Lafora disease (LD) is a teenage-onset progressive neurologic disorder caused by biallelic mutations in EPM2A (laforin) or EPM2B (malin) encoding laforin phosphatase and malin ubiquitin E3 ligase, respectively, both involved in glycogen structural integrity. Defective laforin or malin results in neuronal accumulation of malformed insoluble glycogen termed Lafora bodies (LBs).1 Histologic reports indicate LB accumulation in the inner retinal layers with evidence of bipolar cell atrophy.2 Funduscopic retinal examination has been reported unremarkable except in one patient with unilateral optic atrophy that may have been unrelated to LD.3,4 Recently, retinitis pigmentosa was reported in a 21-year-old patient diagnosed only on skin biopsy, and was highlighted on the journal cover.5 A subsequent letter to the editor suggested that this may represent an instance of false-positive interpretation of skin biopsy, but the debate remains unsettled.1,5,6 We aimed to address this issue by performing multimodal imaging and electrophysiology (e-Methods in links.lww.com/WNL/A580) to characterize the eye phenotype in 4 patients with genetically confirmed LD, concurrently setting the stage to identify potentially useful ophthalmologic biomarkers in LD.
e-Methods and tables e-1 and e-2, links.lww.com/WNL/A578, summarize the genetic results and clinical phenotype of the patients.
Patient 1
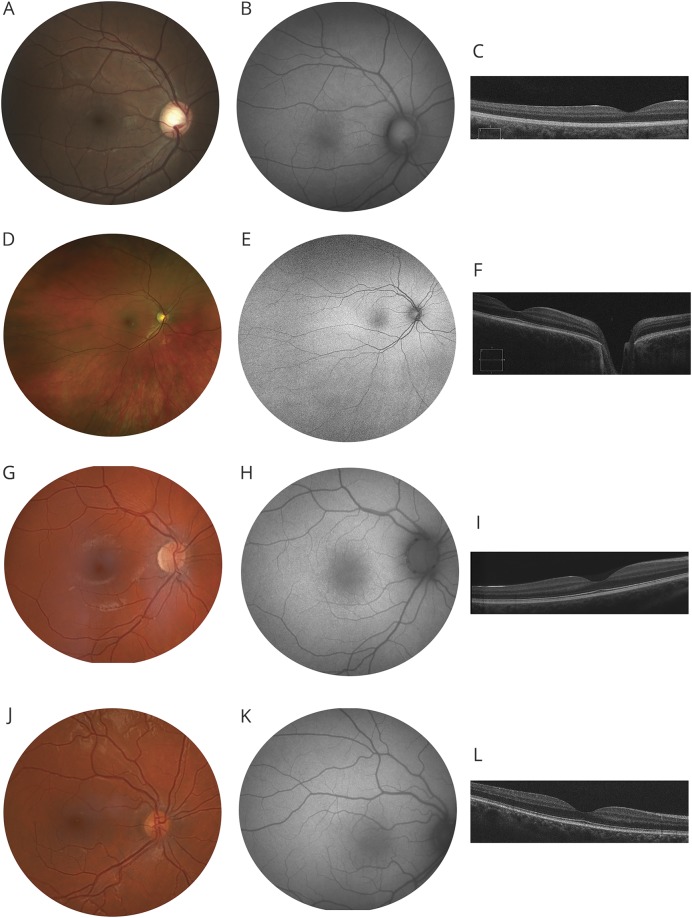
This 21-year-old man (homozygous deletion of EPM2A exons 1 and 2) had first seizure episode at 14 years of age. Best-corrected visual acuity (BCVA) was 20/40 (right eye) and 20/25 (left eye). Retinal examination, fundus autofluorescence (FAF) imaging, and central retinal thickness and lamination were normal (figure, A–C). Full-field electroretinogram (ERG) testing showed rod bipolar cell impairment with mild cone system dysfunction (figure e-1, links.lww.com/WNL/A579).
Figure. Retinal imaging findings in Lafora disease.
(A–C) Images from the right eye of patient 1. (A) Normal fundus photograph. (B) Normal fundus autofluorescence (FAF) image suggests normal lipofuscin levels in the retinal pigment epithelium. (C) Spectral-domain optical coherence tomography (SD-OCT) single line scan through the macula shows normal central retinal thickness and lamination. (D–F) Images from the right eye of patient 2. Fundus and FAF show a wider field of the retina. As described in case 1, fundus, FAF and SD-OCT (D–F) are normal. (G–I) Images from the right eye of patient 3. Fundus, FAF, and SD-OCT (G–I) are normal. (J–L) Images from the right eye of patient 4. Fundus, FAF, and SD-OCT (J–L) are normal.
Patient 2
A 22-year-old woman had first seizure at 14 years of age and harbored compound heterozygous EPM2A mutations (p.Asn163Asp/p.Ala254Metfs*33). BCVA was 20/40 bilaterally. Retinal examination, FAF, and central retinal thickness and lamination were normal (figure, D–F). The ERG showed mild cone system dysfunction; rod function was normal (figure e-1, links.lww.com/WNL/A579).
Patient 3
A 21-year-old woman with homozygous EPM2B missense mutations (p.Pro69Ala) had seizure onset at 14 years of age. BCVA was 20/25 bilaterally with refractive correction of −3.50 D sphere. Retinal examination, FAF, and central retinal thickness and lamination were normal (figure, G–I). The ERG showed rod photoreceptor and bipolar cell impairment with additional cone system dysfunction (figure e-1, links.lww.com/WNL/A579).
Patient 4
A 31-year-old man with homozygous EPM2B missense mutations (p.Asp308Val) had seizure onset at 16 years. BCVA was 20/25 in either eye with refractive correction of −1.00 D sphere. Retinal examination, FAF, and central retinal structure were normal (figure, J–L). The ERG showed rod photoreceptor and bipolar cell impairment with additional cone system dysfunction (figure e-1, links.lww.com/WNL/A579).
Discussion
Newer noninvasive in vivo retinal imaging techniques and electrodiagnostic testing better aid in disease diagnosis and characterization; notably, considering the eyes as windows to the brain, there is marked interest in in vivo retinal imaging in neurodegenerative disorders to identify biomarkers of early disease.
All our patients with LD demonstrated good visual acuity (20/40 or better) and normal retinal examination (including optic disc); none had retinitis pigmentosa. FAF, a noninvasive retinal imaging technique that provides in vivo density map of fluorophores in the retinal pigment epithelium, demonstrated normal pattern in all 4 patients; this observation excludes retinal pigment epithelial atrophy and any significant structural abnormality of the photoreceptor outer segments in the central retina (50°). We did not detect any structural alterations in the macula on spectral-domain optical coherence tomography (in vivo imaging that provides ∼5 μm axial resolution); a prior histologic report observed LB deposition in inner retinal layers of macula.2
Our study revealed abnormal retinal function on ERG testing in all patients. Generalized rod bipolar cell dysfunction as evidenced by reduced b/a ratio of scotopic ERG was present in 3, and generalized cone system dysfunction with cone inner retinal (bipolar) involvement was observed in all 4 patients. The bipolar cell dysfunction observed on ERG is in keeping with the histologic bipolar cell atrophy described in LD.2 The ERG also demonstrated generalized reduction in rod photoreceptor function (decreased rod a-wave amplitude) in both EPM2B patients. Although no abnormality was noted in photoreceptor layers in postmortem eyes (genes unknown), the observed rod a-wave changes in EPM2B patients may indicate functional changes to precede structural changes in specific disease stages or may be unique to EPM2B.2 Reports using early ERG techniques (1978 or earlier) suggested normal responses or transiently subnormal bipolar cell activity in LD3,7; the current techniques are superior, and can better delineate the locus of abnormality.
ERG may be a valuable tool to monitor LD progression, and potentially serve as a useful biomarker to investigate intervention efficacy in gene replacement or other therapeutic clinical trials.
Acknowledgment
The authors thank the families for their participation; Dr. Elise Heon, Toronto, for facilitating collaboration and providing comments; and Associazione Italiana Lafora for support.
Author contributions
Ajoy Vincent: study concept and design, acquisition of data, analysis and interpretation, wrote the draft manuscript. Angelo Macrì: acquisition of data, critical revision of the manuscript for important intellectual content. Anupreet Tumber: acquisition of data, critical revision of the manuscript for important intellectual content. Nikolas Koukas: acquisition of data, critical revision of the manuscript for important intellectual content. Saija Ahonen: analysis and interpretation, critical revision of the manuscript for important intellectual content. Pasquale Striano: acquisition of data, critical revision of the manuscript for important intellectual content. Berge Minassian: study concept and design, study supervision, critical revision of the manuscript for important intellectual content.
Study funding
This work was supported by the Ontario Brain Institute, Foundation Fighting Blindness USA, and the National Institute of Neurological Disorders and Stroke of the NIH under award number P01 NS097197. B.A.M. holds the Jimmy Elizabeth Wescott Chair in Pediatric Neurology at the University of Texas Southwestern.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Andrade DM, Ackerley CA, Minett TS, et al. Skin biopsy in Lafora disease: genotype-phenotype correlations and diagnostic pitfalls. Neurology 2003;61:1611–1614. [DOI] [PubMed] [Google Scholar]
- 2.Berard-Badier M, Pellissier JF, Gambarelli D, de Barsy T, Roger J, Toga M. The retina in Lafora disease: light and electron microscopy. Albrecht Von Graefes Arch Klin Exp Ophthalmol 1980;212:285–294. [DOI] [PubMed] [Google Scholar]
- 3.Van Heycop Ten Ham M. Lafora disease: a form of progressive myoclonus epilepsy. In: Magnus WOC, Haas AMLd, eds. The Epilepsies. Amsterdam, NY: North Holland Pub. Co.; American Elsevier; 1974: xiv, 860 p. [Google Scholar]
- 4.de Graaf AS, Ancker E, Rutherfoord GS, van der Walt JJ, Rossouw DJ. Lafora-body disease with optic atrophy, macular degeneration and cardiac failure. J Neurol Sci 1989;93:69–84. [DOI] [PubMed] [Google Scholar]
- 5.Pinto WB, de Souza PV, Pinheiro JR, Okamoto KY, Enokihara MM, Oliveira AS. Retinitis pigmentosa in Lafora disease: expanding findings of progressive myoclonic epilepsy. Neurology 2015;85:1087. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter S, de Rezende Pinto WB, de Souza PV, Oliveira AS. Retinitis pigmentosa in Lafora disease: expanding findings of progressive myoclonic epilepsy. Neurology 2016;86:1563. [DOI] [PubMed] [Google Scholar]
- 7.Korczyn AD, Ben-Tovim N. Electroretinographic responses in Lafora disease. Electroencephalogr Clin Neurophysiol 1978;45:785–788. [DOI] [PubMed] [Google Scholar]