Summary
Intercellular signaling by extracellular vesicles (EVs) is a route of cell-cell crosstalk that allows cells to deliver biological messages to specific recipient cells. EVs convey these messages through their distinct cargoes consisting of cytokines, proteins, nucleic acids, and lipids, which they transport from the donor cell to the recipient cell. In cardiovascular disease (CVD), endothelial- and immune cell-derived EVs are emerging as key players in different stages of disease development. EVs can contribute to atherosclerosis development and progression by promoting endothelial dysfunction, intravascular calcification, unstable plaque progression, and thrombus formation after rupture. In contrast, an increasing body of evidence highlights the beneficial effects of certain EVs on vascular function and endothelial regeneration. However, the effects of EVs in CVD are extremely complex and depend on the cellular origin, the functional state of the releasing cells, the biological content, and the diverse recipient cells. This paper summarizes recent progress in our understanding of EV signaling in cardiovascular health and disease and its emerging potential as a therapeutic agent.
Key Words: cardiovascular disease, extracellular vesicles, microvesicles
Abbreviations and Acronyms: CVD, cardiovascular disease; EC, endothelial cell; EMV, endothelial cell-derived microvesicles; ESCRT, endosomal sorting complex required for transport; IL, interleukin; miRNA, microRNA; MV, microvesicles; NO, nitric oxide; PEG, polyethylene glycol; TGF, transforming growth factor
Central Illustration
Cardiovascular disease (CVD) still represents the leading cause of mortality worldwide. The underlying disease, atherosclerosis, is initiated and propagated by continuous damage of the vascular endothelium, leading to endothelial activation and apoptosis, the development of endothelial dysfunction, and subsequent atherosclerotic lesion formation (1). Endothelial cell (EC) injury is a key element in the complex pathophysiology of atherogenesis and triggers the release of EC-derived extracellular vesicles (EVs) such as exosomes and microvesicles (MVs) (2). Accordingly, patients with vascular diseases associated with systemic endothelial damage, such as atherosclerosis, show significantly increased levels of circulating EVs 3, 4. However, EVs are not simply inactive debris that reflect cellular activation or injury. EVs can transfer proteins, cytokines, mRNA, or noncoding RNA such as microRNA (miRNA) or long noncoding RNA to target cells and influence their function and phenotype 5, 6. Accordingly, the role of EVs has changed from being only a marker of vascular integrity toward being relevant effectors in intercellular vascular signaling 7, 8. In CVD, EVs have been shown to contribute to disease development and progression by promoting initial lesion formation, intravascular calcifications, plaque progression, and thrombus formation after rupture. In contrast, numerous studies have demonstrated that certain subtypes of EVs can mediate vascular protection and endothelial regeneration (9). In line with these findings, EVs released by progenitor or mesenchymal stem cells have been shown to improve cardiac function after myocardial infarction in experimental studies, highlighting the therapeutic potential of EVs in cardiovascular pathologies (10). This review summarizes current knowledge of EVs as regulators of cardiovascular health and disease and potential opportunities for therapeutic use.
Biogenesis of Extracellular Vesicles and Their Interactions With Target Cells
EVs are membrane vesicles secreted from cells that contain intracellular contents (11). Cells can release a broad range of vesicles with diverse features. This review focuses on 2 major types: MVs and exosomes. MVs are large (>150 nm) vesicles that are released by budding from the plasma membrane, whereas exosomes are smaller (30 to 100 nm) and originate from the endosome (12). However, there is no strict cutoff value that distinguishes MVs from exosomes by vesicle size, which can differ in diverse studies (12).
Exosomes represent a homogeneous population of vesicles that are formed by inward budding of the multivesicular body (MVB) membrane. Exosome biogenesis is mediated mainly by the endosomal sorting complex required for transport (ESCRT) protein (13) or lipid ceramide and neutral sphingomyelinase, the enzyme that converts sphingomyelin to ceramide (14). Cargo sorting into exosomes involves ESCRT and associated proteins such as tumor susceptibility gene 101 protein (TSG101) and ALG-2-interacting protein X (ALIX) and small GTPases such as Rab7a and Rab27b 15, 16, 17. Exosomes are liberated into the extracellular space following fusion of MVBs with the cell membrane, regulated by Rab27A, Rab11, and Rab31 18, 19. MVs represent a relatively heterogeneous population of vesicles formed by outward budding of the cell membrane. This process is regulated by membrane lipid microdomains and regulatory proteins such as ADP-ribosylation factor 6 (ARF6) (20). EVs can be regarded as intercellular messengers for various biological processes. Several routes of interaction between EVs and recipient cells have been described. First, EVs can directly activate target cell surface receptors by bioactive ligands and proteins 21, 22, 23. Second, EVs are able to transfer their biological content by membrane fusion with the recipient cell. The fusion process is regulated by the lipid composition of EV membrane, and several reports indicate that the presence of phosphatidylserine contributes to membrane fusion (24). Third, incorporation of EVs into target cells is mediated by endocytosis, pinocytosis, or phagocytosis (25). Through these interaction routes, EVs transfer their biological contents containing nucleic acids such as mRNA (26), noncoding RNAs (miRNAs [27], long noncoding RNAs [6]), proteins (28), cytokines (29), or bioactive lipids (30) (Figure 1).
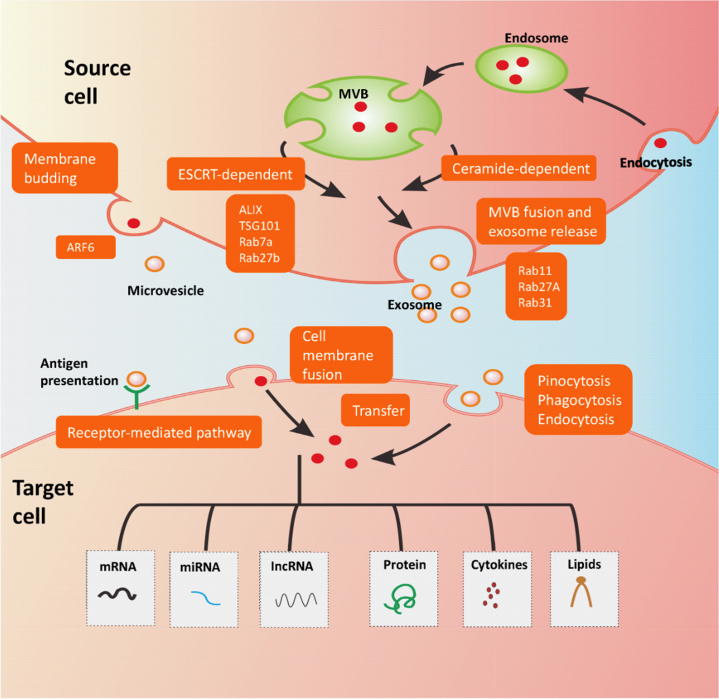
Figure 1.
EV Biogenesis and Interaction With Recipient Cells
Exosome formation starts with endocytosis, a process in which the cell membrane is pinched inward and captures bioactive molecules, resulting in the formation of the endosome. These molecules are sorted into smaller vesicles that bud from the perimeter membrane into the endosome lumen, forming vesicles; this leads to the multivesicular appearance of late endosomes and so they are also known as MVBs. From MSBs, exosome formation occurs by an ESCRT- and ceramide-dependent pathway. Cargo sorting into exosomes involves ESCRT and TSG101, ALIX, and Rab7a, and Rab27b. Exosomes are released into the extracellular space following the fusion of MVBs with the cell membrane, which is regulated by Rab27A, Rab11, and Rab31. Microvesicles are formed by the outward budding of the cell membrane, a process that is regulated by ARF6. Several routes of interaction between EVs and recipient cells have been described. First, EVs can directly activate target cell surface receptors. Second, EVs are able to transfer their biological content by membrane fusion with the recipient cell. Third, incorporation of EVs into target cells is mediated by endocytosis, pinocytosis, or phagocytosis. Using these interaction routes, EVs transfer their biological content containing nucleic acids such as mRNA, noncoding RNAs (microRNAs, long noncoding RNAs), proteins, cytokines, or bioactive lipids. mRNA = messenger RNA; MVB = multivesicular bodies.
Extracellular Vesicles as Effectors of Cardiovascular Diseases
In cardiovascular biology, EVs have various physiological functions, including activation of platelets and ECs, as well as regulation of inflammation and coagulation 31, 32, 33, 34. Therefore, EVs are emerging as key players in different stages of CVD development 31, 32, 35. The effects of EVs in CVD are extremely complex and depend on the cellular origin, the functional state of the releasing cells, the intravesicular content, and the recipient cells 36, 37. The following sections summarize the current knowledge about EVs as effectors of CV disease progression or vascular repair.
Detrimental effects of extracellular vesicles on vascular function
Endothelial dysfunction occurs as a response to cardiovascular risk factors and represents the initial step in atherosclerosis development, the underlying pathology of CVD 38, 39.
Endothelial dysfunction
Endothelial MVs have been shown to impair vasorelaxation by inhibiting nitric oxide (NO) production in target ECs. This phenomenon is mediated through a decrease in endothelial NO synthase phosphorylation and activity (40), local oxidative stress (41), or an increased NADPH oxidase activity with MVs (33) and results in impaired vascular relaxation capacities. MVs in the aforementioned studies are obtained from ECs under physiological 40, 41 or pathological (33) conditions, but they all have detrimental effects on vasorelaxation. Regarding the relation between molecular contents and function of EVs, further investigation should be conducted to clarify and compare the cargoes of EVs derived under different conditions (e.g., by RNA sequencing or proteomic analysis).
In line with the latter findings, MVs isolated from patients with vascular (acute coronary syndrome [42]) or predisposing disease (chronic renal failure or metabolic syndrome 43, 44) were shown to induce endothelial dysfunction ex vivo in rat aortic rings. In contrast, MVs from healthy subjects did not affect endothelial function 42, 43, 44, indicating that the pathophysiological state of the releasing cell determines not only the number of released MVs but also their content and biological function. Which cell-specific MVs mainly influence endothelial function and whether isolated MVs from different cell types may have diverse biological functions need to be addressed in future studies.
Of interest, storage of human blood under standard blood-banking conditions results in accumulation of MV-encapsulated hemoglobin. These erythrocyte-derived MVs react with and degrade NO, inducing endothelial dysfunction (45). Finally, platelet-derived exosomes derived under septic conditions have been shown to mediate septic endothelial dysfunction by inducing endothelial apoptosis involving superoxide, NO, and peroxynitrite production (46). Studying EVs derived from patients represents an important translational approach to exploring the relationship between EVs and certain diseases. However, comorbidities as well as patients’ age and sex should be taken into consideration as possible confounders. Therefore, inclusion and exclusion criteria should be thoughtfully determined, and control groups must be carefully selected.
Endothelial activation, monocyte adhesion/infiltration, and inflammation
Inflammation plays a pivotal role in CVD 38, 47. EVs from various cellular sources contribute to vascular inflammatory processes including endothelial activation, monocyte adhesion, and transmigration 48, 49, 50, 51.
In vitro studies have demonstrated that MVs can induce release of the proinflammatory cytokines interleukin (IL)-6 and IL-8 from ECs and leukocytes 52, 53; and promote expression of adhesion proteins ICAM-1, VCAM-1, and E-selectin, facilitating increased adhesion of monocytes 54, 55 and subsequent transmigration, leading to vascular inflammation and plaque development. Mechanistically, increased adhesion of monocytes to ECs can be mediated by the MV-mediated transfer of proinflammatory molecules such as oxidized phospholipids (56), caspase-3 (57), or RANTES (regulated on activation, normal T cell expressed and secreted) protein, which is transferred from platelet MVs to endothelial target cells (58). Platelet MVs from apoptotic platelets also facilitate differentiation between resident macrophages and professional phagocytes (59). Vascular inflammatory processes involve different cells (e.g., ECs, monocytes, and platelets 48, 49, 50, 51). The aforementioned in vitro studies demonstrated that EVs from diverse parent cells can act on various types of target cells. In summary, vascular inflammation seems to be regulated by complex intercellular communication routes involving a network of cells and EVs. To gain more insight into these multifaceted mechanisms, co-incubation of different EVs with diverse cell types may be helpful to study this “intercellular communication network.” However, there is a lack of adequate in vitro models, which should be addressed in further studies.
Pathological conditions modify EV content and biological functions (60). MVs isolated from atherosclerotic plaques transfer ICAM-1 to ECs and recruit inflammatory cells, suggesting that human plaque MVs promote atherogenesis (24).
Moreover, oxidatively modified, but not native, EC-derived EVs contain proinflammatory oxidized phospholipids that elicit specific responses in ECs, leading to the adhesion of monocytes (56). In line with these findings, EC-derived EVs generated from glucose-treated cells, but not from healthy ECs, facilitated up-regulation of ICAM-1 and VCAM-1 in endothelial target cells by activating p38 in an reactive oxygen species-dependent manner (33). In light of these proinflammatory effects and the increased levels of endothelial MVs in diabetic patients, one may speculate that MVs released under pathological high-glucose conditions might represent a paracrine mediator transporting proinflammatory messages to target cells and thereby foster vascular inflammation 61, 62. However, while studying the functional effects of EVs derived under pathological conditions, one must consider that the quantity and/or content of EVs may vary with different modes and durations of stimulation. Therefore, clearly defined pathological conditions (including standardized concentrations and duration of cell stimulations) as well as a careful selection of an adequate control group are mandatory to elaborate EV functions depending on the parent cell conditions.
Compared with the role of MVs, the role of exosomes in vascular inflammatory processes has been less explored 63, 64. However, monocyte-derived exosomes seem to induce vascular inflammation and cell death by transferring inflammatory miRNAs into ECs resulting in a significant up-regulation of ICAM-1, CCL2, and IL-6 levels (65) and provocation of endothelial apoptosis by tissue factor release (66).
Atherosclerotic plaque development, progression, and rupture
Atherosclerotic plaque rupture with subsequent coronary thrombosis represents the ultimate step in atherosclerotic lesion progression, leading to acute myocardial ischemia. Atherosclerotic plaques can release large amounts of EVs, contributing to plaque progression and instability through various mechanisms. Plaque EVs originate mainly from leucocytes, reflecting the local inflammatory environment (67). Plaque EVs express surface antigens consistent with their leukocyte origin, including major histocompatibility complex classes I and II, and dose-dependently induce T-cell proliferation (68). Antigen-specific activation of CD4+ T cells was also induced by dendritic exosomes, implicating their potential involvement in vascular inflammation and plaque development (69). Furthermore, plaque MVs carry catalytically active tumor necrosis factor (TNF)-α–converting enzyme (TACE/ADAM17) and significantly enhance the processing of its substrates TNF-α and TNF receptor, thereby promoting an inflammatory response (70). Importantly, plaque EVs from patients or in vivo models are not single-component molecules, although mainly of leucocyte origin (67), and their constitutions may also depend on the stage of plaque progression (stable or unstable?). Therefore, standard procedures to derive and analyze plaque EVs should be established.
Local inflammation is enhanced by monocytic MVs fostering leucocyte adhesion to postcapillary venules and T-cell infiltration in atherosclerotic plaques in vivo 71, 72. Exosomes from T cells also can contribute to atherosclerotic plaque development by inducing cholesterol accumulation in human monocytes by the phosphatidylserine-receptor (73).
Vascular smooth muscle cell (VSMC) proliferation plays an important role in atherosclerotic plaque development 39, 74. The effect of MVs on VSMC proliferation depends on the cellular origin. In vitro-generated platelet-derived MVs promoted VSMC proliferation in a platelet-derived growth factor (PDGF)-independent mechanism with minor effects on migratory capacity 75, 76. In turn, monocyte-derived MVs were shown to deliver a lethal message by encapsulated caspase-1–inducing VSMC cell death (77). More recently, calcification-competent EVs derived from smooth muscle cells, valvular interstitial cells, and macrophages have been described as mediators of vascular calcification that modulate heart valve disease and atherogenesis 78, 79, 80. Although EVs show protective effects against heart valve calcification, the potential underlying mechanisms are unknown and should be addressed in future studies.
Angiogenesis is a fundamental process in CVD, contributing to plaque instability by promoting neovascularization. Unstable plaques are characterized by an increased number of vasa vasorum mediating intraplaque hemorrhage (81). MVs isolated from human atherosclerotic plaques were shown to stimulate EC proliferation in vitro after CD40 ligation and to enhance in vivo angiogenesis. Interestingly, the proliferative effect of MVs isolated from atherosclerotic plaques was more pronounced using MVs from symptomatic patients than from patients without symptoms. Therefore, MVs could represent a major determinant of plaque vulnerability (82). Unstable human plaques contain large numbers of procoagulant MVs, originating mostly from leucocytes, erythrocytes, and VSMCs localized within the necrotic core (83). Once plaque rupture occurs, these MVs can initiate the coagulation cascade through different mechanisms 67, 84: first, by the expression of tissue factor (mainly on monocytic MVs), one major initiator of blood coagulation 34, 85, 86; and second, by exposure to phosphatidylserine on their outward membrane layer (87). Procoagulatory effects of MVs have been demonstrated in vitro and in vivo, where they facilitated thrombus formation 88, 89. The deleterious effects of EVs inducing vascular inflammation, plaque progression, and rupture are illustrated in Figure 1.
In summary, a broad body of evidence indicates the active involvement of EVs in plaque development, progression, and thrombus formation after rupture. However, there is an urgent need for additional mechanistic studies to explore how their atheroprone effects can be targeted to decelerate atherosclerotic lesion formation and rupture.
Favorable effects of extracellular vesicles on vascular function
Despite the various deleterious effects of EVs in the pathogenesis of CVD, an increasing body of evidence highlights the beneficial effects of certain EVs on vascular function. The following sections summarize the impact of EV on endothelial repair, their inhibitory effect on vascular inflammation, and their role in plaque stabilization.
Endothelial protection and vascular repair
Given that EC injury is not only a key element in the complex pathophysiology of atherogenesis but also in in-stent restenosis occurring after treatment of coronary stenosis 90, 91, a mechanistic understanding of EC repair is pivotally important to develop therapeutic strategies to preserve endothelial integrity and vascular health. Several studies have shown that EVs particularly of endothelial origin can act as intercellular messenger to promote endothelial regeneration and vascular protection in vitro and in vivo.
A potential contribution of endothelial MVs in EC survival was shown by Abid Hussein et al. (92), who demonstrated that endothelial MV release is cell protective by exporting caspase-3 into MVs and thereby diminishing intracellular levels of proapoptotic caspase-3. Statins seem to facilitate endothelial health by promoting endothelial MV release in vitro (93). Nevertheless, the role of statins in endothelial MV release is still a matter of debate 94, 95. Our group has demonstrated that annexin I/phosphatidylserine receptor-dependent endothelial MV incorporation by ECs protects endothelial and endothelial-regenerating cells against apoptosis (96). Inhibition of p38 activity by annexin I-containing endothelial MV is possibly involved in endothelial MV-mediated protection. However, whether annexin I also mediates endothelial EV uptake in vivo will be explored in additional animal models. Another study showed that platelet-derived MVs induced changes in the early outgrowth cell, secretome, toward a more proangiogenic profile and amplified the early outgrowth cell-mediated induction of endothelial regeneration in vitro and in vivo (97). These studies indicate that MVs may influence the endothelial regeneration by the following 2 mechanisms: they could directly interact with ECs and promote vascular regeneration, or they may activate endothelial progenitor cells, facilitating endothelial repair 96, 98. In line with these findings, endothelial MVs carrying endothelial protein C receptor and activated protein C (APC) could also promote cell survival by induction of cytoprotective effects (99).
Among the biological contents transferred by EVs into target cells, miRNAs seem to play a crucial role by affecting mRNA and protein expression in recipient cells 100, 101, 102. Studies by our group have shown that endothelial MVs promote vascular endothelial repair by delivering functional miRNA-126 into recipient endothelial and vascular smooth muscle cells 103, 104. Of note, endothelial MV-mediated miRNA-126–induced endothelial repair was altered under pathological hyperglycemic conditions (103). These findings emphasize the fact that endothelial MVs can stimulate endothelial repair by functionally influencing migration and proliferation capacities in target cells, in addition to the already described regenerative potential of endothelial MV in interaction with progenitor cells. However, although miRNA-126 plays an important role in vascular health, miRNA-126–mediated downstream signaling and processing are not clearly identified and must be addressed in future research. Of note, EVs could activate EC by mRNA transfer from endothelial progenitor cells stimulating angiogenesis (105). In line with these findings, MVs from ischemic muscle promoted progenitor cell differentiation and subsequent postnatal vasculogenesis (106). Besides MVs, exosomes also play an important role in cardiovascular regeneration. Exosomes derived from mesenchymal stem cells reduced myocardial ischemia/reperfusion injury (107). Furthermore, exosomes from cardiac progenitor cells increased the migratory capacity of ECs in vitro and may contribute to vascular regeneration in vivo (108). Moreover, CD34+ exosomes promoted angiogenesis and preserved cardiac function in ischemic myocardium by delivery of sonic hedgehog (109).
In summary, EVs derived from endothelium, platelets, or endothelium-regenerating cells play a fundamental role by facilitating regenerative processes after vascular or myocardial injury 10, 110, 111.
Anti-inflammatory effects of extracellular vesicles
Several studies have reported anti-inflammatory effects of EVs. Of interest, neutrophils secrete MVs, which in turn promote anti-inflammatory release of transforming growth factor (TGF)-β1 from macrophages. These findings suggest MVs are potent anti-inflammatory effectors, which at an early stage of inflammation could contribute to its resolution (112). This effect seems to be mediated by annexin I expression on the surface of these EVs (113). EVs are also taken up by monocytes and B cells through diverse mechanisms and affect target cells toward an anti-inflammatory phenotype (114). Mesenchymal stem cells contribute to inflammatory repression by releasing exosomes that induce secretion of anti-inflammatory cytokines such as IL-10 and TGF-β (115). Administration of mesenchymal stem cell-derived exosomes in a myocardial ischemia/reperfusion injury model resulted in a significant reduction of local and systemic inflammation after 24 h (116). In a renal ischemia/reperfusion model in rats, intravenously administered mesenchymal stem cell-derived MVs limited inflammation as well as renal fibrosis (117). Finally, endothelial MVs promoted anti-inflammatory effects in vitro and in vivo by reducing endothelial ICAM-1 expression by the transfer of functional miRNA-222 into recipient cells (118). In line with these data, ECs suppressed monocyte activation through secretion of exosomes containing anti-inflammatory miRNAs (119). Importantly, despite a large amount of in vitro cellular studies, more in vivo models exploring local and systemic inflammation should be applied to validate and confirm the anti-inflammatory effects of EVs.
Plaque stabilization and antithrombotic effects
miRNA-containing EVs have been shown to promote vascular protection and plaque stabilization through various mechanisms. Injection of miRNA-126-3p–enriched apoptotic bodies of endothelial origin promoted atheroprotective effects by limiting plaque size, increasing plaque stability, and enhancing progenitor cell recruitment. miRNA-126–dependent inhibition of regulator of G protein signaling (RGS16) and subsequent enhancement of CXCR4 and CXCL12 was elaborated as the underlying mechanism (27). Exosomes from KLF-2–transduced or shear-stress–stimulated ECs are enriched in miRNAs 143 and 145. By transferring functional miRNAs, EVs were shown to control target gene expression in vascular smooth muscle target cells and reduce atherosclerotic lesion formation in the aorta of apolipoprotein E knockout mice. These findings suggest that atheroprotective stimuli induce communication between ECs and vascular smooth muscle cells through miRNA-transferring EVs (120). Similarly, circulating miRNA-223–containing exosomes could penetrate the vascular wall and inhibit vascular smooth muscle cell proliferation and migration, resulting in decreased plaque size (121). Whereas MVs are involved in enhancing blood clotting processes, exosomes seem to suppress platelet aggregation and occlusive thrombosis by inhibiting platelet CD36, inducing antithrombotic effects. However, further research is needed to validate these findings in adequate in vivo models and to understand the opposing roles of exosomes and MVs in this context. Finally, platelet-derived exosomes reduced CD36-dependent oxidized low-density lipoprotein binding and macrophage cholesterol loading, potentially contributing to atheroprotection (122). Figure 2 illustrates the known beneficial effects of EVs in the regulation of vascular integrity. Table 1 summarizes the most important characteristics of studies exploring the effect of EVs on vascular health and disease.
Figure 2.
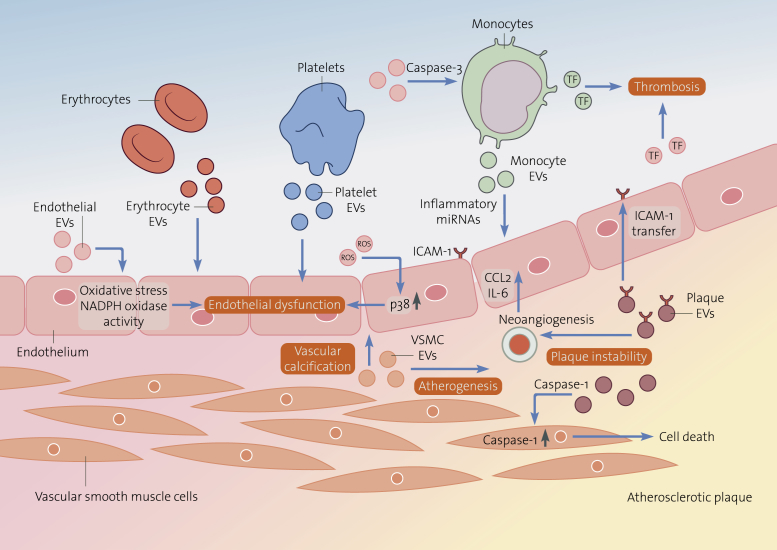
Detrimental Effects of Extracellular Vesicles on Vascular Function
The functional effect of EVs in cardiovascular disease is extremely complex and depends on the cellular origin, the functional state of the releasing cells, the biological content, the distinct recipient cell, and the transfer capacity of intravesicular functional bioactive molecules. Here, we illustrate the role of EVs as active promoters of endothelial dysfunction, vascular calcification, atherogenesis, plaque instability and thrombosis. Endothelial EVs impair vasorelaxation through local oxidative stress or through increased NADPH oxidase activity. EVs released by erythrocytes react with and degrade NO, EVs from platelet and atherosclerotic plaque induce endothelial apoptosis, both mediating endothelial dysfunction. Monocyte-derived EVs transfer inflammatory miRNAs into endothelial cells inducing vascular inflammation. Vascular smooth muscle cell-derived EVs act as mediators of vascular calcification modulating atherogenesis. EVs isolated from atherosclerotic plaques transfer ICAM-1 to endothelial cells and recruit inflammatory cells, contributing to plaque instability by promoting neovascularization. Once plaque rupture occurs, monocyte EVs and endothelial EVs initiate the coagulation cascade by the expression of tissue factor contributing to thrombosis. EV = extracellular vesicles; miRNA = microRNA; NO = nitric oxide.
Table 1.
Detrimental and Favorable Effects of Extracellular Vesicles on Vascular Function
| Effect | EV Type | Isolation Method | Donor Cell/Origin | In Vitro Experiment | In Vivo Experiment | Effects | Mechanisms | Ref. # |
|---|---|---|---|---|---|---|---|---|
| Detrimental effects | ||||||||
| Injured endothelial MVs | Centrifugation 20,000 g | Glucose-treated HCAECs | HCAECs | ApoE−/− mice | Induce EC inflammation | Up-regulate ICAM-1 and VCAM-1 in EC by activating p38 | (33) | |
| Endothelial MVs | Ultracentrifugation 100,000 g | RMVECs | Aortic rings from rats | — | Impair vasorelaxation | Local oxidative stress | (41) | |
| Circulating MVs | Centrifugation 13,000 g | Patients with MI | Aortic rings from rats | — | Vasomotor dysfunction | Impair endothelial NO transduction pathway | (42) | |
| Erythrocyte MVs | Differential centrifugation | Human packed red blood cells under standard blood banking conditions | — | Rat vasoactivity models | Reduce vasoconstrictor effects | Degrade vasodilator NO | (45) | |
| Platelet exosomes | Ultracentrifugation 100,000 g | Platelets from septic patients | ECs | — | Induce ECs apoptosis | Superoxide; NO and peroxynitrite production |
(46) | |
| PMN MVs | Ultracentrifugation 100,000 g | PMNs from healthy volunteers | HUVECs | — | Induce ECs activation | Stimulate EC cytokine release Induction of tissue factor |
(53) | |
| Oxidized MVs | Ultracentrifugation 100,000 g | Oxidatively modified HUVECs | Monocytes | — | Stimulate monocytes adhesion to ECs | Contain oxidized phospholipids | (56) | |
| Plaque MVs | Centrifugation 20,500 g | Human atherosclerotic plaques | HUVECs | — | Promote inflammatory response | Carry catalytically activeTNF-alpha converting enzyme (TACE/ADAM17) Enhance the processing of TNF-α and TNF receptor |
(70) | |
| Monocyte MVs | Ultracentrifugation 100,000 g | Human peripheral blood monocytes | VSMCs | — | Induce VSMCs cell death | Deliver cell death message via encapsulated caspase-1 | (77) | |
| CD40 ligand plus plaque MPs | Centrifugation 20,500 g | Human atherosclerotic plaques | HUVECs | Wild-type and BalbC/Nude mice | Stimulate endothelial proliferation and angiogenesis | CD40L signaling | (82) | |
| Favorable effects | ||||||||
| Endothelial apoptotic bodies | Centrifugation 16,000 g | HUVECs | HUVECs | Mice models of atherosclerosis | Promote atheroprotective effects Increase plaque stability Enhance progenitor cells recruitment |
MiRNA-126-dependent inhibition of RGS16 Enhance CXCR4 and CXCL12 |
(27) | |
| Endothelial MVs | Centrifugation 20,000 g | HCAECs | HCAECs | — | Prevent HCAECs apoptosis | Annexin I/phosphatidylserine receptor–dependent inhibition of p38 activation | (96) | |
| Platelet MVs | Centrifugation 20,000 g | Platelets | EOCs | Mice models of arterial wire-induced injury | Enhance vasoregenerative potential of EOCs | Enhance EOCs recruitment, migration, differentiation Release proangiogenic factors |
(97) | |
| Endothelial MVs | Centrifugation 20,000 g | HCAECs | HCAECs | Electric injury of murine carotid artery | Promote ECs migration and proliferation Accelerate re-endothelialization |
Inhibit SPRED-1 via EMV-mediated transfer of miRNA-126 | (103) | |
| Endothelial MVs | Centrifugation 20,000 g | HCAECs | VSMCs | Wire injury of murine carotid artery | Reduce neointima formation Diminished VSMCs proliferation and migration |
Inhibit LRP6 via EMPs-mediated transfer of miRNA-126-3p | (104) | |
| Exosomes | Differential centrifugation | CMPCs | HMECs | — | Stimulate HMECs migration | EMMPRIN-mediated | (108) | |
| Exosomes | HPLC | Mesenchymal stem cells | — | Mice models of myocardial I/R injury | Reduce local and systemic inflammation | Restore bioenergetics Reduce oxidative stress Activate pro-survival signaling |
(116) | |
| Endothelial MVs | Centrifugation 20,000 g | HCAECs | Monocytes | ApoE-deficient mice | Promote anti-inflammatory effects | Reduce endothelial ICAM-1 expression via the transfer of functional miRNA-222 | (118) | |
| Endothelial exosomes | Centrifugation 20,500 g | KLF2-transduced or shear-stress-stimulated HUVECs | HASMCs | Aorta of ApoE knockout mice | Atheroprotection | EV-mediated transfer of miRNA-143/145 | (120) | |
| Circulating MVs | Ultracentrifugation | Blood | VSMCs | ApoE-deficient mice | Penetrate the vascular wall Inhibit VSMCs proliferation and migration |
miRNA-223-mediated IGF-1R/PI3K-Akt pathway | (121) |
ApoE = apolipoprotein E; CMPC = cardiomyocyte progenitor cell; EC = endothelial cell; EMMPRIN = extracellular matrix metalloproteinase inducer; EMV = endothelial MV; HASMC = human aortic smooth muscle cell; HCAECs = human coronary artery endothelial cells; HMEC = human microvascular endothelial cell; HPLC = high-performance liquid chromatography; HUVEC = human umbilical vein endothelial cell; I/R = ischemia/reperfusion; ICAM = intercellular adhesion molecule; KLF = Krüppel-like factor; MI = myocardial infarction; MV = microvesicles; NO = nitric oxide; PMNs = polymorphonuclear leukocytes; RGS16 = regulator of G-protein signaling; RMVEC = rat renal microvascular endothelial cell; SPRED = sprouty-related EVH1 domain-containing protein; TNF = tumor necrosis factor; VSMC = vascular smooth muscle cell.
In conclusion, beneficial and detrimental effects of EVs have been described in the regulation of vascular health and disease. However, there are no constant rules showing a clear relationship between origin and function of EVs. EVs derived under pathological conditions can induce cardiovascular harm (e.g., plaque EVs promote inflammatory response [70]) but also demonstrate atheroprotective functions (e.g., MVs from ischemic muscle induce progenitor cell differentiation [106]). Even the same original EVs can show both detrimental and favorable effects (e.g., endothelial EVs impairing vasorelaxation on one hand [41] and reducing neointima formation on the other hand [104]). In order to clarify the multifaceted character of EVs and make data more comparable, additional efforts should be put into standardized EV generation techniques. Once they are established, in-depth exploration of EV-incorporated and transferred biological molecules and their intracellular processing is necessary to gain more clarity in the understanding of EV function.
Therapeutic Potential of Extracellular Vesicles in Cardiovascular Diseases
Extracellular vesicles as novel therapeutic tool?
EVs have emerged as vectors for transferring biological information by proteins or genetic material, thereby maintaining vascular homeostasis, favoring endothelial repair, or even limiting atherosclerosis.
Due to these beneficial effects, there has been a rising interest in the potential use of EVs as therapeutic vectors in the field of cardiovascular medicine and regenerative therapy. Multiple studies have shown that the transfer of functional miRNAs into target tissue by EVs promotes vascular regeneration and atheroprotection 27, 103, 119, 120, 123, highlighting the therapeutic potential of miRNA-transferring EVs. In addition to miRNA-containing EVs, many reports describing nanoparticles as a new approach to transport miRNAs or anti-miRNAs to recipient cells have been published recently 124, 125, 126 (Figure 4).
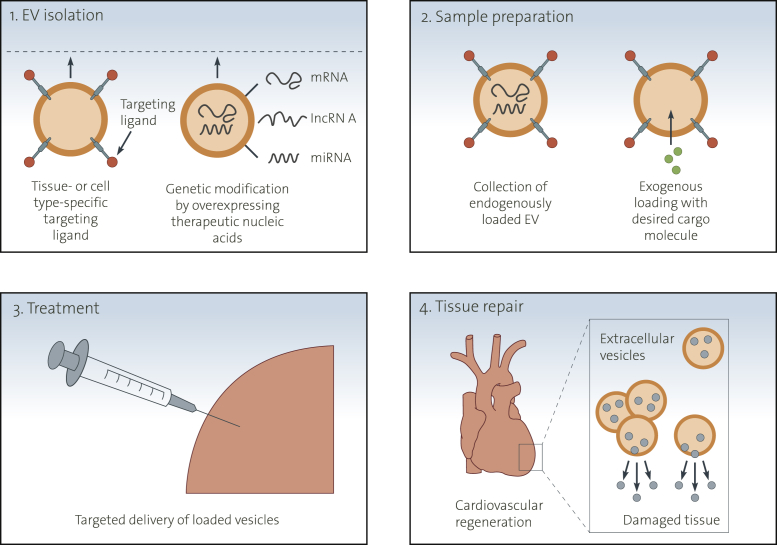
Figure 4.
Potential Therapeutic Use of Extracellular Vesicles
Potential therapeutic application of extracellular vesicles includes the following 4 critical steps: 1) Extracellular vesicles can be modified by using tissue- or cell-type-specific ligands present on their surface. Endogenously expressed molecules such as miRNA and noncoding RNAs can be genetically engineered for therapeutic use (e.g., genetic modification by overexpression therapeutic nucleic acids). 2) Exogenous loading permits the collection of extracellular vesicles with desired cargo molecules. The collection and purification of extracellular vesicles can be carried out by various methods, including differential ultracentrifugation, ultrafiltration, sucrose gradient centrifugation, or immunoprecipitation. 3) Extracellular vesicles, loaded by any of these strategies, can be delivered into target cells or tissues with different delivery methods (e.g., intravenously injection or intracellular injection). 4) The loaded vesicles can function as favorable effectors in intercellular vascular signaling, contributing to the cardiovascular regeneration in damaged tissue.
Chen et al. (124) developed miRNA-34a–containing liposome-polycationhyaluronic acid (LPH) nanoparticle for systemic delivery of miRNA-34a into lung metastasis of murine melanoma, resulting in significant down-regulation of surviving expression in the metastatic tumors, as well as reduced tumor. Furthermore, biodegradable polymer nanoparticles coated with cell-penetrating peptides for an effective delivery of chemically modified oligonucleotide analogues have been described. This nanoparticle system was used to block the activity of the oncogenic miRNA-155, as well as to attenuate the expression of the proto-oncogene Mcl-1, leading to reduced cell viability and pro-apoptotic effects in the recipient cells (127). Another approach targeting miRNA-155 described decelerated tumor growth after application of polymer nanoparticles containing antisense peptide nucleic acids with subsequent miRNA-155 inhibition (125). An integrin αvβ3-targeted nanoparticle was used by Anand et al. (128) to deliver anti-miRNA-132 to the tumor endothelium of human breast carcinoma in mice, causing restored p120RasGAP expression in the tumor endothelium, thereby suppressing angiogenesis and decreasing tumor burden. Although these studies focused on miRNA or anti-miRNA delivery using nanoparticles mainly as a therapeutic tool to combat cancer, it is reasonable that nanoparticles can also be used to deliver miRNAs to recipient vascular cells for tackling inflammation and development of atherosclerosis (129). In this context, magnetic nanoparticle-assisted (circumferential) gene transfer into the vascular endothelium has recently been described as a promising novel strategy to transfer biological messages into the diseased vasculature 130, 131, 132.
Translation into clinical use
EVs have multiple advantages over currently available drug delivery vehicles, such as their ability to overcome natural barriers, their intrinsic cell-targeting properties, protection of their biological cargo from degrading enzymes, and stability in the circulation (133). EV subpopulations could be used as a cargo system for efficient and selective drug delivery to a distinct cell type within diseased tissues. This approach offers the additional advantages of low immunogenicity because patient-derived tissue could be used as the source of individualized and biocompatible drug delivery vehicles 134, 135 (Figure 3).
Figure 3.
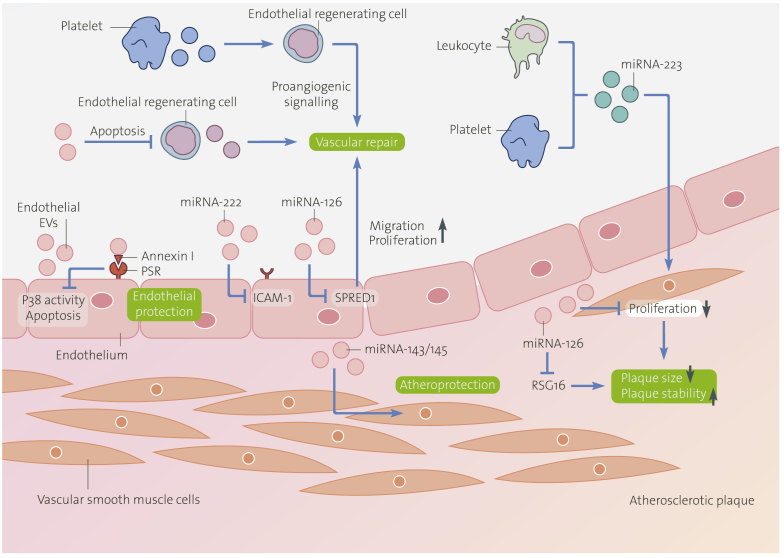
Beneficial Effects of Extracellular Vesicles on Vascular Function
An increasing body of evidence points out the beneficial influence of certain EVs of diverse cellular sources in cardiovascular biology. This figure illustrates the favorable effects of EVs on endothelial and vascular function, atherosclerosis, and plaque stabilization. Endothelial EVs reduce endothelial apoptosis by inhibition of p38 activity mediated by an annexin I/phosphatidylserine receptor-dependent mechanism, contributing to endothelial protection. Moreover, endothelial EVs decrease endothelial regenerating cell apoptosis, facilitating endothelial repair. Platelet-derived MVs induce alterations in the endothelial-regenerating cell secretome toward a more proangiogenic profile and amplify vascular protection. Among the biological content transferred by EVs into target cells, miRNAs play a crucial role. Endothelial EVs promote vascular endothelial repair by inhibition of SPRED1 by delivering functional miRNA-126. Endothelial EVs promote anti-inflammatory effects by reducing endothelial ICAM-1 expression by the transfer of functional miRNA-222 into recipient cells. Exosomes from KLF-2-transduced or shear-stress-stimulated endothelial cells attribute to atheroprotection by transferring miRNA-143/145. Circulating leukocyte- and platelet-derived miRNA-223-containing exosomes penetrate the vascular wall, inhibit vascular smooth muscle cell proliferation and migration, resulting in decreased plaque size. Endothelial apoptotic bodies decrease vascular smooth muscle cell proliferation, limit plaque size, and increase plaque stability by miRNA-126-dependent inhibition of G-protein signaling (RGS16) pathway. MV = microvesicle; other abbreviations as in Figures 1 and 2.
Interestingly, tumor cells incubated with chemotherapeutic drugs are able to package these drugs into EVs, which can be collected and used to effectively kill tumor cells in murine tumor models without typical side effects (136). Moreover, tumor cell-derived EVs were used as a unique carrier system to deliver oncolytic adenoviruses to human tumors, leading to highly efficient cytolysis of tumor cells. These findings highlight a novel adenovirus delivery system with promising clinical applications (137).
An important issue regarding EV therapeutics is the biodistribution of EVs. Intravenously injected EVs are of particular interest in the treatment of cardiovascular alterations, as the entire vascular network would be exposed to EVs. However, to selectively direct EVs to target cells or tissue, cell-specific ligands must be stably expressed on the surface of EVs. Using an innovative approach of donor cell engineering with target cell-specific ligand expression resulted in targeted delivery of short interfering RNA (siRNA) and miRNA-loaded EVs to target neurons (138) and breast cancer cells (139).
Despite promising perspectives for the treatment of cardiovascular pathologies, EV-based therapies still need more investigation to translate experimental data into clinical application. One challenge would be to control the fragile equilibrium between the harmful and beneficial effects reported for EVs in the context of CVD. Furthermore, the off-side effects and clearing mechanisms of EVs need to be better explored before they can be seriously considered as a novel therapeutic tool for combatting CVD (140).
Study limitations and methodological obstacles in EV research
Despite the emerging role of EVs as regulators of health and disease, there are still general limitations in EV research. Within this paragraph, we highlight relevant obstacles, which need to be addressed to better understand EV functions and move the EV field forward.
Methodological obstacles in isolating EVs
Isolation and purification of EVs vary between different research groups and also depend on the donor cells from which they are derived. Therefore, EV classification, isolation, and purification needs to be standardized to ensure that EV analysis is reproducible and internationally comparable among different research groups (141). Furthermore, within EV populations, many distinct subtypes of vesicles exist. However, the currently used methods (differential ultracentrifugation, polymer-based precipitation, density gradients, microfiltration, size-exclusion–based approaches, or polyethylene glycol [PEG]-mediated isolation techniques) all have different pros and cons in attempting to isolate pure EVs with a distinct size and surface markers (142). The most commonly used isolation methods are differential centrifugation, followed by ultracentrifugation. Two major problems with these techniques are the relatively long procedure times and low throughput, limiting their application in the clinical setting (143). Moreover, the yield depends on the viscosity of biological fluids, so the samples with a relatively high viscosity such as plasma would significantly reduce production (144). Density gradient isolation can promote both the yield and purity of EVs compared with ultracentrifugation; however, the procedure is time-consuming and hard to standardize (145). Ultrafiltration is time-efficient and can concentrate EVs up to 240-fold, but the low purity of EVs is an obstacle (146). Commercial EV precipitation kits are based mainly on PEG. Although the PEG-mediated technique provides a high-yield, rapid, and inexpensive EV isolation method from both culture media and body fluids, some other contaminants are also copurified, leading to the low purity of EVs 142, 147. In the clinical setting, size-exclusion chromatography and affinity capture are the 2 methods most often applied to isolate EVs. Size-exclusion chromatography can remove most soluble components and is a relatively quick procedure with good reproducibility (148), but it also faces the possible problem of protein or RNA contamination (143). Compared with other methods, affinity capture can produce subpopulations of EVs with relatively high purity, but the cost of preparation (antibody based) may limit its applicability and may damage surface proteins and functionality of EVs (143). Therefore, the development of new, more selective isolation techniques is urgently needed to increase the purity of each vesicle subpopulation (149). The pros and cons of each available method are summarized in Table 2.
Table 2.
Overview of Commonly Used EV Isolation Methods
| Method | Principle of Separation | Advantages | Disadvantages | Ref. # |
|---|---|---|---|---|
| UC | Size and density | Widely used | Relatively long procedure Low throughput Depends on viscosity of biological fluids |
(143) |
| DG | Size and density | High purity of EVs | Time-consuming | (145) |
| Ultrafiltration | Size | Time efficient Effective to concentrate EVs |
Low purity of EVs | (146) |
| Precipitation kits | PEG-mediated | High yield Rapid |
Low purity of EVs | 142, 147 |
| SEC | Size | Quick procedure Reproducibility |
Low purity of EVs | 143, 148 |
| Affinity capture | Binding with EVs surface components | Production of subpopulations of EVs Relatively high purity |
High cost (antibody-based) May damage surface components of EVs |
(143) |
DG = density gradient; PEG = polyethylene glycol; SEC = size-exclusion chromatography; UC = ultracentrifugation; other abbreviations as in Table 1.
Size measurement techniques for EV characterization
Size is an important defining property of EVs, and measurement of diameter to determine a size distribution is a critical step for EV studies. Size distribution measurement technologies include electron microscopy (EM), flow cytometry (FC), nanoparticle tracking analysis, resistive pulse sensing, and atomic force microscopy (AFM). Although these techniques are commonly used in practice (142), some issues are still unsolved. First, the ideal size measurement should detect EVs with a diameter of 50 nm and larger (150), but most methods, except for EM, cannot detect the smallest EVs (151). Second, the results of size distribution, even for the same EV subpopulation, may show different results depending on the method used (152). Furthermore, there are no standard protocols (e.g., optimal EM-EV measurement protocols) (142). Importantly, some methods, such as nanoparticle-tracking analysis or resistive pulse sensing, cannot distinguish membrane vesicles from nonmembranous particles of similar size, so the results should be compared by using EM, AFM, or other microscopy technique (153).
Regulation of EV packaging
Although some key players in sorting of cargoes into EVs are revealed (e.g., sumoylated hnRNPA2B1 is reported to control the location of miRNAs into exosomes through binding to specific motifs [154]), the underlying mechanisms mediating the packaging and loading of selected molecules into EVs remain largely unknown. Therefore, additional studies exploring EV biogenesis and mechanisms regulating EV packaging are important to understand cardiovascular injury and repair induced by EVs (155).
Methodological issues on EV uptake experiments in vitro
EV uptake experiments are usually performed by direct visualization. Therefore, fluorescent lipid membrane dyes, such as PKH26 (156), PKH68 (157), or rhodamine B (158), are used to stain EV membranes. One potential issue with membrane-binding dyes is that fluorescent molecules could potentially affect the uptake and biological behavior of EVs. However, EV incorporation has been observed with many different lipid-binding dyes, suggesting that such molecules do not affect internalization of vesicles; nevertheless, additional studies are needed to verify whether the biological behavior of EVs is affected by dyes. Another potential limitation of the use of lipophilic dyes is leaching of the fluorescent molecules from EVs into cellular membranes, potentially leading to a pattern of internalization that is due to normal membrane recycling rather than EV uptake. However, direct measurement of the fluorescence transfer rate between EVs and recipient cells support the idea that the increased fluorescence in cells is due to specific uptake of EVs rather than nonspecific dye leaching (159). Another issue which must be considered is the fact that most EV uptake studies have relied on fluorescence microscopy, which has limited resolution because the wavelength of visible light is approximately 390 to 700 nm; therefore, single EVs or aggregated vesicles, which are <390 nm in diameter, cannot be distinguished. This should not affect the assessment of EV uptake in general but may affect the visualization and dynamic localization analysis of individual EVs. Nevertheless, the increasing use of confocal microscopy has confirmed that EVs smaller than 390 nm such as exosomes can be incorporated into recipient cells (160).
Lack of EV secretion and uptake models in vivo
The secretion of EVs by parent cells and uptake by recipient cells are precisely regulated. However, few data are available studying concrete mechanisms regulating MV release and clearance. Moreover, physiological models are not well established (e.g., considering time-dependent release kinetics of EVs from parent cells). Finally, the absence of adequate in vivo models to explore the generation and uptake of cell-specific EVs limits the experimental opportunities to study EV functions in vivo.
Unclear processing of EVs and their content after cellular uptake
EVs interact with recipient cells by transferring their biological contents through membrane fusion in a ligand-receptor-mediated way or by endocytosis, pinocytosis, or phagocytosis (12) (Figure 1). However, processing of incorporated EVs and their intravesicular content after cellular uptake is entirely unknown. To address this point, tracking experiments using fluorescence labeleling represent a possible option to gain further insight into time-dependent cellular processing mechanisms of biological contents transferred by EVs (161). Nevertheless, stable labeling of intravesicular contents is highly demanding technically, and further efforts are needed to improve these techniques (162).
Taken together, there are still serious methodological issues that need to be addressed. Importantly, recent position research papers from the International Society of Extracellular Vesicles have made a first step in the right direction to standardize EV analysis internationally among different laboratories 141, 147, 153. In addition, EV-TRACK (163), a novel crowd-sourcing database, has recently been implemented, centralizing knowledge about EV biology and methodology with the goal of stimulating authors, reviewers, editors, and funders to put experimental guidelines into practice (164).
Conclusions
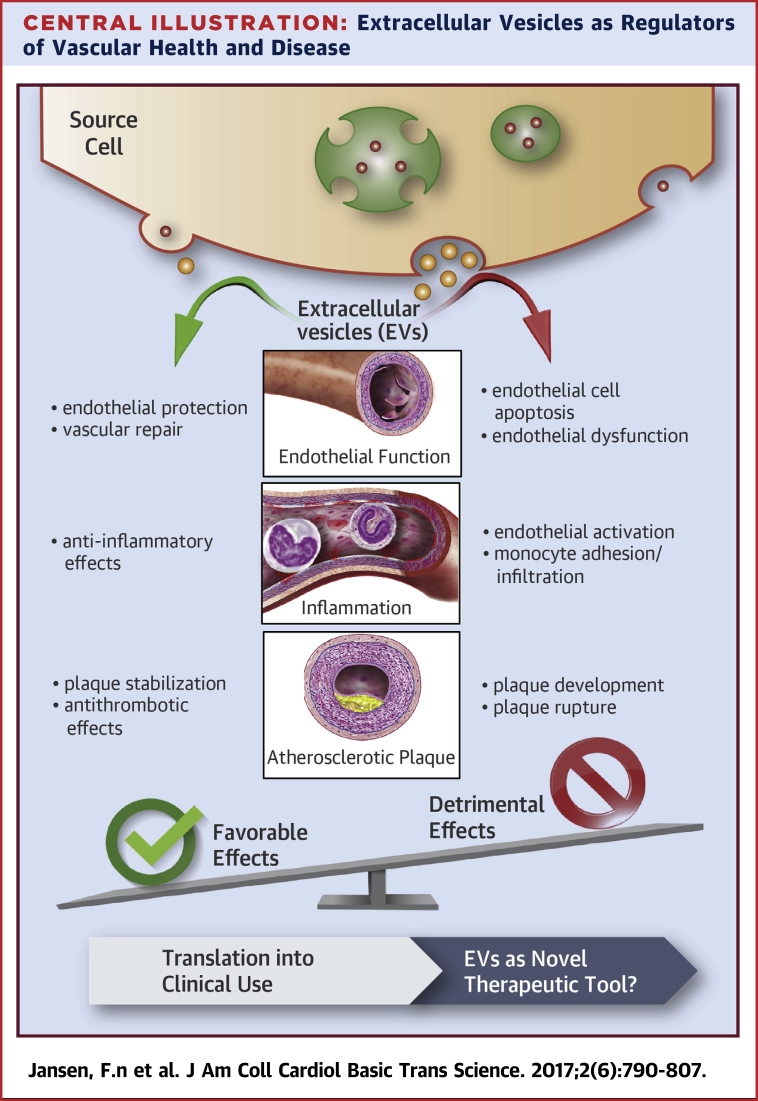
In the regulation of cardiovascular health and disease, EVs act as urgent effectors by transferring bioactive molecules into adjacent and distant recipients. EV-mediated intercellular vascular signaling results in detrimental and favorable effects on vascular integrity. Studies illustrate that EVs can contribute to atherosclerosis development and progression. In contrast, EVs also emerge as crucial regulators of vascular homeostasis and mediate vascular protection. Finally, the use of genetically modified EV might represent a novel therapeutic tool in the field of cardiovascular medicine and regenerative therapy (Central Illustration).
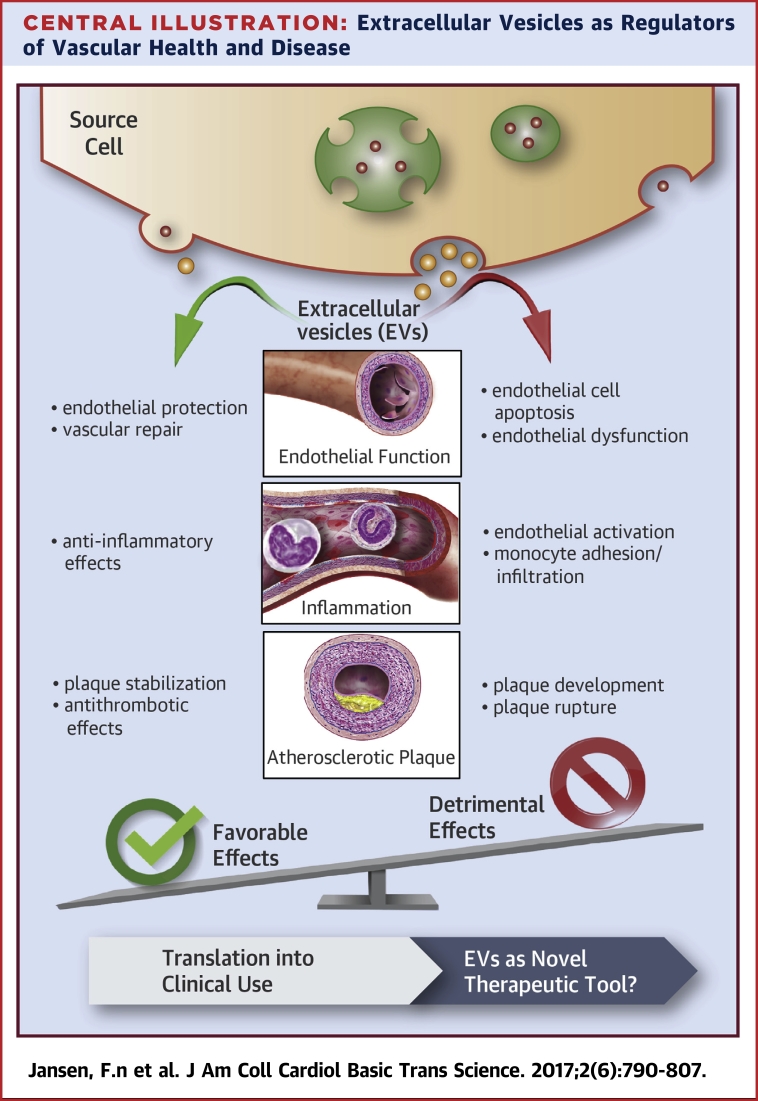
Central Illustration.
Extracellular Vesicles as Regulators of Vascular Health and Disease
Many types of cells release EVs, such as exosomes and microvesicles, by different mechanisms. EVs have both favorable and detrimental effects on vascular integrity. The use of genetically modified EVs might represent a novel therapeutic tool in the field of cardiovascular medicine and regenerative therapy. EV = extracellular vesicles; miRNA = microRNA.
Footnotes
Drs. Werner, Pfeifer, and Jansen are supported by Deutsche Forschungsgemeinschaft (WE 4139/8-1, JA2352/2-1, DFG GRK1873). Dr. Jansen received support from Medical Faculty of the Rheinische Friedrich-Wilhelms-University Bonn, the Familie Schambach foundation and German Society of Cardiology. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Drs. Jansen and Li contributed equally to this study.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Felix Jansen, Email: felix.jansen@ukbonn.de.
Nikos Werner, Email: nwerner@uni-bonn.de.
References
- 1.Libby P. Changing concepts of atherogenesis. J Intern Med. 2000;247:349–358. doi: 10.1046/j.1365-2796.2000.00654.x. [DOI] [PubMed] [Google Scholar]
- 2.Dignat-George F., Boulanger C.M. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 3.Koga H., Sugiyama S., Kugiyama K. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 4.Jansen F., Nickenig G., Werner N. Extracellular vesicles in cardiovascular disease: potential applications in diagnosis, prognosis, and epidemiology. Circ Res. 2017;120:1649–1657. doi: 10.1161/CIRCRESAHA.117.310752. [DOI] [PubMed] [Google Scholar]
- 5.Mause S.F., Weber C. Microparticles: protagonists of a novel communication network for intercellular information exchange. Circ Res. 2010;107:1047–1057. doi: 10.1161/CIRCRESAHA.110.226456. [DOI] [PubMed] [Google Scholar]
- 6.Kogure T., Yan I.K., Lin W.-L., Patel T. Extracellular vesicle-mediated transfer of a novel long noncoding RNA TUC339: a mechanism of intercellular signaling in human hepatocellular cancer. Genes Cancer. 2013;4:261–272. doi: 10.1177/1947601913499020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rautou P.-E., Vion A.-C., Amabile N. Microparticles, vascular function, and atherothrombosis. Circ Res. 2011;109:593–606. doi: 10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- 8.Boon R.A., Vickers K.C. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulanger C.M., Loyer X., Rautou P.-E., Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259–272. doi: 10.1038/nrcardio.2017.7. [DOI] [PubMed] [Google Scholar]
- 10.Barile L., Moccetti T., Marbán E., Vassalli G. Roles of exosomes in cardioprotection. Eur Heart J. 2017;38:1372–1379. doi: 10.1093/eurheartj/ehw304. [DOI] [PubMed] [Google Scholar]
- 11.Colombo M., Raposo G., Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 12.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raiborg C., Stenmark H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature. 2009;458:445–452. doi: 10.1038/nature07961. [DOI] [PubMed] [Google Scholar]
- 14.Trajkovic K., Hsu C., Chiantia S. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 15.Baietti M.F., Zhang Z., Mortier E. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol. 2012;14:677–685. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 16.Nabhan J.F., Hu R., Oh R.S., Cohen S.N., Lu Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A. 2012;109:4146–4151. doi: 10.1073/pnas.1200448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaé N., McEwan D.G., Manavski Y., Boon R.A., Dimmeler S. Rab7a and Rab27b control secretion of endothelial microRNA through extracellular vesicles. FEBS Lett. 2015;589:3182–3188. doi: 10.1016/j.febslet.2015.08.040. [DOI] [PubMed] [Google Scholar]
- 18.Bobrie A., Colombo M., Raposo G., Théry C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 19.Ostrowski M., Carmo N.B., Krumeich S. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12(Suppl 1–13):19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 20.Muralidharan-Chari V., Clancy J., Plou C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr Biol. 2009;19:1875–1885. doi: 10.1016/j.cub.2009.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lo S.-C., Hung C.-Y., Lin D.-T., Peng H.-C., Huang T.-F. Involvement of platelet glycoprotein Ib in platelet microparticle mediated neutrophil activation. J Biomed Sci. 2006;13:787–796. doi: 10.1007/s11373-006-9107-5. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta S.K., Le A., Chavakis T., Rumbaut R.E., Thiagarajan P. Developmental endothelial locus-1 (Del-1) mediates clearance of platelet microparticles by the endothelium. Circulation. 2012;125:1664–1672. doi: 10.1161/CIRCULATIONAHA.111.068833. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta S.K., Abdel-Monem H., Niravath P. Lactadherin and clearance of platelet-derived microvesicles. Blood. 2009;113:1332–1339. doi: 10.1182/blood-2008-07-167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rautou P.-E., Leroyer A.S., Ramkhelawon B. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res. 2011;108:335–343. doi: 10.1161/CIRCRESAHA.110.237420. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:24641. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 27.Zernecke A., Bidzhekov K., Noels H. Delivery of MicroRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2 doi: 10.1126/scisignal.2000610. ra81–ra81. [DOI] [PubMed] [Google Scholar]
- 28.Skog J., Würdinger T., van Rijn S. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J.-G., Williams J.C., Davis B.K. Monocytic microparticles activate endothelial cells in an IL-1β-dependent manner. Blood. 2011;118:2366–2374. doi: 10.1182/blood-2011-01-330878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yáñez-Mó M., Siljander P.R.-M., Andreu Z. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ray D.M., Spinelli S.L., Pollock S.J. Peroxisome proliferator-activated receptor gamma and retinoid X receptor transcription factors are released from activated human platelets and shed in microparticles. Thromb Haemost. 2008;99:86–95. doi: 10.1160/TH07-05-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brill A., Dashevsky O., Rivo J., Gozal Y., Varon D. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res. 2005;67:30–38. doi: 10.1016/j.cardiores.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Jansen F., Yang X., Franklin B.S. High glucose condition increases NADPH oxidase activity in endothelial microparticles that promote vascular inflammation. Cardiovasc Res. 2013;98:94–106. doi: 10.1093/cvr/cvt013. [DOI] [PubMed] [Google Scholar]
- 34.Owens A.P., Mackman N. Microparticles in hemostasis and thrombosis. Circ Res. 2011;108:1284–1297. doi: 10.1161/CIRCRESAHA.110.233056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratajczak J., Wysoczynski M., Hayek F., Janowska-Wieczorek A., Ratajczak M.Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 36.Gupta S.K., Bang C., Thum T. Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet. 2010;3:484–488. doi: 10.1161/CIRCGENETICS.110.958363. [DOI] [PubMed] [Google Scholar]
- 37.Bang C., Thum T. Exosomes: new players in cell-cell communication. Int J Biochem Cell Biol. 2012;44:2060–2064. doi: 10.1016/j.biocel.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 39.Libby P., Ridker P.M., Hansson G.K., Ducluzeau P.H. Transatlantic network on atherothrombosis. inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Densmore J.C., Signorino P.R., Ou J. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock. 2006;26:464–471. doi: 10.1097/01.shk.0000228791.10550.36. [DOI] [PubMed] [Google Scholar]
- 41.Brodsky S.V., Zhang F., Nasjletti A., Goligorsky M.S. Endothelium-derived microparticles impair endothelial function in vitro. Am J Physiol Heart Circ Physiol. 2004;286:H1910–H1915. doi: 10.1152/ajpheart.01172.2003. [DOI] [PubMed] [Google Scholar]
- 42.Boulanger C.M., Scoazec A., Ebrahimian T. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–2652. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 43.Amabile N., Guérin A.P., Leroyer A. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 44.Agouni A., Lagrue-Lak-Hal A.H., Ducluzeau P.H. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donadee C., Raat N.J.H., Kanias T. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124:465–476. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gambim M.H., do Carmo A de O., Marti L., Veríssimo-Filho S., Lopes L.R., Janiszewski M. Platelet-derived exosomes induce endothelial cell apoptosis through peroxynitrite generation: experimental evidence for a novel mechanism of septic vascular dysfunction. Crit Care. 2007;11:R107. doi: 10.1186/cc6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Willerson J.T., Ridker P.M. Inflammation as a cardiovascular risk factor. Circulation. 2004;109:II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto S., Niida S., Azuma E. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Sci Rep. 2015;5:8505. doi: 10.1038/srep08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buzás E.I., György B., Nagy G., Falus A., Gay S. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol. 2014;10:356–364. doi: 10.1038/nrrheum.2014.19. [DOI] [PubMed] [Google Scholar]
- 50.Robbins P.D., Morelli A.E. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robbins P.D., Dorronsoro A., Booker C.N. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126:1173–1180. doi: 10.1172/JCI81131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mesri M., Altieri D.C. Endothelial cell activation by leukocyte microparticles. J Immunol. 1998;161:4382–4387. [PubMed] [Google Scholar]
- 53.Mesri M., Altieri D.C. Leukocyte microparticles stimulate endothelial cell cytokine release and tissue factor induction in a JNK1 signaling pathway. J Biol Chem. 1999;274:23111–23118. doi: 10.1074/jbc.274.33.23111. [DOI] [PubMed] [Google Scholar]
- 54.Nomura S., Tandon N.N., Nakamura T., Cone J., Fukuhara S., Kambayashi J. High-shear-stress-induced activation of platelets and microparticles enhances expression of cell adhesion molecules in THP-1 and endothelial cells. Atherosclerosis. 2001;158:277–287. doi: 10.1016/s0021-9150(01)00433-6. [DOI] [PubMed] [Google Scholar]
- 55.Barry O.P., Praticò D., Savani R.C., FitzGerald G.A. Modulation of monocyte-endothelial cell interactions by platelet microparticles. J Clin Invest. 1998;102:136–144. doi: 10.1172/JCI2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huber J., Vales A., Mitulovic G. Oxidized membrane vesicles and blebs from apoptotic cells contain biologically active oxidized phospholipids that induce monocyte-endothelial interactions. Arterioscler Thromb Vasc Biol. 2002;22:101–107. doi: 10.1161/hq0102.101525. [DOI] [PubMed] [Google Scholar]
- 57.Edrissi H., Schock S.C., Hakim A.M., Thompson C.S. Microparticles generated during chronic cerebral ischemia increase the permeability of microvascular endothelial barriers in vitro. Brain Res. 2016;1634:83–93. doi: 10.1016/j.brainres.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 58.Mause S.F., Hundelshausen von P., Zernecke A., Koenen R.R., Weber C. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 59.Vasina E.M., Cauwenberghs S., Feijge M.A.H., Heemskerk J.W.M., Weber C., Koenen R.R. Microparticles from apoptotic platelets promote resident macrophage differentiation. Cell Death Dis. 2011;2 doi: 10.1038/cddis.2011.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Jong O.G., Verhaar M.C., Chen Y. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles. 2012;1 doi: 10.3402/jev.v1i0.18396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alexandru N., Badila E., Weiss E., Cochior D., Stępień E., Georgescu A. Vascular complications in diabetes: microparticles and microparticle associated microRNAs as active players. Biochem Biophys Res Commun. 2016;472:1–10. doi: 10.1016/j.bbrc.2016.02.038. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y., Chen L.-M., Liu M.-L. Microvesicles and diabetic complications–novel mediators, potential biomarkers and therapeutic targets. Acta Pharmacol Sin. 2014;35:433–443. doi: 10.1038/aps.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr Opin Lipidol 2015;26:412–9. [DOI] [PMC free article] [PubMed]
- 64.Liu M.-L., Williams K.J. Microvesicles: potential markers and mediators of endothelial dysfunction. Curr Opin Endocrinol Diabetes Obes. 2012;19:121–127. doi: 10.1097/MED.0b013e32835057e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang N., Sun B., Gupta A., Rempel H., Pulliam L. Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 2016;30:3097–3106. doi: 10.1096/fj.201600368RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aharon A., Tamari T., Brenner B. Monocyte-derived microparticles and exosomes induce procoagulant and apoptotic effects on endothelial cells. Thromb Haemost. 2008;100:878–885. doi: 10.1160/th07-11-0691. [DOI] [PubMed] [Google Scholar]
- 67.Leroyer A.S., Isobe H., Leseche G. Cellular origins and thrombogenic activity of microparticles isolated from human atherosclerotic plaques. J Am Coll Cardiol. 2007;49:772–777. doi: 10.1016/j.jacc.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 68.Mayr M., Grainger D., Mayr U. Proteomics, metabolomics, and immunomics on microparticles derived from human atherosclerotic plaques. Circ Cardiovasc Genet. 2009;2:379–388. doi: 10.1161/CIRCGENETICS.108.842849. [DOI] [PubMed] [Google Scholar]
- 69.Théry C., Duban L., Segura E., Véron P., Lantz O., Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nat Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 70.Canault M., Leroyer A.S., Peiretti F. Microparticles of human atherosclerotic plaques enhance the shedding of the tumor necrosis factor-alpha converting enzyme/ADAM17 substrates, tumor necrosis factor and tumor necrosis factor receptor-1. Am J Pathol. 2007;171:1713–1723. doi: 10.2353/ajpath.2007.070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu M.-L., Scalia R., Mehta J.L., Williams K.J. Cholesterol-induced membrane microvesicles as novel carriers of damage-associated molecular patterns: mechanisms of formation, action, and detoxification. Arterioscler Thromb Vasc Biol. 2012;32:2113–2121. doi: 10.1161/ATVBAHA.112.255471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoyer F.F., Giesen M.K., Franca C., Lütjohann D., Nickenig G., Werner N. Monocytic microparticles promote atherogenesis by modulating inflammatory cells in mice. J Cell Mol Med. 2012;16:2777–2788. doi: 10.1111/j.1582-4934.2012.01595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zakharova L., Svetlova M., Fomina A.F. T cell exosomes induce cholesterol accumulation in human monocytes via phosphatidylserine receptor. J Cell Physiol. 2007;212:174–181. doi: 10.1002/jcp.21013. [DOI] [PubMed] [Google Scholar]
- 74.Yahagi K., Kolodgie F.D., Otsuka F. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- 75.Weber A., Köppen H.O., Schrör K. Platelet-derived microparticles stimulate coronary artery smooth muscle cell mitogenesis by a PDGF-independent mechanism. Thromb Res. 2000;98:461–466. doi: 10.1016/s0049-3848(00)00192-4. [DOI] [PubMed] [Google Scholar]
- 76.Pakala R. Serotonin and thromboxane A2 stimulate platelet-derived microparticle-induced smooth muscle cell proliferation. Cardiovasc Radiat Med. 2004;5:20–26. doi: 10.1016/j.carrad.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 77.Sarkar A., Mitra S., Mehta S., Raices R., Wewers M.D. Monocyte derived microvesicles deliver a cell death message via encapsulated caspase-1. PLoS One. 2009;4 doi: 10.1371/journal.pone.0007140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krohn J.B., Hutcheson J.D., Martínez-Martínez E., Aikawa E. Extracellular vesicles in cardiovascular calcification: expanding current paradigms. J Physiol (Lond) 2016;594:2895–2903. doi: 10.1113/JP271338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goettsch C., Hutcheson J.D., Aikawa M. Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest. 2016;126:1323–1336. doi: 10.1172/JCI80851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hutcheson J.D., Goettsch C., Bertazzo S. Genesis and growth of extracellular-vesicle-derived microcalcification in atherosclerotic plaques. Nat Mater. 2016;15:335–343. doi: 10.1038/nmat4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Virmani R., Kolodgie F.D., Burke A.P. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–2061. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 82.Leroyer A.S., Rautou P.-E., Silvestre J.-S. CD40 ligand+ microparticles from human atherosclerotic plaques stimulate endothelial proliferation and angiogenesis a potential mechanism for intraplaque neovascularization. J Am Coll Cardiol. 2008;52:1302–1311. doi: 10.1016/j.jacc.2008.07.032. [DOI] [PubMed] [Google Scholar]
- 83.Hoyer F.F., Nickenig G., Werner N. Microparticles—messengers of biological information. J Cell Mol Med. 2010;14:2250–2256. doi: 10.1111/j.1582-4934.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mallat Z., Hugel B., Ohan J., Lesèche G., Freyssinet J.M., Tedgui A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation. 1999;99:348–353. doi: 10.1161/01.cir.99.3.348. [DOI] [PubMed] [Google Scholar]
- 85.Geddings J.E., Mackman N. Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood. 2013;122:1873–1880. doi: 10.1182/blood-2013-04-460139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zwicker J.I., Trenor C.C., Furie B.C., Furie B. Tissue factor-bearing microparticles and thrombus formation. Arterioscler Thromb Vasc Biol. 2011;31:728–733. doi: 10.1161/ATVBAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao L., Bi Y., Kou J., Shi J., Piao D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J Exp Clin Cancer Res. 2016;35:54. doi: 10.1186/s13046-016-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chou J., Mackman N., Merrill-Skoloff G., Pedersen B., Furie B.C., Furie B. Hematopoietic cell-derived microparticle tissue factor contributes to fibrin formation during thrombus propagation. Blood. 2004;104:3190–3197. doi: 10.1182/blood-2004-03-0935. [DOI] [PubMed] [Google Scholar]
- 89.Biró E., Sturk-Maquelin K.N., Vogel G.M.T. Human cell-derived microparticles promote thrombus formation in vivo in a tissue factor-dependent manner. J Thromb Haemost. 2003;1:2561–2568. doi: 10.1046/j.1538-7836.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- 90.Libby P., Sukhova G., Lee R.T., Liao J.K. Molecular biology of atherosclerosis. Int J Cardiol. 1997;62(Suppl 2):S23–S29. doi: 10.1016/s0167-5273(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 91.King S.B., Marshall J.J., Tummala P.E. Revascularization for coronary artery disease: stents versus bypass surgery. Annu Rev Med. 2010;61:199–213. doi: 10.1146/annurev.med.032309.063039. [DOI] [PubMed] [Google Scholar]
- 92.Abid Hussein M.N., Nieuwland R., Hau C.M., Evers L.M., Meesters E.W., Sturk A. Cell-derived microparticles contain caspase 3 in vitro and in vivo. J Thromb Haemost. 2005;3:888–896. doi: 10.1111/j.1538-7836.2005.01240.x. [DOI] [PubMed] [Google Scholar]
- 93.Diamant M., Tushuizen M.E., Abid Hussein M.N. Simvastatin-induced endothelial cell detachment and microparticle release are prenylation dependent. Thromb Haemost. 2008;100:489–497. [PubMed] [Google Scholar]
- 94.Tramontano A.F., O’Leary J., Black A.D., Muniyappa R., Cutaia M.V., El-Sherif N. Statin decreases endothelial microparticle release from human coronary artery endothelial cells: implication for the rho-kinase pathway. Biochem Biophys Res Commun. 2004;320:34–38. doi: 10.1016/j.bbrc.2004.05.127. [DOI] [PubMed] [Google Scholar]
- 95.Nomura S. Statin and endothelial cell-derived microparticles. Thromb Haemost. 2008;100:377–378. [PubMed] [Google Scholar]
- 96.Jansen F., Yang X., Hoyer F.F. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1925–1935. doi: 10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- 97.Mause S.F., Ritzel E., Liehn E.A. Platelet microparticles enhance the vasoregenerative potential of angiogenic early outgrowth cells after vascular injury. Circulation. 2010;122:495–506. doi: 10.1161/CIRCULATIONAHA.109.909473. [DOI] [PubMed] [Google Scholar]
- 98.França C.N., Izar MC de O., Amaral J.B.D., Tegani D.M., Fonseca F.A.H. Microparticles as potential biomarkers of cardiovascular disease. Arq Bras Cardiol. 2015;104:169–174. doi: 10.5935/abc.20140210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pérez-Casal M., Downey C., Cutillas-Moreno B., Zuzel M., Fukudome K., Toh C.H. Microparticle-associated endothelial protein C receptor and the induction of cytoprotective and anti-inflammatory effects. Haematologica. 2009;94:387–394. doi: 10.3324/haematol.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Andaloussi El S., Mäger I., Breakefield X.O., Wood M.J.A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 101.Eldh M., Ekström K., Valadi H. Exosomes communicate protective messages during oxidative stress; possible role of exosomal shuttle RNA. PLoS One. 2010;5 doi: 10.1371/journal.pone.0015353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Diehl P., Fricke A., Sander L. Microparticles: major transport vehicles for distinct microRNAs in circulation. Cardiovasc Res. 2012;93:633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jansen F., Yang X., Hoelscher M. Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles. Circulation. 2013;128:2026–2038. doi: 10.1161/CIRCULATIONAHA.113.001720. [DOI] [PubMed] [Google Scholar]
- 104.Jansen F., Stumpf T., Proebsting S. Intercellular transfer of miR-126-3p by endothelial microparticles reduces vascular smooth muscle cell proliferation and limits neointima formation by inhibiting LRP6. J Mol Cell Cardiol. 2017;104:43–52. doi: 10.1016/j.yjmcc.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 105.Deregibus M.C., Cantaluppi V., Calogero R. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 106.Leroyer A.S., Ebrahimian T.G., Cochain C. Microparticles from ischemic muscle promotes postnatal vasculogenesis. Circulation. 2009;119:2808–2817. doi: 10.1161/CIRCULATIONAHA.108.816710. [DOI] [PubMed] [Google Scholar]
- 107.Lai R.C., Arslan F., Lee M.M. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 108.Vrijsen K.R., Sluijter J.P.G., Schuchardt M.W.L. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med. 2010;14:1064–1070. doi: 10.1111/j.1582-4934.2010.01081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mackie A.R., Klyachko E., Thorne T. Sonic hedgehog-modified human CD34+ cells preserve cardiac function after acute myocardial infarction. Circ Res. 2012;111:312–321. doi: 10.1161/CIRCRESAHA.112.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khan M., Nickoloff E., Abramova T. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res. 2015;117:52–64. doi: 10.1161/CIRCRESAHA.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sahoo S., Losordo D.W. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114:333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 112.Gasser O., Schifferli J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood. 2004;104:2543–2548. doi: 10.1182/blood-2004-01-0361. [DOI] [PubMed] [Google Scholar]
- 113.Dalli J., Norling L.V., Renshaw D., Cooper D., Leung K.-Y., Perretti M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood. 2008;112:2512–2519. doi: 10.1182/blood-2008-02-140533. [DOI] [PubMed] [Google Scholar]
- 114.Köppler B., Cohen C., Schlöndorff D., Mack M. Differential mechanisms of microparticle transfer toB cells and monocytes: anti-inflammatory propertiesof microparticles. Eur J Immunol. 2006;36:648–660. doi: 10.1002/eji.200535435. [DOI] [PubMed] [Google Scholar]
- 115.Mokarizadeh A., Delirezh N., Morshedi A., Mosayebi G., Farshid A.-A., Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147:47–54. doi: 10.1016/j.imlet.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 116.Arslan F., Lai R.C., Smeets M.B. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. doi: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 117.Zou X., Zhang G., Cheng Z. Microvesicles derived from human Wharton's jelly mesenchymal stromal cells ameliorate renal ischemia-reperfusion injury in rats by suppressing CX3CL1. Stem Cell Res Ther. 2014;5:40. doi: 10.1186/scrt428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jansen F., Yang X., Baumann K. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J Cell Mol Med. 2015;19:2202–2214. doi: 10.1111/jcmm.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Njock M.-S., Cheng H.S., Dang L.T. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood. 2015;125:3202–3212. doi: 10.1182/blood-2014-11-611046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hergenreider E., Heydt S., Tréguer K. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 121.Shan Z., Qin S., Li W. An Endocrine genetic signal between blood cells and vascular smooth muscle cells: role of microRNA-223 in smooth muscle function and atherogenesis. J Am Coll Cardiol. 2015;65:2526–2537. doi: 10.1016/j.jacc.2015.03.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Srikanthan S., Li W., Silverstein R.L., McIntyre T.M. Exosome poly-ubiquitin inhibits platelet activation, downregulates CD36 and inhibits pro-atherothombotic cellular functions. J Thromb Haemost. 2014;12:1906–1917. doi: 10.1111/jth.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang Y., Liu D., Chen X. Secreted monocytic miR-150 enhances targeted endothelial cell migration. Mol Cell. 2010;39:133–144. doi: 10.1016/j.molcel.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 124.Chen Y., Zhu X., Zhang X., Liu B., Huang L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol Ther. 2010;18:1650–1656. doi: 10.1038/mt.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Babar I.A., Cheng C.J., Booth C.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc Natl Acad Sci U S A. 2012;109:E1695–E1704. doi: 10.1073/pnas.1201516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Muthiah M., Park I.-K., Cho C.-S. Nanoparticle-mediated delivery of therapeutic genes: focus on miRNA therapeutics. Exp Opin Drug Deliv. 2013;10:1259–1273. doi: 10.1517/17425247.2013.798640. [DOI] [PubMed] [Google Scholar]
- 127.Cheng C.J., Saltzman W.M. Polymer nanoparticle-mediated delivery of microRNA inhibition and alternative splicing. Mol Pharm. 2012;9:1481–1488. doi: 10.1021/mp300081s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Anand S., Majeti B.K., Acevedo L.M. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nouraee N., Mowla S.J. miRNA therapeutics in cardiovascular diseases: promises and problems. Front Genet. 2015;6:232. doi: 10.3389/fgene.2015.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vosen S., Rieck S., Heidsieck A. Improvement of vascular function by magnetic nanoparticle-assisted circumferential gene transfer into the native endothelium. J Control Release. 2016;241:164–173. doi: 10.1016/j.jconrel.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 131.Vosen S., Rieck S., Heidsieck A. Vascular repair by circumferential cell therapy using magnetic nanoparticles and tailored magnets. ACS Nano. 2016;10:369–376. doi: 10.1021/acsnano.5b04996. [DOI] [PubMed] [Google Scholar]
- 132.Hofmann A., Wenzel D., Becher U.M. Combined targeting of lentiviral vectors and positioning of transduced cells by magnetic nanoparticles. Proc Natl Acad Sci U S A. 2009;106:44–49. doi: 10.1073/pnas.0803746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vader P., Mol E.A., Pasterkamp G., Schiffelers R.M. Extracellular vesicles for drug delivery. Adv Drug Deliv Rev. 2016;106:148–156. doi: 10.1016/j.addr.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 134.Lakhal S., Wood M.J.A. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays. 2011;33:737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 135.Andaloussi El S., Lakhal S., Mäger I., Wood M.J.A. Exosomes for targeted siRNA delivery across biological barriers. Adv Drug Deliv Rev. 2013;65:391–397. doi: 10.1016/j.addr.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 136.Tang K., Zhang Y., Zhang H. Delivery of chemotherapeutic drugs in tumour cell-derived microparticles. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. [DOI] [PubMed] [Google Scholar]
- 137.Ran L., Tan X., Li Y. Delivery of oncolytic adenovirus into the nucleus of tumorigenic cells by tumor microparticles for virotherapy. Biomaterials. 2016;89:56–66. doi: 10.1016/j.biomaterials.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 138.Alvarez-Erviti L., Seow Y., Yin H., Betts C., Lakhal S., Wood M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 139.Ohno S.-I., Takanashi M., Sudo K. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21:185–191. doi: 10.1038/mt.2012.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yin M., Loyer X., Boulanger C.M. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol. 2015;763:90–103. doi: 10.1016/j.ejphar.2015.06.047. [DOI] [PubMed] [Google Scholar]
- 141.Witwer K.W., Buzás E.I., Bemis L.T. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Coumans F.A.W., Brisson A.R., Buzás E.I. Methodological Guidelines to Study Extracellular Vesicles. Circ Res. 2017;120:1632–1648. doi: 10.1161/CIRCRESAHA.117.309417. [DOI] [PubMed] [Google Scholar]
- 143.Stremersch S., De Smedt S.C., Raemdonck K. Therapeutic and diagnostic applications of extracellular vesicles. J Control Release. 2016;244:167–183. doi: 10.1016/j.jconrel.2016.07.054. [DOI] [PubMed] [Google Scholar]
- 144.Momen-Heravi F., Balaj L., Alian S. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front Physiol. 2012;3:162. doi: 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kalra H., Adda C.G., Liem M. Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. doi: 10.1002/pmic.201300282. [DOI] [PubMed] [Google Scholar]
- 146.Lobb R.J., Becker M., Wen S.W. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mateescu B., Kowal E.J.K., van Balkom B.W.M. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J Extracell Vesicles. 2017;6:1286095. doi: 10.1080/20013078.2017.1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Böing A.N., van der Pol E., Grootemaat A.E., Coumans F.A.W., Sturk A., Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ridger V.C., Boulanger C.M., Angelillo-Scherrer A. Microvesicles in vascular homeostasis and diseases. Position paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost. 2017;117:1296–1316. doi: 10.1160/TH16-12-0943. [DOI] [PubMed] [Google Scholar]
- 150.Brisson A.R., Tan S., Linares R., Gounou C., Arraud N. Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study. Platelets. 2017;28:263–271. doi: 10.1080/09537104.2016.1268255. [DOI] [PubMed] [Google Scholar]
- 151.Coumans F.A.W., van der Pol E., Böing A.N. Reproducible extracellular vesicle size and concentration determination with tunable resistive pulse sensing. J Extracell Vesicles. 2014;3:25922. doi: 10.3402/jev.v3.25922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.van der Pol E., Coumans F.A.W., Grootemaat A.E. Particle size distribution of exosomes and microvesicles determined by transmission electron microscopy, flow cytometry, nanoparticle tracking analysis, and resistive pulse sensing. J Thromb Haemost. 2014;12:1182–1192. doi: 10.1111/jth.12602. [DOI] [PubMed] [Google Scholar]
- 153.Lötvall J., Hill A.F., Hochberg F. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. Extracellular vesicles in angiogenesis. Circ Res. 2017;120:1658–1673. doi: 10.1161/CIRCRESAHA.117.309681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Atay S., Gercel-Taylor C., Taylor D.D. Human trophoblast-derived exosomal fibronectin induces pro-inflammatory Il-1β production by macrophages. Am J Reprod Immunol. 2011;66:259–269. doi: 10.1111/j.1600-0897.2011.00995.x. [DOI] [PubMed] [Google Scholar]
- 157.Fitzner D., Schnaars M., van Rossum D. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124:447–458. doi: 10.1242/jcs.074088. [DOI] [PubMed] [Google Scholar]
- 158.Tian T., Zhu Y.-L., Hu F.-H., Wang Y.-Y., Huang N.-P., Xiao Z.-D. Dynamics of exosome internalization and trafficking. J Cell Physiol. 2013;228:1487–1495. doi: 10.1002/jcp.24304. [DOI] [PubMed] [Google Scholar]
- 159.Christianson H.C., Svensson K.J., van Kuppevelt T.H., Li J.-P., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc Natl Acad Sci U S A. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bang C., Batkai S., Dangwal S. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest. 2014;124:2136–2146. doi: 10.1172/JCI70577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qu L., Ding J., Chen C. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 162.Tkach M., Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164:1226–1232. doi: 10.1016/j.cell.2016.01.043. [DOI] [PubMed] [Google Scholar]
- 163.Van Deun J. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]
- 164.EV-TRACK Consortium. Van Deun J., Mestdagh P. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods. 2017;14:228–232. doi: 10.1038/nmeth.4185. [DOI] [PubMed] [Google Scholar]