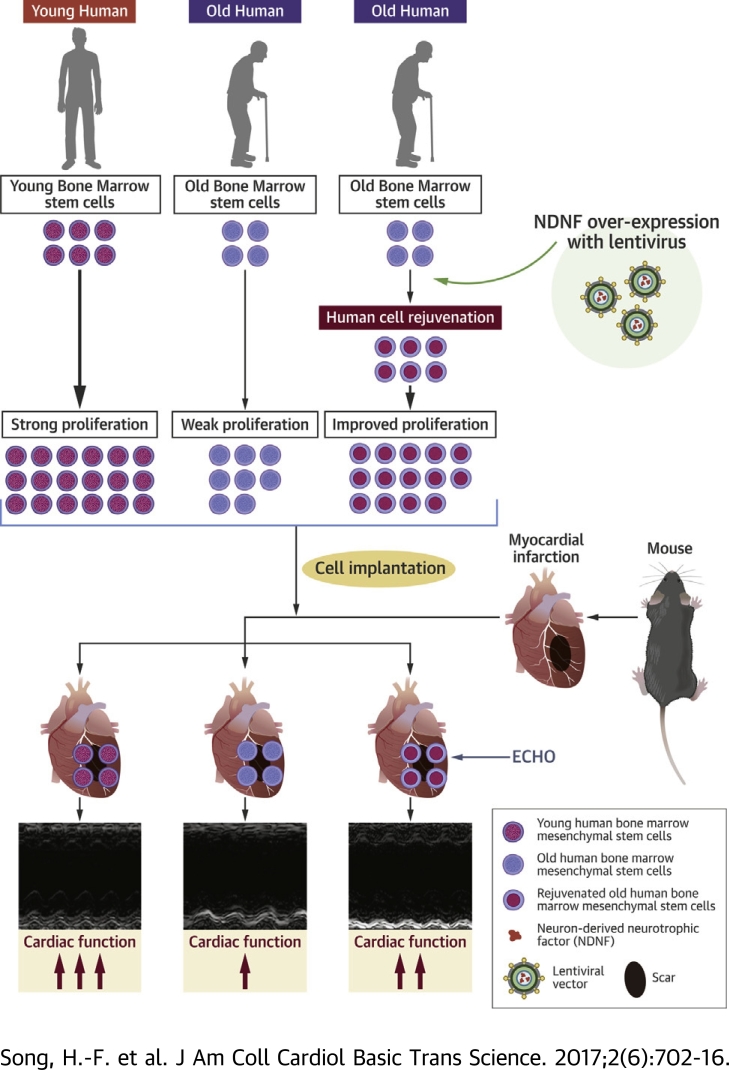
Visual Abstract
Key Words: aging, heart function, human stem cells, myocardial infarction, NDNF, rejuvenation
Abbreviations and Acronyms: CDC, cardiosphere-derived cell; hBM, human bone marrow; MI, myocardial infarction; mRNA, messenger ribonucleic acid; MSC, multipotent mesenchymal stromal cell; NDNF, neuron-derived neurotrophic factor; p-Akt, phosphorylated Akt; RGN, regucalcin
Highlights
-
•
The benefits of cell transplantation for cardiac repair are diminished in older individuals and effective methods to rejuvenate aged stem cells are needed to treat the increasing number of older patients with heart failure.
-
•
Over-expressing NDNF in old hBM-MSCs rejuvenated the cells, increasing their proliferative capacity and reducing cellular apoptosis.
-
•
In vivo engraftment of NDNF-overexpressing old hBM-MSCs into the ischemic area of mouse hearts improved cardiac function after myocardial infarction, while promoting engrafted stem cell survival and proliferation and decreasing cell senescence.
-
•
NDNF rejuvenated aged human stem cells, improving their capability to repair the aged heart after ischemic injury through Activation of Akt singling.
Summary
Reduced regenerative capacity of aged stem cells hampers the benefits of autologous cell therapy for cardiac regeneration. This study investigated whether neuron-derived neurotrophic factor (NDNF) could rejuvenate aged human bone marrow (hBM)- multipotent mesenchymal stromal cells (MSCs) and whether the rejuvenated hBM-MSCs could improve cardiac repair after ischemic injury. Over-expression of NDNF in old hBM-MSCs decreased cell senescence and apoptosis. Engraftment of NDNF over-expressing old hBM-MSCs into the ischemic area of mouse hearts resulted in improved cardiac function after myocardial infarction, while promoting implanted stem cell survival. Our findings suggest NDNF could be a new factor to rejuvenate aged stem cells and improve their capability to repair the aged heart after injury.
Ischemic heart disease leads to very high morbidity and mortality despite existing treatment options 1, 2, 3. Autologous cell transplantation has been developed as a promising new therapy for cardiac repair 4, 5. Multipotent mesenchymal stromal cells (MSCs) from bone marrow represent a robust and accessible stem cell resource characterized by cells with great capacity for self-renewal and multipotent differentiation 6, 7. Transplantation of MSCs into the ischemic heart has been shown to stimulate endogenous cardiac stem cell proliferation and tissue regeneration 8, 9. However, the benefits of cardiac cell therapy are diminished in aged individuals due to the reduced proliferative and self-renewal capacities of aged stem cells and increased cell senescence 10, 11, 12, 13, 14, 15. Allogeneic stem cells have been shown to have the similar early benefits as autologous cells (16), but the long term effects of allogeneic cells have not been established and concerns have been expressed that allogeneic cells may be rejected and lose their benefit late after engraftment (17). Therefore, effective methods to rejuvenate aged human stem cells to improve their regenerative capability are needed to help treat the increasing number of elderly patients with ischemic heart disease and heart failure.
First described in the nervous system 18, 19, neuron-derived neurotrophic factor (NDNF) has several biological functions that align with the goals of stem cell functional restoration, including the promotion of cell growth and the inhibition of apoptosis (19). Recently, secretion of NDNF from endothelial cells was found to promote endothelial cell function and survival following ischemic limb injury in mice (20), and systemically increasing NDNF levels in mice improved cardiac function, increased angiogenesis, and reduced cardiomyocyte apoptosis following myocardial infarction (MI) (21). Although these studies provide evidence that NDNF can facilitate cardiomyocyte function and cardiac repair after injury, they are limited by the fact that NDNF expression was experimentally increased only in mouse cells. Thus, the extent to which NDNF’s proangiogenic and antiapoptotic effects may apply to human cells and specifically to human stem cells remains unknown. Moreover, the effect of age on the expression level of NDNF in human stem cells and its implications for stem cell rejuvenation have not been explored.
In the current study, we investigated whether increasing the expression of NDNF could rejuvenate aged human bone marrow mesenchymal stromal cells (hBM-MSCs). hBM-MSCs were harvested from infant, young, and old patients undergoing bone marrow biopsies and NDNF expression was measured along with cellular proliferation and migration. A lentiviral expression vector carrying the NDNF gene was used to overexpress NDNF in old hBM-MSCs. The effects of NDNF overexpression on hBM-MSC proliferation, survival, senescence, and angiogenesis were investigated in vitro. In vivo, NDNF overexpressing old hBM-MSCs were implanted into the border region of mouse hearts following MI and the effects on cardiac and cellular function were investigated.
Methods
In vitro hBM-MSC harvesting, culture, and analyses
hBM was harvested from infant (n = 16, 11 boys, age 3.8 ± 0.5 years), young (n = 21, 9 men, age 23.3 ± 1.1 years), and old (n = 31, 17 men, age 73.8 ± 1.2 years) patients after giving written informed consent during bone marrow aspiration for subsequent biopsy at the First Hospital of Shanxi Medical University, Taiyuan, China. Samples from patients with no genetic disease or malignancy based on the primary diagnosis were used. This study was approved by the research ethics board of the Shanxi Medical University. hBM was also obtained from patients undergoing cardiovascular surgery at Toronto General Hospital, Toronto, Canada. All the procedures were approved by the Research Ethics Board (REB#CCR001), and patients provided written informed consent.
Overexpression of NDNF in old hBM-MSCs was achieved using a lentiviral expression vector carrying the NDNF gene (pLenti-Puro-EF1α-NDNF-Homo-IRES-eGFP, Cyagen Biosciences Inc., Santa Clara, California) according to the manufacturer’s instructions. NDNF overexpression were confirmed by reverse transcription polymerase chain reaction for messenger ribonucleic acid (mRNA) and Western blot for protein in old hBM-MSCs.
Detailed methods for the in vitro experiments can be found in the Supplemental Appendix.
In vivo mouse MI model using hBM stem cells
All animal procedures were approved by the University Health Network Animal Care Committee and all animals received humane care in compliance with the Canadian Council on Animal Care. Female c57bl/6 mice 12 months of age were used for the following procedures. The left coronary artery of mice was permanently ligated to induce MI. During this procedure, mice were intubated and ventilated with 2% isoflurane (Pharmaceutical Partners of Canada Inc., Richmond Hill, Ontario, Canada). Mice with infarct sizes between 30% and 35% of the left ventricular free wall were used in the following experiments. Young hBM-MSCs (young; n = 25, 1 × 106 cells in 20 μl serum-free Iscove’s modified Dulbecco’s medium per mouse), old NDNF-transduced hBM-MSCs (old+NDNF; n = 27), or empty vector–transduced hBM-MSCs (old; n = 26) were transplanted into 3 sites around the border zone immediately after MI. Serum-free Iscove’s modified Dulbecco’s medium (n = 25) was injected into the border zone of a group of mice, which served as a negative control group. A group of mice implanted with NDNF-transduced human dermal fibroblasts (fibro+NDNF; n = 11) was used to evaluate the effect of NDNF alone and human dermal fibroblasts (fibro; n = 11) were injected into the border zone of a group of mice as cellular negative control animals. Beginning 3 days before MI and cell transplantation and continuing until the end of the study, mice were given daily doses of cyclosporine A (5 mg/kg) to induce immunosuppression. Cardiac function was measured with echocardiography before and 7, 14, 21, and 28 days after MI and with a pressure-volume catheter 28 days after MI. Scar tissue was detected by Masson's trichrome staining, and the scar size was measured by planimetry.
Statistical analyses
All values are expressed as mean ± SEM. Analyses were performed using SPSS software version 15 (SPSS Inc., Chicago, Illinois). Student’s t-test was used for 2-group comparisons. For small sample numbers (n = 4 to 5), we used Mann-Whitney test. Comparisons of parameters among 3 or more groups were analyzed using 1-way analysis of variance followed by Tukey or 2-way analysis of variance with repeated measures over time, followed by Bonferroni post hoc tests. Differences were considered statistically significant at p < 0.05.
Results
NDNF expression in hBM-MSCs decreased with age
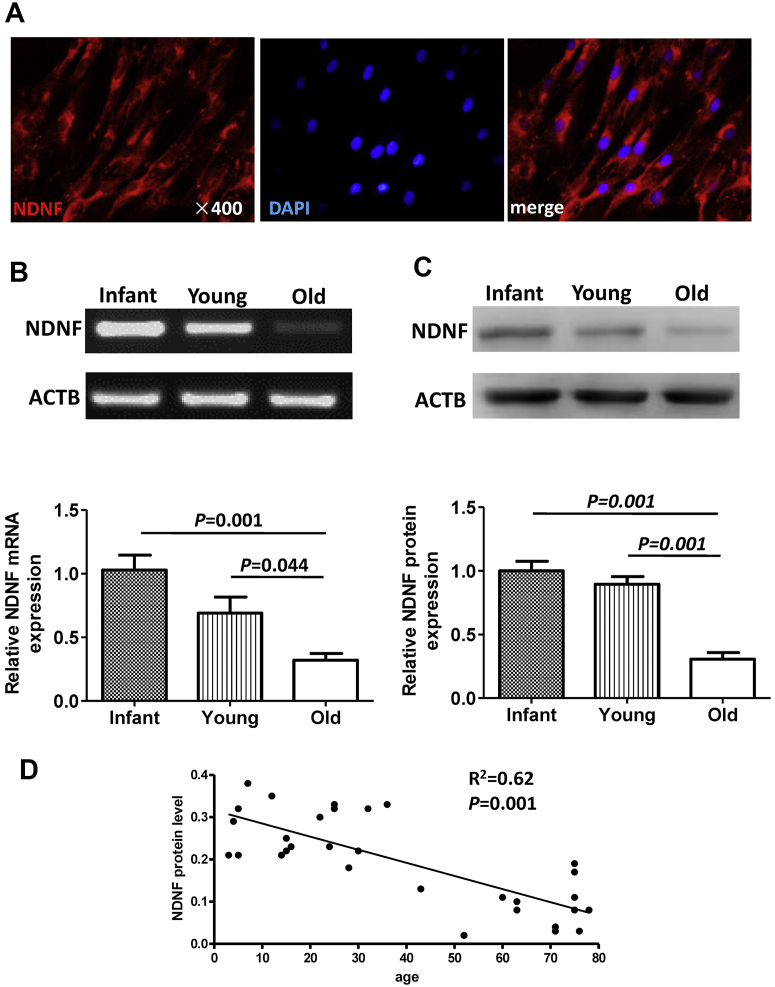
NDNF protein was detected in hBM-MSCs and it was located mostly perinuclear in the cytoplasm (Figure 1A). NDNF mRNA (Figure 1B) and protein (Figure 1C) levels were significantly lower in hBM-MSCs from old patients than from infant and young patients. There was a significant negative correlation between NDNF protein level and age (Figure 1D).
Figure 1.
NDNF Expression in hBM-MSCs Decreased With Age
(A) Representative micrographs of immunofluorescent staining for neuron-derived neurotrophic factor (NDNF) (red) with nuclei stained blue with 4′,6-diamidino-2-phenylindole (DAPI). NDNF messenger ribonucleic acid (mRNA) and protein expression were significantly decreased in old human bone marrow multipotent mesenchymal stromal cells (hBM-MSCs) compared with infant and young hBM-MSCs as measured by (B) reverse transcription polymerase chain reaction and (C) Western blot, respectively. (D) The NDNF protein level negatively correlated with age. The list of X (age) and Y (NDNF protein level) was analyzed using a correlation regression analysis. n = 8 to 13/group. ACTB = beta-actin.
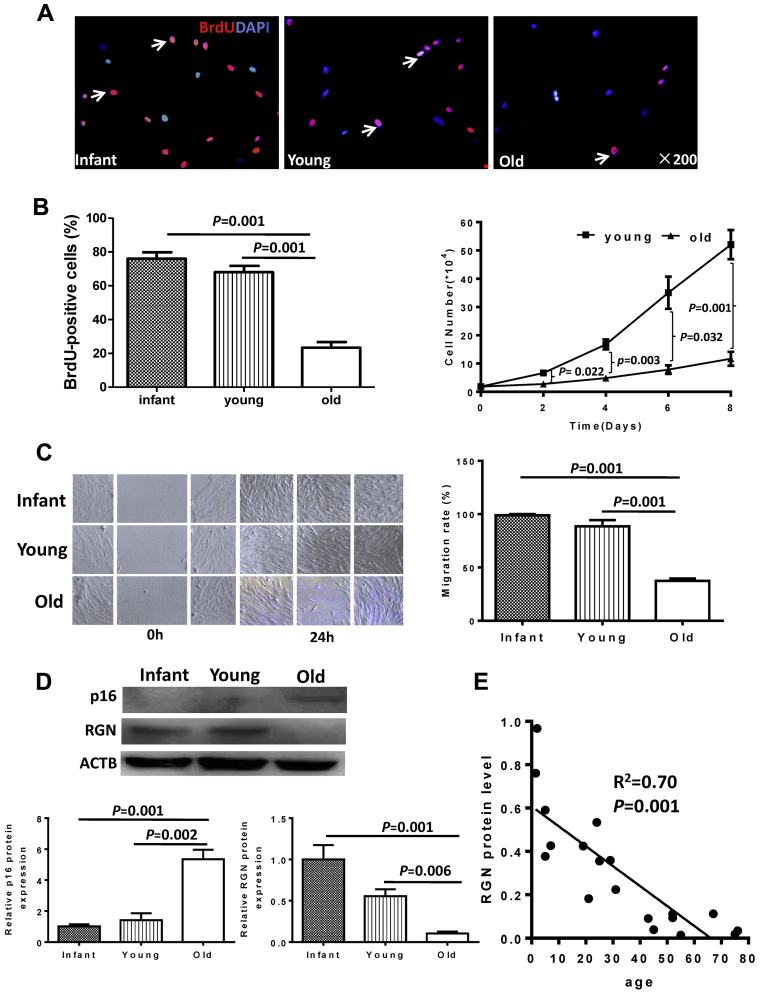
Decreased proliferation and migration and increased cell senescence in old hBM-MSCs
The percentage of bromodeoxyuridine-positive cells was more than 2-fold higher in hBM-MSCs from infant and young patients than in old patients (Figure 2A). The growth from young patients (Figure 2B) and migration (Figure 2C) rates of hBM-MSCs from infant and young patients were significantly higher than those from old patients were. Western blotting showed that the expression of senescence-associated protein cyclin-dependent kinase inhibitor 2A was significantly increased whereas expression of regucalcin (RGN) was significantly decreased in hBM-MSCs from old patients compared with those from infant and young patients (Figure 2D). There was an inverse correlation between RGN protein level and age, showing decreasing expression level of the protein with increasing age (Figure 2E).
Figure 2.
hBM-MSC Proliferation and Migration Decreased and the Senescence Phenomenon Increased With Age
(A) Representative micrographs of immunofluorescent staining for bromodeoxyuridine (BrdU) (red) with nuclei stained blue with DAPI. Arrows indicate BrdU+ cells. (B) The percentage of BrdU+ cells significantly decreased with age, n = 5/group. The rate of cell growth was significantly higher in young hBM-MSCs compared with old cells, n = 5/group. (C) Representative images show the cell migration of hBM-MSCs from the wound-scratch assay. Migration rates were significantly higher in infant and young cells compared with old cells, n = 3/group. (D) Western blot analyses of protein cyclin-dependent kinase inhibitor 2A (p16) and regucalcin (RGN) protein levels in hBM-MSCs revealed an obvious senescence phenomenon with significantly increased p16 and significantly decreased RGN expression in old compared with infant and young cells, n = 3/group. (E) The RGN protein level negatively correlated with age. The list of x (age) and y (RGN [regucalcin] protein level) was analyzed using a correlation regression analysis. Abbreviations as in Figure 1.
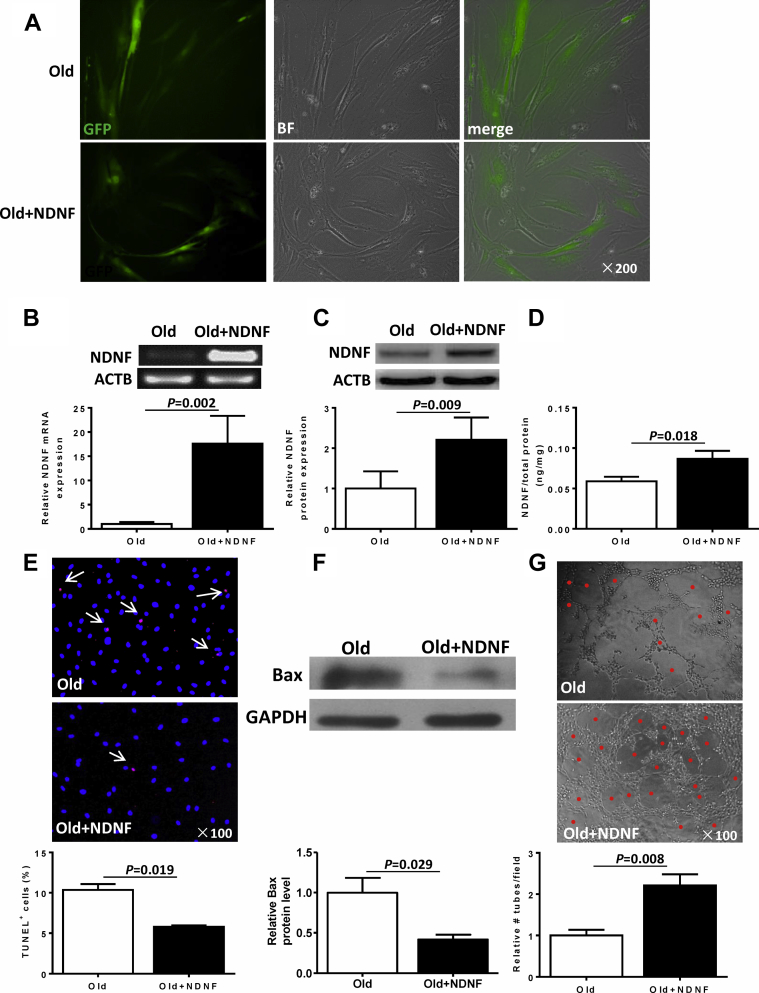
Overexpression of NDNF in old hBM-MSCs decreased hypoxia-induced apoptosis and increased angiogenesis
The transduction efficiency by a lentiviral-vector carrying the NDNF gene in old hBM-MSCs was detected by green fluorescent protein expression 72 h after transduction (Figure 3A). Transduction efficiency when quantified by green fluorescent protein–positive cells was comparable between the 2 groups (empty vector [old]: 70.1 ± 4.2%; NDNF [old+NDNF]: 68.2 ± 3.5%). NDNF mRNA expression was significantly greater in NDNF-transduced hBM-MSCs than in empty vector–transduced hBM-MSCs (Figure 3B). Expression of NDNF protein measured in cell lysates by Western blotting (Figure 3C) and in the supernatant of transduced cells by enzyme-linked immunosorbent assay (Figure 3D) were also significantly higher in NDNF-transduced cells. These results indicate that the NDNF gene was effectively transduced into old hBM-MSCs and these transduced cells were fully capable of secreting the NDNF protein. The percentage of apoptotic cells (terminal deoxynucleotidyl transferase dUTP nick end labeling positive) was almost 2-fold lower in NDNF-transduced old hBM-MSCs than in empty vector–transduced old hBM-MSCs that were cultured for 48 h under hypoxia conditions (Figure 3E). Proapoptosis-related protein Bax was significantly lower in NDNF-transduced cells under hypoxia conditions for 48 h (Figure 3F). Analysis of angiogenesis using the tube formation assay showed that the number of tubes formed was significantly higher in the human umbilical vein endothelial cells treated with the conditioned medium from NDNF-transduced cells (Figure 3G).
Figure 3.
Lentiviral Vector–Induced Overexpression of NDNF Reduced Apoptosis of Old hBM-MSCs and Increased Angiogenesis
(A) Representative fluorescent micrographs show green fluorescent protein–positive (GFP+) cells (green) to indicate transduced cells. (B) NDNF mRNA expression was significantly higher in NDNF-transduced (NDNF+old) than in empty vector–transduced (old) hBM-MSCs, n = 6/group. The expression of NDNF protein (C) in cell lysates evaluated by Western blotting (n = 5/group) and (D) in the supernatant of cells evaluated by enzyme-linked immunosorbent assay (n = 6/group) was significantly higher in NDNF-transduced hBM-MSCs. (E) Representative immunofluorescent staining for terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) (red) with nuclei stained blue with DAPI. Arrows indicate TUNEL+ cells. The percentage of TUNEL+ cells was significantly lower in NDNF-transduced cells than in empty vector–transduced cells, n = 3/group. (F) Western blot showed that the expression of the proapoptosis-related protein Bax was significantly lower in NDNF-transduced cells, n = 4/group. (G) Representative micrographs from the tube formation assay (red dots indicate tubular structure). The number of tubes formed was significantly higher in NDNF-transduced cells, n = 6/group. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; other abbreviations as in Figure 1.
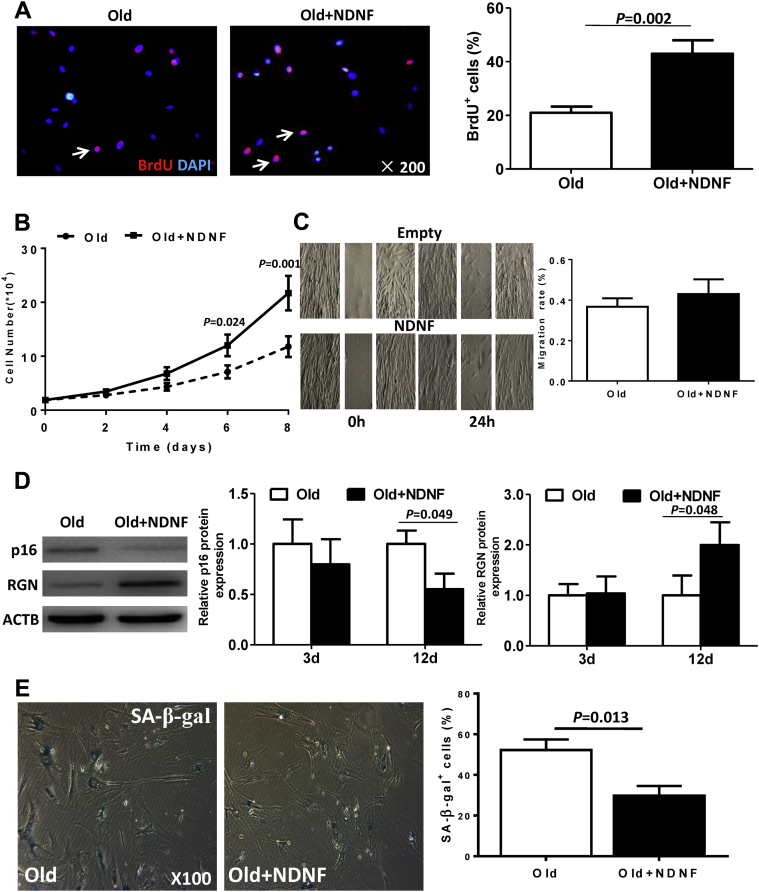
NDNF overexpression rejuvenated old hBM-MSCs by increasing proliferation and suppressing the senescence phenomenon
The percentage of bromodeoxyuridine-positive cells (Figure 4A) and the cell growth rate (Figure 4B) were significantly higher in NDNF-transduced old hBM-MSCs than empty vector–transduced old hBM-MSCs. However, there was no significant difference in migration rates between cells in the 2 groups (Figure 4C). The expression of senescence-associated protein cyclin-dependent kinase inhibitor 2A was significantly decreased while the expression of RGN was significantly increased in NDNF-transduced cells 12 days after transduction (Figure 4D). Further evaluation of senescence status with senescence-associated β-galactosidase staining revealed that NDNF-transduced old hBM-MSCs had fewer β-galactosidase–positive cells than empty vector–transduced old hBM-MSCs (Figure 4E). Expression of deoxyribonucleic acid damage proteins and cyclin-dependent kinase inhibitors was lower in NDNF-transduced old hBM-MSCs compared with control old hBM-MSCs (Supplemental Figure 1). Cell function examined in terms of self-renewal ability using clonogenic assay and in terms of differentiation potential to form adipocytic and osteocytic lineages also showed that NDNF-transduced old hBM-MSCs had a greater number of clonogenic cells and greater differentiation potentials compared with empty vector–transduced old hBM-MSCs (Supplemental Figure 2). On the other hand, knockdown of NDNF in young hBM-MSCs decreased cell proliferative capacity and increased the expression of deoxyribonucleic acid damage proteins and cyclin-dependent kinase inhibitors compared with control young hBM-MSCs (Supplemental Figure 3). Furthermore, knockdown NDNF decreased the number of clonogenic cells, though it did not alter the differentiation potential in young hBM-MSCs compared with young control hBM-MSCs (Supplemental Figure 4).
Figure 4.
NDNF Transduction Rejuvenated Old hBM-MSCs by Increasing Proliferation and Suppressing the Senescence Phenomenon
(A) Representative micrographs of immunofluorescent staining for BrdU (red) with nuclei stained blue with DAPI. Arrows indicate BrdU+ cells. The percentage of BrdU+ cells was significantly higher in NDNF-transduced (NDNF+old) compared with empty vector–transduced (old) hBM-MSCs, n = 5/group. (B) Cell growth curves showed that the growth rate was significantly higher in NDNF-transduced cells, n = 5/group. (C) Representative images show the cell migration of hBM-MSCs from the wound-scratch assay. There was no significant difference in migration rate between the 2 groups, n = 6/group. (D) Western blot showed that the expression of the senescence-associated (SA) protein p16 significantly decreased and RGN significantly increased in NDNF-transduced cells 12 days after transduction, n = 5/group. (E) Representative micrographs of senescence-associated β-galactosidase (β-gal) staining and quantification of β-gal+ cells. The percentage of β-gal+ cells was significantly higher in empty vector–transduced (old) hBM-MSCs compared with NDNF-transduced (NDNF+old) hBM-MSCs, n = 5/group. Abbreviations as in Figures 1 and 2.
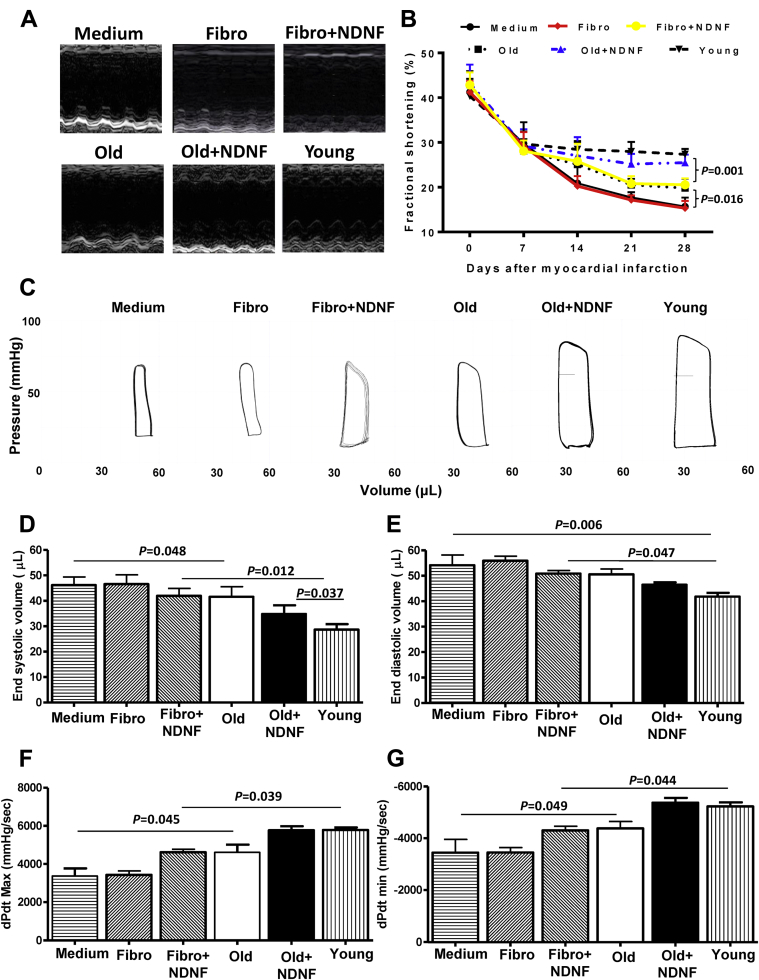
Engraftment of NDNF overexpressing old hBM-MSCs into the ischemic area of mouse hearts improved cardiac function after MI
Cardiac function was determined by echocardiography and pressure-volume loop analysis in mice that received implantation of control medium (medium), empty vector–transduced human dermal fibroblasts (fibro), NDNF-transduced human dermal fibroblasts (fibro+NDNF), empty vector–transduced old hBM-MSCs (old), NDNF-transduced old hBM-MSCs (old+NDNF), or untransduced young hBM-MSCs (young) into the border region immediately following MI. Representative M-mode echocardiographic images were captured 28 days after MI in mice (Figure 5A). Fractional shortening was similar among all 6 groups before MI and cell transplantation (day 0) and decreased by a similar degree 7 days following MI. However, 28 days after MI, fractional shortening was significantly greater in the old+NDNF group than in the fibro+NDNF and old groups (Figure 5B). Representative pressure-volume loop images taken 28 days after MI are depicted in Figure 5C. The end-systolic volume of old+NDNF mice was significantly smaller than that of the fibro+NDNF and old groups 28 days after MI (Figure 5D), while end-diastolic volume was smallest in young mice (Figure 5E). Systolic function as measured by dP/dt max (Figure 5F) and diastolic function as measured by dP/dt min (Figure 5G) was significantly better in the old+NDNF group than in the fibro+NDNF and old groups 28 days after MI.
Figure 5.
In Vivo Engraftment of NDNF Overexpressing Old hBM-MSCs Improved Cardiac Function After MI
(A) Representative M-mode echocardiographic images taken 28 days after myocardial infarction (MI) in mice that received implantation of control medium (medium; n = 5), empty vector–transduced human dermal fibroblasts (fibro; n = 6), NDNF-transduced human dermal fibroblasts (fibro+NDNF; n = 6), empty vector–transduced old hBM-MSCs (old; n = 6), NDNF-transduced old hBM-MSCs (old+NDNF; n = 7), or untransduced young hBM-MSCs (young; n = 5) into the border region immediately following MI. (B) Fractional shortening was significantly higher in old+NDNF than in old mice 28 days after MI. (C) Representative pressure-volume loop images taken 28 days after MI. (D) End-systolic and (E) end-diastolic volumes were smallest in young and old+NDNF mice after MI. (F) Systolic function as measured by dP/dt max and (G) diastolic function as measured by dP/dt min were significantly better in old+NDNF mice than in old mice. dP/dt max = maximal rate of pressure increase during isovolumic contraction; dP/dt min = maximal rate of pressure decrease during isovolumic relaxation; other abbreviations as in Figure 1.
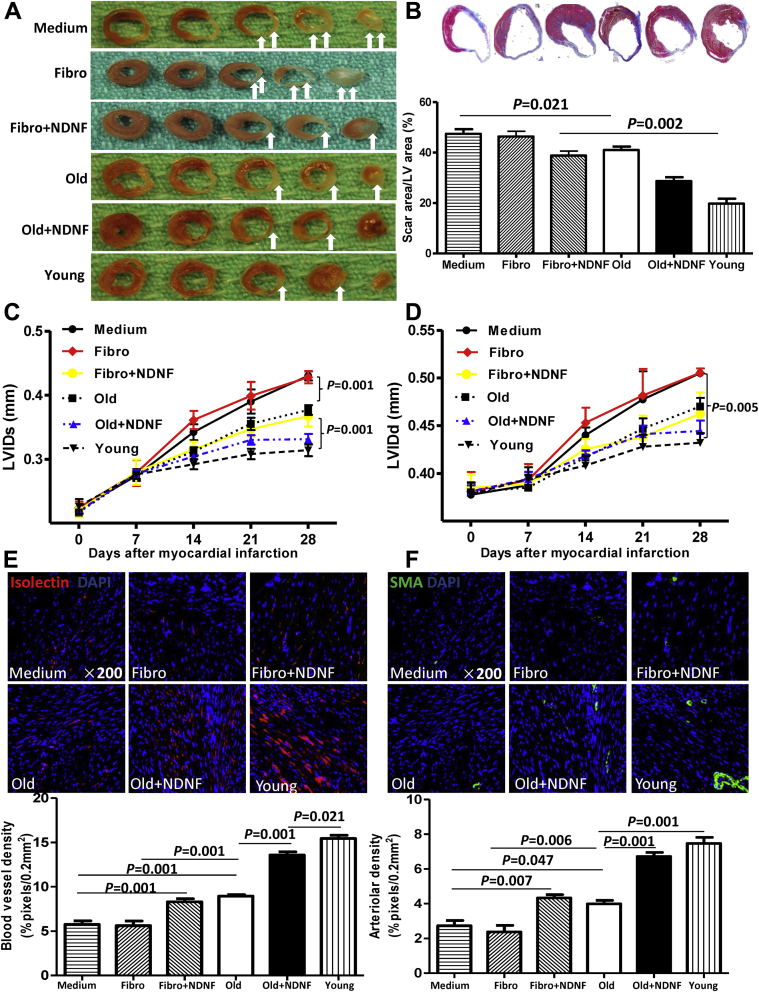
Engraftment of NDNF overexpressing old hBM-MSCs into the ischemic area of mouse hearts decreased scar size and increased angiogenesis after MI
Computerized planimetry on explanted hearts fixed at physiological pressure (Figures 6A and 6B) showed that the scar area was largest in mice from the medium and fibro groups and smallest in mice from the young and old+NDNF groups. Most important, the scar size was significantly smaller in old+NDNF mice than in NDNF+fibro and old mice (Figure 6B). Left ventricular dimensions were also assessed at various time points after MI by echocardiography (Figures 6C and 6D). Left ventricular internal end-systolic dimension (Figure 6C) was significantly lower in old+NDNF mice than in NDNF+fibro and old mice 28 days after MI. Both capillary (stained with isolectin) (Figure 6E) and arteriole (stained with alpha smooth muscle actin) (Figure 6F) densities were significantly higher in the old+NDNF group than in the NDNF+fibro and old groups. Taken together, these functional results suggest that NDNF-transduced aged hBM-MSCs improved ventricular function and reduced adverse left ventricular remodeling after MI. Furthermore, NDNF alone improved cardiac function and angiogenesis. However, NDNF and hBM-MSCs act synergistically to further enhance the effects on cardiac function and angiogenesis.
Figure 6.
In Vivo Engraftment of NDNF Overexpressing Old hBM-MSCs Decreased Scar Size and Increased Angiogenesis After MI
(A) Representative heart sections 28 days after myocardial infarction (MI) (white arrows indicate scar area) in mice that received implantation of control medium (medium), empty vector–transduced human dermal fibroblasts (fibro), NDNF-transduced human dermal fibroblasts (fibro+NDNF), empty vector–transduced old hBM-MSCs (old), NDNF-transduced old hBM-MSCs (old+NDNF), or untransduced young hBM-MSCs (young) into the border region immediately following MI. (B) Representative heart slides stained with Masson’s trichrome and planimetry-based quantification revealed that the scar size area was largest in the medium and fibro groups 28 days after MI. The scar size was significantly larger in the fibro+NDNF and old groups 28 days after MI than in the young and old+NDNF groups. (C) Left ventricular (LV) internal end-systolic dimension (LVIDs) was significantly lower in the young and old+NDNF groups 28 days after MI and (D) LV internal end-diastolic dimension (LVIDd) was significantly lower in the young group. Representative micrographs of heart tissue showing (E) capillary (stained with isolectin, red) and (F) arteriole (stained with isolectin, red) densities. Densities were significantly higher in the old+NDNF group than in the old group, n = 5/group. SMA = smooth muscle actin. Abbreviations as in Figure 1.
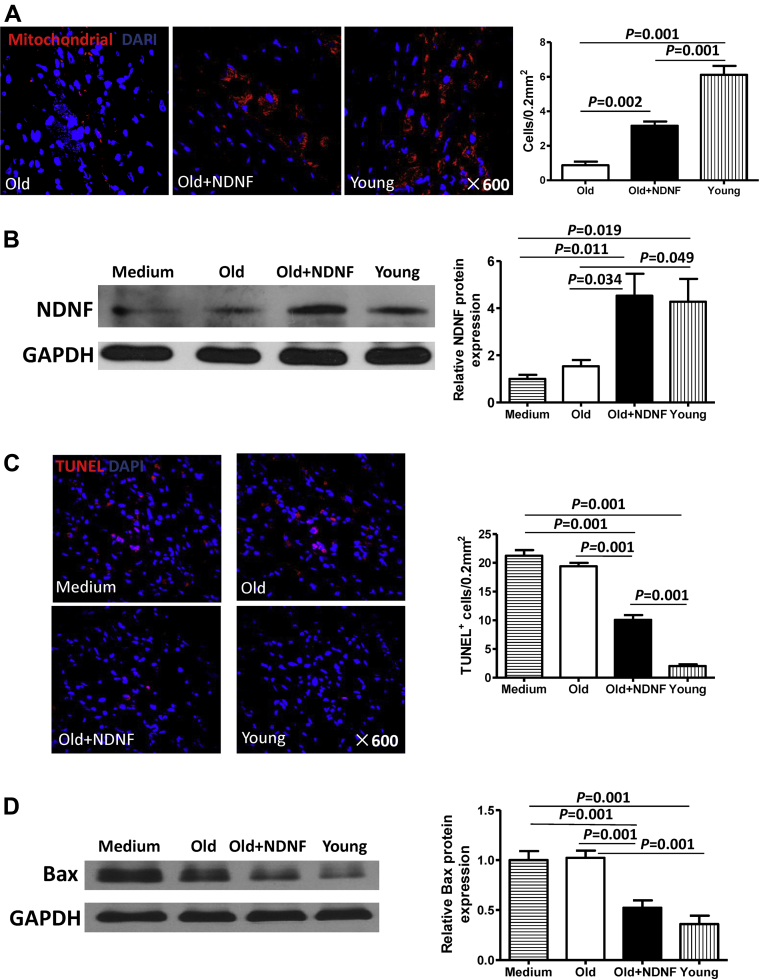
NDNF overexpression increased donor cell survival and decreased apoptosis in the mouse heart after MI
Quantification of surviving donor stem cells in heart slices stained for human-specific mitochondria showed that NDNF overexpression improved donor cell survival following MI (Figure 7A). Although the number of surviving donor cells was highest in hearts from the young group, cell survival was significantly higher in the old+NDNF group than in the old group (Figure 7A). Evaluation of NDNF protein expression in the border area of hearts from mice in the 4 experimental groups revealed significantly increased expression in the old+NDNF and young groups compared with the medium and old groups (Figure 7B). Quantification of terminal deoxynucleotidyl transferase dUTP nick end labeling–positive cells showed that the number of apoptotic cells in the ischemic area of mouse hearts was the lowest in the young group (Figure 7C). Significantly fewer apoptotic cells were found in the old+NDNF mouse hearts than in the old mouse hearts (Figure 7C). Consistent with this finding, Western blotting revealed significantly lower expression of the proapoptotic protein Bax in the old+NDNF infarct tissue samples compared with the old infarct tissue samples (Figure 7D).
Figure 7.
In Vivo Engraftment of NDNF Overexpressing Old hBM-MSCs Increased Cell Survival and Decreased Apoptosis in the Heart After MI
(A) Representative heart tissue micrographs of immunofluorescent staining for human mitochondria (red) from mice that received implantation of empty vector–transduced old hBM-MSCs (old), NDNF-transduced old hBM-MSCs (old+NDNF), or untransduced young hBM-MSCs (young) into the border region immediately following myocardial infarction (MI). Nuclei are stained blue with DAPI. Quantification of surviving donor cells showed that NDNF overexpression improved cell survival. (B) Western blot analysis of the expression of NDNF protein in the border area of mouse hearts revealed significantly higher expression in the old+NDNF and young groups compared with the old and medium control groups. (C) Representative heart tissue micrographs of immunofluorescent staining for TUNEL (red) with nuclei stained blue with DAPI. Significantly fewer TUNEL+ cells were found in the ischemic area of old+NDNF hearts than in old hearts. (D) Western blot analysis of the expression of the proapoptotic protein Bax in the ischemic area of mouse hearts revealed significantly lower expression in the old+NDNF group than in the old group, n = 5/group. Abbreviations as in Figures 1 and 3.
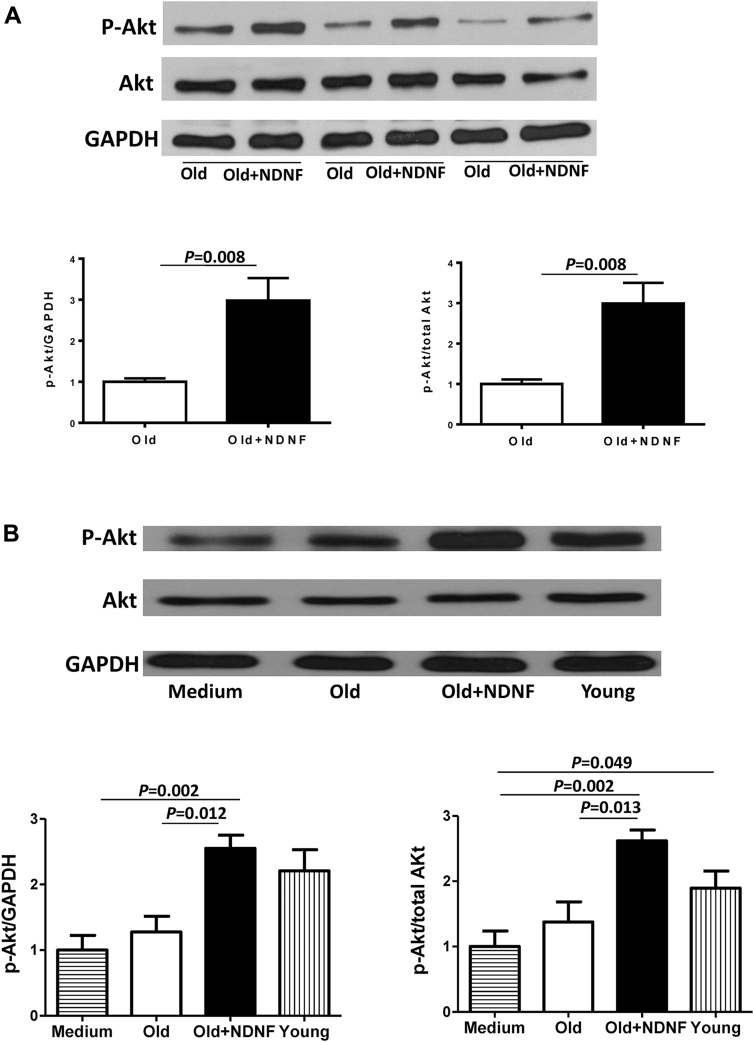
NDNF overexpression activated phosphorylated Akt pathway in vitro and in vivo
Phosphorylated Akt (p-Akt) expression was significantly higher in NDNF-transduced old hBM-MSCs than in empty vector–transduced old hBM-MSCs in vitro, whereas total Akt expression was similar in the 2 groups (Figure 8A). Consistent with this finding, Western blotting conducted using heart tissue from the border region of mice in the 4 experimental groups revealed significantly greater p-Akt expression in the old+NDNF group than in the old group (Figure 8B).
Figure 8.
NDNF Rejuvenated Old hBM-MSCs and Improved Cardiac Function Through Activation of p-Akt
(A) The phosphorylated Akt (p-Akt) level in old NDNF-transduced hBM-MSCs (old+NDNF) was significantly higher than in empty vector–transduced cells (old). (B) Western blotting showed the expression of p-Akt protein in the border area of mouse heart tissue after implantation of control medium (medium), empty vector–transduced old hBM-MSCs (old), NDNF-transduced old hBM-MSCs (old+NDNF), or untransduced young hBM-MSCs (young) into the border region immediately following myocardial infarction (MI). The p-Akt level was significantly higher in the old+NDNF group than in the old group, n = 5/group. Abbreviations as in Figures 1 and 3.
Discussion
Several clinical and preclinical studies have shown that autologous and allogeneic cells (both MSCs and heart-derived cells) are equally potent (16). Hare et al. (16) performed one of the first major MSC therapy trials, a phase I, randomized, double-blinded, placebo-controlled test of allogeneic bone marrow–derived MSCs in patients with acute MI. This study not only demonstrated the safety of MSCs, but also found that the MSC-treated groups experienced fewer arrhythmias and improved left ventricular ejection fraction that was sustained for 6 months after treatment. These findings led to a subsequent phase II study that established that an infusion of MSCs within 7 days of an acute MI significantly reduced cardiac hypertrophy, arrhythmia, progression to heart failure, and rehospitalization for cardiac complications (22). Hare et al. (17) also conducted the phase I/II randomized POSEIDON (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis) trial to determine the effects on neomyogenesis with the purpose of comparing autologous and allogeneic bone marrow MSCs in the treatment of ischemic cardiomyopathy. Though the study was limited by the lack of a placebo control group and a small participant population (n = 30), the results were encouraging. Injected transendocardially, both autologous and allogeneic MSCs showed equally low rates of adverse events and were associated with reversal of left ventricular remodeling, reduction in myocardial scarring, and better quality of life. On the other hand, studies of cardiosphere-derived cells (CDCs) suggested that the benefits stem cells were not sustained. Malliaras et al. (23) conducted a preclinical study to compare among syngeneic, allogeneic, and xenogeneic CDCs for cardiac regeneration in a rat model of MI. They found that syngeneic and allogeneic CDCs survived at similar levels in rat hearts 1 week after cell delivery, but few syngeneic (and even fewer allogeneic) CDCs persisted at 3 weeks. However, improvements in cardiac structure and function were comparable with syngeneic and allogeneic CDCs up to 6 months after cell delivery. Allogeneic CDCs stimulated endogenous regenerative mechanisms and increased myocardial angiogenic factors equally with syngeneic CDCs. The persistence of benefit despite a transient survival of the transplanted cells suggested an indirect mechanism of action involving paracrine effects. They believed that allogeneic CDC therapy without immunosuppression was safe and improved heart function. However, we performed a preclinical study in a rat MI model that suggested that the benefits of syngeneic stem cell therapy were sustained longer than allogeneic cells. We implanted allogeneic or syngeneic MSCs into infarcted rat myocardium. We found that MSCs (vs. media) significantly improved ventricular function for at least 3 months after engraftment. However, allogeneic, but not syngeneic, cells were eliminated from the heart by 5 weeks after engraftment, and their functional benefits were lost within 5 months. We concluded that the long-term ability of allogeneic MSCs to preserve function in the infarcted heart is limited by a biphasic immune response whereby they transition from an immunoprivileged to an immunogenic state after differentiation, which is associated with an alteration in major histocompatibility complex–immune antigen profile (24). We believe that the use of autologous cells still has the inherent advantage that cellular rejection is obviated by default. However, the benefits of autologous cells are diminished in older patients, as both the quantity and regenerative capacity of stem cells decreases significantly with age 10, 12, 13. Therefore, it is still worthwhile to explore the possibility of rejuvenating of the autologous cells obtained from old patients by identifying factors to further amplify the efficacy of cell therapy.
Our recent gene expression analyses on young and old hBM cells (J.G. Guo, unpublished data, December 2013) showed that the expression of NDNF was significantly affected by age. Such findings suggest that NDNF may play an important role in human tissue rejuvenation and repair. Indeed, hBM-MSCs from older patients expressed significantly lower levels of NDNF than hBM-MSCs from younger patients that were associated with lower proliferation and migration rates compared with young cells. Overexpression of NDNF in old hBM-MSCs using a lentiviral expression vector increased cell proliferation, survival, and angiogenesis and decreased apoptosis in vitro. In vivo implantation of these NDNF overexpressing old hBM-MSCs immediately following MI into the cardiac border area of mice decreased scar size and improved cardiac function while increasing implanted cell survival and NDNF level. The engraftment of rejuvenated old cells promoted angiogenesis and induced resistance to hypoxia-induced apoptosis.
Establishing that the expression level of stem cell rejuvenation factors diminishes naturally with age and supports the logical supposition that increasing its availability will facilitate rejuvenation. Consistent with this notion, we found a significant negative correlation between age and hBM-MSC NDNF expression. We then proved that increasing NDNF expression in old hBM-MSCs promoted rejuvenation by increasing cell proliferation and attenuating the senescence phenomenon. These NDNF overexpressed old hBM-MSCs implanted into the infarct region of mouse hearts were less susceptible to hypoxia-induced apoptosis and had better survival after MI, thereby facilitating improved cardiac functional recovery. These results are in agreement with studies showing that NDNF can promote growth and elicit neurite outgrowth of mouse hippocampal neurons (19), as well as reduce apoptosis of cardiac fibroblasts and neonatal rat ventricular cardiomyocytes (21).
Previous studies have shown that NDNF is proangiogenic, improving blood flow recovery and increasing capillary density following ischemia in the limbs of mice (20) and increasing angiogenesis in the heart after MI (21). In this study we demonstrated that cardiac engraftment of NDNF overexpressing hBM-MSCs acted as a proangiogenic cytokine carrier, allowing increased secretion of the proangiogenic factor NDNF into the infarct region and promoting angiogenesis and increasing capillary and arteriole densities.
Akt is a key cell survival factor. Our results indicate that NDNF activated the p-Akt pathway in vitro and in vivo, suggesting that the rejuvenation effects of NDNF may be mediated by this pathway. This finding is consistent with a recent study showing that systemic up-regulation of NDNF promoted the phosphorylation of Akt in cardiomyocytes (21). The next steps are to identify the precise mechanisms by which NDNF improves the function of aged human stem cells, including the particular receptors and upstream and downstream targets involved.
Study limitations
Finally, it is noteworthy to mention that a disease-free aged matched control group was not included because of the limited availability of such samples. Therefore, interpretation of data generated from such complex cohort of samples was difficult, particularly when the focus of this study was aging and rejuvenation. It is plausible to consider that patients who suffered from cardiovascular disease had an acceleration of aging or premature aging caused by disease. Further validation of the current findings to exclude the influence of confounding factors such as cardiovascular disease will be required.
Conclusions
Our findings suggest that NDNF could be a new factor that contributes to the rejuvenation of aged human stem cells and NDNF-rejuvenated stem cells may be a potent vehicle to combine cell and gene therapies to improve cardiac repair of the aged heart after ischemic injury.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Autologous stem cell transplantation is a promising therapy for cardiac repair. Bone marrow MSCs represent an accessible resource with great capacity for self-renewal and multipotent differentiation. However, the reduced regenerative capacity of aged stem cells hampers the benefits of cardiac cell therapy. NDNF rejuvenated aged human stem cells, and improved their capability to repair the aged heart after ischemic injury. NDNF could be a new factor to rejuvenate aged stem cells and improve their capability to repair the aged heart after injury.
TRANSLATIONAL OUTLOOK: Autologous cell therapy for cardiac regeneration is an effective therapy, but aging is a factor that limits the quality and quantity of stem cells. Our findings suggest that simple modification of aged stem cells may rejuvenate their function. It will be important to determine if the beneficial effects of cardiac engraftment of NDNF overexpressing aged stem cells can be shown in large animal models to provide further support for the use of NDNF for clinical applications.
Acknowledgment
The authors thank Dr. Leigh Botly for assistance with manuscript writing, preparation, and editing.
Footnotes
This work was supported by the 331 Early Career Researcher Grant from the Basic Medical School, Shanxi Medical University (no. 201217) awarded to Dr. Xie; and a Foundation grant from the Canadian Institutes of Health Research (no. 332652) award to Dr. Li. Dr. Li holds a Tier 1 Canada Research Chair in Cardiac Regeneration. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Jun Xie, Email: junxie@sxmu.edu.cn.
Ren-Ke Li, Email: renkeli@uhnresearch.ca.
Appendix
References
- 1.Newton J.N., Briggs A.D.M., Murray C.J.L. Changes in health in England, with analysis by English regions and areas of deprivation, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2257–2274. doi: 10.1016/S0140-6736(15)00195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Go A.S., Mozaffarian D., Roger V.L. Executive summary: heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 3.Moran A.E., Forouzanfar M.H., Roth G.A. The global burden of ischemic heart disease in 1990 and 2010: the Global Burden of Disease 2010 study. Circulation. 2014;129:1493–1501. doi: 10.1161/CIRCULATIONAHA.113.004046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heldman A.W., DiFede D.L., Fishman J.E. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karantalis V., DiFede D.L., Gerstenblith G. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: the Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanina C., Hare J.M. Mesenchymal stem cells as a biological drug for heart disease: where are we with cardiac cell-based therapy? Circ Res. 2015;117:229–233. doi: 10.1161/CIRCRESAHA.117.306306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James S., Fox J., Afsari F. Multiparameter analysis of human bone marrow stromal cells identifies distinct immunomodulatory and differentiation-competent subtypes. Stem Cell Rep. 2015;4:1004–1015. doi: 10.1016/j.stemcr.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatzistergos K.E., Quevedo H., Oskouei B.N. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loffredo F.S., Steinhauser M.L., Gannon J., Lee R.T. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H., Fazel S., Tian H. Increasing donor age adversely impacts beneficial effects of bone marrow but not smooth muscle myocardial cell therapy. Am J Physiol Heart Circ Physiol. 2005;289:H2089–H2096. doi: 10.1152/ajpheart.00019.2005. [DOI] [PubMed] [Google Scholar]
- 11.Kan C.D., Li S.H., Weisel R.D., Zhang S., Li R.K. Recipient age determines the cardiac functional improvement achieved by skeletal myoblast transplantation. J Am Coll Cardiol. 2007;50:1086–1092. doi: 10.1016/j.jacc.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 12.Dimmeler S., Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimmeler S., Vasa-Nicotera M. Aging of progenitor cells: limitation for regenerative capacity? J Am Coll Cardiol. 2003;42:2081–2082. doi: 10.1016/j.jacc.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Fan M., Chen W., Liu W. The effect of age on the efficacy of human mesenchymal stem cell transplantation after a myocardial infarction. Rejuvenation Res. 2010;13:429–438. doi: 10.1089/rej.2009.0986. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y., Liu T., Han J. Advanced age impairs cardioprotective function of mesenchymal stem cell transplantation from patients to myocardially infarcted rats. Cardiology. 2014;128:209–219. doi: 10.1159/000360393. [DOI] [PubMed] [Google Scholar]
- 16.Hare J.M., Traverse J.H., Henry T.D. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hare J.M., Fishman J.E., Gerstenblith G. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lein E.S., Hawrylycz M.J., Ao N. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 19.Kuang X.L., Zhao X.M., Xu H.F., Shi Y.Y., Deng J.B., Sun G.T. Spatio-temporal expression of a novel neuron-derived neurotrophic factor (NDNF) in mouse brains during development. BMC Neurosci. 2010;11:137. doi: 10.1186/1471-2202-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohashi K., Enomoto T., Joki Y. Neuron-derived neurotrophic factor functions as a novel modulator that enhances endothelial cell function and revascularization processes. J Biol Chem. 2014;289:14132–14144. doi: 10.1074/jbc.M114.555789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joki Y., Ohashi K., Yuasa D. Neuron-derived neurotrophic factor ameliorates adverse cardiac remodeling after experimental myocardial infarction. Circ Heart Fail. 2015;8:342–351. doi: 10.1161/CIRCHEARTFAILURE.114.001647. [DOI] [PubMed] [Google Scholar]
- 22.Telukuntla K.S., Suncion V.Y., Schulman I.H., Hare J.M. The advancing field of cell-based therapy: insights and lessons from clinical trials. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malliaras K., Li T.-S., Luthringer D. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation. 2012;125:100–112. doi: 10.1161/CIRCULATIONAHA.111.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X.P., Sun Z., Miyagi Y. Differentiation of allogeneic mesenchymal stem cells induces immunogenicity and limits their long-term benefits for myocardial repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.