Abstract
Skeletal muscle dysfunction occurs in patients with chronic obstructive pulmonary disease (COPD) and affects both ventilatory and nonventilatory muscle groups. It represents a very important comorbidity that is associated with poor quality of life and reduced survival. It results from a complex combination of functional, metabolic, and anatomical alterations leading to suboptimal muscle work. Muscle atrophy, altered fiber type and metabolism, and chest wall remodeling, in the case of the respiratory muscles, are relevant etiological contributors to this process. Muscle dysfunction worsens during COPD exacerbations, rendering patients progressively less able to perform activities of daily living, and it is also associated with poor outcomes. Muscle recovery measures consisting of a combination of pulmonary rehabilitation, optimized nutrition, and other strategies are associated with better prognosis when administered in stable patients as well as after exacerbations. A deeper understanding of this process’ pathophysiology and clinical relevance will facilitate the use of measures to alleviate its effects and potentially improve patients’ outcomes. In this review, a general overview of skeletal muscle dysfunction in COPD is offered to highlight its relevance and magnitude to expert practitioners and scientists as well as to the average clinician dealing with patients with chronic respiratory diseases.
Keywords: chronic obstructive pulmonary disease, skeletal muscle dysfunction, muscle wasting, ventilatory muscles, fiber switch
Skeletal muscle dysfunction is a major problem in many patients with chronic obstructive pulmonary disease (COPD). It affects both ventilatory and nonventilatory muscle groups, leading to worse outcomes, including increased mortality and hospitalization rates (1–3). Although not universally present in patients with COPD, muscle wasting is more prevalent in individuals with emphysema than in those with airway-type COPD (4), which is reminiscent of the historical description of the pink puffer emphysematous type mostly associated with body and muscle mass loss (5). Muscle dysfunction is also more significant in lower than in upper extremities (6, 7), which compromises the patients’ ambulatory capacity and has devastating effects on their daily lives. Muscle integrity mirrors general well-being and nutritional status and thus indirectly associates with elements that are relevant to COPD outcomes such as susceptibility to infections, bone mineral density, or exacerbation rate.
Despite its representing an important comorbidity, many healthcare providers have a poor understanding of skeletal muscle dysfunction, in part owing to overlapping and confusing definitions to describe the process and also the wrong perception that there are no useful interventions to alleviate it. In this short review, we describe these fundamental aspects to facilitate clinicians’ better understanding of the topic and improve their day-to-day clinical practice. We first discuss the common features of ventilatory and nonventilatory muscles and later elaborate on the specific aspects pertinent to each group. We also introduce readers to the major controversies in the field; however, owing to space constraints, we defer the description of their more technical elements to good clinical guidelines and statements published over the last two decades (8–11). In the next section, we delve into the topic’s complexities and clarify definitions to facilitate the reading later in the text.
Characteristics of COPD-associated Muscle Dysfunction
In general, the magnitude of muscle dysfunction correlates to the severity of lung disease (12); yet, there is variable expression of this process, with some patients with advanced COPD having relatively preserved muscle integrity and vice versa (12). With the same degree of airway obstruction, patients with the emphysematous phenotype have relatively more muscle wasting than patients with chronic bronchitis (4). We start by introducing important definitions used to characterize the process. Some of them are often applied interchangeably, but their precise use facilitates the understanding of the current literature and the process of muscle dysfunction. In general, there are three domains used to characterize muscle integrity: 1) clinical/functional, which is used to appreciate muscle work, generally in the form of endurance and strength; 2) metabolic, which refers to the ability of the muscle fibers to transform chemical energy into mechanical work generated by the myosin molecular motor (13) and is closely related to oxygen use, ATP generation, and intracellular calcium handling; and 3) anatomical, which refers to the total amount of muscle mass available to generate work.
Although often overlapping (14), these domains can be disrupted in a relatively independent way. For instance, muscle mass may decrease without having a significant impact on force generation capacity, owing to relatively better-preserved metabolic efficiency, whereas metabolic and electrophysiological factors may come into play before muscle wasting develops (15). Thus, combinations of clinical/functional, metabolic, and anatomical disturbances may lead to unique signatures of muscle dysfunction.
Clinical/Functional Muscle Disruption
Muscle work is the result of movement generated through the application of force. Thus, lower muscle work is caused by less force generation capacity, which is due to lower strength, lower endurance, or both. Muscle strength is the ability to generate maximal force at a single time point such as weight lifting, and, compared with muscle endurance, it is relatively more dependent on muscle mass and cross-sectional area (8). In contrast, muscle endurance is the ability to generate submaximal force sustained over time, which, compared with muscle strength, is relatively more dependent on the fiber-type composition of the affected muscles and their oxidative capacity (16, 17). Identification of muscle dysfunction using strength and endurance is very useful for determining actual muscle work in the clinical setting. However, endurance is relatively more complicated to measure than strength, given that it is potentially confounded by the cardiopulmonary limitation on endurance exercises such as climbing stairs. A device known as a “dynamometer” can be used to measure isolated muscle strength and endurance, although detailed description of these determinations is beyond the scope of the present review and can be found elsewhere (9, 18, 19).
Metabolic Muscle Disruption
The muscle metabolic profile is partly influenced by the type of myosin heavy chain (MyHC) expressed by the fibers, which depend primarily on their motoneuronal innervation (20). Type I fibers express MyHC type I, are slow-twitch fibers, are innervated by slow motoneurons, have a predominantly oxidative metabolism, and are more fatigue resistant than type II fibers. Type II fibers express MyHC type II, are fast-twitch fibers, are innervated by fast motoneurons, depend more on anaerobic metabolism, and are more fatigable (20). Importantly, the fiber type is closely associated with its calcium sensitivity: Type I fibers are relatively more calcium sensitive than type II fibers and generate a greater fraction of their maximal force for a given amount of mobilized intracellular calcium (21, 22)
There are subtypes of type II fibers—IIa, IIx, and IIb (20)—that are in general less oxidative, calcium sensitive, and fatigue resistant than type I fibers. As discussed later in the text, fiber-type composition may change in COPD in a process known as “fiber switch” or “transformation,” rendering the diaphragm metabolically more efficient and the lower extremity muscles less efficient (23). The process of fiber switch is contributed by two distinct phenomena: 1) Some fibers undergo selective atrophy, which causes an increase in the relative abundance of the unaffected type (20, 23); and 2) even in the absence of fiber atrophy, the expression of a given MyHC isoform’s gene can be downregulated and a different one upregulated in the same fiber, resulting in a change of its metabolic profile (20). The process of fiber transformation is demonstrated by the presence of hybrid fibers expressing, in a single fiber, different MyHC isoforms that represent the transition occurring during different pathological states (24, 25). Other relevant factors that impact myofibers’ metabolism are oxygen transport and use driven by cardiac output, capillary and mitochondrial densities, and the expression of oxidative enzymes (20, 26).
Anatomical Muscle Disruption
“Muscle anatomy” refers mostly to muscle size and total protein content, which can be decreased because of phenotypic variability, female sex, and other nonpathological factors (12, 27). Muscle anatomy can also be pathologically altered in atrophy, cachexia, and sarcopenia, all of which are possible in COPD. “Muscle atrophy,” or “wasting,” is a general term defining a reduction in the size of the muscle fibers that is usually a sign of net protein catabolism (8). Cachexia is a specific metabolic syndrome associated with an ongoing underlying disease, such as COPD, that is characterized by muscle wasting and weight loss (28). Sarcopenia is also a specific form of muscle loss that occurs with advanced age and is not associated with weight loss (29).
Atrophy, cachexia, and sarcopenia are intertwined categories in patients with COPD, given that many of them are at an advanced age and can have heart failure, diabetes, cancer, and/or other conditions associated with different causes of muscle dysfunction (30). Few studies have addressed the trajectory of muscle loss in COPD (31, 32), and it is uncertain whether patients evaluated were actively losing muscle during the studies and thus were cachectic or instead had lower muscle mass without active catabolism and thus were simply atrophic (8).
Mechanisms of Skeletal Muscle Dysfunction in Patients with COPD
The mechanisms of skeletal muscle dysfunction constitute an expanding area of research, and we introduce the readers to it in the following paragraphs. A more detailed description of these biological processes can be found elsewhere (33–36). There is consensus that no single cellular process leading to muscle wasting exists and that different phenomena converge in the COPD phenotype (37). Different muscle groups, such as the diaphragm, the lower extremities, the abdominal muscles, and the upper extremities, present variable and sometimes divergent characteristics (38), which suggests the nonsystemic nature of the process. However, the fact that muscle groups in different regions are exposed to variable loads makes possible the selective atrophy of some groups and not others despite common mechanisms. Controversy surrounds the role of inflammatory signals as potential drivers of COPD-associated muscle wasting (39), with some groups reporting its relevance (40) and others showing no role (41) or even some protective effect demonstrated by a negative correlation between local inflammation and muscle weakness (42). An issue that remains unclear is whether locomotor muscle dysfunction in COPD is driven by chronic immobility or if there is a distinct COPD myopathy (8). In support of the immobility hypothesis is the fact that many hallmarks of COPD muscle dysfunction resemble the ones associated with immobility, including atrophy, loss of type I fibers, lower oxidative enzyme activity, and others (43), as well as the fact that exercise can potentially restore a normal muscle phenotype to some extent (44). Behind the myopathy theory is evidence that some aspects of muscle dysfunction are poorly correlated with the level of physical activity of patients with COPD (45) and that even when matched by physical activity, COPD and healthy subjects display differences in muscle integrity (46).
Some basic mechanisms leading to muscle dysfunction are well established, whereas others are emerging as potential targets to interfere with this process. We define intrinsic mechanisms as the ones taking place primarily in the neuromuscular unit and leading to clinical/functional, metabolic, or anatomical disruption; extrinsic mechanisms as the ones originating in other structures, such as the chest wall; and mixed mechanisms as the ones combining the preceding categories.
Intrinsic Mechanisms
Net protein loss via catabolic activation
Different signals, including hypoxia, hypercapnia, smoking, malnutrition, and immobilization, eventually lead to accelerated intracellular protein degradation (47–49), which is the hallmark of muscle atrophy and occurs via two major mechanisms: the ubiquitin–proteasome (50) and lysosomal (51) pathways, which coexist in COPD and operate in a coordinated manner. Proteasomal degradation requires the regulated ubiquitin “tagging” of specifically targeted proteins, such as myosin (52), that are subsequently degraded by the 26S proteasome (52). This specificity is conferred by muscle-specific E3-ubiquitin ligases, such as MuRF1 (muscle-specific Ring finger 1 protein), atrogin 1, and NEDD4, which are upregulated in muscle biopsies from patients with COPD (50, 53, 54). Experimental interference with this mechanism prevents muscle loss in the context of chronic hypercapnia (48). Importantly, because the proteasome can degrade only proteins in monomeric form, the muscle sarcomere needs first to be destabilized and disassembled, which is accomplished by calcium-dependent proteases named calpains (55). Moreover, the autophagy–lysosomal pathway is induced in locomotor muscles of stable patients with COPD, and the degree of autophagy correlates with the severity of muscle atrophy and lung function impairment (51, 56).
Net protein loss via anabolic suppression
Two major signaling pathways control skeletal muscle growth: 1) the insulin-like growth factor pathway, which induces muscle growth and is boosted during pulmonary rehabilitation (PR) (57, 58); and 2) the myostatin–Smad3 pathway, which acts as a negative regulator of muscle growth (37, 54), and although it has been reported to be downregulated in noncachectic patients during PR (57), another group found no effect of exercise training on it (59). There is evidence of relative anabolic suppression of locomotor muscles compared with the diaphragm in patients with COPD (60), which is consistent with the adaptation of ventilatory muscles as described below. Also, there is evidence of subnormal testosterone levels in some patients with COPD, which could contribute to depressed anabolism and thus to wasting (61).
Calcium desensitization
As mentioned above, the fast-twitch fibers have a lower calcium sensitivity relative to the slow-twitch ones. Thus, slow-to-fast fiber transformation taking place in lower extremity muscles associates with lower calcium signaling efficiency (21, 22). Altered sarcoplasmic calcium reuptake compromising relaxation efficiency (62) and differential calcium sensitivity of contractile proteins (20) could also contribute to worse performance, although this has not been demonstrated specifically in patients with COPD.
Muscle injury, structural damage, and inadequate repair
Cycles of muscle injury and repair have been suggested in the context of COPD (63) and during eccentric/lengthening exercise (64) commonly used in rehabilitation protocols. Indeed, potential dysfunction of muscle stem cells (satellite cells), which are typically engaged after muscle injury, has been suggested as a contributor to suboptimal muscle repair (65–67).
Others
Alternative splicing of the gene codifying for the giant protein titin (68), oxidative stress (56, 69), and mitochondrial dysfunction (70) are reported to contribute to worse ventilatory and nonventilatory muscle work in COPD.
Extrinsic Mechanisms
Chest wall remodeling
In patients with COPD, the diaphragm chronically adapts to the increased inspiratory load and reduced elastic recoil force of the lungs, leading to a relative preservation from anatomical and metabolic disruptions of the muscle. In fact, at very high lung volumes, the diaphragm from patients with COPD can generate more force than control subjects’ (71). Despite that, from the clinical/functional standpoint, ventilatory dysfunction is very significant in COPD and is caused mainly by changes in the chest wall geometry. Hyperinflation leads to decreased length and area of apposition of the diaphragm with the rib cage (72, 73), and also to a chest wall bone configuration (74), which leads to less efficient ventilatory work. Studies of patients before and after lung volume reduction surgery showed increased postoperative diaphragm length and strength, as well as improved exercise capacity and maximum voluntary ventilation (75). These changes are also associated with chest wall remodeling resulting from bony thorax configuration (74). In contrast to the ventilatory muscles, extrinsic factors leading to peripheral muscle dysfunction have not been described in COPD.
Mixed Mechanisms
Interdependence of locomotor and ventilatory muscles during exercise
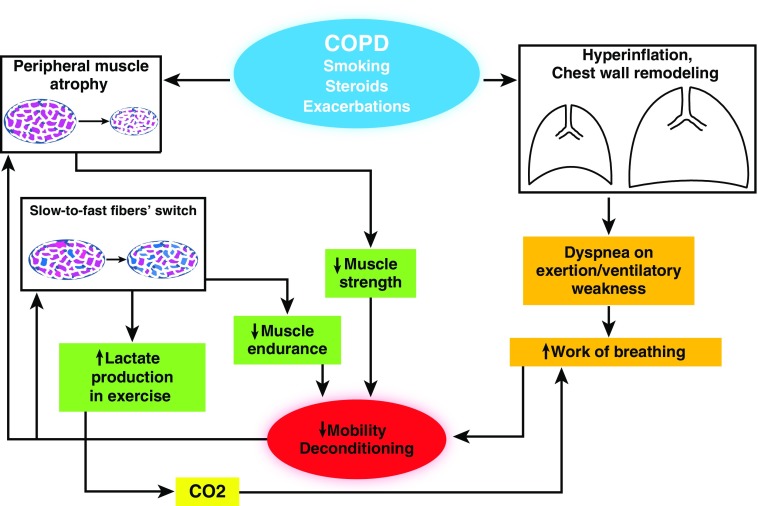
The interaction between locomotor and ventilatory muscle dysfunction in patients with COPD is relevant to the pathophysiology of the process (Figure 1). Indeed, the increased lactic acid production during exercise is a main factor associated with lower exercise tolerance (76). Because of slow- to fast-twitch fiber-type transformation in peripheral muscles, patients with COPD during acute exercise performance produce more lactic acid and CO2 for a given exercise load, which requires increased compensatory work of breathing and could potentially exhaust ventilatory muscle capacity in a patient with very limited physiological reserve (77, 78). A summary of the general mechanisms contributing to muscle dysfunction is presented in Table 1.
Figure 1.
Pathophysiology of chronic obstructive pulmonary disease (COPD)-associated muscle dysfunction. Cigarette smoking and other factors, as well as exacerbations, are the main causes of COPD progression, which is associated with peripheral muscle atrophy and fiber switch. Hyperinflation and loss of elastic recoil lead to chest wall geometrical changes that cause diaphragmatic dysfunction. All these events contribute to immobilization and deconditioning, which further cause peripheral muscle dysfunction and deconditioning.
Table 1.
General Mechanisms Contributing to Skeletal Muscle Dysfunction in Chronic Obstructive Pulmonary Disease
| Signals and stimuli that initiate the muscle loss and wasting processes |
| 1. Immobilization |
| 2. Malnutrition |
| 3. Smoking |
| 4. Infections/exacerbations |
| 5. Hypoxemia |
| 6. Hypercapnia |
| 7. Corticosteroids |
| Intrinsic cellular processes that mediate muscle dysfunction |
| 1. Ubiquitin–proteasome pathway |
| 2. Lysosomal–autophagy pathways |
| 3. Anabolic suppression |
| 4. Calcium desensitization |
| 5. Muscle injury |
| 6. Oxidative stress and mitochondrial dysfunction |
| Extrinsic processes that lead to muscle dysfunction |
| 1. Hyperinflation-associated diaphragmatic dysfunction (diaphragm) |
| 2. Bony thoracic remodeling (diaphragm) |
| 3. Lactate hyperproduction and lower exercise tolerance |
Different signals, including immobilization, malnutrition, and smoking, activate cellular processes leading to net protein loss in skeletal muscle and also alter the chest wall geometry, compromising ventilatory muscle function.
Muscle Dysfunction and COPD Prognosis
Muscle dysfunction is associated with worse COPD prognosis, and two important elements suggest that it could indeed contribute to COPD outcomes. First, the association of survival of patients with COPD with their muscle integrity persists even after correcting for surrogates of pulmonary function (1–3). In other words, patients with similar lung function will be more or less likely to die in the long term based on the presence or absence of muscle dysfunction, respectively (1–3). Second, PR, which has a beneficial effect on muscle dysfunction, is associated with better outcomes without affecting the history of the pulmonary disease (79, 80). It is important to emphasize that although skeletal muscle wasting is associated with lower COPD survival, PR’s beneficial effects on skeletal muscle have not been associated with overall lower mortality (81). The reason for that disconnect is unclear but could be due to heterogeneous responses to PR among different patient groups (8) or to other factors. In the following subsections, we summarize clinical evidence supporting the relevance of muscle dysfunction and recovery to the prognosis of COPD, and we clarify the significance of weight changes in relation to muscle mass and patients’ outcomes.
Effects of Clinical/Functional Disruption and Recovery on COPD Outcomes
Peripheral muscle weakness.
Peripheral muscle weakness, defined as poor muscle strength (3), is associated with increased mortality (3). Similarly, underperformance in 6-minute walk distance (6MWD) (82) and on the handgrip strength test (83) also predicts mortality. Recovery of muscle force in the context of PR is associated with improved symptoms, limb muscle function, exercise capacity, quality of life, and other outcomes (84–86).
Ventilatory muscle weakness.
Ventilatory muscle weakness has consistently been demonstrated in patients with COPD, leading to reduced maximum inspiratory pressure and transdiaphragmatic pressure (9, 87, 88). During an exacerbation, ventilatory muscle dysfunction is a predictor of generalized muscle weakness (89) and of mechanical ventilation requirements (90). Indeed, ventilatory support with noninvasive ventilation can prevent intubation and reduces length of hospital stay and mortality (91, 92).
Effects of Metabolic Disruption and Recovery on COPD Outcomes
Peripheral muscle metabolic disruption
In quadriceps muscles of patients with COPD, the relative number of type I fibers decreases compared with type II fibers (93), a phenomenon known as “slow- to fast-twitch fiber transformation,” which is associated with worse lung function (94, 95), exercise capacity (96), and functional performance (17, 97) as well as mortality (98). Indeed, PR associates with higher expression of the relatively more oxidative types I and IIa fibers (94, 99).
Metabolic disruption in ventilatory muscles
In contrast to peripheral muscles, diaphragm muscles of patients with COPD undergo fast- to slow-twitch fiber transformation (33, 100), which is associated with increased oxygen consumption (101, 102) and resistance to fatigue (88); yet, the force generation capacity of individual fibers seems to be subnormal (100, 103). Indeed, inspiratory muscle training in the context of PR is associated with a higher proportion of type I fibers and the size of type II fibers in accessory ventilatory muscles (104).
Effects of Anatomical Disruption and Recovery on COPD Outcomes
Peripheral muscle wasting.
Peripheral muscle wasting has been associated with worse prognosis. Indeed, computed tomography–measured midthigh muscle cross-sectional areas in stable patients with COPD correlated with mortality and was a better predictor than body mass index (BMI) (1). Also, smaller pectoralis muscle area was associated with higher disease severity as measured by Global Initiative for Chronic Obstructive Lung Disease stage and lower resting oxygen saturation, BODE score (based on BMI, airflow obstruction, dyspnea, and exercise), quality of life score, and exercise capacity (12). A recent report indicated that lesser erector spinae muscle mass is associated with worse dyspnea score, higher BMI, worse emphysema, lower FEV1, and higher mortality (105). During COPD exacerbations, there is an accelerated loss of locomotor muscle mass (106, 107) that is often associated with immobilization (108) and fails to recover to the preexacerbation baseline, similar to what is observed with pulmonary function (109) and the degree of emphysema (110). Indeed, lesser quadriceps mass at the time of exacerbation predicts higher chances of need for readmission and death (111), and in-hospital rehabilitation starting on the second day of admission attenuates the muscle mass decline observed during exacerbations (112).
Ventilatory muscle wasting.
Ventilatory muscle wasting is relatively less relevant than wasting of peripheral muscles. Both autopsy- (113) and ultrasound-based studies (114) indicate that diaphragmatic mass of patients with COPD remained similar to that of healthy control subjects, which may be due to the reported greater firing rate of diaphragm motor unit potentials (70) leading to a hypertrophic signal (33, 100) that compensates the ongoing atrophy (114). In contrast, low intercostal muscle mass predicts future COPD exacerbations (115).
Effects of Body Weight on COPD Prognosis
Lower BMI is a predictor of higher mortality in COPD (116). That effect is independent of lung function and is partially determined by skeletal muscle mass (117). Although there is agreement that underweight patients with COPD have worse outcomes (116, 118), the effect of obesity-driven higher BMI is a subject of controversy (119). An analysis of outcomes in patients who were followed for 8 years after hospital admission for an acute exacerbation showed that overweight was a predictor of survival (120); however, a recent analysis of 3,600 patients from the COPDGene study indicated that obesity was associated with worse outcomes, including quality of life, dyspnea, 6MWD, and severe exacerbations (121). These associations were even stronger when obesity was analyzed as a dose-dependent response (121). Moreover, muscle lipid content correlated with decreased oxidative enzyme activity (122), exercise capacity (96), and lower survival (98), and fat content of intercostal muscles was associated with COPD exacerbation rates (115). Thus, although weight loss is associated most of the time with muscle wasting, weight gain is not necessarily indicative of adequate muscle performance and or mass.
Treatment of Skeletal Muscle Dysfunction in Patients with COPD
In general, the cornerstones of treatment of COPD-associated muscle dysfunction are rehabilitation-based exercises, optimized nutrition, and electrical stimulation. We summarize the evidence and controversies surrounding these measures in the following subsections.
Pulmonary Rehabilitation
Exercise training, which is a formal component of PR, is the most significant currently available intervention for treating muscle dysfunction in COPD (84). Training of the ambulatory muscles is a mandatory component of PR (123). The reported magnitude of muscle recovery after PR has been variable, ranging from modest or nonrecovery (124) to very robust improvement (44), and that variability can be due to different tolerance to exercise (8), genetic and epigenetic factors, regenerative potential, and oxidative metabolism (125). Also, some evidence indicates that localized training focused on specific muscle groups could be more beneficial than exercise targeting multiple groups (126).
Exercise protocols in PR
The ideal rehabilitation program should 1) tailor the training protocol to the specific patient’s requirements to accommodate comorbid conditions and avoid exercise-induced muscle injury, particularly in regard to eccentric/lengthening exercises (64); 2) exceed the loads typically encountered by the patient during his or her daily life; and 3) progress and become more challenging as improvement occurs (81). There are basically two types of exercises: resistance/strength and endurance exercise. Endurance exercise in the form of cycling or walking is a common modality of PR (81). Typically, endurance and strength training of the lower and upper extremities is combined with ventilatory muscle training. In the latter case, inspiratory (80, 127), and less commonly expiratory (128), muscle training led to improvements in dyspnea and other quality-of-life parameters. Details on the technical aspects of PR can be found in excellent resources (81), and a summary of its exercise training principles is presented in Table 2.
Table 2.
Basic Aspects of Exercise in Pulmonary Hypertension
| General principles |
| 1. Exercise must be tailored to specific patient’s needs |
| 2. Exercise must exceed the regular loads patient is used to overcoming |
| 3. Exercise must progress as patient improves performance |
| Specific strategies |
| 1. Endurance training |
| Goal: To improve general aerobic capacity and not any specific muscle group |
| Mode: Cycling and walking are commonly used. |
| 2. Interval training (99) |
| Goal: To provide training for patients unable to tolerate regular endurance exercises (previous point) |
| Mode: High intensity interspersed with lower intensity or rest |
| 3. Resistance/strength training |
| Goal: To target specific local muscle groups and improve their function |
| Mode: Repetitive lifting of heavy weights |
| 4. Upper limb training |
| Goal: To improve activities of daily living (e.g., dressing, bathing) |
| Mode: Endurance (cycle ergometer) and/or resistance (weights) exercises |
| 5. Inspiratory muscle training |
| Goal: To improve exercise capacity and dyspnea |
| Mode: Use of loads ≥30% of maximal inspiratory pressure |
Timing of PR
Traditionally, PR is offered to patients with severe COPD, but there is evidence that muscle dysfunction (129) and its PR-associated recovery (94) also occur in early stages. PR should be provided during or shortly after an exacerbation because it accelerates functional recovery and decreases the chances of readmission (81). Also, in stable patients, it improves health-related quality of life, readmission rate, and other outcomes (123).
PR implementation problems and maintenance strategies
PR should be provided to patients who are maximally treated with appropriate medications, including bronchodilators and supplemental oxygen, but limited recognition of its benefits and the lack of available programs for its delivery preclude massive implementation (84). During clinical encounters, healthcare providers should engage patients and families to facilitate recognition and adherence to PR by reviewing its benefits (Table 3). Another area of interest is the duration of PR, which is typically recommended for a minimum of 8 weeks (130). Although experts recommend that patients should continue to exercise beyond the PR program period, there is controversy regarding the ideal maintenance plan. Some studies have shown no benefits of maintenance technique beyond 1 year (131), whereas others have demonstrated that longer protocols extend the benefits of PR (132). A recent trial involving stable patients with COPD revealed that more prolonged and intensive PR was associated with better BODE index and 6MWD than a standard strategy. These effects were significant at 2 years, but they vanished after that (133).
Table 3.
Benefits of Pulmonary Rehabilitation
| • Reduced hospitalization |
| • Reduced unscheduled healthcare visits |
| • Improved exercise capacity |
| • Reduced symptoms of dyspnea and leg discomfort |
| • Improved limb muscle strength and endurance |
| • Improved health-related quality of life |
| • Improved functional capacity (e.g., activities of daily living) |
| • Improved emotional function |
| • Enhanced self-efficacy and knowledge |
| • Enhanced collaborative self-management |
| • Potential for increased daily physical activity levels |
Practitioners should dedicate time to discussing the general benefits of exercise and nutrition, and specifically pulmonary rehabilitation, with patients with chronic obstructive pulmonary disease and their families. Adapted from Reference 84.
Nutritional Support
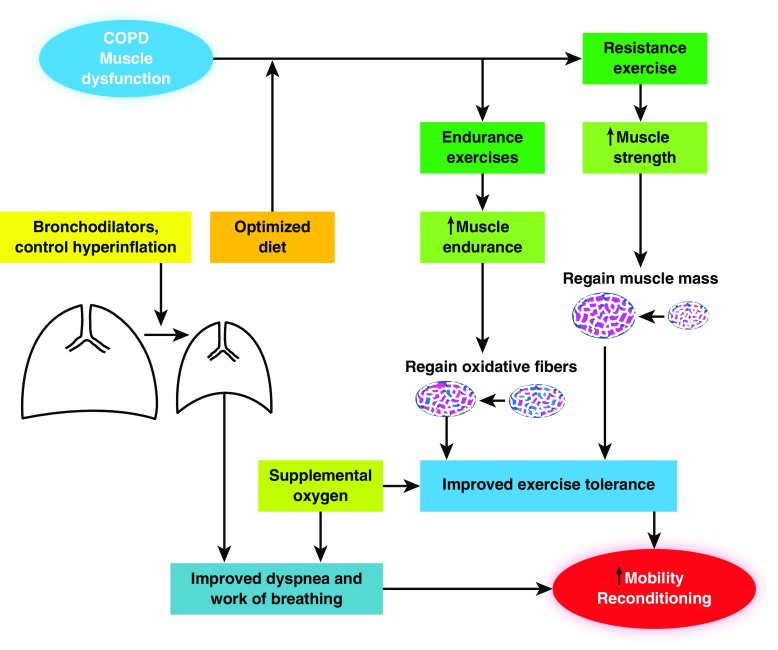
The central relevance of adequate nutrition in COPD has been recognized for all patients, not only for those with weight loss and muscle wasting (134). Indeed, obesity has been identified as a potential risk factor for COPD (135), and age-related sarcopenia seems to be associated with the development of metabolic syndrome in these patients (136). Patients with COPD demonstrate some muscle ultrastructural and molecular changes typically found in starvation, such as upregulation of the lysosomal–autophagy pathway and oxidative stress (51). Importantly, severe malnutrition associated with cachexia still portends significant potential for recovery, although some aspects of it are different from those exhibited by patients without cachexia undergoing the same treatment (57). Some of the PR benefits consist of transforming the nutritional substrates provided by an optimized diet into larger muscle mass as well as regaining oxidative capacity and exercise tolerance in the form of both strength and endurance (Figure 2).
Figure 2.
Holistic approach to prevent and reverse chronic obstructive pulmonary disease (COPD)-associated muscle dysfunction. Combination of optimized diet with resistance and endurance exercise training programs is associated with improved muscle mass and oxidative capacity in locomotor muscles, which in turn decrease fatigue upon exercise. Bronchodilators, cigarette smoking cessation, and occasionally lung volume reduction surgery decrease hyperinflation and lead to better dyspnea control. All these measures contribute to reconditioning and eventually to regaining functional capacity in these patients.
Evidence centered on nutrition in COPD is controverted: Whereas some reports indicate the benefits of nutritional optimization as a strategy to improve PR outcome (137–139), others show no clear benefits (140, 141) or identify specific subgroups with better potential (142). There is agreement that nutritional support with no exercise has a very limited effect. Authors of a meta-analysis of 17 studies involving more than 600 patients found that nutritional supplementation alone led to a marginal weight gain in patients with COPD and normal nutritional status, whereas a benefit of about 2 kg was seen in those patients who were initially malnourished (143). There is evidence that low dietary fiber intake is associated with higher risk of COPD (144), and some reports indicate that the composition of the ideal diet for a malnourished patient with COPD should include branched-chain amino acids (145) and polyunsaturated fatty acids (146); and a potential benefit of whey supplementation on exercise tolerance and quality of life has been suggested (147). Researchers in a recent trial found that optimizing nutrition with leucine, vitamin D, and omega-3 fatty acids significantly improved the effects of high-intensity exercise on lower limb muscle strength and exercise performance in COPD (148).
Neuromuscular Electrical Stimulation
Patients with severe exercise limitation are sometimes unable to undergo PR and can be good candidates for an alternative mode of muscle stimulation via transcutaneous electrical probes (81). Dyspnea, functional capacity, and muscle strength improved using this approach in stable patients (149–152) and during exacerbations (153). In a recent double-blind, placebo-controlled trial, neuromuscular electrical stimulation improved functional exercise capacity in patients with severe COPD by enhancing quadriceps muscle mass and function. The effects are maximal at 6 weeks and wane over time (154). An alternative approach that is less frequently used but could be better tolerated is quadriceps magnetic stimulation. This approach was shown to improve strength, exercise capacity, and metabolic profile as reflected by an increase in type I fiber size (155, 156).
Anabolic Steroids
Based on observations that low testosterone is present in many male patients with COPD (61), a trial involving its administration to men with severe COPD with low basal levels led to improved lean body mass and strength, which was amplified by concomitant resistant training (157). Oxandrolone increased lean body mass in an open-label, prospective, multicenter trial of a stable outpatient COPD population with cachexia; however, the trial had the limitation of lacking a control group (158). Researchers in another trial evaluated the potential benefit of nandrolone during respiratory rehabilitation and found no significant differences in outcomes between groups as measured by muscle function, exercise capacity, and health status (139). The main concern regarding anabolic steroids is related to their side effects in the long term, which created interest in the recently developed nonsteroidal selective androgen receptor modulators. These drugs have preliminarily been shown to lead to improvement in lean body mass and physical function in different forms of cachexia, although they have not been tried in COPD so far (159). The appetite stimulant megestrol acetate led to an improvement in body weight that was not reflected in improved respiratory muscle function or exercise tolerance (160).
Other Potential Therapeutic Strategies
There is also some evidence supporting the possible benefit of N-acetylcysteine (161) and the calcium sensitizer levosimendan (162) on diaphragmatic function. Also, there is interest in the muscle anabolic effects of growth hormone secretagogues such as ghrelin, as well as in myostatin inhibitors, although the data supporting them is very preliminary or not confirmed, and thus they are beyond the scope of the present review (19, 163).
Conclusions
Skeletal muscle dysfunction is a relevant comorbidity in COPD that is associated with worse outcomes, including greater hospitalizations rates, worse quality of life, and lower survival. It is often considered a minor aspect of this disease; yet, abundant evidence supports its significance and the role of PR and other measures in dealing with the problem. Although it still represents an expanding territory in COPD research, enough information is already available to help healthcare providers improve their day-to-day clinical practice to alleviate their patients’ symptoms and make them less vulnerable to worse outcomes. Muscle dysfunction in COPD should be regarded as a systemic phenomenon that demands a holistic approach aimed at exercise tolerance and nutritional status, which should be routinely implemented to attempt to reverse the pathophysiology of the process (Figure 2). Early detection of muscle dysfunction and wasting, as well as engagement of patients and their families in strategies to deal with the problem, could prevent disease progression and improve prognosis, regardless of the degree of pulmonary disease. The present review represents a useful tool for the average clinician to improve management of patients with major chronic respiratory conditions.
Footnotes
Some of the results reported in this review were funded by NHLBI–NIH award K01-HL130704-01 and the Collins Family Foundation Endowment (A.J.), as well as by Instituto de Salud Carlos III contract grants from Centro de Investigación en Red de Enfermedades Respiratorias and FIS 14/00713 (Fondo Europeo de Desarrollo Regional), Spanish Ministry of Economy and Competitiveness, Spanish Respiratory Society 2016, Catalan Foundation of Pulmonology 2016, and an unrestricted grant from Menarini SA 2018 (E.B.).
Author Contributions: A.J. and E.B. wrote and edited the manuscript.
CME will be available for this article at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201710-2140CI on March 19, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, Carrier G, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166:809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 2.Shrikrishna D, Patel M, Tanner RJ, Seymour JM, Connolly BA, Puthucheary ZA, et al. Quadriceps wasting and physical inactivity in patients with COPD. Eur Respir J. 2012;40:1115–1122. doi: 10.1183/09031936.00170111. [DOI] [PubMed] [Google Scholar]
- 3.Swallow EB, Reyes D, Hopkinson NS, Man WD, Porcher R, Cetti EJ, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62:115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, Bruijnzeel PL, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187:728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 5.Filley GF, Beckwitt HJ, Reeves JT, Mitchell RS. Chronic obstructive bronchopulmonary disease. II. Oxygen transport in two clinical types. Am J Med. 1968;44:26–38. doi: 10.1016/0002-9343(68)90234-9. [DOI] [PubMed] [Google Scholar]
- 6.Gea JG, Pasto M, Carmona MA, Orozco-Levi M, Palomeque J, Broquetas J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J. 2001;17:939–945. doi: 10.1183/09031936.01.17509390. [DOI] [PubMed] [Google Scholar]
- 7.Gosselink R, Troosters T, Decramer M. Distribution of muscle weakness in patients with stable chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2000;20:353–360. doi: 10.1097/00008483-200011000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, et al. ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society statement: update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;189:e15–e62. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Thoracic Society/European Respiratory Society. ATS/ERS Statement on Respiratory Muscle Testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 10.Barreiro E, Gea J. Molecular and biological pathways of skeletal muscle dysfunction in chronic obstructive pulmonary disease. Chron Respir Dis. 2016;13:297–311. doi: 10.1177/1479972316642366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barreiro E, Bustamante V, Cejudo P, Gáldiz JB, Gea J, de Lucas P, et al. SEPAR. Guidelines for the evaluation and treatment of muscle dysfunction in patients with chronic obstructive pulmonary disease. Arch Bronconeumol. 2015;51:384–395. doi: 10.1016/j.arbres.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 12.McDonald ML, Diaz AA, Ross JC, San Jose Estepar R, Zhou L, Regan EA, et al. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schliwa M, Woehlke G. Molecular motors. Nature. 2003;422:759–765. doi: 10.1038/nature01601. [DOI] [PubMed] [Google Scholar]
- 14.Vermeeren MA, Creutzberg EC, Schols AM, Postma DS, Pieters WR, Roldaan AC, et al. COSMIC Study Group. Prevalence of nutritional depletion in a large out-patient population of patients with COPD. Respir Med. 2006;100:1349–1355. doi: 10.1016/j.rmed.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Berg HE, Larsson L, Tesch PA. Lower limb skeletal muscle function after 6 wk of bed rest. J Appl Physiol (1985) 1997;82:182–188. doi: 10.1152/jappl.1997.82.1.182. [DOI] [PubMed] [Google Scholar]
- 16.Barreiro E, Gea J. Respiratory and limb muscle dysfunction in COPD. COPD. 2015;12:413–426. doi: 10.3109/15412555.2014.974737. [DOI] [PubMed] [Google Scholar]
- 17.Allaire J, Maltais F, Doyon JF, Noël M, LeBlanc P, Carrier G, et al. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax. 2004;59:673–678. doi: 10.1136/thx.2003.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nyberg A, Saey D, Maltais F. Why and how limb muscle mass and function should be measured in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2015;12:1269–1277. doi: 10.1513/AnnalsATS.201505-278PS. [DOI] [PubMed] [Google Scholar]
- 19.Abdulai RM, Jensen TJ, Patel NR, Polkey MI, Jansson P, Celli BR, et al. Deterioration of limb muscle function during acute exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197:433–449. doi: 10.1164/rccm.201703-0615CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91:1447–1531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 21.Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieck GC, Prakash YS. Cross-bridge kinetics in respiratory muscles. Eur Respir J. 1997;10:2147–2158. doi: 10.1183/09031936.97.10092147. [DOI] [PubMed] [Google Scholar]
- 23.Ciciliot S, Rossi AC, Dyar KA, Blaauw B, Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Schiaffino S, Ausoni S, Gorza L, Saggin L, Gundersen K, Lomo T. Myosin heavy chain isoforms and velocity of shortening of type 2 skeletal muscle fibres. Acta Physiol Scand. 1988;134:575–576. doi: 10.1111/j.1748-1716.1998.tb08539.x. [DOI] [PubMed] [Google Scholar]
- 25.Klitgaard H, Bergman O, Betto R, Salviati G, Schiaffino S, Clausen T, et al. Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflugers Arch. 1990;416:470–472. doi: 10.1007/BF00370757. [DOI] [PubMed] [Google Scholar]
- 26.Maltais F, LeBlanc P, Whittom F, Simard C, Marquis K, Bélanger M, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax. 2000;55:848–853. doi: 10.1136/thorax.55.10.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr. 2008;27:793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Evans WJ. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50:5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 30.Fabbri LM, Luppi F, Beghé B, Rabe KF. Complex chronic comorbidities of COPD. Eur Respir J. 2008;31:204–212. doi: 10.1183/09031936.00114307. [DOI] [PubMed] [Google Scholar]
- 31.Hopkinson NS, Tennant RC, Dayer MJ, Swallow EB, Hansel TT, Moxham J, et al. A prospective study of decline in fat free mass and skeletal muscle strength in chronic obstructive pulmonary disease. Respir Res. 2007;8:25. doi: 10.1186/1465-9921-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Borst B, Koster A, Yu B, Gosker HR, Meibohm B, Bauer DC, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66:961–969. doi: 10.1136/thoraxjnl-2011-200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337:1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- 34.Debigaré R, Côté CH, Maltais F. Ubiquitination and proteolysis in limb and respiratory muscles of patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2010;7:84–90. doi: 10.1513/pats.200906-051JS. [DOI] [PubMed] [Google Scholar]
- 35.Caron MA, Debigaré R, Dekhuijzen PN, Maltais F. Comparative assessment of the quadriceps and the diaphragm in patients with COPD. J Appl Physiol (1985) 2009;107:952–961. doi: 10.1152/japplphysiol.00194.2009. [DOI] [PubMed] [Google Scholar]
- 36.Sanders KJ, Kneppers AE, van de Bool C, Langen RC, Schols AM. Cachexia in chronic obstructive pulmonary disease: new insights and therapeutic perspective. J Cachexia Sarcopenia Muscle. 2016;7:5–22. doi: 10.1002/jcsm.12062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013;280:4294–4314. doi: 10.1111/febs.12253. [DOI] [PubMed] [Google Scholar]
- 38.Polkey MI, Rabe KF. Chicken or egg: physical activity in COPD revisited. Eur Respir J. 2009;33:227–229. doi: 10.1183/09031936.00176808. [DOI] [PubMed] [Google Scholar]
- 39.Sin DD, Reid WD. Is inflammation good, bad or irrelevant for skeletal muscles in COPD? Thorax. 2008;63:95–96. doi: 10.1136/thx.2007.088575. [DOI] [PubMed] [Google Scholar]
- 40.Debigaré R, Marquis K, Côté CH, Tremblay RR, Michaud A, LeBlanc P, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003;124:83–89. doi: 10.1378/chest.124.1.83. [DOI] [PubMed] [Google Scholar]
- 41.Crul T, Spruit MA, Gayan-Ramirez G, Quarck R, Gosselink R, Troosters T, et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest. 2007;37:897–904. doi: 10.1111/j.1365-2362.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 42.Barreiro E, Schols AM, Polkey MI, Galdiz JB, Gosker HR, Swallow EB, et al. ENIGMA in COPD project. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax. 2008;63:100–107. doi: 10.1136/thx.2007.078030. [DOI] [PubMed] [Google Scholar]
- 43.Larsson L, Ansved T. Effects of long-term physical training and detraining on enzyme histochemical and functional skeletal muscle characteristic in man. Muscle Nerve. 1985;8:714–722. doi: 10.1002/mus.880080815. [DOI] [PubMed] [Google Scholar]
- 44.Brønstad E, Rognmo O, Tjonna AE, Dedichen HH, Kirkeby-Garstad I, Håberg AK, et al. High-intensity knee extensor training restores skeletal muscle function in COPD patients. Eur Respir J. 2012;40:1130–1136. doi: 10.1183/09031936.00193411. [DOI] [PubMed] [Google Scholar]
- 45.Bossenbroek L, de Greef MH, Wempe JB, Krijnen WP, Ten Hacken NH. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8:306–319. doi: 10.3109/15412555.2011.578601. [DOI] [PubMed] [Google Scholar]
- 46.Couillard A, Prefaut C. From muscle disuse to myopathy in COPD: potential contribution of oxidative stress. Eur Respir J. 2005;26:703–719. doi: 10.1183/09031936.05.00139904. [DOI] [PubMed] [Google Scholar]
- 47.Barreiro E, Sznajder JI, Nader GA, Budinger GR. Muscle dysfunction in patients with lung diseases: a growing epidemic. Am J Respir Crit Care Med. 2015;191:616–619. doi: 10.1164/rccm.201412-2189OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jaitovich A, Angulo M, Lecuona E, Dada LA, Welch LC, Cheng Y, et al. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1) J Biol Chem. 2015;290:9183–9194. doi: 10.1074/jbc.M114.625715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Degens H, Gayan-Ramirez G, van Hees HW. Smoking-induced skeletal muscle dysfunction: from evidence to mechanisms. Am J Respir Crit Care Med. 2015;191:620–625. doi: 10.1164/rccm.201410-1830PP. [DOI] [PubMed] [Google Scholar]
- 50.Doucet M, Russell AP, Léger B, Debigaré R, Joanisse DR, Caron MA, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176:261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 51.Guo Y, Gosker HR, Schols AM, Kapchinsky S, Bourbeau J, Sandri M, et al. Autophagy in locomotor muscles of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;188:1313–1320. doi: 10.1164/rccm.201304-0732OC. [DOI] [PubMed] [Google Scholar]
- 52.Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. doi: 10.1152/physrev.00027.2001. [DOI] [PubMed] [Google Scholar]
- 53.Crul T, Testelmans D, Spruit MA, Troosters T, Gosselink R, Geeraerts I, et al. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell Physiol Biochem. 2010;25:491–500. doi: 10.1159/000303054. [DOI] [PubMed] [Google Scholar]
- 54.Plant PJ, Brooks D, Faughnan M, Bayley T, Bain J, Singer L, et al. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2010;42:461–471. doi: 10.1165/rcmb.2008-0382OC. [DOI] [PubMed] [Google Scholar]
- 55.Goll DE, Thompson VF, Taylor RG, Christiansen JA. Role of the calpain system in muscle growth. Biochimie. 1992;74:225–237. doi: 10.1016/0300-9084(92)90121-t. [DOI] [PubMed] [Google Scholar]
- 56.Puig-Vilanova E, Rodriguez DA, Lloreta J, Ausin P, Pascual-Guardia S, Broquetas J, et al. Oxidative stress, redox signaling pathways, and autophagy in cachectic muscles of male patients with advanced COPD and lung cancer. Free Radic Biol Med. 2015;79:91–108. doi: 10.1016/j.freeradbiomed.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Vogiatzis I, Simoes DC, Stratakos G, Kourepini E, Terzis G, Manta P, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36:301–310. doi: 10.1183/09031936.00112909. [DOI] [PubMed] [Google Scholar]
- 58.Vogiatzis I, Stratakos G, Simoes DC, Terzis G, Georgiadou O, Roussos C, et al. Effects of rehabilitative exercise on peripheral muscle TNFα, IL-6, IGF-I and MyoD expression in patients with COPD. Thorax. 2007;62:950–956. doi: 10.1136/thx.2006.069310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lewis MI, Fournier M, Storer TW, Bhasin S, Porszasz J, Ren SG, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol (1985) 2007;103:1299–1310. doi: 10.1152/japplphysiol.00150.2007. [DOI] [PubMed] [Google Scholar]
- 60.Doucet M, Dubé A, Joanisse DR, Debigaré R, Michaud A, Paré MÈ, et al. Atrophy and hypertrophy signalling of the quadriceps and diaphragm in COPD. Thorax. 2010;65:963–970. doi: 10.1136/thx.2009.133827. [DOI] [PubMed] [Google Scholar]
- 61.Kamischke A, Kemper DE, Castel MA, Lüthke M, Rolf C, Behre HM, et al. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998;11:41–45. doi: 10.1183/09031936.98.11010041. [DOI] [PubMed] [Google Scholar]
- 62.Ferguson DG, Franzini-Armstrong C. The Ca2+ ATPase content of slow and fast twitch fibers of guinea pig. Muscle Nerve. 1988;11:561–570. doi: 10.1002/mus.880110607. [DOI] [PubMed] [Google Scholar]
- 63.Martínez-Llorens J, Casadevall C, Lloreta J, Orozco-Levi M, Barreiro E, Broquetas J, et al. Activation of satellite cells in the intercostal muscles of patients with chronic obstructive pulmonary disease [in Spanish] Arch Bronconeumol. 2008;44:239–244. [PubMed] [Google Scholar]
- 64.Patel TJ, Cuizon D, Mathieu-Costello O, Fridén J, Lieber RL. Increased oxidative capacity does not protect skeletal muscle fibers from eccentric contraction-induced injury. Am J Physiol. 1998;274:R1300–R1308. doi: 10.1152/ajpregu.1998.274.5.R1300. [DOI] [PubMed] [Google Scholar]
- 65.Polkey MI, Griffiths MJ, Kemp PR. Muscle regeneration after critical illness: are satellite cells the answer? Am J Respir Crit Care Med. 2016;194:780–782. doi: 10.1164/rccm.201603-0633ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pomiès P, Rodriguez J, Blaquière M, Sedraoui S, Gouzi F, Carnac G, et al. Reduced myotube diameter, atrophic signalling and elevated oxidative stress in cultured satellite cells from COPD patients. J Cell Mol Med. 2015;19:175–186. doi: 10.1111/jcmm.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thériault ME, Paré ME, Maltais F, Debigaré R. Satellite cells senescence in limb muscle of severe patients with COPD. PLoS One. 2012;7:e39124. doi: 10.1371/journal.pone.0039124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ottenheijm CA, Heunks LM, Hafmans T, van der Ven PF, Benoist C, Zhou H, et al. Titin and diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:527–534. doi: 10.1164/rccm.200507-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barreiro E, de la Puente B, Minguella J, Corominas JM, Serrano S, Hussain SN, et al. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1116–1124. doi: 10.1164/rccm.200407-887OC. [DOI] [PubMed] [Google Scholar]
- 70.Ottenheijm CA, Heunks LM, Dekhuijzen PN. Diaphragm muscle fiber dysfunction in chronic obstructive pulmonary disease: toward a pathophysiological concept. Am J Respir Crit Care Med. 2007;175:1233–1240. doi: 10.1164/rccm.200701-020PP. [DOI] [PubMed] [Google Scholar]
- 71.Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325:917–923. doi: 10.1056/NEJM199109263251304. [DOI] [PubMed] [Google Scholar]
- 72.Rochester DF, Braun NM. Determinants of maximal inspiratory pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;132:42–47. doi: 10.1164/arrd.1985.132.1.42. [DOI] [PubMed] [Google Scholar]
- 73.Rochester DF, Braun NM, Arora NS. Respiratory muscle strength in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1979;119:151–154. doi: 10.1164/arrd.1979.119.2P2.151. [DOI] [PubMed] [Google Scholar]
- 74.Lando Y, Boiselle P, Shade D, Travaline JM, Furukawa S, Criner GJ. Effect of lung volume reduction surgery on bony thorax configuration in severe COPD. Chest. 1999;116:30–39. doi: 10.1378/chest.116.1.30. [DOI] [PubMed] [Google Scholar]
- 75.Lando Y, Boiselle PM, Shade D, Furukawa S, Kuzma AM, Travaline JM, et al. Effect of lung volume reduction surgery on diaphragm length in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159:796–805. doi: 10.1164/ajrccm.159.3.9804055. [DOI] [PubMed] [Google Scholar]
- 76.Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143:9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 77.Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153:288–293. doi: 10.1164/ajrccm.153.1.8542131. [DOI] [PubMed] [Google Scholar]
- 78.Saey D, Michaud A, Couillard A, Côté CH, Mador MJ, LeBlanc P, et al. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1109–1115. doi: 10.1164/rccm.200408-1005OC. [DOI] [PubMed] [Google Scholar]
- 79.Franssen FM, Broekhuizen R, Janssen PP, Wouters EF, Schols AM. Effects of whole-body exercise training on body composition and functional capacity in normal-weight patients with COPD. Chest. 2004;125:2021–2028. doi: 10.1378/chest.125.6.2021. [DOI] [PubMed] [Google Scholar]
- 80.Spruit MA, Gosselink R, Troosters T, De Paepe K, Decramer M. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J. 2002;19:1072–1078. doi: 10.1183/09031936.02.00287102. [DOI] [PubMed] [Google Scholar]
- 81.Spruit MA, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. ATS/ERS Task Force on Pulmonary Rehabilitation. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 82.Dajczman E, Wardini R, Kasymjanova G, Préfontaine D, Baltzan MA, Wolkove N. Six minute walk distance is a predictor of survival in patients with chronic obstructive pulmonary disease undergoing pulmonary rehabilitation. Can Respir J. 2015;22:225–229. doi: 10.1155/2015/280187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Burtin C, Ter Riet G, Puhan MA, Waschki B, Garcia-Aymerich J, Pinto-Plata V, et al. Handgrip weakness and mortality risk in COPD: a multicentre analysis. Thorax. 2016;71:86–87. doi: 10.1136/thoraxjnl-2015-207451. [DOI] [PubMed] [Google Scholar]
- 84.Rochester CL, Vogiatzis I, Holland AE, Lareau SC, Marciniuk DD, Puhan MA, et al. ATS/ERS Task Force on Policy in Pulmonary Rehabilitation. An Official American Thoracic Society/European Respiratory Society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med. 2015;192:1373–1386. doi: 10.1164/rccm.201510-1966ST. [DOI] [PubMed] [Google Scholar]
- 85.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;(10):CD005305. doi: 10.1002/14651858.CD005305.pub3. [DOI] [PubMed] [Google Scholar]
- 86.Puhan MA, Lareau SC. Evidence-based outcomes from pulmonary rehabilitation in the chronic obstructive pulmonary disease patient. Clin Chest Med. 2014;35:295–301. doi: 10.1016/j.ccm.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 87.Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154:1310–1317. doi: 10.1164/ajrccm.154.5.8912741. [DOI] [PubMed] [Google Scholar]
- 88.Levine S, Bashir MH, Clanton TL, Powers SK, Singhal S. COPD elicits remodeling of the diaphragm and vastus lateralis muscles in humans. J Appl Physiol (1985) 2013;114:1235–1245. doi: 10.1152/japplphysiol.01121.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vilaró J, Ramirez-Sarmiento A, Martínez-Llorens JM, Mendoza T, Alvarez M, Sánchez-Cayado N, et al. Global muscle dysfunction as a risk factor of readmission to hospital due to COPD exacerbations. Respir Med. 2010;104:1896–1902. doi: 10.1016/j.rmed.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 90.Antenora F, Fantini R, Iattoni A, Castaniere I, Sdanganelli A, Livrieri F, et al. Prevalence and outcomes of diaphragmatic dysfunction assessed by ultrasound technology during acute exacerbation of COPD: a pilot study. Respirology. 2017;22:338–344. doi: 10.1111/resp.12916. [DOI] [PubMed] [Google Scholar]
- 91.Brochard L, Isabey D, Piquet J, Amaro P, Mancebo J, Messadi AA, et al. Reversal of acute exacerbations of chronic obstructive lung disease by inspiratory assistance with a face mask. N Engl J Med. 1990;323:1523–1530. doi: 10.1056/NEJM199011293232204. [DOI] [PubMed] [Google Scholar]
- 92.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 93.Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 1998;30:1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Vogiatzis I, Terzis G, Stratakos G, Cherouveim E, Athanasopoulos D, Spetsioti S, et al. Effect of pulmonary rehabilitation on peripheral muscle fiber remodeling in patients with COPD in GOLD stages II to IV. Chest. 2011;140:744–752. doi: 10.1378/chest.10-3058. [DOI] [PubMed] [Google Scholar]
- 95.Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax. 2007;62:944–949. doi: 10.1136/thx.2007.078980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Natanek SA, Gosker HR, Slot IG, Marsh GS, Hopkinson NS, Man WD, et al. Heterogeneity of quadriceps muscle phenotype in chronic obstructive pulmonary disease (COPD); implications for stratified medicine? Muscle Nerve. 2013;48:488–497. doi: 10.1002/mus.23784. [DOI] [PubMed] [Google Scholar]
- 97.Patel MS, Mohan D, Andersson YM, Baz M, Samantha Kon SC, Canavan JL, et al. Phenotypic characteristics associated with reduced short physical performance battery score in COPD. Chest. 2014;145:1016–1024. doi: 10.1378/chest.13-1398. [DOI] [PubMed] [Google Scholar]
- 98.Patel MS, Natanek SA, Stratakos G, Pascual S, Martínez-Llorens J, Disano L, et al. Vastus lateralis fiber shift is an independent predictor of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:350–352. doi: 10.1164/rccm.201404-0713LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vogiatzis I, Terzis G, Nanas S, Stratakos G, Simoes DC, Georgiadou O, et al. Skeletal muscle adaptations to interval training in patients with advanced COPD. Chest. 2005;128:3838–3845. doi: 10.1378/chest.128.6.3838. [DOI] [PubMed] [Google Scholar]
- 100.Levine S, Nguyen T, Kaiser LR, Rubinstein NA, Maislin G, Gregory C, et al. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med. 2003;168:706–713. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- 101.Wijnhoven JH, Janssen AJ, van Kuppevelt TH, Rodenburg RJ, Dekhuijzen PN. Metabolic capacity of the diaphragm in patients with COPD. Respir Med. 2006;100:1064–1071. doi: 10.1016/j.rmed.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 102.Sánchez J, Bastien C, Medrano G, Riquet M, Derenne JP. Metabolic enzymatic activities in the diaphragm of normal men and patients with moderate chronic obstructive pulmonary disease. Bull Eur Physiopathol Respir. 1984;20:535–540. [PubMed] [Google Scholar]
- 103.Geiger PC, Cody MJ, Macken RL, Sieck GC. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol (1985) 2000;89:695–703. doi: 10.1152/jappl.2000.89.2.695. [DOI] [PubMed] [Google Scholar]
- 104.Ramirez-Sarmiento A, Orozco-Levi M, Guell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166:1491–1497. doi: 10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 105.Tanimura K, Sato S, Fuseya Y, Hasegawa K, Uemasu K, Sato A, et al. Quantitative assessment of erector spinae muscles in patients with chronic obstructive pulmonary disease: novel chest computed tomography–derived index for prognosis. Ann Am Thorac Soc. 2016;13:334–341. doi: 10.1513/AnnalsATS.201507-446OC. [DOI] [PubMed] [Google Scholar]
- 106.Spruit MA, Gosselink R, Troosters T, Kasran A, Gayan-Ramirez G, Bogaerts P, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58:752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Physical activity and hospitalization for exacerbation of COPD. Chest. 2006;129:536–544. doi: 10.1378/chest.129.3.536. [DOI] [PubMed] [Google Scholar]
- 108.Donaldson GC, Wilkinson TM, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:446–452. doi: 10.1164/rccm.200408-1054OC. [DOI] [PubMed] [Google Scholar]
- 109.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanabe N, Muro S, Hirai T, Oguma T, Terada K, Marumo S, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1653–1659. doi: 10.1164/rccm.201009-1535OC. [DOI] [PubMed] [Google Scholar]
- 111.Greening NJ, Harvey-Dunstan TC, Chaplin EJ, Vincent EE, Morgan MD, Singh SJ, et al. Bedside assessment of quadriceps muscle by ultrasound after admission for acute exacerbations of chronic respiratory disease. Am J Respir Crit Care Med. 2015;192:810–816. doi: 10.1164/rccm.201503-0535OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Troosters T, Probst VS, Crul T, Pitta F, Gayan-Ramirez G, Decramer M, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:1072–1077. doi: 10.1164/rccm.200908-1203OC. [DOI] [PubMed] [Google Scholar]
- 113.Arora NS, Rochester DF. COPD and human diaphragm muscle dimensions. Chest. 1987;91:719–724. doi: 10.1378/chest.91.5.719. [DOI] [PubMed] [Google Scholar]
- 114.Baria MR, Shahgholi L, Sorenson EJ, Harper CJ, Lim KG, Strommen JA, et al. B-mode ultrasound assessment of diaphragm structure and function in patients with COPD. Chest. 2014;146:680–685. doi: 10.1378/chest.13-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Güerri R, Gayete A, Balcells E, Ramirez-Sarmiento A, Vollmer I, Garcia-Aymerich J, et al. Mass of intercostal muscles associates with risk of multiple exacerbations in COPD. Respir Med. 2010;104:378–388. doi: 10.1016/j.rmed.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 116.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:1005–1012. doi: 10.1056/NEJMoa021322. [DOI] [PubMed] [Google Scholar]
- 117.Schols AM, Broekhuizen R, Weling-Scheepers CA, Wouters EF. Body composition and mortality in chronic obstructive pulmonary disease. Am J Clin Nutr. 2005;82:53–59. doi: 10.1093/ajcn.82.1.53. [DOI] [PubMed] [Google Scholar]
- 118.Hallin R, Gudmundsson G, Suppli Ulrik C, Nieminen MM, Gislason T, Lindberg E, et al. Nutritional status and long-term mortality in hospitalised patients with chronic obstructive pulmonary disease (COPD) Respir Med. 2007;101:1954–1960. doi: 10.1016/j.rmed.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 119.Galesanu RG, Bernard S, Marquis K, Lacasse Y, Poirier P, Bourbeau J, et al. Obesity in chronic obstructive pulmonary disease: is fatter really better? Can Respir J. 2014;21:297–301. doi: 10.1155/2014/181074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Stoll P, Foerster S, Virchow JC, Lommatzsch M. Overweight is a predictor of long-term survival in hospitalised patients with exacerbations of COPD. Respir Med. 2016;116:59–62. doi: 10.1016/j.rmed.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 121.Lambert AA, Putcha N, Drummond MB, Boriek AM, Hanania NA, Kim V, et al. COPDGene Investigators. Obesity is associated with increased morbidity in moderate to severe COPD. Chest. 2017;151:68–77. doi: 10.1016/j.chest.2016.08.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.He J, Watkins S, Kelley DE. Skeletal muscle lipid content and oxidative enzyme activity in relation to muscle fiber type in type 2 diabetes and obesity. Diabetes. 2001;50:817–823. doi: 10.2337/diabetes.50.4.817. [DOI] [PubMed] [Google Scholar]
- 123.Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007;131(5) Suppl:4S–42S. doi: 10.1378/chest.06-2418. [DOI] [PubMed] [Google Scholar]
- 124.Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from nonresponders. J Cardiopulm Rehabil. 2001;21:10–17. doi: 10.1097/00008483-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 125.Bouchard C, Sarzynski MA, Rice TK, Kraus WE, Church TS, Sung YJ, et al. Genomic predictors of the maximal O2 uptake response to standardized exercise training programs. J Appl Physiol (1985) 2011;110:1160–1170. doi: 10.1152/japplphysiol.00973.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dolmage TE, Goldstein RS. Effects of one-legged exercise training of patients with COPD. Chest. 2008;133:370–376. doi: 10.1378/chest.07-1423. [DOI] [PubMed] [Google Scholar]
- 127.Larson JL, Kim MJ, Sharp JT, Larson DA. Inspiratory muscle training with a pressure threshold breathing device in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1988;138:689–696. doi: 10.1164/ajrccm/138.3.689. [DOI] [PubMed] [Google Scholar]
- 128.Mota S, Güell R, Barreiro E, Solanes I, Ramírez-Sarmiento A, Orozco-Levi M, et al. Clinical outcomes of expiratory muscle training in severe COPD patients. Respir Med. 2007;101:516–524. doi: 10.1016/j.rmed.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 129.Spruit MA, Pennings HJ, Janssen PP, Does JD, Scroyen S, Akkermans MA, et al. Extra-pulmonary features in COPD patients entering rehabilitation after stratification for MRC dyspnea grade. Respir Med. 2007;101:2454–2463. doi: 10.1016/j.rmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:19–38. doi: 10.1164/rccm.200408-1109SO. [DOI] [PubMed] [Google Scholar]
- 131.Ries AL, Kaplan RM, Myers R, Prewitt LM. Maintenance after pulmonary rehabilitation in chronic lung disease: a randomized trial. Am J Respir Crit Care Med. 2003;167:880–888. doi: 10.1164/rccm.200204-318OC. [DOI] [PubMed] [Google Scholar]
- 132.Güell R, Casan P, Belda J, Sangenis M, Morante F, Guyatt GH, et al. Long-term effects of outpatient rehabilitation of COPD: A randomized trial. Chest. 2000;117:976–983. doi: 10.1378/chest.117.4.976. [DOI] [PubMed] [Google Scholar]
- 133.Güell MR, Cejudo P, Ortega F, Puy MC, Rodríguez-Trigo G, Pijoan JI, et al. Benefits of long-term pulmonary rehabilitation maintenance program in patients with severe chronic obstructive pulmonary disease: three-year follow-up. Am J Respir Crit Care Med. 2017;195:622–629. doi: 10.1164/rccm.201603-0602OC. [DOI] [PubMed] [Google Scholar]
- 134.Schols AM. Nutritional advances in patients with respiratory diseases. Eur Respir Rev. 2015;24:17–22. doi: 10.1183/09059180.00010914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Behrens G, Matthews CE, Moore SC, Hollenbeck AR, Leitzmann MF. Body size and physical activity in relation to incidence of chronic obstructive pulmonary disease. CMAJ. 2014;186:E457–E469. doi: 10.1503/cmaj.140025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chung JH, Hwang HJ, Han CH, Son BS, Kim DH, Park MS. Association between sarcopenia and metabolic syndrome in chronic obstructive pulmonary disease: the Korea National Health and Nutrition Examination Survey (KNHANES) from 2008 to 2011. COPD. 2015;12:82–89. doi: 10.3109/15412555.2014.908835. [DOI] [PubMed] [Google Scholar]
- 137.Pison CM, Cano NJ, Chérion C, Caron F, Court-Fortune I, Antonini MT, et al. IRAD Investigators. Multimodal nutritional rehabilitation improves clinical outcomes of malnourished patients with chronic respiratory failure: a randomised controlled trial. Thorax. 2011;66:953–960. doi: 10.1136/thx.2010.154922. [DOI] [PubMed] [Google Scholar]
- 138.van Wetering CR, Hoogendoorn M, Broekhuizen R, Geraerts-Keeris GJ, De Munck DR, Rutten-van Mölken MP, et al. Efficacy and costs of nutritional rehabilitation in muscle-wasted patients with chronic obstructive pulmonary disease in a community-based setting: a prespecified subgroup analysis of the INTERCOM trial. J Am Med Dir Assoc. 2010;11:179–187. doi: 10.1016/j.jamda.2009.12.083. [DOI] [PubMed] [Google Scholar]
- 139.Creutzberg EC, Wouters EF, Mostert R, Pluymers RJ, Schols AM. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124:1733–1742. doi: 10.1378/chest.124.5.1733. [DOI] [PubMed] [Google Scholar]
- 140.Constantin D, Menon MK, Houchen-Wolloff L, Morgan MD, Singh SJ, Greenhaff P, et al. Skeletal muscle molecular responses to resistance training and dietary supplementation in COPD. Thorax. 2013;68:625–633. doi: 10.1136/thoraxjnl-2012-202764. [DOI] [PubMed] [Google Scholar]
- 141.Deacon SJ, Vincent EE, Greenhaff PL, Fox J, Steiner MC, Singh SJ, et al. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;178:233–239. doi: 10.1164/rccm.200710-1508OC. [DOI] [PubMed] [Google Scholar]
- 142.Steiner MC, Barton RL, Singh SJ, Morgan MD. Nutritional enhancement of exercise performance in chronic obstructive pulmonary disease: a randomised controlled trial. Thorax. 2003;58:745–751. doi: 10.1136/thorax.58.9.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;12:CD000998. doi: 10.1002/14651858.CD000998.pub3. [DOI] [PubMed] [Google Scholar]
- 144.Fonseca Wald ELA, van den Borst B, Gosker HR, Schols AMWJ. Dietary fibre and fatty acids in chronic obstructive pulmonary disease risk and progression: a systematic review. Respirology. 2014;19:176–184. doi: 10.1111/resp.12229. [DOI] [PubMed] [Google Scholar]
- 145.Engelen MP, Rutten EP, De Castro CL, Wouters EF, Schols AM, Deutz NE. Supplementation of soy protein with branched-chain amino acids alters protein metabolism in healthy elderly and even more in patients with chronic obstructive pulmonary disease. Am J Clin Nutr. 2007;85:431–439. doi: 10.1093/ajcn/85.2.431. [DOI] [PubMed] [Google Scholar]
- 146.Broekhuizen R, Wouters EF, Creutzberg EC, Weling-Scheepers CA, Schols AM. Polyunsaturated fatty acids improve exercise capacity in chronic obstructive pulmonary disease. Thorax. 2005;60:376–382. doi: 10.1136/thx.2004.030858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Laviolette L, Lands LC, Dauletbaev N, Saey D, Milot J, Provencher S, et al. Combined effect of dietary supplementation with pressurized whey and exercise training in chronic obstructive pulmonary disease: a randomized, controlled, double-blind pilot study. J Med Food. 2010;13:589–598. doi: 10.1089/jmf.2009.0142. [DOI] [PubMed] [Google Scholar]
- 148.van de Bool C, Rutten EPA, van Helvoort A, Franssen FME, Wouters EFM, Schols AMWJ. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle. 2017;8:748–758. doi: 10.1002/jcsm.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Neder JA, Sword D, Ward SA, Mackay E, Cochrane LM, Clark CJ. Home based neuromuscular electrical stimulation as a new rehabilitative strategy for severely disabled patients with chronic obstructive pulmonary disease (COPD) Thorax. 2002;57:333–337. doi: 10.1136/thorax.57.4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bourjeily-Habr G, Rochester CL, Palermo F, Snyder P, Mohsenin V. Randomised controlled trial of transcutaneous electrical muscle stimulation of the lower extremities in patients with chronic obstructive pulmonary disease. Thorax. 2002;57:1045–1049. doi: 10.1136/thorax.57.12.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sillen MJH, Speksnijder CM, Eterman RA, Janssen PP, Wagers SS, Wouters EFM, et al. Effects of neuromuscular electrical stimulation of muscles of ambulation in patients with chronic heart failure or COPD: a systematic review of the English-language literature. Chest. 2009;136:44–61. doi: 10.1378/chest.08-2481. [DOI] [PubMed] [Google Scholar]
- 152.Jones S, Man WD, Gao W, Higginson IJ, Wilcock A, Maddocks M. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease. Cochrane Database Syst Rev. 2016;10:CD009419. doi: 10.1002/14651858.CD009419.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Abdellaoui A, Préfaut C, Gouzi F, Couillard A, Coisy-Quivy M, Hugon G, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J. 2011;38:781–788. doi: 10.1183/09031936.00167110. [DOI] [PubMed] [Google Scholar]
- 154.Maddocks M, Nolan CM, Man WD, Polkey MI, Hart N, Gao W, et al. Neuromuscular electrical stimulation to improve exercise capacity in patients with severe COPD: a randomised double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:27–36. doi: 10.1016/S2213-2600(15)00503-2. [DOI] [PubMed] [Google Scholar]
- 155.Bustamante V, Casanova J, López de Santamaría E, Mas S, Sellarés J, Gea J, et al. Redox balance following magnetic stimulation training in the quadriceps of patients with severe COPD. Free Radic Res. 2008;42:939–948. doi: 10.1080/10715760802555569. [DOI] [PubMed] [Google Scholar]
- 156.Bustamante V, López de Santa María E, Gorostiza A, Jiménez U, Gáldiz JB. Muscle training with repetitive magnetic stimulation of the quadriceps in severe COPD patients. Respir Med. 2010;104:237–245. doi: 10.1016/j.rmed.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 157.Casaburi R, Bhasin S, Cosentino L, Porszasz J, Somfay A, Lewis MI, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170:870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 158.Yeh SS, DeGuzman B, Kramer T, Group MS M012 Study Group. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest. 2002;122:421–428. doi: 10.1378/chest.122.2.421. [DOI] [PubMed] [Google Scholar]
- 159.Dalton JT, Barnette KG, Bohl CE, Hancock ML, Rodriguez D, Dodson ST, et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J Cachexia Sarcopenia Muscle. 2011;2:153–161. doi: 10.1007/s13539-011-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weisberg J, Wanger J, Olson J, Streit B, Fogarty C, Martin T, et al. Megestrol acetate stimulates weight gain and ventilation in underweight COPD patients. Chest. 2002;121:1070–1078. doi: 10.1378/chest.121.4.1070. [DOI] [PubMed] [Google Scholar]
- 161.Travaline JM, Sudarshan S, Roy BG, Cordova F, Leyenson V, Criner GJ. Effect of N-acetylcysteine on human diaphragm strength and fatigability. Am J Respir Crit Care Med. 1997;156:1567–1571. doi: 10.1164/ajrccm.156.5.96-09133. [DOI] [PubMed] [Google Scholar]
- 162.Doorduin J, Sinderby CA, Beck J, Stegeman DF, van Hees HW, van der Hoeven JG, et al. The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med. 2012;185:90–95. doi: 10.1164/rccm.201107-1268OC. [DOI] [PubMed] [Google Scholar]
- 163.Nagaya N, Itoh T, Murakami S, Oya H, Uematsu M, Miyatake K, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128:1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]