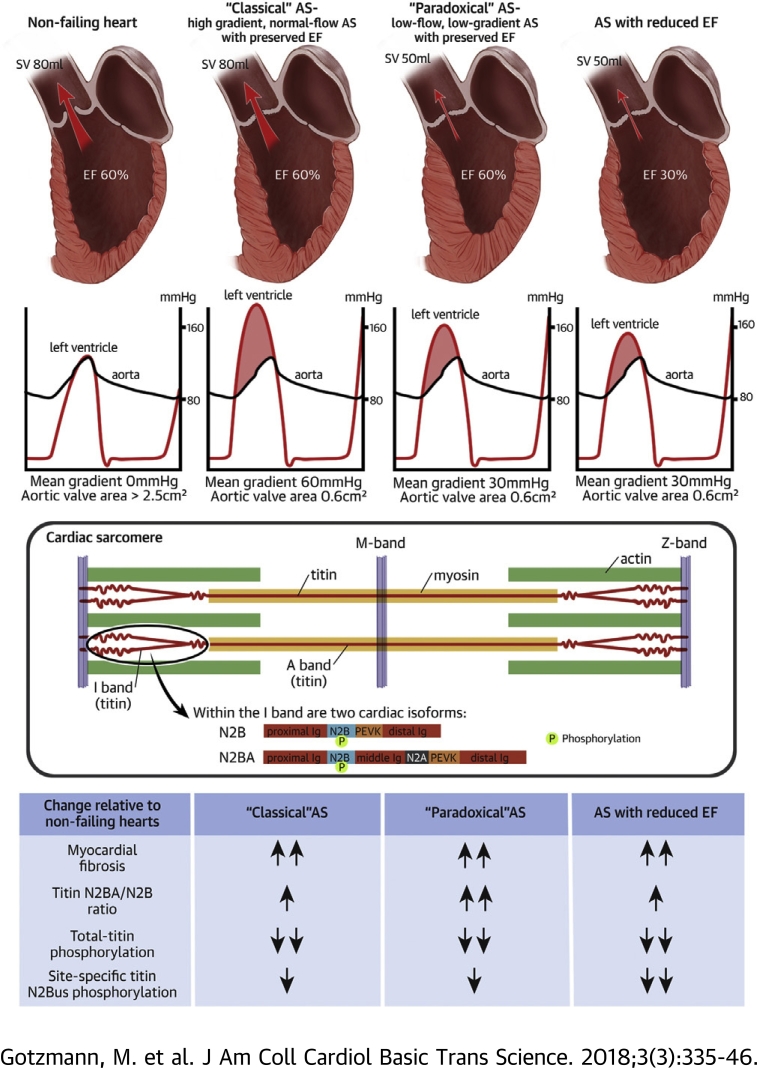
Visual Abstract
Key Words: myocardial fibrosis, myocardial stiffness paradoxical aortic stenosis, titin isoforms, titin phosphorylation
Abbreviations and Acronyms: AS, aortic stenosis; AVA, aortic valve area; BNP, B-type natriuretic peptide; EF, ejection fraction; LV, left ventricular; MHC, myosin heavy chain; N2Bus, unique sequence within the cardiac-specific N2B titin domain; NYHA, New York Heart Association; Z, valvuloarterial impedance
Highlights
-
•
The extent of myocardial fibrosis and the degree of isoform-expression and phosphorylation changes in cardiomyocyte titin were unknown in different hemodynamic subgroups of AS, including “paradoxical” low-flow, low-gradient AS with preserved ejection fraction.
-
•
Hemodynamic subtypes of AS were found to exhibit increased cardiac fibrosis, titin-isoform transition toward more compliant N2BA variants, and both total and site-specific titin (N2Bus) hypophosphorylation compared with donor heart controls.
-
•
A significant shift toward N2BA titin appeared in “paradoxical” AS, whereas alterations in total-titin phosphorylation and cardiac fibrosis were similar in all hemodynamic subtypes of AS, suggesting increased myocardial passive stiffness.
-
•
The unfavorable prognosis of “paradoxical” AS could be explained by the pronounced myocardial remodeling, which is no less severe than in other AS subtypes.
Summary
Titin-isoform expression, titin phosphorylation, and myocardial fibrosis were studied in 30 patients with severe symptomatic aortic stenosis (AS). Patients were grouped into “classical” high-gradient, normal-flow AS with preserved ejection fraction (EF); “paradoxical” low-flow, low-gradient AS with preserved EF; and AS with reduced EF. Nonfailing donor hearts served as controls. AS was associated with increased fibrosis, titin-isoform switch toward compliant N2BA, and both total and site-specific titin hypophosphorylation compared with control hearts. All AS subtypes revealed titin and matrix alterations. The extent of myocardial remodeling in “paradoxical” AS was no less severe than in other AS subtypes, thus explaining the unfavorable prognosis.
Severe symptomatic aortic stenosis (AS) is a common cardiac disease associated with poor prognosis in the absence of heart valve replacement (1). In AS, chronically elevated afterload induces left ventricular (LV) hypertrophy, increased myocardial stiffness, and impaired relaxation 2, 3. This myocardial remodeling is associated with progressive dyspnea, the most common symptom of AS, and poor prognosis (1).
Patients with severe, high-gradient, normal-flow AS with preserved ejection fraction (“classical” AS) benefit with a high probability from aortic valve replacement (1). However, there are other hemodynamic types of AS, such as low-flow, low-gradient severe AS with preserved ejection fraction (EF) (“paradoxical” AS) and aortic stenosis with reduced ejection fraction (ASrEF), which occur in more than 30% of all cases 4, 5. Probably due to advanced myocardial remodeling, the positive effect of aortic valve replacement is more uncertain in these patients 1, 5.
In particular, the pathophysiology of “paradoxical” AS is not fully understood. There is controversy about whether it is merely a moderate form of AS with favorable prognosis or an advanced form of AS. It could be a pseudo-severe AS due to inaccuracies in the measurements or inconsistencies in the guidelines 6, 7, 8, or it could be a true AS associated with pronounced myocardial stiffening, severe intrinsic myocardial damage, impaired relaxation, and therefore, worse prognosis than “classical” AS 5, 9, 10.
Pathological myocardial relaxation and stiffness in AS are determined, in part, by fibrosis 11, 12, 13. More recently, the giant sarcomere protein titin has emerged as a main contributor to myocardial relaxation and stiffness (14). Titin properties can be severely altered in heart failure, including AS (14). Titin is expressed in 2 major isoforms in adult hearts: stiff N2B and compliant N2BA isoforms (15). In recent years, there has been growing evidence for a titin isoform switch toward the more compliant N2BA isoform in various cardiac diseases, for example, heart failure with reduced or preserved EF (14). This isoform transition may be compensatory, to counteract the increased stiffening from myocardial fibrosis. In contrast, for AS patients, it is not clear whether titin isoforms switch toward N2BA, N2B, or not at all 16, 17, 18. In addition, there are no studies of titin isoform expression in different hemodynamic subtypes of AS. Phosphorylation of titin is another parameter that determines myocardial passive stiffness (14), but likewise, has not been studied in hemodynamic subtypes of AS. To our knowledge, site-specific titin phosphorylation has never been investigated in human AS, but would be important to measure in particular at 2 mechanically active regions within the titin springs present in both the N2B and N2BA isoforms, the N2B-unique sequence (N2Bus) and the proline/glutamate/valine/lysine-rich (PEVK) region, because the stiffness of these segments is altered when they become phosphorylated (14) or dephosphorylated (19).
In the present study, we investigated titin-isoform expression, total-titin and site-specific N2Bus/PEVK phosphorylation, and the degree of fibrosis in endomyocardial biopsy samples from AS patients compared with nonfailing donor heart samples. We examined these properties in 3 hemodynamic subgroups of AS: “paradoxical” AS, “classical” AS, and ASrEF. We aimed to establish whether such indexes of structural and functional cardiac remodeling with relevance for myocardial passive stiffness are similar or different among those patients and compared with the nonfailing control hearts.
Methods
Patient cohort
In this prospective study, we examined patients with severe AS and an indication for surgical aortic valve replacement. Inclusion criteria were: aortic valve area (AVA) <1 cm2, according to continuity formula; AVA index <0.6 cm2/m2; and symptoms of severe AS, such as dyspnea (New York Heart Association [NYHA] functional class ≥II), angina pectoris (Canadian Cardiovascular Society class ≥II), or syncope. Exclusion criteria were: 1) more than moderate valve regurgitation or stenosis; 2) previous cardiac surgery; and 3) a previous myocardial infarction. A previous myocardial infarction was defined according to current recommendations (20). Coronary angiography was performed pre-operatively. Coronary heart disease was diagnosed when coronary artery stenosis was ≥50%. The presence of coronary artery occlusions, including those that were subclinical, was considered an exclusion criterion for the study. All patients gave written consent to participate in this study. The study protocol was approved by the local ethics committee (approval 4379-12, Ethics Committee of the Ruhr University Bochum). The study protocol has been published (BOREAS [BOchum heaRt failurE in Aortic Stenosis] study, DRKS-ID: DRKS00005623).
Echocardiography and B-type natriuretic peptide
Transthoracic echocardiography was performed with the Vivid 7 or 9 System (General Electric, Horton, Norway) within 48 h before aortic valve replacement. Established parameters were used to quantify AS. Patients were divided into 3 groups using LVEF and mean gradient: 1) classical AS with a high gradient, normal flow, and preserved LVEF, defined as a mean transaortic gradient of ≥40 mm Hg, stroke volume index >35 ml/m2, and LVEF >50%; 2) paradoxical AS with low flow, low gradient, and preserved LVEF, diagnosed according to current recommendations as mean transaortic gradient <40 mm Hg, stroke volume index <35 ml/m2, and LVEF ≥50% (5); and 3) ASrEF, defined as reduced LVEF (<50%), regardless of transaortic gradient and stroke volume. Dobutamine echocardiography was performed in all patients with AS and reduced EF. All of these patients had a flow reserve. Patients with AS and preserved EF did not undergo dobutamine echocardiography. LV mass was calculated according to the modified method of Devereux and was related to the body surface. Valvuloarterial impedance Z (Z = SAP · MG/SVI, where SAP is the systolic arterial pressure, MG is the mean gradient, and SVI is the stroke volume index) was calculated. Measurements of global longitudinal strain were performed by speckle tracking. Quantification of valve regurgitation was performed according to current recommendations (21). The volume of the left atrium was measured and related to the body surface. LV diastolic dysfunction with abnormal relaxation and diastolic stiffness was assumed when the criteria of the European Society of Cardiology consensus statement of 2007 were met (22). On the day of the echocardiographic examination, B-type natriuretic peptide (BNP) was measured (normal value <100 pg/ml). The blood samples were collected in tubes containing ethylenediaminetetraacetic acid. After immediate centrifugation, BNP was measured using a chemiluminescent immunoassay kit (Biosite Triage, San Diego, California).
Aortic valve replacement and biopsy sampling
Aortic valve replacement was performed in the Department of Cardiothoracic Surgery, Ruhr University Hospital Bergmannsheil–Bochum. During surgery, endomyocardial biopsies (30 to 40 mg) were taken from the basal septum. Samples were immediately separated into one-half stored in formaldehyde, to be used for histochemistry, and one-half frozen in liquid nitrogen, to be used for titin protein analysis. The study protocol was approved by the Ethics Committee of the Ruhr University Bochum (approval 4379-12).
Control heart samples
Nonfailing human LV samples (control group) were obtained from brain-dead human donors for whom normal LV function had been confirmed by echocardiographic evidence. Sample collection was done in full accordance with Australian National Health Medical Research guidelines and approved by the Human Research Ethics Committee of the University of Sydney (HREC approval: 2012/2814).
Quantification of fibrosis
The quantification of myocardial fibrosis was performed by an experienced pathologist (A.T.) according to the method of Tanaka et al. (23). Fibrosis was classified as no/mild, moderate, or severe.
Titin protein chemistry
A total of 20 to 25 μg of protein prepared from frozen human heart tissue were loaded onto 1.8% polyacrylamide/1% agarose gels for the detection of titin isoforms by Coomassie-blue staining, as previously described (24). All-titin phosphorylation was quantified by Western blotting using antiphosphoserine/-threonine antibodies (Biotrend Chemicals, Cologne, Germany) (25). In lieu of a protein marker in the size range of titin, Coomassie-stained polyvinylidenfluoride membranes served as markers of protein loading for immunoblots. Site-specific titin phosphorylation was measured within the unique N2Bus element of cardiac titin at 3 phosphoserines and within the PEVK domain of titin at 1 phosphoserine (both titin regions are mechanically active). The following custom-made, affinity-purified polyclonal antibodies against the phosphosites in human titin were used (24): anti-P-S4010, anti-P-S4099, anti-P-S4185 (all N2Bus), and P-S11878 (PEVK). Except for anti-P-S4185 (generated by Immunoglobe, Himmelstadt, Germany), these antibodies were made by Eurogentec (Brussels, Belgium). To measure the titin to myosin heavy chain (MHC) ratio, Coomassie-stained 2.5% polyacrylamide/1% agarose gels were prepared. We note that a substantial number of AS biopsies revealed no or barely measurable signals in the titin isoform/phosphorylation analysis, which was probably due to the fact that the biopsy material sometimes contained a low number of cardiomyocytes, whereas fibrotic regions were abundant.
Bands were visualized using the LAS-4000 Image Reader (Fuji Science Imaging Systems, Stamford, Connecticut), and densitometry was performed using Multi Gauge version 3.2 software (Fuji Science Imaging Systems). Titin N2B (≈3,000 kDa) and N2BA (≈3,300 kDa) isoforms were quantified by setting total N2B + N2BA band density to 100% and calculating percentages of the 2 isoforms. Titin/MHC ratios were directly calculated from the densitometric values measured for the respective protein bands. Quantification of titin phosphorylation was done by normalizing immunoblot signals to corresponding signals on (Coomassie-stained) polyvinylidenfluoride transfer membranes. Because total phospho-titin signals were consistently detected only in the N2B isoform, the N2BA isoform was excluded from the analysis of total-titin phosphorylation.
Follow-up
Three months after aortic valve replacement, the patients were evaluated at a clinical follow-up visit with renewed echocardiography. Another clinical follow-up was carried out 12 months after aortic valve replacement to determine NYHA functional class and survival.
Statistics
Numerical values were expressed as the mean ± SD. Continuous variables without normal distribution were summarized by the median (first quartile, third quartile). Continuous variables were compared between groups using an unpaired Student’s t test or analysis of variance test (for normally distributed variables) or a Mann-Whitney U test or Kruskal-Wallis test (for non-normally distributed variables), when appropriate. Chi-square analysis was used to compare categorical variables between groups. Adjusted results were obtained by multivariable analysis via generalized linear models and analysis of variance with age and sex as additional predictors. Post-hoc multiple pairwise comparisons were made using Bonferroni correction. Continuous variables were compared between patients before and after aortic valve replacement via paired Student’s t test (for normally distributed variables) or Wilcoxon test (for non-normally distributed variables). For outcome analysis, Kaplan-Meier survival curves were generated and differences between all-cause mortality were assessed using log-rank test. All reported probability values are 2-tailed, and p < 0.05 was considered statistically significant. Analyses were performed with the SPSS statistical software package version 22.0 (IBM, Armonk, New York) and with custom S scripts for R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
A total of 30 patients with severe symptomatic AS were included in this study. The mean age of the patients was 74 ± 7 years, and the mean AVA was 0.7 ± 0.1 cm2. Diastolic dysfunction was found in 26 of 30 patients (87%). Of the patients with AS, 14 patients had classical AS, 8 had paradoxical AS, and 8 had ASrEF. Patients with paradoxical AS had a significantly lower mean gradient and a lower stroke volume index compared with patients with classical AS (Table 1). Moreover, the E/A ratio, valvuloarterial impedance Z, and left atrial volume index were higher in the paradoxical than in the classical AS group. Compared with classical AS, patients with ASrEF had significantly increased BNP values and larger LV diameter, valvuloarterial impedance Z, LV mass index, and E/A ratio. Additional characteristics of the patients are listed in Table 1. Donor heart subjects had a mean age of 64 ± 8 years (5 female, 3 male), significantly lower that the AS group (p = 0.009). The proportion of females differed between the AS (7 of 30; 23%) and control groups (5 of 8; 63%; p = 0.034).
Table 1.
Characteristics of Different Subgroups of Patients With Severe Aortic Stenosis
| cAS (n = 14) | pAS (n = 8) | ASrEF (n = 8) | p Value | |
|---|---|---|---|---|
| Age, yrs | 77 ± 3 | 71 ± 9 | 72 ± 10 | 0.264 |
| Female | 29 | 38 | 0 | 0.170 |
| NYHA functional class III/IV | 29 | 63 | 75 | 0.079 |
| Coronary artery disease | 50 | 50 | 75 | 0.474 |
| Atrial fibrillation | 7 | 38 | 25 | 0.212 |
| Diabetes mellitus | 29 | 13 | 25 | 0.687 |
| B-type natriuretic peptide, pg/ml | 146 (107, 192) | 222 (169, 331) | 1,399 (477, 1,788)∗† | <0.001 |
| Systolic blood pressure, mm Hg | 132 ± 15 | 139 ± 22 | 126 ± 18 | 0.463 |
| Diastolic blood pressure, mm Hg | 75 ± 10 | 80 ± 7 | 81 ± 7 | 0.325 |
| Aortic valve area, cm2 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.6 ± 0.2 | 0.226 |
| Aortic mean gradient, mm Hg | 50 ± 7 | 34 ± 4‡ | 46 ± 14† | 0.001 |
| SVI, ml/m2 | 40 ± 7 | 30 ± 4‡ | 30 ± 11∗ | 0.008 |
| Z, mm Hg/ml · m2 | 4.8 ± 1.1 | 5.9 ± 1.1‡ | 6.7 ± 2.8∗ | 0.040 |
| Left ventricular ejection fraction, % | 65 ± 4 | 60 ± 6 | 36 ± 6∗† | <0.001 |
| LVEDDI, cm/m2 | 2.4 ± 0.3 | 2.2 ± 0.4 | 2.8 ± 0.3∗† | 0.005 |
| LVESDI, cm/m2 | 1.6 ± 0.3 | 1.6 ± 0.4 | 2.3 ± 0.3∗† | 0.002 |
| Global longitudinal strain, % | 14 ± 4 | 13 ± 3 | 9 ± 3∗† | 0.007 |
| LVMI, g/m2 | 130 ± 25 | 154 ± 82 | 192 ± 50∗ | 0.017 |
| Mitral regurgitation, grade 0/1/2 | 65/35/0 | 50/50/0 | 0/75/25 | 0.017 |
| LAVI, ml/m2 | 32 ± 8 | 41 ± 11‡ | 39 ± 13∗ | 0.117 |
| Diastolic dysfunction | 71 | 100 | 100 | 0.072 |
| E/A ratio | 0.9 ± 0.3 | 2.2 ± 1.5‡ | 3.4 ± 2.1∗ | 0.115 |
| sPAP, mm Hg | 24 ± 11 | 30 ± 12 | 41 ± 11∗ | 0.011 |
Values are mean ± SD, %, or median (first quartile, third quartile).
ASrEF = aortic stenosis with reduced ejection fraction; cAS = classical aortic stenosis; LAVI = left atrial volume index; LMVI = left ventricular mass index; LVEDVI = left ventricular end-diastolic diameter index; LVESDI = left ventricular end-systolic diameter index; NYHA = New York Heart Association; pAS = paradoxical aortic stenosis; sPAP = systolic pulmonary artery pressure; SVI = stroke volume index; Z = valvuloarterial impedance.
cAS vs. ASrEF (p < 0.05).
pAS vs. ASrEF (p < 0.05).
cAS vs. pAS (p < 0.05).
Clinical follow-up
Clinical and echocardiographic follow-up evaluations were performed after 3 months. During this time, 6 patients died due to acute cardiogenic shock (n = 1), sepsis (n = 2), ventricular fibrillation (n = 1), and heart pump failure (n = 2). The 30-day mortality rate was 3%. There was no significant difference in outcome between patients with or without coronary artery bypass grafting (p = 0.750). In the 24 surviving patients, NYHA functional class improved; aortic mean gradient, valvuloarterial impedance Z, and LV mass index decreased; and AVA increased significantly (Table 2). Among the different AS subgroups, significant differences were found only for LVEF and aortic mean gradient (Table 3). The 1-year survival rates were 93% in patients with classical AS, 75% in patients with ASrEF, and 63% in patients with paradoxical AS. Although the differences in survival were not significant, there was a trend toward worse survival in patients with paradoxical AS.
Table 2.
Follow-Up Evaluation Before and 3 Months After AVR
| Before AVR | After AVR | p Value | |
|---|---|---|---|
| NYHA functional class III/IV, % | 44 | 9 | 0.021 |
| B-type natriuretic peptide, pg/ml | 338 ± 377 | 279 ± 143 | 0.568 |
| Aortic valve area, cm2 | 0.7 ± 0.1 | 1.7 ± 0.2 | <0.001 |
| Aortic mean gradient, mm Hg | 45 ± 9 | 9 ± 3 | <0.001 |
| Z, mm Hg/ml · m2 | 5.7 ± 2.1 | 4.2 ± 1.2 | 0.015 |
| Left ventricular ejection fraction, % | 59 ± 12 | 58 ± 8 | 0.749 |
| LVEDDI, cm/m2 | 2.5 ± 0.4 | 2.4 ± 0.3 | 0.086 |
| LVESDI, cm/m2 | 1.8 ± 0.5 | 1.6 ± 0.3 | 0.077 |
| LVMI, g/m2 | 139 ± 34 | 117 ± 30 | 0.002 |
Values are % or mean ± SD.
AVR = aortic valve replacement; other abbreviations as in Table 1.
Table 3.
Changes in NYHA Functional Class, B-Type Natriuretic Peptide and Hemodynamics in Different Subgroups of Patients With Severe Aortic Stenosis
| cAS (n = 13) | pAS (n = 5) | ASrEF (n = 6) | p Value | |
|---|---|---|---|---|
| Δ NYHA functional class III/IV, % | −31 | −40 | −33 | 0.717 |
| Δ B-type natriuretic peptide, pg/ml | 39 ± 154 | 112 ± 18 | −523 ± 750∗ | 0.110 |
| Δ Aortic valve area, cm2 | 1.0 ± 0.2 | 0.9 ± 0.3 | 1.1 ± 0.4 | 0.571 |
| Δ Aortic mean gradient, mm Hg | −41 ± 8 | −24 ± 7† | −36 ± 9 | 0.006 |
| Δ Z, mm Hg/ml · m2 | −1.2 ± 1.0 | −0.7 ± 2.4 | −3.3 ± 4.1 | 0.571 |
| Δ Left ventricular ejection fraction, % | −6 ± 5 | 1.6 ± 8.2† | 13 ± 12∗ | 0.012 |
| Δ LVEDDI, cm/m2 | −0.1 ± 0.3 | −0.1 ± 0.3 | −0.3 ± 0.3 | 0.349 |
| Δ LVESDI, cm/m2 | 0.1 ± 0.6 | −0.3 ± 0.2 | −0.5 ± 0.4 | 0.086 |
| Δ LVMI, g/m2 | −19 ± 32 | −19 ± 22 | −35 ± 30∗ | 0.612 |
Values are % or mean ± SD.
Abbreviations as in Table 1.
cAS vs. ASrEF (p < 0.05).
cAS vs. pAS (p < 0.05). Δ indicates comparison of baseline parameters to parameters at 3-month follow-up.
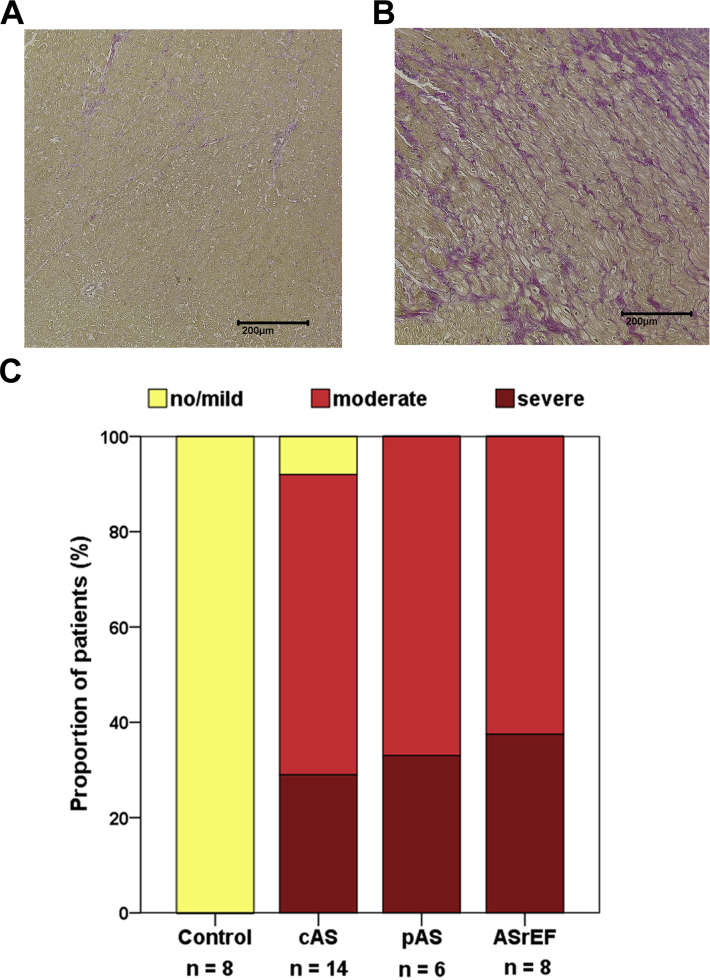
Fibrosis is increased in AS
In endomyocardial biopsies, significantly more cases of severe fibrosis were found in patients with severe AS than in the control group (severe AS: no/mild fibrosis, n = 1; moderate fibrosis, n = 13; severe fibrosis, n = 14; vs. control group: no/mild fibrosis, n = 8; p = 0.001). After adjusting for age and sex, aortic stenosis was the only predictor for the degree of fibrosis. The degree of myocardial fibrosis appeared to be similar among patients with classical AS, paradoxical AS, and ASrEF (Figure 1). In patients with or without coronary heart disease, there was no significant difference in the degree of fibrosis (coronary artery disease: no/mild fibrosis, n = 3; moderate fibrosis, n = 5; severe fibrosis, n = 8; vs. no coronary artery disease: no/mild fibrosis, n = 4; moderate fibrosis, n = 7; severe fibrosis, n = 6; p = 0.231).
Figure 1.
Myocardial Fibrosis in Subtypes of Aortic Stenosis
(A) No/mild myocardial fibrosis in a patient with “classical” aortic stenosis. (B) Severe myocardial fibrosis in a patient with “paradoxical” aortic stenosis. (C) Distribution of different degrees of myocardial fibrosis. Mann-Whitney U test revealed significant differences between control group and all AS patients (p = 0.001). Comparison was made between study patients’ subgroups using Kruskal-Wallis test (p = 0.007). ASrEF = aortic stenosis with reduced left ventricular ejection fraction; cAS = “classical” aortic stenosis; pAS = “paradoxical” aortic stenosis.
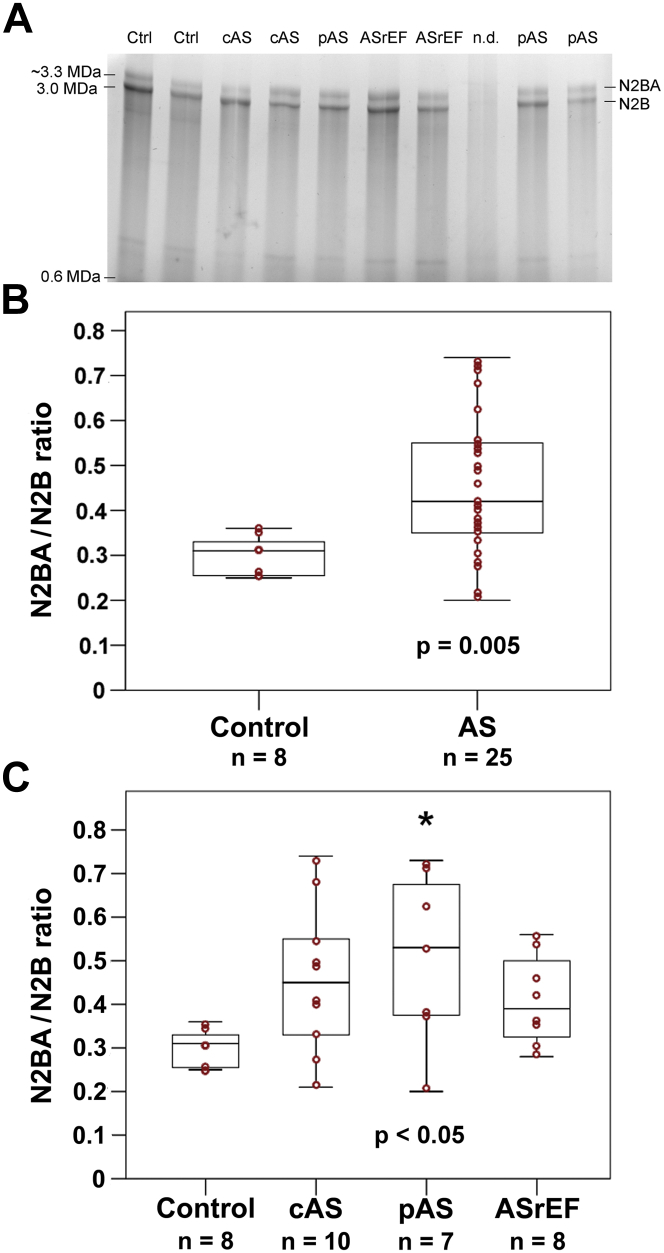
Titin isoforms switch toward N2BA in AS
A relatively large variability in titin-isoform composition was observed among the biopsies of patients with AS (n = 25), but not among the donor samples (n = 8) (Figure 2). The median (first quartile, third quartile) N2BA/N2B titin isoform ratio was significantly higher in patients with AS than in the control group (0.42 [0.34, 0.56] vs. 0.31 [0.25, 0.34]; p = 0.005) (Figure 2B). After adjusting for age and sex, aortic stenosis was the only predictor for N2BA/N2B ratio.
Figure 2.
Titin Isoform Expression in Nonfailing Donor (Control) and AS Patient Hearts
(A) Representative loose gel (1.8% polyacrylamide/1% agarose) resolving the titin isoforms N2BA and N2B. (B and C) Summary of results of densitometric analysis of titin protein composition, demonstrated as N2BA/N2B isoform expression ratio. Shown are box plots (median [first quartile, third quartile]) with whiskers and individual data points (red circles). (B) Mann-Whitney U test revealed statistically significant differences between control hearts and all AS patients (p = 0.005). (C) Comparison was made between study patients’ subgroups using the Kruskal-Wallis test (p = 0.042). Post hoc multiple pairwise comparisons revealed significant differences between the control group and paradoxical AS (*p < 0.05). AS = aortic stenosis; other abbreviations as in Figure 1.
Comparing the AS subgroups to the donor group, the median (first quartile, third quartile) N2BA/N2B ratio was significantly higher only for patients with paradoxical AS (0.53 [0.37, 0.68] vs. 0.31 [0.25, 0.34]; p = 0.013) (Figure 2C). The median (first quartile, third quartile) titin/MHC ratio showed no significant differences between the donor and AS groups (0.21 [0.17, 0.24] vs. 0.20 [0.19, 0.22]; p = 0.949) and there were no significant differences among the AS subgroups or between a given AS subgroup and the control group (Supplemental Figure 1). In patients with or without coronary heart disease, there was no significant difference in the median (first quartile, third quartile) N2BA/N2B ratio (0.50 [0.36, 0.63] vs. 0.38 [0.26, 0.51]; p = 0.131) and the median titin/MHC ratio (0.20 [0.17, 0.25] vs. 0.22 [0.17, 0.23]; p = 0.542).
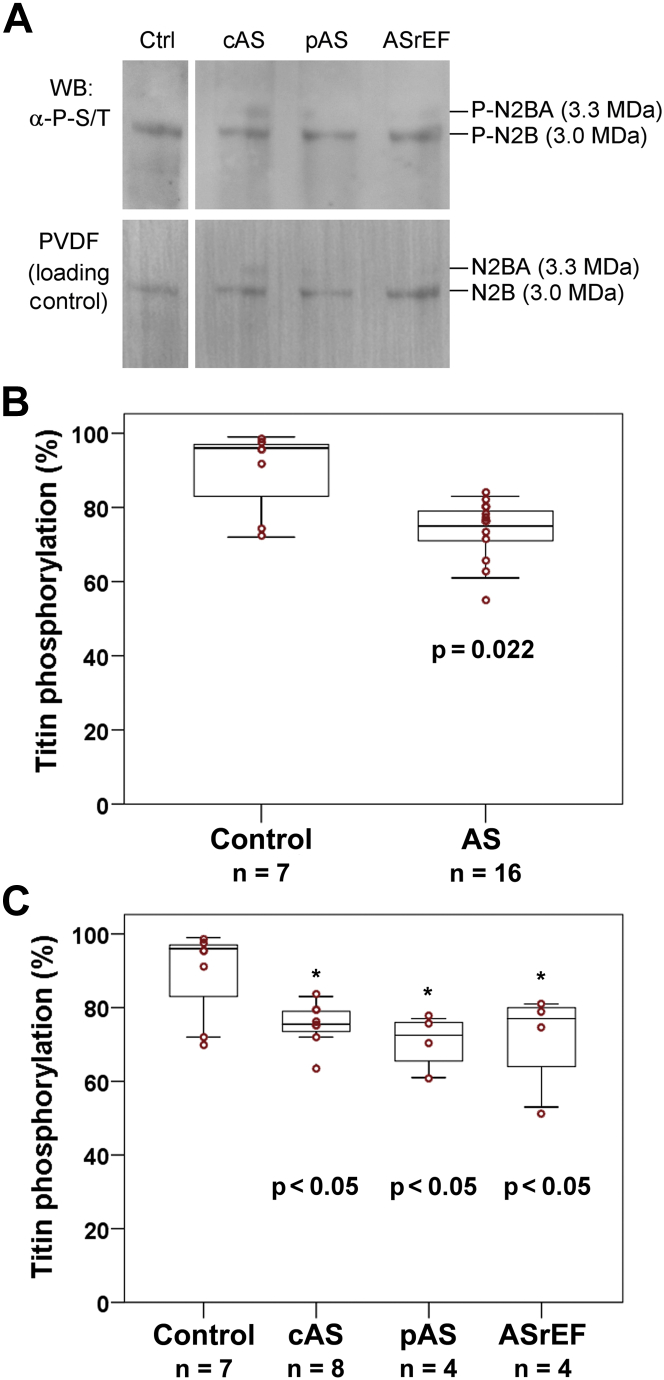
Total titin phosphorylation is reduced in AS
Analysis of total-titin phosphorylation by Western blot using antiphosphoserine/-threonine antibodies revealed signals from the N2B isoform of 7 of the 8 donor samples and 16 of the 30 AS samples (Figure 3). Compared with the donor cohort, a significantly lower degree of phosphorylation was observed in the AS hearts (median [first quartile, third quartile] phosphorylation 75% [71%, 79%] vs. 96% [74%, 98%]; p = 0.022) (Figure 3B). After adjusting for age and sex, aortic stenosis was the only predictor for total-titin phosphorylation. Titin-phosphorylation signals were obtained for 8 of 14 patients with classical AS, 4 of 8 patients with paradoxical AS, and 4 of 8 patients with ASrEF. All 3 AS subgroups showed significant hypophosphorylation compared with donor hearts (p < 0.05), including patients with paradoxical AS, which tended to have a relatively high degree of titin hypophosphorylation (Figure 3C). In patients with or without coronary heart disease, there was no significant difference in the median (first quartile, third quartile) total-titin phosphorylation level (75% [73%, 78%] vs. 75% [64%, 81%]; p = 0.689).
Figure 3.
Phosphorylation of Total Titin in Nonfailing Donor (Control) and AS Patient Hearts
(A) Representative Western blot (WB) from 1.8% polyacrylamide/1% agarose gel, using antiphosphoserine/-threonine antibodies, as well as Coomassie-stained polyvinylidenfluoride (PVDF) membrane indicating protein load. All bands shown were from the same gel/blot. (B and C) Summary of results of densitometric analysis of titin phosphorylation (N2B isoform), indexed to protein load. Shown are box plots (median [first quartile, third quartile]) with whiskers and individual data points (red circles). (B) Mann-Whitney U test revealed statistically significant differences between control hearts and all AS patients (p = 0.022). (C) Comparison was made between AS subgroups using Kruskal-Wallis test (p > 0.05). Post hoc multiple pairwise comparisons revealed significant differences between control group and cAS, pAS, and ASrEF (*p < 0.05). Abbreviations as in Figure 1.
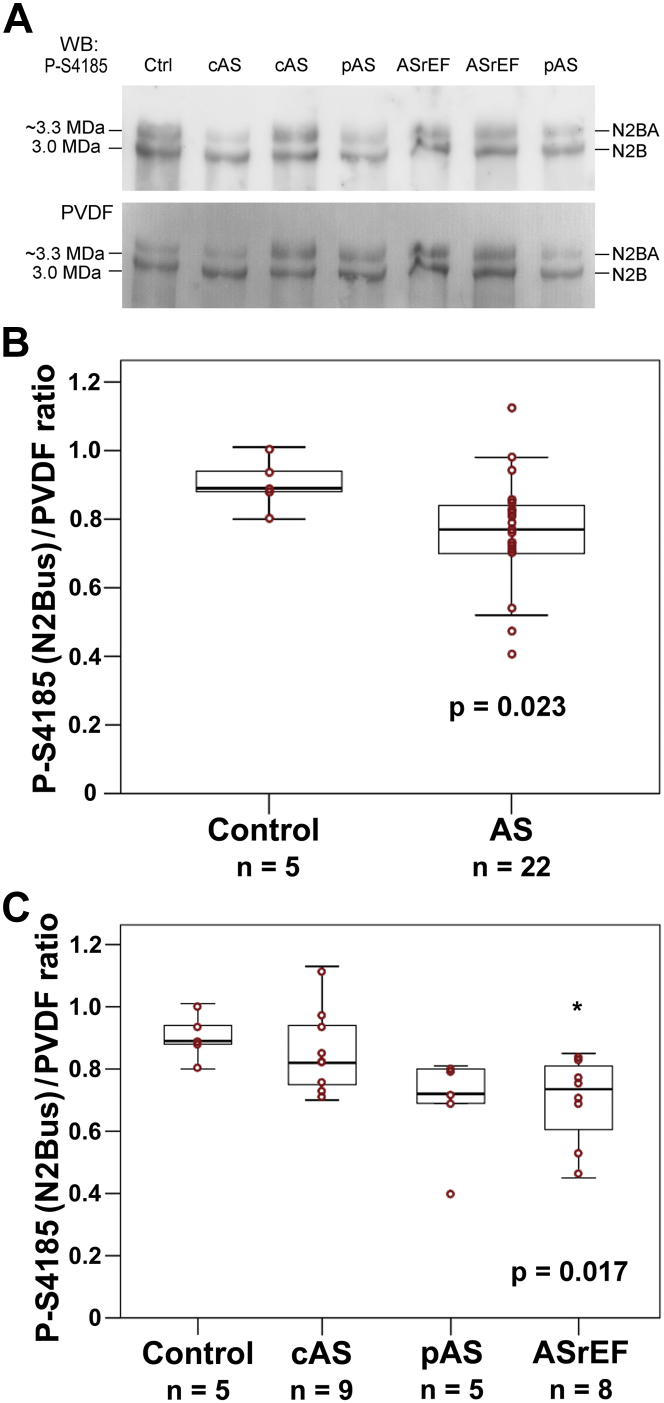
Mechanically relevant phosphoserine at titin N2Bus is hypophosphorylated in AS
Site-specific titin phosphorylation was measured for several phosphosites within the elastic spring regions N2Bus and PEVK, using a panel of phospho-specific antibodies (Table 4, Figure 4). Phosphorylation of these titin spring elements affects cardiomyocyte and myocardial passive stiffness (14). The phosphoserines within N2Bus measured here are known to be phosphorylated by protein kinase (PK)A or extracellular signal-regulated protein kinase 2 (P-S4010), cGMP-activated PKG (P-S4099), and PKG or PKA (P-S4185), and they can be dephosphorylated by protein phosphatase 5 (19), whereas phosphoserine P-S11878 within the PEVK domain can be phosphorylated by PKCα (14). In AS versus donor samples, no difference in median phosphorylation was observed for P-S4010, P-S4099, and P-11878 (Table 4). However, N2Bus phosphosite P-S4185 was significantly less phosphorylated in AS than in donors (median [first quartile, third quartile] 0.77 [0.70, 0.84] vs. 0.89 [0.88, 0.94]; p = 0.023) (Figure 4B). Using multivariable analysis, both aortic stenosis and age were found to predict phosphorylation of the titin phosphosite P-S4185 (p < 0.01 for both). The subgroup pAS showed a trend for hypophosphorylation at S4185, compared with donor hearts and cAS, whereas the ASrEF subgroup was significantly hypophosphorylated at S4185 versus donors (Table 4, Figure 4C). In summary, the PKA/PKG-dependent phosphoserine P-S4185 within human N2Bus was hypophosphorylated in AS, explaining part of the total titin phosphorylation deficit in AS versus nonfailing control hearts.
Table 4.
Site-Specific Titin Phosphorylation
| Control | AS | cAS | pAS | ASrEF | p Value∗ | |
|---|---|---|---|---|---|---|
| P-S4010/PVDF (N2Bus) | 0.80 (0.76, 0.82) (n = 5) | 0.75 (0.65, 0.81) (n = 20) | 0.80 (0.69, 0.83) (n = 8) | 0.80 (0.68, 0.84) (n = 4) | 0.70 (0.65, 0.72) (n = 8) | 0.216 0.156 |
| P-S4099/PVDF (N2Bus) | 0.86 (0.83, 0.92) (n = 6) | 0.85 (0.78, 0.90) (n = 22) | 0.85 (0.78, 0.90) (n = 9) | 0.79 (0.76, 0.80) (n = 5) | 0.85 (0.78, 0.88) (n = 8) | 0.214 0.353 |
| P-S4185/PVDF (N2Bus) | 0.89 (0.88, 0.94) (n = 5) | 0.77 (0.70, 0.84) (n = 22) | 0.82 (0.74, 0.94) (n = 9) | 0.72 (0.69, 0.80) (n = 5) | 0.74 (0.61, 0.81) (n = 8) | 0.023 0.017 |
| P-S11878/PVDF (PEVK) | 0.91 (0.78, 1.00) (n = 6) | 0.94 (0.87, 0.98) (n = 21) | 0.94 (0.89, 0.98) (n = 9) | 0.97 (0.92, 1.02) (n = 4) | 0.88 (0.81, 0.98) (n = 8) | 0.712 0.570 |
Values are median (1st quartile, 3rd quartile).
Upper p value compares control group and all AS subgroups using the Mann-Whitney U test. Lower p value compares control and subgroups of AS using the Kruskal-Wallis test.
Figure 4.
Site-Specific Phosphorylation of N2Bus Titin in Nonfailing Donor (Control) and AS Patient Hearts
(A) Representative WBs from 1.8% polyacrylamide/1% agarose gels, using antititin antibodies against phosphoserine P-S4185 within the N2Bus element. Coomassie-stained PVDF membrane is also shown to indicate protein load. (B and C) Summary of results of densitometric analysis for the P-S4185 phosphosite in N2Bus titin, which was the only phosphosite showing significant differences in AS versus nonfailing donor hearts. Titin phosphorylation was indexed to protein load. Shown are box plots (median [first quartile, third quartile]) with whiskers and individual data points (red circles). (B) Mann-Whitney U test revealed statistically significant differences between control hearts and all AS patients (p = 0.023). (C) Comparison was made between the control group and AS subgroups using the Kruskal-Wallis test, revealing significant differences within the study groups (p = 0.017). A post hoc test (pairwise Wilcoxon Mann-Whitney U test) suggested a significant difference between donor and ASrEF hearts (*p < 0.05). Abbreviations as in Figures 1 and 3.
Discussion
In this study, we examined patients with severe symptomatic AS who underwent surgical aortic valve replacement. There were no significant differences between the subgroups of AS in terms of clinical symptoms and AVA. However, the hemodynamic analysis revealed fundamental differences between classical high-gradient, normal-flow AS; paradoxical low-flow, low-gradient AS with preserved LV ejection fraction; and AS with reduced EF. In particular, there were significant differences in aortic mean gradient, stroke volume, valvuloarterial impedance Z, LVEF, and BNP (Table 1). A trend toward worse outcome was observed in patients with paradoxical AS after aortic valve replacement (1-year survival was 63%, compared with 93% in classical AS). This trend is consistent with earlier findings (9).
Myocardial fibrosis
The myocardium in AS is known to be characterized by increased cell mass and increased fibrosis 1, 2, 3, 11, 12, 13. In our study, we confirmed that patients with severe symptomatic AS have significant myocardial fibrosis. Patients with paradoxical AS showed a degree of fibrosis similar to that of patients with classical AS or ASrEF (Figure 1). Our findings are consistent with those of Herrmann et al. (12), who examined different forms of AS (12), and underscore the view that paradoxical AS is a disease with severe intrinsic myocardial damage 9, 10.
Titin N2BA/N2B isoform expression ratio
Diverging published data exist regarding the ratio of the more compliant titin isoform N2BA to the stiffer isoform N2B in patients with AS. One study demonstrated a reduced N2BA/N2B expression ratio in 19 patients with AS, compared to donor hearts (18). Another study reported no significant alterations in titin-isoform composition in 14 AS patients versus healthy hearts (17). Yet, another study with 9 AS patients, from the same group, detected a significant shift of the titin-isoform expression ratio toward more compliant N2BA (16). The reason for these diverging results is unknown. We investigated the largest number of AS patients yet for titin-isoform expression. In our patient group, we found a significant shift toward the more compliant N2BA titin isoform versus nonfailing donor heart samples (Figure 2). The magnitude and direction of this isoform transition were similar to those seen in end-stage failing human hearts with ischemic or dilated cardiomyopathy, compared with nonfailing donor hearts 26, 27. Such a transition toward compliant titin isoforms may be a compensatory mechanism accompanying the increase in myocardial stiffness through fibrosis or titin-hypophosphorylation. Similar observations have been made in various other human myocardial diseases, including HF with preserved EF (14).
Our study examined, for the first time, the expression of titin isoforms in different hemodynamic subgroups of AS. The titin-isoform switch toward N2BA was significant in patients with paradoxical AS (Figure 2C). This could be an indication of substantial remodeling taking place in the myocardium in this AS subtype. In classical AS, there was a strong trend toward increased N2BA proportions, whereas in ASrEF, a relatively low degree of titin-isoform shift was found, despite pronounced fibrosis in all AS subtypes. However, the low sample sizes in the individual AS subgroups precludes a generalization of these findings. Collectively, our data support the view that the titin-isoform transition in human AS typically is in the direction of the more compliant N2BA variant.
Titin phosphorylation
We also demonstrated hypophosphorylation of total titin in patients with severe AS compared with healthy donor hearts (Figure 3). Phosphorylation is an important factor in the modification of myocardial protein function 14, 28. Previous studies demonstrated hypophosphorylation of titin at the cardiac-specific N2B element in human heart failure 29, 30, 31 and in animal models of heart failure with preserved EF 32, 33. Some studies also showed an increased ratio of phosphorylated N2BA over phosphorylated N2B in human heart failure with reduced or preserved ejection fraction 16, 17. Incubation of isolated (skinned) failing human cardiomyocytes with PKA or PKG corrected a pathologically elevated titin-based passive stiffness 17, 34. Here, we provided evidence that human AS patients have a phosphorylation deficit at the elastic N2Bus domain, specifically, at residue S4185 (Figure 4). Phosphorylation of this serine, as well as other residues within the N2Bus region, is known to reduce titin-based myocardial passive stiffness (29). Conversely, dephosphorylation (19) or hypo-phosphorylation of N2Bus is predicted to increase titin-based stiffness. P-S4185 in human titin can be phosphorylated by both PKA and PKG 29, 30, and low activity of these protein kinases in AS may be a reason for the hypophosphorylation of N2Bus. Other known phosphoserines within the N2Bus and PEVK regions were unaltered in AS versus nonfailing hearts. Notably, hypophosphorylation of total titin and P-S4185 within N2Bus were also present or tended to be present in patients with paradoxical AS (Figures 3 and 4). These results provided additional evidence that this type of AS is similarly affected by remodeling as classical AS or ASrEF. In conclusion, although we did not measure titin-based passive tension, the reduced titin phosphorylation, especially at the N2Bus element, could be a factor underlying the increased passive stiffness and diastolic dysfunction in AS.
Study limitations
In the relatively small population group, a subdivision into a group with low-flow, low-gradient AS with reduced LVEF was not possible. However, the present study is the largest yet to investigate the expression of titin isoforms and phosphorylation in AS, and to our knowledge is the only study to look at site-specific titin phosphorylation. Notably, it is not settled whether or not the basal septum endocardial biopsy site is representative of the entire LV. However, it was not possible in this study to take a sample from other regions of the LV, as this would have meant a much higher risk for the patients. In our study, more than 50% of all patients had coronary heart disease. The characteristics of our study cohort thus correspond to the usual characteristics of patients with severe AS 1, 2. However, we excluded patients with previous myocardial infarction to prevent bias in the analysis of titin and fibrosis. Due to the small size of the myocardial biopsies available, functional studies at the cellular level (e.g., passive tension measurements) were not feasible. Thus, it remains open whether the titin-based passive stiffness was altered in the AS versus donor cohorts, considering that the titin isoform shift toward more compliant N2BA in AS and the hypophosphorylation at N2Bus would be expected to change this stiffness in opposite directions.
Conclusions
Our study demonstrated an increase in myocardial fibrosis and titin-isoform shift toward the more compliant N2BA variants in AS. This titin-isoform switch could be a compensatory mechanism following or accompanying the increased matrix stiffness, or it could compensate for increased cardiomyocyte stiffness due to chronic titin hypophosphorylation. There was significant hypophosphorylation of total titin and N2Bus-titin residue P-S4185 in AS compared with healthy hearts. This study is the first to investigate the expression of titin isoforms (N2BA/N2B), titin phosphorylation, and titin/MHC ratio in different hemodynamic subtypes of AS. Alterations in titin phosphorylation and myocardial fibrosis were found to be similar in paradoxical AS, classical high-gradient AS, and AS with reduced EF. “Paradoxical” AS, but not cAS or ASrEF, showed a significant shift toward compliant N2BA titin compared with control hearts. Findings suggest that paradoxical AS is a severe AS with pronounced myocardial remodeling. These changes could explain the unfavorable prognosis of the disease.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE 1: Myocardial relaxation and stiffness in AS are determined by myocardial fibrosis and the giant cytoskeletal protein titin.
COMPETENCY IN MEDICAL KNOWLEDGE 2: Paradoxical low-flow, low-gradient AS with preserved ejection is a common subtype of severe AS. It is characterized by increased myocardial fibrosis, titin-isoform transition toward compliant variants, and significant hypophosphorylation of titin compared with healthy hearts.
TRANSLATIONAL OUTLOOK: Additional research, such as measurements of cardiomyocyte function in AS and invasive hemodynamics, may also improve our understanding of the pathophysiology of paradoxical AS.
Footnotes
This work was supported by the German Heart Foundation/German Foundation of Heart Research. Dr. Dietrich has received funding from Sanofi-Henning, Hexal AG, Bristol-Myers Squibb, and Pfizer; and is co-owner of the intellectual property rights for the patent “System and Method for Deriving Parameters for Homeostatic Feedback Control of an Individual” (Singapore Institute for Clinical Sciences, Biomedical Sciences Institutes, Application Number 201208940-5, WIPO number WO/2014/088516). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Michael Gotzmann, Email: michael.gotzmann@ruhr-uni-bochum.de.
Wolfgang A. Linke, Email: wlinke@uni-muenster.de.
Appendix
References
- 1.Otto C.M., Prendergast B. Aortic-valve stenosis—from patients at risk to severe valve obstruction. N Engl J Med. 2014;371:744–756. doi: 10.1056/NEJMra1313875. [DOI] [PubMed] [Google Scholar]
- 2.Carabello B.A., Paulus W.J. Aortic stenosis. Lancet. 2009;373:956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 3.Hess O.M., Ritter M., Schneider J., Grimm J., Turina M., Krayenbuehl H.P. Diastolic stiffness and myocardial structure in aortic valve disease before and after valve replacement. Circulation. 1984;69:855–865. doi: 10.1161/01.cir.69.5.855. [DOI] [PubMed] [Google Scholar]
- 4.Hachicha Z., Dumesnil J.G., Bogaty P., Pibarot P. Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation. 2007;115:2856–2864. doi: 10.1161/CIRCULATIONAHA.106.668681. [DOI] [PubMed] [Google Scholar]
- 5.Clavel M.A., Magne J., Pibarot P. Low-gradient aortic stenosis. Eur Heart J. 2016;37:2645–2657. doi: 10.1093/eurheartj/ehw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jander N., Minners J., Holme I. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation. 2011;123:887–895. doi: 10.1161/CIRCULATIONAHA.110.983510. [DOI] [PubMed] [Google Scholar]
- 7.Chin C.W., Ding Z.P., Lam C.S., Ling L.H. Paradoxical low-gradient aortic stenosis: the HFpEF of aortic stenosis. J Am Coll Cardiol. 2016;67:2447–2448. doi: 10.1016/j.jacc.2016.02.070. [DOI] [PubMed] [Google Scholar]
- 8.Chhabra L. Inconsistency of hemodynamic data in low-gradient severe aortic stenosis. J Am Coll Cardiol. 2016;67:2446–2447. doi: 10.1016/j.jacc.2016.01.089. [DOI] [PubMed] [Google Scholar]
- 9.Clavel M.A., Dumesnil J.G., Capoulade R., Mathieu P., Sénéchal M., Pibarot P. Outcome of patients with aortic stenosis, small valve area, and low-flow, low-gradient despite preserved left ventricular ejection fraction. J Am Coll Cardiol. 2012;60:1259–1267. doi: 10.1016/j.jacc.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 10.Dayan V., Vignolo G., Magne J., Clavel M.A., Mohty D., Pibarot P. Outcome and impact of aortic valve replacement in patients with preserved LVEF and low-gradient aortic stenosis. J Am Coll Cardiol. 2015;66:2594–2603. doi: 10.1016/j.jacc.2015.09.076. [DOI] [PubMed] [Google Scholar]
- 11.Krayenbuehl H.P., Hess O.M., Monrad E.S., Schneider J., Mall G., Turina M. Left ventricular myocardial structure in aortic valve disease before, intermediate, and late after aortic valve replacement. Circulation. 1989;79:744–755. doi: 10.1161/01.cir.79.4.744. [DOI] [PubMed] [Google Scholar]
- 12.Herrmann S., Störk S., Niemann M. Low-gradient aortic valve stenosis myocardial fibrosis and its influence on function and outcome. J Am Coll Cardiol. 2011;58:402–412. doi: 10.1016/j.jacc.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 13.Milano A.D., Faggian G., Dodonov M. Prognostic value of myocardial fibrosis in patients with severe aortic valve stenosis. J Thorac Cardiovasc Surg. 2012;144:830–837. doi: 10.1016/j.jtcvs.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Linke W.A., Hamdani N. Gigantic business: titin properties and function through thick and thin. Circ Res. 2014;114:1052–1068. doi: 10.1161/CIRCRESAHA.114.301286. [DOI] [PubMed] [Google Scholar]
- 15.Freiburg A., Trombitas K., Hell W. Series of exon-skipping events in the elastic spring region of titin as the structural basis for myofibrillar elastic diversity. Circ Res. 2000;86:1114–1121. doi: 10.1161/01.res.86.11.1114. [DOI] [PubMed] [Google Scholar]
- 16.Borbély A., Falcao-Pires I., van Heerebeek L. Hypophosphorylation of the stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 17.Falcão-Pires I., Hamdani N., Borbély A. Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation. 2011;124:1151–1159. doi: 10.1161/CIRCULATIONAHA.111.025270. [DOI] [PubMed] [Google Scholar]
- 18.Williams L., Howell N., Pagano D. Titin isoform expression in aortic stenosis. Clin Sci (Lond) 2009;117:237–242. doi: 10.1042/CS20080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krysiak J., Unger A., Beckendorf L. Protein phosphatase 5 regulates titin phosphorylation and function at a sarcomere-associated mechanosensor complex in cardiomyocytes. Nat Commun. 2018;9:262. doi: 10.1038/s41467-017-02483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thygesen K., Alpert J.S., Jaffe A.S., for the Writing Group on the Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction. ESC Committee for Practice Guidelines (CPG) Third universal definition of myocardial infarction. Eur Heart J. 2012;33:2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 21.Lancellotti P., Tribouilloy C., Hagendorff A., for the Scientific Document Committee of the European Association of Cardiovascular Imaging Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2013;14:611–644. doi: 10.1093/ehjci/jet105. [DOI] [PubMed] [Google Scholar]
- 22.Paulus W.J., Tschöpe C., Sanderson J.E. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M., Fujiwara H., Onodera T., Wu D.J., Hamashima Y., Kawai C. Quantitative analysis of myocardial fibrosis in normals, hypertensive hearts, and hypertrophic cardiomyopathy. Br Heart J. 1986;55:575–581. doi: 10.1136/hrt.55.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamdani N., Krysiak J., Kreusser M.M. Crucial role for Ca2(+)/calmodulin-dependent protein kinase-II in regulating diastolic stress of normal and failing hearts via titin phosphorylation. Circ Res. 2013;112:664–674. doi: 10.1161/CIRCRESAHA.111.300105. [DOI] [PubMed] [Google Scholar]
- 25.Mohamed B.A., Schnelle M., Khadjeh S. Molecular and structural transition mechanisms in long-term volume overload. Eur J Heart Fail. 2016;18:362–371. doi: 10.1002/ejhf.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neagoe C., Kulke M., del Monte F. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–1341. doi: 10.1161/01.cir.0000029803.93022.93. [DOI] [PubMed] [Google Scholar]
- 27.Makarenko I., Opitz C.A., Leake M.C. Passive stiffness changes caused by upregulation of compliant titin isoforms in human DCM hearts. Circ Res. 2004;95:708–716. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 28.Hudson B., Hidalgo C., Saripalli C., Granzier H. Hyperphosphorylation of mouse cardiac titin contributes to transverse aortic constriction-induced diastolic dysfunction. Circ Res. 2011;109:858–866. doi: 10.1161/CIRCRESAHA.111.246819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krüger M., Kötter S., Grützner A. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104:87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 30.Kötter S., Gout L., Von Frieling-Salewsky M. Differential changes in titin domain phosphorylation increase myofilament stiffness in failing human hearts. Cardiovasc Res. 2013;99:648–656. doi: 10.1093/cvr/cvt144. [DOI] [PubMed] [Google Scholar]
- 31.Zile M.R., Baicu C.F., Ikonomidis J.S. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–1259. doi: 10.1161/CIRCULATIONAHA.114.013215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamdani N., Bishu K.G., von Frieling-Salewsky M., Redfield M.M., Linke W.A. Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res. 2013;97:464–471. doi: 10.1093/cvr/cvs353. [DOI] [PubMed] [Google Scholar]
- 33.Hamdani N., Franssen C., Lourenço A. Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail. 2013;6:1239–1249. doi: 10.1161/CIRCHEARTFAILURE.113.000539. [DOI] [PubMed] [Google Scholar]
- 34.van Heerebeek L., Borbély A., Niessen H.W.M. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113:1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.