To the Editor:
Reduced BMPR2 (bone morphogenetic protein receptor 2) signaling is central to the pathobiology of pulmonary arterial hypertension (PAH). However, the reduced penetrance of BMPR2 mutations in families suggests that other factors are required to establish disease (1). To date, it has proved difficult to elucidate these factors because of a lack of appropriate models. Sa and colleagues (2) developed an induced pluripotent stem cell (iPSC)-derived endothelial cell (iPSC-EC) model of PAH that recapitulated some of the previously described phenotypes of patient-derived pulmonary artery endothelial cells (PAECs), as well as appropriate responsiveness to Elafin and FK506 (2). This demonstrated a potential utility of iPSCs in modeling PAECs in PAH. However, other phenotypes such as inner mitochondrial membrane (IMM) hyperpolarization could not be recapitulated. Therefore, there is a need to better understand the contribution of BMPR2 mutations to PAH-associated phenotypes and the requirement for other factors in this process. Two advantages of iPSCs in disease modeling are their amenability to genome editing and their differentiation into specific cell types under serum-free, chemically defined conditions. This allows the assessment of the effect of a BMPR2 mutation without the confounding effects of genetic differences between cell lines, and the determination of the effect of controlled exposure to extrinsic factors that may influence the acquisition of a diseased state. In addition, no iPSC–smooth muscle cell (SMC) model of PAH has yet been described. We have addressed these issues.
Methods
Using clustered regularly interspaced short palindromic repeats–Cas9–mediated homologous recombination in a wild-type iPSC line, two isogenic sublines carrying either a known causal BMPR2 mutation (W9X; referred to as C2 W9X+/−) or a deletion of exon 1 (C2 ΔExon1) were generated. Serum-free, chemically defined iPSC differentiation protocols were used to generate iPSC-derived SMCs (iPSC-SMCs) and iPSC-ECs. This was achieved by differentiating iPSCs into iPSC-SMCs via a lateral plate mesoderm, paraxial mesoderm, or neural ectoderm lineage followed by 12 days in TGF-β1 (transforming growth factor β 1) and PDGF-BB (platelet-derived growth factor BB) ± BMP4 (bone morphogenetic protein 4) (Figure 1A) (3), and into ECs via FGF-2 (fibroblast growth factor 2)–induced, BMP4-induced, and LY294002-induced mesoderm followed by FGF-2 and VEGF (vascular endothelial growth factor) ± BMP4. iPSC-SMCs were compared with adult distal and proximal pulmonary artery smooth muscle cells (PASMCs) by microarray analysis. Cells were used postdifferentiation and in chemically defined conditions, and exposed to additional factors such as serum, BMP4, and TNFα (tumor necrosis factor α). Key PAH-associated cellular phenotypes, including altered apoptosis (via caspase cleavage and annexin/propidium iodide staining), proliferation (via DNA content and cell counts), and IMM polarization (via tetramethylrhodamine ethyl ester staining), which are cellular changes common to both SMCs and ECs (4), were assessed.
Figure 1.
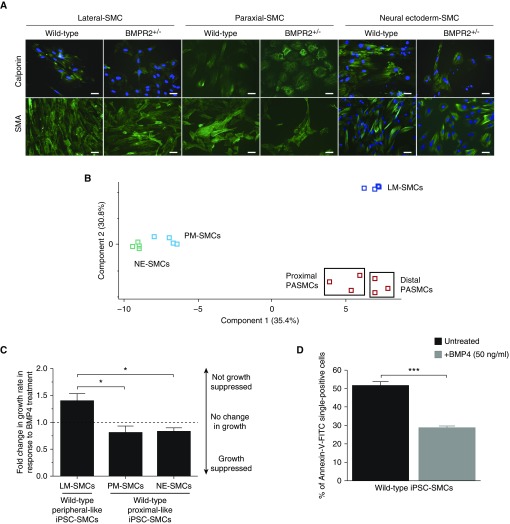
Generation of pulmonary artery smooth muscle cell (PASMC)-like induced pluripotent stem cell (iPSC)-derived smooth muscle cells (SMCs). (A) Wild-type and BMPR2+/− iPSCs differentiated into iPSC-SMCs from lateral plate mesoderm (LM), paraxial mesoderm (PM), and neuroectoderm (NE) express SMA (smooth muscle actin) and calponin (green), (DAPI; blue) (scale bars, 20 μm). (B) Gene expression patterns of all samples (human PASMCs, PM-SMCs, NE-SMCs, and LM-SMCs) were analyzed using Illumina HumanHT-12 v4 Expression BeadChip microarrays. Gene expression patterns of samples were sorted based on similarity by hierarchical clustering. For this analysis, 171 SMC-specific genes were selected based on the wikipathway database (WikiPathway WP2064 revision 47071). The two-dimensional principal component analysis for differential gene expression is plotted in B. The red squares inside black boxes represent PASMCs, dark blue squares represent LM-SMCs, green squares represent NE-SMCs, and light blue squares represent PM-SMCs. (C) LM-SMCs are not growth suppressed by BMP4 (50 ng/ml), unlike PM- and NE-SMCs. (D) The apoptotic response in wild-type LM-SMCs is reduced in the presence of exogenous BMP4 (50 ng/ml), as previously described for distal PASMCs. Data in C and D are presented as mean ± SEM of three biological replicates (*P < 0.05; ***P < 0.001; one-way ANOVA [C] and Student’s t test [D]). BMP = bone morphogenetic protein; BMPR = BMP receptor; FITC = fluorescein isothiocyanate.
Results and Discussion
The BMPR2 mutations introduced into C2-iPSCs resulted in BMPR2 haploinsufficiency in an otherwise isogenic background compared with the wild-type parent iPSC line. This approach removed the effects that different genetic modifiers (5) may have on the penetrance of cellular phenotypes.
Derivation of iPSC-SMCs and iPSC-ECs that perfectly represent adult PASMCs and PAECs is yet to be achieved. Therefore, our goal was to generate iPSC-SMCs that recapitulated some of the important functional responses of adult-derived distal PASMCs, as well as iPSC-ECs with enhanced expression of arterial markers, that could be used as surrogates for adult pulmonary vascular cells. The lineage-specific differentiation protocols used generated iPSC-SMCs expressing SMA (smooth muscle actin), CALPONIN, and MYH11 (myosin heavy chain 11) (Figure 1A and data not shown) that had a contractile phenotype (data not shown), as well as iPSC-ECs, which were enriched for arterial-specific EC markers including ACVRL1 (activin A receptor like type 1), CLDN5 (claudin 5), EFNB2 (ephrin B2), NOTCH1, JAG (Jagged)-1, and JAG2 and able to form vascular networks (data not shown) (6). Principle component analysis of microarray gene expression data for 171 SMC-associated genes showed that lateral plate mesoderm-SMCs were more similar to distal PASMCs compared with paraxial mesoderm-derived and neural ectoderm-derived SMCs (Figure 1B). In addition, lateral plate mesoderm-SMCs were not growth suppressed by BMP4 (50 ng/ml) and were less apoptotic when treated with BMP4, similar to the responses previously described in distal PASMCs from donors (Figures 1C and 1D) (7).
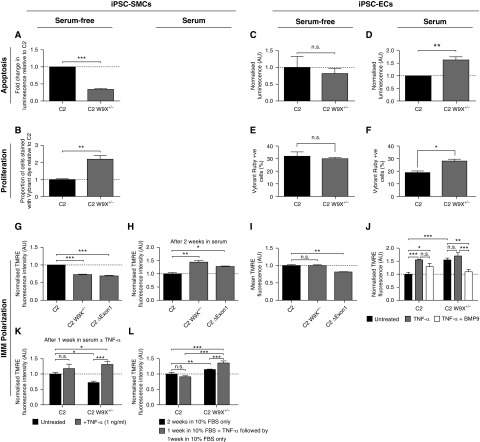
Under these serum-free, chemically defined conditions, BMPR2 heterozygosity alone was sufficient to cause reduced apoptosis and increased proliferation in iPSC-SMCs (Figures 2A and 2B). However, BMPR2 heterozygosity in iPSC-ECs required additional exposure to serum to manifest increased proliferation and apoptosis (Figures 2C–2F). These findings were confirmed by performing cell counts to assess proliferation, and annexin-V–fluorescein isothiocyanate propidium iodide staining to measure apoptosis (data not shown). Taken together, these results demonstrate a clear difference in the contribution of BMPR2 heterozygosity to establishing disease phenotypes in SMCs and ECs, and therefore highlight an important difference between these cell types. In contrast, neither cell type displayed hyperpolarized IMMs as they emerged from the serum-free iPSC differentiation protocols (Figures 2G and 2I). Only after serum ± TNFα exposure for 1 week for iPSC-ECs (Figure 2J), and serum + TNFα exposure for 1 week or serum-only for 2 weeks for iPSC-SMCs (Figures 2H, 2K, and 2L), did these cells acquire IMM hyperpolarization. IMM hyperpolarization is a key factor in pulmonary vascular remodeling, but how a hyperpolarized state is established in the context of BMPR2 heterozygosity was not known. Recently, BMP9 was shown to reverse PAH in rodent models, mainly via its action on PAECs (8). In iPSC-ECs, IMM hyperpolarization could be prevented by exposure to BMP9 (1 ng/ml; Figure 2J), potentially exposing one of the possible modes of action of BMP9 in reversing PAH. Remarkably, BMPR2+/− iPSC-SMCs demonstrated prolonged hyperpolarization despite withdrawal of TNFα (Figure 2L). This suggests that transient exposure to a disease-triggering agent may be sufficient to drive the progression of disease in a BMPR2 mutation carrier.
Figure 2.
Effect of BMPR2 (bone morphogenetic protein receptor 2) heterozygosity on proliferation, apoptosis, and inner mitochondrial membrane (IMM) polarization of induced pluripotent stem cell (iPSC)-derived smooth muscle cells (SMCs) and iPSC–endothelial cells (ECs). (A and B) C2 W9X+/− iPSC-SMCs are (A) significantly less apoptotic and (B) more proliferative than wild-type C2 iPSC-SMCs under serum-free conditions, assessed using the Caspase-3/7 Glo assay (A) and by measuring double-stranded DNA content by Vybrant DyeCycle Ruby staining (B). (C and E) In contrast, there was no significant difference in (C) apoptosis and (E) proliferation between C2 and C2 W9X+/− iPSC-ECs under serum-free conditions. (D and F) C2 W9X+/− iPSC-ECs became significantly more (D) apoptotic and (F) proliferative relative to isogenic wild-type C2 iPSC-ECs after exposure to 10% FBS for 1 week. (G) Serum-free BMPR2+/− iPSC-SMCs display a hypopolarized IMM compared with isogenic wild-type cells, assessed using tetramethylrhodamine ethyl ester (TMRE) staining and flow cytometry. (H and K) BMPR2+/− iPSC-SMCs became hyperpolarized compared with isogenic wild-type cells (H) after 2 weeks of exposure to serum or (K) after 1 week of exposure to serum and TNFα (tumor necrosis factor α) (1 ng/ml). (L) After 1 week of exposure to serum + TNFα, TNFα was removed and all cells were cultured for 1 further week in serum only to see whether the polarization state would recover. The polarization state of BMPR2+/− iPSC-SMCs did not normalize and was significantly higher than exposure to serum only for 2 weeks, and was also significantly higher than in isogenic wild types treated the same way. (I and J) BMPR2+/− iPSC-ECs (I) do not have a hyperpolarized IMM relative to the isogenic wild-type under serum-free conditions but (J) become significantly hyperpolarized after exposure to serum for 1 week. Wild-type iPSC-ECs showed significantly higher TMRE fluorescence after TNFα treatment (1 ng/ml), suggesting that TNFα increases IMM hyperpolarization. Cotreatment of C2 W9X+/− iPSC-ECs with TNFα (1 ng/ml) and BMP9 (1 ng/ml) for 1 week resulted in significantly reduced TMRE fluorescence, and hence IMM polarization, compared with the effect of TNFα alone. Data are presented as mean ± SEM of the results from three independent differentiations (A–F) and three technical replicates per differentiation (*P < 0.05; **P < 0.01; ***P < 0.001; Student’s t test [A–F], one-way ANOVA [G–I], or two-way ANOVA [J–L]). AU = arbitrary units; BMP9 = bone morphogenetic protein 9; C2 = control 2, patient cell line; C2 δExon 1 = C2 carrying deletion of exon 1 in the gene BMPR2; C2 W9X = C2 carrying a W9X mutation in the gene BMPR2; FBS = fetal bovine serum; n.s. = not significant.
The significance of these findings is that this iPSC system can be used to address the controversial question of whether genetic reduction of BMPR2 alone is necessary and/or sufficient for establishing the major cellular phenotypes associated with PAH. This would be extremely difficult to address in patient-derived primary cells. The use of specialized differentiation protocols with minimal interference from extrinsic factors allowed the effect of BMPR2 heterozygosity in SMCs and ECs to be shown definitively. Extrinsic factors were then added in a highly controlled manner to show their effect on establishing PAH-associated cellular phenotypes. In essence, it was possible to transition cells from a prediseased to a diseased state, opening the way to discovering new druggable pathways to prevent or reverse PAH. Although the generation of pulmonary SMCs and ECs from iPSCs is yet to be achieved, the differentiation protocols used in this study produced cells that recapitulated key phenotypes found in diseased adult PASMCs and PAECs. Thus, these protocols will have a broad effect for those modeling pulmonary vascular diseases, and also for those using pulmonary organoids and pulmonary artery–on-chip technologies to study epithelial–endothelial cell interactions in the alveoli and for drug screening. Finally, this study defines an iPSC-derived SMC model of PAH. Only EC and mesenchymal iPSC models of PAH have been described previously, and the mesenchymal model did not recapitulate the pro-proliferative phenotype of SMCs from patients with PAH (9).
Footnotes
Supported by funding from the British Heart Foundation (BHF; project grant PG/14/31/30786 and program grant RG/13/4/30107), the Cambridge National Institute for Health Research Biomedical Research Centre, the Dinosaur Trust, Fondation Leducq, the Medical Research Council (MRC Experimental Challenge Award MR/KO20919/1), Pulmonary Hypertension Association UK, and Fight for Sight and the Robert McAlpine Foundation. N.W.M. was supported by a BHF Chair Award (CH/09/001/25945) and F.N.K. was supported by a BHF PhD studentship (FS/13/51/30636) and a travel grant from St. Catharine’s College Cambridge. N.W.M. and A.A.R. would also like to acknowledge support from the BHF Centre of Regenerative Medicine, Oxford and Cambridge (RM/13/3/30159); the BHF Centre for Research Excellence (RE/13/6/30180); the BHF IPAH cohort grant (SP/12/12/29836); Selwyn and St. Catharine’s Colleges, Cambridge; and a Pfizer European Young Researcher of the Year award (A.A.R.).
Author Contributions: F.N.K. and C-H.C. designed and performed experiments, analyzed data, and wrote the manuscript; C.J.Z.H. designed and performed experiments and analyzed data; B.K., C.C., and B.J.D. performed experiments; F.S. and S.S. analyzed data; N.W.M. designed experiments and wrote the manuscript; A.A.R. designed and performed experiments, analyzed data, and wrote the manuscript; F.N.K., C-H.C., and C.J.Z.H. contributed equally to the work; N.W.M. and A.A.R. supervised the work; and all authors read the manuscript and approved the final version.
Originally Published in Press as DOI: 10.1164/rccm.201801-0049LE on March 16, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Soon E, Crosby A, Southwood M, Yang P, Tajsic T, Toshner M, et al. Bone morphogenetic protein receptor type II deficiency and increased inflammatory cytokine production: a gateway to pulmonary arterial hypertension. Am J Respir Crit Care Med. 2015;192:859–872. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sa S, Gu M, Chappell J, Shao NY, Ameen M, Elliott KA, et al. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med. 2017;195:930–941. doi: 10.1164/rccm.201606-1200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung C, Bernardo AS, Trotter MW, Pedersen RA, Sinha S. Generation of human vascular smooth muscle subtypes provides insight into embryological origin-dependent disease susceptibility. Nat Biotechnol. 2012;30:165–173. doi: 10.1038/nbt.2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pullamsetti SS, Savai R, Seeger W, Goncharova EA. Translational advances in the field of pulmonary hypertension: from cancer biology to new pulmonary arterial hypertension therapeutics: targeting cell growth and proliferation signaling hubs. Am J Respir Crit Care Med. 2017;195:425–437. doi: 10.1164/rccm.201606-1226PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, et al. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell. 2017;20:490–504. doi: 10.1016/j.stem.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcelo KL, Goldie LC, Hirschi KK. Regulation of endothelial cell differentiation and specification. Circ Res. 2013;112:1272–1287. doi: 10.1161/CIRCRESAHA.113.300506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, et al. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 8.Long L, Ormiston ML, Yang X, Southwood M, Gräf S, Machado RD, et al. Selective enhancement of endothelial BMPR-II with BMP9 reverses pulmonary arterial hypertension. Nat Med. 2015;21:777–785. doi: 10.1038/nm.3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.West JD, Austin ED, Gaskill C, Marriott S, Baskir R, Bilousova G, et al. Identification of a common Wnt-associated genetic signature across multiple cell types in pulmonary arterial hypertension. Am J Physiol Cell Physiol. 2014;307:C415–C430. doi: 10.1152/ajpcell.00057.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]