Visual Abstract
Key Words: ambulatory blood pressure monitoring, blood pressure, diagnosis
Abbreviations and Acronyms: AAMI, Association for the Advancement of Medical Instrumentation; ABPM, ambulatory blood pressure monitoring; BP, blood pressure; CB, cuff-based blood pressure measurement; CI, confidence interval; CLB, cuff-less blood pressure estimation; DBP, diastolic blood pressure; ECG, electrocardiogram; HR, heart rate; HF, high-frequency; ICC, intraclass correlation coefficient; IEEE, Institute of Electrical and Electronics Engineers; LF, low-frequency; MAD, mean absolute difference; PTG, photoplethysmogram; SBP, systolic blood pressure
Highlights
-
•
As a joint project between industry and academia, we are developing a CLB that enables BP measurement continuously and noninvasively by capturing photoplethysmographical biosignals.
-
•
To validate the estimation of BP using a CLB in accordance with the latest wearable device standard issued by the Institute of Electrical and Electronics Engineers (IEEE 1708-2014).
-
•
We found that CLB is technically comparable to the ordinary cuff-based BP-measuring device.
-
•
CLB will apply for wearable health care monitoring device that may change landscape of BP measurements in terms of continuous and stress-free monitoring.
Summary
Ordinary cuff-based blood pressure–monitoring devices remain a technical limitation that disturbs activities of daily life. Here we report a novel system for the cuff-less blood pressure estimation (CLB) that requires only 1 sensor for photoplethysmography. The present study is the first report to validate and assess the clinical application of the CLB in accordance with the latest wearable device standard (issued by the Institute of Electrical and Electronics Engineers, standard 1708-2014). Our CLB is expected to offer a flexible and wearable device that permits blood pressure monitoring in more continuous and stress-free settings.
Since the invention of a rubber cuff for the compression of the brachial artery by Dr. Riva-Rocci in 1896 (1) and the development of the auscultatory method of blood pressure (BP) reading by Dr. Korotkoff in 1905 (2), cuff-based blood pressure measurement (CB) has been used as the gold standard method for blood pressure monitoring (3). The widespread use of the CB has inestimably contributed to the clinical management of BP; however, the decline in the use of mercury sphygmomanometers due to environmental contamination has led to the development of various alternatives (4). Since the 1970s, oscillometric devices have become popular because they can be used to take multiple and automated measurements (5). However, the oscillometric method still requires a cuff to compress the artery for BP measurement, which can disturb daily activities, particularly during ambulatory blood pressure monitoring (ABPM) (6).
The most recent guidelines for the care of hypertension have emphasized home BP monitoring and ABPM 7, 8. However, an ordinary CB allows only a “snapshot” or intermittent assessment of BP, and the inflation of a cuff can induce discomfort, especially during ABPM, which often affects the BP data 6, 9 and disturbs examinees’ daily activities (6). Many technical innovations have been developed for BP monitoring, such as BP estimation sensors, mobile apps, and wearable devices (10). Among these, a BP sensor measuring pulse transit time (i.e., the time interval from left ventricular contraction to pulse waveform acquisition at the extremity) is one of the most promising candidates (11). However, no system has been developed to enable BP estimation and continuous monitoring by a single sensor that has comparable fidelity to the CB (12).
The goal of the present study was to develop a new device for recording BP without a cuff. We report a novel system for cuff-less blood pressure estimation (CLB) that requires only 1 sensor for photoplethysmogram (PTG). Our original algorithm enables this simple system and expands the possibility of measuring BP comfortably and flexibly in various settings. This study is the first report to validate the CLB with a single sensor and to assess the clinical application of the CLB in accordance with the latest wearable device standard issued by the Institute of Electrical and Electronics Engineers (IEEE), IEEE 1708-2014 (13).
Methods
Study population
This study conforms to the principles outlined in the Declaration of Helsinki, and the Ethical Committee of Nagoya University School of Medicine approved this study. All examinees provided informed consent before the measurements were conducted. Written informed consent was obtained from all subjects. A summary of the enrolled participants is shown in Table 1, and their distributions of sex and age are summarized in Supplemental Figure 1A. Subjects with arrhythmias or who were pregnant were excluded.
Table 1.
Data Overview for BP Validation Tests (N = 172)
| Male, % | 115 (66.9) | ||
| Age, yrs | 47.6 ± 17.3 | ||
| Hypertension (%)∗ | 53 (30.8) | ||
| Total data number | 932 | ||
| Static | 386 | ||
| BP rise | 182 | ||
| BP lowering | 102 | ||
| Reproducibility | 262 | ||
| BP differences, mm Hg | SBP | DBP | |
| Total | −0.4 ± 8.0 [6.1] | −1.5 ± 6.4 [4.9] | |
| Static | 0.3 ± 6.6 [5.2] | −1.0 ± 5.4 [4.1] | |
| BP rise | −2.1 ± 7.5 [6.0] | −4.0 ± 5.6 [5.3] | |
| BP lowering | 1.0 ± 10.2 [8.0] | −0.6 ± 7.7 [5.8] | |
| Reproducibility | −0.8 ± 9.2 [6.8] | −0.9 ± 7.3 [5.5] | |
| PR, beats/min | |||
| Total | 71.4 ± 10.3 | ||
| Static | 68.7 ± 9.6 | ||
| BP rise | 75.9 ± 10.0 | ||
| BP lowering | 75.0 ± 12.6 | ||
| Reproducibility | 71.0 ± 8.8 |
Values are mean ± SD, n (%), or mean ± SD [MAD†].
Participant who has been diagnosed and treated with antihypertensive medication.
Mean absolute difference (MAD) between the blood pressure (BP) value of the test device (CLB) and the reference device (CB), the cuff-wearing sphygmomanometer. According to the Institute of Electrical and Electronics Engineers standard, the accuracy of the cuff-less BP devise was assessed by the sufficient number of systolic blood pressure (SBP) rise and decline in the range of 0 to 30 mm Hg. The accuracy limit of BP estimation was determined by the MAD of BP (<7 mm Hg difference between the CLB and the reference device. DBP = diastolic blood pressure; PR = pulse rate recorded by CLB.
Principles and procedure for BP estimation by CLB
Our novel CLB uses only PTG to estimate BP (Figure 1A, Supplemental Figure 1B). Each examinee wore a sensor measuring PTG on his or her right index finger. The PTG sensor was equipped with a light-emitting diode (940-nm wavelength) on the side facing the nail and a photodetector on the other side to monitor changes in peripheral blood volume (14). Changes in light absorption were monitored by the photodetector at a sampling frequency of 200 Hz with a resolution of 12 bits. Analog PTG data obtained during 5 contiguous pulse wave intervals were digitalized and averaged, followed by the calculation estimated by using a specific algorithm (Figure 1B) 15, 16. As a reference, BP was simultaneously measured by CB (both by auscultatory [Korotkoff] and electronic [oscillometric] sphygmomanometers) on the left arm (Figure 1C). A bladder-type cuff (8.6 inches wide and 12.6 inches long) was placed on the left upper arm of each participant, and BP readings were taken at 30-s intervals by using an oscillometric method (UA-1020G, A&D Company, Tokyo, Japan) and an auscultatory method. To obtain the auscultatory BP values, a microphone was attached to the left upper arm to capture the Korotkoff sounds of the brachial artery on the left side. The recorded Korotkoff sounds were digitalized by using a data logger (midi LOGGER GL900, Graphtec, Yokohama, Japan). Calibration of CLB values by using CB was performed 3 times at 60-s intervals before the validation test (Figure 1E).
Figure 1.
Circuit Diagram for the PW Sensor and BP Estimation System
(A) The light-emitting diode (LED) emits light with a wavelength of 940 nm. The light penetrates the finger and arrives at the photo detector (PD). The PD detects blood flow changes, which correspond to the natural pulsation of the blood flow. Baseline fluctuation is removed by a high-pass filter (HPF) with a cutoff frequency of 0.3 Hz, and noise is removed by a low-pass filter (LPF) with a cutoff frequency of 30 Hz. The output signal is digitized at a sampling frequency of 200 Hz and a resolution of 12 bits. (B to E) Schematic diagram (B and D) and illustration (C and E) used to develop the BP estimation algorithm. ∗Leg clamp (E). ∗∗Arithmetic calculation. A total of 887 participants were enrolled (a histogram of participant age and gender is displayed in Supplemental Figure 1A). The obtained pulse waves were analyzed, and feature parameters were extracted and collected. A database of feature parameters was analyzed to generate a BP estimation algorithm. (D) Validation protocol based on Institute of Electrical and Electronics Engineers (IEEE) standard 1708-2014. BP data were obtained by using cuff-less BP estimation (CLB) with simultaneous recording by a cuff-type sphygmomanometer (CB) as a reference conducted at the time point indicated by the closed circle. Calibration was performed at the beginning of each measurement using a cuff-type sphygmomanometer to take 3 measurements at 60-s intervals. After calibration, simultaneous BP monitoring was performed by using CLB and CB under (C) static conditions followed by dynamic measurements using (E) leg stretching and a clamp. ADC = analog to digital converter; Amp = amplifier; PC = personal computer.
Validation of CLB and the IEEE standard
The IEEE has issued a validation guideline (IEEE 1708-2014) for a wearable or cuff-less BP estimating device (13). IEEE 1708-2014 reflects the established clinical guidelines for BP monitoring issued by the Association for the Advancement of Medical Instrumentation (AAMI) (17) and the European Society of Hypertension (18). In brief, this guideline describes the fidelity requirements that are determined by the mean absolute difference (MAD) of BP between values estimated by using a test device (i.e., CLB) and those measured by using an ordinary CB under 3 different conditions. An overview of the study protocol is displayed in Figure 1B and in Supplemental Figure 1C. The illustrated procedures used are shown in Figures 1C to 1E. The experimental criteria and conditions are summarized in Supplemental Figure 1D.
To validate the CLB according to IEEE 1708-2014 (13), BP validation was performed under 3 different conditions: static (resting BP), dynamic (BP rising and falling), and reproducible (repeating BP estimation after a 1-month interval) (Figure 1E). BP rise was provoked by a simple leg stretch and clamp (Figure 1D). After the calibration, 3 pairs of BP measurements were taken for each subject using both CLB and CB simultaneously, as described in the previous section.
Scoring of the impact of ABPM on sleep quality
Thirty-five participants were subjected to bedtime BP monitoring by either CB (i.e., ABPM) (Study 1) or CLB (Study 2) on 2 separate calendar days. Between Study 1 and Study 2, 2-month intervals were set to avoid any interference that might occur by wearing order, such as the lack of sleep in a previous night induced by CB. Immediately after the device was detached, each examinee completed the sleep quality questionnaire. A questionnaire about sleep quality was used to screen for discomfort during BP monitoring by standard ABPM or CLB. Sleep quality was rated on a scale using 0, 1, and 2. Fair sleep quality during BP monitoring was rated as 2; mildly disturbed sleep was rated as 1; and if sleep quality deteriorated or the subject was unable to sleep, a score of 0 was recorded.
To address the impact of BP-monitoring device on sleep disturbance in a more objective fashion, electrocardiogram (ECG) data were simultaneously obtained and analyzed in terms of the changes in heart rate (HR), the high-frequency (HF) component of HR variability, and the ratio of the low-frequency (LF) component of HR variability and HF (LF/HF).
Statistical analyses
All statistical analyses were performed by using computer software (IBM SPSS version 24.0 [IBM SPSS Statistics, IBM Corporation, Armonk, New York] and JMP Pro 11 [SAS Institute, Inc., Cary, North Carolina]). To evaluate the reproducibility of the measurements, the intraclass correlation coefficient (ICC) and its 95% confidence intervals (CIs) were calculated (Table 2). In general, an ICC >0.8 represents good repeatability of measurements, and an ICC >0.9 represents excellent repeatability.
Table 2.
Intra-Device Repeatability of BP Measurement by CLB and CB
| ICC (95% CI) | Mean Difference ± 2 SDs | |
|---|---|---|
| Total (N = 932) | ||
| SBP | 0.918 (0.907–0.927) | −0.41 ± 16.1 |
| DBP | 0.842 (0.812–0.866) | −1.53 ± 12.8 |
| Static (n = 386) | ||
| SBP | 0.950 (0.940–0.959) | 0.26 ± 13.1 |
| DBP | 0.903 (0.880–0.921) | −1.01 ± 10.8 |
| BP rise (n = 182) | ||
| SBP | 0.920 (0.889–0.942) | −2.07 ± 14.9 |
| DBP | 0.805 (0.514–0.902) | −4.04 ± 11.2 |
| BP lowering (n = 102) | ||
| SBP | 0.856 (0.794–0.900) | 1.00 ± 20.3 |
| DBP | 0.784 (0.697–0.875) | −0.60 ± 15.3 |
| Reproducibility (n = 262) | ||
| SBP | 0.844 (0.805–0.849) | −0.77 ± 18.3 |
| DBP | 0.752 (0.694–0.800) | −0.90 ± 14.5 |
The reproducibility of the measurements, the ICC and its 95% confidence interval (CI) value between the BP value of test device (CLB) and the reference device (CB; cuff-wearing sphygmomanometer), were calculated. In general, an ICC >0.8 represents good repeatability, and an ICC >0.9 represents excellent repeatability of measurements.
ICC = intraclass correlation coefficient; other abbreviations as in Table 1.
To address the agreement (i.e., interchangeability) of a new measurement technique with an established one, the Bland-Altman plot is useful because direct comparison of the measured value or correlation coefficient analysis has a limitation 19, 20. The Bland-Altman plot statistically determines whether cuff-less estimation is sufficient to replace ordinary CB. Agreement between the 2 distinct devices was assessed by the data distribution pattern of SDs plotted along the ordinate, with the mean BP value plotted along the abscissa (19). The agreement limits were defined by the mean ± 2 SDs of the measured differences. The McNemar test was used to determine the existence of differences in a dichotomous variable between 2 related groups. Values of p < 0.05 were considered statistically significant.
Results
Correlation of BP values between the CLB estimation and cuff-type auscultatory measurement during static, dynamic, and 1-month follow-up conditions
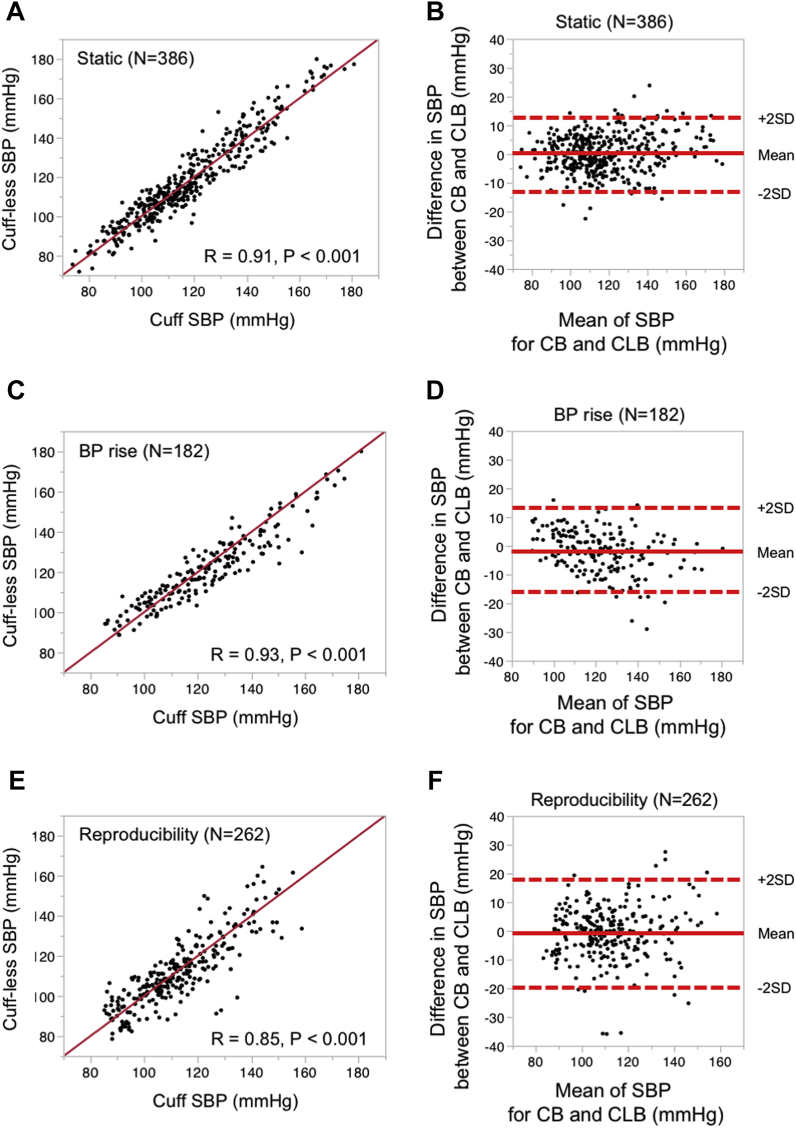
Illustrated images of the CLB with a PTG sensor and the principle and algorithm used to estimate BP are detailed by flowcharts (Figure 1, Supplemental Figure 1C). In accordance with IEEE 1708-2014, we conducted a test to validate the CLB by simultaneously monitoring BP with the CLB and with a cuff-type auscultatory device under 3 different conditions: static, dynamic, and 1-month follow-up (Figure 1E). The correlation coefficients of systolic blood pressure (SBP) data between the CLB and the CB were highly significant in SBP (Figures 2A, 2C, and 2E). MAD values were <8 mm Hg (MAD; 6.1 for SBP), suggesting that the BP values measured by using CLB meet the IEEE 1708-2014 standard.
Figure 2.
Validation and Reproducibility of CLB According to the IEEE 1708-2014 Standard Under Static and Dynamic Conditions
Cumulative systolic blood pressure (SBP) dataset was obtained under (A and B) static (n = 386), (C and D) BP rise (n = 182), and (E) reproducible (retaking BP measurements on the same examinee 1 month later, n = 262) conditions. Linear correlation analysis was used to assess the association between the 2 measures. The correlation coefficient is given by r. The results of analyzing diastolic BP data are shown in Supplemental Figure 2. To assess the agreement of BP data measured by using CLB with those recorded by using a cuff-based sphygmomanometer, all BP data were assessed by using a Bland-Altman plot. Scatter plots of difference in SBP between CB and CLB under (B) static conditions, (D) dynamic conditions, and (F) for reproducibility assessment are shown. The average difference in SBP between the CLB and the CB is indicated by solid lines. Dotted lines indicate the 95% limits of agreement (mean ± 2 SDs of the difference in SBP) between the CB and the CLB. The agreement limits of SBP were: (in mean ± 2 SDs): (B) static, −0.3 ± 13.2; (D) BP rise, −2.1 ± 15.0; and (F) reproducibility, −0.8 ± 18.4. Abbreviations as in Figure 1.
To assess the reproducibility of the measurements, the ICC and its 95% CIs were calculated (Table 2). The ICC indicated good repeatability at all conditions (0.918 for overall SBP, 0.950 for static SBP, and 0.920 for BP rise [SBP]). In the clinical setting, a new measurement technique (in this paper, CLB) is needed to determine whether they agree sufficiently for the new to replace the old one, and the Bland-Altman plot is widely used to address the agreement 19, 21. The Bland-Altman plot analysis (Figures 2B, 2D, and 2F) consistently indicated that the CLB method is sufficient to replace the cuff-based device method (the mean difference and 95% limits of agreement of CLB for the reference device [in mean ± 2 SD] were −0.4 ± 16.1 in SBP). Correlation and the Bland-Altman plot analysis for diastolic blood pressure (DBP) were highly credible under various conditions (Supplemental Figure 2).
Correlation of BP between CLB estimation and cuff-type device estimations under falling BP conditions
The IEEE issued a validity requirement for CLB devices by indicating requirements for reproducibility and for measuring static BP, rising BP, and falling BP in the ranges of 0 to 15 and 15 to 30 mm Hg (13). To verify the precision of BP estimation by the CLB under falling BP conditions, we simultaneously recorded from the CLB and the CB during coronary angiography after nitroglycerin administration (Figure 3A and Supplemental Table 1 for baseline characteristics of participants [n = 29]). As expected, the intracoronary administration of nitroglycerin (1.5 mg) (protocol is displayed in Figure 3A) induced a decrease in BP of approximately 30 mm Hg (Figures 3B and 3C). Despite the rapid response occurring over a few minutes, the CLB measurements were highly correlated with the changes in BP (R = 0.86, p < 0.001 for SBP [Figure 3D]; R = 0.78, p < 0.0001, for DBP [Supplemental Figure 2G]) and exhibited sufficient agreement (agreement limit of 1.0 ± 20.3 for SBP [Figure 3E]: −0.6 ± 15.3 for DBP [Supplemental Figure 2H]). All BP estimation data at the phase of static, BP rise, BP lowering, and 1 month internal study were plotted together to summarize and verify the precision of the cuff-less measurement independently of BP variability in Supplemental Figure 3.
Figure 3.
Validation of the Cuff-less BP Monitoring Device Under Decreasing BP Conditions During Coronary Arterial Angiography
To address the precision requirements outlined by IEEE 1708-2014 for decreasing BP, we conducted simultaneous BP recording using CLB and a standard CB (A). Image of the recording system used during coronary angiography is shown in the left panel in A. (B) Representative recording of changes in intra-arterial BP during coronary angiography (B). Intracoronary arterial administration of nitroglycerin (1.5 mg/bolus shot) reduced arterial BP by >15 mm Hg in accordance with the validation criteria described in IEEE 1708-2014. (C) Typical results from the simultaneous BP recordings of CLB (red line) and CB (blue line). (D and E) Correlation and agreement of SBP data measured by using CB and CLB. Agreement of CLB with CB was analyzed by using a Bland-Altman plot (E) during coronary arterial angiography. Average difference in SBP between the CLB and the CB is indicated by the solid line. The 95% limits of agreement (mean ± 2 SDs of the difference) between CLB and CB are indicated by dotted lines. The agreement limit for SBP was 1.00 ± 20.3. Assessments of DBP are shown in Supplemental Figure 2. Abbreviations as in Figure 1.
To address whether CLB sensitivity may be altered in response to these dynamic BP changes, the BP distribution patterns measured by CB and CLB were compared by using a histogram. In SBP, the BP distribution pattern appeared identical to that measured by CB under independent recording conditions (Supplemental Figures 4A to 4C). In contrast, the histogram of DBP from CLB was not identical to that measured by CB under dynamic conditions (i.e., rising and falling BP).
Effects of the CLB and ordinary ABPM on sleep quality
One of the expected advantages of the CLB is its use in the continuous recording of BP variability (6). We therefore conducted a pilot study to compare the CLB and cuff-type ABPM during sleep. Thirty-five participants (Supplemental Table 1) were subjected to BP monitoring during sleep using an ordinary automated CB (i.e., ABPM; Study 1). The next morning, they scored their discomfort and sleep quality using a simple questionnaire (details noted in the Methods section). A second study using CLB was performed on a separate calendar day, after an interval of >2 months (Figure 4A). There were no differences in the mean SBP or DBP measurements between the 2 devices (Supplemental Figure 5A). The questionnaire revealed that >70% of participants felt uncomfortable when wearing an ordinary cuff-type ABPM (Figure 4B), and this rate was significantly reduced when wearing a CLB, suggesting that sleep quality was significantly preserved during overnight BP monitoring with a CLB.
Figure 4.
Effect of Ambulatory BP Measurement on Sleep Quality
The effect of BP measurement during sleep was evaluated in terms of discomfort using (B) a questionnaire about sleep quality and (C to F) physiological parameters (heart rate [HR], high-frequency [HF], and low-frequency [LF]/HF). (A) Examinees were randomly assigned and subjected to a bedtime BP monitoring study using standard ambulatory BP monitoring (ABPM) (Study 1). More than 2 months later, the same examinees were subjected to bedtime BP monitoring using the CLB (Study 2). To avoid any bias resulting from device order, the second study was performed after a long interval. There were no significant differences in the mean SBP or DBP recorded by using either of these devices (Supplemental Figure 5A). (B) The effects on sleep quality of standard ABPM and CLB were compared with a questionnaire. The effect of ABPM and CLB on the sleep quality of 35 participants was assessed using ratings provided on a scale of 0 to 2. A higher score indicates better sleep quality during BP monitoring. A score of 2 (white area) indicates fair or usual sleep quality; a score of 1 (gray area) indicates sleep that was mildly disturbed by BP measurement; and a score of 0 (black area) indicates sleep that was significantly disturbed. P < 0.001 according to the McNemar test. (C to F) Changes in physiological parameters. To assess the effect of a BP cuff on sleep quality, HR variability was analyzed. Typical recordings of HR, HF, and LF/HF during (C) Study 1 and (D) Study 2 are shown. (E) Time course of changes in mean HR during sleep by CB (Study 1, blue line) and by CLB (Study 2, red line) are displayed. The mean HR was significantly lower when using CLB (line) during the first hour after going to bed (F), presumably indicating the time to sleep onset. Abbreviations as in Figure 1.
To address the objective impact of CLB on sleep quality, we measured HR variability (Figures 4C to 4E) by using the ECG data simultaneously obtained from the CLB device. These variables were measured in terms of the changes in HR, the HF component of HR variability, and the ratio of the LF component of HR variability and HF (i.e., LF/HF). Notably, HR, HF, and LF/HF were significantly lower in the CLB group exclusively in the first hour after going to bed (i.e., the time to onset of sleep). In contrast, there were no significant differences in HR or the LF/HF ratio during the total duration of sleep (8 h) (Supplemental Figures 5B and 5C).
Discussion
Following the accumulating clinical evidence 22, 23, greater attention has been paid to BP management, resulting in an increased need for ambulatory BP monitoring (12). Experts have emphasized the clinical significance of BP recording at home as a surrogate for the prevention of cardiovascular events in patients with hypertension 12, 24. To record BP at home, CB is the most popular method, but it requires the use of a cuff that limits the self-recording of BP. In the present study, on the basis of innovative sensor assemblies and an algorithm, CLB demonstrated high precision and agreement with standard CB through the validation of BP measurements under static and dynamic conditions (Figures 1, 2, and 3). We further certified that the CLB met the validation and reproducibility criteria through follow-up measurements (Figures 2E and 2F).
Interestingly, the CLB exhibited high fidelity in response to rapid changes in BP, both increases (Figure 2) and decreases (Figure 3), that were recorded during the intracoronary injection of nitroglycerin. We carefully reviewed these BP data by comparing histograms to assess the agreement of CB and CLB and to visualize the differences in the distribution patterns of the recorded BP data (Supplemental Figure 4). The histograms showed high consistency between CB and CLB, except for DBP under dynamic conditions. Previous reports have consistently reported similar evidence that exercise alters the relationship between pulse transit time and arterial blood pressure (25), which is more sensitive to the case of peripheral measurement of DBP than to the measurement of SBP by pulse transit time 25, 26. This alteration is believed to result from changes in the correlation between pulse transit time (i.e., pulse wave velocity) and arterial wall distensibility in response to exercise 26, 27. To overcome this physiological limitation, further improvement in the CLB is necessary.
To conduct validation tests, we devised original protocols that produce sufficient changes in BP to meet the most recent guidelines for wearable devices used for BP monitoring issued by the IEEE (13). To date, the AAMI (17), the European Society of Hypertension (18), and the British Hypertension Society (28) have issued clinical recommendations for validating a cuff-type automated BP-monitoring device 18, 29. The IEEE guideline (13) is the most recently published and complies with the previous guidelines. More specifically, an AAMI position paper recommend criteria to follow when comparing any new automatic device versus the cuff-based auscultatory method: average differences no >5 mm Hg and SDs no >8 mm Hg in groups of no fewer than 85 subjects (5). Addressing the AAMI standard, all the data from the present study met these criteria for average difference, SDs, and sample number.
Regardless of device features, BP measurements are easily affected by various conditions, including environmental factors, such as ambient temperature, exercise, and body posture 30, 31. Therefore, to validate the accuracy of any BP-reading device, a universal standard protocol is essential. Technical innovation in the field of wearable devices increases the practical demands for a validation protocol for these new modalities. Surprisingly, there is no universal standard for calibrating oscillometric BP-reading devices, which have become more popular worldwide than mercury manometers 5, 32. The present study sheds light on the critical gap between technical innovation and practical demands for the validation of clinical BP.
Various attempts have been made to estimate BP by using a pulse waveform; however, unsolved critical issues of low sensitivity that demand the use of supplementary biosignals, such as an ECG, remain 15, 33. Various attempts have been made to develop clinically relevant cuff-free devices for BP estimation, and previous reports have indicated the pitfalls and limitations associated with these devices 11, 12, 33. One such limitation is calibration. To convert the PTG signal into BP, calibration using CB is unavoidable. We are not yet free from the cuff, and our device is therefore termed “cuff-less,” not “cuff-free.” However, our CLB has the advantage of using a single sensor for BP recording, unlike previous devices that require multiple sensors (15). For cuff-less BP monitoring to be user-friendly (11), the use of multiple sensors should not limit portability or flexibility. Gesche et al. (33) showed that BP estimation using PTG and ECG is regarded as more convenient and less costly because this method requires only an estimation algorithm, ECG, and a finger PTG sensor (33). More recently, a Taiwanese company has developed a cuff-less BP reading device that is already commercially available (34). Notably, compared with our device that requires only a PTG signal for BP estimation, this apparatus requires 2 signals, representing ECG and PTG data, for BP measurement. We preliminarily compared our CLB with this 2-signal-based cuff-less device. Our CLB exhibits higher sensitivity (Hiroshi Yamakita, unpublished observation, March 24, 2017; MAD, 3.8 mm Hg for SBP and 4.6 mm Hg for DBP measured by our CLB, n = 23; MAD, 16.0 mm Hg for SBP and 7.3 mm Hg for DBP measured by the counterpart, n = 23).
Conclusions
We have developed a novel BP-monitoring sensor using innovative digital technology. Although our device has yet to overcome the requirement for pre-calibration using CB, our study shows the high precision and great advantage of CLB as a paradigm shift in BP monitoring in the digital health era.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Cuff-based BP measurement has been the gold standard for the past 120 years. To prevent cardiovascular events, BP monitoring is essential. The most recent clinical guidelines for the care of hypertension have emphasized home BP monitoring and ABPM. However, current standard devices for BP recording still have several hurdles for ambulatory BP monitoring due to the cuff, which causes patient discomfort and disturbs examinees’ daily activities.
TRANSLATIONAL OUTLOOK: The CLB is technically comparable to standard cuff-based devices and provides various advantages for BP recording, such as more comfortable monitoring during a variety of life activities. CLB enables patients to share more accurate and reliable data of ambulatory BP monitoring with their physicians. Collectively, CLB is expected to lower the incidence of cardiovascular events by collecting BP data that are unmeasurable by current diagnostic modalities.
Acknowledgments
The authors extend their appreciation to the staff and volunteers who supported this study.
Footnotes
This research was supported in part by Grant-in-Aid for Scientific Research No. 26-D-Dj10 (to Dr. Murohara) from the Innovative Research Center for Preventive Medical Engineering, Nagoya University. The funding sources had no role in the design and conduct of the study. Dr. Murohara has received lecture fees and research grants from Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi-Sankyo, DENSO CORPORATION, Kowa, MSD, Pfizer, Takeda, and Tanabe-Mitsubishi. Dr. Bando has received lecture fees and research grants from AstraZeneca, Boehringer Ingelheim, MSD, Taisho-Toyama, Elly-Lilly, Astellas, Daiichi-Sankyo, and Tanabe-Mitsubishi. Dr. Ishii has received lecture fees from Astellas Pharma Inc., AstraZeneca, and Daiichi-Sankyo. Drs. Kawachi, Yamakita, Honda, and Futatsuyama are employees of DENSO CORPORATION. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.O'Brien E., Fitzgerald D. The history of blood pressure measurement. J Hum Hypertens. 1994;8:73–84. [PubMed] [Google Scholar]
- 2.Korotkoff N.S. On the subject of methods of measuring blood pressure. Bull Imp Military Med Acad. 1905;11:365–367. [Google Scholar]
- 3.Campbell N.R., Chockalingam A., Fodor J.G., McKay D.W. Accurate, reproducible measurement of blood pressure. CMAJ. 1990;143:19–24. [PMC free article] [PubMed] [Google Scholar]
- 4.Pickering T.G., Hall J.E., Appel L.J. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 5.Smulyan H., Safar M.E. Blood pressure measurement: retrospective and prospective views. Am J Hypertens. 2011;24:628–634. doi: 10.1038/ajh.2011.22. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal R., Light R.P. The effect of measuring ambulatory blood pressure on nighttime sleep and daytime activity—implications for dipping. Clin J Am Soc Nephrol. 2010;5:281–285. doi: 10.2215/CJN.07011009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piper M.A., Evans C.V., Burda B.U., Margolis K.L., O'Connor E., Whitlock E.P. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2015;162:192–204. doi: 10.7326/M14-1539. [DOI] [PubMed] [Google Scholar]
- 8.Pickering T.G., Miller N.H., Ogedegbe G. Call to action on use and reimbursement for home blood pressure monitoring: executive summary: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:1–9. doi: 10.1161/HYPERTENSIONAHA.107.189011. [DOI] [PubMed] [Google Scholar]
- 9.Davies R.J., Jenkins N.E., Stradling J.R. Effect of measuring ambulatory blood pressure on sleep and on blood pressure during sleep. BMJ. 1994;308:820–823. doi: 10.1136/bmj.308.6932.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldberg E.M., Levy P.D. New approaches to evaluating and monitoring blood pressure. Curr Hypertens Rep. 2016;18:49. doi: 10.1007/s11906-016-0650-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mukkamala R., Hahn J.O., Inan O.T. Toward ubiquitous blood pressure monitoring via pulse transit time: theory and practice. IEEE Trans Biomed Eng. 2015;62:1879–1901. doi: 10.1109/TBME.2015.2441951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandit J.A., Batlle D. Snapshot hemodynamics and clinical outcomes in hypertension: precision in the measurements is key. Hypertension. 2016;67:270–271. doi: 10.1161/HYPERTENSIONAHA.115.06818. [DOI] [PubMed] [Google Scholar]
- 13.IEEE Standard Association. IEEE Standard for Wearable Cuffless Blood Pressure Measuring Devices, IEEE Std 1708-2014. 2014;i–38. DOI: 10.1109/IEEESTD.2014.6882122.
- 14.Evans M.L., Geddes L.A. An assessment of blood vessel vasoactivity using photoplethysmography. Med Instrum. 1988;22:29–32. [PubMed] [Google Scholar]
- 15.Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012;8:14–25. doi: 10.2174/157340312801215782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takazawa K., Tanaka N., Fujita M. Assessment of vasoactive agents and vascular aging by the second derivative of photoplethysmogram waveform. Hypertension. 1998;32:365–370. doi: 10.1161/01.hyp.32.2.365. [DOI] [PubMed] [Google Scholar]
- 17.Association for the Advancement of Medical Instrumentation. American National Standard: noninvasive sphygmomanometers -part 2: clinical validation of automated measurement type. ANSI/AAMI/ISO 2009;81060–2.
- 18.O'Brien E., Atkins N., Stergiou G. European Society of Hypertension International Protocol revision 2010 for the validation of blood pressure measuring devices in adults. Blood Press Monit. 2010;15:23–38. doi: 10.1097/MBP.0b013e3283360e98. [DOI] [PubMed] [Google Scholar]
- 19.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 20.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud. 2010;47:931–936. [Google Scholar]
- 21.Ozaki Y., Ohota M., Ismail T.F., Okumura M., Ishikawa M., Muramatsu T. Thin cap fibroatheroma defined as lipid core abutting lumen (LCAL) on integrated backscatter intravascular ultrasound—comparison with optical coherence tomography and correlation with peri-procedural myocardial infarction. Circ J. 2015;79:808–817. doi: 10.1253/circj.CJ-14-0758. [DOI] [PubMed] [Google Scholar]
- 22.Wright J.T., Jr., Whelton P.K., Reboussin D.M. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2016;374:2294. doi: 10.1056/NEJMc1602668. [DOI] [PubMed] [Google Scholar]
- 23.Kario K., Bhatt D.L., Brar S., Bakris G.L. Differences in dynamic diurnal blood pressure variability between Japanese and American treatment-resistant hypertensive populations. Circ J. 2017;81:1337–1345. doi: 10.1253/circj.CJ-16-1237. [DOI] [PubMed] [Google Scholar]
- 24.Siu A.L., U.S. Preventive Services Task Force Screening for high blood pressure in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;163:778–786. doi: 10.7326/M15-2223. [DOI] [PubMed] [Google Scholar]
- 25.Wong M.Y., Pickwell-MacPherson E., Zhang Y.T. The acute effects of running on blood pressure estimation using pulse transit time in normotensive subjects. Eur J Appl Physiol. 2009;107:169–175. doi: 10.1007/s00421-009-1112-8. [DOI] [PubMed] [Google Scholar]
- 26.Wibmer T., Doering K., Kropf-Sanchen C. Pulse transit time and blood pressure during cardiopulmonary exercise tests. Physiological Res. 2014;63:287–296. doi: 10.33549/physiolres.932581. [DOI] [PubMed] [Google Scholar]
- 27.Naka K.K., Tweddel A.C., Parthimos D., Henderson A., Goodfellow J., Frenneaux M.P. Arterial distensibility: acute changes following dynamic exercise in normal subjects. Am J Physiol Heart C. 2003;284:H970–H978. doi: 10.1152/ajpheart.00529.2002. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien E., Petrie J., Littler W. The British Hypertension Society protocol for the evaluation of automated and semi-automated blood pressure measuring devices with special reference to ambulatory systems. J Hypertens. 1990;8:607–619. doi: 10.1097/00004872-199007000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Wan Y., Heneghan C., Stevens R. Determining which automatic digital blood pressure device performs adequately: a systematic review. J Hum Hypertens. 2010;24:431–438. doi: 10.1038/jhh.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peters G.L., Binder S.K., Campbell N.R. The effect of crossing legs on blood pressure: a randomized single-blind cross-over study. Blood Press Monit. 1999;4:97–101. [PubMed] [Google Scholar]
- 31.van Groningen L.F., Adiyaman A., Elving L., Thien T., Lenders J.W., Deinum J. Which physiological mechanism is responsible for the increase in blood pressure during leg crossing? J Hypertens. 2008;26:433–437. doi: 10.1097/HJH.0b013e3282f35276. [DOI] [PubMed] [Google Scholar]
- 32.van Montfrans G.A. Oscillometric blood pressure measurement: progress and problems. Blood Press Monit. 2001;6:287–290. doi: 10.1097/00126097-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Gesche H., Grosskurth D., Küchler G., Patzak A. Continuous blood pressure measurement by using the pulse transit time: comparison to a cuff-based method. Eur J Appl Physiol. 2012;112:309–315. doi: 10.1007/s00421-011-1983-3. [DOI] [PubMed] [Google Scholar]
- 34.Boubouchairopoulou N., Kollias A., Chiu B. A novel cuffless device for self-measurement of blood pressure: concept, performance and clinical validation. J Hum Hypertens. 2017;31:479–482. doi: 10.1038/jhh.2016.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.