Abstract
Background: The purpose of this analysis is to evaluate the effect of institutional accrual volume on clinical outcomes among patients receiving chemoradiation for locally advanced non–small cell lung cancer (LA-NSCLC) on a phase III trial.
Methods: Patients with LA-NSCLC were randomly assigned to 60 Gy or 74 Gy radiotherapy (RT) with concurrent carboplatin/paclitaxel +/- cetuximab on NRG Oncology RTOG 0617. Participating institutions were categorized as low-volume centers (LVCs) or high-volume centers (HVCs) according to the number of patients accrued (≤3 vs > 3). All statistical tests were two-sided.
Results: Range of accrual for LVCs (n = 195) vs HVCs (n = 300) was 1 to 3 vs 4 to 18 patients. Baseline characteristics were similar between the two cohorts. Treatment at a HVC was associated with statistically significantly longer overall survival (OS) and progression-free survival (PFS) compared with treatment at a LVC (median OS = 26.2 vs 19.8 months; HR = 0.70, 95% CI = 0.56 to 0.88, P = .002; median PFS: 11.4 vs 9.7 months, HR = 0.80, 95% CI = 0.65-0.99, P = .04). Patients treated at HVCs were more often treated with intensity-modulated RT (54.0% vs 39.5%, P = .002), had a lower esophageal dose (mean = 26.1 vs 28.0 Gy, P = .03), and had a lower heart dose (median = V5 Gy 38.2% vs 54.1%, P = .006; V50 Gy 3.6% vs 7.3%, P < .001). Grade 5 adverse events (AEs) (5.3% vs 9.2%, P = .09) and RT termination because of AEs (1.3% vs 4.1%, P = .07) were less common among patients treated at HVCs. HVC remained independently associated with longer OS ( P = .03) when accounting for other factors.
Conclusion: Treatment at institutions with higher clinical trial accrual volume is associated with longer OS among patients with LA-NSCLC participating in a phase III trial.
Lung cancer is the second most common cancer type and the leading cause of cancer death in the United States, where there were estimated to be 221 200 new cases and 158 040 lung cancer deaths in 2015 alone ( 1 ). As an important public health concern, understanding quality indicators in lung cancer care is of critical importance. In addition to patient-specific factors known to influence treatment delivery and clinical outcomes ( 2 , 3 ), institutional specific factors, such as hospital volume and physician specialization, have also been associated with quality outcomes in lung cancer care ( 4–9 ).
The association between hospital volume and physician specialization with improved patient outcomes has most consistently been demonstrated in situations where the primary therapy is a technically challenging and high-risk surgical intervention ( 4–11 ). Approximately one-third of non–small cell lung cancers patients have locally advanced disease (LA-NSCLC) at diagnosis and require chemoradiation (CRT) for definitive treatment ( 3 ). CRT for patients with LA-NSCLC is similar to a complex surgical procedure in that it is also complex to deliver and involves risk for treatment-related morbidity and mortality, predominately related to normal tissue toxicity of the visceral thoracic organs. With multiple technological advancements that require extensive physician decision-making, radiation delivery is increasingly dependent upon expertise from the radiation oncology team, which may or may not include a dedicated thoracic radiation oncologist. Furthermore, patients receiving CRT for LA-NSCLC may benefit from the experience of the entire clinical care team in managing unique disease and treatment-related complications. However, data evaluating the effect of institutional factors such as clinical volume or physician expertise with the delivery of CRT for LA-NSCLC is limited.
Active clinical trial participation has been associated with improved patient outcomes in other cancer types ( 12–15 ). As clinical trial accrual volume may be considered a representation of both the degree of research participation as well as clinical volume, it was hypothesized that patients treated on a clinical trial at institutions with a higher clinical trial accrual volume may experience improved clinical outcomes. The purpose of this analysis is to test this hypothesis among patients receiving definitive CRT for LA-NSCLC as specified by the phase III trial NRG Oncology Radiation Therapy Oncology Group (RTOG) 0617 ( 16 ).
Methods
Patients
Patients were enrolled in NRG Oncology RTOG 0617 (NCT00533949), a four-arm randomized study of standard-dose (60 Gy) RT vs escalated-dose (74 Gy) conformal RT delivered with concurrent and consolidation carboplatin and paclitaxel with or without cetuximab in patients with stage III NSCLC ( 16 ). Eligibility criteria included newly diagnosed stage IIIA or IIIB NSCLC ( 17 ), Zubrod performance status of 0-1, and pulmonary function greater than 50% predicted. Patients with supraclavicular or contralateral hilar adenopathy, severe medical comorbidities, weight loss greater than 10% in the previous four weeks, or a history of prior malignancy within the past three years were ineligible.
NRG Oncology RTOG 0617 was open to accrual from November 2007 through November 2011. All patients signed formal consent before enrollment, and the study was approved by each participating institution’s internal review board.
Treatment and Quality Assurance
RT was administered according to guidelines for target volume definition and treatment delivery as previously described ( 16 ). All patients underwent CT simulation for 3D treatment planning. Treatment was delivered with 6-18 MV photons via either 3D conformal radiation therapy (3DCRT) or intensity modulated radiation therapy (IMRT), determined by physician discretion. Importantly, all participating centers were credentialed through the Radiological Physics Center, requiring phantom irradiation, in order to be eligible to participate. The use of IV contrast with simulation, fluorodeoxyglucose/positron emission tomography (FDG/PET)–assisted treatment planning, and four-dimensional assessment of tumor motion for planning and treatment delivery were strongly encouraged but not required.
Quality assurance (QA) of RT treatment plans, including target and organ-at-risk (OAR) volume definition and dose-volume analysis (DVA), was performed by the principal investigators. RT was scored as unacceptable if less than 90% of the planning target volume (PTV) received the prescribed dose, the maximum PTV dose was less than 93% or greater than 125% of prescription dose, or less than 1 cm 3 of tissue outside the PTV received 120% or more of prescription dose.
Weekly concurrent carboplatin/paclitaxel +/-cetuximab was delivered according to protocol and random assignement. Consolidation chemotherapy +/- cetuximab was given for two cycles following completion of CRT. The study medical oncology co-chairs performed QA review of drug delivery and scored chemotherapy and cetuximab, if applicable, as per protocol/acceptable, not per protocol, or not evaluable.
Statistical Methods
Following the methods previously utilized by Wuthrick et al. ( 18 ), institutions were categorized by tertiles into groups of low, medium, or high accrual according to the number of patients they accrued to 0617. NRG Oncology member institutions and their satellite institutions were each considered individually. The range of accrual for low, medium, and high accruing centers was one, two to three, and four to 18 patients, respectively. Given the narrow range of patient accrual for institutions within the low and medium accrual tertiles, the low (n = 72) and medium (n = 123) accruing centers were grouped together into one cohort (low-volume centers [LVCs]; n = 195). This allowed for comparison with outcomes among patients treated at institutions within the highest (n = 300) accrual tertile (high-volume centers [HVCs]) and better approximated the number of patients in each cohort.
Descriptive statistics were compared using Chi-square test or Fisher’s exact test for categorical variables, t test for normally distributed continuous variables, and Wilcoxon test for non-normal continuous variables and ordinal variables. All tests of statistical significance are two-sided. The primary outcome variable was overall survival (OS). Secondary outcome variables included progression-free survival (PFS), local-regional failure (LRF), and study-related adverse events (AEs). OS was recorded as the time from random assignment until time of death from any cause. PFS was defined as the time from random assignment until LRF, distant metastases, or death. LRF was recorded at the time of evidence of local or regional thoracic progression.
The cumulative incidence of LRF was compared with Gray’s test ( 19 ) because of the competing risk of death, and PFS and OS were analyzed with the Kaplan-Meier (KM) method and compared with the log-rank test ( 20 ). Cox proportional hazards models ( 21 ) were used to estimate the effect of multiple covariates, including sex, ethnicity, race, current smoking status, marital status, weight loss, performance status, histology, T-stage, N-stage, American Joint Committee on Cancer stage group, tumor location, cetuximab assignment, RT dose level assignment, PTV volume (cm 3 ), RT technique (IMRT vs 3DCRT), mean esophagus dose, and percent volume of the heart receiving 5 or more Gy (V5 Gy) or 50 or more Gy (V50 Gy), on the outcome variables. Linear hypothesis tests were used to assess the proportionality assumption of Cox models. Because of the number of covariates and exploratory nature of this process, univariate models were conducted first and only covariates with P values of less than .1 were considered in the multivariable model, along with institutional accrual volume group. Stepwise selection was utilized with P values of less than .25 required to enter the model and P values of less than .15 required to remain in the model. A P value of less than .05 was used to determine statistical significance. Cox models were also used in the presence of a competing risk to estimate the cause-specific hazards.
Study-related adverse events were graded according to the Common Terminology Criteria for Adverse Events, version 4.0. The effect of treatment center volume and other variables on acute and late hematologic and nonhematologic AEs was assessed with linear regression models while logistic regression was used for analysis of grade 5 AEs.
Results
Patients and Institutional Accrual
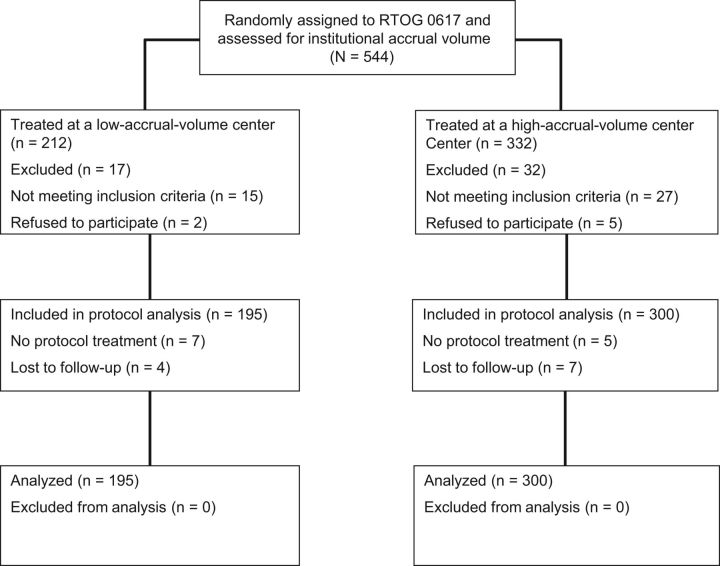
Four hundred and ninety-five eligible patients were randomly assigned and treated at 180 different institutions on NRG Oncology RTOG-0617. Figure 1 depicts the separation of patients into cohorts based on whether they were treated at a LVC or HVC. The range of accrual for LVCs was one to three patients, vs four to 18 patients for HVCs. Descriptive patient characteristics and clinical variables according to institutional accrual volume are listed in Table 1 . Patient cohorts were well balanced with respect to all clinical variables, though patients treated at HVCs were more often stage Tx-T2 (56.3% vs 48.2%, P = .08), less often randomly assigned to the 74 Gy RT dose arm (38.7% vs 46.7%, P = .08), and more likely to be treated at an RTOG full member institution (33.7% vs 11.3%, P < .001) rather than an affiliate or satellite institution.
Figure 1.
CONSORT Diagram of patients randomized on NRG Oncology Radiation Therapy Oncology Group (RTOG) 0617 and separated into cohorts based on whether they were treated at a low accrual volume or high accrual volume institution.
Table 1.
Descriptive patient characteristics and clinical variables by institutional accrual volume
| Clinical variable | Low-volume center (n = 195) | High-volume center (n = 300) | P * |
|---|---|---|---|
| Age, median (range) | 64 (37–83) | 64 (38–83) | .58 † |
| Sex, No. (%) | |||
| Male | 123 (63.1) | 172 (57.3) | .20 |
| Female | 72 (36.9) | 128 (42.7) | |
| Race, No. (%) | |||
| White | 162 (84.4) | 261 (87.6) | .31 |
| Other | 30 (15.6) | 37 (12.4) | |
| Smoking status, No. (%) | |||
| No | 9 (4.9) | 22 (7.7) | .18 |
| Former smoker | 136 (74.7) | 220 (77.5) | |
| Current smoker | 37 (20.3) | 42 (14.8) | |
| Zubrod performance status, No. (%) | |||
| 0 | 118 (60.5) | 167 (55.7) | .29 |
| 1 | 77 (39.5) | 133 (44.3) | |
| Histology, No. (%) | |||
| Squamous cell | 90 (46.2) | 127 (42.3) | .59 |
| Adenocarcinoma | 76 (39.0) | 119 (39.7) | |
| Large cell | 6 (3.1) | 7 (2.3) | |
| NSCLC NOS | 23 (11.8) | 47 (15.7) | |
| T stage, No. (%) | |||
| TX-T2 | 94 (48.2) | 169 (56.3) | .08 |
| T3-T4 | 101 (51.8) | 131 (43.7) | |
| AJCC stage, No. (%) | |||
| IIIA | 136 (69.7) | 188 (62.7) | .11 |
| IIIB | 59 (30.3) | 112 (37.3) | |
| Tumor location, No. (%) | |||
| Upper lobe | 132 (67.7) | 196 (65.3) | .79 |
| Lower lobe | 42 (21.5) | 66 (22.0) | |
| Other | 21 (10.8) | 38 (12.7) | |
| RT random assignment, No. (%) | |||
| 60 Gy | 104 (53.3) | 184 (61.3) | .08 |
| 74 Gy | 91 (46.7) | 116 (38.7) | |
| Cetuximab random assignment, No. (%) | |||
| Yes | 93 (47.7) | 144 (48.0) | .95 |
| No | 102 (52.3) | 156 (52.0) | |
| Institution type, No. (%) | |||
| RTOG full member | 22 (11.3) | 101 (33.7) | <.001 |
| RTOG affiliate member | 57 (29.3) | 60 (20) | |
| RTOG member satellite | 55 (28.2) | 44 (14.7) | |
| CCOP member | 32 (16.4) | 79 (26.3) | |
| Non-RTOG | 29 (14.9) | 16 (5.3) |
* P value from a two-sided Chi-Square test. AJCC = American Joint Committee on Cancer; CCOP = Community Clinical Oncology Programs; RT = radiotherapy; NSCLC NOS = non–small cell lung cancer not otherwise specified; RTOG = Radiation Therapy Oncology Group.
† P value from a two-sided Wilcoxon test assuming a normal approximation.
Overall Survival
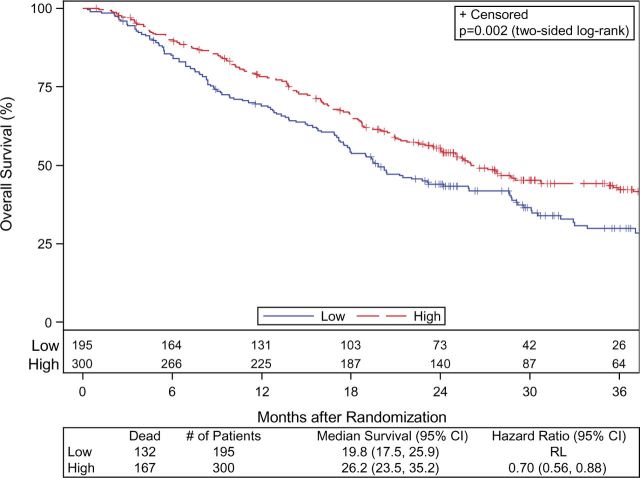
Patients treated at HVCs had statistically significant longer median OS compared with patients treated at LVCs (26.2 vs 19.8 months, hazard ratio [HR] = 0.70, 95% confidence interval [CI] = 0.56 to 0.88, P = .002). KM estimates of OS at two years were 55.5% (95% CI = 0.50 to 0.61) for the HVC cohort compared with 43.9% (95% CI = 0.37 to 0.51) for the LVC cohort ( Figure 2 ). Sex ( P = .07), smoking status ( P = .05), tumor location ( P = .05), RT dose level assignment ( P = .05), PTV volume ( P = .003), mean esophagus dose ( P = .08), and heart V5 Gy ( P < .001) or V50 Gy ( P < .001) were also found to be prognostic for OS in univariate models. On multivariable stepwise analysis ( Table 2 ), HVC remained statistically significantly associated with a lower risk of death, resulting in a hazard ratio of 0.75 (95% CI = 0.59 to 0.97, P = .03).
Figure 2.
Kaplan-Meier curves of overall survival according to whether patients were treated at a high-volume center (dashed line) or low-volume center (solid line). CI = confidence interval; HR = hazard ratio; RL = reference level.
Table 2.
Multivariable Cox proportional hazards model
| Outcome and variable | HR (95% CI) | P * |
|---|---|---|
| OS | ||
| Treatment center volume, HVC vs LVC † | 0.75 (0.59 to 0.97) | .03 |
| Tumor location, lower lobe vs upper lobe † | 0.71 (0.47 to 1.07) | .10 |
| Tumor location, other lobe vs upper lobe † | 0.62 (0.44 to 0.89) | .01 |
| RT dose level, 74 Gy vs 60 Gy † | 1.41 (1.10 to 1.79) | .01 |
| PTV volume, cc | 1.00 (1.00 to 1.00) | .09 |
| Heart V5, % | 1.01 (1.00 to 1.01) | .03 |
| PFS | ||
| Treatment center volume, HVC vs LVC † | 0.85 (0.68 to 1.06) | .14 |
| Current smoker, yes vs no † | 1.33 (1.01 to 1.75) | .04 |
| RT dose level, 74 Gy vs 60 Gy † | 1.23 (0.99 to 1.52) | .07 |
| Heart V5, % | 1.01 (1.00 to 1.02) | .01 |
| LRC | ||
| Treatment center volume, HVC vs LVC † | 0.79 (0.60 to 1.04) | .10 |
| Current smoker, yes vs no † | 1.37 (0.96 to 1.94) | .08 |
| RT dose level, 74 Gy vs 60 Gy † | 1.36 (1.03 to 1.79) | .03 |
| Mean esophagus dose, gray | 1.01 (1.00 to 1.03) | .09 |
* P value from the two-sided Wald Chi-square test. Model derived from stepwise selection using P < .25 for entry and P < .15 to remain in the model. CI = confidence interval; HR = hazard ratio; HVC = high-volume center; LVC = low-volume center; OS = overall survival; PTV = planning target volume; RT = radiotherapy; V5 = volume receiving ≥ 5 Gray.
†Reference level for hazard ratios.
Given the importance of radiation dose level in the primary analysis of 0617 ( 16 ), a subset analysis was performed to evaluate the effect of treatment center volume among patients treated at each RT dose level. There was a statistically significant difference in median OS for HVC vs LVC at the 60 Gy dose level (29 months vs 21.5 months, HR = 0.69, 95% CI = 0.48 to 0.92, P = .01), but differences in median OS at the 74 Gy dose level (22.5 months vs 17.9 months, HR = 0.77, 95% CI = 0.55 to 1.08, P = .13) were not statistically significant.
Disease Control
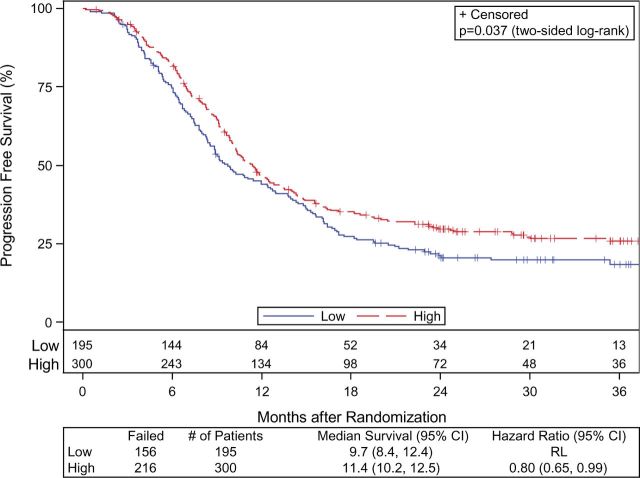
Patients treated at HVCs had a statistically significant longer median PFS compared with patients treated at LVCs (11.4 vs 9.7 months, HR = 0.80, 95% CI = 0.65 to 0.99, P = .04). KM estimates of PFS at two years were 29.7% (95% CI = 0.24 to 0.35) among the HVC cohort and 21.2% (95% CI = 0.15 to 0.27) among the LVC cohort ( Figure 3 ). Smoking status ( P = .02), RT dose level assignment ( P = .08), mean esophagus dose ( P = .02), and heart V5 Gy ( P = .03) or V50 Gy ( P = .002) were found to be prognostic for PFS in univariate models.
Figure 3.
Kaplan-Meier curves of progression-free survival according to whether patients were treated at a high-volume center (solid line) or low-volume center (solid line). CI = confidence interval; HR = hazard ratio; RL = reference level.
There was a trend for reduced LRF among patients treated at HVCs, with two-year cumulative LRF incidence of 41.5% (95% CI = 0.36 to 0.47) at HVCs vs 47.7% (95% CI = 0.41 to 0.55) at LVCs, though this did not reach statistical significance ( P = .08). Current smoker ( P = .04), 74 Gy RT dose level assignment ( P = .03), higher mean esophagus dose ( P = .01), and higher heart V50 Gy ( P = .04) were statistically significantly associated with LRF on univariate analysis while only the association between 74 Gy RT dose and LRF remained statistically significant on multivariable analysis (HR = 1.36, 95% CI = 1.03 to 1.79, P = .03) ( Table 2 ).
Treatment Delivery and Adverse Events
Treatment variables and compliance according to institutional accrual volume are listed in Table 3 . Patients treated at HVCs were more often treated with intensity modulated RT (54.0% vs 39.5%, P = .002), had a lower esophageal dose (mean 26.1 vs 28.0 Gy, P = .03) and heart dose (median V5 Gy 38.2% vs 54.1%, P = .006; V50 Gy 3.6% vs 7.3%, P < .001), and more often had and acceptable/per protocol OAR DVA review (93.1% vs 86.5%, P = .02).
Table 3.
Treatment variables and compliance by accrual volume
| Treatment variable | Low-volume center (n = 195) | High-volume center (n = 300) | P * |
|---|---|---|---|
| RT technique, No. (%) | |||
| 3DCRT | 118 (60.5) | 138 (46.0) | .002 |
| IMRT | 77 (39.5) | 162 (54.0) | |
| Mean esophagus dose (gray), mean (SD) | 28.0 (9.9) | 26.1 (8.7) | .03 † |
| Heart V5, median (range) | 54.1 (0–100) | 38.2 (0–100) | .01 ‡ |
| Heart V50, median (range) | 7.32 (0–56.05) | 3.60 (0–54.77) | <.001 ‡ |
| Overall RT review, No. (%) | |||
| Per protocol/acceptable | 156 (84.8) | 241 (82.5) | .61 |
| Unacceptable deviation | 28 (15.2) | 51 (17.5) | |
| Target volume DVA review, No. (%) | |||
| Per protocol/acceptable | 173 (93.5) | 278 (96.2) | .94 |
| Unacceptable/unknown | 12 (6.5) | 11 (3.8) | |
| OAR DVA review, No. (%) | |||
| Per protocol/acceptable | 160 (86.5) | 269 (93.1) | .02 |
| Unacceptable/unknown | 25 (13.5) | 20 (6.9) | |
| Concurrent chemotherapy review, No. (%) | |||
| Per protocol | 178 (91.3) | 280 (93.3) | .48 |
| Unacceptable deviation | 17 (8.7) | 20 (6.7) | |
| Consolidation chemotherapy review, No. (%) | |||
| Per protocol | 134 (69.1) | 227 (75.7) | .12 |
| Unacceptable deviation | 60 (30.9) | 73 (24.3) | |
| Concurrent cetuximab review, No. (%) | |||
| Per protocol | 87 (93.5) | 139 (96.5) | .35 |
| Unacceptable deviation | 6 (6.5) | 5 (3.5) | |
| Consolidation cetuximab review, No. (%) | |||
| Per protocol | 70 (75.3) | 119 (83.2) | .14 |
| Unacceptable deviation | 23 (24.7) | 24 (16.8) |
* P value from two-sided Fisher’s exact test. 3DCRT = 3-dimensional conformal radiotherapy; DVA = dose-volume analysis; IMRT = intensity modulated radiotherapy; OAR = organs at risk; RT = radiotherapy; V5 = volume receiving ≥ 5 Gray; V50 = volume receiving ≥ 50 Gray.
† P value from a two-sided t test assuming equal variances.
‡ P value from a two-sided Wilcoxon test assuming a normal approximation.
Treatment-related AEs according to accrual volume are listed in Table 4 . There were fewer grade 5 AEs (5.3% vs 9.2%, P = .09) among patients treated at HVCs. Sex, histology, tumor location, RT dose level, and heart V50 were found to be prognostic of experiencing a grade 5 AE. After combining into one model, male sex ( P = .05), squamous cell histology ( P = .04), and heart V50 ( P = .01) were retained as independent prognostic factors for an increases risk of grade 5 AE. Patients treated at HVCs were less likely to have RT interruptions (29.4% vs 37.6%, P = .17) and less likely to have RT terminated because of adverse events (1.3% vs 4.1%, P = .07). Random assignment to cetuximab ( P = .07), not per protocol consolidation chemotherapy ( P = .001), and 74 Gy RT dose level ( P = .03) were also associated with RT termination because of adverse events. On multivariable analysis, only use of cetuximab was statistically significantly associated with RT termination ( P = .04).
Table 4.
Adverse events by accrual volume
| Adverse event | Low-volume center (n = 195) | High-volume center (n = 300) | P * |
|---|---|---|---|
| Grade 3+ esophagitis, No. (%) | |||
| No | 169 (86.7) | 270 (90.0) | .31 |
| Yes | 26 (13.3) | 30 (10.0) | |
| Grade 3+ pneumonitis, No. (%) | |||
| No | 182 (93.3) | 285 (95.0) | .43 |
| Yes | 13 (6.7) | 15 (5.0) | |
| Grade 5 AE, No. (%) | |||
| No | 177 (90.8) | 284 (94.7) | .09 |
| Yes | 18 (9.2) | 16 (5.3) | |
| Acute hematologic grade 3+ AE, median (range) | 1 (0–16) | 1 (0–23) | .30 † |
| Acute nonhematologic grade 3+ AE, median (range) | 2 (0–34) | 1 (0–30) | .39 † |
| Late hematologic grade 3+ AE, median (range) | 0 (0–7) | 0 (0–2) | .48 † |
| Late nonhematologic grade 3+ AE, median (range) | 0 (0–24) | 0 (0–9) | .77 † |
* P value from two-sided Fisher’s exact test. AE = adverse event.
† P value from a two-sided Wilcoxon test assuming a normal approximation.
Discussion
The results of this analysis demonstrate a statistically and clinically significant difference in the primary study outcome variables according to the institutional clinical trial accrual volume. The absolute survival difference observed between patients treated at HVCs and LVCs was greater than 10% at two years, which is a finding of considerable magnitude for LA-NSCLC. On univariate analysis, accrual volume was associated with improved PFS and reduced RT dose delivered to critical structures, and there was a trend for reduced grade 5 AEs and RT treatment interruptions at HVCs. Given these associations, it is suspected that the effect on OS may be a reflection of both improved disease control and better management or prevention of adverse events.
Clinical trial accrual volume may represent lung cancer clinical volume of the institution overall and/or the expertise of the treating physicians and care team. Complex therapies such as CRT for LA-NSCLC require a unique infrastructure and specialized training. Institutions with a larger lung cancer clinical volume may be more likely to have radiation and medical oncologists dedicated to the treatment of thoracic malignancies. Thus, variation with respect to the integrity with which therapies are provided may differ considerably across institutions with different practice patterns, even within the guidelines of the protocol. This analysis allowed for evaluation of the effect of institutional accrual volume on explicit process criteria, such as the delivery of radiation and drug therapy per protocol. While no statistically significant differences in overall systemic therapy review, RT review, or RT target dose-volume analysis review were noted, differences in therapy delivery are evident in the greater use of IMRT as opposed to 3DCRT, as well as the reduced radiation dose delivered to critical structures at HVCs. These findings are particularly important given that the volume of the heart receiving 50 Gy or more was an independent predictor of grade 5 AEs. The differences in treatment technique, however, cannot solely account for the statistically significant longer OS demonstrated at HVCs as IMRT itself was not found to be associated with clinical outcome. Although a greater proportion of patients treated at HVCs were randomly assigned to the 60 Gy dose level, treatment at HVC was associated with longer OS even among the subset of patients randomly assigned to 60 Gy.
Lamont et al. previously evaluated clinical outcomes according to institution type in lung cancer cooperative group trials ( 22 ). Using 10 Cancer and Leukemia Group B (CALGB) trials, patient survival was analyzed in relationship to whether the treating institution was categorized as a main CALGB member, a Veterans Hospital affiliate, or a community practice. Baseline patient characteristics were found to differ substantially across institution type, but after accounting for these patient variables, no statistically significant difference in survival was found. These findings are in contrast to the data presented here, where no differences in baseline patient characteristics according to institutional volume were found, and the effect of institutional volume persisted even after accounting for all clinical and treatment characteristics.
A recent publication from Wang et al. analyzed the effect of institutional clinical volume among patients undergoing CRT for stage III NSCLC registered in the National Cancer Center Database and similarly demonstrated improved survival among patients treated at high-volume centers ( 23 ). In this analysis, high-volume facilities (HVFs) were defined as those in the 90th percentile of annual CRT volume (or ≥ 12 cases/year). In comparison, our analysis is unique in that is it limited to institutions participating in a cooperative group randomized trial, and HVCs were defined as those within the top tertile of clinical trial accrual volume (or ≥ 4 patients accrued). Considering that patients accrued to the trial are only a small sample of total CRT lung cancer volume, the total institutional lung cancer CRT volume is not represented. Our analysis is similar to that of Wuthrick et al, which also found improved OS and PFS among locally advanced head and neck cancer (LA-HNC) patients treated with CRT on NRG Oncology RTOG trials, when patients were treated at institutions with high clinical trial accrual volume ( 18 ). These analyses are alike in that CRT for both LA-NCLSC and LA-HNC is challenging from the prospective of both RT delivery and toxicity management, and both deploy chemoradiation as a potentially curative therapy. Together, the results highlight the great variability in patient outcome that can be related to institutional factors with the delivery of definitive CRT for advanced malignancies.
A great strength of this analysis is that it utilizes a dataset of patients with similar baseline characteristics who are treated and assessed in a uniform manner with prospective collection of outcome data. The results are thus not attributable to major differences in patient characteristics, processes of diagnosis and staging, or treatment strategies at HVCs as compared with LVCs and, therefore, may even be a conservative estimate of differences that exist in clinical outcomes according to institutional volume among patients not treated on a prospective trial. One may question whether effort should be made to concentrate patient care and clinical trial participation to high-volume centers. This concept is fraught with social, financial, and ethical challenges ( 24 ), not limited to the discrimination against patient participation from rural care centers and the likely negative effects on the ability to timely accrue clinical trials. The challenge is to define the relative contribution of specific institutional factors to the differences in outcomes seen, which could facilitate better education and process improvement strategies to create a more uniform delivery of treatment.
The ability to extrapolate the volume-outcome relationship demonstrated here to clinical care outside of a clinical trial is limited in that clinical trial accrual may not accurately reflect the total lung cancer clinical volume of a center and multiple factors unrelated to clinical volume can impact cooperative group trial participation. Additional details about NCI designation or academic vs community practice, or physician specialization, may also be of value but were not the focus of this analysis.
In conclusion, treatment at institutions with high accrual volume is associated with statistically significantly longer OS among patients receiving chemoradiation therapy for LA-NSCLC in a phase III trial. Additionally, differences in care delivery according to institutional accrual volume were demonstrated, and greater accrual volume was associated with more frequent use of IMRT and reduced radiation dose delivered to critical normal structures. This is an important finding regarding quality outcomes with chemotherapy and radiation delivered in a prospective clinical trial. These results suggest institutional accrual volume should be accounted for in future clinical trial study design and analysis and highlight the need for a deeper understanding of the specific processes leading to the differing clinical outcomes seen.
Funding
This work was supported by grants U10CA21661, U10CA180868, U10CA180822, U10CA37422, and UG1CA189867 from the National Cancer Institute (NCI) and Bristol-Myers Squibb.
Notes
Previous presentation: This study was presented in abstract form at ASCO Annual Meeting 2014, Chicago, IL.
NCI Program staff reviewed the protocol document but were not involved in the collection and analysis of the data, nor were they involved in the writing of the manuscript. BMS was not involved in the study design; in the collection, analysis, or interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication. The authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015 . CA Cancer J Clin . 2015. ; 65 ( 1 ): 5 – 29 . [DOI] [PubMed] [Google Scholar]
- 2. Bach PB, Cramer LD, Warren JL, Begg CB. Racial differences in the treatment of early-stage lung cancer . N Engl J Med. 1999. ; 341 ( 16 ): 1198 – 1205 . [DOI] [PubMed] [Google Scholar]
- 3. Potosky AL, Saxman S, Wallace RB, Lynch CF. Population variations in the initial treatment of non-small-cell lung cancer . J Clin Oncol. 2004. ; 22 ( 16 ): 3261 – 3268 . [DOI] [PubMed] [Google Scholar]
- 4. Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care . J Clin Oncol. 2000. ; 18 ( 11 ): 2327 – 2340 . [DOI] [PubMed] [Google Scholar]
- 5. Birkmeyer JD, Sun Y, Wong SL, Stukel TA. Hospital volume and late survival after cancer surgery . Ann Surg. 2007. ; 245 ( 5 ): 777 – 783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bach PB, Cramer LD, Schrag D, Downey RJ, Gelfand SE, Begg CB. The influence of hospital volume on survival after resection for lung cancer . N Engl J Med. 2001. ; 345 ( 3 ): 181 – 188 . [DOI] [PubMed] [Google Scholar]
- 7. Silvestri GA, Handy J, Lackland D, Corley E, Reed CE. Specialists achieve better outcomes than generalists for lung cancer surgery . Chest. 1998. ; 114 ( 3 ): 675 – 680 . [DOI] [PubMed] [Google Scholar]
- 8. Romano PS, Mark DH. Patient and hospital characteristics related to in-hospital mortality after lung cancer resection . Chest. 1992. ; 101 ( 5 ): 1332 – 1337 . [DOI] [PubMed] [Google Scholar]
- 9. Whittle J, Steinberg EP, Anderson GF, Herbert R. Use of Medicare claims data to evaluate outcomes in elderly patients undergoing lung resection for lung cancer . Chest. 1991. ; 100 ( 3 ): 729 – 734 . [DOI] [PubMed] [Google Scholar]
- 10. Grilli R, Minozzi S, Tinazzi A, Labianca R, Sheldon TA, Liberati A. Do specialists do it better? The impact of specialization on the processes and outcomes of care for cancer patients . Ann Oncol. 1998. ; 9 ( 4 ): 365 – 374 . [DOI] [PubMed] [Google Scholar]
- 11. Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature . Ann Intern Med. 2002. ; 137 ( 6 ): 511 – 520 . [DOI] [PubMed] [Google Scholar]
- 12. Paulson EC, Mitra N, Sonnad S , et al. . National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery . Ann Surg. 2008. ; 248 ( 4 ): 675 – 686 . [DOI] [PubMed] [Google Scholar]
- 13. Birkmeyer NJ, Goodney PP, Stukel TA, Hillner BE, Birkmeyer JD. Do cancer centers designated by the National Cancer Institute have better surgical outcomes? Cancer. 2005. ; 103 ( 3 ): 435 – 441 . [DOI] [PubMed] [Google Scholar]
- 14. Du Bois A, Rochon J, Lamparter C, Pfisterer J. Pattern of care and impact of participation in clinical studies on the outcome in ovarian cancer . Int J Gynecol Cancer. 2005. ; 15 ( 2 ): 183 – 191 . [DOI] [PubMed] [Google Scholar]
- 15. Schneider EC, Malin JL, Kahn KL, Emanuel EJ, Epstein AM. Developing a system to assess the quality of cancer care: ASCO's national initiative on cancer care quality . J Clin Oncol. 2004. ; 22 ( 15 ): 2985 – 2991 . [DOI] [PubMed] [Google Scholar]
- 16. Bradley JD, Paulus R, Komaki R , et al. . Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study . Lancet Oncol. 2015. ; 16 ( 2 ): 187 – 199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. AJCC Cancer Staging Manual: Sixth Edition . New York: : Springer-Verlag New York, Inc; .; 2002. . [Google Scholar]
- 18. Wuthrick EJ, Zhang Q, Machtay M , et al. . Institutional clinical trial accrual volume and survival of patients with head and neck cancer . J Clin Oncol. 2015. ; 33 ( 2 ): 156 – 164 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray R. A Class of K-Sample Tests for Comparing the Cummulative Incidence of a Competing Risk . Ann Stat. 1988. ; 16 : 1141 – 1154 . [Google Scholar]
- 20. Kaplan EL MP. Nonparametric estimation from incomplete observations . J Am Stat Assoc. 1958. ; 53 ( 282 ): 457 – 481 . [Google Scholar]
- 21. Cox DR. Regression Models and Life Tables . J R Stat Soc Series B Stat Methodol . 1972. ; B34 ( 2 ): 187 – 220 . [Google Scholar]
- 22. Lamont EB, Landrum MB, Keating NL , et al. . Differences in clinical trial patient attributes and outcomes according to enrollment setting . J Clin Oncol. 2010. ; 28 ( 2 ): 215 – 221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang EH, Rutter CE, Corso CD , et al. . Patients Selected for Definitive Concurrent Chemoradiation at High-volume Facilities Achieve Improved Survival in Stage III Non-Small-Cell Lung Cancer . J Thorac Oncol. 2015. ; 10 ( 6 ): 937 – 943 . [DOI] [PubMed] [Google Scholar]
- 24. Epstein AM. Volume and outcome–it is time to move ahead . N Engl J Med. 2002. ; 346 ( 15 ): 1161 – 1164 . [DOI] [PubMed] [Google Scholar]