Abstract
Pneumonia is a complex pulmonary disease in need of new clinical approaches. Although triggered by a pathogen, pneumonia often results from dysregulations of host defense that likely precede infection. The coordinated activities of immune resistance and tissue resilience then dictate whether and how pneumonia progresses or resolves. Inadequate or inappropriate host responses lead to more severe outcomes such as acute respiratory distress syndrome and to organ dysfunction beyond the lungs and over extended time frames after pathogen clearance, some of which increase the risk for subsequent pneumonia. Improved understanding of such host responses will guide the development of novel approaches for preventing and curing pneumonia and for mitigating the subsequent pulmonary and extrapulmonary complications of pneumonia. The NHLBI assembled a working group of extramural investigators to prioritize avenues of host-directed pneumonia research that should yield novel approaches for interrupting the cycle of unhealthy decline caused by pneumonia. This report summarizes the working group’s specific recommendations in the areas of pneumonia susceptibility, host response, and consequences. Overarching goals include the development of more host-focused clinical approaches for preventing and treating pneumonia, the generation of predictive tools (for pneumonia occurrence, severity, and outcome), and the elucidation of mechanisms mediating immune resistance and tissue resilience in the lung. Specific areas of research are highlighted as especially promising for making advances against pneumonia.
Keywords: pneumonia, bacterial infection, viral infection, host response, host susceptibility
Pneumonia is a persistent, pervasive, and difficult pulmonary disease. Lower respiratory tract infection is the most common infectious cause of death in the world, responsible for almost 2.7 million deaths yearly and 103 million disability-adjusted life-years lost in 2015 (1). The burden of disease consistently ranks first or second largest worldwide, with economic costs in the United States alone amounting to tens of billions of dollars per year. Trends such as increasing antibiotic resistance, emerging pathogens, demographic shifts toward older populations, and changes in medical care resulting in more subjects living in conditions that increase pneumonia risk suggest that the pneumonia problem is poised to worsen rather than improve. Guidelines for community-acquired, hospital-acquired, and ventilator-associated pneumonias have been developed and are being updated to aid in the clinical management of pneumonia. However, even accurate diagnosis can be a challenge in adult and especially pediatric populations. Current clinical practice is insufficient to curtail the acute mortality and long-term consequences of this syndrome. Pneumonia prevention and treatment strategies need to be complemented with novel approaches that incorporate new and emerging knowledge.
The microbes most often triggering pneumonia are ubiquitous and unavoidable pathobionts rather than highly virulent pathogens, growing in the lung to cause pneumonia only under exceptional circumstances. Whether pneumonia ensues depends largely on the quality and quantity of host response activities that defend the lung against pneumonia. Failures of these host defense pathways lead to pneumonia and to poor pneumonia outcomes, including acute respiratory distress syndrome and sepsis (with pneumonia being the leading cause of each syndrome). Pneumonia also leads to pulmonary function decline and the onset or exacerbation of cardiovascular disease (CVD; including heart attacks, strokes, heart failure, and atrial fibrillation), cognitive decline, depression, physical limitation, and shortened lifespan. Although a host–pathogen interaction underlies this disease, key determinants of the susceptibility to, progression of, and outcomes of pneumonia are driven by characteristics of the host. Much focus has been placed on the pathogens causing pneumonia, but a tremendous need for research from the perspective of the host remains.
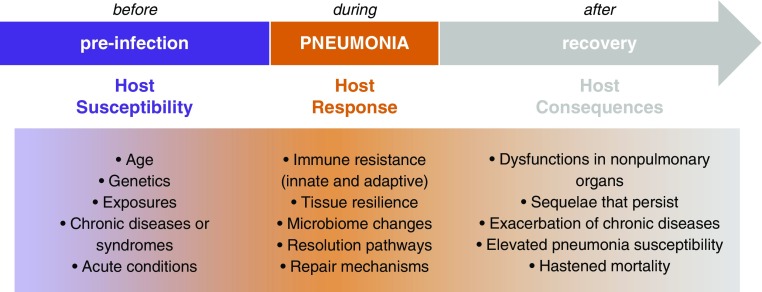
In August 2017, the NHLBI assembled a Working Group for Advancing Pneumonia Research in Heart, Lung, and Blood Diseases, comprised of a multidisciplinary group of experts and thought leaders in pneumonia biology and medicine, including basic, translational, and clinical investigators. The working group was charged with brainstorming about future research opportunities in pneumonia-related heart, lung, and blood research to help inform next steps for the NHLBI Pneumonia Program. A specific mandate was to identify knowledge gaps that, if addressed, will lead to improved understanding of the host factors influencing pneumonia incidence and severity; helping to guide new strategies for lowering pneumonia risk; for preventing or curing acute lower respiratory infections; and for improving heart, lung, and blood health. The working group meeting was divided into three sessions (Figure 1) addressing topics of pneumonia host susceptibility, host responses, and host consequences, followed by group discussions of the common themes and overarching priorities. This summary includes topical recommendations, as well as overarching themes that emerged as having the greatest potential for contributing to progress against pneumonia across all three topics.
Figure 1.
Different biological processes especially relevant to distinct stages of pneumonia biology present unique opportunities for discovery and for intervention. The working group was structured to present the current state of the field in each of these three areas; to identify the knowledge gaps, emerging opportunities, and the most pressing needs in each; and to determine whether and which research priorities span all three divisions. Results are presented in the text and tables.
Host Susceptibility to Pneumonia
Pneumonia associates with many host characteristics, including age and chronic conditions such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, atherosclerosis, heart failure, cancer, arthritis, Parkinson’s disease, depression, and others. Leading risk factors for chronic heart, lung, and other diseases are independent risk factors for pneumonia, including cigarette smoking, obesity, alcohol abuse, and air pollution (2). However, the biological pathways linking pneumonia susceptibility and risk factors such as aging, comorbidities, and exposures are only beginning to be delineated (Figure 1).
Patients at the extremes of age—the pediatric and geriatric populations—have increased susceptibility to pneumonia and increased mortality, with myriad biological changes potentially contributing to an unclear degree (3–8). Cigarette smoke, a major cause of COPD, has significant pathological effects on the lung that impact the host response to pneumonia (9, 10). Ambient air pollution is a major risk factor for pulmonary mortality, and respiratory infections are exacerbated because of exposure to environmental air pollutants, with the greatest effects being due to particulate matter, ozone, and nitrogen oxides (11, 12). The increased risk of pneumonia for patients with chronic lung diseases such as COPD and asthma may result from combinations of anatomic changes, altered immune functions, and inhaled corticosteroids (13–19). Whether susceptibility determinants that underlie infectious exacerbations of these chronic lung diseases overlap those for pneumonia is not known (20). With over one-third of the U.S. adult population being obese (15, 21), substantial need exists to understand the impaired response to respiratory infections in obesity, diabetes, and the metabolic syndrome (22–24). Body mass index is an inadequate surrogate, and studies are needed to determine how analytes such as serum leptin, insulin, hemoglobin A1c, or lipid species associate with pneumonia risk (21). Patients with cancer develop a spectrum of infections that depend on the patient’s specific underlying immunologic defects, local barrier defense defects, and malignancy type (25, 26). Lymphopenia, myeloablative and B- or T-cell–depleting chemotherapies, graft-versus-host disease, and other factors increase the risk of developing life-threatening pneumonia. In patients with lung cancer, localized airway obstruction by tumor and radiation therapy causing mucosal changes can predispose individuals to pneumonia. For patients with cancer, such structural and immunologic abnormalities make the diagnosis of pneumonia challenging and impact the composition and duration of therapy.
Many of the above-mentioned identifiable host susceptibility factors are important in the understanding and management of patients with pneumonia. Research is needed to move beyond generalizations about which broad populations are generally prone to pneumonia in order to develop patient measurements that report an individual’s risk of pneumonia. Although many complex diseases have clear links to gene variants, only a few genetic susceptibility loci are identified for pneumonia (27–29), and these need further genetic, epidemiologic, and functional investigation. Besides genomics, other approaches and platforms, including transcriptomics, epigenetics, proteomics, metabolomics, and microbiomics/metagenomics, should be explored to identify markers and mechanistic causes of pneumonia susceptibility. The diversity and complexity of the patient population require reenvisioning how patients are endotyped for risk profiling. Identifying specific biological biomarkers that indicate levels of pneumonia susceptibility will be enabled by “omics”-based technologies as well as currently available large clinical datasets. Although the relatively low annual incidence of pneumonia in at-risk populations and the ambiguities related to pneumonia diagnosis complicate these endeavors, efforts should be made to link genetic and biologic data to pneumonia rates in ongoing large population studies, as well as to consider creative new experimental designs for revealing biological signs of pneumonia susceptibility. These approaches will need to incorporate current technologies that help better identify and quantitate the microbial pathogen(s) to best understand these host susceptibility factors in the appropriate and clinically relevant context. As etiology becomes better understood, select defects in host defense may emerge that result in susceptibilities to defined subsets of pneumonia pathogens.
To better define the critical determinants of host susceptibility that will help identify novel preventive strategies and support timely initiation and augmentation of therapy in susceptible populations, the working group recommends the following as priority research areas (Table 1):
-
1.
Use biological “omics” profiles (e.g., transcriptomic, metabolomic, proteomic, glycomic, metagenomic, and other unbiased platforms) and clinical information (such as datasets derived from electronic health records) to develop and test a “risk-ome” that predicts susceptibility versus resistance in de novo and recurrent pneumonia, including the important cohort of patients with chronic lung disease.
-
2.
Broaden consideration for and testing of human specimens collected to examine potential biomarkers for host susceptibility to community-acquired or nosocomial pneumonia.
-
3.
Define connections between select elements of host susceptibility and changes in the microbial pathogens (viral, bacterial, or fungal) causing pneumonia in adults or children, including delineation of pneumonia susceptibilities specific to limited types of pathogen.
Table 1.
Recommendations Related to Stages of before, during, or after Pneumonia
| Defining susceptibility to pneumonia (before) | Develop “risk-ome” from biological profiles and/or clinical data |
| Incorporate susceptibilities into diagnoses and severity assessments | |
| Connect susceptibilities to types of microbes | |
| Elucidating host responses (during) | Define lung cell roles in defense |
| Elucidate transition from inflammation to resolution/repair stages | |
| Compare and contrast host responses across pathogens | |
| Derive endotypes from biological and clinical information | |
| Rapidly identify those failing current clinical approach | |
| Understanding consequences (after) | Mechanistically link lung infection to extrapulmonary pathophysiologies |
| Find markers that predict specific types of decline after clinical recovery |
Host Response to Pneumonia
Defense against pneumonia is initiated by and dependent on cells of the lung (Figure 1), which work together to accomplish immune resistance (attacking microbes to reduce pathogen burden) and tissue resilience (protecting the organ’s structure and function against the onslaughts of infection and inflammation) (30–33). The first cells encountered by a microbe in the lung are epithelial cells, macrophages, and dendritic cells, making their responses especially key to rapidly amplifying and modulating effective immune resistance (34–38). Lymphocytes in the lung direct, skew, and modulate these pulmonary defenses, including resident memory lymphocytes (CD4+ T cells, CD8+ T cells, B cells, and distinct subsets thereof), innate lymphoid cells, mucosal-associated invariant T cells, and others making contributions that are beginning to be defined (8, 30). These cells and pathways are in turn influenced by and influence the lung microbiome (39, 40), which may further direct pneumonia defense activities in ways that need to be understood better. Improved understanding of the fundamental mechanisms of immune resistance in the lung will provide new ways to differentiate subjects with increased pneumonia susceptibility and to develop novel therapeutic approaches tailored to these lung defense pathways. Tissue resilience and the resolution of pulmonary inflammation involve cytoprotective pathways within lung cells (8), as well as autocrine and paracrine signaling mediated by proresolving eicosanoids (33, 34). The inflection point between the positive feedback loops amplifying inflammation and the ensuing resolution stages is yet to be defined, and factors differentiating whether resolution is fully effective or instead only partially effective or even deleterious need to be determined.
Emerging areas with special potential for large impact in pneumonia host response include 1) determining how host responses that defend against pneumonia are altered and improved by prior experiences with respiratory infections and 2) using computational biomedical approaches to more meaningfully reveal ongoing host responses in patients with pneumonia. Respiratory infections are ubiquitous and inevitable, and the pulmonary defenses of all but the very youngest of children are influenced by their respiratory infection history. Although lung defenses clearly change dramatically after the resolution of prior infections (8, 41–43), the mechanisms protecting these more experienced lungs are still poorly understood. The lower pneumonia rates in young, healthy adults likely result from naturally acquired heterotypic immune memory (8, 42) and trained innate immunity (44) generated by prior infections, but the mechanisms remain poorly understood. Different cellular components of the lung (particularly resident memory lymphocytes and innate lymphocytes), as well as reprogrammed responses from macrophages and epithelial cells (due to metabolic and epigenetic alterations), are likely responsible for the improved lung defense, but exactly which changes occur and contribute are largely unclear. Dysregulation of these lung-protective mechanisms is inferred to be responsible for the microbial growth and physiological dysfunction that occurs in pneumonia, but which pathways are disrupted in which cases remain speculative. Computational approaches to pneumonia patient samples reveal heterogeneity in host responses during lung infection (45–48), and such heterogeneity can be leveraged to provide insights into alterations in the relevant immunity and physiology pathways. Establishing molecular profiles that report variations in patients’ host responses during pneumonia holds promise for guiding research into mechanisms of lung protection. Such profiles also have great potential for endotyping patients with pneumonia, which will improve diagnosis, prognostication, and clinical approaches.
To incorporate the knowledge of host responses during pneumonia as a means to elucidate pathophysiology and improve patient care, the working group identified several goals (Table 1):
-
1.
Better define the roles of specific lung cells (including but not limited to epithelial cells, macrophages, innate lymphoid cells, and resident memory lymphocytes) in host defense, with a particular emphasis on how lung defense is reprogramed (e.g., altered responses from the cells) and remodeled (e.g., different cellular constituents of the lung) owing to the repeated microbial and nonmicrobial exposures experienced by the lung
-
2.
Identify the critical determinants of the transition from the initial phases of amplifying acute responses to the later phases of resolution and lung-reparative processes in the infected lung, and establish biological and clinical parameters that distinguish between appropriate versus dysregulated tissue response and repair
-
3.
Delineate elements of host responses that are conserved across diverse pneumonias or distinct to pneumonias caused by different types of respiratory pathogens
-
4.
Differentiate pneumonia endotypes on the basis of biological and clinical information, and determine their potential utility for guiding medical approaches (to improve abilities to stage disease, to gauge effects of clinical activities, to distinguish pathophysiologies, etc.)
-
5.
Develop methods for using measures of the host response to predict pneumonia outcomes in adult and pediatric patients, aiming for early detection of clinical failure
Systemic and Long-Term Consequences of Pneumonia
Two erroneous paradigms have limited understanding of the role of pneumonia on health and have compromised the research agenda on pneumonia. The first is that pneumonia is a localized disease. The systemic response defined as sepsis has long been recognized and pneumonia remains the most common site of infection leading to sepsis and septic shock in the general population. Metastatic infection, such as to pleura, bone, joints, brain, heart valves, and the myocardium (49), explains some but not all systemic manifestations. Pneumonia causes systemic muscle weakness, and interactions between acute respiratory infection and age on skeletal muscle stress and repair demand attention (50, 51). During the acute episode, pneumonia affects multiple organ systems (8), often causing delirium, acute kidney injury, liver injury, atrial fibrillation, and more. Mechanisms underlying these extrapulmonary pathophysiologies during pneumonia currently are poorly understood (Figure 1).
The second erroneous concept is that pneumonia is an acute disease. In fact, some of the clinical manifestations during the acute episode may persist (e.g., persistent delirium, skeletal muscle weakness, or acute kidney injury leading to chronic kidney disease), or preexisting chronic diseases may be exacerbated, after hospital discharge (8). Thus, pneumonia accelerates either subclinical or overt chronic diseases and is a leading cause of readmissions (52). The role of pneumonia as a risk factor for chronic diseases is increasingly recognized. For example, the magnitude of risk for CVD, including stroke and myocardial infarction, associated with pneumonia is similar to or higher than the risk of CVD associated with traditional risk factors such as cigarette smoking, diabetes, and hypertension (53). Although amplified in those with chronic diseases, the effect of an episode of pneumonia on younger and previously healthy patients is also significant (54). Mechanisms have yet to be definitively demonstrated for any of the myriad long-term consequences of pneumonia (8). Biomarkers including cytokines associated with pathogenesis are often not normal at the time of discharge or when clinical “cure” has been achieved (55–57). This persistent altered immune state may represent dysregulated resolution and lead to more chronic manifestations such as an abnormal pro- or antiinflammatory state, altered coagulation, or enhanced proteolysis, which may accelerate preexisting chronic diseases or trigger a new chronic impairment. Pneumonia appears to activate the proteasome–ubiquitin pathway and cause muscle fiber degradation that may last beyond the pulmonary syndrome, particularly in elderly patients (50, 51). Alterations in the normal lung microbiome after infection or as a result of treatment may be causative or an amplifier of this dysregulated resolution (58), similar to that documented for the gut microbiome and for respiratory tract infections in chronic respiratory conditions such as asthma, cystic fibrosis, idiopathic pulmonary fibrosis, and bronchiectasis (59–61). How biological changes during pneumonia connect to longer-term consequences of pneumonia is an important question that is only beginning to be addressed (Figure 1). Determining the functional significance and clinical utility of such connections (e.g., as biomarkers and druggable targets) may yield novel approaches to preventing decline after pneumonia.
Finally, a bidirectional relationship between pneumonia and underlying diseases appears to be operative. Interactions with CVD (53, 62), chronic lung disease (63), neurocognitive function (52), and skeletal muscle (50) may lead to an iterative feedforward interaction between pneumonia and chronic diseases that accelerates physiologic decline, drives the process of unhealthy aging, and hastens mortality through these chronic complications (8).
To understand the mechanisms linking pneumonia to extrapulmonary disease and determine its impact on short- and long-term outcomes, the working group defined these goals (Table 1):
-
1.
Elucidate how the lung immune response to pneumonia relates to the systemic inflammatory response and manifests in cardiac, neurological, muscular, psychological and other diseases
-
2.
Identify markers of pneumonia severity and host response that predict long-term mortality and acceleration of associated chronic diseases
Overarching Themes to Direct Future Pneumonia Research
The sections above reflect topics and recommendations particular to each discrete section of the workshop. In addition, themes emerged that spanned all sessions and became prominent topics in the general discussion session that concluded the workshop. These are summarized below.
Host-focused Clinical Approaches to Pneumonia
The optimal treatment for pneumonia should involve identifying the host process(es) dysregulated in the individual patient and providing host-directed therapies that counter the dysregulated process(es). This requires better endotyping of patients with pneumonia combined with better host-directed therapies (Table 2).
Table 2.
Overarching Priority Areas in Pneumonia Research
| Connect endotyping with host-directed therapies | Use clinical and biological data to establish and better delineate endotypes of pneumonia |
| Develop metrics for assigning endotypes to individual patients | |
| Test abilities of already available host-directed therapies to improve pneumonia treatment, guided by endotype | |
| Develop and test novel endotype-directed therapies for pneumonia, based on advances in mechanistic understanding | |
| Generate predictive tools for pneumonia | For those with risk factors, develop measurements that discriminate who will most likely get pneumonia |
| For those with pneumonia, develop measurements that discriminate who will most likely experience clinical failure | |
| For those recovering, develop measurements that discriminate who will most likely have recurrence, cardiovascular events, pulmonary decrements, cognitive decline, or other sequelae | |
| Elucidate defense mechanisms in the protected lung | Refine, improve, or develop alternatives for approaches to better studying the protected lung |
| Determine how lung defense is affected by earlier life experiences, including antecedent respiratory infections | |
| Define defense roles of cells within the lung interstitium |
The current lack of effective host-focused endotyping for pneumonia is a major shortcoming. Current pneumonia typing strategies differentiate patients by the microbes present (e.g., influenza, pneumococcus, or Acinetobacter), by the setting in which pneumonia developed (e.g., community-acquired pneumonia, healthcare-associated pneumonia, or ventilator-associated pneumonia) (64), or by pneumonia severity using any of many available clinical scoring systems (65). These have utility for guiding antimicrobial use, but they do not report on or guide responses to the host biology driving pneumonia pathogenesis. Immunoparalysis has been inferred as one possible endotype of pneumonia, based on blood transcriptome differences interpreted to reflect decreases in antigen presentation, TLR (Toll-like receptor) signaling, or B-cell development, or increases in T-cell exhaustion or endotoxin tolerance (66); its clinical utility is intriguing but speculative. Severe pneumonia with a strong inflammatory response has been proposed as an endotype useful for guiding therapy, defined in one clinical trial by a serum C-reactive protein threshold (67). Additional host endotypes yet to be defined could have important clinical implications for pneumonia. A few possible examples of endotypes that may emerge include dysregulations of innate immune pathways (such as excess or inadequate inflammasome activity), dysfunctions of select cell types (such as airway epithelial antimicrobial activities), or alterations of processes beyond inflammation and immunity (such as repair and regeneration pathways for lung tissue). However, endotyping pneumonia is currently in a nascent stage and needs to be greatly expanded to become more precise, more sophisticated, and more clinically useful. All three sessions of the working group stressed a need for greater abilities to subdivide at-risk subjects, patients with pneumonia, and pneumonia survivors into meaningful endotypes that report underlying physiologic processes responsible for pneumonia susceptibility and/or outcomes at the individual-patient level.
Host-directed therapeutic approaches against pneumonia appear promising but are today largely unrealized. Therapies in clinical practice may prove useful, but when and how they should be used for pneumonia are uncertain. These include antiinflammatory agents (such as corticosteroids, nonsteroidal antiinflammatory drugs, macrolides, inhibitors of proinflammatory cytokines such as tumor necrosis factor or IL-1, and more), immunostimulatory agents (such as checkpoint inhibitors, recombinant proinflammatory cytokines such as IFN-γ, and more), proresolving agents (such as aspirin or polyunsaturated fatty acid–derived specialized proresolving mediators), and others with pneumonia-relevant physiologic effects (such as statins, glitazones, angiotensin-converting enzyme inhibitors, and more). Effectively testing such host-focused agents will require abilities to endotype patients with pneumonia for host processes contributing to pathophysiology. In addition, novel host-directed therapies are needed. Preclinical examples discussed in the workshop included improvements in immune resistance against pneumonia resulting from epithelial stimulation by ligands for TLR2 and TLR9 (68), improvements in tissue resilience against pneumonia in the elderly by transfer of alveolar macrophages (7), and improvements in both immune resistance and tissue resilience during pneumonia resulting from systemic administration of aspirin-triggered resolvin D1 (34). Whether these examples will eventually prove to enhance clinical pneumonia outcomes is yet to be determined, but they illustrate that diverse new types of approaches to enhancing the host response should be considered and tested. Improved understanding of mechanisms of lung defense against pneumonia, and how defenses change in those at risk for or recovering from pneumonia, will yield new host-focused strategies for pharmacologically bolstering immune resistance and tissue resilience.
Predictive Tools for Pneumonia Susceptibility and Outcome
A theme emerging across all three sessions was the need for methods that discriminate those patients likely to decline over time, and in which specific ways, compared with others with a more favorable course (Table 2). This applies to individuals who are at risk for, have, or are recovering from pneumonia. Although temporal perspective was central to the organization of the workshop, all three sessions stressed a frustratingly poor ability to predict the future course for any individual subject, including which of those with risk factors (e.g., COPD, Parkinson’s disease, and diabetes) will later develop pneumonia, which of those with pneumonia will later demand more urgent attention (e.g., critical care or mechanical ventilation) and when, and which of those who recover from pneumonia will later experience adverse outcomes (e.g., infarctions, fibrosis, or depression). Attempts have been made to predict clinical failure during pneumonia, but none yet are satisfactory (69). For subjects at risk for or recovering from pneumonia, attention to generating predictive systems has been limited (55, 70, 71). Much more research is required, with computational and machine learning approaches presenting especially exciting opportunities in this realm. In addition to creative computational approaches, clinical cohorts and biological models especially well suited to these questions are needed. New strategies need to be derived and then tested for predicting pneumonia in those at risk, for predicting progression or clinical failure in those with pneumonia, and for predicting adverse events in those who recover from pneumonia. Such predictive tools will aid in the development and application of host-based interventions designed to prevent pneumonia and its short- and long-term sequelae.
Elucidating Mechanisms of Pneumonia Defense in the Protected Lung of Young, Healthy Adults
A better understanding of the mechanistic pathways that effectively prevent pneumonia in most young adults with respiratory infections (Table 2) will advance all of the goals discussed above. Major knowledge gaps in pneumonia biology stem in part from how pneumonia defenses are traditionally studied using human subjects and animal models. Approaches need to be reconsidered and complemented. Human studies have been driven in large part by accessibility, typically relying on available clinical data or samples collected by venipuncture or bronchoscopy. However, major components of the host defense pivotal to pneumonia prevention are poorly illuminated using these approaches, such as the lymphocytes residing in the lung interstitium (8). More studies are needed of cells located within human lungs, to discover their regulation and functions related to pneumonia defense. Animal studies are driven in large part by analyzing subjects with unnaturally clean histories, often in specific-pathogen-free facilities where they have had few or likely no past infections. However, major components of host defenses pivotal to pneumonia prevention arise only after repeated respiratory infections, such as adaptive immunity driven by central memory and lung-resident memory cells as well as trained innate immunity from lung epithelial cells and macrophages (8). More lung defense research needs to incorporate models with relevant exposure histories to dissect the roles of such acquired defense mechanisms. Although much remains to be learned from traditional avenues, alternative approaches must also be pursued to elucidate mechanisms of pneumonia defense that are lung localized and exposure driven, both of which are poorly understood currently but pivotal to the lower pneumonia rates observed in healthy young adults.
Improved understanding of the normal defenses against pneumonia in young, healthy lungs is key to investigating compromises due to advancing age; chronic diseases and conditions; and noninfectious exposures such as cigarette smoke, air pollution, and alcohol. It will rationally guide studies related to predictive tools, to endotyping, and to host-directed therapies. It will provide new targets for discriminating individual host susceptibility and guide strategies to counteract that. If failures of specific pneumonia defense mechanisms contribute to complications arising later and elsewhere, then addressing these knowledge gaps will raise new opportunities for differentiating which patients are at risk for accelerated decline and for interventions to prevent complications. In short, virtually all of the goals outlined in this workshop would be informed by greater mechanistic understanding of how pneumonia is typically avoided when healthy, young lungs are infected.
Conclusions
A diverse assembly of investigators concluded that now is an exceptionally exciting time for pneumonia research, identifying research priorities that are especially pressing, opportune, and likely to make profound differences. Major suggestions involved reenvisioning pneumonia as more than just an acute lower respiratory tract infection; pneumonia results from dysregulations of host defense that precede and potentially predict infection, and pneumonia sequelae can appear much later and in organs beyond the lung. Although microbes are recognized as essential triggers, the emphasis is that host biology is paramount to developing and recovering from pneumonia. The group made several specific suggestions related to each of the sessions focusing on before (“susceptibility”), during (“host response”), and after (“consequences”) pneumonia. Common themes arose across sessions, including the need for better endotyping of patients with pneumonia as well as those susceptible to or recovered from pneumonia, and for measuring longitudinally host functional or dysfunctional activities before, during, and after pneumonia. A few overarching goals emerged that should be highlighted for special attention from researchers interested in pneumonia: improving the types and targeting of host-focused clinical approaches for preventing and treating pneumonia; generating tools that predict pneumonia occurrence, severity, and outcomes; and defining mechanisms of immune resistance and tissue resilience that normally prevent pneumonia during infection.
Footnotes
Supported by the NHLBI, NIH.
Originally Published in Press as DOI: 10.1164/rccm.201801-0139WS on March 16, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramirez JA, Wiemken TL, Peyrani P, Arnold FW, Kelley R, Mattingly WA, et al. University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis. 2017;65:1806–1812. doi: 10.1093/cid/cix647. [DOI] [PubMed] [Google Scholar]
- 3.Wallihan R, Ramilo O. Community-acquired pneumonia in children: current challenges and future directions. J Infect. 2014;69(Suppl 1):S87–S90. doi: 10.1016/j.jinf.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 4.Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53:e25–e76. doi: 10.1093/cid/cir531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry AM, Shay DK, Holman RC, Curns AT, Anderson LJ. Trends in hospitalizations for pneumonia among persons aged 65 years or older in the United States, 1988-2002. JAMA. 2005;294:2712–2719. doi: 10.1001/jama.294.21.2712. [DOI] [PubMed] [Google Scholar]
- 6.Boyd AR, Orihuela CJ. Dysregulated inflammation as a risk factor for pneumonia in the elderly. Aging Dis. 2011;2:487–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging impairs alveolar macrophage phagocytosis and increases influenza-induced mortality in mice. J Immunol. 2017;199:1060–1068. doi: 10.4049/jimmunol.1700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinton LJ, Walkey AJ, Mizgerd JP. Integrative physiology of pneumonia. Physiol Rev. In press doi: 10.1152/physrev.00032.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stämpfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9:377–384. doi: 10.1038/nri2530. [DOI] [PubMed] [Google Scholar]
- 10.Kang MJ, Lee CG, Lee JY, Dela Cruz CS, Chen ZJ, Enelow R, et al. Cigarette smoke selectively enhances viral PAMP- and virus-induced pulmonary innate immune and remodeling responses in mice. J Clin Invest. 2008;118:2771–2784. doi: 10.1172/JCI32709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurt OK, Zhang J, Pinkerton KE. Pulmonary health effects of air pollution. Curr Opin Pulm Med. 2016;22:138–143. doi: 10.1097/MCP.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5:426–434. doi: 10.1016/S2213-2600(17)30119-4. [DOI] [PubMed] [Google Scholar]
- 14.Farne HA, Johnston SL. Immune mechanisms of respiratory viral infections in asthma. Curr Opin Immunol. 2017;48:31–37. doi: 10.1016/j.coi.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Flegal KM, Kruszon-Moran D, Carroll MD, Fryar CD, Ogden CL. Trends in obesity among adults in the United States, 2005 to 2014. JAMA. 2016;315:2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14:686–698. doi: 10.1038/nri3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sajjan US. Susceptibility to viral infections in chronic obstructive pulmonary disease: role of epithelial cells. Curr Opin Pulm Med. 2013;19:125–132. doi: 10.1097/MCP.0b013e32835cef10. [DOI] [PubMed] [Google Scholar]
- 18.Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med. 2010;16:118–122. doi: 10.1097/MCP.0b013e328334c085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres A, Blasi F, Dartois N, Akova M. Which individuals are at increased risk of pneumococcal disease and why? Impact of COPD, asthma, smoking, diabetes, and/or chronic heart disease on community-acquired pneumonia and invasive pneumococcal disease. Thorax. 2015;70:984–989. doi: 10.1136/thoraxjnl-2015-206780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berenson CS, Kruzel RL, Eberhardt E, Dolnick R, Minderman H, Wallace PK, et al. Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations. Thorax. 2014;69:811–818. doi: 10.1136/thoraxjnl-2013-203669. [DOI] [PubMed] [Google Scholar]
- 21.Ubags ND, Stapleton RD, Vernooy JH, Burg E, Bement J, Hayes CM, et al. Hyperleptinemia is associated with impaired pulmonary host defense. JCI Insight. 2016;1:e82101. doi: 10.1172/jci.insight.82101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neidich SD, Green WD, Rebeles J, Karlsson EA, Schultz-Cherry S, Noah TL, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41:1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang AJ, To KK, Li C, Lau CC, Poon VK, Chan CC, et al. Leptin mediates the pathogenesis of severe 2009 pandemic influenza A(H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 24.Suratt BT. Mouse modeling of obese lung disease: insights and caveats. Am J Respir Cell Mol Biol. 2016;55:153–158. doi: 10.1165/rcmb.2016-0063PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong JL, Evans SE. Bacterial pneumonia in patients with cancer: novel risk factors and management. Clin Chest Med. 2017;38:263–277. doi: 10.1016/j.ccm.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vakil E, Evans SE. Viral pneumonia in patients with hematologic malignancy or hematopoietic stem cell transplantation. Clin Chest Med. 2017;38:97–111. doi: 10.1016/j.ccm.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden LP, Cho MH, McDonald MN, Crapo JD, Beaty TH, Silverman EK, et al. COPDGene Investigators. Susceptibility to childhood pneumonia: a genome-wide analysis. Am J Respir Cell Mol Biol. 2017;56:20–28. doi: 10.1165/rcmb.2016-0101OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zúñiga J, Buendía-Roldán I, Zhao Y, Jiménez L, Torres D, Romo J, et al. Genetic variants associated with severe pneumonia in A/H1N1 influenza infection. Eur Respir J. 2012;39:604–610. doi: 10.1183/09031936.00020611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rautanen A, Mills TC, Gordon AC, Hutton P, Steffens M, Nuamah R, et al. ESICM/ECCRN GenOSept Investigators. Genome-wide association study of survival from sepsis due to pneumonia: an observational cohort study. Lancet Respir Med. 2015;3:53–60. doi: 10.1016/S2213-2600(14)70290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol. 2015;77:407–430. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen K, Kolls JK. T cell-mediated host immune defenses in the lung. Annu Rev Immunol. 2013;31:605–633. doi: 10.1146/annurev-immunol-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker D, Ahn D, Cohen T, Prince A. Innate immune signaling activated by MDR bacteria in the airway. Physiol Rev. 2016;96:19–53. doi: 10.1152/physrev.00009.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basil MC, Levy BD. Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol. 2016;16:51–67. doi: 10.1038/nri.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, et al. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal Immunol. 2016;9:1278–1287. doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coleman FT, Blahna MT, Kamata H, Yamamoto K, Zabinski MC, Kramnik I, et al. Capacity of pneumococci to activate macrophage nuclear factor κB: influence on necroptosis and pneumonia severity. J Infect Dis. 2017;216:425–435. doi: 10.1093/infdis/jix159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamata H, Yamamoto K, Wasserman GA, Zabinski MC, Yuen CK, Lung WY, et al. Epithelial cell–derived secreted and transmembrane 1a signals to activated neutrophils during pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2016;55:407–418. doi: 10.1165/rcmb.2015-0261OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parker D, Planet PJ, Soong G, Narechania A, Prince A. Induction of type I interferon signaling determines the relative pathogenicity of Staphylococcus aureus strains. PLoS Pathog. 2014;10:e1003951. doi: 10.1371/journal.ppat.1003951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, et al. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huffnagle GB, Dickson RP, Lukacs NW. The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol. 2017;10:299–306. doi: 10.1038/mi.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Twigg HL, III, Knox KS, Zhou J, Crothers KA, Nelson DE, Toh E, et al. Effect of advanced HIV infection on the respiratory microbiome. Am J Respir Crit Care Med. 2016;194:226–235. doi: 10.1164/rccm.201509-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith NM, Wasserman GA, Coleman FT, Hilliard KL, Yamamoto K, Lipsitz E, et al. Regionally compartmentalized resident memory T cells mediate naturally acquired protection against pneumococcal pneumonia. Mucosal Immunol. 2018;11:220–235. doi: 10.1038/mi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532:512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holcomb ZE, Tsalik EL, Woods CW, McClain MT. Host-based peripheral blood gene expression analysis for diagnosis of infectious diseases. J Clin Microbiol. 2017;55:360–368. doi: 10.1128/JCM.01057-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsalik EL, Henao R, Nichols M, Burke T, Ko ER, McClain MT, et al. Host gene expression classifiers diagnose acute respiratory illness etiology. Sci Transl Med. 2016;8:322ra11. doi: 10.1126/scitranslmed.aad6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney TE, Khatri P. Generalizable biomarkers in critical care: toward precision medicine. Crit Care Med. 2017;45:934–939. doi: 10.1097/CCM.0000000000002402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sweeney TE, Wong HR, Khatri P. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci Transl Med. 2016;8:346ra91. doi: 10.1126/scitranslmed.aaf7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reyes LF, Restrepo MI, Hinojosa CA, Soni NJ, Anzueto A, Babu BL, et al. Severe pneumococcal pneumonia causes acute cardiac toxicity and subsequent cardiac remodeling. Am J Respir Crit Care Med. 2017;196:609–620. doi: 10.1164/rccm.201701-0104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barreiro E, Sznajder JI, Nader GA, Budinger GR. Muscle dysfunction in patients with lung diseases: a growing epidemic. Am J Respir Crit Care Med. 2015;191:616–619. doi: 10.1164/rccm.201412-2189OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Budinger GRS, Kohanski RA, Gan W, Kobor MS, Amaral LA, Armanios M, et al. The intersection of aging biology and the pathobiology of lung diseases: a joint NHLBI/NIA workshop. J Gerontol A Biol Sci Med Sci. 2017;72:1492–1500. doi: 10.1093/gerona/glx090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mayr FB, Talisa VB, Balakumar V, Chang CH, Fine M, Yende S. Proportion and cost of unplanned 30-day readmissions after sepsis compared with other medical conditions. JAMA. 2017;317:530–531. doi: 10.1001/jama.2016.20468. [DOI] [PubMed] [Google Scholar]
- 53.Corrales-Medina VF, Alvarez KN, Weissfeld LA, Angus DC, Chirinos JA, Chang CC, et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA. 2015;313:264–274. doi: 10.1001/jama.2014.18229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waterer GW, Kessler LA, Wunderink RG. Medium-term survival after hospitalization with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:910–914. doi: 10.1164/rccm.200310-1448OC. [DOI] [PubMed] [Google Scholar]
- 55.Yende S, D’Angelo G, Kellum JA, Weissfeld L, Fine J, Welch RD, et al. GenIMS Investigators. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am J Respir Crit Care Med. 2008;177:1242–1247. doi: 10.1164/rccm.200712-1777OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. PRORATA trial group. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 57.Yende S, D’Angelo G, Mayr F, Kellum JA, Weissfeld L, Kaynar AM, et al. GenIMS Investigators. Elevated hemostasis markers after pneumonia increases one-year risk of all-cause and cardiovascular deaths. PLoS One. 2011;6:e22847. doi: 10.1371/journal.pone.0022847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickson RP, Erb-Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med. 2014;2:238–246. doi: 10.1016/S2213-2600(14)70028-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J, Lesko M, Badri MH, Kapoor BC, Wu BG, Li Y, et al. Lung microbiome and host immune tone in subjects with idiopathic pulmonary fibrosis treated with inhaled interferon-γ. ERJ Open Res. 2017;3:00008-2017. doi: 10.1183/23120541.00008-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fastrès A, Felice F, Roels E, Moermans C, Corhay JL, Bureau F, et al. The lung microbiome in idiopathic pulmonary fibrosis: a promising approach for targeted therapies. Int J Mol Sci. 2017;18:2735. doi: 10.3390/ijms18122735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Green H, Jones AM. The microbiome and emerging pathogens in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin Respir Crit Care Med. 2015;36:225–235. doi: 10.1055/s-0035-1546752. [DOI] [PubMed] [Google Scholar]
- 62.Walkey AJ, Hammill BG, Curtis LH, Benjamin EJ. Long-term outcomes following development of new-onset atrial fibrillation during sepsis. Chest. 2014;146:1187–1195. doi: 10.1378/chest.14-0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rangelov K, Sethi S. Role of infections. Clin Chest Med. 2014;35:87–100. doi: 10.1016/j.ccm.2013.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anand N, Kollef MH. The alphabet soup of pneumonia: CAP, HAP, HCAP, NHAP, and VAP. Semin Respir Crit Care Med. 2009;30:3–9. doi: 10.1055/s-0028-1119803. [DOI] [PubMed] [Google Scholar]
- 65.Singanayagam A, Chalmers JD. Severity assessment scores to guide empirical use of antibiotics in community acquired pneumonia. Lancet Respir Med. 2013;1:653–662. doi: 10.1016/S2213-2600(13)70084-5. [DOI] [PubMed] [Google Scholar]
- 66.Bermejo-Martin JF, Almansa R, Martin-Fernandez M, Menendez R, Torres A. Immunological profiling to assess disease severity and prognosis in community-acquired pneumonia. Lancet Respir Med. 2017;5:e35–e36. doi: 10.1016/S2213-2600(17)30444-7. [DOI] [PubMed] [Google Scholar]
- 67.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 68.Cleaver JO, You D, Michaud DR, Pruneda FA, Juarez MM, Zhang J, et al. Lung epithelial cells are essential effectors of inducible resistance to pneumonia. Mucosal Immunol. 2014;7:78–88. doi: 10.1038/mi.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morley D, Torres A, Cillóniz C, Martin-Loeches I. Predictors of treatment failure and clinical stability in patients with community acquired pneumonia. Ann Transl Med. 2017;5:443. doi: 10.21037/atm.2017.06.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yende S, Tuomanen EI, Wunderink R, Kanaya A, Newman AB, Harris T, et al. Preinfection systemic inflammatory markers and risk of hospitalization due to pneumonia. Am J Respir Crit Care Med. 2005;172:1440–1446. doi: 10.1164/rccm.200506-888OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. GenIMS Investigators. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–1663. doi: 10.1001/archinte.167.15.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]