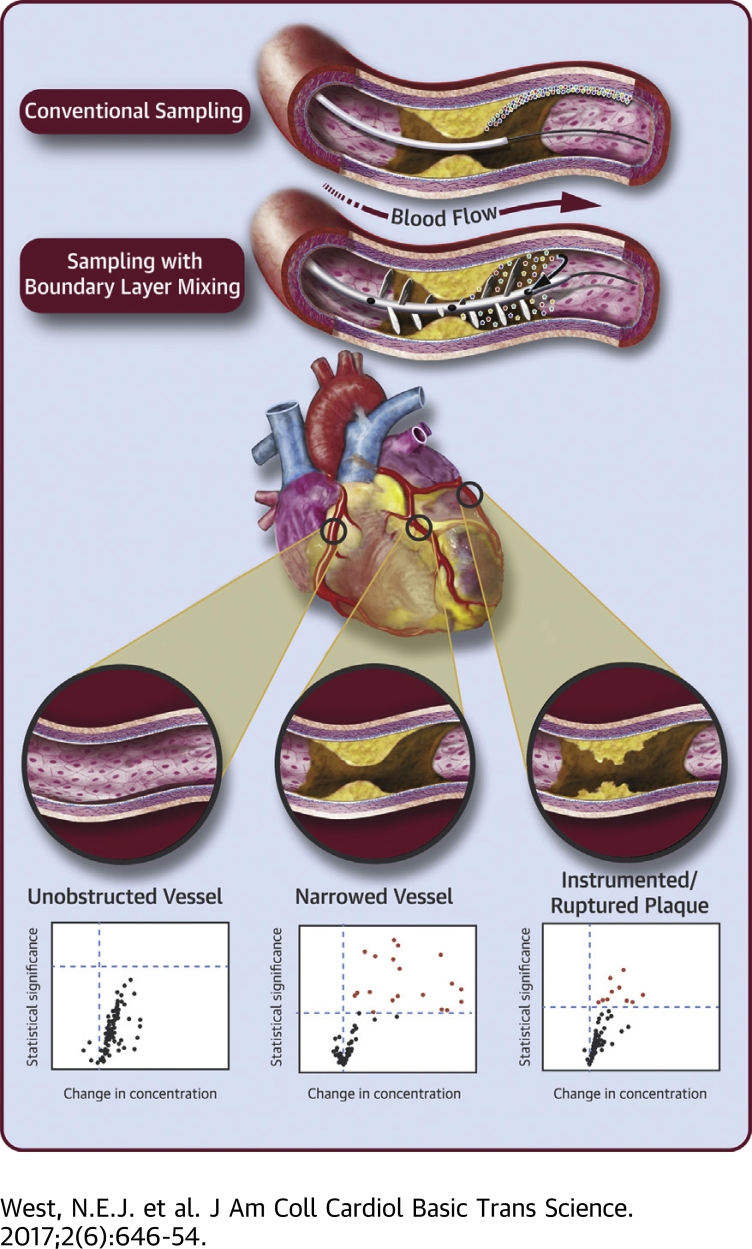
Visual Abstract
Key Words: biomolecule release, coronary atherosclerosis, inflammation, vulnerable plaque
Abbreviations and Acronyms: EC, endothelial cell; FDR, false discovery rate; EC, endothelial cell; MI, myocardial infarction; MMP, matrix metalloproteinase; SMC, smooth muscle cell
Highlights
-
•
Deployment of the Liquid Biopsy System into normal coronary arteries and its use to take 4 simultaneous blood samples at equal spaces along a 10-cm length was shown to be feasible and safe in patients undergoing angioplasty. No gradients of the 92 biomolecules chosen for multiplexed analysis were observed.
-
•
By contrast, stable and disrupted plaques showed biomolecule gradients along the sampling length, which implied that substances were being released from plaques into the boundary layer and flowing blood that was coursing over them. Gradients of molecules likely to be derived from the endothelium overlying plaques, especially the oxidized LDL receptor (OLR1; also known as LOX-1) and inflammatory mediators, were detected, consistent with the inflammatory status of endothelium previously defined by ex vivo and postmortem studies.
-
•
After angioplasty, some molecular gradients persisted, but release of additional substances was observed, perhaps most interestingly, matrix metalloproteinase-12, which had been localized previously to the core of vulnerable plaques and associated with subsequent unstable presentation.
-
•
These experiments establish the feasibility and potential of the Liquid Biopsy System. This new methodology is flexible enough to be used in conjunction with high-content analyses for the definition of prognostic biomarkers or biomarker combinations and for the development of surrogate endpoints in clinical intervention studies aimed at reducing plaque vulnerability and preventing acute coronary syndromes.
Summary
A percutaneous catheter device, the Liquid Biopsy System, was developed to sample the unstirred boundary layer of blood upstream and downstream of intact and disrupted human coronary atherosclerotic plaques. Using multiplexed proximity extension assays, release of 20 biomolecules was simultaneously detected in samples taken across plaques before balloon angioplasty, including the soluble form of the endothelial lectin-like oxidized LDL receptor. Additional biomolecules, including matrix metalloproteinase-12, were released after plaque disruption with angioplasty. These experiments demonstrate the power of the Liquid Biopsy System to yield new scientific insights and its ultimate potential to generate new biomarkers and surrogate endpoints for clinical trials.
Acute, life-threatening complications of coronary artery disease occur unpredictably, although risk factor modification, including the use of lipid-lowering statin drugs, reduces the frequency of myocardial infarction and prolongs life (1). Statins primarily promote plaque stability by altering biological processes including inflammatory activity of plaques (2), rather than through relatively modest reduction in plaque size (3). Percutaneous catheter-based coronary interventions performed to alleviate angina provide an opportunity to examine the local inflammatory status of plaques by using temperature sensors, ultrasound, light of various wavelengths, and cardiac magnetic resonance 4, 5. However, of these techniques, only intravascular ultrasonography imaging has yielded prognostic information 6, 7, albeit limited, and none of these methods is able to identify whether individual inflammatory mediators or other biomolecules involved in the disease process of atherosclerosis are being actively generated from plaques in vivo.
We hypothesized that sampling blood to detect gradients of such biomolecules released from obstructive coronary plaques could provide unique information of scientific and, ultimately, clinical utility. Previously, simple micro-catheters have been used to sample coronary artery blood for comparison with venous samples. However, the local gradients thus derived are subject to dilution with blood from other territories, and biomolecules are likely to suffer uptake or biochemical transformation during passage through the coronary microcirculation. Furthermore, as flow through the coronary arteries is predominantly laminar and much more rapid than radial diffusion, biomolecules entering the circulation from the artery wall would most probably become entrained in a near-wall, unstirred boundary layer, inaccessible to conventional catheters placed directly into the coronary arteries. The Liquid Biopsy System (LBS) (PlaqueTec, Ltd., Cambridge, United Kingdom) (Figure 1A) was therefore designed specifically to overcome boundary layer effects, thereby allowing more effective blood sampling to identify local gradients of biomolecules in the proximal segment of a target coronary artery, the critical region for culprit plaque formation responsible for most myocardial infarctions (8).
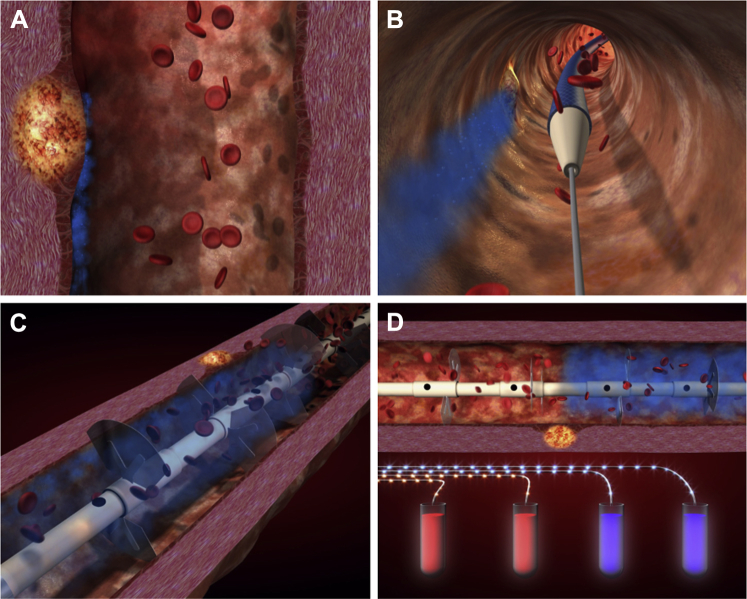
Figure 1.
Boundary Layer Phenomenon and Concept of the Liquid Biopsy System
(A) Atherosclerotic plaque-associated biomolecules remained trapped in a discrete flow layer adjacent to the vessel wall (blue zone). (B) The LBS, designed specifically to mix and sample from this boundary layer, is passed over a standard intracoronary guidewire used in all coronary angioplasty and stent procedures. (C) Once positioned, the LBS sheath is retracted, deploying dedicated mixing structures that disrupt the boundary later and divert entrained biomolecules into the bulk flow from where they may be simultaneously sampled directly by vacuum suction through 1 of 4 discrete ports equally spaced along the catheter. (D) Differential concentrations of such biomolecules may then be measured by multiplex assays to determine transplaque, intracoronary gradients. LBS = Liquid Biopsy System.
Following simulations in silico and in vitro and safety testing in a preclinical porcine model, we now report results from the first human safety study and the first human proof of clinical concept evaluation, which were designed to investigate whether the LBS can detect biomolecular gradients in diseased coronary arteries of symptomatic patients undergoing coronary angioplasty and stent deployment.
Methods
Clinical study design
Patients from the LBS (PlaqueTec) first-in-human study (n = 10; Medicines and Healthcare products Regulatory Agency [MHRA] ref. CI/2012/0031) and from the first human proof-of-concept study (n = 28; NCT02119767) were included in this analysis. Both of the study protocols were approved by local ethics committees (National Research Ethics Service [NRES] East of England, Cambridge Central 12/EE/0004 and NRES London, Chelsea 13/LO/0954, respectively) and the institutional review board at Papworth Hospital, Cambridge, United Kingdom.
Briefly, the PlaqueTec first-in-human study (MHRA ref. CI/2012/0031) was designed as a safety and feasibility study aimed at acquiring Conformité Européene (CE) mark certification. Patients with stable angina pectoris or with stabilized non–ST-segment elevation acute coronary syndromes undergoing percutaneous coronary intervention were included subject to informed written consent and appropriate coronary anatomy for the LBS to be deployed in a nonculprit, unobstructed coronary artery (i.e., not the artery receiving balloon angioplasty and stent insertion). From this study, 10 patients underwent sampling from a lesion-free, unobstructed artery.
The human proof-of-concept study (NCT02119767) used similar inclusion criteria (n = 23 stable angina pectoris), but in this study, the LBS was positioned within the target artery for planned intervention, and sampling was performed before (when lesion severity and geometry allowed) and after balloon dilation of the plaque. From this study, 1 patient’s artery was sampled only before balloon dilation of a target, obstructive, coronary plaque, 11 were sampled before and after balloon dilation, and 15 were sampled only after balloon dilation.
All patients received standard-of-care treatment including dual antiplatelet therapy and periprocedural anticoagulation with heparin; and activated clotting time was maintained at >250 s during LBS sampling. The safety endpoints assessed included incidence of periprocedural myocardial infarction and adverse clinical events at 30-day follow-up, as reviewed by an independent data and safety monitoring committee. There were no major adverse events attributable to LBS sampling in either study.
Operation of the LBS
The LBS catheter consists of discrete lumina exiting at ports equally spaced along the sampling length, which can be advanced and positioned across a target plaque. It has 3 arrays of mixing structures stowed within a retractable sheath (Figure 1B) that can accommodate various artery geometries and are designed to divert the central, faster moving blood flow to break up the boundary layer (Figure 1C); the resulting mixed samples are aspirated into removable sample tubes in the handle of the device (Figure 1D).
The LBS was used as an adjunct to percutaneous coronary intervention procedures in accordance with the instructions for use. Briefly, after passage of a guidewire into the target coronary artery and appropriate administration of anticoagulant and vasodilators (glyceryl trinitrate, 100 to 200 μg), the LBS was negotiated to the area of interest under radiographic guidance. After the device was unsheathed, the dedicated mixing structures were deployed to disrupt the unstirred boundary layer. Samples of mixed blood were then drawn into sample tubes in the LBS handle assembly from the catheter sampling ports through discrete lumina by manual actuation of a lockable vacuum syringe. Typically, sampling was undertaken for 3 mins in the coronary circulation (delivering approximately 1.5 ml/sample tube) before resheathing the LBS and withdrawing it from the patient. Ten patients underwent sampling from a lesion-free unobstructed artery; 12 were sampled before balloon dilation of a target, obstructive, coronary plaque; and 27 were sampled after balloon dilation. From these 49 procedures, 196 samples were collected from a proximal reference and 3 downstream sampling ports. When transplaque sampling was performed, the distance from the minimum lesion diameter to each sampling port was determined by quantitative coronary angiography (CAAS workstation version 5.10.2; Pie Medical Imaging BV, Maastricht, the Netherlands).
Proteomic analysis
Samples were mixed with EDTA to achieve a concentration of 1.75 mg/ml. Plasma was separated by centrifugation, anonymized, and stored at −80°C. Samples failing to separate due to contamination with saline or radiographic contrast medium (5 of 196 samples [2.6%]) were discarded. Plasma samples were assessed for levels of 92 proteins by proximity extension assay (Olink Bioscience, Uppsala, Sweden). Detectable analytes were defined as those for which >75% of samples gave values above the limit of detection plus 0.5 normalized protein expression (NPX) unit. Measurements of the 10 analytes that failed to meet this criterion and spurious data points (>200% of the within-patient baseline; 3 from 17,952 samples [0.0002%]) were rejected from statistical analysis. For each analyte, data were log transformed to achieve a normal distribution, and a mixed effects linear regression predicting the logarithmic concentration as a function of distance from the lesion minimum diameter was fitted, adjusting for the number of mixing arrays deployed, with the patient as a random effect (9). In unobstructed vessels, distance from the proximal port was used instead. The estimate of the effect of relative distance on log-concentration was used to determine the percentage change for each biomarker over 6 cm of the studied artery length.
Statistical analysis
For each analyte, a paired Student's t-test of log-value concentration was performed, and gradients of log concentration were assessed by linear mixed effects regression with the patient as a random effect; p values obtained were corrected for multiple comparisons by using the Benjamini-Hochberg false discovery rate procedure (10). Estimates of change in concentration within the artery were back-converted to linear NPX as a percentage. All analyses were performed using R version 3.2.3 software (R Foundation for Statistical Computing, Vienna, Austria) and were independently validated by Numerus, Ltd. (Wockingham, United Kingdom), using SAS version 9.4 software (SAS, Cary, North Carolina).
Results
The LBS was used to sample blood, including blood from the near-wall, unstirred boundary layers in normal and diseased coronary arteries, as shown schematically in Figure 2A. In unobstructed vessels with no lesion, no significant positive gradients were found between the proximal and distal ports of the device (Figure 2B). Before balloon dilation of obstructive plaques, significant positive gradients were found for 20 analytes (Figure 2C). After balloon dilation and consequent plaque disruption, significant positive gradients were found for 10 analytes (false discovery rate p value (p[FDR]) of <0.05) (Figure 2D). No significant negative gradients were observed. The magnitudes in NPX units and significance, p(FDR) <0.05, of 14 of the gradients observed before balloon dilation are detailed in Table 1 (top). Because the relationship between NPX and concentration is specific for each analyte, small changes in NPX can represent large-percentage gradients between proximal and distal sampling ports. For this reason, and to give a measurement that is easier to compare, we calculated median-percentage differences in concentration across the sampling length of the LBS (Table 1 top, Figure 2C). For selected analytes, we also show the actual values proximal to, at the midpoint, and distal to the lesions (Figure 3, upper row). Details of 9 of the gradients observed after angioplasty are provided in Table 1 (bottom), with data for the same selected analytes expanded in Figure 3 (lower row). Data demonstrate unequivocally the ability of the LBS to detect biomolecular gradients across coronary plaques before and after balloon angioplasty.
Figure 2.
Expression of Plaque-Associated Biomolecules in Unobstructed Coronary Arteries, Across Obstructive/Narrowed Lesions and After Angioplasty of Coronary Atherosclerotic Plaques
(A) A summary is shown of biomarker gradients measured in blood sampled across the proximal to distal ports of the LBS system. (B) In 1 study, samples were taken from unobstructed vessels (green vessel). (C) In a separate study, samples were taken from narrowed or obstructive atherosclerotic plaques, where possible, before balloon dilation (yellow vessel) and in the same and other vessels (D) (red vessel) after balloon dilation. Data are volcano plots, with each identified biomolecular gradient represented by a single point, with effect size (median percentage of change of biomolecule concentration over a distance of 6 cm [PP-DP] within coronary artery) plotted on the x-axis and statistical significance (−log10[p value]) on the y-axis. The dashed horizontal line on each graph represents corrected threshold for significance of each gradient (p[FDR] <0.05); the vertical dashed line represents zero gradient. p(FDR) = false discovery rate p value; NPX = normalized protein expression.
Table 1.
Gradients Detected Across Atherosclerotic Plaques
| HUGO Designation | Molecule | Δ Concentration (NPX) | p(FDR) | Δ(%)/Sampling Length |
|---|---|---|---|---|
| Before balloon dilation | ||||
| OLR1 | Oxidized LDL receptor 1 (LOX-1) | 20.01 (−5.2 to 137.1) | <0.05 | 53.2 |
| EGF | Epidermal growth factor | 23.9 (−19.3 to 62.0) | <0.005 | 47.8 |
| IL-16 | Interleukin-16 | 2.8 (−3.2 to 30.0) | <0.05 | 39.0 |
| CD40LG | sCD40 ligand | 1.6 (−1.3 to 5.6) | <0.005 | 27.6 |
| CXCL1 | C-X-C motif chemokine 1 (Gro-a, GRO1, NAP-3, KC) | 12.3 (1.3 to 60.1) | <0.005 | 26.7 |
| TNFSF14 | Tumor necrosis factor ligand superfamily member 14 | 0.4 (−0.3 to 4.7) | <0.05 | 26.5 |
| CXCL5/6 | C-X-C motif chemokine 6 (GCP2) | 6.1 (0.6 to 23.1) | <0.005 | 24.7 |
| PDGFB | Platelet-derived growth factor subunit B | 12.9 (−4.0 to 41.6) | <0.005 | 24.5 |
| IL-8 | Interleukin-8 | 3.3 (−1.3 to 51.4) | <0.05 | 24.1 |
| IL1RN | Interleukin-1 receptor antagonist protein | 1.8 (0.2 to 7.0) | <0.005 | 15.5 |
| RETN | Resistin | 10.6 (−3.9 to 16.5) | <0.05 | 12.9 |
| DKK1 | Dickkopf-related protein 1 | 2.9 (0.2 to 12.0) | <0.005 | 10.5 |
| PLAUR | Urokinase plasminogen activator surface receptor | 20.9 (−7.0 to 106.1) | <0.05 | 6.9 |
| CCL4 | C-C motif chemokine-4 (MIP-1β) | 4.5 (−1.3 to 10.9) | <0.05 | 6.4 |
| HBEGF | Heparin-binding EGF-like growth factor | 2.6 (−1.2 to 0.1) | <0.05 | 5.4 |
| After balloon dilation | ||||
| HSPB1 | Heat shock 27-kDa protein | 1.1 (−4.5 to 54.2) | <0.05 | 26.11 |
| EGF | Epidermal growth factor | 10.1 (−35.2 to 67.4) | <0.05 | 21.05 |
| PDGFB | Platelet-derived growth factor subunit B | 8.6 (−35.6 to 81.0) | <0.05 | 18.26 |
| CXCL5/6 | C-X-C motif chemokine 6 (GCP2) | 3.2 (−7.3 to 39.2) | <0.05 | 16.59 |
| MMP-12 | Matrix metalloproteinase-12 | 8.7 (−20.3 to 153.2) | <0.05 | 14.48 |
| IL-8 | Interleukin-8 | 1.8 (−6.1 to 14.6) | <0.05 | 10.19 |
| DKK1 | Dickkopf-related protein 1 | 2.8 (−9.4 to 11.2) | <0.05 | 8.60 |
| CCL2 | Monocyte chemotactic protein 1 (MCP-1) | 14.9 (−88.5 to 119.9) | <0.05 | 6.39 |
| HBEGF | Heparin-binding EGF-like growth factor | 2.5 (−12.7 to 10.3) | <0.05 | 4.44 |
Blood sampling across coronary plaques was achieved using the Liquid Biopsy System before balloon dilation and after balloon dilation. Proximal and distal measurements of the biomolecules, identified by their HUGO designations, were used to calculate the change in concentration across plaques, which are shown as median values (range), measured by proximity extension assay multiplex assays and expressed in ΔNPX units. The significance of gradients is indicated by p(FDR) values. To aid comparison between different analytes, NPX values were further used to calculate the median percentage change in concentration across the sampling distance.
p(FDR) = false discovery rate p values; HUGO = Human Genome Organization; LDL = low-density lipoprotein; NPX = normalized protein expression.
Figure 3.
Example Box-and-Whisker Plots Illustrate Changes in Biomolecule Concentrations Across Coronary Atherosclerotic Plaques
Paired summary figures show concentration of biomolecules at proximal (prox), mid, and distal sampling ports of LBS. The upper row shows samples obtained before and the bottom row balloon pre-dilation of plaque. False discovery rate p values are noted at top right of each graph; p(FDR) <0.05 indicates significant changes in biomolecular gradients. Sampled biomolecules labeled by Human Genome Organization designation show significant gradients in oxidized low-density lipoprotein receptor-1/LOX-1 (OLR1) before but not after balloon dilation; in matrix metalloproteinase (MMP)-12 after but not before dilation; in interleukin (IL)-8; and in platelet-derived growth factor B (PDGFB) before and after balloon dilation; and no gradient in growth hormone (GH), acting as a negative control. Box and whisker plots show median, interquartile range, and range, with outliers designated as points. Abbreviations as in Figures 1 and 2.
Discussion
Local release of biomolecules from plaques has been postulated, but measurements of the resulting local gradients of concentration have not been achieved previously. Of the biomolecules showing significant gradients before balloon angioplasty (Table 1 top), 5 are known to be components of blood platelet α granules (11), namely, IL-8, DKK1, CXCL1, CD40LG, and PDGFB. Even though we studied mainly stable angina patients (23 of 28), it is therefore conceivable that these metabolites are derived from adhered platelets. However, IL-8 is produced by activated monocytes, macrophages, and endothelial cells (ECs) (12); DKK1 mRNA is found within atherosclerotic plaques, in ECs, and in leukocytes (13); CXCL1 is secreted by macrophages (14); and CD40LG is prevalent in lymphocytes as well as in other plaque cells (15). Hence, nonplatelet sources of these metabolites are probable. DKK1, CXCL1, CD40LG, and PDGFB can all cause activation of ECs 13, 14, 15, which are likely sources of OLR1, IL-8, PLAUR, CXCL5/6, HBEGF, and PDGFB. In particular, ECs are overwhelmingly the most abundant source of OLR1 in the vasculature (16), and thus the concentration gradient of OLR1 most likely arises from ECs overlying culprit coronary plaques before balloon dilation. Furthermore, production of CXCL5/6 is approximately 100-fold upregulated in ECs treated with the inflammatory mediator tumor necrosis factor-α (TNF-α) (17), and disturbed flow increases the release of HBEGF from ECs (18). CXCL5/6 and HBEGF could also be released from underlying smooth muscle cells (SMCs). All remaining mediators are known to be secreted by blood leukocytes or mature macrophages, which are present in plaques but also potentially trapped within thrombi.
The 9 analytes released from plaques after balloon injury (Table 1 bottom) include 3 of the known platelet products released before pre-dilation of plaques (IL-8, DKK1, and PDGFB) plus CXCL5/6 and HBEGF, which could be derived from ECs or SMCs. Most interestingly, new gradients of CCL2, HSPB1, and MMP-12 were detected after balloon dilation. CCL2 is a secreted chemokine that is localized to macrophages and SMCs in plaques (19). On the other hand, HSPB1 is primarily an intracellular chaperonin with anti-inflammatory activity that is more highly expressed in SMCs of normal vessel walls than in plaques (20). Given its localization, the gradient of HSPB1 is most likely derived from cells injured during plaque disruption by angioplasty. Consistent with this, increased plasma levels of HSP27 have been reported in patients after acute coronary syndromes (20). Release of MMP-12 after angioplasty may be particularly significant because it is normally undetectable by histology in ECs or SMCs and is expressed only in plaque macrophages surrounding the necrotic core of rupture-prone plaques 21, 22. Moreover, genome-wide association studies suggested a causative role for MMP-12 in early strokes (23), and the abundance of MMP-12-expressing cells in carotid artery plaques predicts the subsequent occurrence of strokes and major adverse events (22). It is highly likely, therefore, that the post-angioplasty gradient of MMP-12 is derived from the exposure of the plaque core to the circulation, and the size of gradient may potentially possess diagnostic or prognostic information. In a similar fashion, a recent study demonstrated local release of MMP-9 into coronary blood from spontaneously disrupted plaques and suggested it as a biomarker that distinguishes ST-segment elevation myocardial infarction and non–ST-segment elevation myocardial infarction (24). Unfortunately, MMP-9 was not included in the proximity extension assay panel we used, which precludes a direct comparison. There are also several methodological differences that would have complicated a direct comparison with our new data. First, Nishiguchi et al. (24) compared coronary luminal blood downstream of the culprit site with that from a systemic arterial sample, whereas we sampled the unstirred layer up- and downstream of individual plaques. Second, Nishiguchi et al. (24) studied lesions giving rise to myocardial infarctions, which were mostly spontaneous plaque ruptures where thrombus was detected and would have been present for some time. Given that MMP-9 is abundant in monocytes/macrophages and neutrophils, a significant contribution by the thrombus to local release of MMP-9 cannot be ruled out. In accordance with this, when Nishiguchi et al. (24) studied stable angina patients before angioplasty, they found no MMP-9 release. We used mostly stable angina patients undergoing elective percutaneous intervention, which would have diluted our ability to detect gradients of MMP-9. Instead, we found release of MMP-12 immediately after iatrogenic plaque rupture as part of the angioplasty procedure. MMP-12 is not expressed at detectable levels in human peripheral blood mononuclear cells (25) and may therefore more selectively reflect the status of the plaque.
Conclusions
Our results demonstrate that gradients of bioactive molecules exist across intact and disrupted atherosclerotic plaques, and these may be sampled and assessed using this novel technology. From the data in Table 1, we have already shown that the LBS can yield novel biological insights. Furthermore, our results provide proof-of-concept for even more ambitious studies, for example, to test whether LBS sampling can provide a surrogate marker for activity of plaques that are vulnerable to rupture. In this context, OLR1 and CXCL5/6 seem particularly promising because they are likely to be endothelium-derived before angioplasty. In addition, levels of MMP-12 may be especially informative after balloon dilation for the reasons detailed above. Future studies should address whether gradients of released biomolecules have the potential to monitor the impact of existing and new therapeutics, and when fully validated and coupled to high-content analyses, LBS-detected gradients could be used as biomarkers to identify patients with high-risk plaques. The potential combination of LBS-derived biological information could be used to complement conventional anatomical imaging methods in order to augment and refine plaque/patient risk-stratification and prognostic power, enabling therapeutic changes that may improve patient outcomes.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: There is an unmet clinical need to detect coronary atherosclerotic plaques that are likely to rupture and therefore precipitate acute coronary syndromes. Such vulnerable plaques could be passivated by angioplasty, or the patients could be treated aggressively, for example, by adding newly developed PCSK9 inhibitors to commonly applied statin therapy. Catheter-based imaging and biophysical methods have been able to detect features of vulnerable plaques, including large lipid cores and thin fibrous caps, prevalence of inflammatory cells, and raised temperatures. However, up until now, the ability to define a biochemical signature for vulnerable plaques has been limited to ex vivo and post-mortem methods or to detecting single molecule gradients between coronary and peripheral blood. The Liquid Biopsy System was developed to detect the release of biomolecules across plaques in live patients. It was shown previously by fluid mechanics to effectively mix the barrier layer and bulk flow and therefore harbor the ability to sample blood close to the vessel wall where released biomolecules are likely to be most concentrated. The ability to detect small gradients of released molecules was optimized by collecting blood at the same time upstream and downstream of individual plaques. Furthermore, coupling this optimal sampling to highly multiplexed analysis increased the power of the approach to generate scientifically and clinically valuable information.
TRANSLATIONAL OUTLOOK: The present feasibility studies used 2 relatively small patient cohorts in whom blood was sampled separately from normal coronary arteries or intact plaques before and after disruption by angioplasty. In the future, the Liquid Biopsy System could be used to sample across multiple angiographically significant plaques in the same patient to distinguish vulnerable from stable plaques. Similarly, the panel of biomolecules currently used was simply a small sample from the thousands of disease-related biomolecules that have been identified and for which proximity extension assays could be developed. There is still much to be done to identify the optimal biochemical signature to define a vulnerable plaque. Single biomarkers or combinations will hopefully be defined by future explorations using the device. The sampling technique could also be coupled to high-content “omic” analyses of, for example, proteins or microRNAs. Longitudinal studies could be conducted to investigate the ability of drug interventions to normalize the release of biomolecules, the goal being to define reliable surrogate endpoints for clinical intervention studies.
Acknowledgments
The authors thank Li-Ming Gan and Carl Whatling, AstraZeneca, for selection and coordination of multiplexed assays for biomolecule analysis.
Footnotes
Supported by PlaqueTec, Ltd. Dr. Newby was supported by grant CH95001 from the British Heart Foundation. Drs. Corrigan, Owen, and Blatcher are employees of PlaqueTec, Ltd. Drs. West, Hoole, Brown, and Newby are consultants for PlaqueTec, Ltd.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Baigent C., Keech A., Kearney P.M. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 2.Tang T.Y., Howarth S.P., Miller S.R. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Libby P., Sasiela W. Plaque stabilization: Can we turn theory into evidence? Am J Cardiol. 2006;98:26P–33P. doi: 10.1016/j.amjcard.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Brown A.J., Costopoulos C., West N.E., Bennett M.R. Contemporary invasive imaging modalities that identify and risk-stratify coronary plaques at risk of rupture. Expert Rev Cardiovasc Ther. 2015;13:9–13. doi: 10.1586/14779072.2015.989836. [DOI] [PubMed] [Google Scholar]
- 5.Toutouzas K., Benetos G., Karanasos A., Chatzizisis Y.S., Giannopoulos A.A., Tousoulis D. Vulnerable plaque imaging: updates on new pathobiological mechanisms. Eur Heart J. 2015;36:3147–3154. doi: 10.1093/eurheartj/ehv508. [DOI] [PubMed] [Google Scholar]
- 6.Calvert P.A., Obaid D.R., O'Sullivan M. Association between IVUS findings and adverse outcomes in patients with coronary artery disease: the VIVA (VH-IVUS in Vulnerable Atherosclerosis) study. J Am Coll Cardiol. 2011;4:894–901. doi: 10.1016/j.jcmg.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Stone G.W., Maehara A., Lansky A.J. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 8.Cheruvu P.K., Finn A.V., Gardner C. Frequency and distribution of thin-cap fibroatheroma and ruptured plaques in human coronary arteries: a pathologic study. J Am Coll Cardiol. 2007;50:940–949. doi: 10.1016/j.jacc.2007.04.086. [DOI] [PubMed] [Google Scholar]
- 9.Bates D., Machler M., Bolker B.M., Walker S.C. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 10.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 11.Nurden A.T. Platelets, inflammation and tissue regeneration. Thromb Haemost. 2011;105(Suppl 1):S13–S33. doi: 10.1160/THS10-11-0720. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann E., Dittrich-Breiholz O., Holtmann H., Kracht M. Multiple control of interleukin-8 gene expression. J Leukoc Biol. 2002;72:847–855. [PubMed] [Google Scholar]
- 13.Ueland T., Otterdal K., Lekva T. Dickkopf-1 enhances inflammatory interaction between platelets and endothelial cells and shows increased expression in atherosclerosis. Arterioscler Thromb Vasc Biol. 2009;29:1228–1234. doi: 10.1161/ATVBAHA.109.189761. [DOI] [PubMed] [Google Scholar]
- 14.Amiri K.I., Richmond A. Fine tuning the transcriptional regulation of the CXCL1 chemokine. Prog Nucleic Acid Res Mol Biol. 2003;74:1–36. doi: 10.1016/s0079-6603(03)01009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smeets E., Meiler S., Lutgens E. Lymphocytic tumor necrosis factor receptor superfamily co-stimulatory molecules in the pathogenesis of atherosclerosis. Curr Opin Lipidol. 2013;24:518–524. doi: 10.1097/MOL.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 16.Sawamura T., Kakino A., Fujita Y. LOX-1: a multiligand receptor at the crossroads of response to danger signals. Curr Opin Lipidol. 2012;23:439–445. doi: 10.1097/MOL.0b013e32835688e4. [DOI] [PubMed] [Google Scholar]
- 17.Rajashekhar G., Grow M., Willuweit A., Patterson C.E., Clauss M. Divergent and convergent effects on gene expression and function in acute versus chronic endothelial activation. Physiol Genomics. 2007;31:104–113. doi: 10.1152/physiolgenomics.00157.2006. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H., Sunnarborg S.W., McNaughton K.K., Johns T.G., Lee D.C., Faber J.E. Heparin-binding epidermal growth factor-like growth factor signaling in flow-induced arterial remodeling. Circ Res. 2008;102:1275–1285. doi: 10.1161/CIRCRESAHA.108.171728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tedgui A., Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 20.Park H.K., Park E.C., Bae S.W. Expression of heat shock protein 27 in human atherosclerotic plaques and increased plasma level of heat shock protein 27 in patients with acute coronary syndrome. Circulation. 2006;114:886–893. doi: 10.1161/CIRCULATIONAHA.105.541219. [DOI] [PubMed] [Google Scholar]
- 21.Halpert I., Sires U.I., Roby J.D. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholtes V.P.W., Johnson J.L., Jenkins N. Carotid atherosclerotic plaque matrix metalloproteinase-12–positive macrophage subpopulation predicts adverse outcome after endarterectomy. J Am Heart Assoc. 2012;1:e001040. doi: 10.1161/JAHA.112.001040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traylor M., Makela K.M., Kilarski L.L. A novel MMP12 locus is associated with large artery atherosclerotic stroke using a genome-wide age-at-onset informed approach. PLoS Genet. 2014;10:e1004469. doi: 10.1371/journal.pgen.1004469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishiguchi T., Tanaka A., Taruya A. Local matrix metalloproteinase 9 level determines early clinical presentation of ST-segment-elevation myocardial infarction. Arterioscler Thromb Vasc Biol. 2016;36:2460–2467. doi: 10.1161/ATVBAHA.116.308099. [DOI] [PubMed] [Google Scholar]
- 25.Bar-Or A., Nuttall R.K., Duddy M. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126:2738–2749. doi: 10.1093/brain/awg285. [DOI] [PubMed] [Google Scholar]