions (15).
Although these major phenotypes of PH are statically classified by the World Symposium on Pulmonary Hypertension, they are dynamic over time. A major challenge facing practitioners is that individual patients may have elements that cross phenotypes.
Should You Treat HFpEF-PH with PAH-Specific Therapies?
WHO group 2 PH is by far the most common form of PH, accounting for more than 65% of all PH seen in the clinical arena (16, 17). Examining prevalence data from the perspective of heart failure, between 50% and 80% of patients will have some echocardiographic evidence of PH (18–24). If there are 5.7 million patients with heart failure in the United States, then 2.9 to 4.6 million have some degree of PH (25). In addition, mortality rates in heart failure are related both to the presence and degree of PH (3, 18, 26, 27). These numbers and mortality trends emphasize the clinical burden of group 2 PH in our society. Yet, there are currently no approved evidence-based medical therapies for PH complicating heart failure. The search for such therapies has been confounded by the phenotypic diversity of the left ventricular (LV) dysfunction and the uncertainty of the degree to which PH is a marker of severity of the underlying LV dysfunction versus a target for treatment. In light of this, it makes logical sense to first identify amenable phenotypes of HFpEF and then develop strategies to target the PH remaining once the underlying substrate has been optimally managed.
Although group 2 PH arises from elevations in left atrial pressure (LAP), it may present in an occult fashion with a relatively normal baseline pulmonary artery occlusion pressure (PAOP) of less than 15 mm Hg. Provocative testing, such as a fluid challenge or exercise during right heart catheterization, could reveal an elevated PAOP, and thus may provide additional information toward accurate diagnosis and classification (28, 29). Yet, several unanswered questions regarding these tests remain. How best to implement the fluid challenge, and what defines an abnormal response given that increases in filling pressures with volume loading have been reported in healthy volunteers (30, 31)? Determining an optimal PAOP cutoff will have important therapeutic implications. For example, 7% of patients with PAH and 8% of control subjects without suspected PH were reclassified as having postcapillary PH using a PAOP cutoff of greater than or equal to 18 mm Hg after rapid saline infusion (29). In comparison, 15% to 22% of patients with precapillary PH were reclassified as postcapillary PH using a fluid challenge–induced PAOP cutoff of greater than 15 mm Hg (28, 29). Questions also remain when considering exercise as a provocative challenge, including the appropriate mode and intensity as well as rigorously defining normal responses across various age and body mass index strata (32, 33). Finally, investigations comparing acute fluid loading to exercise are an important first step to determining the sensitivity, specificity, and predictive values of these techniques (31, 33).
Hemodynamic evaluation can further parse group 2 PH into two distinct phenotypes defined by the recent World Symposium on Pulmonary Hypertension and highlighted in the recent European societal PH guidelines (3, 34): isolated postcapillary PH (Ipc-PH), in which the PAOP is 15 mm Hg or greater and the diastolic pressure gradient (difference between the pulmonary arterial diastolic and occlusion pressures) is less than 7 mm Hg; and combined pre- and postcapillary PH (Cpc-PH), in which both the PAOP and diastolic pressure gradient are elevated. We are just starting to understand the broader phenotypes encompassed in these hemodynamic definitions. In terms of clinical phenotypes, although patients with Cpc-PH, Ipc-PH, and PAH frequently have diabetes and systemic hypertension, these metabolic syndrome comorbidities were more prevalent in patients with Cpc-PH than in patients with PAH (35). In addition, genotyping data in patients with Cpc-PH revealed that these patients share a large number of genetic variants commonly identified in patients with PAH. When subjected to a gene ontology analysis, these variants were found to be enriched in cytoskeletal genes and genes regulating inflammation, potentially suggesting a shared molecular etiology between PAH and Cpc-PH that is different from Ipc-PH (35).
Although the principal recommendation for managing group 2 PH is to aggressively treat the underlying etiology (i.e., manage the substrate) (3), there has been recent interest in the use of PAH-specific pharmacotherapies to treat PH in patients with HFpEF-PH. However, PAH-specific drugs target the endothelin, nitric oxide, and prostacyclin signaling pathways that are dysfunctional in PAH and have been implicated in the adverse precapillary pulmonary arteriole remodeling and plexiform lesion formation pathognomonic for the disease. In contrast to PAH, the pathophysiology underlying HFpEF-PH is related to left heart dysfunction with left atrial overload, left ventricular hypertrophy, fibrosis, and increased stiffness. Impaired left heart function, in turn, promotes postcapillary pulmonary venule remodeling as a result of backward transmission of elevated filling pressures (36). Although significant knowledge gaps remain in understanding the pathobiology of pulmonary vascular remodeling in HFpEF-PH, the recent development of HFpEF-PH preclinical animal models is an important initial step forward (37, 38).
Recently, data from the COMPERA (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension) registry evaluating PAH therapy in HFpEF found these patients were most frequently prescribed a phosphodiesterase-5 inhibitor, and 7% were on combination therapy at 1 year (39). Further analysis examining the tolerability and efficacy of PAH-specific drugs prescribed off-label to patients with HFpEF-PH found that among patients with follow-up data, 18.4% discontinued a phosphodiesterase-5 inhibitor and 42.9% discontinued use of an endothelin receptor antagonist because of either efficacy failure or side effects. In addition, compared to idiopathic PAH (IPAH) patients treated with targeted PAH therapy, HFpEF-PH patients were less likely to be WHO functional class I/II at 1 year (39). Thus, off-label use of PAH-specific drugs in patients with HFpEF-PH was less effective and often discontinued (39).
Patients with systemic sclerosis (SSc) warrant specific mention, because they can present with varying elements of parenchymal lung disease, pulmonary vasculopathy, and left heart disease. In the PHAROS (Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma) cohort, 20% (59 of 298) of the patients with SSc-PH had a PAOP of greater than 15 mm Hg (40). Nearly all of these patients had HFpEF-PH, and a third were classified as Cpc-PH. The fact that most patients with Cpc-PH (86%) and half of the patients with Ipc-PH were treated with PAH medications likely reflects practitioners’ awareness of the potential for pulmonary arteriopathy in SSc, and therefore standard HFpEF-PH treatment paradigms may not be sufficient.
Randomized, controlled trials of PAH-specific drugs in patients with HFpEF-PH have yielded mixed results. Contemporary, single-center studies have reported conflicting effects of inhaled prostanoids on the PAOP in stable patients with HFpEF-PH, with one showing a reduction and the other showing an increase in PAOP (41, 42). On the other hand, acute administration of inhaled nitrite increased pulmonary artery compliance and lowered right atrial pressure, mPAP, and PAOP, more so in patients with HFpEF-PH than in patients with group 1 or 3 PH (43). Endothelin receptor antagonists have also been studied in patients with HFpEF-PH in two clinical trials, with disappointing results. The Safety and Efficacy Trial to Treat Diastolic Heart Failure Using Ambrisentan was terminated early because of poor enrollment, and the BADDHY (Safety and Efficacy of Bosentan in Patients with Diastolic Heart Failure and Secondary Pulmonary Hypertension) trial was stopped early when an interim analysis favored placebo (44). In the RELAX (Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Heart Failure with Preserved Ejection Fraction) trial, sildenafil for 24 weeks compared with placebo had no effect on the change in peak oxygen consumption, and there was no difference in 6-minute-walk distance (6MWD) (45). However, in a single-center study of patients with HFpEF with Cpc-PH and severe RV dysfunction documented by right heart catheterization, sildenafil significantly improved hemodynamics, RV function, and quality of life (46). Notably, the RELAX study included a broader population of patients with HFpEF and did not specifically examine patients with HFpEF-PH or those with Cpc-PH and severe RV dysfunction (45, 47). In line with the RELAX study, a recent single-center study in patients with HFpEF with predominantly Ipc-PH documented by right heart catheterization, sildenafil failed to improve hemodynamics, exercise capacity, or quality of life (48). In addition, riociguat, a soluble guanylate cyclase stimulator, failed to acutely lower mPAP compared with placebo in patients with HFpEF-PH (49).
To date, there is no multicenter, randomized trial of a PAH-specific drug in HFpEF-PH that has met their primary endpoint (13). Failure to adequately address patient volume status (i.e., control LAP) and account for prominent pulmonary venous remodeling likely contributed to the suboptimal results of these pulmonary vasodilator trials in HFpEF-PH. Does this mean there are no patients with HFpEF-PH who could benefit from PAH-specific therapies, or does it instead imply that we have yet to identify the appropriate HFpEF-PH phenotype to study in these trials? The latter concept is supported by the observation that patients with HFpEF-PH with more advanced disease were more likely to respond to PAH-specific therapy in these trials (46, 50). Interestingly, although the initial trigger (i.e., elevated LAP) for Cpc-PH is distinct from that of PAH, the secondary mediators and mechanisms underpinning this disease, as well as the pathological remodeling of the pulmonary arteries, share some similarities (35, 51). For example, it is reasonable to hypothesize that the Cpc-PH phenotype of HFpEF-PH may represent a unique subgroup that would benefit from PAH therapies once volume status and LAP have been optimized. However, this approach needs to be examined in carefully controlled clinical trials.
At present, HFpEF-PH should be managed according to guideline-accepted therapies (52) with diuretics, risk factor modification to impact the underlying substrate, and treatment of comorbidities (e.g., sleep-disordered breathing). The question now is, “What algorithm can be developed to study the effect of PAH-specific drugs in HFpEF-PH in the context of optimal control of LAP?” Choosing the right timing to introduce a PAH-specific therapy may be critical. Novel and aggressive unloading strategies at the level of the left atrium (53) may pave the way for safe, effective pulmonary vasodilator trials in Cpc-PH. Aggressive left atrial decompression has the potential to drive favorable venous remodeling. In conjunction with modification of risk factors and comorbidities, long-term decompression of LAP might allow for adequate pulmonary venous and arterial unloading and gradual venous remodeling. The subsequent introduction of PAH-specific therapy may promote further arterial remodeling in an “unloaded” venous system, thus mitigating the likelihood of alveolar leak and pulmonary edema. It is in this context that PAH therapies might hold promise for improving the disease process and RV performance.
Should You Treat ILD-PH with PAH-Specific Therapies?
The prevalence of severe ILD-PH varies by the underlying cause of parenchymal lung disease, method of detection (echocardiography vs. cardiac catheterization), and cohort studied (unselected ILD clinic vs. patients referred for lung transplantation) (3, 14, 54–57). Yet, when detected, even mild PH is associated with increased morbidity and mortality in patients with ILD (58–63). Unfortunately, despite the explosion over the past two decades of new medications for PAH, there are currently no approved evidence-based therapies for PH complicating chronic lung diseases. Pirfenidone and nintedanib, recently approved by the U.S. Food and Drug Administration for the treatment of idiopathic pulmonary fibrosis (IPF), modestly slow loss of lung function, although their effects on PH in these patients was not specifically examined (64–66). Therefore, in patients with advanced ILD and established PH, where lung transplant remains the only effective treatment option, PAH-specific drugs become a tempting therapeutic opportunity. However, because of the lack of convincing data that pulmonary vasodilators are beneficial in this patient population (67, 68), the most recent PH treatment guidelines clearly state that “the use of drugs approved for PAH is not recommended in patients with PH due to lung disease” (3). This recommendation appears justified after the recent termination of a phase 2 ILD-PH study because of a higher rate of death and other serious adverse events noted in patients randomized to riociguat compared with placebo (69). However, extrapolating these results to all other subtypes of patients with ILD-PH is problematic, because this study included only patients with symptomatic PH associated with idiopathic interstitial pneumonias.
So, how to treat a patient with chronic lung disease who develops PH, or even more importantly, what constitutes clinically significant PH that warrants therapy in these patients? A closer look at the studies examining the efficacy of pulmonary vasodilators in patients with ILD leaves one wondering whether there is truly “evidence of absence” or merely “absence of evidence.” Studies published to date have largely included an unselected IPF patient cohort (i.e., those with and without PH) (70–75) or were uncontrolled (76–82). Notably, only one randomized, double-blind, placebo-controlled trial of PAH-specific therapy has been completed exclusively in patients with ILD-PH, and although this study failed to demonstrate efficacy, it was rather small and included only patients with IPF with PH (83). In light of these shortcomings in the available data, a survey of current practice patterns suggests that PAH-specific therapies are prescribed to patients with WHO group 3 PH at most U.S. PH referral centers despite current guidelines (84). The majority of PH physician specialists, with an average (±SD) of 16.5 ± 5.8 years of experience treating patients with PH, reported that evidence of RV dysfunction or failure was a significant factor influencing their decision to treat patients with WHO group 3 PH with PAH-specific therapy (84). In support of this practice, a post hoc subgroup analysis of the STEP-IF (Sildenafil Trial of Exercise Performance in Idiopathic Pulmonary Fibrosis) trial demonstrated that patients with evidence of RV dysfunction by echocardiography were the most likely to benefit from therapy, with a mean increase of 99.3 m (95% confidence interval, 22.3–176.2 m) in their 6MWD at 12 weeks compared with baseline (85). Therefore, evidence of a possible circulatory limitation to functional capacity, as opposed to predominantly a ventilatory limitation, may identify a subset of patients in whom studies of pulmonary vasodilator therapy should be focused. However, such an approach needs to be validated prospectively. In addition to the limitations of the available evidence, there is also a concern for potential hypoxemia due to worsening / mismatch with systemically administered pulmonary vasodilators in patients with advanced parenchymal lung disease (76, 77). However, oxygenation was not adversely affected in an uncontrolled study of patients with advanced ILD-PH awaiting lung transplant treated for 12 weeks with intravenous treprostinil (82). Whether the impact of / mismatch is offset by improvements in RV performance and cardiac output in a subset of patients with ILD-PH with symptoms and exercise intolerance due to circulatory limitations should be tested in a properly controlled clinical trial.
Indeed, current guidelines acknowledge the need to further subtype patients with group 3 PH (3, 67). On the basis of hemodynamics obtained by right heart catheterization, “severe” PH is defined as mPAP greater than 35 mm Hg or greater than or equal to 25 mm Hg in the presence of a low cardiac index (<2.5 L/min/m2) (3). Likewise, guidelines suggest that pulmonary vasodilator therapy may be considered when “severe” PH is present in the setting of “mild” lung disease (3). However, these distinctions are easily blurred in clinical practice, when patients present with other relevant comorbidities associated with the development of PH (e.g., HFpEF and obstructive sleep apnea). Similarly, patients with connective tissue disease, especially those with SSc, represent a unique challenge because of the potential coexistence of parenchymal lung disease and pulmonary vasculopathy. Thus, a “one-size-fits-all” approach for determining those patients with ILD-PH who are appropriate to treat with pulmonary vasodilators may be inadequate (86). For instance, in a retrospective cohort study of treatment-naive incident cases of chronic lung disease–associated severe precapillary PH, as defined above, PAH-specific therapy did not result in significant improvements in New York Heart Association functional class or 6MWD (87). Moreover, data from the COMPERA registry did not demonstrate any significant difference in the proportion of patients who improved their 6MWD or functional class with PAH-specific therapy in patients with ILD-PH who fulfilled the definition for severe PH versus those who did not (88). In addition, treatment of patients with SSc ILD-PH with PAH therapy has demonstrated mixed results in retrospective cohort studies (89, 90). However, given their dismal prognosis and the possibility of a separate and potentially “responsive” pulmonary arteriopathy, patients with SSc ILD-PH are often given a trial of pulmonary vasodilators with close follow-up at expert centers.
Perhaps we should be asking not when is it appropriate to treat PH associated with ILD, but rather what are we treating? Although hypoxia was traditionally believed to be the major driver of PH in patients with ILD, there is evidence that PH can develop independent of hypoxemia (91, 92). In addition, the fact that PH only occurs in a subset of patients with established ILD and is weakly associated with either lung function or radiographic fibrosis scores also hints at the complexity of this disease (56, 93). Currently recognized subtypes of ILD-PH are based on the degree of elevation in mPAP and the presence of RV dysfunction but do not further delineate between patients with reduced functional capacity due to a circulatory versus a primarily restrictive ventilatory impairment. Evidence of circulatory limitation by cardiopulmonary exercising testing has been previously reported in patients with ILD-PH (94, 95) and offers a means for further enriching clinical trials with subjects who may have the most to gain from pulmonary vasodilator therapy. Furthermore, recent technical advances have increased the feasibility of cardiac magnetic resonance imaging in patients with significant pulmonary disease (96–98), thus enabling more precise clinical phenotyping in patients with ILD-PH through high-resolution quantification of RV function as well as noninvasive determination of mPAP and pulmonary vascular resistance (99–103).
Whether a subset of patients with ILD-PH will benefit from PAH-specific therapy remains an untested hypothesis. Thus, at this time, optimizing oxygen delivery, treating the underlying lung condition as well as associated comorbidities (e.g., left heart disease, volume overload), and referral of eligible patients for lung transplant remain the only valid recommendations for patients with ILD-PH (3).
Are You Treating Unrecognized PVOD with PAH-Specific Therapies?
Idiopathic and heritable PVOD and PCH are rare, difficult-to-diagnose, overlapping variants with an estimated prevalence of 1 to 2 cases per million (104, 105). However, this estimate does not account for PVOD associated with connective tissue disease (mainly SSc) or other associated risk factors (e.g., chemotherapy or exposure to organic solvents), nor does it account for patients misclassified with IPAH (104–106). Indeed, PVOD can masquerade as IPAH, and distinguishing between the two on clinical grounds is challenging because of broadly similar physical and hemodynamic findings (107). PVOD is characterized by a progressive clinical course, the lack of long-term response to PAH medical therapies, and possible occurrence of vasodilator-induced pulmonary edema, resulting in the need for early lung transplantation in eligible patients (104). On the basis of these observations, PVOD is classified under a separate subgroup category in the PH classification (3). The recent identification of an autosomal recessive form of PVOD and PCH due to biallelic mutations of the EIF2AK4 (eukaryotic translation initiation factor 2 α kinase 4) gene (15, 108, 109) further supports these entities as overlapping variants in the current classification.
Although histology is necessary to make a definitive diagnosis of PVOD, histological confirmation is usually only available on postmortem or explanted lung specimens because of the inherent risks of lung biopsy in this patient population. Therefore, a systematic, noninvasive strategy integrating results from high-resolution computed tomography, DlCO, arterial blood gas analysis, and 6MWD testing with pulse oximetry is necessary to identify PVOD as the cause of precapillary PH (107). Importantly, each of these clinical tests is currently recommended as part of the diagnostic workup in all patients with PH (3). In the largest case series to date, patients with histologically proven PVOD had significantly lower DlCO and PaO2 as well as more severe exertional oxygen desaturation than patients with histologically confirmed IPAH, heritable PAH, and anorexigen-associated PAH (107). Furthermore, the presence of two or more abnormalities on high-resolution computed tomography, including centrilobular ground-glass opacities, thickened interlobular septa, and mediastinal lymphadenopathy, was strongly associated with PVOD, although their absence did not rule out PVOD. Importantly, patients with PVOD have a normal PAOP and are otherwise hemodynamically indistinguishable from patients with other forms of precapillary PH (15, 104, 107). Although acute vasodilator testing with relatively low concentrations of inhaled nitric oxide (10 ppm) did not cause pulmonary edema in patients with PVOD, it also did not identify the approximately 20% to 40% of patients who would eventually develop pulmonary edema with chronic use of PAH-specific therapy (15, 107).
Although encountered much less frequently in clinical practice than ILD-PH, HFpEF-PH, and even PAH, a high index of suspicion for PVOD is essential to avoid potential adverse effects (e.g., pulmonary edema with pulmonary vasodilators) and delays in definitive therapy (i.e., lung transplantation) (104). Similarly, in patients with presumed IPAH who are not responding well to therapy, clinicians should consider whether they have adequately ruled out PVOD. This is particularly true in the setting of PH due to SSc, where PVOD has been recently described in a significant proportion of patients (110, 111). Notably, up to 10% of patients diagnosed clinically with IPAH are ultimately found to have histological evidence of PVOD on lung biopsy or at autopsy (112). Thus, in contrast to the quandary of whether there is a subset of patients with HFpEF-PH or ILD-PH who could benefit from PAH-specific therapy, here the challenge for clinicians is whether or not the patients with “PAH” they are treating have been thoroughly evaluated for suspected PVOD before initiating therapy. Recently, whole-genome sequencing of more than 800 patients diagnosed clinically with IPAH or familial PAH revealed a small subset of patients with biallelic EIF2AK4 mutations (113). Compared with patients with PAH without EIF2AK4 mutations, those with mutations had a lower transfer coefficient for carbon monoxide and were diagnosed with PAH at a younger age (median [interquartile range], 29 [23–38] years). Similar to patients clinically diagnosed with PVOD, patients with PAH with biallelic EIF2AK4 mutations (n = 9) did not improve their functional status with pulmonary vasodilators and had a shortened survival. Therefore, genetic testing for biallelic EIF2AK4 mutations should be considered in younger patients clinically diagnosed with IPAH but with a low diffusing capacity to prompt consideration of early referral for lung transplantation. Reduced expression of GCN2 (general control nonderepressible 2), the protein product of the EIF2AK4 gene, in patients with IPAH and heritable PAH with BMPR2 (bone morphogenetic protein receptor type 2) mutations, further highlights a previously unrecognized link between PVOD and PAH pathobiology (114).
Similar to HFpEF-PH and ILD-PH, there is no currently approved medical therapy for PVOD. However, in addition to supportive therapy with diuretics and oxygen, the approach to treatment of patients with PVOD with PAH-specific therapy is complex and potentially life threatening and therefore should only be initiated at a PH center with experience in caring for these patients (3).
Conclusions
Although finding promise for treatments that might eventually cross PH WHO groups, the symposium clearly pointed out that improved phenotyping is still needed to better determine who will benefit from these therapies. In addition to well-planned, randomized, double-blind, placebo-controlled trials, efforts such as the NHLBI Pulmonary Vascular Disease Phenomics Program (PVDOMICS) are needed to move the field forward from our “one-size-fits-all” PH strategy toward a precision medicine approach that will lay the groundwork for the development of appropriate and effective therapeutic interventions. Similarly, large, contemporary epidemiological studies are providing further evidence that pulmonary vascular disease exists on a continuum rather than simply above a threshold resting mPAP (115). In conjunction with more refined clinical and physiological phenotyping, integrated “omics” approaches and large data analytics offer the opportunity to 1) deconstruct our current classification of PH subtypes; 2) improve our understanding of molecular mechanisms leading to pulmonary vascular disease in the context of complex, multifaceted conditions, such as left heart disease and chronic lung diseases; and 3) characterize treatment responders versus nonresponders in future clinical trials (86, 116). As the field moves from population to precision medicine (86, 116, 117) and focuses future resources on deep phenotyping (advanced imaging, transcriptomics, proteomics, and metabolomics), we may eventually realize our potential to identify relevant clinical and molecular features, shattering our icebergs into more granular groupings of phenotypically distinct crystals of varying shapes and sizes.
Acknowledgments
Acknowledgment
The authors thank Kelly Byrne (NIH Clinical Center) for her support and assistance with figure preparation.
Footnotes
Supported in part by the Intramural Research Program of the NIH.
Originally Published in Press as DOI: 10.1164/rccm.201710-2093PP on February 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Galiè N, Simonneau G. The Fifth World Symposium on Pulmonary Hypertension. J Am Coll Cardiol. 2013;62:D1–D3. doi: 10.1016/j.jacc.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D34–D41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 4.Smith CM. Origin and uses of primum non nocere: above all, do no harm! J Clin Pharmacol. 2005;45:371–377. doi: 10.1177/0091270004273680. [DOI] [PubMed] [Google Scholar]
- 5.Xamoterol in Severe Heart Failure Study Group Xamoterol in severe heart failure: the Xamoterol in Severe Heart Failure Study Group Lancet 19903361–6.[Published erratum appears in Lancet 336:698.] [PubMed] [Google Scholar]
- 6.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure: the PROMISE Study Research Group. N Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure: Vesnarinone Trial Investigators. N Engl J Med. 1998;339:1810–1816. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 8.Gladwin MT. Translational advances in the field of pulmonary hypertension bench to bedside: how fundamental discoveries in science are advancing our understanding and therapy of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:1–3. doi: 10.1164/rccm.201608-1637ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chun HJ, Bonnet S, Chan SY. Translational advances in the field of pulmonary hypertension: translating microRNA biology in pulmonary hypertension: it will take more than “miR” words. Am J Respir Crit Care Med. 2017;195:167–178. doi: 10.1164/rccm.201604-0886PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension: from cancer biology to new pulmonary arterial hypertension therapeutics: targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicolls MR, Voelkel NF. The roles of immunity in the prevention and evolution of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;195:1292–1299. doi: 10.1164/rccm.201608-1630PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guazzi M. Pulmonary hypertension in heart failure preserved ejection fraction: prevalence, pathophysiology, and clinical perspectives. Circ Heart Fail. 2014;7:367–377. doi: 10.1161/CIRCHEARTFAILURE.113.000823. [DOI] [PubMed] [Google Scholar]
- 13.Hoeper MM, Lam CSP, Vachiery JL, Bauersachs J, Gerges C, Lang IM, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a plea for proper phenotyping and further research. Eur Heart J. 2017;38:2869–2873. doi: 10.1093/eurheartj/ehw597. [DOI] [PubMed] [Google Scholar]
- 14.Panagiotou M, Church AC, Johnson MK, Peacock AJ. Pulmonary vascular and cardiac impairment in interstitial lung disease. Eur Respir Rev. 2017;26:160053. doi: 10.1183/16000617.0053-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montani D, Girerd B, Jais X, Levy M, Amar D, Savale L, et al. Clinical phenotypes and outcomes of heritable and sporadic pulmonary veno-occlusive disease: a population-based study. Lancet Respir Med. 2017;5:125–134. doi: 10.1016/S2213-2600(16)30438-6. [DOI] [PubMed] [Google Scholar]
- 16.Lam CS, Borlaug BA, Kane GC, Enders FT, Rodeheffer RJ, Redfield MM. Age-associated increases in pulmonary artery systolic pressure in the general population. Circulation. 2009;119:2663–2670. doi: 10.1161/CIRCULATIONAHA.108.838698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strange G, Playford D, Stewart S, Deague JA, Nelson H, Kent A, et al. Pulmonary hypertension: prevalence and mortality in the Armadale echocardiography cohort. Heart. 2012;98:1805–1811. doi: 10.1136/heartjnl-2012-301992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 19.Grigioni F, Potena L, Galie N, Fallani F, Bigliardi M, Coccolo F, et al. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–1246. doi: 10.1016/j.healun.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Shalaby A, Voigt A, El-Saed A, Saba S. Usefulness of pulmonary artery pressure by echocardiography to predict outcome in patients receiving cardiac resynchronization therapy heart failure. Am J Cardiol. 2008;101:238–241. doi: 10.1016/j.amjcard.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 21.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khush KK, Tasissa G, Butler J, McGlothlin D, De Marco T, Investigators E. Effect of pulmonary hypertension on clinical outcomes in advanced heart failure: analysis of the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) database. Am Heart J. 2009;157:1026–1034. doi: 10.1016/j.ahj.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Leung CC, Moondra V, Catherwood E, Andrus BW. Prevalence and risk factors of pulmonary hypertension in patients with elevated pulmonary venous pressure and preserved ejection fraction. Am J Cardiol. 2010;106:284–286. doi: 10.1016/j.amjcard.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 24.Gerges M, Gerges C, Pistritto AM, Lang MB, Trip P, Jakowitsch J, et al. Pulmonary hypertension in heart failure: epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 25.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. American Heart Association Statistics Committee, Stroke Statistics Subcommittee. Executive summary: heart disease and stroke statistics–2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 26.Cappola TP, Felker GM, Kao WH, Hare JM, Baughman KL, Kasper EK. Pulmonary hypertension and risk of death in cardiomyopathy: patients with myocarditis are at higher risk. Circulation. 2002;105:1663–1668. doi: 10.1161/01.cir.0000013771.30198.82. [DOI] [PubMed] [Google Scholar]
- 27.Szwejkowski BR, Elder DH, Shearer F, Jack D, Choy AM, Pringle SD, et al. Pulmonary hypertension predicts all-cause mortality in patients with heart failure: a retrospective cohort study. Eur J Heart Fail. 2012;14:162–167. doi: 10.1093/eurjhf/hfr159. [DOI] [PubMed] [Google Scholar]
- 28.Robbins IM, Hemnes AR, Pugh ME, Brittain EL, Zhao DX, Piana RN, et al. High prevalence of occult pulmonary venous hypertension revealed by fluid challenge in pulmonary hypertension. Circ Heart Fail. 2014;7:116–122. doi: 10.1161/CIRCHEARTFAILURE.113.000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Alto M, Romeo E, Argiento P, Motoji Y, Correra A, Di Marco GM, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest. 2017;151:119–126. doi: 10.1016/j.chest.2016.08.1439. [DOI] [PubMed] [Google Scholar]
- 30.Fujimoto N, Borlaug BA, Lewis GD, Hastings JL, Shafer KM, Bhella PS, et al. Hemodynamic responses to rapid saline loading: the impact of age, sex, and heart failure. Circulation. 2013;127:55–62. doi: 10.1161/CIRCULATIONAHA.112.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersen MJ, Olson TP, Melenovsky V, Kane GC, Borlaug BA. Differential hemodynamic effects of exercise and volume expansion in people with and without heart failure. Circ Heart Fail. 2015;8:41–48. doi: 10.1161/CIRCHEARTFAILURE.114.001731. [DOI] [PubMed] [Google Scholar]
- 32.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3:588–595. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–D50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 34.Vachiéry JL, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100–D108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 35.Assad TR, Hemnes AR, Larkin EK, Glazer AM, Xu M, Wells QS, et al. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–2536. doi: 10.1016/j.jacc.2016.09.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk-Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954. doi: 10.1093/eurheartj/ehv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai YC, Tabima DM, Dube JJ, Hughan KS, Vanderpool RR, Goncharov DA, et al. SIRT3-AMP-activated protein kinase activation by nitrite and metformin improves hyperglycemia and normalizes pulmonary hypertension associated with heart failure with preserved ejection fraction. Circulation. 2016;133:717–731. doi: 10.1161/CIRCULATIONAHA.115.018935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng Q, Lai YC, Kelly NJ, Bueno M, Baust JJ, Bachman TN, et al. Development of a mouse model of metabolic syndrome, pulmonary hypertension, and heart failure with preserved ejection fraction. Am J Respir Cell Mol Biol. 2017;56:497–505. doi: 10.1165/rcmb.2016-0177OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke-Zaba J, Coghlan JG, et al. Pre-capillary, combined, and post-capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;68:368–378. doi: 10.1016/j.jacc.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 40.Lammi MR, Saketkoo LA, Gordon JK, Lauto P, Fagan K, Steen VD, et al. Clinical characteristics and survival of systemic sclerosis patients with pulmonary hypertension and elevated wedge pressure: Observations from the PHAROS cohort. Respirology. 2017;22:1386–1392. doi: 10.1111/resp.13067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Preston IR, Sagliani KD, Roberts KE, Shah AM, Desouza SA, Howard W, et al. Comparison of acute hemodynamic effects of inhaled nitric oxide and inhaled epoprostenol in patients with pulmonary hypertension. Pulm Circ. 2013;3:68–73. doi: 10.4103/2045-8932.109916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grossman NL, Fiack CA, Weinberg JM, Rybin DV, Farber HW. Pulmonary hypertension associated with heart failure with preserved ejection fraction: acute hemodynamic effects of inhaled iloprost. Pulm Circ. 2015;5:198–203. doi: 10.1086/679725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon MA, Vanderpool RR, Nouraie M, Bachman TN, White PM, Sugahara M, et al. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI Insight. 2016;1:e89620. doi: 10.1172/jci.insight.89620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koller B, Steringer-Mascherbauer R, Ebner CH, Weber T, Ammer M, Eichinger J, et al. Pilot study of endothelin receptor blockade in heart failure with diastolic dysfunction and pulmonary hypertension (BADDHY-Trial) Heart Lung Circ. 2017;26:433–441. doi: 10.1016/j.hlc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 47.Forfia PR, Borlaug BA. Letter by Forfia and Borlaug regarding article, “Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study”. Circulation. 2012;125:e408. doi: 10.1161/CIRCULATIONAHA.111.064584. author reply e409–410. [DOI] [PubMed] [Google Scholar]
- 48.Hoendermis ES, Liu LC, Hummel YM, van der Meer P, de Boer RA, Berger RM, et al. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 49.Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Borlaug BA. Taking aim at pulmonary hypertension in heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2574–2575. doi: 10.1093/eurheartj/ehv280. [DOI] [PubMed] [Google Scholar]
- 51.Leopold JA. Biological phenotyping of combined post-capillary and pre-capillary pulmonary hypertension: focus on pulmonary vascular remodeling. J Am Coll Cardiol. 2016;68:2537–2539. doi: 10.1016/j.jacc.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 53.Burkhoff D, Maurer MS, Joseph SM, Rogers JG, Birati EY, Rame JE, et al. Left atrial decompression pump for severe heart failure with preserved ejection fraction: theoretical and clinical considerations. JACC Heart Fail. 2015;3:275–282. doi: 10.1016/j.jchf.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 54.Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008;31:1357–1367. doi: 10.1183/09031936.00171307. [DOI] [PubMed] [Google Scholar]
- 55.Nathan SD, Shlobin OA, Ahmad S, Koch J, Barnett SD, Ad N, et al. Serial development of pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respiration. 2008;76:288–294. doi: 10.1159/000114246. [DOI] [PubMed] [Google Scholar]
- 56.Shorr AF, Wainright JL, Cors CS, Lettieri CJ, Nathan SD. Pulmonary hypertension in patients with pulmonary fibrosis awaiting lung transplant. Eur Respir J. 2007;30:715–721. doi: 10.1183/09031936.00107206. [DOI] [PubMed] [Google Scholar]
- 57.Raghu G, Nathan SD, Behr J, Brown KK, Egan JJ, Kawut SM, et al. Pulmonary hypertension in idiopathic pulmonary fibrosis with mild-to-moderate restriction. Eur Respir J. 2015;46:1370–1377. doi: 10.1183/13993003.01537-2014. [DOI] [PubMed] [Google Scholar]
- 58.Andersen CU, Mellemkjaer S, Hilberg O, Nielsen-Kudsk JE, Simonsen U, Bendstrup E. Pulmonary hypertension in interstitial lung disease: prevalence, prognosis and 6 min walk test. Respir Med. 2012;106:875–882. doi: 10.1016/j.rmed.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 59.Modrykamien AM, Gudavalli R, McCarthy K, Parambil J. Echocardiography, 6-minute walk distance, and distance-saturation product as predictors of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Respir Care. 2010;55:584–588. [PubMed] [Google Scholar]
- 60.Swigris JJ, Olson AL, Shlobin OA, Ahmad S, Brown KK, Nathan SD. Heart rate recovery after six-minute walk test predicts pulmonary hypertension in patients with idiopathic pulmonary fibrosis. Respirology. 2011;16:439–445. doi: 10.1111/j.1440-1843.2010.01877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lettieri CJ, Nathan SD, Barnett SD, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 62.Patel NM, Lederer DJ, Borczuk AC, Kawut SM. Pulmonary hypertension in idiopathic pulmonary fibrosis. Chest. 2007;132:998–1006. doi: 10.1378/chest.06-3087. [DOI] [PubMed] [Google Scholar]
- 63.Kimura M, Taniguchi H, Kondoh Y, Kimura T, Kataoka K, Nishiyama O, et al. Pulmonary hypertension as a prognostic indicator at the initial evaluation in idiopathic pulmonary fibrosis. Respiration. 2013;85:456–463. doi: 10.1159/000345221. [DOI] [PubMed] [Google Scholar]
- 64.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 65.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 66.Noble PW, Albera C, Bradford WZ, Costabel U, du Bois RM, Fagan EA, et al. Pirfenidone for idiopathic pulmonary fibrosis: analysis of pooled data from three multinational phase 3 trials. Eur Respir J. 2016;47:243–253. doi: 10.1183/13993003.00026-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Seeger W, Adir Y, Barbera JA, Champion H, Coghlan JG, Cottin V, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol. 2013;62:D109–D116. doi: 10.1016/j.jacc.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 68.Prins KW, Duval S, Markowitz J, Pritzker M, Thenappan T. Chronic use of PAH-specific therapy in World Health Organization group III pulmonary hypertension: a systematic review and meta-analysis. Pulm Circ. 2017;7:145–155. doi: 10.1086/690017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nathan SD, Behr J, Collard HR, Cottin V, Hoeper MM, Martinez F, et al. RISE-IIP: riociguat for the treatment of pulmonary hypertension associated with idiopathic interstitial pneumonia [abstract] Eur Respir J. 2017;50:OA1985. [Google Scholar]
- 70.King TE, Jr, Behr J, Brown KK, du Bois RM, Lancaster L, de Andrade JA, et al. BUILD-1: a randomized placebo-controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:75–81. doi: 10.1164/rccm.200705-732OC. [DOI] [PubMed] [Google Scholar]
- 71.Idiopathic Pulmonary Fibrosis Clinical Research Network. Zisman DA, Schwarz M, Anstrom KJ, Collard HR, Flaherty KR, et al. A controlled trial of sildenafil in advanced idiopathic pulmonary fibrosis. N Engl J Med. 2010;363:620–628. doi: 10.1056/NEJMoa1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jackson RM, Glassberg MK, Ramos CF, Bejarano PA, Butrous G, Gomez-Marin O. Sildenafil therapy and exercise tolerance in idiopathic pulmonary fibrosis. Lung. 2010;188:115–123. doi: 10.1007/s00408-009-9209-8. [DOI] [PubMed] [Google Scholar]
- 73.King TE, Jr, Brown KK, Raghu G, du Bois RM, Lynch DA, Martinez F, et al. BUILD-3: a randomized, controlled trial of bosentan in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:92–99. doi: 10.1164/rccm.201011-1874OC. [DOI] [PubMed] [Google Scholar]
- 74.Raghu G, Behr J, Brown KK, Egan JJ, Kawut SM, Flaherty KR, et al. AREMIS-IPF Investigators. Treatment of idiopathic pulmonary fibrosis with ambrisentan: a parallel, randomized trial. Ann Intern Med. 2013;158:641–649. doi: 10.7326/0003-4819-158-9-201305070-00003. [DOI] [PubMed] [Google Scholar]
- 75.Raghu G, Million-Rousseau R, Morganti A, Perchenet L, Behr J, Music Study Group Macitentan for the treatment of idiopathic pulmonary fibrosis: the randomised controlled MUSIC trial. Eur Respir J. 2013;42:1622–1632. doi: 10.1183/09031936.00104612. [DOI] [PubMed] [Google Scholar]
- 76.Olschewski H, Ghofrani HA, Walmrath D, Schermuly R, Temmesfeld-Wollbruck B, Grimminger F, et al. Inhaled prostacyclin and iloprost in severe pulmonary hypertension secondary to lung fibrosis. Am J Respir Crit Care Med. 1999;160:600–607. doi: 10.1164/ajrccm.160.2.9810008. [DOI] [PubMed] [Google Scholar]
- 77.Ghofrani HA, Wiedemann R, Rose F, Schermuly RT, Olschewski H, Weissmann N, et al. Sildenafil for treatment of lung fibrosis and pulmonary hypertension: a randomised controlled trial. Lancet. 2002;360:895–900. doi: 10.1016/S0140-6736(02)11024-5. [DOI] [PubMed] [Google Scholar]
- 78.Collard HR, Anstrom KJ, Schwarz MI, Zisman DA. Sildenafil improves walk distance in idiopathic pulmonary fibrosis. Chest. 2007;131:897–899. doi: 10.1378/chest.06-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Minai OA, Sahoo D, Chapman JT, Mehta AC. Vaso-active therapy can improve 6-min walk distance in patients with pulmonary hypertension and fibrotic interstitial lung disease. Respir Med. 2008;102:1015–1020. doi: 10.1016/j.rmed.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Corte TJ, Gatzoulis MA, Parfitt L, Harries C, Wells AU, Wort SJ. The use of sildenafil to treat pulmonary hypertension associated with interstitial lung disease. Respirology. 2010;15:1226–1232. doi: 10.1111/j.1440-1843.2010.01860.x. [DOI] [PubMed] [Google Scholar]
- 81.Hoeper MM, Halank M, Wilkens H, Gunther A, Weimann G, Gebert I, et al. Riociguat for interstitial lung disease and pulmonary hypertension: a pilot trial. Eur Respir J. 2013;41:853–860. doi: 10.1183/09031936.00213911. [DOI] [PubMed] [Google Scholar]
- 82.Saggar R, Khanna D, Vaidya A, Derhovanessian A, Maranian P, Duffy E, et al. Changes in right heart haemodynamics and echocardiographic function in an advanced phenotype of pulmonary hypertension and right heart dysfunction associated with pulmonary fibrosis. Thorax. 2014;69:123–129. doi: 10.1136/thoraxjnl-2013-204150. [DOI] [PubMed] [Google Scholar]
- 83.Corte TJ, Keir GJ, Dimopoulos K, Howard L, Corris PA, Parfitt L, et al. Bosentan in pulmonary hypertension associated with fibrotic idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2014;190:208–217. doi: 10.1164/rccm.201403-0446OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Trammell AW, Pugh ME, Newman JH, Hemnes AR, Robbins IM. Use of pulmonary arterial hypertension-approved therapy in the treatment of non-group 1 pulmonary hypertension at US referral centers. Pulm Circ. 2015;5:356–363. doi: 10.1086/681264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Han MK, Bach DS, Hagan PG, Yow E, Flaherty KR, Toews GB, et al. Sildenafil preserves exercise capacity in patients with idiopathic pulmonary fibrosis and right-sided ventricular dysfunction. Chest. 2013;143:1699–1708. doi: 10.1378/chest.12-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dweik RA, Rounds S, Erzurum SC, Archer S, Fagan K, Hassoun PM, et al. ATS Committee on Pulmonary Hypertension Phenotypes. An official American Thoracic Society Statement: pulmonary hypertension phenotypes. Am J Respir Crit Care Med. 2014;189:345–355. doi: 10.1164/rccm.201311-1954ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brewis MJ, Church AC, Johnson MK, Peacock AJ. Severe pulmonary hypertension in lung disease: phenotypes and response to treatment. Eur Respir J. 2015;46:1378–1389. doi: 10.1183/13993003.02307-2014. [DOI] [PubMed] [Google Scholar]
- 88.Hoeper MM, Behr J, Held M, Grunig E, Vizza CD, Vonk-Noordegraaf A, et al. Pulmonary hypertension in patients with chronic fibrosing idiopathic interstitial pneumonias. PLoS One. 2015;10:e0141911. doi: 10.1371/journal.pone.0141911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Le Pavec J, Girgis RE, Lechtzin N, Mathai SC, Launay D, Hummers LK, et al. Systemic sclerosis-related pulmonary hypertension associated with interstitial lung disease: impact of pulmonary arterial hypertension therapies. Arthritis Rheum. 2011;63:2456–2464. doi: 10.1002/art.30423. [DOI] [PubMed] [Google Scholar]
- 90.Volkmann ER, Saggar R, Khanna D, Torres B, Flora A, Yoder L, et al. Improved transplant-free survival in patients with systemic sclerosis-associated pulmonary hypertension and interstitial lung disease. Arthritis Rheumatol. 2014;66:1900–1908. doi: 10.1002/art.38623. [DOI] [PubMed] [Google Scholar]
- 91.Ryu JH, Krowka MJ, Pellikka PA, Swanson KL, McGoon MD. Pulmonary hypertension in patients with interstitial lung diseases. Mayo Clin Proc. 2007;82:342–350. doi: 10.4065/82.3.342. [DOI] [PubMed] [Google Scholar]
- 92.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol. 2011;45:1–15. doi: 10.1165/rcmb.2010-0365TR. [DOI] [PubMed] [Google Scholar]
- 93.Zisman DA, Ross DJ, Belperio JA, Saggar R, Lynch JP, III, Ardehali A, et al. Prediction of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2007;101:2153–2159. doi: 10.1016/j.rmed.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hansen JE, Wasserman K. Pathophysiology of activity limitation in patients with interstitial lung disease. Chest. 1996;109:1566–1576. doi: 10.1378/chest.109.6.1566. [DOI] [PubMed] [Google Scholar]
- 95.Boutou AK, Pitsiou GG, Trigonis I, Papakosta D, Kontou PK, Chavouzis N, et al. Exercise capacity in idiopathic pulmonary fibrosis: the effect of pulmonary hypertension. Respirology. 2011;16:451–458. doi: 10.1111/j.1440-1843.2010.01909.x. [DOI] [PubMed] [Google Scholar]
- 96.Fischer SE, Wickline SA, Lorenz CH. Novel real-time R-wave detection algorithm based on the vectorcardiogram for accurate gated magnetic resonance acquisitions. Magn Reson Med. 1999;42:361–370. doi: 10.1002/(sici)1522-2594(199908)42:2<361::aid-mrm18>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 97.Abd-Elmoniem KZ, Obele CC, Sibley CT, Matta JR, Pettigrew RI, Gharib AM. Free-breathing single navigator gated cine cardiac magnetic resonance at 3 T: feasibility study in patients. J Comput Assist Tomogr. 2011;35:382–386. doi: 10.1097/RCT.0b013e31821b0ade. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Piehler KM, Wong TC, Puntil KS, Zareba KM, Lin K, Harris DM, et al. Free-breathing, motion-corrected late gadolinium enhancement is robust and extends risk stratification to vulnerable patients. Circ Cardiovasc Imaging. 2013;6:423–432. doi: 10.1161/CIRCIMAGING.112.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanz J, Kuschnir P, Rius T, Salguero R, Sulica R, Einstein AJ, et al. Pulmonary arterial hypertension: noninvasive detection with phase-contrast MR imaging. Radiology. 2007;243:70–79. doi: 10.1148/radiol.2431060477. [DOI] [PubMed] [Google Scholar]
- 100.Swift AJ, Rajaram S, Condliffe R, Capener D, Hurdman J, Elliot CA, et al. Diagnostic accuracy of cardiovascular magnetic resonance imaging of right ventricular morphology and function in the assessment of suspected pulmonary hypertension results from the ASPIRE registry. J Cardiovasc Magn Reson. 2012;14:40. doi: 10.1186/1532-429X-14-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swift AJ, Wild JM, Nagle SK, Roldan-Alzate A, Francois CJ, Fain S, et al. Quantitative magnetic resonance imaging of pulmonary hypertension: a practical approach to the current state of the art. J Thorac Imaging. 2014;29:68–79. doi: 10.1097/RTI.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, et al. Magnetic resonance imaging in the prognostic evaluation of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2017;196:228–239. doi: 10.1164/rccm.201611-2365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johns CS, Rajaram S, Capener DA, Oram C, Elliot C, Condliffe R, et al. Non-invasive methods for estimating mPAP in COPD using cardiovascular magnetic resonance imaging. Eur Radiol. 2018;28:1438–1448. doi: 10.1007/s00330-017-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Montani D, Lau EM, Dorfmuller P, Girerd B, Jais X, Savale L, et al. Pulmonary veno-occlusive disease. Eur Respir J. 2016;47:1518–1534. doi: 10.1183/13993003.00026-2016. [DOI] [PubMed] [Google Scholar]
- 105.Chaisson NF, Dodson MW, Elliott CG. Pulmonary capillary hemangiomatosis and pulmonary veno-occlusive disease. Clin Chest Med. 2016;37:523–534. doi: 10.1016/j.ccm.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 106.Harch S, Whitford H, McLean C. Failure of medical therapy in pulmonary arterial hypertension: is there an alternative diagnosis? Chest. 2009;135:1462–1469. doi: 10.1378/chest.08-2006. [DOI] [PubMed] [Google Scholar]
- 107.Montani D, Achouh L, Dorfmuller P, Le Pavec J, Sztrymf B, Tcherakian C, et al. Pulmonary veno-occlusive disease: clinical, functional, radiologic, and hemodynamic characteristics and outcome of 24 cases confirmed by histology. Medicine (Baltimore) 2008;87:220–233. doi: 10.1097/MD.0b013e31818193bb. [DOI] [PubMed] [Google Scholar]
- 108.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014;46:65–69. doi: 10.1038/ng.2844. [DOI] [PubMed] [Google Scholar]
- 109.Best DH, Sumner KL, Austin ED, Chung WK, Brown LM, Borczuk AC, et al. EIF2AK4 mutations in pulmonary capillary hemangiomatosis. Chest. 2014;145:231–236. doi: 10.1378/chest.13-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dorfmüller P, Humbert M, Perros F, Sanchez O, Simonneau G, Muller KM, et al. Fibrous remodeling of the pulmonary venous system in pulmonary arterial hypertension associated with connective tissue diseases. Hum Pathol. 2007;38:893–902. doi: 10.1016/j.humpath.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 111.Günther S, Jais X, Maitre S, Berezne A, Dorfmuller P, Seferian A, et al. Computed tomography findings of pulmonary venoocclusive disease in scleroderma patients presenting with precapillary pulmonary hypertension. Arthritis Rheum. 2012;64:2995–3005. doi: 10.1002/art.34501. [DOI] [PubMed] [Google Scholar]
- 112.Mandel J, Mark EJ, Hales CA. Pulmonary veno-occlusive disease. Am J Respir Crit Care Med. 2000;162:1964–1973. doi: 10.1164/ajrccm.162.5.9912045. [DOI] [PubMed] [Google Scholar]
- 113.Hadinnapola C, Bleda M, Haimel M, Screaton N, Swift A, Dorfmuller P, et al. Phenotypic characterization of EIF2AK4 mutation carriers in a large cohort of patients diagnosed clinically with pulmonary arterial hypertension. Circulation. 2017;136:2022–2033. doi: 10.1161/CIRCULATIONAHA.117.028351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nossent EJ, Antigny F, Montani D, Bogaard H, Ghigna M, Lambert M, et al. Pulmonary vascular remodeling patterns and expression of general control nonderepressible 2 (GCN2) in pulmonary veno-occlusive disease. J Heart Lung Transplant. 2018;37:647–655. doi: 10.1016/j.healun.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 115.Maron BA, Abman SH. Translational advances in the field of pulmonary hypertension: focusing on developmental origins and disease inception for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2017;195:292–301. doi: 10.1164/rccm.201604-0882PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Newman JH, Rich S, Abman SH, Alexander JH, Barnard J, Beck GJ, et al. Enhancing insights into pulmonary vascular disease through a precision medicine approach: a joint NHLBI-Cardiovascular Medical Research and Education Fund Workshop Report. Am J Respir Crit Care Med. 2017;195:1661–1670. doi: 10.1164/rccm.201701-0150WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Austin ED, West J, Loyd JE, Hemnes AR. Translational advances in the field of pulmonary hypertension molecular medicine of pulmonary arterial hypertension: from population genetics to precision medicine and gene editing. Am J Respir Crit Care Med. 2017;195:23–31. doi: 10.1164/rccm.201605-0905PP. [DOI] [PMC free article] [PubMed] [Google Scholar]