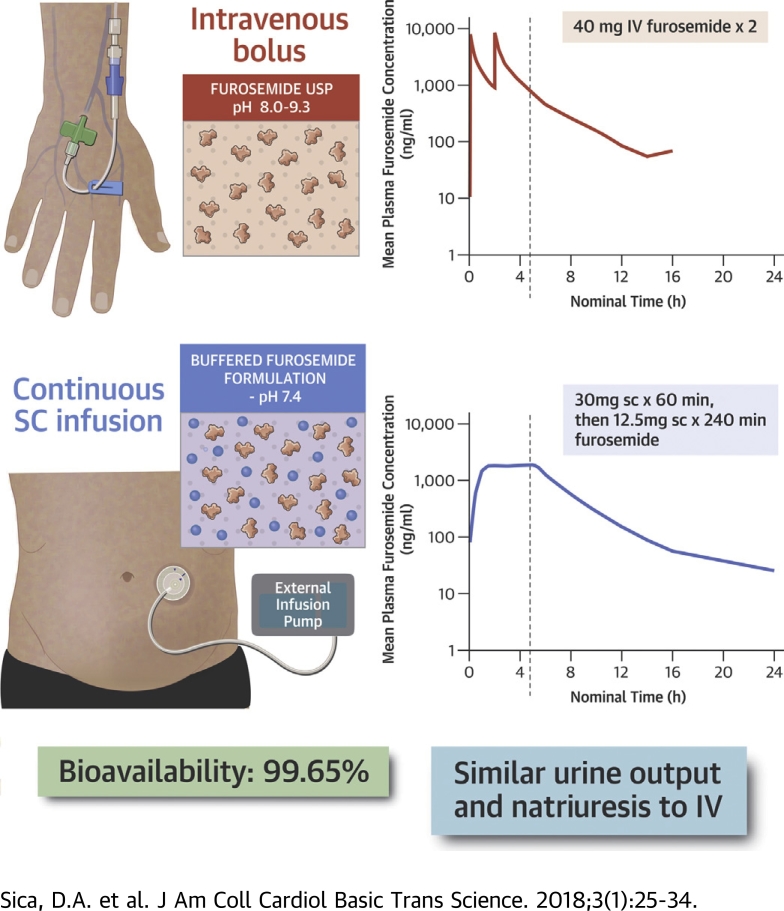
Visual Abstract
Key Words: diuresis, furosemide, heart failure, pharmacokinetics, subcutaneous
Abbreviations and Acronyms: ANOVA, analysis of variance; AUC, area under the curve; AUC∞, plasma concentration to infinity; AUClast, last measurable plasma concentration; CI, confidence interval; Cmax, peak plasma concentration; HF, heart failure; IV, intravenous; LSM, least squares mean; SC, subcutaneous; t½, terminal phase elimination half-life
Highlights
-
•
Parenteral diuretics form the cornerstone of treatment of fluid overload in heart failure. The alkaline pH of furosemide USP precludes SC administration.
-
•
A novel buffered formulation of furosemide (pH 7.4) was developed for SC infusion using a wearable patch pump.
-
•
A 5-h biphasic SC infusion (30 mg in hour 1 followed by 12.5 mg/h for 4 h) of the novel formulation was tested in 2 clinical studies.
-
•
Following SC administration, therapeutic levels of furosemide were reached within 30 min and maintained for up to 6 h. This resulted in complete bioavailability and equivalent diuresis when compared with 80 mg of intravenous furosemide.
-
•
A novel strategy using SC administration of buffered furosemide is introduced for outpatient use, including self-administration at home. This may help to reduce the patient and economic burden of heart failure.
Summary
Parenteral diuretics form the cornerstone of decongestion in heart failure. However, parenteral therapy routinely requires emergency room or inpatient care. A novel buffered furosemide formulation with neutral pH was developed to offer “hospital-strength” diuresis for outpatient use, including self-administration at home. Subcutaneous infusion using a biphasic delivery profile resulted in complete bioavailability (99.65%) and equivalent diuresis when compared with intravenous administration. Subcutaneous administration of buffered furosemide was well tolerated with no evidence of any drug-induced skin reactions. Subcutaneous infusion of buffered furosemide in the outpatient setting or home may help to reduce the burden of heart failure.
Heart failure (HF) is a chronic disease associated with considerable morbidity, mortality, and health care expenditures. According to the American College of Cardiology/American Heart Association, 5.7 million Americans were diagnosed with HF in 2012 (1), and this number is expected to increase 46% by 2030 (2). Patients with HF make up 11% of the Medicare population and are responsible for 34% of the total annual Medicare expenditures. Expenditures related to HF are expected to increase 127% by 2030 alone (2). It is noteworthy that the HF population constitutes 41.5%, 55.3%, and 49.5% of total Medicare inpatient admissions, readmissions, and admissions to skilled nursing facilities, respectively (3).
Although HF management has improved over the years, many patients experience repeated hospital admissions for fluid overload marked by worsening of congestive symptoms. Intravenous (IV) furosemide is the foundation of treatment for patients with decompensated HF (4). However, IV therapy may only be administered by a certified health care professional, typically done in emergency rooms or in a hospital setting, which limits more timely access and inherently drives up the cost of treatment.
There is a large gap between chronic oral diuretic therapy given at home versus IV diuretics administered in the naturally more expensive hospital setting. A potential intermediate step would be that of access to an “IV-like furosemide” for use outside the hospital. This would enable development of a new care model with access to a more predictable and/or intensive diuresis in the outpatient setting, thereby preventing (re-)hospitalizations and reducing length of hospital stay.
Several small studies have reported that furosemide administration via the subcutaneous (SC) route can result in significant diuresis in both healthy volunteers (5), as well as patients with advanced stage HF 6, 7, 8, 9. However, furosemide is insoluble at physiological pH, and commercially available furosemide injectable products (furosemide injection USP or BP) have a pH of approximately 9.0. Alkaline products can cause significant irritation and discomfort, which currently precludes SC administration of available furosemide solutions.
A novel proprietary furosemide formulation was developed with a pH of 7.4 to minimize the risk of tissue irritation and discomfort. The aim of this report is to present the first pharmacokinetic and pharmacodynamic results of this novel furosemide formulation administered via the SC route using a biphasic delivery profile.
Methods
Subjects and study design
This article reports on 2 separate studies designed to: 1) characterize the pharmacokinetic profile of a novel formulation of furosemide administered SC and measure the resulting diuresis and natriuresis; and 2) estimate the bioavailability of SC furosemide compared with an equivalent dose of oral or IV furosemide in subjects with chronic stable HF.
In the first study, a first-in-man, proof-of-concept study (FUROPHARM-HF [Furosemide Pharmacodynamics and Pharmacokinetics After Subcutaneous or Oral Administration]; NCT02350725), eligible subjects (n = 10) were randomized (1:1) to receive 80 mg of oral furosemide (Lasix, Sanofi Belgium, Diegem, Belgium) or 80 mg (8 mg/ml in 10 ml) of a novel furosemide buffered solution administered SC with an external infusion pump using a biphasic pattern (30 mg over the first 60 min followed by 12.5 mg/h for 4 h). After a 14-day fluid re-equilibration washout, all subjects received the alternate treatment. Plasma furosemide levels were evaluated over 8 h.
In the second study (PK/PD Pivotal study) (Crossover Study to Compare the Pharmacokinetics and Bioavailability of a Novel Furosemide Regimen Administered Subcutaneously vs. the Same Dose Administered Intravenously in Subjects With Chronic Heart Failure; NCT02329834), eligible subjects (n = 16) were randomized (1:1) to receive 80 mg of furosemide administered IV (40 mg over 2 min followed by a second 40-mg dose 2 h later) or 80 mg (8 mg/ml in 10 ml) of a novel furosemide buffered solution administered SC with an external infusion pump using a biphasic pattern (30 mg over the first 60 min followed by 12.5 mg/h for 4 h). After a 7-day fluid re-equilibration washout, all subjects received the alternate treatment. During the course of both studies, all subjects discontinued oral furosemide therapy at least 24 h before the administration of the study treatment. Furosemide therapy was reinitiated at the end of each study period.
Both studies aimed to recruit similar patient populations of men and women of at least 18 years of age, who were on chronic oral loop diuretic therapy with a documented history of chronic HF. Patients had to be symptomatic with the presence of moderate symptoms of chronic fluid overload as the baseline for oral diuretic therapy (see the Supplemental Table for a full list of inclusion and exclusion criteria).
The 2 studies were conducted in accordance with the Declaration of Helsinki. The study protocols were reviewed and approved by an institutional review board or ethical committee, and all patients provided written informed consent before enrollment.
SC furosemide
The SC furosemide formulation (scPharmaceuticals, Burlington, Massachusetts) consists of furosemide reconstituted in a buffered solution containing tromethamine at a physiological pH of approximately 7.4 (range 7.2 to 7.6). To achieve a rapid onset of diuresis lasting 8 h and equivalent to that of IV furosemide, SC furosemide is delivered according to a biphasic dosing profile of 30 mg over the first 60 min followed by 12.5 mg/h for 4 h. For both studies, drug delivery was achieved by means of an external infusion pump (Perfusor Space Infusion Pump, B. Braun Medical, Bethlehem, Pennsylvania) and standard infusion set with the needle placed on the upper lateral abdominal wall.
Pharmacokinetic assessment
Programming of tables, figures, and listings was performed using R version 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria), as validated per Nuventra (Research Triangle Park, North Carolina) validation VAL.002.03. Pharmacokinetic parameters were calculated by Nuventra using Phoenix WinNonlin 6.3 (Pharsight, St. Louis, Missouri), as validated per Nuventra validation VAL.001.03. Data management and generation of the pharmacokinetic input file was performed using R version 3.0.3 (R Foundation for Statistical Computing). To quantify furosemide in plasma, venous blood samples were collected immediately before and throughout the entire time period of study drug administration. For IV furosemide, samples were collected at 5 (immediately after the first IV bolus injection), 15, 30, 45, 60, 90, 120, 125 (immediately after the second bolus injection), 135, 150, 165, 180, and 210 min, and at 4, 5, 6, 8, 10, 12, 14, and 16 h post-dose. For SC furosemide in the PK/PD Pivotal study, samples were collected at 30, 60, 90, 120, 180, 240, 300, 305 (immediately after completion of the infusion), 315, 330, and 345 min, and at 6, 8, 10, 12, 14, 16, and 24 h post-dose. For the First-in-Man study, a modified sampling procedure was used with a reduced number of sampling points. All plasma samples were processed and stored at −70° C until assayed using a validated liquid chromatography tandem-mass spectrometry analytical method (range 5.00 to 5,000.00 ng/ml, Algorithme Pharma, Laval, Quebec, Canada).
For the PK/PD Pivotal study of both the IV and SC administration routes, the following pharmacokinetic parameters were determined: the peak plasma concentration (Cmax), the time to Cmax, the area under the plasma concentration time curve (AUC) from time 0 (pre-dose) to the last measurable plasma concentration (AUClast) and to infinity (AUC∞), the apparent terminal phase elimination rate constant, the terminal phase elimination half-life (t½), the apparent systemic clearance (for SC furosemide only), the systemic clearance (for IV furosemide only), the apparent systemic volume of distribution (for SC furosemide only), and the systemic volume of distribution (for IV furosemide only). The following equation was used to calculate the absolute bioavailability of SC furosemide: (AUC∞ / dose of SC furosemide) / (AUC∞ / dose of IV furosemide). All parameters were calculated using a noncompartmental analysis (Nuventra). In the First-in-Man study, the plasma levels were plotted for proof-of-principal evaluation.
Pharmacodynamic assessment
For the PK/PD Pivotal study of both the SC and IV administration routes, urine samples were collected from spontaneous voids during the time periods (0 to 1 h), (1 to 2 h), (2 to 4 h), (4 to 6 h), (6 to 8 h), (8 to 10 h), (10 to 12 h), (12 to 18 h), and (18 to 24 h) after initiation of each furosemide treatment. Aliquots of urine samples were processed and then stored under refrigeration until assayed for sodium concentration, and therein an excretion amount, using standard biochemistry testing.
Assessment of local tolerance
In the PK/PD study, pain was assessed on a 0 to 10 point scale with 0 representing “No Pain” and 10 representing to the “Worst Pain Imaginable.” Additionally, the skin at the SC infusion site was evaluated and photographed at various time points. Erythema was scored on a 0- to 4-point scale with 0 representing “No Erythema” and 4 representing “Severe Erythema to Slight Eschar Formation.” Edema formation was scored using a 0- to 4-point scale with 0 representing “No Edema” and 4 representing “Severe Edema (raised more than 1 mm and beyond exposure area).”
Follow-up
For the PK/PD Pivotal study, clinical follow-up was scheduled per protocol at 7 ± 1 day from discharge and included changes in physical status (physical exam and electrocardiogram), HF symptoms, concomitant medications, and the occurrence of adverse or serious adverse events. The monitoring of the PK/PD study was overseen by an independent clinical research organization (Cardiovascular Clinical Studies, Boston, Massachusetts).
Sample size calculation and statistical analysis
Assuming the average coefficients of variation for furosemide AUC∞ following a 20-mg dose administered by the IV, oral, and sublingual routes are 50%, 25%, and 33%, respectively (10), and assuming the relative bioavailability is equal to unity and the intrasubject variability for AUC would fall within 25% to 50%, a sample size of 16 subjects completing the PK/PD Pivotal study in each treatment group would provide reasonably precise estimates of the systemic exposure of furosemide (AUC and Cmax).
All pharmacokinetic analyses of the PK/PD Pivotal study were performed by Nuventra using Phoenix WinNonlin 6.3 (Pharsight, St. Louis, Missouri). Furosemide concentrations were tabulated by treatment group and summarized for each sampling time points as descriptive statistics (mean, SD, coefficient of variation, median, minimum, and maximum). For the calculation of mean concentrations and mean concentration–time profiles, all below the limit of quantification values were set to zero except when a subject fell between 2 quantifiable values, in which case it was treated as missing data.
An analysis of variance (ANOVA) was performed on the ln-transformed furosemide AUClast and AUC∞ to assess the difference in overall systemic exposure between the IV and SC furosemide treatments. The ANOVA model included treatment as a fixed effect, and subject as a random effect. Each ANOVA included the calculation of least squares means (LSM) and the difference between treatment LSM. The 90% confidence intervals (CIs) for the ratios were derived by exponentiation of the CIs obtained for the difference between treatment LSM (SC furosemide/IV furosemide) resulting from the analyses on the furosemide ln-transformed AUClast and AUC∞. A p value <0.05 was considered statistically significant. The statistical analysis was performed by an independent clinical research organization (Nuventra). The first-in-man study was a proof-of-concept study to gain initial experience with SC administration of the novel formulation. No formal statistical analysis was applied, and the number of participants was based on empirical considerations.
Results
In both the First-in-Man and PK/PD Pivotal studies, the SC administration of furosemide was well tolerated with no evidence of any drug-induced skin reactions. In the First-in-Man study, all 10 subjects completed each of the cross-over treatments. The mean age of the subjects was 69.9 ± 8.6 years, 80% were male, the body mass index was 27.5 ± 4.5 kg/m2, and all were New York Heart Association (NYHA) functional class II (Table 1).
Table 1.
Subject Demographics and Clinical Characteristics From the First-in-Man and PK/PD Pivotal Studies
| First-in-Man (n = 10) | PK/PD Pivotal (n = 17∗) | |
|---|---|---|
| Age, yrs | 69.9 ± 8.6 | 68.0 ± 9.5 |
| Male, % | 80.0 | 88.2 |
| Weight, kg | 83.3 ± 17.2 | 96.6 ± 15.6 |
| BMI, kg/m2 | 27.5 ± 4.5 | 31.0 ± 4.6 |
| Systolic BP, mm Hg | 115.6 ± 15.3 | 134.0 ± 16.1 |
| Diastolic BP, mm Hg | 68.2 ± 7.7 | 78.4 ± 6.5 |
| Heart rate, beats/min | 75.4 ± 19.5 | 67.8 ± 10.9 |
| NYHA functional class, % | ||
| II | 100 | 76.5 |
| III | 0.0 | 23.5 |
| Sodium, mmol/l | 140.0 (138.2–141.5) | 142.2 (135–147) |
| Potassium, mmol/l | 4.5 (4.3–4.7) | 4.7 (4.0–5.6) |
| Creatinine, μmol/l | 120.0 (102.5–131.2) | 105.5 (80.5–143.24) |
| eGFR, ml/min/1.73 m2 | 53.8 (49.5–58.7) | 63.4 (41–97) |
| proBNP, pg/ml | 1,130 (732–2,115) | 897 (41–2,514) |
| Arrhythmia, % | 50.0 | 82.4 |
| AMI, % | 50.0 | 70.6 |
| Diabetes, % | 10.0 | 35.3 |
| Maintenance diuretic use, furosemide equivalent dose mg/day | 44.0 ± 12.6 | 40 ± 0 |
Values are mean ± SD, %, or median (interquartile range).
AMI = acute myocardial infarction; BMI = body mass index; BNP = brain natriuretic peptide; eGFR = estimated glomerular filtration rate; IV = intravenous; N/A = not available; NYHA = New York Heart Association; PK/PD = Pharmacokinetic and pharmacokinetic; proBNP = pro–B-type natriuretic protein; SC = subcutaneous.
17 patients were enrolled, 1 patient withdrew before dosing. One subject was found to have high pre-dose concentrations of furosemide and was not included in the PK, PD, or statistical analysis.
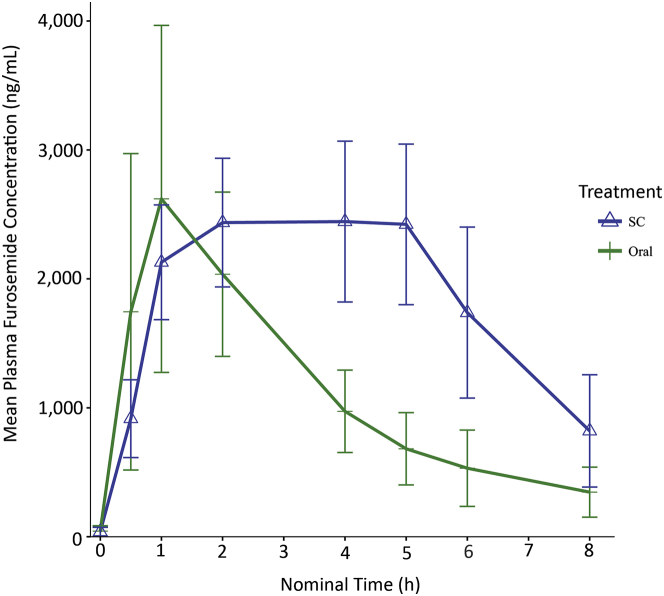
Following SC administration, all subjects (n = 10) achieved typically therapeutic plasma furosemide levels within 30 min of the start of administration (mean 916 ± 302 ng/ml, range 617 to 1,548 ng/ml), with levels of around 2,000 ng/ml (mean 2,129 ± 445 ng/ml, range 1,615 to 3,085 ng/ml) at 60 min (Figure 1). The plasma furosemide levels in the plateau phase (60 to 300 min) were maintained in a narrow range from the Cmax (67% to 93%). Following oral administration, marked intersubject variation was observed, with a 70-fold difference between the highest and lowest furosemide levels at 30 min (mean 1,745 ± 1,227 ng/ml, range 43 to 2,989 ng/ml) and a 10-fold difference at 60 min (mean 2,620 ± 1,346 ng/ml, range 447 to 4,406 ng/ml). Most subjects experienced a peak level at 60 min, and only 10% maintained plasma levels in the plateau phase of a minimum of 1,000 ng/ml compared with 100% following SC administration (Figure 1). Mean bioavailability (oral to SC) was 61% (range 33% to 96%). Fluid intake during the study was ad libitum (range 1,175 to 2,000 ml over the 8-h time interval). Total urine output was 1,550 ml (range 1,353 to 1,866 ml) after oral administration, and 1,833 ml (range 1,623 to 2,726 ml) after SC delivery.
Figure 1.
Plasma Furosemide Levels Following Oral and SC Administration (First-in-Man Study)
All subjects achieved therapeutic plasma furosemide levels of over 1,000 ng/ml within 1 h after subcutaneous (SC) administration, which were maintained for at least 5 h.
In the PK/PD Pivotal study, a total of 17 subjects were enrolled, but 1 subject that was randomized to receive IV furosemide first was withdrawn by the investigators before the start of drug administration. All remaining subjects (n = 16) completed the cross-over treatments, and complete pharmacokinetics, pharmacodynamic, and safety data were available for all subjects. One subject was found to have an unexplained high concentration of furosemide in a pre-dose sample (927.6 ng/ml, >35% of Cmax). In accordance with Food and Drug Administration guidance, the subject was excluded from the analyses because the pre-dose concentration was >5% of Cmax. Therefore, 15 subjects were included in the pharmacokinetic, pharmacodynamic, and statistical analyses. The mean age of the participants (n = 15) was 68 ± 9.6 years, 93.3% were male, and 86.7% were in NYHA functional class II. Overall, 100% of subjects had hypertension, 80.0% had various arrhythmias, 70.6% had history of acute myocardial infarction, and 40.0% had diabetes (Table 1).
Pharmacokinetics
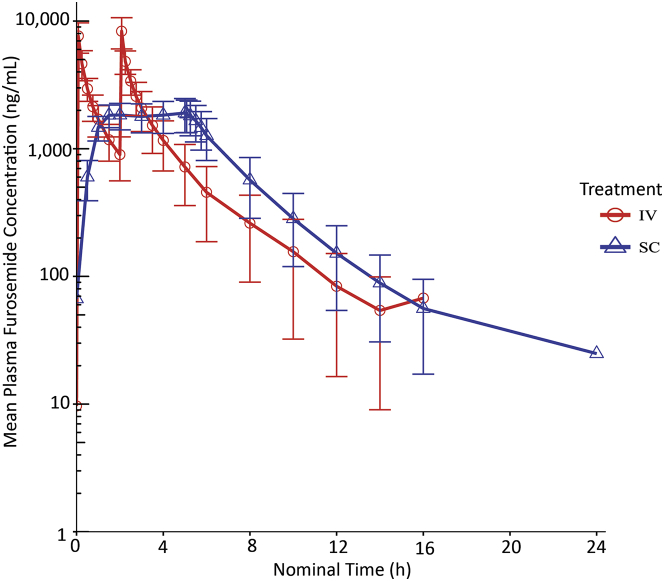
The time course of plasma furosemide concentrations for both the IV and SC administration routes are presented in Figure 2. Following IV administration, the mean furosemide concentrations were highest after the first (7,650 ± 2,050 ng/ml) and second (8,340 ± 2,290 ng/ml) IV bolus injections, for a median tmax of 125 min (range 5 to 125 min) and a geometric mean Cmax of 8,270 ng/ml. The geometric mean AUClast and AUC∞ estimates were 12,400 and 12,600 h ∙ ng/ml, respectively, the geometric mean of the t½ estimates was 151.8 min, and therapeutic levels of furosemide of 1,000 ng/ml were maintained for 90 and 240 min after the first and second IV bolus injections, respectively. All subjects receiving IV furosemide had quantifiable concentrations of furosemide up to 16 h post-dose. The summary statistics of noncompartmental pharmacokinetic parameters for the IV furosemide administration are presented in Table 2.
Figure 2.
Plasma Furosemide Levels Following IV and SC Administration (PK/PD Pivotal Study)
All subjects achieved therapeutic plasma furosemide levels of over 1,000 ng/ml within 1 h after subcutaneous (SC) administration, which were maintained for at least 6 h. The figure is in semi-log scale. IV = intravenous; PK/PD = Pharmacokinetic and Pharmacodynamic.
Table 2.
Plasma Furosemide Noncompartmental PK Parameters Following IV Administration
| Cmax (ng/ml) | Tmax (h) | AUClast (h ∙ ng/ml) | AUC∞ (h ∙ ng/ml) | AUCext (%) | λz (1/h) | t½ (h) | V (l) | CL (l/h) | |
|---|---|---|---|---|---|---|---|---|---|
| n | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Mean | 8,580 | N/A | 13,000 | 13,200 | 0.912 | 0.277 | 2.55 | 24.4 | 6.71 |
| SD | 2,540 | N/A | 4050 | 4,170 | 0.631 | 0.0365 | 0.339 | 9.15 | 2.31 |
| % CV | 29.5 | N/A | 31.1 | 31.6 | 69.2 | 13.2 | 13.3 | 37.5 | 34.5 |
| Geometric mean | 8,270 | N/A | 12,400 | 12,600 | 0.729 | 0.274 | 2.53 | 23.2 | 6.37 |
| Geometric % CV | 28.4 | N/A | 33.3 | 33.7 | 82.2 | 13.3 | 13.3 | 31.2 | 33.7 |
| Minimum | 5,060 | 0.08 | 6,540 | 6,600 | 0.199 | 0.218 | 2.00 | 15.8 | 3.83 |
| Median | 8,230 | 2.08 | 12,800 | 12,900 | 0.666 | 0.275 | 2.52 | 23.0 | 6.20 |
| Maximum | 13,800 | 2.08 | 20,500 | 20,900 | 2.47 | 0.347 | 3.18 | 53.1 | 12.1 |
λz = apparent terminal phase elimination rate constant; AUC = area under the curve; AUC∞ = plasma concentration to infinity; AUCext = percentage of AUC that is extrapolated beyond the last measurable concentration; AUClast = last measurable plasma concentration; CL = systemic clearance; Cmax = peak plasma concentration; CV = coefficient of variation; N/A = not available; t½ = terminal phase elimination half-life; Tmax = time to peak plasma concentration; V = systemic volume of distribution.
Following SC administration, therapeutic furosemide concentrations were observed as early as 30 min (600 ± 209 ng/ml) and 1 h (1,470 ± 321 ng/ml) after the initiation of the infusion, and increased steadily to 1,910 ± 570 ng/ml by 5 h (immediately before removal of the SC infusion), for a median tmax of approximately 4 h (range 1 to 5.05 h) and a geometric mean Cmax of 1,990 ng/ml. The geometric mean AUClast and AUC∞ estimates were 12,400 and 12,500 h ∙ ng/ml, respectively, the geometric mean of the t½ estimates was 3.04 h, and therapeutic levels of furosemide of 1,000 ng/ml were maintained up to 6 h. Most subjects (75%) receiving SC furosemide had a quantifiable concentration of furosemide up to 24 h post-dose. The summary statistics of noncompartmental pharmacokinetic parameters for the SC furosemide are presented in Table 3.
Table 3.
Plasma Furosemide Noncompartmental PK Parameters Following Subcutaneous Administration
| Cmax (ng/ml) | Tmax (h) | AUClast (h ∙ ng/ml) | AUC∞ (h ∙ ng/ml) | AUCext (%) | λz (1/h) | t½ (h) | F (%) | V/F (L) | CL/F (L/h) | |
|---|---|---|---|---|---|---|---|---|---|---|
| n | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 | 15 |
| Mean | 2,040 | N/A | 13,000 | 13,100 | 1.05 | 0.238 | 3.16 | 100.0 | 28.5 | 6.71 |
| SD | 449 | N/A | 4,000 | 4,010 | 1.29 | 0.0732 | 0.911 | 10.5 | 5.28 | 2.21 |
| % CV | 22.0 | N/A | 30.8 | 30.6 | 123.0 | 30.8 | 28.8 | 10.5 | 18.5 | 32.9 |
| Geometric mean | 1,990 | N/A | 12,400 | 12,500 | 0.710 | 0.228 | 3.04 | 99.6 | 28.0 | 6.4 |
| Geometric % CV | 23.0 | N/A | 33.3 | 32.8 | 99.0 | 30.4 | 30.4 | 10.5 | 19.8 | 32.8 |
| Minimum | 1,340 | 1.00 | 7,110 | 7,520 | 0.218 | 0.148 | 1.78 | 81.0 | 17.7 | 3.84 |
| Median | 2,040 | 4.00 | 12,900 | 12,900 | 0.614 | 0.217 | 3.20 | 100 | 28.8 | 6.2 |
| Maximum | 2,780 | 5.08 | 20,700 | 20,800 | 5.42 | 0.390 | 4.69 | 121 | 36.5 | 10.6 |
Abbreviations as in Table 2.
The geometric LSM of AUClast and AUC∞ for subjects receiving SC furosemide was similar to the reference IV furosemide, with ratios of 99.50% and 99.65%, respectively. The absolute bioavailability of furosemide was 99.65% with 90% CIs of 94.79% to 104.75% for the AUC∞, and 99.50% with 90% CIs of 94.77% to 104.46% for the AUClast. The geometric mean absolute bioavailability for SC furosemide was 99.60%.
Pharmacodynamics
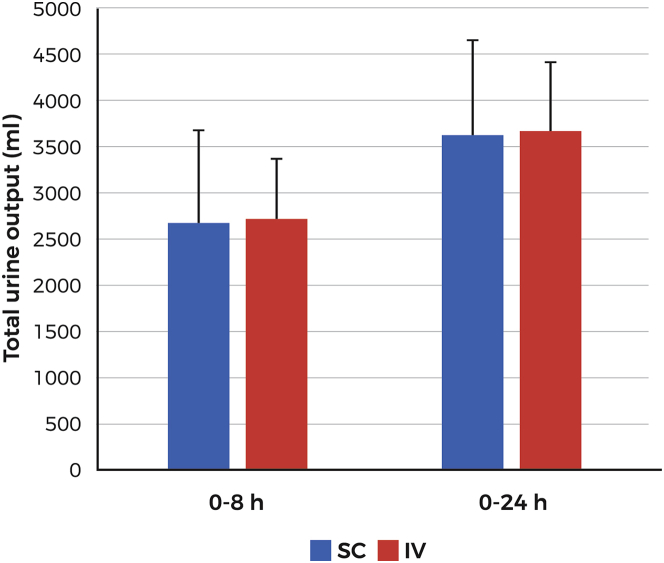
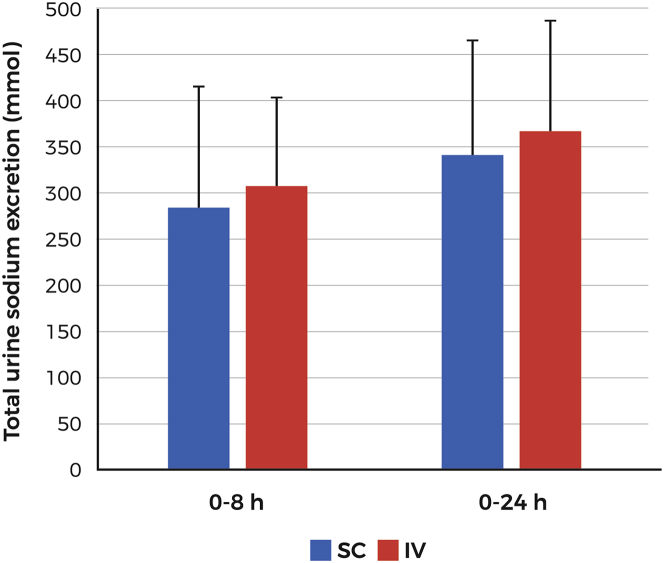
In the PK/PD Pivotal study, fluid intake was controlled and restricted to 100 ml of water every hour starting 2 h before dosing. Administration of IV and SC furosemide resulted in similar degrees of diuresis at both the 0- to 8-h interval (2,718 ± 654 ml and 2,663 ± 1,021 ml for the IV and SC routes, respectively) and the 0- to 24-h interval (3,672 ± 740 ml and 3,614 ± 1,045 ml for the IV and SC routes, respectively) (Figure 3). Mean diuresis (0 to 8 h) was comparable for both treatment periods (2,578 ± 854 ml vs. 2,803 ± 846 ml) without an indication of possible sequence or period effect. Urine sodium excretion at 0 to 8 h was 307 mmol and 286 mmol for the IV and SC routes, respectively, and at 0 to 24 h, 367 mmol and 341 mmol for the IV and SC routes, respectively (Figure 4).
Figure 3.
Diuresis Following SC or IV Furosemide Administration (PK/PD Pivotal Study)
Urine output over the periods 0 to 8 and 0 to 24 following administration of 80-mg furosemide by 5-h SC infusion or IV administration of 2 doses of 4 0 mg 2 h apart. Time 0 indicates the start of diuresis therapy. Abbreviations as in Figure 2.
Figure 4.
Sodium Excretion Following SC or IV Furosemide Administration (PK/PD Pivotal Study)
Sodium excretion (mmol) over the periods 0 to 8 and 0 to 24 h following administration of 80-mg furosemide by 5-h subcutaneous infusion or IV administration of 2 doses of 40 mg 2 h apart. Time 0 indicates the start of diuresis therapy. Abbreviations as in Figure 2.
Safety
Irrespective of the administration routes, all vital signs remained stable and similar throughout the study period. None of the subjects reported pain at the injection site (maximum pain score of 0 on a scale of 0 to 10) during or after SC administration. Only 1 subject reported transient “very slight erythema” (Score 1) during SC furosemide administration. An additional 8 subjects were found to have minimal erythema (rated as “very slight erythema) after completion of the infusion and removal of the infusion set and adhesive. One subject experienced “Well Defined Erythema” (Score 2) during IV administration. A total of 6 subjects were found to have minimal swelling during or following SC administration (maximum severity rate as “Very Slight Edema,” Score 1). All events resolved shortly after SC furosemide administration without treatment or further complications.
Discussion
Herein, we report on 2 studies that provide the first experiences with a novel injectable furosemide formulation suitable for SC infusion. The First-in-Man study was primarily designed to test the feasibility of a novel 80-mg buffered furosemide solution administered via the SC route in subjects with HF and chronic fluid overload. The main findings were: 1) SC furosemide using a biphasic delivery profile resulted in therapeutic levels within 30 min, which were maintained for the entire drug administration period (5 h) within a narrow therapeutic (diuretic) concentration range; and 2) SC furosemide was well tolerated with minimal erythema and edema at the site of injection. The PK/PD Pivotal study was designed to characterize the pharmacokinetics of the 80-mg buffered furosemide solution administered via the SC route and to estimate the bioavailability of SC furosemide compared with an equivalent 80-mg dose of IV furosemide in a similar subject population. The main findings of this study were: 1) typical therapeutic levels of furosemide were reached within 30 min of SC administration and maintained for more than 5 h; 2) absolute bioavailability of SC furosemide was complete at 99.65%; 3) SC furosemide was as effective as IV furosemide in achieving diuresis; and 4) SC furosemide was well tolerated, with minimal erythema and edema at the site of injection. These results suggest that SC furosemide administration may offer a “hospital-strength” diuretic option for patients with HF who require a shift of their oral diuretic treatment, when hospitalization is not strictly warranted. These results also suggest that the reformulation combined with the slow infusion rate has resulted in a product that is free from the stinging and discomfort that had been reported with the administration of conventional furosemide (furosemide injection USP).
Parenteral loop diuretics form the cornerstone of treatment of acute decompensated HF when patients respond insufficiently to oral diuretics alone. It is well known that the HF syndrome is characterized by unpredictable periods of decompensation, which require escalation of oral diuretic doses and/or frequency of dosing. When such oral treatment adjustment fails, IV diuretics are usually prescribed. Furthermore, many patients appear to temporarily have a reduced response to oral medication due to impaired absorption because of fluid overload and other factors affecting gastric and intestinal absorptive functions 11, 12. Indeed, the average bioavailability after oral administration of furosemide is highly variable and has been reported to range between 49% and 72% 11, 13, 14, 15, while individual differences range from below 20% to over 90%. Parenteral furosemide therapy not only reduces hypervolemia, but in many patients, it also restores oral bioavailability, allowing them to transition more readily back to oral maintenance therapy.
Our 2 studies show that the bioavailability of SC furosemide is complete (>99%) and that therapeutic plasma levels are achieved soon after initiation, with peak levels generally achieved within 30 to 60 min. In the PK/PD study, we chose to administer 2 doses of 40-mg IV furosemide, 2 h apart. Although rarely used clinically, it is the recommended dosage in the furosemide injection prescribing information implemented with the first U.S. introduction in 1968. The curves show that 5 h of SC furosemide infusion results in plasma levels that are maintained in the therapeutic range for at least as long as the repeated IV administration.
The question arises as to how might a SC delivery mode for furosemide be advantageous to the patient with HF? Several patient categories may benefit from this treatment such as those hospitalized for IV diuretics who may then be discharged earlier and can then continue SC and thus parenteral diuretic administration for several additional days in the outpatient setting. Second, patients who are slowly deteriorating and not responding to dose escalation with oral furosemide may use SC furosemide. This may lessen the need for hospitalization.
With similar diuresis as conventional IV furosemide, our results demonstrate the feasibility and safety of administering a novel buffered furosemide formulation via the SC route using the biphasic delivery profile. Because placing and starting IV drug administrations may only be performed by a certified health care professional, the SC route with an easy-to-use patch pump may allow for use by patients or lay caregivers as directed by a health care professional. As with all medical devices, qualifying the patient or caregiver and operator training by a health care professional will be a requirement for safe and effective use. Clinicians and institutions have adopted a range of strategies in the treatment of fluid overload in HF. These typically depend upon the available resources and the organization of practice or clinic. Furosemide is among the best-known and most widely used medications, and this familiarity will probably lead clinicians and practices to experiment with workflows specific to their setup that take advantage of this new option. Experimentation by individual clinicians and structured research are likely to focus on testing specific workflows with an aim to reduce the burden for the health care system and patient. These novel workflows may rise to the level of a paradigm change such as at-home treatment with the help of home health services as an alternative to inpatient care, a marked shortening of length of stay with at-home treatment to achieve euvolemia, a closed-loop kind of model with a reliable method to monitor fluid overload. These new care strategies would require adequately designed controlled studies to gain support and provide compelling information to support changes in reimbursement that now often support the in-patient care model.
Study limitations
First, it was designed and conducted as an experimental PK/PD study, resulting in a highly artificial setup not representative of the clinical setting in which furosemide might be used. Also, the study was conducted in patients with stable HF who discontinued oral medication on the day before the investigation to induce slight hypervolemia, not patients with clinical hypervolemia in need of parenteral diuretics. Additionally, the study was only powered for its pharmacokinetic endpoint, resulting in a small sample size. A prospective, randomized trial (Sub-Q Versus IV Furosemide in Acute Heart Failure; NCT02579057) is currently underway to evaluate the effectiveness and safety of this route of administration in patients with decompensated HF.
Conclusions
The SC administration of a novel buffered furosemide formulation is feasible and safe, and achieves diuresis equivalent to conventional IV furosemide. SC furosemide may offer a new therapeutic modality for outpatient use with equivalent diuresis to hospital- or emergency room–administered IV diuretics.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Furosemide is the cornerstone of heart failure treatment. Because many patients become unresponsive to oral medication, they often are hospitalized for parenteral furosemide. Subcutaneous administration of a novel formulation of furosemide with a physiological pH appears feasible and safe, has complete bioavailability, and results in therapeutic diuresis as conventional intravenous furosemide. An outpatient strategy of subcutaneous furosemide therapy may help reduce the burden of heart failure by reducing the need for inpatient care.
TRANSLATIONAL OUTLOOK: Additional studies that mirror real-word clinical use will be required to determine the role of subcutaneous furosemide in routine management of heart failure. Such studies may investigate its role in reducing index and readmission hospitalizations for heart failure, the length of in-hospital stay, costs of care, and to better understand the risks associated with at-home treatment of mild or moderate decompensated heart failure as compared with the current in-hospital use of IV furosemide. Particular areas of interest and future clinical investigation include: patient selection criteria; parameters to determine the efficacy or the failure of this approach; clinical and laboratory monitoring protocols; when to initiate transition to oral furosemide; and the use of potassium supplementation.
Acknowledgments
The authors wish to express their gratitude to the patients who took part in our studies. The authors the staff from CardioResearch (University Medical Center Groningen) for conducting the First-in-Man study, and the staff of Avail Clinical Research LLC (DeLand, Florida) for conducting the PK/PD study. The authors also thank Danielle Libersan, PhD, for her help in preparing the manuscript.
Footnotes
These studies were supported by scPharmaceuticals. Dr. Sica has been a consultant to scPharmaceuticals. Drs. Muntendam, de Boer, and Pitt are consultants to and shareholders of scPharmaceuticals. Dr. Myers is an employee and shareholder of scPharmaceuticals. Dr. Sale is an employee of Nuventra. Dr. ter Maaten has reported that she has no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Mozaffarian D., Benjamin E.J., Go as Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Albert N.M., Allen L.A. Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitch K., Pelizzari P.M., Pyenson B. Inpatient utilization and costs for Medicare fee-for-service beneficiaries with heart failure. Am Health Drug Benefits. 2016;9:96–104. [PMC free article] [PubMed] [Google Scholar]
- 4.Owen D.R., MacAllister R., Sofat R. Intravenous furosemide for acute decompensated congestive heart failure: what is the evidence? Clin Pharmacol Ther. 2015;98:119–121. doi: 10.1002/cpt.120. [DOI] [PubMed] [Google Scholar]
- 5.Verma A.K., da Silva J.H., Kuhl D.R. Diuretic effects of subcutaneous furosemide in human volunteers: a randomized pilot study. Ann Pharmacother. 2004;38:544–549. doi: 10.1345/aph.1D332. [DOI] [PubMed] [Google Scholar]
- 6.Zatarain-Nicolás E., López-Diaz J., de la Fuente-Galán L., Garcia-Pardo H., Recio-Platero A., San Román-Calvar J.A. Subcutaneous infusion of furosemide administered by elastomeric pumps for decompensated heart failure treatment: initial experience. Rev Esp Cardiol (Engl Ed) 2013;66:1002–1004. doi: 10.1016/j.rec.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Zacharias H., Raw J., Nunn A., Parsons S., Johnson M. Is there a role for subcutaneous furosemide in the community and hospice management of end-stage heart failure? Palliat Med. 2011;25:658–663. doi: 10.1177/0269216311399490. [DOI] [PubMed] [Google Scholar]
- 8.Farless L.B., Steil N., Williams B.R., Bailey F.A. Intermittent subcutaneous furosemide: parenteral diuretic rescue for hospice patients with congestive heart failure resistant to oral diuretic. Am J Hosp Palliat Care. 2013;30:791–792. doi: 10.1177/1049909112465795. [DOI] [PubMed] [Google Scholar]
- 9.Goenaga M.A., Millet M., Sánchez E., Garde C., Carreta J.A., Arzellus E. Subcutaneous furosemide. Ann Pharmacother. 2004;38:1751. doi: 10.1345/aph.1E172. [DOI] [PubMed] [Google Scholar]
- 10.Haegeli L., Brunner-La Rocca H.P., Wenk M., Pfisterer M., Drewe J., Krähenbühl S. Sublingual administration of furosemide: new application of an old drug. Br J Clin Pharmacol. 2007;64:804–809. doi: 10.1111/j.1365-2125.2007.03035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vargo D.L., Kramer W.G., Black P.K., Smith W.B., Serpas T., Brater D.C. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–609. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 12.Gottlieb S.S., Khatta M., Wentworth D., Roffman D., Fisher M.L., Kramer W.G. The effects of diuresis on the pharmacokinetics of the loop diuretics furosemide and torsemide in patients with heart failure. Am J Med. 1998;104:533–538. doi: 10.1016/s0002-9343(98)00111-9. [DOI] [PubMed] [Google Scholar]
- 13.Hammarlund M.M., Paalzow L.K., Odlind B. Pharmacokinetics of furosemide in man after intravenous and oral administration. Application of moment analysis. Eur J Clin Pharmacol. 1984;26:197–207. doi: 10.1007/BF00630286. [DOI] [PubMed] [Google Scholar]
- 14.Grahnén A., Hammarlund M., Lundqvist T. Implications of intraindividual variability in bioavailability studies of furosemide. Eur J Clin Pharmacol. 1984;27:595–602. doi: 10.1007/BF00556898. [DOI] [PubMed] [Google Scholar]
- 15.Murray M.D., Haag K.M., Black P.K., Hall S.D., Brater D.C. Variable furosemide absorption and poor predictability of response in elderly patients. Pharmacotherapy. 1997;17:98–106. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.