To the Editor:
The use of thoracic computed tomographic (CT) imaging in current and former smokers is becoming increasingly more common because of its widespread availability and utility in lung cancer screening. Prior studies have demonstrated associations between CT measures of emphysema and respiratory symptoms, exacerbations, and mortality (1–5). However, thus far, practical guidance on how to incorporate increasingly available quantitative imaging data into clinical practice is lacking. In this analysis, we use data from two large NIH-sponsored cohort studies, SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study) and COPDGene (COPD Genetic Epidemiology), to examine the relationship between a readily available metric, CT-based emphysema, and risk for important patient outcomes including symptoms, exacerbations, and mortality. The goal of our analysis is not to advocate for routine CT imaging in chronic obstructive pulmonary disease (COPD) but, rather, to provide clinicians with guidance on how to translate increasingly available quantitative imaging data into information that can guide patient decision-making.
We used data from COPDGene and SPIROMICS, both NIH-funded multicenter cohort studies of smokers with and without airflow obstruction. All subjects were age 40–80 years with 10 pack-years or more of exposure (6, 7). Exacerbations were defined by use of an antibiotic and/or steroid, emergency room visit, or hospital admission for a respiratory “flare-up” on the American Thoracic Society Respiratory Disease questionnaire. Exacerbation data for both cohorts were captured via longitudinal follow-up protocols. For both studies, all participants underwent spirometry before and after the administration of a short-acting bronchodilator; postbronchodilator values were used in this analysis. We analyzed lung parenchyma on volumetric CT scans of the chest obtained at full inspiration, using VIDA software (VIDA Diagnostics), with percentage emphysema defined by the percentage of voxels less than −950 Hounsfield units (7, 8).
Statistical analyses were performed in R version 3.1.3 (www.r-project.org). Analyses were performed first in the COPDGene cohort, with validation conducted using the SPIROMICS cohort. Plots for average event rates were created using raw averages for event rates grouped by nearest emphysema percentile. Overlaid are age-adjusted event rates by emphysema percentile, estimated using linear splines. Visual inspection of plots suggested 5% emphysema as a significant threshold for several clinically important metrics. The threshold of 5% is also roughly the 90th percentile value of emphysema for normal individuals within the COPDGene cohort (9). P value for average follow-up was based on restricted mean test. The statistical analysis of event rates (exacerbation rates and death) were conducted using quasi-Poisson regression models unadjusted and adjusted for age.
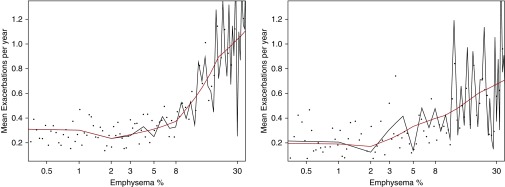
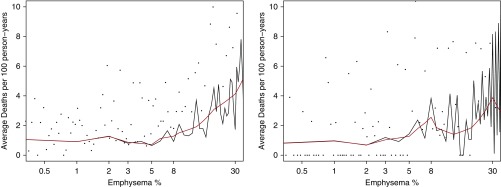
We created plots of emphysema versus relevant outcomes in COPD. In Figure 1, we examine mean prospective moderate and severe exacerbations in linear splines (black) and smoothed splines (red). A distinct increase in exacerbation frequency appears to occur roughly at 5% of emphysema. We saw similar findings for mean severe exacerbations (data not shown). In Figure 2, we compare emphysema percentage against average death rates in COPDGene and SPIROMICS. Again, a linear, positive association between increasing emphysema and deaths also appears at roughly 5% emphysema. Similar associations were seen with increases in St. George’s Respiratory Questionnaire beginning at approximately 5% emphysema (data not shown).
Figure 1.
Plot of mean exacerbations per year by percentage emphysema in COPDGene (COPD Genetic Epidemiology; left) and SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study; right). Black dots represent raw averages for exacerbation rates grouped by nearest emphysema percentile. Age-adjusted estimates for average exacerbations per year shown via linear splines in black (using every percentile of emphysema) and smoothed splines in red. Mean follow-up time was 4.06 years.
Figure 2.
Plot of average deaths per 100 person-years by percentage emphysema in COPDGene (COPD Genetic Epidemiology; left) and SPIROMICS (Subpopulations and Intermediate Outcomes in COPD Study; right). Black dots represent raw averages for death rates grouped by nearest emphysema percentile. Age-adjusted estimates for average deaths per 100 person-years are shown via linear splines in black (using every percentile of emphysema) and smoothed splines in red. The y-axis of each graph is truncated at 10 average deaths per 100 person-years to allow better visual comparison of both cohorts.
To confirm the visual findings, we split the cohort into individuals above and below a 5% emphysema threshold. In COPDGene, 5,366 subjects had less than 5% emphysema (72% with FEV1/FVC ≥ 0.7 and 28% with FEV1/FVC ≥ 0.7) versus 2,601 with 5% or more emphysema (17% with FEV1/FVC ≥ 0.7 and 83% with FEV1/FVC ≥ 0.7). A similar breakdown was seen in SPIROMICS. The 5% or more emphysema threshold was significantly associated with greater risk and St. George’s Respiratory Questionnaire score (35.1 vs. 22.4; P < 0.001) in COPDGene and SPIROMICS (39.6 vs. 27.1; P < 0.001). We also found significantly higher mean exacerbation frequency in those with 5% or more emphysema (0.69 vs. 0.29 exacerbations/yr; P = 0.03) in COPDGene and SPIROMICS (0.46 vs. 0.21 exacerbations/yr; P < 0.001). The 5% or more emphysema threshold was also associated with greater mortality (3.18 vs. 0.98 deaths per 100 person-years; P < 0.001) compared with those with emphysema <5% in COPDGene, as well as SPIROMICS (2.66 vs. 0.89 deaths per 100 person-years; P = 0.01). Additional analyses, however, stratified by presence of airflow obstruction, demonstrated that these relationships were maintained in those with airflow obstruction, but not in those without.
We also noted that in COPDGene, of the individuals with 5% or more emphysema and FEV1/FVC < 0.70, 26% had no prior diagnosis of COPD. In SPIROMICS, of 994 individuals with 5% or more emphysema and FEV1/FVC < 0.70, 15% similarly had no prior diagnosis of COPD. Furthermore, among these participants meeting criteria for FEV1/FVC < 0.70 and 5% or more emphysema, 33.9% (COPDGene) and 33.0% (SPIROMICS) were receiving no inhaled maintenance therapy for COPD (long-acting β agonist, long-acting β agonist/inhaled corticosteroid combination, or long-acting muscarinic antagonist).
Conversely, it is interesting to note that we did not see greater symptoms or exacerbations in those with emphysema less than 5%. It may be that in such subjects, the CT features capturing symptoms are actually more related to airways disease, which has been suggested in prior analyses (10).
These results have significant advantages over previously published algorithms using CT data and clinical variables to determine their predictive value in identifying COPD. One is that our proposed approach uses percentage emphysema, which can be obtained via software readily available on the majority of commercial CT scanners. We have also intentionally chosen to use a simplified approach, reporting a single threshold that can be used by clinicians as a guidepost to point toward the need for additional testing and/or treatment. Importantly, we found that the strongest signal for all-cause mortality occurs in those with airflow obstruction.
The clinical implication is that a combination of CT and spirometric criteria identify individuals at highest risk for symptoms, future risk for exacerbations, and death. These data suggest that for individuals with known COPD, a CT with 5% or more emphysema should signal the clinician that this individual is at greater risk for poor outcomes, and directed therapy would be indicated if it has not already been initiated. For individuals without a prior COPD diagnosis, the presence of 5% or more emphysema suggests spirometry should be considered, as presence of airflow obstruction identifies a subgroup of patients with COPD at higher risk for poor outcomes.
Footnotes
COPDGene is supported by NHLBI grants R01 HL089897 and R01 HL089856. The COPDGene project is also supported by the COPD Foundation through contributions made to an industry advisory board consisting of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pfizer, Siemens, and Sunovion. SPIROMICS was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici SpA; Forest Research Institute, Inc.; GSK; Grifols Therapeutics, Inc.; Ikaria, Inc.; Nycomed GmbH; Takeda Pharmaceutical Co.; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc.; and Sanofi. M.K.H., F.J.M., B.D.R., C.J.G., and G.C. are supported by NHLBI grant R01 HL122438. M.K.H. is also supported by NHLBI grant K24 HL138118.
Author Contributions: M.K.H., N.T., S.M., P.G.W., and F.J.M. contributed to conceptualization of the study; M.K.H., V.K., J.L.C., G.C., and F.J.M. were involved in data collection; M.K.H., N.T., S.M., and F.J.M. contributed to data analysis; and all authors participated in manuscript writing and editing.
Originally Published in Press as DOI: 10.1164/rccm.201801-0051LE on February 27, 2018
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: Fernando J. Martinez, M.D., M.S.1,8; For the COPDGene and SPIROMICS Investigators
References
- 1.Martinez CH, Chen YH, Westgate PM, Liu LX, Murray S, Curtis JL, et al. COPDGene Investigators. Relationship between quantitative CT metrics and health status and BODE in chronic obstructive pulmonary disease. Thorax. 2012;67:399–406. doi: 10.1136/thoraxjnl-2011-201185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grydeland TB, Dirksen A, Coxson HO, Eagan TM, Thorsen E, Pillai SG, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181:353–359. doi: 10.1164/rccm.200907-1008OC. [DOI] [PubMed] [Google Scholar]
- 3.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zulueta JJ, Wisnivesky JP, Henschke CI, Yip R, Farooqi AO, McCauley DI, et al. Emphysema scores predict death from COPD and lung cancer. Chest. 2012;141:1216–1223. doi: 10.1378/chest.11-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MK, Kazerooni EA, Lynch DA, Liu LX, Murray S, Curtis JL, et al. COPDGene Investigators. Chronic obstructive pulmonary disease exacerbations in the COPDGene study: associated radiologic phenotypes. Radiology. 2011;261:274–282. doi: 10.1148/radiol.11110173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zach JA, Newell JD, Jr, Schroeder J, Murphy JR, Curran-Everett D, Hoffman EA, et al. COPDGene Investigators. Quantitative computed tomography of the lungs and airways in healthy nonsmoking adults. Invest Radiol. 2012;47:596–602. doi: 10.1097/RLI.0b013e318262292e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]