To the Editor:
The club cell secretory protein CC16 is a pneumoprotein present in circulation but mainly produced by club cells and other nonciliated airway epithelial cells (1–3). Its biological functions have not been conclusively elucidated, but findings from in vitro studies (4) and mouse models (5) support antiinflammatory and antitoxicant properties of CC16 in the lung.
In line with this scenario, CC16 deficits in blood and airways have been associated with asthma (6), chronic obstructive pulmonary disease (7), impaired growth of FEV1 in children (8), and accelerated FEV1 decline in adults (8). Whether CC16 plays a direct role in lung health or it represents an early indicator of alterations in airway epithelial cell functions, understanding the trajectories of serum CC16 from childhood into adult life and the early factors that control such trajectories is a critical initial step to evaluate the possible use of this protein in risk stratification and/or early prevention of lung disease.
In the present study, we used data from the Tucson Children’s Respiratory Study, a prospective birth cohort study that recruited 1,246 healthy infants between 1980 and 1984. Questionnaires were completed by the primary caregiver at enrollment within weeks after the child’s birth, and again when the child was 6 years old. Blood samples were collected from umbilical cord at birth and by venipuncture at ages 6, 11, 16, 22, 26, and 32 years. Circulating CC16 levels were measured in serum, using a commercially available ELISA kit (BioVendor). For 78 participants at Year 6, only plasma was available and a correction factor was applied to their CC16 concentrations to enable comparison with serum samples, as described previously (8).
We tested multiple early-life factors for association with serum CC16, including participant’s sex, ethnicity/race, gestational age at birth, type of delivery, ponderal index, feeding practices in the first 9 months of life, and genotype for the SNP rs3741240, which is located in the 5′ untranslated region of the CC16-encoding gene SCGB1A1 (secretoglobin family 1A member 1) and is a known protein quantitative trait locus for serum CC16 levels (9). Genetic analyses were restricted to participants with both white (either Hispanic or non-Hispanic) parents. We also evaluated maternal age at delivery, parental education, and maternal and paternal smoking both at enrollment (child’s birth) and at Year 6. Participants completed confidential questionnaires on active smoking starting at age 11 years. To rule out the possibility that the effects of some early-life factors (particularly parental smoking) on later serum CC16 levels were a result of confounding by active smoking later in life, sensitivity analyses were completed with further adjustment for cumulative pack-years of participants up to age 32 years.
CC16 levels were log10 transformed to approximate normal distribution. Longitudinal random effects models were used to assess early-life factors for association with CC16 levels measured at multiple ages up to 32 years. In these models, we used CC16 z-scores so that coefficients of determinants represented their effects on CC16 in SDs. An interaction term with survey year was tested for each factor to determine whether its association with CC16 levels varied at different ages.
Overall, 1,168 Tucson Children’s Respiratory Study participants had CC16 measurements from at least one survey. As compared with the remaining 78 participants, these participants were more likely to be white and to have older mothers and more educated parents. No other significant differences were found.
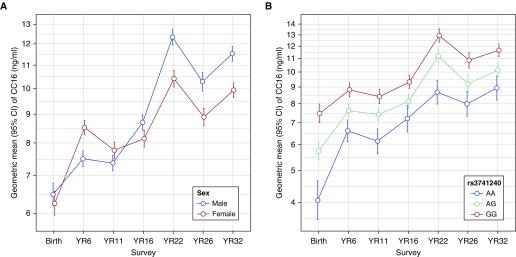
CC16 levels increased from birth (geometric mean, 6.36 ng/ml) to childhood (7.98, 7.57, and 8.42 ng/ml at Year 6, Year 11, and Year 16, respectively), and to adult life (11.28, 9.54, and 10.61 ng/ml at Year 22, Year 26, and Year 32, respectively). We found intrasubject tracking of CC16 levels across all ages. CC16 levels at birth were weakly correlated with CC16 levels at all ages (Pearson correlation coefficients between 0.33 and 0.38; all P values < 0.001). Postnatal CC16 levels were moderately correlated across all ages (correlation coefficients between 0.40 for Year 6–with–Year 32 correlation and 0.58 for Year 22–with–Year 26 correlation; all P values < 0.001).
We found sex and rs3741240 to have the strongest effects on CC16 levels (Figure 1). Sex was the only factor with a significant interaction with survey year: females had higher levels than males at age 6 years, but this relation reversed in adult life. In contrast, the effects of rs3741240 on serum CC16 were consistent from birth to age 32 years and explained between 10% (at birth) and 5% (at Year 16) of the variability in CC16 levels.
Figure 1.
Geometric means of serum CC16 concentrations from birth to age 32 years by sex (A; all participants, N subjects = 1,168; N observations = 3,721) and by rs3741240 genotypes (B; only white participants, N subjects = 661; N observations = 2,815). Error bars indicate the 95% confidence intervals. CI = confidence interval; YR = year.
Among other significant predictors, participants with both Hispanic parents had lower CC16 levels than those with both non-Hispanic white parents. In addition, we found direct effects of maternal age at delivery and parental education and inverse effects of maternal smoking at Year 6 (but not at birth) on CC16 levels. These effects were relatively consistent across ages 6–32 years. Apart from rs3741240, no other factor was associated with CC16 levels at birth. Therefore, we limited the final multivariable random effects models to postnatal CC16 levels between ages 6 and 32 years. Table 1 summarizes results from the model including significant early-life factors (model 1), the model further adding rs3741240 among covariates (model 2), and the model further adding the time-dependent covariates height and cumulative pack-years (model 3). Interestingly, in model 3, the inclusion of height as a covariate affected the main effects of years, suggesting that the increase of serum CC16 with age may be related to increasing body/lung size with age. In contrast, although pack-years were significantly associated with reduced serum CC16, their inclusion in the model did not affect the coefficient of maternal smoking, indicating that confounding by active smoking is unlikely. Overall, determinants from models 2 and 3 explained 11% of the variability of CC16 levels across ages 6–32 years.
Table 1.
Longitudinal Random Effects Models for Early-Life Determinants of CC16 Levels as Measured from Age 6 to Age 32 Years
| Model 1: Base Model, All Participants (N Subjects = 762; N Observations = 2,589) |
Model 2: Base Model plus rs3741240*, White† Participants (N Subjects = 603; N Observations = 2,208) |
Model 3: Model 2 plus Height and Cumulative Pack-Years*, White† Participants (N Subjects = 586; N Observations = 2,049) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coef | 95% CI | P Value | Coef | 95% CI | P Value | Coef | 95% CI | P Value | ||
| Sex (ref: male) | ||||||||||
| Female | 0.310 | 0.149 to 0.471 | <0.001 | 0.273 | 0.095 to 0.451 | 0.003 | 0.259 | 0.072 to 0.446 | 0.007 | |
| Years (ref: Year 6) | ||||||||||
| Year 11 | 0.095 | −0.025 to 0.214 | 0.120 | 0.112 | −0.018 to 0.242 | 0.090 | −0.101 | −0.337 to 0.135 | 0.403 | |
| Year 16 | 0.238 | 0.111 to 0.365 | <0.001 | 0.248 | 0.110 to 0.385 | <0.001 | −0.202 | −0.647 to 0.243 | 0.373 | |
| Year 22 | 0.342 | 0.212 to 0.473 | <0.001 | 0.377 | 0.236 to 0.517 | <0.001 | −0.063 | −0.521 to 0.395 | 0.789 | |
| Year 26 | 0.308 | 0.171 to 0.444 | <.001 | 0.316 | 0.170 to 0.461 | <0.001 | −0.100 | −0.565 to 0.364 | 0.672 | |
| Year 32 | 0.331 | 0.169 to 0.493 | <0.001 | 0.331 | 0.159 to 0.503 | <0.001 | −0.043 | −0.517 to 0.431 | 0.857 | |
| Sex by year‡ | ||||||||||
| Female: Year 11 | −0.192 | −0.362 to −0.022 | 0.027 | −0.190 | −0.375 to −0.006 | 0.043 | −0.185 | −0.385 to 0.016 | 0.071 | |
| Female: Year 16 | −0.503 | −0.685 to −0.322 | <0.001 | −0.471 | −0.667 to −0.275 | <0.001 | −0.371 | −0.589 to −0.153 | 0.001 | |
| Female: Year 22 | −0.650 | −0.834 to −0.466 | <0.001 | −0.620 | −0.818 to −0.422 | <0.001 | −0.522 | −0.745 to −0.300 | <0.001 | |
| Female: Year 26 | −0.574 | −0.768 to −0.381 | <0.001 | −0.522 | −0.729 to −0.314 | <0.001 | −0.440 | −0.674 to −0.207 | <0.001 | |
| Female: Year 32 | −0.606 | −0.830 to −0.383 | <0.001 | −0.594 | −0.835 to −0.352 | <0.001 | −0.526 | −0.790 to −0.262 | <0.001 | |
| Parental ethnicity (ref: NHW/NHW) | ||||||||||
| NHW/Hispanic | 0.017 | −0.161 to 0.196 | 0.848 | 0.029 | −0.148 to 0.206 | 0.748 | 0.043 | −0.141 to 0.228 | 0.645 | |
| Hispanic/Hispanic | −0.197 | −0.386 to −0.008 | 0.041 | −0.193 | −0.384 to −0.002 | 0.048 | −0.162 | −0.362 to 0.037 | 0.111 | |
| Other | −0.096 | −0.283 to 0.090 | 0.312 | N/A | N/A | |||||
| Maternal age, yr | 0.020 | 0.007 to 0.034 | 0.003 | 0.016 | 0.001 to 0.031 | 0.034 | 0.016 | 0.001 to 0.032 | 0.037 | |
| Mother smoked at Year 6 (ref: no) | ||||||||||
| Yes | −0.188 | −0.341 to −0.034 | 0.017 | −0.205 | −0.369 to −0.041 | 0.014 | −0.210 | −0.381 to −0.038 | 0.017 | |
| Parental education (ref: both >12 yr) | ||||||||||
| Only mother >12 yr | −0.052 | −0.252 to 0.148 | 0.612 | −0.148 | −0.363 to 0.067 | 0.176 | −0.139 | −0.360 to 0.082 | 0.217 | |
| Only father >12 yr | −0.001 | −0.215 to 0.212 | 0.989 | −0.112 | −0.351 to 0.126 | 0.355 | −0.113 | −0.358 to 0.132 | 0.366 | |
| Neither >12 yr | −0.174 | −0.347 to −0.001 | 0.048 | −0.207 | −0.395 to −0.019 | 0.031 | −0.237 | −0.432 to −0.041 | 0.018 | |
| rs3741240 (additive model per A allele) | N/A | −0.381 | −0.470 to −0.292 | <0.001 | −0.383 | −0.474 to −0.291 | <0.001 | |||
| Height, cm | N/A | N/A | 0.007 | 0.0002 to 0.014 | 0.042 | |||||
| Cumulative smoking, pack-years | N/A | N/A | −0.030 | −0.052 to −0.007 | 0.009 | |||||
Definition of abbreviations: CI = confidence interval; Coef = coefficient; N/A = not applicable; NHW = non-Hispanic white; ref = reference.
Circulating CC16 levels were expressed as z-scores in the model. N values in Table 1 are smaller than N values in Figure 1 because the former includes CC16 levels between ages 6 and 32 years; the latter also includes CC16 levels at birth.
Models including rs3741240 were restricted to white participants to control for population stratification. No differences in minor allele frequencies and genotype distributions of rs3741240 were present across the ethnic groups NHW/NHW, NHW/Hispanic, and Hispanic/Hispanic. In addition, significant effects of rs3741240 on CC16 levels were found in all three ethnic groups.
Both Hispanic and non-Hispanic white participants.
Coefficients represent interaction between sex and year.
This is the first study to model the natural trajectory of serum CC16 from birth to 32 years, and to address systematically its determinants in early life. In our population-based cohort, serum CC16 levels increased from birth to childhood and into adulthood, and significant intrasubject tracking of serum CC16 was present across all ages. However, this tracking explained a minority of the variability in serum CC16. Thus, other factors related to environmental exposures, developmental processes, and/or lung diseases are likely to also play a role in controlling serum CC16 levels.
The effects of host and parental factors on CC16 appeared relatively consistent across all ages, with the notable exception of sex: females had higher serum CC16 levels than males in childhood, but the association reversed after adolescence. The reasons for these differences are unclear, although they may be related to different patterns of lung growth, the higher degree of dysanapsis in boys during childhood (10), and/or regulation of SCGB1A1 by sex hormones (2). In any case, it is noteworthy that this trend mirrors the inversion of the sex ratio in asthma prevalence that occurs in the transition from childhood (male > female) into adult life (male < female) (11).
In conclusion, in the largest prospective study on serum CC16 to date, we have observed significant intrasubject tracking from birth up to age 32 years and identified a set of genetic, host, and parental factors that are associated with CC16 trajectories. Although the biological functions and role of CC16 remain to be elucidated, the effects of these early determinants should be taken into account when evaluating CC16 in early risk stratification for lung diseases.
Footnotes
This study was funded by AI135108 from the National Institute of Allergy and Infectious Diseases and by HL132523 and HL095021 from the NHLBI.
Originally Published in Press as DOI: 10.1164/rccm.201712-2398LE on February 27, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–475. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev. 2007;28:707–725. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 3.Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12:445–467. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 4.Gamez AS, Gras D, Petit A, Knabe L, Molinari N, Vachier I, et al. Supplementing defect in club cell secretory protein attenuates airway inflammation in COPD. Chest. 2015;147:1467–1476. doi: 10.1378/chest.14-1174. [DOI] [PubMed] [Google Scholar]
- 5.Harrod KS, Mounday AD, Stripp BR, Whitsett JA. Clara cell secretory protein decreases lung inflammation after acute virus infection. Am J Physiol. 1998;275:L924–L930. doi: 10.1152/ajplung.1998.275.5.L924. [DOI] [PubMed] [Google Scholar]
- 6.Guerra S, Vasquez MM, Spangenberg A, Halonen M, Martin RJ. Club cell secretory protein in serum and bronchoalveolar lavage of patients with asthma. J Allergy Clin Immunol. 2016;138:932–934. doi: 10.1016/j.jaci.2016.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laucho-Contreras ME, Polverino F, Tesfaigzi Y, Pilon A, Celli BR, Owen CA. Club cell protein 16 (CC16) augmentation: a potential disease-modifying approach for chronic obstructive pulmonary disease (COPD) Expert Opin Ther Targets. 2016;20:869–883. doi: 10.1517/14728222.2016.1139084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guerra S, Halonen M, Vasquez MM, Spangenberg A, Stern DA, Morgan WJ, et al. Relation between circulating CC16 concentrations, lung function, and development of chronic obstructive pulmonary disease across the lifespan: a prospective study. Lancet Respir Med. 2015;3:613–620. doi: 10.1016/S2213-2600(15)00196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim DK, Cho MH, Hersh CP, Lomas DA, Miller BE, Kong X, et al. ECLIPSE, ICGN, and COPDGene Investigators. Genome-wide association analysis of blood biomarkers in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186:1238–1247. doi: 10.1164/rccm.201206-1013OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghavan D, Jain R. Increasing awareness of sex differences in airway diseases. Respirology. 2016;21:449–459. doi: 10.1111/resp.12702. [DOI] [PubMed] [Google Scholar]
- 11.Kynyk JA, Mastronarde JG, McCallister JW. Asthma, the sex difference. Curr Opin Pulm Med. 2011;17:6–11. doi: 10.1097/MCP.0b013e3283410038. [DOI] [PubMed] [Google Scholar]