ABSTRACT
N6-(2-Hydroxyethyl)-adenosine (HEA), which was the first calcium antagonist derived from biological sources, has been intensively investigated because of its ability to inhibit tumour cell proliferation, restrain inflammation, protect kidneys and function as a sedative and insecticide. Up to now, the production of HEA has been detected only in a few species such as Cordyceps pruinosa, C. militaris and Isaria tenuipes. Here, we found a new HEA-producing fungus, which was identified as Beauveria bassiana based on morphological and phylogenetic characteristics. The HEA production was verified by reversed-phase high-performance liquid chromatography/mass spectrometry in this fungus. Moreover, HEA was also detected in the mycelia of two other B. bassiana strains from different origins, but not in the culture medium of all tested B. bassiana strains. The maximum production of HEA (0.8483 ± 0.0439 mg/g mycelia dry weight) was achieved on day 7. The fungal biomass and kinetics of HEA production exhibited similar trends over the duration of the culture period. This is the first report describing HEA production in B. bassiana, which suggests that this fungal strain may have new applications as a source of HEA.
KEYWORDS: Nucleosides, N-(2-hydroxyethyl)-adenosine, Beauveria bassiana, Cordyceps
Introduction
Nucleosides are important for regulating diverse physiological processes in human and for preventing or treating diseases (Hu and Yang 2014). Additionally, nucleosides, including adenosine, cordycepin and N6-(2-hydroxyethyl)-adenosine (HEA), represent the major active components in Cordyceps sensu lato (Gong et al. 2004; Li et al. 2006). HEA was the first calcium antagonist derived from biological sources, and can be used as an inotropic agent (Furuya et al. 1983). Recent studies have revealed HEA has various biological activities. For example, it can inhibit the proliferation of tumour cells in vitro, including Lewis lung cancer and K562 erythroleukemia cells (Lu et al. 2013; Zhu et al. 2013). HEA regulates cerebral and coronary circulation and appears to function as a sedative in pharmacological tests (Wang et al. 2013). The mechanism underlying the analgesic activity of HEA differs from opioids, a widely used drug for analgesic. More importantly, HEA is neither addictive nor affected by pepsin, which is the advantage over opioids (Chai et al. 2004). Peng et al. (2015) reported that HEA protected mice from renal ischemia reperfusion injury. Studies confirmed that HEA and human serum albumin interacted to form a complex by hydrophobic interaction (Cui et al. 2007; Meng et al. 2015b). HEA also attenuated lipopolysaccharide-induced pro-inflammatory responses by suppressing the toll-like receptor 4-mediated nuclear factor-κB signalling pathway (Lu et al. 2015). A recent study revealed that HEA exhibited insecticidal activity against Plutella xylostella larvae by targeting an adenosine receptor (Fang et al. 2016), suggesting an environmentally friendly pesticide (Chai et al. 2015).
HEA was firstly isolated from Cordyceps species. Furuya et al. (1983) tested for the presence of Ca2+ antagonists and inotropic agents in the water–ethanol extracts from mycelia of 17 Cordyceps species and 6 Isaria species using the left atrium of a guinea pig heart in an in vitro system. The mycelial extracts from two Cordyceps species (i.e. C. pruinosa and C. coccidiiocola) and four Isaria species (i.e. I. japonica and three incertae sedis species) were highly active for inotropic. This active substance was identified as HEA. The production of HEA by C. pruinosa mycelia in static liquid and solid-state cultures was verified by high-performance liquid chromatography/mass spectrometry (HPLC-MS) (Meng et al. 2014).
HEA has also been identified in several Cordyceps sensu lato species, such as I. cicadae, C. militaris and I. tenuipes. Content of HEA in I. cicadae mycelia cultured in barley was higher than those cultured in other substrates (Ke and Lee 2016). C. militaris mycelia and fruiting bodies produce HEA (Zhu et al. 2013; Zhang et al. 2016), as does I. tenuipes grown under submerged culture conditions (Liu et al. 2012; Lei et al. 2014). It was reported that the amount of HEA in Ophiocordyceps sinensis (Berk.) G. H. Sung, J. M. Sung, Hywel-Jones & Spatafora [≡ C. sinensis (Berk.) Sacc.] is a reliable indicator of the overall health benefits of the medicinal fungus (Phillip et al. 2008). However, HEA production in the stroma and submerged mycelia of O. sinensis has not been detected (Liu et al. 2017).
About 538 Cordyceps sensu lato species have been identified by now (http://www.indexfungorum.org/names/Names.asp, 10 July 2017). However, HEA has been detected in only a few species such as C. pruinosa, C. militaris and I. tenuipes. Consequently, identifying new sources of HEA from Cordyceps sensu lato species is warranted (Liu et al. 2017). In this study, we detected HEA in the entomopathogenic fungi collected in our laboratory and found a new HEA-producing fungus, which was identified as Beauveria bassiana. We monitored the HEA production in a liquid culture of B. bassiana. This is the first report of B. bassiana producing HEA, suggesting a new source of HEA.
Materials and methods
Fungal strains and culture conditions
More than 20 entomogenous fungal strains collected in our laboratory were used to screen HEA-producing fungi. These strains were maintained on potato dextrose agar (PDA) at 4°C. They were inoculated in the same medium in Petri dishes and incubated at 20°C or 25°C for 7–15 days. Liquid cultures were subsequently prepared by inoculating 80 mL potato dextrose broth (PDB) in 250-mL Erlenmeyer flasks with 5-mm mycelial plugs. The flasks were incubated at 20°C or 25°C with shaking (150 rpm) for 7–15 days.
Screening for HEA-producing fungi
Samples were extracted by deionised water using the ultrasonic extraction method for 0.5 h and filtered through a 0.45-mm filter membrane prior to injection into the HPLC system.
The production of HEA was analysed by reversed-phase HPLC using a Waters 2695 Separations Module (Waters Corporation, Milford, CT, USA) equipped with a built-in quaternary pump, a 120 autosampler, the Waters 2998 Photodiode Array Detector and the Empower program for analysing data. A pre-packed Puritex C18 column (4.6 × 250 mm, 5 μm particle size; Beijing Greenherbs Science and Technology Development Co. Ltd., Beijing, China) was used. Samples were eluted in the mobile phase, which consisted of acetonitrile: double-distilled H2O (0.1% acetic acid) (5:95), for 20 min at 25°C. The flow rate was 1 mL/min and the injection volume was 10 μL. Nucleosides were monitored and quantified at 260 nm. Commercially available adenosine (Sigma, Munich, Germany) and HEA (Shanghai PureOne Biotechnology, Pudong, Shanghai) were dissolved in distilled water and used as calibrators.
Verification of HEA production
The production of HEA was verified by HPLC-MS. An Agilent 6520 Accurate-Mass Quadrupole Time-of-Flight mass spectrometer (Santa Clara, CA, USA) was equipped with an electrospray ionisation interface. Peaks were detected in the positive ionisation mode. The HPLC-MS conditions were as follows: capillary voltage, 3500 V; drying gas temperature, 300°C; drying gas, 11.0 L/min; fragmentor voltage, 130 V; and nebuliser pressure, 30 psi. Data were collected on an HP ChemStation. Spectra were scanned over the mass range 80–2000 m/z at 1.03 spectra/s. The HEA standard was dissolved in distilled water and used as a calibrator.
Identification of an HEA-producing strain
Strains were identified based on morphological characteristics and phylogenetic analysis. The HEA-producing fungal strain 351 was grown on malt extract agar (MEA; 20 g malt extract, 1.0 g peptone, 20 g glucose, 20 g agar and 1 L distilled water) and PDA. Morphological characteristics were examined using a Nikon Eclipse 80i microscope (Nikon Instruments Inc., Tokyo, Japan) and a preliminary identification was made based on fungal colony morphology and spore characteristics.
For strain identification, genomic DNA was prepared with the cetyltrimethylammonium bromide method (Doyle and Doyle 1987) and the Internal Transcribed Spacer (ITS1-5.8S-ITS2) of the nuclear ribosomal DNA was amplified. The nucleotide sequence has been deposited in GenBank under accession number MF417575 for strain 351.
Phylogenetic analysis based on the ITS sequence was performed to ascertain the identity of the strain. Reference sequences downloaded from GenBank and the sequences obtained in this study were aligned with Clustal X program. Maximum-likelihood analysis was performed using Mega 6.06 with Kimura’s 2-parameter model (Tamura et al. 2013). Bootstrap analyses were conducted with 1000 replicates. Trees were figured in FigTree v1.4.2, with bootstrap values greater than 50% provided. The ITS sequences of Aspergillus terreus (GU256759.1) and A. fumigatus (KU612366.1) were used as out groups.
Determination of HEA production by the other B. bassiana strains
Two other B. bassiana strains were analysed for HEA production, namely CGMCC 3.3575 (from the China General Microbiological Culture Collection Center) and strain 627 (a gift from Prof. Xingzhong Liu, Institute of Microbiology, Chinese Academy of Sciences). These two B. bassiana strains were cultured for 7 days at 25°C in enriched PDB medium (200 g potato, 3.0 g peptone, 20 g glucose, 1.0 g KH2PO4, 0.5 g MgSO4 and 1 L distilled water). The mycelia were pelleted by centrifuging the cultures at 4500 rpm for 15 min. The mycelial pellets were washed with distilled water and dried at 45°C to a constant weight, after which the mycelial biomass was determined. The mycelium and culture medium were analysed for HEA content. All experiments were conducted in triplicate.
HEA production curve during the liquid culture
Twenty-four Erlenmeyer flasks were used to monitor HEA production in liquid cultures. The 250-mL flasks containing 80 mL medium were inoculated with a seed culture (10% volume) and then incubated in darkness with shaking (150 rpm). The mycelia from three flasks were harvested every 2 days to determine the mycelia dry weight and measure the HEA content.
Statistical analysis
The data are expressed as mean ± SD and were analysed by one-way analysis of variance. Significant differences were determined by Duncan’s multiple range tests (P < 0.05). Data analyses were completed with SPSS 23.0 (SPSS Inc., Chicago, IL, USA) and OriginPro 8.5 (OriginLab Corporation, Northampton, MA, USA).
Results
Identification and verification of HEA production
Of the tested strains, only strain 351 isolated from a Clitocybe sp. fruiting body was detected to produce HEA by HPLC spectrogram. The HPLC analysis of the water extract for the strain 351 mycelium revealed a peak at a retention time of 13.533 min (Figure 1(b)), which was consistent with the retention time of the peak for the HEA standard (13.724 min; Figure 1(a)). The strain 351 HEA content was 0.8483 ± 0.0439 mg/g mycelia dry weight.
Figure 1.
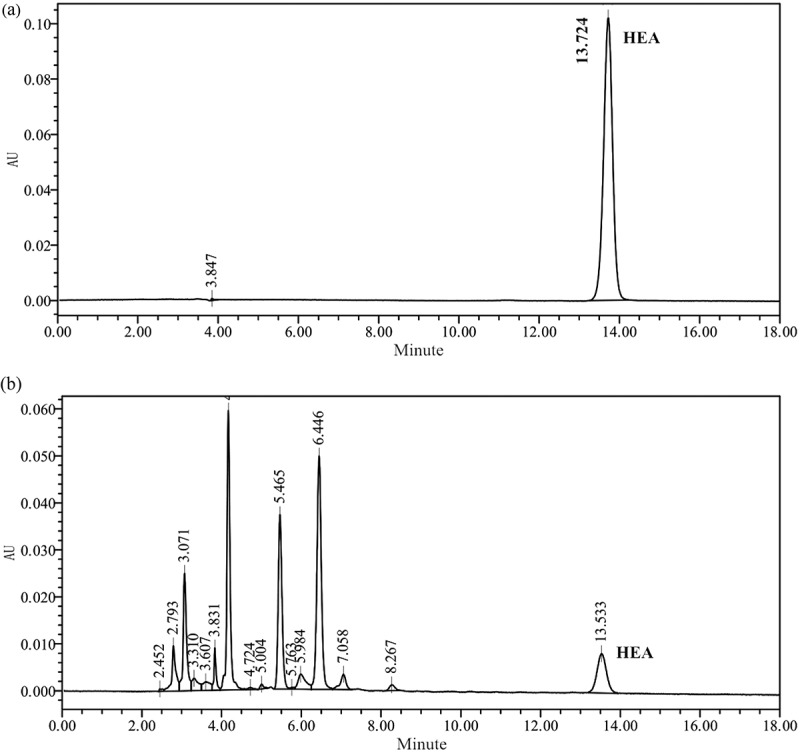
HPLC chromatograms of HEA standard solution and the water extract from mycelium of strain 351.
Samples were eluted in the mobile phase, which consisted of acetonitrile: double-distilled H2O (0.1% acetic acid) (5:95), for 20 min at 25°C. The flow rate was 1 mL/min and the injection volume was 10 μL. HEA was monitored and quantified at 260 nm.
The production of HEA by strain 351 was verified by LC-MS. The mass fragmentation pattern of the peak at the retention time of 13.533 min is presented in Figure 2(a), with the m/z spectrum of the peak revealing dominant ions [M + H]+ at 312.1 under positive ionisation conditions (Figure 2(a)). Thus, the deduced molecular weight was 311, which was similar to the molecular weight of the HEA standard (Figure 2(b)).
Figure 2.
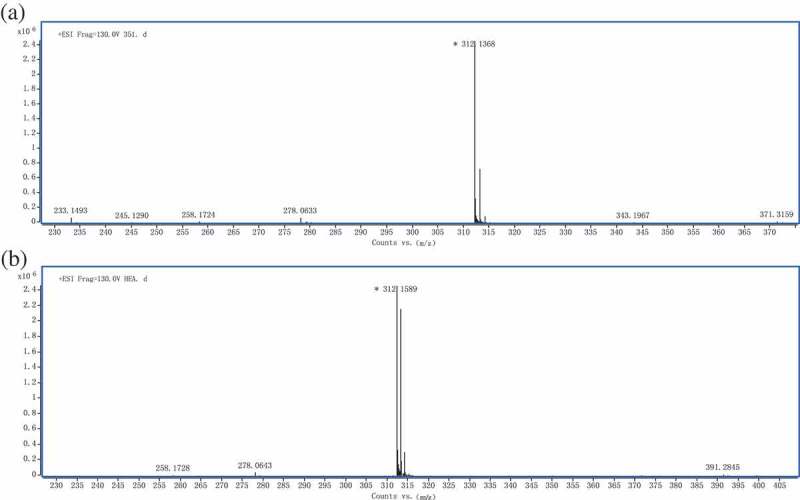
Electrospray mass spectra of HEA isolated from strain 351 and standard solution of HEA.
Spectra were scanned over the mass range 80–2000 m/z at 1.03 spectra/s. The mass fragmentation pattern of the peak at the retention time of 13.533 min is presented with the m/z spectrum of the peak revealing dominant ions [M + H]+ at 312.1 under positive ionisation conditions.
Identification of the HEA-producing strain
The morphological characteristics of the HEA-producing strain 351 colonies were observed on MEA and PDA (Figure 3). The mycelia growth rates were approximately 0.325 mm/day. The strain 351 mycelium generally appeared densely cottony to flocculent, and grew as a yellowish–white, raised and dome-shaped mould on PDA (Figure 3(a)). In contrast, the mycelium on MEA seemed thicker or more swollen. Mycelial fragments were placed on glass slides and a subsequent microscopic observation indicated the hyphae diameter were about 1.2–3.1 μm (Figure 3(b)). The hyphae were branched and formed bottle-like conidiogenous cells with a small neck. The branches were 8.0–23.0 μm long and 1.0–2.0 μm wide (Figure 3(b)). Conidia were oval to subglobose and apiculate, and were 2.0–4.5 μm or (2.0–5.0) × (2.5–4.5) μm (Figure 3(b)). Additionally, strain 351 produced considerably more conidia on MEA than on PDA.
Figure 3.
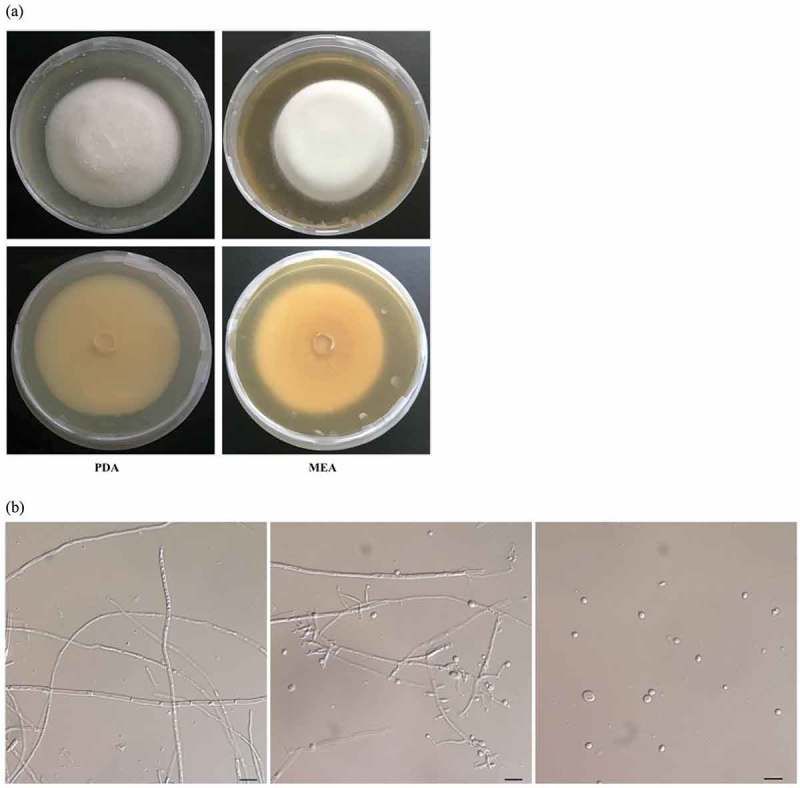
Morphology of the HEA-producing strain 351 (CGMCC 3.18729).
(a) Morphology of colonial growth on MEA and PDA medium.The plates in the top show the front view and bottom show the reverse view of the growth after incubation for 10 days at 25°C.(b) Microscopic features of strain 351 taken from MEA showing hypha, conidiophore and conidia (scale bar = 10 μm). Strain 351 was deposited in China General Microbiological Culture Collection Center (CGMCC 3.18729).
In addition to morphological characteristics in cultures, a molecular method was used to confirm the strain identity. The ITS1–5.8S–ITS2 nuclear ribosomal DNA sequence was amplified for the cultures. The BLAST searches of the NCBI nucleotide database indicated that ITS sequence of strain 351 exhibited a 100% similarity with B. bassiana isolate ArgB14 (KT378231.1). In the phylogenetic analyses (Figure 4), strain 351 formed a clade with B. bassiana isolate ArgB14 (KT378231.1) and EABb08_03po (KC753379.1) with 100% bootstrap support. These results confirmed that strain 351 was a B. bassiana strain. The fungus was deposited in the collection maintained by the China General Microbiological Culture Collection Center (CGMCC 3.18729).
Figure 4.
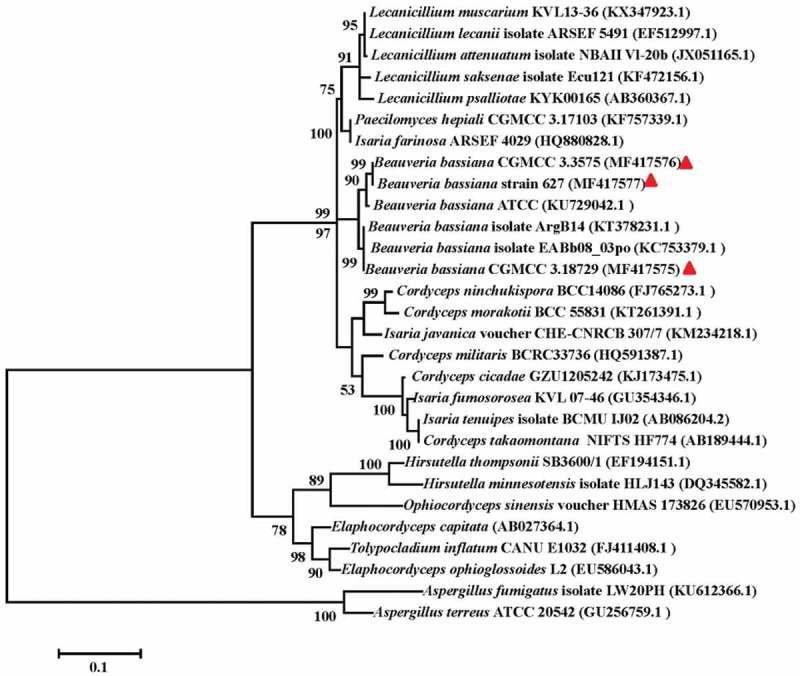
Phylogenetic relationship of CGMCC 3.18729 and the other related species inferred from analysis of ITS rDNA sequences.
The numbers at each node represent the percentage of bootstrap support calculated from 1000 replicates. The sequences of strain CGMCC 3.18729, CGMCC 3.3575 and strain 627 indicated with solid triangle were generated in this study, while others were retrieved from GenBank. The ITS sequences of Aspergillus terreus (GU256759.1) and A. fumigatus (KU612366.1) were used as the out groups.
Production of HEA in other B. bassiana isolates
To ascertain whether the other analysed B. bassiana strains also produce HEA, strain 627 and CGMCC 3.3575 were cultured in enriched PDB medium, after which the mycelial extract and culture medium were analysed by HPLC. The HEA content was 0.0089 ± 0.0000 and 0.2673 ± 0.0009 mg/g mycelia dry weight for strain 627 and CGMCC 3.3575, respectively. No HEA was detected in the fermentation broth of all the three tested B. bassiana strains. However, adenosine was detected in the mycelia and culture medium for all tested strains (Table 1). The identities of strain 627 and CGMCC 3.3575 were verified by phylogenetic analyses based on the ITS sequences (Figure 4).
Table 1.
Adenosine and HEA production by different strains of Beauveria bassiana.
| Strain number | Adenosine (mg/g)a | HEA (mg/g) | |
|---|---|---|---|
| CGMCC 3.18729 (strain 351) |
Mycelium | 2.9149 ± 0.0254 | 0.8483 ± 0.0439 |
| Broth | 1.8027 ± 0.2113 | 0 | |
| CGMCC 3.3575 | Mycelium | 2.6194 ± 0.0195 | 0.2673 ± 0.0009 |
| Broth | 1.2325 ± 0.0886 | 0 | |
| Strain 627 | Mycelium | 2.2000 ± 0.1790 | 0.0089 ± 0.0000 |
| Broth | 0.2128 ± 0.1071 | 0 |
aThe unit of adenosine content in broth is μg/ml. “g” indicates mycelia dry weight.
HEA production curve during the liquid culture of strain CGMCC 3.18729
To study kinetics of HEA production, a typical time course analysis of strain CGMCC 3.18729 grown in a liquid culture was conducted in Erlenmeyer flasks. In submerged cultures, the highest mycelia dry weight (i.e. 12.78 g/L) was accumulated on day 7 after inoculation (Figure 5), while the stationary phase lasted 4 days before shifting to the death phase. An analysis of the kinetics of HEA content revealed a similar time course as that for the biomass production during the culture. The production of HEA was highest on day 7 (i.e. 0.8483 ± 0.0439 mg/g mycelia dry weight) and then decreased slightly by the end of the growth period. Meanwhile, adenosine production decreased over the growth period, with a maximum production of 3.1625 ± 0.1463 mg/g mycelia dry weight on day 3.
Figure 5.
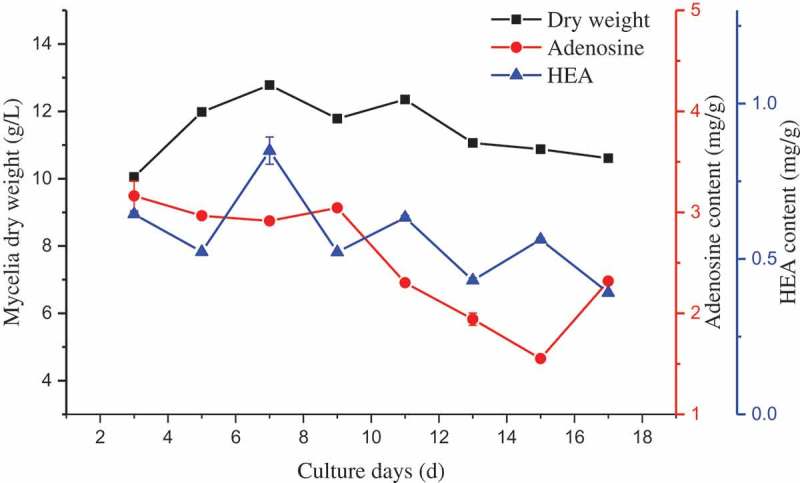
Mycelial biomass and HEA production by strain CGMCC 3.18729 during the liquid culture.
The culture was performed in 25°C with 150 rpm for 17 days. The mycelia from three flasks were harvested every 2 days to determine the mycelia dry weight and measure the HEA content.
Discussion
HEA was originally isolated from cultured mycelia of Cordyceps species. There has been increasing interest in HEA because of its potential medicinal properties, including its ability to inhibit tumour cell proliferation, prevent inflammation, protect kidneys and function as a sedative. Because the production of HEA has been detected only in some Cordyceps species, searching for new HEA-producing resources is warranted. In the present study, we observed HEA production in B. bassiana under submerged culture conditions. It will provide a new HEA-producing resource and may explore a new application for B. bassiana in future.
B. bassiana is a ubiquitous fungal pathogen of insects which is used to control a variety of insect pests as a biocontrol agent (Singh et al. 2015). B. bassiana is also widely used to treat infantile convulsions, epilepsy, stroke, sore throat and different types of wounds (Madsen et al. 2007). The fungal species produces a plethora of secondary metabolites such as bassiacridin, beauvericin, bassianolide and bassianolone (Singh et al. 2015). Additionally, C. bassiana, the teleomorph of B. bassiana, produces nucleosides, such as 1,9-dimethylauanine, adenosine, uridine, 3-methyluracil and 1,7-dimethylxanthine (Park et al. 2013; Suh et al. 2017). In this study, we found that B. bassiana also produce HEA.
The HEA content in the submerged mycelia of B. bassiana strain CGMCC 3.18729 was analysed by HPLC-MS. Moreover, the mycelial HEA contents were examined for two other strains, namely strain 627 and CGMCC 3.3575. The HEA concentration in CGMCC 3.18729 was 0.8483 ± 0.0439 mg/g mycelia dry weight, which is much higher than that reported for I. cicadae (Liu et al. 2012) and C. pruinosa (Meng et al. 2014). Because HEA is associated with several bioactivities, new applications for B. bassiana may be developed in the future.
In this study, we detected HEA in the B. bassiana mycelium, but not in the culture medium, unlike adenosine, which was detected either in the mycelia or the culture media. Similarly, HEA has been detected in the mycelia of other Cordyceps species, including I. cicadae, C. pruinosa and I. tenuipes, with very little in the culture media (Li 2011). These results imply that HEA is an intracellular compound.
Some Cordyceps species have been used as traditional Chinese medicine for centuries and the manufacturing of Cordyceps-derived products represents a major industry in China (Dong et al. 2015, 2016). Adenosine, 3′-deoxyadenosine (cordycepin) and HEA are the major active constituents of Cordyceps species. Unlike adenosine, which is widely distributed among Cordyceps species (Liu et al. 2012), cordycepin and HEA have been detected in only some Cordyceps species. Cordycepin is produced only by C. militaris (Meng et al. 2015a), while HEA has been detected in C. militaris, C. pruinosa, I. tenuipes and B. bassiana. Obviously, there is no correlation between cordycepin and HEA production. Furthermore, the cordycepin biosynthesis pathway, with adenosine as the starting substrate, has been characterised (Xia 2014). We are currently attempting to clarify the HEA biosynthesis pathway.
Conclusion
In summary, we identified a new HEA-producing fungal strain, which was subsequently identified as B. bassiana based on morphological and phylogenetic analyses. The mycelia of two other B. bassiana strains from different origins were also detected HEA production. The fungal biomass and kinetics of HEA production exhibited similar trends over the culture period. To the best of our knowledge, this is the first report describing HEA production in a B. bassiana strain. This new source of HEA may lead to novel applications for B. bassiana.
Funding Statement
This study was funded by the National Natural Science Foundation of China [31572179], [31600054], Coal-based Key Scientific and Technological Project from Shanxi Province [FT2014-03-01] and Key Research and Development Program from Guangxi Province [2016AB05317].
Acknowledgements
We are grateful to Prof. Xingzhong Liu (Institute of Microbiology, Chinese Academy of Sciences) for providing B. bassiana strain 627. We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English text of a draft of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chai YQ, Chen GJ, Jin YW, Liu YG, Li XL, Dang XL, 2015. Use of N6-(2-hydroxyethyl)-adenosine in preparation of crop pesticide. USA, US9198431B2 2015 Dec 01.
- Chai YQ, Wei ZM, Chen ZA, Li XL, Liu YG, Wang GE, 2004. N6-(2-hydroxyethyl)-adenosine’ application in the preparation of analgesic drugs. China, ZL200410094511.0. in Chinese.
- Cui FL, Wang JL, Cui YR, Yao XJ, Qu GR, Lu Y.. 2007. Investigation of interaction between human serum albumin and N6-(2-hydroxyethyl)-adenosine by fluorescence spectroscopy and molecular modelling. Luminescence. 22:546–553. [DOI] [PubMed] [Google Scholar]
- Dong CH, Guo SP, Wang WF, Liu XZ.. 2015. Cordyceps industry in China. Mycology. 6(2):121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong CH, Li WJ, Li ZZ, Yan WJ, Li TH, Liu XZ, et al. 2016. Cordyceps industry in China: current status, challenges and perspectives—Jinhu declaration for cordyceps industry development. Mycosystema. 35(1):1–15. in Chinese. [Google Scholar]
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19:11–15. [Google Scholar]
- Fang M, Chai YQ, Chen GJ, Wang HD, Huang B. 2016. N6-(2-Hydroxyethyl)-adenosine exhibits insecticidal activity against Plutella xylostella via adenosine receptors. PLoS One. 11(9):e0162859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya T, Hirotani M, Matsuzawa M. 1983. N6-(2-hydroxyethyl)-adenosine, a biologically active compound from cultured mycelia of Cordyceps and Isaria species. Phytochemistry. 22(11):2509–2512. [Google Scholar]
- Gong YX, Li SP, Li P, Liu JJ, Wang YT. 2004. Simultaneous determination of six main nucleosides and bases in natural and cultured Cordyceps by capillary electrophoresis. J Chromatogr A. 1055(1):215–221. [DOI] [PubMed] [Google Scholar]
- Hu G, Yang FQ. 2014. Biological activities of nucleosides and their analogues in dietary foods. Chem Rapid Commun. 2(2):22–28. [Google Scholar]
- Ke BJ, Lee CL. 2016. Investigation on the fermentation for high adenosine and N6-(2-hydroxyethyl)-adenosine (HEA) productions of Cordyceps cicadae. New Biotechnol. 33:S206–S207. [Google Scholar]
- Lei BX, He J, Kang JC, Yu X, Gui JX, Wen TC. 2014. Optimization of selected parameters affecting N6-(2-hydroxyethyl)-adenosine production by Isaria tenuipes strain E3 grown in submerged culture. Acta Edulis Fungi. 21(2):63–66; in Chinese. [Google Scholar]
- Li SP, Yang FQ, Tsim KW. 2006. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J Pharm Biomed Anal. 41(5):1571–1584. [DOI] [PubMed] [Google Scholar]
- Li XL, 2011. Study on liquid fermentation of Paecilomyces cicadae (Miquel.) Samon. Master Thesis, Nanjing Agricultural University, Nanjing: 21–22. in Chinese. [Google Scholar]
- Liu B, Kang JC, Lei BX, He J, Wen TC, Meng ZB, Pan LL. 2012. Analysis of adenosines in anamorphic mycelia of several species of Cordyceps. Mycosystema. 31(3):405–412; in Chinese. [Google Scholar]
- Liu KB, Wang F, Chai YQ, Dong CH. 2017. Research and exploitation of N6-(2-hydroxyethyl)-adenosine from Cordyceps s.l.: progress and problems. Mycosystema. 36(1):6–13; in Chinese. [Google Scholar]
- Lu LL, Shu TJ, Chen JQ, Wu XF, Zhang YZ, 2013. Study on the isolation and purification of N6-(2-hydroxyethyl)-adenosine from Cordyceps militaris and its inhibitory effect on the Lewis lung cancer. Sciencepaper Online [accessed 2016 June 20]. http://www.paper.edu.cn. in Chinese.
- Lu MY, Chen CC, Lee LY, Lin TW, Kuo CF. 2015. N6-(2-hydroxyethyl)-adenosine in the medicinal mushroom Cordyceps cicadae attenuates lipopolysaccharide-stimulated pro-inflammatory responses by suppressing TLR4-mediated NF-kB signaling pathways. J Nat Prod. 78(10):2452–2460. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Hansen VM, Meyling NV, Eilenberg J. 2007. Human exposure to airborne fungi from genera used as biocontrol agents in plant production. Ann Agric Environ Med. 14(1):5–24. [PubMed] [Google Scholar]
- Meng ZB, Chen LH, Han JY, Jiang JZ, Wen TC, Kang JC. 2015a. Research advances of the chemical active constituents of Cordyceps militaris. Mol Plant Breed. 13(9):2147–2154. [Google Scholar]
- Meng ZB, Kang JC, Wen TC, Lei BX, Hyde KD. 2015b. Cordycepin and N6-(2-hydroxyethyl)-adenosine from Cordyceps pruinosa and their interaction with human serum albumin. PLoS One. 10(3):e0121669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng ZB, Wen TC, Kang JC, Lei BX, Hyde KD. 2014. Cordyceps pruinosa produces cordycepin and N6-(2-hydroxyethyl)-adenosine in culture. Arch Biol Sci. 66(4):1411–1421. [Google Scholar]
- Park SJ, Hyun SH, Suh HW, Lee SY, Sung GH, Kim SH, Choi HK. 2013. Biochemical characterization of cultivated Cordyceps bassiana mycelia and fruiting bodies by 1H nuclear magnetic resonance spectroscopy. Metabolomics. 9:236–246. [Google Scholar]
- Peng XX, Chai YQ, Zhu BC, Jin YW, Li XL, Yu LS. 2015. The protective effects of N6-(2-hydroxyethyl)-adenosine extracted from Ophiocordyceps sobolifera on renal ischemia reperfusion injury (IRI) in mice. Mycosystema. 34(02):311–320; in Chinese. [Google Scholar]
- Phillip DC, John CH, Megan LP, 2008. Method for growing Cordyceps sinensis on a substrate and novel method for hybridizing different strains of Cordyceps sinensis. USA, 7407795B2.
- Singh HB, Keswani C, Ray S, Yadav SK, Singh SP, Singh S, Sarma BK. 2015. Beauveria bassiana: biocontrol beyond lepidopteran pests. Soil Biol. 45: 219–235. [Google Scholar]
- Suh W, Nam G, Yang WS, Sung GH, Shim SH, Cho JY. 2017. Chemical constituents identified from fruit body of Cordyceps bassiana and their anti-inflammatory activity. Biomol Ther. 25(2):165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 30(12):2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DM, Liu XH, Guo H, Huang JH, Wang L. 2013. Design, synthesis and biological activity evaluation of adenosine analogues. Acta Pharm Sinica. 48(06):881–886; in Chinese. [PubMed] [Google Scholar]
- Xia YL, 2014. Unraveling the biosynthetic mechanism of cordycepin in the medicinal fungus. Ph.D thesis, University of Chinese Academy of Sciences, Beijing. in Chinese. [Google Scholar]
- Zhang Z, Tudi T, Liu YF, Zhou S, Feng N, Tang Y, Tang CH, Tang QJ, Zhang JS. 2016. Preparative isolation of cordycepin, N6-(2-hydroxyethyl)-adenosine and adenosine from Cordyceps militaris by macroporous resin and purification by recycling high-speed counter-current chromatography. J Chromatogr. B. 1033-1034: 218–225. [DOI] [PubMed] [Google Scholar]
- Zhu LN, Xue JJ, Liu YF, Zhou S, Zhang JS, Tang QJ. 2013. Isolation, purification and anti-tumor activity of N6-(2-hydroxyethyl)-adenosine from the fruiting body Cordyceps militaris cultured. Acta Edulis Fungi. 20:62–65. in Chinese. [Google Scholar]


