Abstract
Background
Patients considering postmastectomy radiation and reconstruction require information regarding expected outcomes to make preference-concordant decisions.
Methods
A prospective multicenter cohort study of women diagnosed with breast cancer at 11 centers between 2012 and 2015 compared complications and patient-reported outcomes of 622 irradiated and 1625 unirradiated patients who received reconstruction. Patient characteristics and outcomes between irradiated and unirradiated patients were analyzed using ttests for continuous variables and chi-square tests for categorical variables. Multivariable mixed-effects regression modelsassessed the impact of reconstruction type and radiotherapy on outcomes after adjusting for relevant covariates. All statistical tests were two-sided.
Results
Autologous reconstruction was more commonly received by irradiated patients (37.9% vs 25.0%, P < .001). Immediate reconstruction was less common in irradiated patients (83.0% vs 95.7%, P < .001). At least one breast complication had occurred by two years in 38.9% of irradiated patients with implant reconstruction, 25.6% of irradiated patients with autologous reconstruction, 21.8% of unirradiated patients with implant reconstruction, and 28.3% of unirradiated patients with autologous reconstruction. Multivariable analysis showed bilateral treatment and higher body mass index to be predictive of developing a complication, with a statistically significant interaction between radiotherapy receipt and reconstruction type. Among irradiated patients, autologous reconstruction was associated with a lower risk of complications than implant-based reconstruction at two years (odds ratio [OR] = 0.47, 95% confidence interval [CI] = 0.27 to 0.82, P = .007); no between-procedure difference was found in unirradiated patients. The interaction was also statistically significant for satisfaction with breasts at two years (P = .002), with larger adjusted difference in satisfaction between autologous vs implant approaches (63.5, 95% CI = 55.9 to 71.1, vs 47.7, 95% CI = 40.2 to 55.2, respectively) in irradiated patients than between autologous vs implant approaches (67.6, 95% CI = 60.3 to 74.9, vs 60.5, 95% CI = 53.6 to 67.4) in unirradiated patients.
Conclusions
Autologous reconstruction appears to yield superior patient-reported satisfaction and lower risk of complications than implant-based approaches among patients receiving postmastectomy radiotherapy.
Women diagnosed with early-stage breast cancer face challenging decisions that will impact both their long-term disease control and quality of life. To participate optimally in shared decision-making and ensure that these decisions reflect their own preferences and values, these women must have a sophisticated understanding of the outcomes of various options, including information about patients’ own satisfaction with different approaches. For women undergoing mastectomy as the primary oncologic surgery, high-quality evidence to guide the optimal integration of postmastectomy radiotherapy and breast reconstruction is particularly important.
After all, many women who receive mastectomy for breast cancer are expected to derive substantial benefits from adjuvant radiotherapy (1–3). A recent meta-analysis revealed a substantial improvement in breast cancer mortality from postmastectomy radiotherapy even in women treated with complete axillary dissection and systemic therapy who had only one to three lymph nodes involved (4). Updated guidelines reflect the growing evidence supporting postmastectomy radiotherapy in appropriate patients (5,6), but many patients still must decide whether they feel that the benefits given their particular circumstances outweigh the risks.
One of the risks of radiotherapy is that it may affect the options and outcomes for breast reconstruction (7,8), which many women who receive mastectomy desire. Especially because many women with early-stage breast cancer become long-term survivors, reconstruction can have lasting benefits for quality of life (9–12). Unfortunately, the optimal approach to the integration of postmastectomy radiotherapy and breast reconstruction is not well established. Different institutions have embraced specific approaches to try to minimize complications and optimize cosmesis and satisfaction. Yet because randomized trials of different approaches have not proven feasible, practice has largely been driven by historical traditions and institutional culture.
In order to generate the sort of high-quality evidence regarding outcomes with different approaches that patients require to make informed decisions in this context, we conducted a prospective, multicenter cohort study that collected both medical data and patient-reported outcomes using validated instruments. We sought to investigate the outcomes of different types of breast reconstruction, both with and without radiotherapy, in order to inform patient decision-making. Because some patients desire information regarding the impact of radiotherapy upon reconstruction outcomes when deciding whether or not to pursue radiotherapy, we sought to quantify the impact of radiotherapy on autologous and implant-based reconstruction by comparing outcomes between irradiated and unirradiated patients treated with each approach. In addition, because patients who have decided to pursue both radiotherapy and reconstruction often desire information on the expected outcomes with autologous vs implant-based approaches in the setting of radiotherapy, we also compared outcomes by type of reconstruction among irradiated patients specifically.
Methods
Sample and Data Collection
We conducted a prospective multicenter cohort study (the Mastectomy Reconstruction Outcomes Consortium [MROC]) of women treated with breast reconstruction who provided written informed consent at 11 participating centers between 2012 and 2015 with institutional review/research ethics board approval at all centers (ClinicalTrials.gov NCT01723423). Inclusion criteria for this study were female sex, age 18 years or older, and undergoing first-time, immediate, or delayed reconstruction following mastectomy. Reconstruction options were tissue expander/implant, direct-to-implant, latissimus dorsi flap procedures with and without implant, pedicle transverse rectus abdominis musculocutaneous (TRAM) flap, free TRAM flap including muscle-sparing, deep inferior epigastric perforator flap, superficial inferior epigastric perforator flap, superior gluteal artery perforator, and inferior gluteal artery perforator. For the current analysis, we excluded any patients treated with prophylactic mastectomy (without cancer diagnosis), women who had received radiotherapy prior to mastectomy (for example, in the context of a prior lumpectomy), patients who received bilateral reconstruction with autologous reconstruction techniques on one side and implant-based reconstruction techniques on the other, those who received two-staged crossover of procedure types, or those who received immediate reconstruction after mastectomy of one breast but delayed reconstruction of the other (see Supplementary Figure 1, available online). All patients with complete one-year follow-up (n = 2247), as specified above, were included for the one-year analyses of complications and failure (the subset of 2146 who provided patient-reported outcomes were included in analyses of those outcomes). From these, those who also had complete two-year follow-up (n = 1778) were further included for the two-year analyses (n = 1679 for patient-reported outcomes).
Measures
The primary dependent variables of interest included development of any breast complication, reconstruction failure, and four patient-reported outcomes: satisfaction with breasts, satisfaction with outcome, psychosocial well-being, and physical well-being. Information on breast complications was collected by site coordinators from patients’ electronic medical records one year and two years following breast reconstruction. Specific complications included in this analysis were hematoma, wound infection, wound dehiscence, mastectomy skin flap necrosis, reconstructive flap necrosis, total flap loss, capsular contracture, implant malposition, implant leakage/rupture/deflation, and seroma. For analysis, we considered “any complication,” as well as the subgroup of “major complications,” defined as requiring rehospitalization or re-operation. Complications data were reviewed and then categorized by a single certified plastic surgeon (EGW). Reconstructive failure was defined as complete loss or necrosis of an autologous flap, or implant explantation or unplanned expander removal without immediate replacement.
Patient-reported outcomes measures were restricted to patients who did not experience reconstruction failure. Satisfaction with breasts, satisfaction with outcomes, psychosocial well-being, and physical well-being were measured using the BREAST-Q questionnaire, which is a validated, condition-specific patient-reported outcome (PRO) instrument that has been used to measure health-related quality of life and satisfaction in breast reconstruction patients (13,14). Satisfaction with breasts measures breast appearance, how bras fit, and how the breasts look when clothed and unclothed. Satisfaction with outcome measures the overall evaluation of the outcome of a woman's surgery and whether or not her expectations were met. For each scale measure, response scores can range from 0 to 100, with higher numbers reflecting better outcomes. Study patients completed the web-based questionnaire (the questionnaires are presented in the Supplementary Materials, available online) preoperation, and at one and two years postoperation.
The primary independent variables of interest were reconstruction type (autologous or implant-based) and radiation receipt status (irradiated or not). Patients who received latissimus dorsi flap procedures combined with implants (n = 20) were categorized as having implant-based reconstruction for analyses, along with those who received expander-implant and direct-to-implant procedures.
Covariates included reconstruction timing (immediate, at the time of mastectomy, vs delayed to any time thereafter), age (continuously measured and categorized into five groups: <30, 30–39, 40–49, 50–59, ≥60 years), extent of disease (local, regional, or metastatic), bilateral vs unilateral reconstruction, chemotherapy receipt, nodal management (sentinel lymph node biopsy alone, axillary lymph node dissection, or no surgical axillary evaluation), BMI (<30 vs 30 or more), smoking (nonsmoker, previous smoker, current smoker), diabetes, race (white, black, or other), ethnicity (Hispanic or not), education (college degree or not), employment (full-time, part-time, or none), income (<$50 000, $50 000–$99 999, >$100 000/year), and site (hospital).
Statistical Analysis
Patient characteristics and outcomes between irradiated and unirradiated patients were analyzed using t tests for continuous variables and chi-square tests for categorical variables. For all the analyses, the patient was the analytic unit. Given the relatively small number of failures, failure rates were analyzed only descriptively. To compare complication rates between the groups, a multivariable mixed-effects logistic regression model was employed, with the outcome being the presence of any breast complication. Primary predictors included an indicator for autologous reconstruction (vs implant reconstruction), an indicator for radiotherapy, and the interaction between radiotherapy and autologous reconstruction. The interaction term allowed for the assessments of radiotherapy effect within each procedure type and the procedure effect within irradiated patients. Patient demographic and clinical characteristics were included as covariates, and random intercepts were included for treatment sites (hospitals) to account for between-hospital variability.
Mean PRO scale scores were summarized by radiotherapy status and procedure types. To compare two-year PROs, separate mixed-effects regression models were employed for each PRO scale. As with complication analysis, each model included indicators for autologous reconstruction and radiotherapy, as well as their interaction term. Each model was further adjusted for the baseline value of the corresponding outcome variable and for clinical and demographic characteristics and was adjusted for between-center variability by including sites (hospitals) as random intercepts. To reduce potential bias from nonresponse or missing PROs at one and two years postoperation, analyses were weighted by the inverse of the probability of response. The probability of response was estimated based on data from all included study participants, where a separate logistic regression model was fit for each outcome measure at each follow-up time, with nonmissing response status as the dependent variable and baseline patient characteristics and baseline values of the outcome variable as predictors. All P values were calculated using either two-tailed t tests or F tests, and statistical analyses were performed using SAS 9.4 (Cary, NC). A P value of less than .05 was considered statistically significant.
Results
A total of 2247 patients were beyond one year of follow-up from their reconstructions in July 2016 and were included in the one-year analytic study sample. Among them, 1604 received implant-based reconstruction and 643 received autologous reconstruction (with the most common types being DIEP flaps in 347 and TRAM flaps in 145). Table 1 shows the characteristics of the sample by radiotherapy receipt status. The sample included 622 irradiated and 1625 unirradiated patients. The median patient age was 50 years. Bilateral reconstruction was received by 45.0% of irradiated and 53.6% of unirradiated patients (P < .001). Autologous reconstruction was more commonly received by irradiated patients (37.9% vs 25.0%, P < .001). Immediate reconstruction was less common in irradiated patients (83.0% vs 95.7%, P < .001).
Table 1.
Clinical and demographic characteristics of patients by radiotherapy receipt status (n = 2247)
| Variable | Irradiated (n = 622) No. (%) | Unirradiated (n = 1625) No. (%) | P* |
|---|---|---|---|
| Procedure type | |||
| Implant | 386 (62.1) | 1218 (75.0) | <.001 |
| Autologous | 236 (37.9) | 407 (25.0) | |
| Age, y | |||
| <30 | 19 (3.1) | 24 (1.5) | .003 |
| 30–39 | 99 (15.9) | 181 (11.1) | |
| 40–49 | 211 (33.9) | 586 (36.1) | |
| 50–59 | 191 (30.7) | 542 (33.4) | |
| ≥60 | 102 (16.4) | 292 (18.0) | |
| Extent of disease | |||
| Local | 124 (20.1) | 1392 (85.8) | <.001 |
| Regional | 490 (79.3) | 216 (13.3) | |
| Metastatic | 4 (0.6) | 14 (0.9) | |
| Laterality | |||
| Unilateral | 342 (55.0) | 754 (46.4) | <.001 |
| Bilateral | 280 (45.0) | 871 (53.6) | |
| Timing | |||
| Immediate | 516 (83.0) | 1555 (95.7) | <.001 |
| Delayed | 106 (17.0) | 70 (4.3) | |
| Chemotherapy | |||
| Yes | 585 (94.1) | 588 (36.2) | <.001 |
| No | 37 (5.9) | 1037 (63.8) | |
| Lymph node biopsy | |||
| SLNB | 130 (20.9) | 995 (61.2) | <.001 |
| ALND | 367 (59.0) | 326 (20.1) | |
| None | 125 (20.1) | 304 (18.7) | |
| Obesity status by BMI, kg/m2 | |||
| <30 | 431 (69.3) | 1251 (77.0) | <.001 |
| ≥30 | 191 (30.7) | 374 (23.0) | |
| Smoking status | |||
| Nonsmoker | 387 (62.8) | 1049 (65.2) | .46 |
| Previous smoker | 213 (34.6) | 513 (31.9) | |
| Current smoker | 16 (2.6) | 47 (2.9) | |
| Diabetes | |||
| Yes | 34 (5.5) | 63 (3.9) | .10 |
| No | 588 (94.5) | 1562 (96.1) | |
| Race | |||
| White | 545 (89.1) | 1399 (87.0) | .17 |
| Black | 39 (6.4) | 101 (6.3) | |
| Other | 28 (4.6) | 108 (6.7) | |
| Ethnicity | |||
| Hispanic/Latino | 39 (6.3) | 89 (5.6) | .50 |
| Non-Hispanic/Latino | 576 (93.7) | 1502 (94.4) | |
| Education | |||
| No college | 196 (31.7) | 415 (25.6) | .004 |
| College | 422 (68.3) | 1204 (74.4) | |
| Employment status | |||
| Full-time (include student) | 353 (57.6) | 924 (57.6) | .31 |
| Part-time | 72 (11.7) | 223 (13.9) | |
| Unemployed | 188 (30.7) | 456 (28.4) | |
| Household annual income | |||
| <$50 000 | 123 (20.3) | 273 (17.3) | .13 |
| $50 000–$99 999 | 202 (33.4) | 507 (32.1) | |
| ≥$100 000 | 280 (46.3) | 798 (50.6) |
Based on a two-sided chi-square test. ALND = axillary lymph node dissection; BMI = body mass index; SLNB = sentinel lymph node biopsy.
As shown in Table 2, among the 1778 patients who were beyond two years of follow-up, at least one breast complication had occurred by two years in 38.9% of irradiated patients with implant reconstruction, 25.6% of irradiated patients with autologous reconstruction, 21.8% of unirradiated patients with implant reconstruction, and 28.3% of unirradiated patients with autologous reconstruction. Supplementary Table 1 (available online) details the specific complications experienced.
Table 2.
One- and two-year postoperative breast complication and reconstructive failure rates by radiotherapy status and procedure type
| Group | No. of patients | Any complication, No. (%) | Major* complication, No. (%) | Reconstructive failure, No. (%) | Median time to the first complication (IQR), mo |
|---|---|---|---|---|---|
| 1 y postop (n = 2247) | |||||
| Irradiated | 622 | 180 (28.9) | 134 (21.5) | 48 (7.7) | 1.0 (2.8) |
| Implant | 386 | 123 (31.9) | 99 (25.7) | 47 (12.2) | 1.1 (3.1) |
| Autologous | 236 | 57 (24.2) | 35 (14.8) | 1 (0.4) | 0.8 (1.1) |
| Unirradiated | 1625 | 374 (23.0) | 280 (17.2) | 53 (3.3) | 0.7 (1.6) |
| Implant | 1218 | 254 (20.9) | 182 (14.9) | 43 (3.5) | 0.7 (1.6) |
| Autologous | 407 | 120 (29.5) | 98 (24.1) | 10 (2.5) | 0.6 (1.7) |
| 2 y postop (n = 1778)† | |||||
| Irradiated | 482 | 161 (33.4) | 129 (26.8) | 55 (11.4) | 1.3 (6.7) |
| Implant | 283 | 110 (38.9) | 94 (33.2) | 53 (18.7) | 1.8 (8.9) |
| Autologous | 199 | 51 (25.6) | 35 (17.6) | 2 (1.0) | 0.8 (1.1) |
| Unirradiated | 1296 | 304 (23.5) | 226 (17.4) | 44 (3.4) | 0.8 (3.2) |
| Implant | 964 | 210 (21.8) | 150 (15.6) | 36 (3.7) | 1.0 (3.6) |
| Autologous | 332 | 94 (28.3) | 76 (22.9) | 8 (2.4) | 0.6 (2.6) |
Major complications were defined as those requiring rehospitalization or re-operation. IQR = interquartile range.
Complication rates are cumulative for the entire two-year postoperative period.
Rates of reconstruction failure by two years were 18.7% among irradiated patients with implants, 1.0% among irradiated patients with autologous reconstruction, 3.7% among unirradiated patients with implants, and 2.4% among unirradiated patients with autologous reconstruction. Of the 53 irradiated implant patients whose reconstructions failed, 30 underwent a second attempt at reconstruction, of which 29 were with a different approach; of the 36 unirradiated implant patients whose reconstructions failed, 20 underwent a second attempt (10 with a different approach). Most (8/10) patients whose autologous reconstructions failed underwent a second attempt, all with a different approach.
Multivariable analysis showed that bilateral treatment and higher BMI were predictive of developing a breast complication by both one and two years, and there was a statistically significant interaction between radiotherapy and reconstruction type (Table 3). Radiotherapy effect differed by reconstruction type; by one year postoperation, radiotherapy was associated with 2.12 (95% confidence interval [CI] = 1.48 to 3.03, P < .001) times higher odds of complication in patients receiving implant reconstruction, while showing no difference in patients receiving autologous reconstruction (odds ratio [OR] = 1.04, 95% CI = 0.64 to 1.67, P = .89). Similarly, by two years postoperation, radiotherapy among implant patients was associated with 2.64 (95% CI = 1.77, 3.94, P < .001) times higher odds of complication, but yielded comparable risks among autologous patients (OR = 1.12, 95% CI = 0.66 to 1.92, P = .67). Among irradiated patients, autologous reconstruction was associated with a lower risk of complications than implant-based reconstruction, which was statistically significantly different at year 2 (OR = 0.47, 95% CI = 0.27 to 0.82, P = .007) though not statistically significantly different at year 1 (OR = 0.66, 95% CI = 0.4 to 1.08, P = .10); odds ratios are not included in Table 3.
Table 3.
Adjusted odds ratio estimates of postoperative breast complications
| Variable | 1 y postop |
2 y postop |
||||||
|---|---|---|---|---|---|---|---|---|
| Any complication |
Major complication |
Any complication |
Major complication |
|||||
| OR (95% CI) | P* | OR (95% CI) | P* | OR (95% CI) | P* | OR (95% CI) | P* | |
| Radiation in implant cohort | ||||||||
| No | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Yes | 2.12 (1.48 to 3.03) | <.001 | 2.60 (1.73 to 3.91) | <.001 | 2.64 (1.77 to 3.94) | <.001 | 3.36 (2.17 to 5.22) | <.001 |
| Radiation in autologous cohort | ||||||||
| No | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Yes | 1.04 (0.64 to 1.67) | .89 | 0.76 (0.44 to 1.32) | .34 | 1.12 (0.66 to 1.92) | .67 | 0.92 (0.51 to 1.66) | .78 |
| Age, y | (.02) | (<.001) | (.17) | (.04) | ||||
| 40–49 | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| <30 | 0.47 (0.19 to 1.16) | .10 | 0.64 (0.24 to 1.73) | .38 | 0.41 (0.15 to 1.16) | .09 | 0.48 (0.15 to 1.48) | .20 |
| 30–39 | 0.67 (0.46 to 0.97) | .03 | 0.56 (0.36 to 0.87) | .01 | 0.70 (0.47 to 1.04) | .08 | 0.56 (0.35 to 0.89) | .01 |
| 50–59 | 1.13 (0.88 to 1.46) | .34 | 1.15 (0.86 to 1.52) | .35 | 1.00 (0.75 to 1.32) | .98 | 1.02 (0.75 to 1.39) | .90 |
| >60 | 1.25 (0.91 to 1.72) | .17 | 1.44 (1.01 to 2.04) | .04 | 1.11 (0.78 to 1.59) | .56 | 1.23 (0.83 to 1.82) | .29 |
| Extent of disease | (.92) | (.21) | (.68) | (.40) | ||||
| Local | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Regional | 1.04 (0.76 to 1.44) | .79 | 0.78 (0.54 to 1.12) | .17 | 1.03 (0.72 to 1.48) | .87 | 0.83 (0.56 to 1.24) | .36 |
| Metastatic | 1.22 (0.41 to 3.63) | .73 | 1.71 (0.56 to 5.26) | .35 | 1.68 (0.53 to 5.35) | .38 | 1.78 (0.51 to 6.22) | .37 |
| Reconstruction laterality | ||||||||
| Unilateral | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Bilateral | 1.45 (1.16 to 1.81) | .001 | 1.43 (1.11 to 1.83) | .005 | 1.40 (1.09 to 1.80) | .008 | 1.39 (1.05 to 1.83) | .02 |
| Reconstruction timing | ||||||||
| Immediate | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Delayed | 0.59 (0.34 to 1.02) | .06 | 0.79 (0.44 to 1.43) | .44 | 0.58 (0.32 to 1.05) | .07 | 0.77 (0.40 to 1.48) | .44 |
| Chemotherapy | ||||||||
| No | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Yes | 0.59 (0.34 to 1.02) | .40 | 1.01 (0.75 to 1.35) | .95 | 0.95 (0.71 to 1.28) | .74 | 1.15 (0.83 to 1.59) | .41 |
| Nodal management | (.60) | (.56) | (.61) | (.88) | ||||
| None | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| SLNB | 1.07 (0.75 to 1.52) | .70 | 1.24 (0.83 to 1.86) | .28 | 0.86 (0.58 to 1.27) | .44 | 1.12 (0.71 to 1.75) | .63 |
| ALND | 0.92 (0.61 to 1.38) | .69 | 1.17 (0.74 to 1.85) | .50 | 0.80 (0.50 to 1.26) | .33 | 1.06 (0.63 to 1.77) | .82 |
| BMI, kg/m2 | ||||||||
| <30 | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| ≥30 | 1.84 (1.44 to 2.34) | <.001 | 1.68 (1.29 to 2.21) | <.001 | 1.77 (1.35 to 2.33) | <.001 | 1.77 (1.31 to 2.38) | <.001 |
| Smoking | (.40) | (.01) | (.16) | (.02) | ||||
| Nonsmoker | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Previous smoker | 0.96 (0.77 to 1.21) | .74 | 1.02 (0.79 to 1.31) | .91 | 0.89 (0.69 to 1.15) | .39 | 0.84 (0.63 to 1.11) | .22 |
| Current smoker | 1.44 (0.81 to 2.55) | .21 | 2.40 (1.33 to 4.32) | .004 | 1.67 (0.87 to 3.21) | .12 | 2.24 (1.14 to 4.41) | .02 |
| Diabetes | ||||||||
| No | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Yes | 1.48 (0.90 to 2.42) | .12 | 2.05 (1.22 to 3.43) | .007 | 1.32 (0.75 to 2.32) | .33 | 1.48 (0.81 to 2.70) | .20 |
| Race | (.90) | (.49) | (.67) | (.74) | ||||
| White | 1.00 (reference | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | ||||
| Black | 0.91 (0.58 to 1.41) | .66 | 0.90 (0.55 to 1.48) | .67 | 0.84 (0.51 to 1.39) | .49 | 0.93 (0.54 to 1.60) | .79 |
| Other | 0.96 (0.60 to 1.53) | .85 | 0.71 (0.40 to 1.27) | .25 | 1.15 (0.69 to 1.92) | .60 | 0.79 (0.42 to 1.48) | .46 |
| Ethnicity | ||||||||
| Non-Hispanic/Latino | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Hispanic/Latino | 0.62 (0.36 to 1.07) | .09 | 0.62 (0.32 to 1.18) | .14 | 0.71 (0.40 to 1.27) | .25 | 0.77 (0.40 to 1.47) | .43 |
| Education | ||||||||
| No college degree | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| College degree | 0.94 (0.73 to 1.21) | .61 | 0.99 (0.74 to 1.31) | .92 | 0.88 (0.67 to 1.16) | .37 | 0.88 (0.65 to 1.20) | .43 |
| Employment status | (.70) | (.58) | (.53) | (.88) | ||||
| Unemployed | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| Full-time (including students) | 0.95 (0.74 to 1.22) | .70 | 1.09 (0.82 to 1.45) | .56 | 0.85 (0.64 to 1.13) | .26 | 0.93 (0.68 to 1.26) | .64 |
| Part-time | 1.08 (0.77 to 1.53) | .64 | 1.22 (0.83 to 1.80) | .30 | 0.90 (0.62 to 1.31) | .60 | 0.93 (0.61 to 1.41) | .72 |
| Income | (.21) | (.11) | (.33) | (.25) | ||||
| <50 000 | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – | 1.00 (reference) | – |
| 50 000–99 999 | 1.10 (0.80 to 1.50) | .56 | 1.36 (0.96 to 1.93) | .09 | 1.00 (0.72 to 1.41) | .98 | 1.27 (0.87 to 1.86) | .22 |
| >100 000 | 0.88 (0.64 to 1.21) | .43 | 1.07 (0.74 to 1.54) | .72 | 0.83 (0.59 to 1.17) | .29 | 1.02 (0.69 to 1.51) | .92 |
P value for each category compared with the reference is based on a two-sided t test; P value from a global test of the variable (shown in parentheses) is based on a two-sided F test. ALND = axillary lymph node dissection; BMI = body mass index; CI = confidence interval; OR = odds ratio; SLNB = sentinel lymph node biopsy.
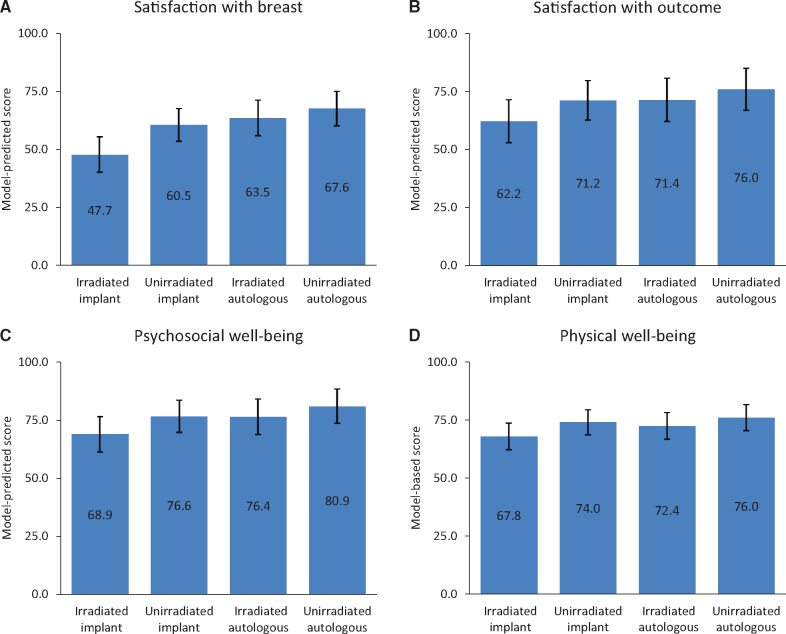
Table 4 describes the BREAST-Q patient-reported satisfaction and well-being scores among patients who responded and had not experienced reconstruction failure, by reconstruction type and radiotherapy status, and Figure 1 shows the adjusted mean scores from multivariable models (Supplementary Tables 2 and 3, available online, present model details). The interaction between radiotherapy and reconstruction type was statistically significant for the outcome of patient-reported satisfaction with breasts, both at one (P = .008) and two years (P = .002). As shown in Figure 1, at two years, in irradiated patients, the adjusted mean satisfaction with breast score was 63.5 (95% CI = 55.9 to 71.1) in women who received autologous reconstruction while it was only 47.7 (95% CI = 40.2 to 55.2) in those who received implant reconstruction. On the other hand, the difference in mean satisfaction with breast scores between procedure type was smaller in unirradiated patients, with a mean of 67.6 (95% CI = 60.3 to 74.9) for autologous vs 60.5 (95% CI = 53.6 to 67.4) for implant patients.
Table 4.
Summary of BREAST-Q patient-reported outcomes by radiation status and procedure type
| Radiation status | Procedure type | Preop (n = 2146*) |
1 y postop (n = 2146*) |
2 y postop (n = 1679) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N† | n‡ | Mean (SD) | N† | n‡ | Mean (SD) | N† | n‡ | Mean (SD) | ||
| Satisfaction with breast | ||||||||||
| Irradiated | Implant | 339 | 339 | 64.6 (21.3) | 339 | 224 | 55.6 (17.1) | 230 | 126 | 54.2 (19) |
| Autologous | 235 | 234 | 48.7 (21.2) | 235 | 185 | 63.9 (16.9) | 197 | 149 | 65.1 (18.2) | |
| Unirradiated | Implant | 1175 | 1167 | 63.9 (21.9) | 1175 | 811 | 65 (17.4) | 928 | 571 | 65.4 (17.5) |
| Autologous | 397 | 396 | 58.1 (21.9) | 397 | 312 | 68.5 (17.5) | 324 | 241 | 69 (17.8) | |
| Satisfaction with outcome§ | ||||||||||
| Irradiated | Implant | – | – | – | 339 | 216 | 67.9 (19.7) | 230 | 124 | 64.8 (22) |
| Autologous | – | – | – | 235 | 184 | 71.8 (21.6) | 197 | 147 | 70.1 (22.9) | |
| Unirradiated | Implant | – | – | – | 1175 | 803 | 71.5 (20.2) | 928 | 567 | 71.3 (21.4) |
| Autologous | – | – | – | 397 | 309 | 74.3 (20.3) | 324 | 238 | 73.6 (20) | |
| Psychosocial well-being | ||||||||||
| Irradiated | Implant | 339 | 339 | 71.1 (17.8) | 339 | 220 | 66.7 (18.5) | 230 | 123 | 66.4 (19.2) |
| Autologous | 235 | 234 | 58.7 (16.7) | 235 | 184 | 70.4 (18.6) | 197 | 149 | 71.8 (19.4) | |
| Unirradiated | Implant | 1175 | 1166 | 72 (17.9) | 1175 | 809 | 72.6 (19.2) | 928 | 567 | 75.2 (18.8) |
| Autologous | 397 | 397 | 67.5 (18.9) | 397 | 310 | 75.4 (19.6) | 324 | 238 | 77.7 (18.9) | |
| Physical well-being | ||||||||||
| Irradiated | Implant | 339 | 338 | 79.6 (14.8) | 339 | 219 | 70 (13.8) | 230 | 122 | 71.3 (14.1) |
| Autologous | 235 | 235 | 72.5 (15) | 235 | 184 | 71.2 (13.9) | 197 | 148 | 72 (15.3) | |
| Unirradiated | Implant | 1175 | 1169 | 79.7 (14.1) | 1175 | 809 | 77.1 (14.1) | 928 | 564 | 77.6 (14.1) |
| Autologous | 397 | 397 | 77.2 (14.9) | 397 | 310 | 76.3 (15.8) | 324 | 239 | 77.2 (15.1) | |
A total of 2146 patients were included in the analytic cohort for the one-year analyses, that is, women for whom one or more years had elapsed since breast reconstruction and who did not fail.
Number of patients overall.
Number of patients with complete patient-reported outcomes measures.
Satisfaction with outcome of reconstruction was deliberately not assessed preoperatively.
Figure 1.
Model-predicted scores for BREAST-Q domains. Model-predicted scores are shown for the BREAST-Q domain of (A) satisfaction with breast, (B) satisfaction with outcome, (C) psychosocial well-being, and (D) physical well-being. A, C, and D) Scores were derived from the models in Supplementary Table 2 (available online) and adjust for baseline score, reconstruction timing, age, extent of disease, bilateral vs unilateral reconstruction, chemotherapy receipt, nodal management, body mass index, smoking, diabetes, race, ethnicity, education, employment, income, and hospital site. B) Scores were derived from the models in Supplementary Table 2 (available online) and adjust for reconstruction timing, age, extent of disease, bilateral vs unilateral reconstruction, chemotherapy receipt, nodal management, body mass index, smoking, diabetes, race, ethnicity, education, employment, income, and hospital site. Error bars represent the 95% confidence intervals.
Discussion
In this large, prospective, multicenter cohort study, after adjusting for a number of key covariates, we observed radiotherapy to increase complications and impair patient-reported satisfaction with breasts among patients receiving implant reconstruction but not those receiving autologous reconstruction. Although not all patients are candidates for autologous reconstruction, these findings have important implications for those who have decided to receive postmastectomy radiotherapy and must select a type of reconstruction. Although women must still weigh multiple factors, including the differences in operative time and rehabilitation required for different approaches, when selecting their preferred type of reconstruction, those who plan to receive postmastectomy radiotherapy should be informed of the substantial and statistically significant impact of radiotherapy observed in the current study among those who received implant reconstruction. Conversely, those who plan to pursue autologous reconstruction and are debating whether or not to receive radiotherapy may derive some reassurance from the current study findings that outcomes among patients receiving autologous reconstruction did not appear substantially worse than those of unirradiated patients by two years.
The impact of radiotherapy on breast reconstruction has long been studied (15,16). Radiation toxicity, including skin changes, vascular compromise, and fibrosis, can compromise the viability and cosmesis of the reconstruction and may require repeated intervention. In patients who pursue implant reconstruction, complications related to radiotherapy may include scarring, capsular contracture, infection, pain, skin necrosis, fibrosis, and impaired wound healing (17–21). Nevertheless, certain institutions have published promising outcomes with specific, carefully designed approaches that integrate implant reconstruction with postmastectomy radiotherapy (22,23). Patients who pursue autologous reconstruction may also face radiotherapy-related complications, including fat necrosis, fibrosis, atrophy, and flap contracture (17,24,25). Yet it has been hypothesized based on evidence from single institutional retrospective analyses that patients who receive radiotherapy may have better outcomes after autologous reconstruction than after implants (26).
Because the estimates of the frequency of complications with different approaches to the integration of radiotherapy and different types of reconstructive procedures vary widely across different institutional series, outcomes of patients treated in a variety of settings, such as in the current multicenter study, and including patient-reported satisfaction, are critically important. A previous study of reconstruction outcomes in the United States that included patient-reported outcomes measurement found higher aesthetic satisfaction at two years in patients who had received autologous tissue-based reconstruction rather than implant techniques (27), with those differences increasing over time (28). However, that study lacked sufficient numbers of irradiated patients to provide definitive conclusions about the impact of radiotherapy or outcomes in irradiated patients. A population-based study of patients identified through two Surveillance, Epidemiology, and End Results (SEER) registries that included 60 irradiated and 160 unirradiated patients who received postmastectomy breast reconstruction suggested that outcomes were influenced both by reconstruction type and radiotherapy receipt. Specifically, adjusted scaled cosmetic satisfaction scores on the CanSORT instrument were best for patients receiving autologous reconstruction without radiotherapy (4.39 out of 5), but not substantially different for patients receiving autologous reconstruction and radiotherapy (4.09) or patients receiving implant reconstruction without radiotherapy (3.86); scores were dramatically lower at 2.71 for patients receiving implant reconstruction and radiotherapy. Our current study results are consistent with the findings from that smaller study and now provide strong evidence from a large, multicenter data set, including prospective collection of rigorously validated patient-reported outcomes measures, supporting the idea that the impact of radiotherapy on reconstruction is most pronounced and clinically significant among patients receiving implant reconstruction.
Of note, although a higher proportion of women who received postmastectomy radiotherapy received autologous reconstruction (37.9%) than unirradiated women (25.0%), implant techniques were still the most common approach observed in this multicenter cohort, even in the setting of radiotherapy. Other studies have demonstrated a dramatic increase in the use of implant reconstruction over time (29). This trend may be driven by patient preferences to minimize the acute morbidity, operative time, hospitalization, and recovery time, but perhaps also by financial disincentives to perform complex, resource-intensive autologous reconstruction procedures (30). Of course, not all patients are candidates for autologous techniques, and not all patients would choose an autologous approach over implants even if aware of the complication and outcomes data we have collected. However, the availability of the information collected in the current study is critical for patients to be able to make optimally informed decisions that reflect their own values and preferences.
These findings, which constitute the sole analysis from MROC focused on the impact of radiotherapy on outcomes by reconstruction type, complement others derived from the same large data set. Analysis of one-year outcomes from MROC suggested that overall among patients receiving immediate reconstruction, those who received autologous reconstruction were more likely to experience complications (31) but also more satisfied with their breasts and had greater psychosocial and sexual well-being than those who underwent implant reconstruction (32). Other one-year analyses have examined whether outcomes differ by race/ethnicity (33), age (34), and whether both breasts were removed (35)—all factors for which we controlled in the current analysis. Other studies focus on issues of timing of reconstruction for patients receiving radiotherapy (36,37), as well as timing of surgical site infections (38). Together, these studies provide women considering breast reconstruction with the detailed estimates of risk and benefit information necessary to make optimally individualized decisions.
Although this study has multiple strengths, including its prospective design, large size, inclusion of numerous irradiated patients, and rigorous outcomes measures, it also has limitations. As in any observational study, associations may not indicate causation, and selection bias may exist. Nevertheless, given that randomized trials to investigate these issues have not been feasible, a carefully designed observational study is the best source of data available to inform patients making these critical decisions. As part of the study design, we have carefully collected and adjusted for potential confounding factors. Still, if patients selected for autologous reconstruction were generally healthier in unmeasured ways than patients receiving implants, this might be an underlying reason for the observation that implant patients had a higher risk of complications with radiotherapy than those undergoing autologous reconstruction. Another limitation is that complications were determined through chart review, which may not capture all events. Another potential limitation for the analyses of patient-reported outcomes is the large percentage (35.3%) of missing outcomes of the patients who were missing patient-reported outcomes at two years postreconstruction. While we used the approach of weighting by the inverse of the probability of response to account for missing outcomes, this relies on the assumption that missingness does not depend on the actual value of missing outcome after conditioning on the observed patient information. In the process of formulating the weights, we included a wide range of baseline patient characteristics so that the assumption is more likely to be met, although we also note that the assumption cannot be verified. We have also excluded those who had a reconstructive failure and thus excluded those who were most likely to have worse outcomes. This, however, also means our PRO findings cannot be generalized to those whose reconstruction failed. Because those patients who failed presumably had worse outcomes, the PRO analysis likely provides an overestimate of satisfaction for the overall population. Because failure rates were higher among irradiated patients receiving implant reconstruction, this would also bias against detecting further differences in patient-reported outcomes between groups.
In sum, although physicians and patients alike have long worried about the impact of radiotherapy on breast reconstruction, the evidence regarding outcomes to date has been limited. The current study provides much-needed quantitative information about complication rates and patient-reported outcomes of satisfaction and well-being among patients receiving implant or autologous reconstruction, either with or without radiotherapy. This information (see Box 1) is essential for informed patient decision-making in the increasingly common scenario where postmastectomy radiotherapy is being considered and postmastectomy reconstruction is desired.
Box 1.
Summary of clinical implications
Radiotherapy appears to increase breast complications and impair patient-reported satisfaction with breasts among patients receiving implant reconstruction but not those receiving autologous reconstruction.
At two years, major breast complications occurred in 33.2% of irradiated patients receiving implant-based reconstruction, 17.6% of irradiated patients receiving autologous reconstruction, 15.6% of unirradiated patients receiving implant-based reconstruction, and 22.9% of unirradiated patients receiving autologous reconstruction.
Rates of reconstruction failure by two years were 18.7% among irradiated patients with implants, 1.0% among irradiated patients with autologous reconstruction, 3.7% among unirradiated patients with implants, and 2.4% among unirradiated patients with autologous reconstruction.
Although women must weigh multiple factors, those who plan to receive postmastectomy radiotherapy should be informed of the substantial impact of radiotherapy observed in the current study among those who received implant reconstruction.
Conversely, those who plan to pursue autologous reconstruction and are debating whether or not to receive radiotherapy may derive some reassurance from the current study findings that outcomes among patients receiving autologous reconstruction did not appear substantially worse than those of unirradiated patients by two years.
Funding
This work was supported by the National Institutes of Health/National Cancer Institute (grant number R01 CA152192). ALP was supported, in part, by National Institutes of Health/National Cancer Institute Cancer Center Support (grant number P30 CA008748), funded by the National Cancer Institute (grant number 1RO1 CA152192).
Notes
The funders had no role in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The BREAST-Q is owned by Memorial Sloan Kettering Cancer Center and the University of British Columbia. Dr. Pusic is a codeveloper of the BREAST-Q and receives royalties when it is used in “for profit” industry-sponsored clinical trials.
This material was orally presented at the San Antonio Breast Cancer Symposium, December 2016.
We would like to gratefully acknowledge all the participating patients and all our colleagues at the following centers who contributed greatly to this multicenter trial: University of Michigan Health System, Ann Arbor, MI; Memorial Sloan-Kettering Cancer Center, New York City, NY; St. Joseph Mercy Hospital, Ypsilanti, MI; Northwestern Memorial Hospital, Chicago, IL; Ohio State Medical Center, Columbus, OH; Brigham and Women’s Hospital, Boston, MA; Georgetown University Medical Center, Washington, DC; Georgia Institute of Plastic Surgery, Savannah, GA; M.D. Anderson Cancer Center, Houston, TX; University of Manitoba, Winnipeg, MB; University of British Columbia, Vancouver, BC. Additionally, we would like to thank the Mastectomy Reconstruction Outcomes Consortium site investigators for their expertise and collaboration: Yoon S. Chun, MD, Richard Greco, MD, Troy A. Pittman, MD, Mark W. Clemens, MD, John Kim, MD, Gayle Gordillo, MD, Daniel Sherick, MD, Nancy VanLaeken, MD, and Edward Buchel, MD.
Supplementary Material
References
- 1. Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Co-operative Group 82b Trial. N Engl J Med. 1997;337(14):949–955. [DOI] [PubMed] [Google Scholar]
- 2. Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–1648. [DOI] [PubMed] [Google Scholar]
- 3. Ragaz J, Jackson SM, Le N, et al. Adjuvant radiotherapy and chemotherapy in node-positive premenopausal women with breast cancer. N Engl J Med. 1997;337(14):956–962. [DOI] [PubMed] [Google Scholar]
- 4. Early Breast Cancer Trialists' Collaborative Group, McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Recht A, Comen EA, Fine RE, et al. Postmastectomy radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology focused guideline update. J Clin Oncol. 2016;34(36):4431–4442. [DOI] [PubMed] [Google Scholar]
- 6.NCCN guidelines for breast cancer. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed July 20, 2017.
- 7. Kelley BP, Ahmed R, Kidwell KM, et al. A systematic review of morbidity associated with autologous breast reconstruction before and after exposure to radiotherapy: Are current practices ideal? Ann Surg Oncol. 2014;21(5):1732–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Momoh AO, Ahmed R, Kelley BP, et al. A systematic review of complications of implant-based breast reconstruction with prereconstruction and postreconstruction radiotherapy. Ann Surg Oncol. 2014;21(1):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilkins EG, Cederna PS, Lowery JC, et al. Prospective analysis of psychosocial outcomes in breast reconstruction: One-year postoperative results from the Michigan Breast Reconstruction Outcome Study. Plast Reconstr Surg. 2000;106(5):1014–1025. [DOI] [PubMed] [Google Scholar]
- 10. Atisha D, Alderman AK, Lowery JC, et al. Prospective analysis of long-term psychosocial outcomes in breast reconstruction: Two-year post- operative results from the Michigan Breast Reconstruction Outcomes Study. Ann Surg. 2008;247(6):1019–1028. [DOI] [PubMed] [Google Scholar]
- 11. Rowland JH, Desmond KA, Meyerowitz BE, et al. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. J Natl Cancer Inst. 2000;92(17):1422–1429. [DOI] [PubMed] [Google Scholar]
- 12. Al-Ghazal SK, Fallowfield L, Blamey RW.. Comparison of psychological aspects and patient satisfaction following breast conserving surgery, simple mastectomy and breast reconstruction. Eur J Cancer. 2000;36(15):1938–1943. [DOI] [PubMed] [Google Scholar]
- 13. Cano SJ, Klassen AF, Scott AM, et al. The BREAST-Q: Further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293–302. [DOI] [PubMed] [Google Scholar]
- 14. Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: The BREAST-Q. Plast Reconstr Surg. 2009;124(2):345–353. [DOI] [PubMed] [Google Scholar]
- 15. Kronowitz S. Current Status of implant-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(4):513e–523e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kronowitz S. Current status of autologous tissue-based breast reconstruction in patients receiving postmastectomy radiation therapy. Plast Reconstr Surg. 2012;130(2):282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jagsi R, Jiang J, Momoh AO, et al. Complications after mastectomy and immediate breast reconstruction for breast cancer: A claims-based analysis. Ann Surg. 2016;263(2):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krueger EA, Wilkins EG, Strawderman M, et al. Complications and patient satisfaction following expander/implant breast reconstruction with and without radiotherapy. Int J Radiat Oncol Biol Phys. 2001;49(3):713–721. [DOI] [PubMed] [Google Scholar]
- 19. Contant CME, van Geel AN, van der Holt B, et al. Morbidity of immediate breast reconstruction (IBR) after mastectomy by a subpectorally placed silicone prosthesis: The adverse effect of radiotherapy. Eur J Surg Oncol. 2000;26(4):344–350. [DOI] [PubMed] [Google Scholar]
- 20. Tallet AV, Salem N, Moutardier V, et al. Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant: Complications and esthetic results. Int J Radiat Oncol Biol Phys. 2003;57(1):136–142. [DOI] [PubMed] [Google Scholar]
- 21. Ascherman JA, Hanasono MM, Newman MI, et al. Implant reconstruction in breast cancer patients treated with radiation therapy. Plast Reconstr Surg. 2006;117(2):359–365. [DOI] [PubMed] [Google Scholar]
- 22. Ho A, Cordeiro P, Disa J, et al. Long-term outcomes in breast cancer patients undergoing immediate 2-stage expander/implant reconstruction and postmastectomy radiation. Cancer. 2012;118(9):2252–2259. [DOI] [PubMed] [Google Scholar]
- 23. Kronowitz SJ, Lam C, Terefe W, et al. A multidisciplinary protocol for planned skin-preserving delayed breast reconstruction for patients with locally advanced breast cancer requiring postmastectomy radiation: 3-year follow-up. Plast Reconstr Surg. 2011;127(6):2154–2166. [DOI] [PubMed] [Google Scholar]
- 24. Williams JK, Carlson GW, Bostwick J, et al. The effects of radiation treatment after TRAM flap breast reconstruction. Plast Reconstr Surg. 1997;100(5):1153–1160. [DOI] [PubMed] [Google Scholar]
- 25. Rogers NE, Allen RJ.. Radiation effects on breast reconstruction with the deep inferior epigastric perforator flap. Plast Reconstr Surg. 2002;109(6):1919–1924. [DOI] [PubMed] [Google Scholar]
- 26. Chawla AK, Kachnic L, Taghian AG, et al. Radiotherapy and breast reconstruction: Complications and cosmesis with TRAM versus tissue expander/implant. Int J Radiat Oncol Biol Phys. 2002;54(2):520–526. [DOI] [PubMed] [Google Scholar]
- 27. Alderman AK, Kuhn LE, Lowery JC, et al. Does patient satisfaction with breast reconstruction change over time? Two-year results of the Michigan Breast Reconstruction Outcomes Study. J Am Coll Surg. 2007;204(1):7–12. [DOI] [PubMed] [Google Scholar]
- 28. Hu ES, Pusic AL, Waljee JF, et al. Patient-reported aesthetic satisfaction with breast reconstruction during the long-term survivorship period. Plast Reconstr Surg. 2009;124(1):1–8. [DOI] [PubMed] [Google Scholar]
- 29. Jagsi R, Jiang J, Momoh AO, et al. Trends and variation in use of breast reconstruction in patients with breast cancer undergoing mastectomy in the United States. J Clin Oncol. 2014;32(9):919–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alderman AK, Storey AF, Nair NS, et al. Financial impact of breast reconstruction on an academic surgical practice. Plast Reconstr Surg. 2009;123(5):1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilkins EG, Hamill JB, Kim HM, et al. Complications in post-mastectomy breast reconstruction: One year outcomes of the Mastectomy Reconstruction Outcomes Consortium (MROC) study. Ann Surg. 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pusic AL, Matros E, Fine N, et al. Patient-reported outcomes 1 year after immediate breast reconstruction: Results of the Mastectomy Reconstruction Outcomes Consortium study. J Clin Oncol. 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Berlin NL, Momoh AO, Hamill JB, Kim HM, Pusic AL, Wilkins EG.. Racial and ethnic variations in one-year clinical and patient-reported outcomes following breast reconstruction. Am J Surg. 2017; in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Santosa KB, Qi J, Kim HM, Hamill JB, Pusic AL, Wilkins EG.. Effect of patient age on outcomes in breast reconstruction: Results from a multicenter prospective study. J Am Coll Surg. 2016;223(6):745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Momoh AO, Cohen WA, Kidwell KM, et al. Tradeoffs associated with contralateral prophylactic mastectomy in women choosing breast reconstruction: Results of a prospective multicenter cohort. Ann Surg. 2017;266(1):158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Billig J, Jagsi R, Qi J, et al. Should immediate autologous breast reconstruction be considered in women who require post-mastectomy radiation therapy? A prospective analysis of outcomes. Plast Reconstr Surg. 2017;139(6):1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Santosa KB, Chen X, Qi J, et al. Postmastectomy radiation therapy and two-stage implant-based breast reconstruction: Is there a better time to irradiate? Plast Reconstr Surg. 2016;138(4):761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sinha I, Pusic AL, Wilkins EG, et al. Late surgical-site infection in immediate implant-based breast reconstruction. Plast Reconstr Surg. 2017;139(1):20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.