Summary
With drug approval times taking an average of 8 years from entry into clinical trials to full U.S. Food and Drug Administration (FDA) approval, patients with life-threatening and severely debilitating disease and no reasonable therapeutic options are advocating for expanded access (EA) to investigational drugs prior to approval. Special investigational new drug (IND) application categories allow patients who meet specific criteria to receive treatment with non-approved drugs. The FDA approves over 99% of all single-patient INDs, providing emergency approval within hours, and non-emergency approval within an average of 4 days. “Right-to-try” laws passed in 38 states would allow patients to bypass FDA processes altogether, but contain controversial provisions that some claim risk more harm than benefit to desperate and vulnerable patients. This review focuses on FDA EA to non-approved drugs through a special category of IND—the single-patient IND—and “right-to-try” (R2T) access outside of the FDA.
Key Words: compassionate use, EIND, emergency IND, expanded access, right-to-try, single-patient IND
Abbreviations and Acronyms: EAP, expanded access program; EIND, emergency investigational new drug; FDA, U.S. Food and Drug Administration; IND, investigational new drug (filing); IRB, institutional review board; LOA, letter of authorization; R2T, right-to-try; TIND, treatment investigational new drug
Answering a call to curb interstate market for adulterated and/or mishandled food and pharmaceuticals, the Federal Food, Drug and Cosmetics Act of 1938 required safety approval of all drugs by the FDA prior to marketing (1). Later amendments to federal law required that a drug must be proven effective as well as safe before marketing in the United States (2). The FDA is now among the largest of consumer protection agencies in the world, balancing increasing pressure to expedite the development and release of new and effective therapies to patients, against a mission to simultaneously minimize harm. Meanwhile, drug innovation, at least for some classes of drugs such as cardiovascular therapies, is slowing (3), and the costs of drug development are skyrocketing. Public pressure to expedite the deployment of new medical therapeutics has led to numerous recent legislative interventions.
Once drugs have passed preclinical conceptualization, manufacture, in vitro testing and in vivo animal testing, they enter the phase of development that requires in-human demonstration of safety and efficacy for their intended purpose. In order to enter this critical and costly development stage, drugs must be filed with the FDA (the investigational new drug [IND] filing), so that the agency can monitor in-human testing via periodic reports, inspections and audits as the entity progresses through clinical trials before an application is submitted for FDA marketing approval and the drug becomes generally available to the public.
Recent federal legislation seeking to expedite drug deployment to patients may significantly affect how such therapeutics enter the FDA processes and how soon they reach the public. This article discusses the FDA process for individual patient access to non-approved drugs, and explores “right-to-try” (R2T) legislation that is intended to facilitate the entry of new therapeutics into clinical use prior to full FDA approval.
Investigational New Drug INDs
The basic IND filing is a request for FDA authorization A to administer a non-approved drug or biological product to humans so that evidence of efficacy and safety can be obtained for marketing approval. In addition, federal law requires that a drug be the subject of an approved marketing application before it can be transported or distributed across state lines. Long before FDA approval, the drug developer will usually want to ship the drug to clinical investigators in multiple states, and they must obtain an exemption from that law. The IND application provides the means by which a drug sponsor can acquire this exemption (4). Thirty days after the sponsor files an IND application, unless prevented by a directive from the FDA, they may begin the long process of clinical testing. The average time to complete all phases of clinical trials is approximately 8 years (1).
In many cases, drugs may be able to enter clinical use before achieving full FDA approval, through special expanded access (EA) IND filings. In addition, most states have now passed R2T legislation aimed at guaranteeing that certain patients can receive experimental therapies without going through the FDA, before full FDA approval has been granted.
INDs have 2 major classifications—research or commercial—depending upon the sponsor for the drug development and intended destination of the drug. The FDA defines an IND as commercial if the sponsor is a corporate entity or 1 of the institutes of the National Institutes of Health (NIH), or if it is clear that the drug may eventually become commercialized (5). The IND is called a research IND if the drug is sponsored by an individual. Within both the research and commercial INDs are IND subcategories. The “investigator IND” is the most common type of research or commercial IND, in which the investigator initiates and conducts studies and provides immediate supervision of the study drug. A more detailed review of the Investigator IND application process has been published previously (1).
FDA Expanded Access INDs for Nonapproved Drugs
The FDA began facilitating access to non-approved drugs in the 1970s, although it took until 1987 for a specific pathway for such access to be developed (6). Revised regulations regarding EA access to investigational drugs were published in 2009, with revised guidance for industry update as recently as October 2017 (7).
FDA EA falls into 3 categories: 1) individual access to INDs, including emergency use (EIND); 2) access for intermediate-size patient populations; and 3) widespread treatment use, or treatment investigational new drugs (TIND) 7, 8. Certain requirements apply to all 3 categories: 1) the patient must have a serious or immediately life-threatening disease or condition, and have no comparable therapy or satisfactory alternative therapy; 2) the potential benefit must justify the potential risks of the treatment; and 3) providing the treatment must not interfere with or compromise the drug development program (e.g., by critically depleting a supply of a drug that is needed for conducting clinical studies) (9).
The “widespread” TIND is typically obtained to bridge the gap between completion of clinical trials and full FDA approval. Patients in phase 3 clinical trials who are benefitting from the new drug, for example, may be allowed to continue treatment after study completion while full approval is obtained (10). An intermediate-size treatment IND has no specific population size definition, but is used when more than 1 patient will be treated, or when the drug is not being actively developed for market. An intermediate-size early access IND might be created by consolidating multiple single-patient IND requests for the same drug. An individual, or single-patient IND allows treatment of a single patient with a non-approved drug. The EIND is a subcategory of the individual patient IND, for when a patient requires emergency treatment and cannot wait for the FDA 30-day review period. The evidentiary threshold to prove to the FDA’s satisfaction that benefits outweigh the harms are higher as more patients are involved in the IND. Thus, a widespread or intermediate-size treatment IND may require significant clinical evidence in the form of studies, while for a single-patient IND the physician need only conclude that the drug does not pose greater risk than the disease itself (10).
The Single Patient IND
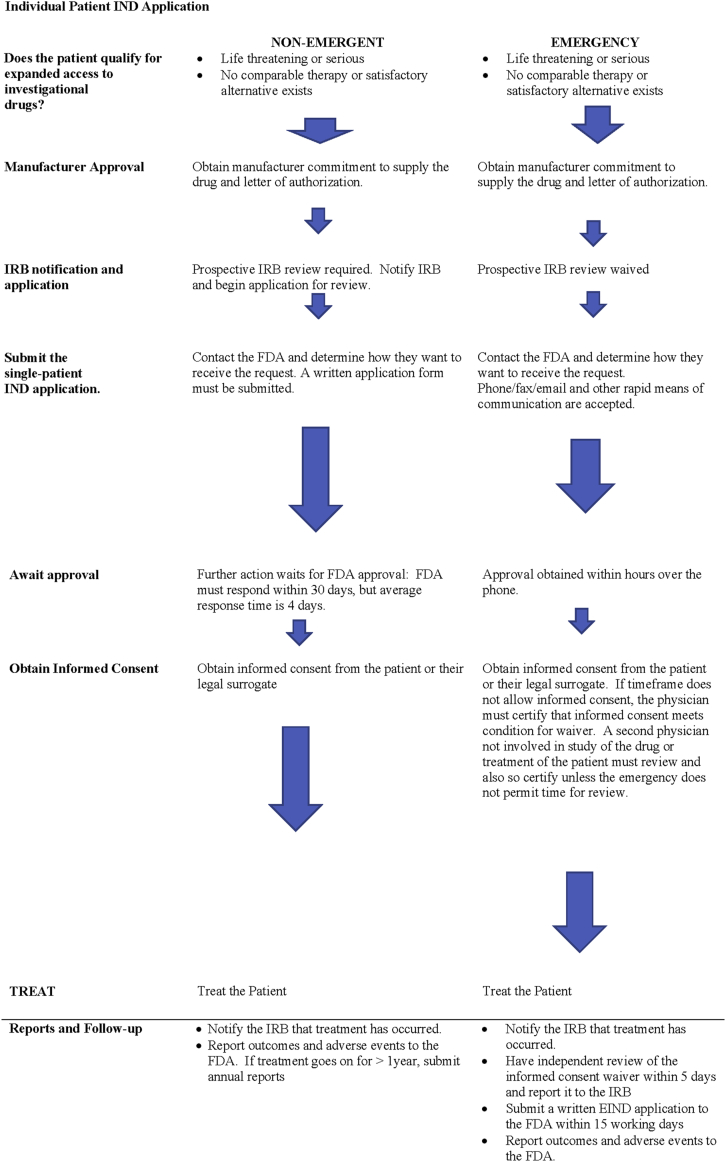
With rare exceptions, commercial sponsors do not apply for individual patient INDs, since it is rare for a commercial entity to directly oversee the treatment of an individual patient. Although the manufacturer will have an IND filing with the FDA to conduct clinical trials, in single patient use, the treating physician must usually obtain a separate IND. Obtaining an individual patient IND, follows similar steps, whether in an emergency or not (Figure 1). The treating physician must first obtain the drug company’s agreement to provide the drug, since the FDA cannot compel the company to do so. Once company approval has been obtained, the physician agrees to obtain local prospective institutional review board (IRB) approval to administer the drug, or else to certify to the FDA that they meet conditions for a waiver of prospective IRB review. Once the manufacturer is willing to supply the drug, and IRB approval is underway (or a waiver is claimed), a single patient IND application must be made to the FDA. In nonemergent cases, the individual patient IND filing starts the clock on a 30-day review period in which the FDA is required to respond. If there is no objection within 30 days, or if the FDA serves affirmative notification sooner, treatment may begin. In emergency conditions when immediate treatment is sought, EIND approval can be obtained from the FDA over the phone. A written individual patient IND application is still required to be filed for the EIND within 15 days of initial FDA notification.
Figure 1.
Application Flow for Single Patient Non-Emergency and Emergency IND
FDA = U.S. Food and Drug Administration; IND = investigational new drug; IRB = institutional review board.
The FDA receives on average 1,000 requests for individual patient INDs annually. Recently published information by the FDA: 1) provides instructions on applying for individual patient expanded access (11); 2) describe circumstances under which the FDA will permit charging for the drug in clinical trials and in expanded access use, and which costs can be recovered (12); and 3) give detailed information about the EAP, such as what information is needed for expanded access requests (9).
Applying for an Individual Patient IND
Step 1: Obtain manufacturer agreement to supply the drug
Although advocates for early access to investigational drugs often cite the FDA as the cause of excessive delays in obtaining drugs for compassionate use, in fact the early rate limiting step in obtaining approval for individual patient INDs is agreement to supply the drug by manufacturer. Historically, the initial step in evaluating compassionate use requests has rested exclusively or predominantly with drug company employees prior even to FDA review (13).
For a single patient IND, whether emergent or not, the manufacturer must supply a letter of authorization (LOA) for the investigator to submit along with the FDA application. This LOA not only confirms that manufacturer will make the drug available, but authorizes the FDA to access any of the FDA’s existing files on the drug on behalf of the individual patient IND. Since investigational drugs sought after for individual patient use are already the subject of an existing, investigator IND, accessing this information significantly streamlines the application process. An example of appropriate wording of the LOA is provided by the FDA (14).
If for any reason the manufacturer refuses to supply an LOA, the FDA instructs treating physicians to contact them directly to determine if other existing information can be used for the individual patient IND. However, such a refusal would certainly delay, if not entirely prevent, individual patient access (15).
Step 2: Initiate contact with the institutional IRB
The FDA requires prospective IRB review and approval before a non-emergent individual patient IND can be approved (7). Simple notification of the IRB is not to be construed as approval. However, the FDA recognizes that it may be difficult to convene an IRB review board under the emergency conditions that warrant an EIND, and agrees that for EINDs, prospective IRB approval can be waived. In order to so, however, conditions for waiver must meet all of the requirements described in FDA regulations for “life-threatening” or “severely debilitating” condition (Table 1). Any “subsequent use” of the drug after initial emergency treatment is subject to prospective IRB review and approval. The FDA defines “use” and “subsequent use” as either a single dose, or a single course of treatment (16). If the investigator anticipates a second course of treatment, IRB review is required, however the FDA also states that “in spite of the best efforts of the clinical investigator and the IRB,” the need may arise for a second emergency use. The FDA states that it believes “it is inappropriate to deny emergency treatment to an individual when the only obstacle is lack of time for the IRB to convene, review the use and give approval” (16).
Table 1.
FDA Definitions for Immediately Life-Threatening or Serious Disease or Condition for Single Patient IND (8)
Immediately life-threatening disease or condition
|
Serious disease or condition
|
The physician should always determine what local institutional IRB processes are regarding emergency use of investigational agents. Some institutions define a time frame that is considered too short for full review—i.e., treatment is needed in 7 days—and provide emergency or alternate review processes in lieu of convening a full IRB committee, such as review by the head of the IRB or the Medical Director of the institution. Local institutional requirements must always be met for emergency use.
When prospective IRB review is waived by the FDA in an EIND, investigator must file a full report with the institutional IRB no more than 5 days after use of the drug. Applicants should take note of the fact that the FDA does not accept an IRB “approval” that is anything less than full IRB approval, even in an emergency. This means that “interim”, “compassionate”, “temporary” or other terms for any local “expedited” IRB approval process will not be accepted as “IRB approval”. Rather, the FDA explicitly states that “An IRB must either convene and give “full board approval” of the emergency use, or if the conditions [for exemption from IRB approval] are met and it is not possible to convene a quorum within the time available, the use may proceed without any IRB approval” (17).
Step 3: Contact the FDA for a single-patient IND approval
For drugs and biologics, only a licensed physician can submit a request for EA for an individual patient, emergency or otherwise. Unless specified otherwise, the physician will then be the designated holder of the IND and will be responsible for overseeing administration of the drug and treatment of the patient. The physician should contact the specific division of the FDA overseeing the drug’s development to receive instructions about how they wish to receive the request. Contact information for FDA review divisions, and emergency contact information for EINDs are found on the FDA website and are summarized in Table 2 (18).
Table 2.
Contact Information for the FDA for Single Patient IND∗
| General resources (4) |
|
| Non-emergency single patient IND∗ |
|
| Emergency single patient IND, Division Contacts (17) |
|
| Emergency Single Patient IND (17): Weekdays 8:00 PM to 4:30 PM ET |
|
| Emergency Single Patient IND (17): Nights, Weekends and Holidays |
|
Contact information accurate as of November 10, 2017.
In the case of non-emergent individual patient INDs, a written request for IND must be received by the FDA before shipment of the drug can occur and/or treatment begun. The FDA has recently streamlined the individual patient use application form, which now takes only approximately 45 min to complete, including time to read the instructions, search existing data sources, gather the data needed, and complete the review and information collection (19). In addition, an application for IRB approval must be on file with the originating institution.
EIND applications can be submitted over the phone or other means of rapid communication, and authorization for shipping and treatment may be given by the FDA over the phone. Contact numbers that can be used at any time of day or night are available at the FDA website 17, 18. Typically, approval for emergency use can be obtained in a matter of hours 20, 21. Shipping and use of the drug occurs in that case before actual FDA receipt of the written application (which must be received by the FDA in no case more than 15 working days after initiation of the EIND by phone or other method) 7, 22, and the institutional IRB must be notified within 5 days of administration of the drug (7).
Step 4: Obtain written informed consent from the patient or their legal surrogate
The FDA requires written informed consent for any EA drug use prior to treatment, including emergency use. Exemptions can occur, but require that both the investigator, and a physician who is not otherwise participating in a clinical investigation of the drug or treatment of the patient certify in writing all of the necessary conditions, which are listed in Table 3 (7). If time does not permit independent review, then the investigator must certify all of the required conditions in writing, and then obtain review and evaluation from an independent physician within 5 days of use of the drug, and notify the IRB that this has been done.
Table 3.
Conditions for Waiver of Informed Consent for Single Patient IND (17)
| Waiver of prospective informed consent is only allowed in the single patient emergency IND and not in the non-emergent single patient IND |
Waiver of informed consent can occur under the following conditions:
|
Informed consent can be waived in planned research in life-threatening situations in which the subjects will not generally be able to consent. However, the research plan and waiver of consent must be approved in advance by the FDA and the institutional IRB as part of the research protocol (23).
Step 5: Treat the patient
For non-emergent INDs, treatment cannot take place until either 30 days has passed without FDA objection, or the FDA provides affirmative approval prior to that. Currently, average time to approval of non-emergent individual patient INDs is 4 days (6). For EINDs, approval may be granted over the phone within hours.
Step 6: Immediate follow-up procedures
In the case of EINDs, emergency treatment of the patient takes priority and can occur after verbal (over the phone) approval by the FDA, but must be followed within 15 days by submission of the full single patient IND application 7, 24. In addition to completion of a written individual patient access EIND application, the investigator must send a report to the IRB within 5 days of treatment, and must submit a written report to the institutional IRB if treatment was undertaken under exemption from IRB approval. If the investigator was unable to obtain an independent physician’s review of a certification of conditions to waive informed consent prior to treatment, he or she must still obtain that review within 5 days after treatment of the patient and also submit that to the institutional IRB. If fatal or life-threatening adverse reactions occur, the physician must report them to the FDA within 7 days; serious and unexpected adverse reactions must be reported within 15 days (11).
Step 7: Follow-up reports
At the conclusion of treatment, provide a written summary to the FDA of the results of the individual patient use, including adverse effects. Any adverse event that might be related to the drug, even if only suspected, must be included in the report (7).
Accessing Investigational Drugs Without the FDA: “Right-To-Try” Legislation
The plight of patients seeking access to unapproved treatment has been heavily covered in the media 25, 26, and even forms the basis of a Hollywood movie, the Dallas Buyers Club (27). R2T has also been both publicized and heavily supported by political organizations that concentrate on states’ rights, such as the Goldwater Institute, a libertarian think tank (28). In addition, the use by patients of social media and multimedia formats to petition drug companies for access to investigational drugs has been on the rise (29).
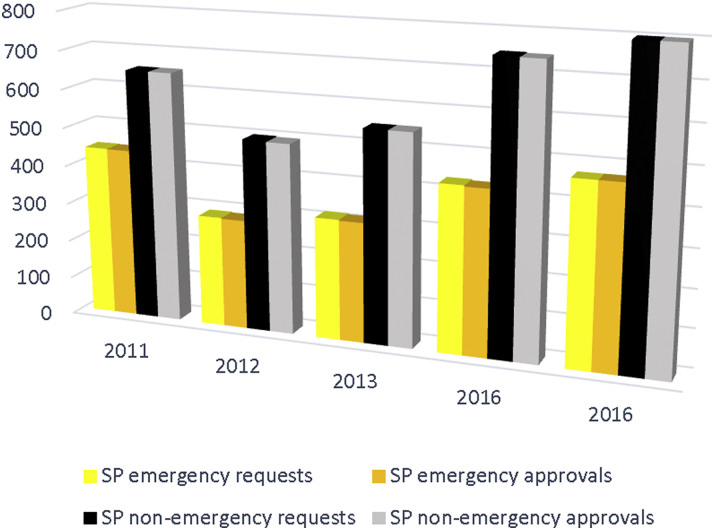
Unfortunately, both the public debate surrounding R2T legislation and the laws themselves demonstrate significant gaps in the public understanding of FDA pathways by which investigational treatments can already be released to patients in desperate circumstances on virtually a moment’s notice. The FDA currently approves over 99% of all “compassionate use” requests, and turns around emergency requests in 1 day or less (Figure 2) 22, 25, 30. Nonemergency requests are approved on average within 4 days 6, 23, 30. Recently, a review of a full decade of new expanded access IND applications to the FDA demonstrated that of 8,922 requests, only 38 EIND requests were denied or not allowed to proceed (22). Despite the high approval rate, however, the FDA is not merely “rubber stamping” these requests, but serves an important review function, as reasons for the denials demonstrate. The most common reason for denial of the EIND was that the patient was already stable on current therapy and thus the situation was not deemed to be an emergency. Of the 24 nonemergent single-patient INDs that were not allowed, the most common reasons were incomplete application (usually a lack of a LOA from the manufacturer), unsafe dosing, proven lack of efficacy for intended use, availability of adequate alternative therapies, and insufficient information provided by the applicant on which to base a decision (22).
Figure 2.
U.S. Food and Drug Administration Individual Patient IND Requests and Approvals by Year
The FDA approves over 99% of all SP IND requests, whether non-emergent or emergency. Abbreviations: IND = investigational new drug; SP = single patient.
R2T legislation took center stage in May of 2006, when a panel of the U.S. Court of Appeals for the District of Columbia issued a startling opinion in Abigail Alliance for Better Access to Developmental Drugs v. Von Eschenbach, that terminally ill patients who have exhausted all other available options have a fundamental constitutional right to experimental therapy not yet approved by the FDA 31, 32. The Abigail Alliance was founded in 2001 by survivors of 2 cancer patients who died after being been excluded from clinical trials for several investigational new drugs because they did not meet study inclusion criteria. The ruling was of serious significance: a fundamental constitutional right, as established in Washington v. Glucksberg, is a much deeper, less assailable right than a mere constitutional right. A fundamental right must pass a 3-part test: 1) it must be “firmly rooted” in the nation’s history and traditions; 2) must be “implicit in the rights concept of ordered liberty;” and 3) must be “carefully described.” Fundamental constitutional rights receive the highest constitutional protection against government and other interference 32, 33.
The court victory was short-lived; a scant 16 months later, the ruling was reversed by the D.C. Circuit Court 27, 34, 35, 36, and summarily denied review thereafter by the U.S. Supreme Court (34). Judge Thomas Griffeth of the D.C. Circuit Court noted in the ruling that “although terminally ill patients desperately need curative treatments…deaths can certainly be hastened by the use of a potentially toxic drug with no proven therapeutic benefit” (37).
The case was extremely important, however. In 2006, prior to judicial reversal of the Abigail Alliance case and at least partially in response to the case, the FDA amended regulations to expand its “compassionate use” exception, which previously had allowed only individual patients to apply on a case-by-case basis for permission to access experimental therapies. The new rules allowed access to such compassionate use to entire classes of patients (the “intermediate” and “widespread” treatment INDs), and, in order to prevent price gouging, set out rules for how companies could charge for such treatments.
Public initiatives continue: as of 2017, R2T legislation had been enacted in 38 states, and sobering stories of individual patients have become integrated in the current, highly-polarized podium of health care politics (38).
In addition to state-based R2T legislation, the U.S. Senate unanimously approved the Trickett Wendler, Frank Mongiello, Jordan McLinn and Matthew Bellina Right to Try Act this last August 39, 40. Similar bills have been filed in the U.S. House of Representatives. However, it is unclear whether these laws affect any real change. The Trickett Act in its current version requires that, for a patient to access an investigational drug they must already be under an FDA IND application and have completed phase I clinical trials. FDA approval for single patient use INDs do not require that a drug must have completed phase I trials, although meeting the FDA requirement that the risks of treatment are outweighed by the benefits might prove difficult without at least some preliminary clinical studies. R2T advocacy groups insist that right to try drugs are “safe” 41, 42 because of the requirement for completed phase I clinical testing. However, recalling that 90% of drugs that have completed Phase I trials fail in Phases II and III due to safety issues and lack of efficacy 1, 43, there are obvious concerns that the drugs that will be made available under R2T laws are much more probably than not going to be ineffective, or even harmful (44).
In R2T rules, the physician must certify that the patient has exhausted approved treatment options and are unable to participate in a clinical trial of the drug, and the patient must provide written informed consent (39)—all of which are the same as the FDA requirements for single-patient IND access. The Senate bill protects manufacturers from all but “willful” acts of negligence, and limits the amount the company can charge the patient to the company’s “direct costs”. However, this latter condition is probably unenforceable, since companies are not required to inform regulators of what they provide to the patients, nor what they charged them.
Companies are not required by law to provide investigational drugs upon request, and thus far, no pharmaceutical company has guaranteed that they will make drugs available under the legislation. In fact, some pharmaceutical companies actively oppose the measure. Merck and Co has stated, “While well-intentioned, current R2T legislation is not in the best interest of patients and is unlikely to help us bring forward innovative, safe and effective medicines to all patients as quickly as possible” (45). Although the former CEO of Neuralstem, Inc. testified in favor of the bill, he appears to be alone among pharmaceutical executives: even his own company management opposed it, feeling it would ‘delay or jeopardize the approval of therapies by reducing the supply of study agents or adversely affecting the data collection process’ 45, 46.
In anticipation that increased numbers of both individual patient IND applications and attempts to obtain drugs via R2T laws would lead to increased demand for both LOAs and drugs, pharmaceutical organizations and companies have had to address a number of concerns, such as whether sufficient evidence exists to warrant an individual patient claim that risks outweigh potential benefits, whether allowing individual patient access will deplete critical supplies of investigational drugs that are needed for clinical trials, and how access to a limited drug is managed and distributed if there are multiple applicants (47). Many companies, such as Janssen, Pfizer and Johnson&Johnson, have adopted a uniform internal “compassionate use” process, which involves formal application, review by a panel of company medical professionals, and the obtaining of approval by the appropriate regulatory body within the country originating the request, such as the FDA 13, 48, 49. Pfizer and Eli Lilly commit to respond to such requests in no more than 5 days after receiving the required documentation 48, 50. Lycera promises a response within 3 to 5 “business days” (51). In the case of some specific therapeutics, such as Catalyst Pharma’s therapeutic, amifampridine phosphate, for Lambert-Eaton syndrome, the response may take up to 30 days (52).
Although companies have developed internal pathways by which individual patients can achieve access to investigational drugs, the majority of such requests are denied. In 2015, Janssen, after consultation with the Division of Medical Ethics at NYU Langone Medical Center, developed a 10-person committee to review requests for compassionate use of its investigational drug daratumumab. In the last 6 months of 2015, they received 160 requests, of which they approved only 62 (13).
Obtaining Access to Drugs Under R2T
The steps to obtaining an investigational drug from the manufacturer under R2T are less clear than expanded access processes at the FDA, largely because each company has their own internal rules to handle such requests. By law, any drug manufacturer or distributor must make the company’s policy for evaluating and responding to expanded access requests both public and readily available, such as by posting its policy on its website (7). The policy must include contact information, procedures for making expanded access requests, the general criteria by which the manufacturer will evaluate and respond to such requests, the length of time the manufacturer will take to acknowledge receipt of the request, and a hyperlink or other reference to the clinical trial record that is reported in ClinicalTrials.gov about expanded access for the drug (7).
Janssen’s Compassionate Use Advisory Committee (CompAC) illustrates 1 manufacturer’s process. The 10-person CompAC is a committee of physicians, bioethicists, patients, and patient advocates from 5 different countries. Most, but not all members of the committee are compensated by Janssen (some members refused compensation). When a physician submits a request from anywhere in the world, Janssen physicians determine which patients are medically eligible for trials or other expanded use programs and directs such requests to the appropriate division. The remaining requests are directed to the CompAC, where they are evaluated in a uniform process. All requests must provide similar information, and requests are anonymized to prevent bias based on income, nationality, sex, race or celebrity status. Expertise and technical facts regarding the request is provided by subject experts within and outside of the company. If a request is denied, the physician can appeal the request by reintroducing standardized patient information. The committee responds to all requests within 5 business days (13). The Janssen website does not indicate how or whether emergency requests would be processed more quickly.
Concerns About R2T
While it is hard not to empathetically side with the plaintiffs in Abigail, bypassing the FDA approval process is fraught with risks, both for the general public, and for patients seeking desperate therapies 31, 32. Understanding those risks can provide insights about existing FDA regulations surrounding the various INDs, and provide guidance for any potential future regulatory and legislative actions. R2T laws engage a number of interested parties, many of which have competing interests. These include the patients, the general public, the FDA, the manufacturer, physicians, and researchers and the scientific community as a whole. Each stakeholder presents different questions and problems.
Patients
Who should be able to access non-approved therapeutics?
The interests of terminally ill patients in controlling their own bodies and determining whether they have a right to ingest potentially dangerous, possibly useless, and uniformly costly substances, are compelling. However, finding ethical and constitutional grounds for distinguishing the limits of autonomy for terminally ill patients as being different from autonomy for other groups of patients is problematic. While legal rules can be drawn to define R2T as applying only to the terminally ill, that approach is likely to face serious ethical and legislative challenges in the future. What would patients do, for example, who suffer from extremely debilitating, painful, or disfiguring, but not fatal, illnesses? Suffering and quality of life are defined by the patient’s personal perceptions and characteristics. Why should one group of patients who are suffering in 1 specific way (terminal illness) be treated differently with respect to their quality of life than another who, for example, is in perpetual pain? Powerful grounds are required to ethically justify differential treatment, and that is particularly true if the aggrieved group claims not merely statutory relief, but a constitutionally-based right.
Desperation and exploitation
The FDA was founded as a means to prevent a vulnerable public from being exploited by quackery, and to prevent the commerce of adulterated, possibly dangerous, inefficacious, and unsafe substances. Desperation creates vulnerability, which in turn creates opportunity for exploitation. The public as a whole has an interest in preventing the indiscriminate hocking of unproven therapeutics. Indeed, the FDA was established for this express purpose. Desperate patients have desperate hopes, and yet failure rates for drugs (e.g., 90% failure for anticancer drugs reaching phase I trials) 1, 53 suggests that there is actually little reason to assume that most patients would benefit from receiving drugs in their earliest stages of development, and much more substantial reason to anticipate that many patients would be harmed.
Creation of a “guinea pig class” of patients
The opening of the market to sale of investigational drugs raises the specter of creating a 2-tiered treatment system, with the wealthiest payers able to pay for and access expensive treatments ahead of approval, and poor patients relegated to only having access if they agree to become the “guinea pigs” of clinical trials. The ability of some patients to pay for drug access may reduce also the number of patients available for clinical trials, slowing progress towards full drug approval.
Patient benefits and harms
The likelihood that an investigational drug that is early in clinical phase testing will be beneficial and not harmful is actually quite small. Only about 10% of drugs completing Phase I testing will eventually be approved, and the most common reasons they fail in clinical trials are proven lack of efficacy and/or problems with drug safety 1, 32. Harms to patients from untested or incompletely tested therapies can be considerable: an historical example was high-dose chemotherapy and bone marrow transplant for advanced breast cancer, which physicians were so convinced was effective therapy that clinical trials were delayed for years. Clinical studies eventually proved that the therapy was ineffective, but not before countless women were harmed, and dozens died as a direct result of treatment toxicity (32).
The FDA
Undermining FDA approval
Early access to drugs, even in controlled trials can actually undermine the ability to gain full FDA approval; without some validated method of clinical trials anecdotal adverse reactions, heightened safety concerns, and worries about product liability could cause a manufacturer to prematurely discontinue trials before they are completed. Patients seeking expanded access are likely to be sicker than most study patients, and adverse events that occur in that population may have an adverse effect on ultimate approval. Furthermore, scientific validity of trials is compromised on both ends of the equation: via a clamor by patients to enroll despite disqualifying characteristics, and via patient reluctance to enroll in controlled studies since the drug is already obtainable outside of the approval system.
The manufacturer/distributor
Public relations
Public relations issues cause problems both ways for drug developers. Denying access to desperate patients gives the impression that the developer is greedy, and worried about liability and loss of market share. It bespeaks a lack of compassion for desperately ill patients. And yet, permitting access to unproven therapies can be motivated by opportunism rather than compassion. Quoting the case of the United States v. Rutherford: “Since the turn of the century, resourceful entrepreneurs have advertised a wide variety of purportedly simple and painless curse for cancer, including liniments of turpentine, mustard, oil, eggs and ammonia; peat moss and arrangements of colored floodlamps pastes made from glycerin and limburger cheese…” (54).
Manufacturer liability
Legislative efforts to expand access to experimental drugs have generally included clauses to limit or entirely eliminate liability for drug manufacturers, thereby denying compensation to patients who might otherwise have a right to it, for injuries that they received in the course of receiving an investigational drug. Protecting manufacturers from legal challenges potentially shields manufacturers from collateral issues that might arise unrelated to the drug’s efficacy or toxicity, such as negligent manufacture, handling, or administration of the medication, and thus removes important consumer protections unrelated to the medical efficacy of the treatment.
Charging the patient
Both the FDA and R2T legislation allow the manufacturer to charge for the drug (55). In the case of the FDA, charges must be approved, but can include not only the direct costs, handling and shipping charges, but in some cases monitoring and reporting costs. Even so, the charge for investigational drugs is likely to be less than the drug charge will be if full FDA approval is achieved (10). Publicizing a lower investigational drug charge is likely to create adverse publicity, and some companies have expressed concerns that it may negatively impact post-approval pricing (56).
Administrative burdens
The FDA estimates that 120 h of human effort go into the development of an intermediate-size IND protocol (10). While fewer hours are likely involved in individual-patient compassionate use consideration and approval, that burden is not negligible. On a systematic scale, manufacturers face the potential of having to create committees and review processes, much like that described for Janssen, and may bear a financial burden to keep it established and running (56). Such burdens are likely to be felt most acutely by smaller companies dedicated to fewer, but desirable therapeutics, such as biologicals for cancer treatment.
Depleting the supply of drugs for clinical studies
Early in manufacture, supplies are limited, and barely enough drug is produced to meet the requirements of clinical studies. This is because widespread marketing of the substance is not yet approved, and in fact is unlikely to occur (since most drugs fail in clinical trials). Manufacturers limit the amount of drug produced in order to limit costs in the early phase of commercialization, and many have expressed concerns that R2T will stress an already-limited supply of investigational drugs (46). Particularly when a drug company is small or the drug involves complex manufacturing procedures, there may be little drug to spare, and the availability of an investigational drug outside of clinical studies is therefore completely dependent on the individual manufacturer’s willingness to supply it 29, 36.
Negative impact on drug approval
Manufacturers have voiced concerns that adverse events occurring during patient “compassionate use” might be used by the FDA to delay drug approval. Although there is little evidence that this would be the case. Out of 11,000 expanded access requests at the FDA over a 10-year period, only 2 drug development programs were placed on clinical hold due to an adverse event observed during compassionate use, and those holds were temporary 10, 22.
Physicians
Reluctance to prescribe untested therapeutics
Physicians face both ethical and legal and issues in prescribing untested, or inadequately tested drugs. The practice of medicine is not merely the administration of substances that patients request or desire. It is defined by adherence to principles of practice, which include having a rational and tested basis that establish a reasonable belief that such therapy is used to treat disease, and will be more beneficial than harmful. Otherwise, there would be no substantial difference between the administration of medications by a physician, and the injection of heroin by a drug pusher, for example. Expanded access laws place the burden on the physician to determine whether the benefits of the treatment outweigh burdens—and that may be very difficult to estimate if there is limited or no clinical data. Prescribing untested therapies to desperate patients who are demanding them despite a lack of evidence of efficacy and safety, with reason to believe that the drug will more likely prove ineffective or harmful, violates the very basis of ethical medical practice (57).
Reachers and the quality of clinical research
The validity of scientific research
Legislative efforts have included proposals to expand inclusion criteria for clinical studies and to eliminate control groups. Currently, double-blind controlled trials are considered the “gold standard” of scientific method. Without controls and reasonable inclusion and exclusion criteria to reduce heterogeneity in study groups, the reliability of study results may be questionable. In fact, manufacturers are concerned that adverse events that occur in the treatment of dying patients may improperly lumped with patients who met trial inclusion criteria, skewing data and possibly interfering with final drug approval.
The concept of clinical equipoise
A randomized controlled trial is only ethically justifiable if there exists a state of genuine uncertainty about the relative merits of each arm of the study, i.e. if both arms are equally likely to be beneficial, and all arms are compatible with competent medical care. This concept is called clinical “equipoise” 58, 59. Ethical standards of clinical research exist to prevent patient harm and exploitation, but they sometimes create barriers for suffering for individuals. None probably illustrates this better than the story of Thomas McLaughlin and Brandon Ryan, cousins who suffered from advanced BRAF-mutated metastatic melanoma and were enrolled in a late-phase premarket study of the drug vemurafenib, that had shown early promising results for their type of melanoma. McLaughlin, randomized to the treatment arm, initially improved significantly within 2 months of starting the study. Ryan was randomized to the control arm of the study in which he received standard chemotherapy and declined clinically. McLaughlin pleaded for his cousin to be switched to the treatment arm, and even offered to switch places with him. His campaign was to no avail, and Ryan died in 2010 with advancing pain while in the study (60).
Why did researchers deny Ryan access to their study drug even though it appeared to be effective? At the time of the trial, no drug that had looked promising in early trials had ever proven to prolong the lives of melanoma patients. When faced with this dilemma, researchers responded that despite early tumor response to vemurafenib, there was no guarantee that McLaughlin would experience improved life expectancy from the drug—what if early response was followed by accelerated tumor advancement thereafter? As some pointed out, without controlled trials, doctors were prescribing “unsubstantiated hope” and expecting the patient/public to pick up the cost.
Reports in the medical literature (58) and lay press (60) to the effect that Ryan had been denied “life-saving” therapy and that Thomas had survived were premature. The clinical efficacy of vemurafenib did in fact regress in later studies, just as some researchers believed it might. McLaughlin also succumbed to melanoma in 2014 (61).
Such well-worn arguments as clinical equipoise for traditional controlled trials are being increasingly challenged, in an era of new biological therapeutics. A significant reason for clinical trials has always been to test the ever-present danger of significant clinical toxicity, particularly with chemotherapeutic agents. But many new anticancer drugs and biologicals have been showing minimal side effects, combined with dramatic early response rates. Should such drugs be subjected to prolonged clinical trials before terminally ill patients facing certain poor outcomes can try them? In the case of terminal illness, even if a drug is not shown to prolong life, couldn’t tumor response, even if temporary, be relevant to patients?
Comparing Single Patient IND Versus R2T Legislation
Differences between regulations for FDA EIND approval and use and legislative R2T access are summarized in Table 4. FDA EIND eligibility is broader than that for R2T, encompassing both “serious” and “immediately life-threatening” disease, rather than being limited to terminal disease. R2T requires that the drug in question have completed Phase I clinical trials, which is not a requirement of the FDA for individual patient INDs 62, 63. In contrast with the FDA, R2T legislation removes oversight of the manufacturer with regard to quality and production, does not require that outcomes of treatment be reported to the FDA, does not require an independent (IRB) review of the rationale for emergency use, and does not limit the amount or how a patient can be charged for the therapy, raising the specter of predatory pricing (29). Manufacturers impose their own “approval” process for early access to their study drugs, a step which limits both the FDA process and R2T.
Table 4.
A Comparison of FDA Single-Patient IND and Federal Right-to-Try Legislation (Trickett Wendler Right to Try Act)∗
| FDA Single-Patient IND | Federal Right-to-Try | |
|---|---|---|
| How many patients is the IND for? | 1 | 1 |
| Are prior clinical in-human studies required? | No prior in-human studies are required to be complete, but drug must be subject of an IND application with the FDA. | Drug must have completed phase I trials and must be under active commercial development (i.e., cannot be on an FDA clinical hold) |
| Informed consent | Required unless waived due to patient condition | Required |
| Regulates how the patient can be charged for the drug? | Yes | No |
| Is independent review (e.g., IRB) required? | Yes, but can be waived in an emergency. If waived, IRB notification is required. | No |
| Patient eligibility | Serious, or immediately life-threatening disease or condition, no reasonable alternative therapy available, supplying the drug will not interfere with drug studies and development. | Terminal illness only; must have exhausted other treatment options and are ineligible for ongoing trials |
| Benefit/risk requirements | Physician determines that risk of treatment likely does not exceed risk of illness | Not explicitly spelled out |
| Duration of therapy | Usually limited to a single course or specified duration of therapy | No limits specified |
| Costs to patient | Manufacturer may charge the patient if FDA parameters are met; FDA must approve patient billing | No specifications or limitations to what the manufacturer can charge |
| Insurance coverage | Insurance companies not required to cover | Insurance companies not required to cover |
| Liability | Not addressed | Bars medical licensing agents from taking action against the physician. It is a misdemeanor to block access to the drug. Indemnifies manufacturer against all but “willful” misconduct or gross negligence”. Protects the manufacturer against liability for not supplying the drug |
| Definition of investigational drug | New agent that is being used in an investigation (phase not designated) | Must have completed phase I testing, and must be under investigation in a current FDA trial |
| Drug/device quality | Must meet manufacturing standards | Not addressed |
| Drug/device information that must be supplied | Physician and IRB of record must be given investigator’s brochure, if available: drug toxicities, info on administration | Not addressed |
| Availability | Determined by the manufacturer | Determined by the manufacturer |
| Impact on future research | Must not interfere with initiation, conduct or completion of marketing investigations | Not addressed |
| Can outcome data from expanded use be used? | FDA requires report of outcomes and adverse effects, but it is not clear how this might be used in drug approval | Yes. The HHS secretary may use outcome data if they see it as critical to determining the safety of the investigational drug or if the sponsor requests the data be used |
| Is post-treatment reporting required | Yes. Must report adverse events, drug deposition, and file an end-of-treatment summary report to the FDA | Must report annually to the HHS secretary—not the FDA—the number of patients treated, the number of doses given, the reasons for treatment and any adverse events. The Secretary determines if and when adverse events used by the FDA. The secretary is required to post on the FDA website when the drug was allowed EA, and when it was not. |
| Time frame | For emergency use: usually hours. For non-emergency use, average of 4 days | “No delay” otherwise not specified, manufacturer websites specify time frames (e.g., “3 to 5 business days” and many do not indicate whether emergency requests will be handled differently than non-emergency requests |
Information regarding the Tricket Wendler Act reflects the August 2017 version. The legislation has been submitted to the House of Representatives, which have several similar bills in session, and the final version of any of these is yet to be determined.
McKee et al. (64) found that between fiscal year (FY) 2010 and FY 2014, 5,394 individual patient EA INDs were filed with the FDA, 52% of which were individual patient INDs and 48% individual patient EINDs. The FDA allowed 99% and 97% of EINDs and individual patient INDs to proceed, respectively. Overall, 3,365 (64%) of drugs allowed to be used under a EIND or individual patient IND went on to be approved by September of 2015 (within 1 to 5 years of EA IND approval). This latter statistic must be interpreted with caution, however, since for many of the single patient IND applications, the drugs were already far along in the approval process and increasingly likely to be approved.
No information has been published thus far about how manufacturer responses to requests for investigational drugs compare to the FDA in both the rates of compassionate use of investigational drugs and the time from request to treatment. However, the rapid FDA turnaround time suggests that the manufacturers are unlikely to be able to provide clinically relevant improvements. While almost all FDA single patient IND requests are approved, statistics from some pharmaceutical companies indicate that the majority of R2T requests are denied. Evidence is emerging that some companies have rejected more compassionate use requests for their single drug than the FDA has rejected for all drugs requested between 2010 and 2014 combined (64). Janssen turned down 98 of 160 requests for compassionate use of daratumumab in 2015 alone through its CompAC process, while the FDA denied only 62 requests over a 10-year period (22). The most common reasons for manufacturer denial was poor risk-to-benefit ratio of the drug, failure to exhaust other possibilities and/or eligibility for a clinical trial (13). Such findings have led some commentators to suggest that the new legislation be renamed “right to ask” rather than “right to try” (65).
Conclusions
Once a drug enters clinical trials, it takes an average of 8 years to achieve market approval, and over 90% of drugs entering Phase I clinical trials fail to obtain approval. For patients with life-threatening or severely debilitating disease and limited treatment options, the right to access investigational drugs prior to approval has become a priority. Regulatory and legislative efforts have resulted in earlier access to investigational drugs, through FDA EA programs, and outside of the FDA via R2T legislation. Critics worry that R2T puts vulnerable and desperate patients at risk by removing FDA oversight of drug access, and by limiting liability of drug manufacturers. Pharmaceutical companies face significant challenges in developing fair processes for patients seeking early access that do not expose vulnerable patients to potential harms, and preserves the sponsor’s ability to carry out clinical trials. Overall, the public appears to underestimate FDA mechanisms for individual access, which approve over 99% of applications in an average of 4 days. Whether R2T efforts will substantially impact drug availability, manufacturer willingness to release drugs for compassionate use, or the timeframe in which such requests are met, remains to be seen.
Footnotes
Dr. Van Norman has received financial support from the Journal of the American College of Cardiology.
The author attests he is in compliance with human studies committees and animal welfare regulations of the authors' institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Van Norman G. Drugs, devices, and the FDA: Part 1: an overview of approval processes for drugs. J Am Coll Cardiol Basic Trans Science. 2016;1:170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gieringer D.H. The safety and efficacy of new drug approval. Cato J. 1985;5:177–201. [PubMed] [Google Scholar]
- 3.Van Norman G.A. Overcoming the declining trends in innovation and investment in cardiovascular therapeutics. Beyond Eroom’s law. J Am Coll Cardiol Basic Trans Science. 2017;2:613–625. doi: 10.1016/j.jacbts.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U.S. FDA. Investigational new drug (IND) application. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/InvestigationalNewDrugINDApplication/default.htm#Introduction. Accessed October 4, 2017.
- 5.Holbein M.E. Understanding FDA regulatory requirements of investigational new drug applications for sponsor-investigators. J Investig Med. 2009;57:688–694. doi: 10.231/JIM.0b013e3181afdb26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarow J.P., Lurie P., Ikenberry S.C., Lemery S. Overview of the FDA’s expanded access program for investigational new drugs. Ther Innov Regul Sci. 2017;52:177–179. doi: 10.1177/2168479017694850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. FDA. Expanded access to investigational drugs for treatment use—questions and answers; guidance for industry. U.S. FDA Center for Drug Evaluation and Research (CDER), Silver Spring, MD. 2017. Available at: https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm351261.pdf. Accessed October 30, 2017.
- 8.U.S. FDA. Investigational new drug application, subpart I—expanded access to investigational drugs for treatment use. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=312&showFR=1&subpartNode=21:5.0.1.1.3.9. Accessed November 7, 2017.
- 9.U.S. FDA. Expanded access (compassionate use). Available at: https://www.fda.gov/NewsEvents/PublicHealthFocus/ExpandedAccessCompassionateUse/default.htm. Accessed November 7, 2017.
- 10.Darrow J.J., Sarpatwari A., Avorn J., Kesselheim A.S. Practical, legal and ethical issues in expanded access to investigational drugs. N Engl J Med. 2015;372:279–286. doi: 10.1056/NEJMhle1409465. [DOI] [PubMed] [Google Scholar]
- 11.U.S. FDA. Expanded access: information for physicians. Available at: https://www.fda.gov/NewsEvents/PublicHealthFocus/ExpandedAccessCompassionateUse/ucm429624.htm. Accessed November 7, 2017.
- 12.U.S. FDA. Charging for investigational drugs under an IND—questions and answers. Guidance for industry. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM351264.pdf. Accessed November 7, 2017.
- 13.Caplan A., Ray A. The ethical challenges of compassionate use. JAMA. 2016;315:979–980. doi: 10.1001/jama.2016.0416. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Example of wording for letter of authorization (LOA) for individual patient expanded access IND. U.S. FDA. Available at: https://www.fda.gov/NewsEvents/PublicHealthFocus/ExpandedAccessCompassionateUse/ucm432575.htm. Accessed November 1, 2017.
- 15.U.S. FDA. Form 3926. Available at: https://www.fda.gov/downloads/AboutFDA/ReportsManualsForms/Forms/UCM504574.pdf. Accessed November 2, 2017.
- 16.U.S. FDA. Institutional review boards frequently asked questions—information sheet. Available at: https://www.fda.gov/RegulatoryInformation/Guidances/ucm126420.htm. Accessed November 2, 2017.
- 17.U.S. FDA. Emergency use of an investigational drug or biologic—information sheet. Available at: https://www.fda.gov/RegulatoryInformation/Guidances/ucm126491.htm. Accessed November 2, 2017.
- 18.U.S. FDA. For physicians: How to request single patient expanded access (“compassionate use”) Available at: https://www.fda.gov/drugs/developmentapprovalprocess/howdrugsaredevelopedandapproved/approvalapplications/investigationalnewdrugindapplication/ucm107434.htm. Accessed November 7, 2017.
- 19.U.S. Food and Drug Administration. Individual patient expanded access applications: Form FDA 3926. Guidance for industry. Available at: https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM432717.pdf. Accessed November 2, 2017.
- 20.U.S. Government Printing Office, July 2017. United States Government Accountability Office Report to Congressional Addressees. Investigational new drugs. FDA has taken steps to improve the expanded access program but should further clarify how adverse events data are used. Available at: https://www.gao.gov/products/GAO-17-564. Accessed November 10, 2017.
- 21.Gottleif S. Expanded access: FDA describes efforts to ease application process. FDA Voice. October 3, 2017. Available at: https://blogs.fda.gov/fdavoice/index.php/tag/expanded-access/. Accessed November 7, 2017.
- 22.Jarow J.P., Lemery S., Buigin K., Khosin S., Moscicki R. Expanded access of investigational drugs: the experience of the Center for Drug Evaluation and Research over a 10-year period. Ther Innov Regul Sci. 2016;50:705–709. doi: 10.1177/2168479016656030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.U.S. FDA. Exception from informed consent for studies conducted in emergency settings: regulatory language and excerpts from preamble—information sheet. Available at: https://www.fda.gov/RegulatoryInformation/Guidances/ucm126482.htm. Accessed November 7, 2017.
- 24.Holbein M.E., Berglund J., Weatherwax K., Gerber D.E., Adamo J.E. Access to investigational drugs: FDA expanded access programs or “right to try” legislation? Clin Transl Sci. 2015;8:526–532. doi: 10.1111/cts.12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanghavi D. The pills of last resort. How dying patients get access to experimental drugs. The New York Times Magazine. October 13, 2013. Available at: http://www.nytimes.com/2013/11/03/magazine/how-dying-patients-get-access-to-experimental-drugs.html?pagewanted=all. Accessed October 7, 2017.
- 26.Dennis B, Cha AE. ‘Right to try’ laws spur debate over dying patients’ access to experimental drugs. The Washington Post. May 15, 2014. Available at: https://www.washingtonpost.com/national/health-science/right-to-try-laws-spur-debate-over-dying-patients-access-to-experimental-drugs/2014/05/16/820e08c8-dcfa-11e3-b745-87d39690c5c0_story.html?utm_term=.19bda7185125. Accessed October 7, 2017.
- 27.Trasatti MA, Grago SM. To try or not to try. Who decides is the question. FDCC Quarterly, Summer 2015;311–337. Available at; http://c.ymcdn.com/sites/thefederation.site-ym.com/resource/resmgr/docs/Quarterly/Archive/V64N4_Trasatti.pdf. Accessed November 7, 2017.
- 28.The Goldwater Institute. Right to Try. January 20, 2015. Available at: http://goldwaterinstitute.org/en/work/topics/healthcare/right-to-try/right-try/. Accessed October 6, 2017.
- 29.Mackey T.K., Schoenfeld V. Going “social” to access experimental and potentially life-saving treatment: an assessment of the policy and online patient advocacy environment for expanded access. BMC Med. 2016;14:17. doi: 10.1186/s12916-016-0568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.U.S. FDA. Expanded access INDs and protocols. Available at: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/DrugandBiologicApprovalReports/INDActivityReports/ucm373560.htm. Accessed November 2, 2017.
- 31.Kearns L. 17 real, workable ways to improve patient access to experimental drugs. The Hill. April 28, 2017. Available at: http://thehill.com/blogs/congress-blog/healthcare/331145-17-real-workable-ways-to-improve-patient-access-to. Accessed October 25, 2017.
- 32.Shah S., Zettler P. From a constitutional right to a policy of exceptions: Abigail Alliance and the future of access to experimental therapy. Yale J Health Policy Law Ethics. 2010;10:135–196. [PubMed] [Google Scholar]
- 33.Washington v. Glucksberg, 521 U.S. 702 (1997). Available at: https://supreme.justia.com/cases/federal/us/521/702/case.html. Accessed November 10, 2017.
- 34.Leonard E.W. Right to experimental treatment; FDA new drug approval, constitutional rights, and the public’s health. J Law Med Ethics. 2009;37:269–279. doi: 10.1111/j.1748-720X.2009.00371.x. [DOI] [PubMed] [Google Scholar]
- 35.FDA Law Blog. D.C. Circuit Court rules in Abigail Alliance case; affirms district court ruling that there is no fundamental right of access to experimental drugs for the terminally ill. Available at: http://www.fdalawblog.net/fda_law_blog_hyman_phelps/2007/08/dc-circuit-cour.html. Accessed October 7, 2017.
- 36.Editorial A delicate balancing act. Nature Rev Drug Discov. 2007;6:685. doi: 10.1038/nrd2413. [DOI] [PubMed] [Google Scholar]
- 37.United States Court of Appeals for the District of Columbia Circuit. Abigail Alliance et al vs. Andrew von Eschenback, et al. Available at: http://www.scotusblog.com/archives/07-444_ob.pdf. Accessed December 2, 2017.
- 38.McGinely L. Are right to try laws a last hope for dying patients—or a false hope? The Washington Post. March 26, 2017. Available at: https://www.washingtonpost.com/national/health-science/are-right-to-try-laws-a-last-hope-for-dying-patients–or-a-cruel-sham/2017/03/26/1aa49c7c-10a2-11e7-ab07-07d9f521f6b5_story.html?utm_term=.a0be15f0da57. Accessed October 27, 2017.
- 39.Trickett Wendler, Frank Mangiello, Jordan McLinn and Matthew Bellina Right to Try Act. 115th Congress 1st session. S.204(115). Available at: https://www.congress.gov/115/bills/s204/BILLS-115s204rfh.pdf. Accessed November 7, 2017.
- 40.Reese-Coulbourne J. Expanded access to experimental medications—where is federal legislation taking us? Mark Krueger and Associates, Inc. 2017 Available at: http://mkanda.com/expanded-access-to-experimental-medicines-where-is-federal-legislation-taking-us/. Accessed November 7, 2017.
- 41.RighttoTry. FAQs. Available at: http://righttotry.org/faq/. Accessed November 7, 2017.
- 42.DeTora L.M. What is safety? Miracles, benefit-risk assessments, and the “right to try”. Int J Clin Pract. 2017 June 8 doi: 10.1111/ijcp.12966. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 43.Yag Y.T., Chen B., Bennett C. “Right to try” legislation: progress or peril? J Clin Oncol. 2015;33:2597–2599. doi: 10.1200/JCO.2015.62.8057. [DOI] [PubMed] [Google Scholar]
- 44.Cha Arian E. The dark side of ‘compassionate use’ of experimental drugs. The Washington Post. August 3, 2016. Available at: https://www.washingtonpost.com/news/to-your-health/wp/2016/08/31/the-dark-side-of-compassionate-use-of-experimental-drugs/?utm_term=.4961ec9c8063. Accessed November 2, 2017.
- 45.Firth S. Will ‘right-to-try’ bill actually help anyone?—Little effect from state laws, and industry largely uninterested. Medpage. Public Health and Policy. August 11, 2017. Available at: https://www.medpagetoday.com/publichealthpolicy/fdageneral/67222. Accessed October 27, 2017.
- 46.Zettler P.J., Greely H.T. The strange allure of “right to try” laws. JAMA Intern Med. 2014;174:1885–1886. doi: 10.1001/jamainternmed.2014.5767. [DOI] [PubMed] [Google Scholar]
- 47.Fox, R. Expanded access: get ready. September 15, 2015. PharmExec.com. Available at: http://www.pharmexec.com/expanded-access-get-ready. Accessed November 2, 2017.
- 48.Pfizer. Compassionate use and expanded access: frequently asked questions. How do I submit a request for compassionate use of an investigational drug? Available at: https://www.pfizer.com/purpose/medicine-access/compassionate-use. Accessed November 1, 2017.
- 49.Young K. Johnson & Johnson to have bioethics panel 2, 2017 weigh patients’ requests for investigational drugs. NEJM Journal Watch. May 8, 2015. Available at: http://www.jwatch.org/fw110175/2015/05/08/johnson-johnson-have-bioethics-panel-weigh-patients. Accessed November 2, 2017.
- 50.Eli Lilly. Expanded Access. Available at: https://www.lilly.com/discovery/clinical-trials/expanded-access. Accessed November 7, 2017.
- 51.Lycera. Expanded Access. Available at: http://www.lycera.com/expanded-access. Accessed November 7, 2017.
- 52.Catalyst Pharma. Amifampridine phosphate expanded access. Available at: http://www.catalystpharma.com/firdapse-expanded-access.shtm. Accessed November 1, 2017.
- 53.Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates? Nature Rev Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 54.United States v. Rutherford, 442 U.S. 544, 558 (1979). Available at: https://supreme.justia.com/cases/federal/us/442/544/case.html. Accessed November 10, 2017.
- 55.U.S. FDA Charging for investigational drugs under an investigator new drug application. Final rule. Fed Regist. 2009;74:40871–40900. [PubMed] [Google Scholar]
- 56.Gaffney A. Regulatory explainer: FDA’s expanded access (compassionate use) program. Regulatory Affairs Professionals Society. February 4, 2014. Available at: http://www.raps.org/Regulatory-Focus/News/2015/02/04/18343/Regulatory-Explainer-FDAs-Expanded-Access-Compassionate-Use-Program/ Accessed November 7, 2017.
- 57.Van Norman G., Rosen J. Michael Jackson, medical ethics, and what went wrong. In: Pediatric Sedation Outside of the Operating Room. Springer. New York, NY. 2014:685–698. [Google Scholar]
- 58.Hey S.P., Truog R.D. The question of clinical equipoise and patients’ best interests. AMA J Ethics. 2015;17:1108–1115. doi: 10.1001/journalofethics.2015.17.12.ecas1-1512. [DOI] [PubMed] [Google Scholar]
- 59.Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317:141–145. doi: 10.1056/NEJM198707163170304. [DOI] [PubMed] [Google Scholar]
- 60.Harmon A. New drugs stir debate on rules of clinical trials. NY Times. September 18, 2010. Available at: http://www.nytimes.com/2010/09/19/health/research/19trial.html. Accessed October 2, 2017.
- 61.Thomas James McLaughlin. Obituary, Santa Maria Times, February 16, 2014. Available at: http://santamariatimes.com/lifestyles/announcements/obituaries/thomas-james-mclaughlin/article_ef9cced7-3145-585a-90b7-686d6296c6ba.html. Accessed November 7, 2017.
- 62.Dresser R. “Right to try laws: the gap between experts and advocates. Hastings Ctr Rep. 2015;45:9–10. doi: 10.1002/hast.442. [DOI] [PubMed] [Google Scholar]
- 63.FDA “Expanded access” to investigational drugs for treatment use. Final rule. Fed Regist. 2009;74:40900–40945. [PubMed] [Google Scholar]
- 64.McKee A.E., Markon A.O., Chan-Tack K.M., Lurie P. How often are drugs made available under the Food and Drug Administration’s expanded access process approved? J Clin Pharmacol. 2017;57(Suppl 10):S136–S142. doi: 10.1002/jcph.960. [DOI] [PubMed] [Google Scholar]
- 65.Karlin-Smith S. Libertarians score big victory in ‘right-to-try ‘drug bill. Politico. August 3, 2017. Available at: https://www.politico.com/story/2017/08/03/libertarians-score-big-victory-drug-bill-241314. Accessed November 2, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.