Abstract
Brain-derived neurotrophic factor (BDNF) is an important modulator of constitutive stress responses mediated by limbic frontotemporal circuits, and its gene contains a functional polymorphism (Val66Met) that may influence trait stress sensitivity. Reports of an association of this polymorphism with anxiety-related personality traits have been controversial and without clear neurophysiological support. We, therefore, determined the relationship between resting regional cerebral blood flow (rCBF) and a well-validated measure of anxiety-related personality, the TPQ Harm Avoidance (HA) scale, as a function of BDNF Val66Met genotype. Sixty-four healthy participants of European ancestry underwent resting H215O positron emission tomography scans. For each genotype group separately, we first determined the relationship between participants' HA scores and their resting rCBF values in each voxel across the entire brain, and then directly compared these HA–rCBF relationships between Val66Met genotype groups. HA–rCBF relationships differed between Val homozygotes and Met carriers in several regions relevant to stress regulation: subgenual cingulate, orbital frontal cortex, and the hippocampal/parahippocampal region. In each of these areas, the relationship was positive in Val homozygotes and negative in Met carriers. These data demonstrate a coupling between trait anxiety and basal resting blood flow in frontolimbic neurocircuitry that may be determined in part by genetically mediated BDNF signaling.
Keywords: BDNF genotype, frontolimbic circuitry, harm avoidance, PET, resting rCBF
Introduction
Twin and family studies have shown that temperament and personality traits are highly heritable (Floderus-Myrhed et al. 1980; Bouchard and Loehlin 2001), but the specific genes and neurobiology underlying this heritability are largely unknown. Resolving this question is important because anxiety-related personality traits, such as harm avoidance (Cloninger et al. 1991), may determine resilience to stress and susceptibility to a range of psychiatric disorders (Hansenne et al. 1999; Jylha and Isometsa 2006).
One likely candidate gene is BDNF, which codes for brain-derived neurotrophic factor (BDNF), a molecule that is highly expressed in the hippocampus (Conner et al. 1997) and throughout the cortex, including prefrontal cortex (Hofer et al. 1990; Huntley et al. 1992). BDNF is crucial throughout development in maintaining neural integrity and plasticity (Messaoudi et al. 1998; Drake et al. 1999; Lu 2003), and several lines of evidence suggest that it may be important in determining an individual's resilience to stress. For instance, experimentally disrupting BDNF function in hippocampal and prefrontal regions results in exaggerated stress-induced neural atrophy and inhibition of neurogenesis (Duman et al. 1997; Shirayama et al. 2002; Warner-Schmidt and Duman 2006; Forbes et al. 2011; Gerritsen et al. 2012), whereas the administration of BDNF attenuates behavioral responses to stress in animals (Schmidt and Duman 2010; Ye et al. 2011).
A frequent and uniquely human single-nucleotide polymorphism (SNP) in the 5′ pro-region of BDNF (Val66Met, rs6265) affects activity-dependent intracellular trafficking and secretion of BDNF (Egan et al. 2003; Chen et al. 2004). Transgenic mice with the human Met allele demonstrate more anxious temperaments: impaired extinction learning (Chen et al. 2006; Soliman et al. 2010), elevated endocrinological reactivity to stress (Yu et al. 2012), and increased avoidant behaviors (Chen et al. 2006; Soliman et al. 2010). In humans, Met carriers also show impaired fear extinction (Soliman et al. 2010), propensity for affective disorders (Frodl et al. 2007), and greater trait harm avoidance (Jiang et al. 2005; Montag et al. 2010; but see Frustaci et al. 2008). In parallel with these observations, neuroimaging experiments have linked this SNP to structural (Pezawas et al. 2004), neurochemical (Egan et al. 2003), and functional (Egan et al. 2003; Hariri et al. 2003) attributes of the hippocampus, a region consistently implicated in stress adaptation (Oler et al. 2010). Moreover, basal resting-state neural activity in the hippocampus—but also in prefrontal regions involved in stress regulation, such as the subgenual cingulate cortex (Mayberg et al. 2005), medial orbital frontal cortex (Shin and Liberzon 2010), and parahippocampal gyrus (Papagni et al. 2011)—have shown strong BDNF Val66Met effects (Wei et al. 2012).
In light of the convergence of BDNF effects and stress regulation on the same neural circuitry and evidence of nonlinear dynamics of both BDNF and stress systems in the brain (Salehi et al. 2010; D'Amore et al. 2013), the possibility that BDNF–stress regulation interactions are functionally meaningful in the human brain merits investigation. Specifically, if BDNF genotype impacts the operation of these stress-regulatory regions in a manner relevant to anxiety-related traits, then there may be a modulatory effect of BDNF on trait–activity relationships in these circuits. In the context of full clinical anxiety disorders, for instance, this would suggest that BDNF genotype may dictate the nature of illness-related brain phenotypes. Providing credence to this hypothesis, an interaction between BDNF Val66Met genotype and anxiety disorder diagnosis has recently been identified in the hippocampus and amygdala, where only Met carrying patients (but not Val homozygotes) showed much greater neural responses to facial stimuli than controls (Lau et al. 2009). Whether such interaction effects might exist for BDNF and subclinical anxiety-related traits remains an open question.
In the present study, we examined a well-characterized cohort of healthy adults with resting-state H215O positron emission tomography (PET) and a self-report test of harm avoidance (Cloninger et al. 1991) to assess regional cerebral blood flow (rCBF) in correlation with TPQ Harm Avoidance (HA) scores during rest as a function of BDNF genotype. Because we were interested in obtaining reliable, absolute measurements of basal neural activity in regions near air-fluid-tissue interfaces that may introduce artifacts during magnetic resonance based imaging (e.g., medial orbitofrontal cortex), H215O PET provided an ideal method to test these hypothesized gene–personality physiological relationships.
Materials and Methods
Participants
Sixty-four healthy, right-handed participants of European ancestry, aged 18–50, provided written informed consent, as per procedures approved by the National Institutes of Health CNS Institutional Review Board and Radiation Safety Committee. The ethnicity of our participants was assessed based on self-report data. To ensure that the participants were free from confounding past or present psychiatric illness, they were screened with the Structured Clinical Interview for DSM-IV Axis I Disorders and DSM-IV Axis II Disorders Research Version (SCID-II; Spitzer et al. 1996). In addition, to rule out major medical illness, substance abuse, centrally active medications, and neurostructural abnormalities, each subject underwent physical examination, medical history review, laboratory tests, and a clinical magnetic resonance imaging scan series.
We restricted subject selection to Caucasians of European ancestry because BDNF allele frequencies vary across different populations. Notably, Met allele frequency varies from 55% to 19.9% and 43.6% in Sub-Saharan Africans, Europeans, and Asians, respectively (Petryshen et al. 2010).
BDNF Val66Met Genotyping
All subjects underwent blood draws and DNA was extracted using standard methods. BDNF genotyping was determined by TaqMan 5′ exonuclease assay using a custom-designed assay (Applied Biosystems, Foster City, CA, USA). To test for occult genetic stratification, participants were also genotyped for a functional polymorphism in COMT Val158Met (rs4680), and no significant COMT-by-BDNF interaction was found in the study populations. As is commonly done in investigations of genotype–phenotype associations with the BDNF Val66Met SNP, BDNF Val/Met heterozygotes and Met/Met homozygotes were combined into a “Met carrier” group because of the rarity of Met homozygotes (<5% in Caucasian samples [Shimizu et al. 2004]).
Self-Report Measure of Characterological Anxiety
The Tridimensional Personality Questionnaire (TPQ; Cloninger 1986), version IV, was administered to all participants as part of a larger assessment battery. The TPQ is a 100-item, self-administered, true/false questionnaire that measures 3 higher order temperament/personality dimensions—HA, novelty seeking (NS), and reward dependence (RD).
PET Data Acquisition
Participants were instructed to refrain from nicotine and caffeine for 4h prior to the PET session, and they were then instructed to lie still in the scanner and rest with their eyes closed. Following an 8-min transmission scan to correct for attenuation of radiation counts by the skull and intracranial tissues, two 60-s emission scans separated by 6 min and each preceded by an intravenous bolus of 10 mCi of [15O]-H2O were carried out. Data were collected with a GE Advance (Milwaukee) 3D PET scanner (septa retracted, in plane resolution 6- to 6.5-mm, 4.25-mm slice separation, 35 slices, axial field of view 15.3 cm).
PET Data Preprocessing
Scans were corrected for attenuation and were reconstructed into 32 axial planes (6.5 mm full-width at half-maximum [FWHM]). The reconstructed PET data were processed and analyzed with SPM5 (Wellcome Department of Cognitive Neurology, http://www.fil.ion.ucl.ac.uk/spm/). Scans were corrected for background activity, scaled proportionally to remove variations in global counts, co-registered (by first creating a mean [nonaligned] PET image for every participant and then co-registering all images to the single image), anatomically normalized to the SPM standard PET template, and smoothed using a 10-mm Gaussian kernel. After these steps, the 2 resting scans of each subject were averaged to enter into group-level analysis.
Statistical Procedures
Demographic, Genetic, and TPQ Analyses
General linear modeling, Pearson's correlations, and χ2 tests for analysis of demographics and TPQ score data were performed with PASW Statistics 18, Release Version 18.0.0 (SPSS, Inc., 2009, Chicago, IL, USA, www.spss.com). Haldane's exact test for evaluation of Hardy–Weinberg equilibrium was implemented in R (http://cran.r-project.org/web/packages/HardyWeinberg/index.html).
rCBF Analyses
First, to address our primary research objective, characterizing the relationship between trait anxiety and basal regional neural activity as a function of the BDNF Val66Met polymorphism, we determined rCBF–HA relationships within BDNF Val homozygotes and Met carrier groups separately with a voxel-wise, brain-wide linear regression analysis of resting rCBF using HA scores for each genotype group as regressors. Next, to test for between-group differences in this relationship, a brain-wide, linear regression model was estimated with 2 regressors: one representing the HA subtest scores for Val homozygotes, and the other representing the HA subtest scores for Met carriers. A planned contrast test was then performed to compare the beta weights for these 2 regressors, thereby directly testing whether BDNF genotype predicted differential relationships between HA scores and rCBF.
Within- and between-group voxel-wise correlation results were examined with a set of 3 regions of interest (ROIs) chosen a priori on the basis of previous demonstrations of differential neurophysiology by both BDNF genotype (Hariri et al. 2003; Wei et al. 2012) and in anxious and affective phenotypes (Mayberg et al. 2005; Oler et al. 2010; Shin and Liberzon 2010; Papagni et al. 2011) as well as being part of the resting-state network: the hippocampus/parahippocampus, subgenual cingulate cortex (BA25), and medial orbital frontal cortex (OFC). These ROIs were anatomically defined using Wake Forest University's Pick-Atlas software, and a correction for false-discovery rate (FDR) was applied for results within a single volume including all 3 ROIs. The FDR-corrected voxel values are calculated using the SPM software package, which incorporates this standard statistical approach to provide P-values corrected for the expected proportion of false positives among suprathreshold voxels that are within SPM5's implicit gray matter mask (Genovese et al. 2002). Additionally, within- and between-genotype results outside the ROIs that met a whole brain, voxel-wise statistical threshold of P = 0.05 FDR-corrected and a cluster threshold of 10 voxels are reported in Tables 2 and 3, where both uncorrected and FDR-corrected P values are given.
Table 2.
Within-genotype HA–rCBF correlation analysis
| Brain region | MNI coordinates |
Cluster size | T values | Puncorrected | PFDR-corrected | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A priori ROI analysis | |||||||
| L subgenual cingulate (BA25) | −14 | 16 | −20 | 480 | 5.29 | 0.000001 | 0.009 |
| R subgenual cingulate (BA25) | 8 | 18 | −2 | 100 | 4.76 | 0.000006 | 0.009 |
| R orbital frontal cortex (BA11) | 34 | 62 | −14 | 65 | 4.50 | 0.00002 | 0.009 |
| L orbital frontal cortex (BA11) | −14 | 48 | −14 | 149 | 4.29 | 0.00003 | 0.01 |
| L parahippocampal gyrus (BA36) | −32 | 0 | −36 | 90 | 3.79 | 0.0002 | 0.01 |
| L parahippocampal gyrus (BA28) | −18 | −6 | −32 | 76 | 3.74 | 0.0002 | 0.01 |
| R parahippocampal gyrus (BA28) | 22 | −2 | −30 | 80 | 3.64 | 0.0003 | 0.01 |
| Whole-brain voxel-wise analysis | |||||||
| R anterior cingulate (BA24) | 12 | 34 | 4 | 310 | 5.37 | 0.0000007 | 0.009 |
| Anterior cingulate (BA24) | −2 | 24 | 22 | 151 | 4.27 | 0.00004 | 0.01 |
| Anterior cingulate (BA32) | 0 | 44 | 12 | 155 | 4.21 | 0.00004 | 0.01 |
| L superior frontal gyrus (BA10) | −6 | 62 | 10 | 115 | 5.33 | 0.0000008 | 0.009 |
| L superior frontal gyrus (BA9) | −12 | 50 | 40 | 78 | 3.79 | 0.0002 | 0.01 |
| R superior frontal gyrus (BA10) | 22 | 66 | −4 | 85 | 4.84 | 0.000005 | 0.009 |
| R superior frontal gyrus (BA8) | 6 | 26 | 62 | 126 | 3.51 | 0.0004 | 0.01 |
| L middle frontal gyrus (BA8) | −48 | 14 | 48 | 60 | 3.62 | 0.0003 | 0.01 |
| R middle frontal gyrus (BA10) | 36 | 54 | 26 | 94 | 4.60 | 0.00001 | 0.009 |
| L middle temporal gyrus (BA21) | −48 | −30 | −18 | 249 | 4.90 | 0.000004 | 0.009 |
| L inferior temporal gyrus (BA21) | −46 | −14 | −26 | 78 | 4.15 | 0.00005 | 0.01 |
| L middle temporal gyrus (BA21) | −60 | −24 | −14 | 69 | 3.76 | 0.0002 | 0.01 |
| R inferior temporal gyrus (BA21) | 56 | −4 | −30 | 73 | 4.32 | 0.00003 | 0.01 |
| R temporopolar (BA38) | 24 | 8 | −42 | 84 | 4.60 | 0.00001 | 0.009 |
| L fusiform gyrus (BA37) | −48 | −62 | −24 | 98 | 3.79 | 0.0002 | 0.02 |
| R fusiform gyrus (BA20) | 52 | −26 | −30 | 74 | 3.78 | 0.0002 | 0.01 |
| R fusiform gyrus (BA37) | 58 | −56 | −24 | 54 | 3.49 | 0.0005 | 0.02 |
Positive HA–rCBF relationships in BDNF Val homozygotes during rest in regions chosen a priori, as well as those not hypothesized but identified in whole-brain analysis. Peak voxel coordinates meeting a threshold of P ≤ 0.05, FDR-corrected, and their exact uncorrected and corrected P values, T values, and cluster sizes are listed. There were no statistically significant negative HA–rCBF relationship in the Val homozygotes, and Met carriers showed no positive or negative HA–rCBF relationships at the P ≤ 0.05 FDR-corrected level. L, left; R, right; MNI, Montreal Neurological Institute.
Table 3.
Between-genotype differences in HA–rCBF correlations
| Brain region | MNI coordinates |
Cluster size | T values | Puncorrected | PFDR-corrected | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| A priori ROI analysis | |||||||
| Val ≥ Met | |||||||
| L subgenual cingulate (BA25) | −12 | 14 | −20 | 495 | 4.43 | 0.00002 | 0.05 |
| R orbital frontal cortex (BA11) | 14 | 18 | −22 | 248 | 4.46 | 0.00002 | 0.05 |
| L parahippocampal gyrus (BA28) | −20 | −2 | −28 | 368 | 4.48 | 0.00002 | 0.05 |
| Whole-brain voxel-wise analysis | |||||||
| Val ≥ Met | |||||||
| L dorsal anterior cingulate (BA32) | −8 | 20 | −8 | 549 | 4.77 | 0.000006 | 0.05 |
| R inferior frontal gyrus (BA45) | 12 | 34 | 6 | 106 | 4.67 | 0.00001 | 0.05 |
| R superior frontal gyrus (BA10) | 12 | 54 | 28 | 73 | 4.07 | 0.00007 | 0.05 |
Regions where HA and rCBF relationships differ between BDNF Val homozygotes and Met carriers during rest, showing results in brain areas chosen a priori as well as those not hypothesized but identified in whole-brain analysis. Peak voxel coordinates meeting a threshold of P ≤ 0.05, FDR corrected, and their exact uncorrected and FDR-corrected P values, T values, and cluster sizes are listed. There were no regions in which the correlation was greater in Met Carriers than in Val homozygotes at the P < 0.05 FDR-corrected level. L, left; R, right; MNI, Montreal Neurological Institute.
Finally, we performed 2 separate exploratory analyses to test the specificity of the HA–rCBF findings by examining the effect of BDNF genotype on the relationship between resting rCBF and the other personality measures derived from the TPQ (NS and RD). For these 2 TPQ measures, the same steps described above were carried out both within- and between-genotype groups. To further ensure that results are not solely due to other main or interactive effects of no interest, we conducted additional analyses that controlled for main effects of genotype, HA, covariates of no-interest (sex, age, and smoking), as well as all genotype × covariates of no interest and HA × covariates of no interest interactions.
Post hoc Analyses Determining TPQ–rCBF Relationships
To determine the direction of TPQ HA–, NS–, RD–rCBF relationships within genotype groups, rCBF values for each subject were extracted from spheres with 5 mm radii drawn around the most significant voxel showing differential TPQ–rCBF associations by genotype within each ROI. Pearson's correlation was used to relate the extracted values to higher order temperament/personality dimensions scores.
Results
BDNF Val66Met Genotypes and Demographics
The participants in the current study were a subset of participants examined in a previous study (Wei et al. 2012). Six participants reported being smokers. There were 43 Val homozygotes (mean age 31.7 ± 8.8 years) consisting of 21 males and 22 females (mean ages 31.4 ± 8.6 and 31.9 ± 9.1 years, respectively), as well as 21 Met carriers (mean age 35.4 ± 8.3 years; 2 Met homozygotes) consisting of 10 males and 11 females (mean ages 34.9 ± 9.8 and 35.9 ± 8.5, respectively). Haldane's exact test (Haldane 1954) confirmed that genotype frequencies were in Hardy–Weinberg equilibrium (D = 0.0664; P = 1). Neither age nor gender distribution differed between Val homozygotes and Met carriers (t(62) = 0.11, P = 0.27 and 0.008 [df = 1, N = 64], P = 0.927, respectively).
BDNF Val66Met Genotype and Harm Avoidance Scores
There was no significant difference between HA scores of Val homozygotes (8.09 ± [standard deviation (SD)] 4.44) and those of Met carriers (8.76 ± 4.67; t(63) = 0.58, P = 0.56). HA did not correlate with age across the whole sample (Pearson's r = 0.01, P = 0.92) or in either genotype group (Val/Val: Pearson's r = −0.008, P = 0.961; Met carriers: Pearson's r = −0.03, P = 0.884), and there was no age-by-genotype interaction (F2,62 = 0.09, P = 0.76). Across the whole sample, women showed significantly higher HA scores than men (10.09 ± 4.22 vs. 6.42 ± 4.02; t(62) = 3.56, P = 0.0007). Post hoc uncorrected two-sample t-tests revealed higher TPQ HA scores in women than in men for the Val homozygotes (10.18 ± 0.90 vs. 5.90 ± 0.78; t(63) = 3.57, P = 0.0009). In the Met carrier group, HA scores were higher in women than men, but not significantly so (9.80 ± 1.46 vs. 7.50 ± 1.54; t(63) = 1.19, P = 0.24). The interaction between BDNF Val66Met genotype and gender on HA scores did not reach statistical significance (F2,62 = 1.99, P = 0.07).
BDNF Val66Met Genotype and Novelty Seeking/Reward Dependence
There was no significant genotype effect on NS or RD scores (Table 1). Correlations of age with NS and RD did not reach statistical significance in the sample as a whole (NS and RD Pearson's r's ≤ |0.25|, P's ≥ 0.06) or within either genotype group (Val/Val: NS and RD Pearson's r ≤ |0.21|; P ≥ 0.18; Met Carriers: NS and RD Pearson's r ≤ |0.31|; P ≥ 0.17), and there was no age-by-genotype interaction (NS and RD: F2,62 ≤ 0.64, P ≥ 0.88). NS and RD scores did not differ significantly between men and women in the sample as a whole (NS and RD: t(62) ≤ 0.96, P ≥ 0.34) or within either genotype group (NS and RD Val/Val and Met Carriers: t(62) ≤ 1.86, P ≥ 0.07), and there were no sex-by-genotype interactions (NS and RD: F2,62 ≤ 2.13, P ≥ 0.15).
Table 1.
TPQ HA, novelty seeking, and reward dependence scores (Cloninger 1986) by genotype
| Val homozygotes (mean ± SD), range | Met carriers (mean ± SD), range | |
|---|---|---|
| Harm avoidance (max score: 34) | 8.09 ± 4.44, 0–21 | 8.76 ± 4.67, 0–15 |
| Novelty seeking (34) | 14.76 ± 4.44, 6–25 | 13.9 ± 4.44, 3–23 |
| Reward dependence (32) | 19.77 ± 3.74, 10–25 | 17.19 ± 5.78, 6–25 |
No statistically significant genotype effects were seen.
Between- and Within-BDNF Genotype Effects on the Relationship of rCBF to Harm Avoidance
In Val homozygotes, within-group, voxel-wise, whole-brain regression analyses demonstrated significant positive relationships between rCBF and HA in several brain areas, including regions chosen a priori, particularly those in medial OFC and medial temporal regions (Table 2). Post hoc examination of these data revealed extension of the parahippocampal cluster well into the amygdala. In contrast, there were no such positive relationships in the Met carriers. Additionally, there were no regions in which there were significant negative relationships at P = 0.05, FDR corrected, within either genotype group, although trends in this direction were observed in Met carriers.
Between-genotype analysis demonstrated a significantly different HA–rCBF relationship in Val homozygotes compared with Met carriers in several of the regions chosen a priori: left parahippocampal gyrus, subgenual cingulate, and right medial OFC (all P's = 0.00002 uncorrected, P = 0.05 FDR corrected). Details of these results are given in Table 3. Several additional loci in the medial OFC were significant at the P < 0.00007, uncorrected level and the P = 0.05 FDR-corrected level: (right OFC: 12, 62, −20; left: −10. 40, −22 and −34, 60, −10). When analyses were repeated including sex, age, smoking status, BDNF × sex and BDNF × age interaction in the model, the findings were unchanged.
When an expanded model analysis controlling for main effects of genotype, personality characteristics, covariates of no-interest (sex and age), as well as all genotype × covariates of no interest and HA × covariates of no interest interactions results were less robust (P< 0.001, uncorrected) but otherwise unchanged.
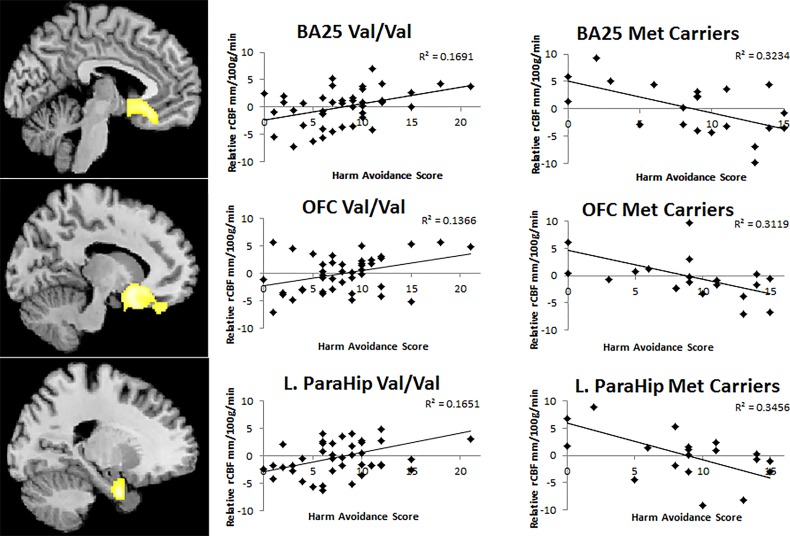
Post hoc examination using Pearson's correlation to examine and decompose the statistically significant genotype-by-HA rCBF interaction (Table 3) revealed a consistent pattern within genotype groups: all observed associations between HA and rCBF were positive in Val homozygotes (P's < 0.006) and negative in Met carriers (P's < 0.01). These findings in the a priori ROIs, BA25 (subgenual cingulate), medial OFC, and left parahippocampus, are shown in Figure 1.
Figure 1.
BDNF genotype and HA interactions affect rCBF. Statistical parametric map and graphs of differential BDNF genotype × HA relationships with resting rCBF in BA25, OFC, and the left parahippocampus (figure at left shown at P = 0.001, uncorrected for display). Associated plots show post hoc linear fits of HA scores versus extracted mean rCBF values from a sphere with 5 mm radius drawn around the seed voxel at BA25 (MNI x, y, z = −12, 14, −20), BA 11 (MNI x, y, z = 14, 18, −22), and the left parahippocampus (MNI x, y, z = −20, −2, −18) demonstrating positive relationships with HA for the Val homozygotes and negative relationships with HA for the Met carriers. Between-genotype group differences at these regions of interest t = 4.43, 4.46, 4.48, respectively, P = 0.006, FDR corrected (Table 3). BDNF, brain-derived neurotrophic factor; HA, harm avoidance; rCBF, regional cerebral blood flow; BA25, subgenual cingulate; OFC, medial orbital frontal cortex.
Between- and Within-BDNF Genotype Effects on the Relationship of rCBF to Novelty Seeking and Reward Dependence
In contrast to the HA–rCBF findings, there were no significant relationships between rCBF and NS or RD within genotype groups. Nor were there significant genotype-related differences in correlations between rCBF and NS or RD in ROI's chosen a priori (all P's > 0.06, uncorrected). Whole-brain, voxel-wise analyses revealed a single, unpredicted region in which there was a differential NS-rCBF correlation between Val homozygotes and Met Carriers: BA 7 (P = 0.04, FDR corrected). Post hoc analyses in this region revealed the same pattern of relationships within genotype groups that was observed for HA: a positive NS-rCBF relationship was seen in the Val homozygotes (r = 0.41, P = 0.007), whereas there was a negative NS-rCBF correlation in the Met carriers (r = −0.68, P = 0.001).
Discussion
Our data support differential relationships between the trait anxiety measure of harm avoidance and resting rCBF in BDNF Val homozygotes and Met carriers in brain regions previously shown to be modulated by BDNF genotype and involved in anxiety regulation. However, at the behavioral level, the present study did not confirm several previous reports of association between the BDNF Val66Met polymorphism and HA scores (Jiang et al. 2005; Montag et al. 2010), which is consistent with other negative findings in the literature (Frustaci et al. 2008), suggesting that if there is a reliable genotype–HA relationship, its effect size may be relatively small. Nonetheless, our finding of higher HA scores in women than in men is consistent with past research, suggesting greater female bias toward negative valence, such as susceptibility to some anxiety-related personality traits (Feingold 1994) as well as anxiety and depressive disorders (WHO 2008).
H215O PET, a gold-standard method for measuring rCBF, provides a direct, well-validated, perfusion-based estimate of regional neural activity and metabolism, which [unlike canonical functional magnetic resonance imaging (fMRI) based approaches) does not rely on comparisons with control conditions or on interregional covariance of fluctuating hemodynamic signal variation over time (as does resting-state fMRI). Taking advantage of this methodology, we have demonstrated the existence of statistically robust relationships between HA and basal neural activity in several brain regions that are implicated in anxiety and affect regulation, and have even been identified as potential treatment targets (Nestler et al. 2002; Mayberg et al. 2005; Martin et al. 2009), including the hippocampus/parahippocampus, subgenual anterior cingulate, and medial OFC. These findings align well with the notion of HA as a trait closely associated with the development of anxiety and depressive illnesses (Ongur et al. 2005; Nery et al. 2008, 2009). Additionally, in the context of this study's cohort—individuals without current or past anxiety or affective disorders—such an observation highlights the likelihood that similar neural circuits might underlie subclinical, characterological anxiety phenomena.
Furthermore, we have shown that these HA–rCBF relationships are very different depending on BDNF genotype, with strongly positive relationships in Val homozygotes and trends toward negative relationships in Met carriers. This is in line with a large body of evidence supporting the influence of the BDNF Val66Met polymorphism on the development, operation, and structure of these same regions, most notably the hippocampus (Smith et al. 1995; Chen et al. 2001; Nestler et al. 2002), but also the subgenual cingulate (Gerritsen et al. 2012; Wei et al. 2012) and the OFC (Lotfipour et al. 2009; Forbes et al 2011). Though associative in nature, these results make plausible the possibility that either developmental or ongoing functional effects of the Val66Met polymorphism modulate the neural substrates of trait anxiety. The Met allele is associated with heightened basal neural activity in anxiety circuit nodes (Wei et al. 2012); impaired extinction learning, a phenotype commonly observed in anxiety disorders (Soliman et al. 2010); and, in BDNF Met knock-in mice, increased anxiety-like behavior (Chen et al. 2006; Soliman et al. 2010). Taken together with the present data, these findings encourage speculation that with greater trait anxiety, Met carriers increasingly shunt neural resources away from frontotemporal limbic structures already operating at capacity, to ancillary networks, whereas Val homozygotes are able to more flexibly engage these same circuits in the predicted manner (Oler et al. 2010). Determining whether this is, in fact, the case and whether this interaction is relevant to the greater risk of stress-related affective illness in Met carriers must await further research.
Several additional caveats warrant mention regarding the present work. First, although many important factors including age, race, sex, and past psychiatric illness were carefully controlled, other factors such as hormones (e.g., menstrual cycle phase), early life stress, and the environment could interact with genes and influence the underlying neurobiology of the intermediate phenotype. Moreover, the HA scores were acquired from self-administered questionnaires, and the measure may be susceptible to reporting bias. Additionally, rCBF measurements, although a well-validated “snap-shot” of metabolic topography, are dynamic and sensitive to changes in emotion and cognitive activity, as are all functional neuroimaging methods. Though increasingly utilized, resting-state paradigms are subject to the vagaries of the thought processes enacted by participants as well as their experiences. Thus, we cannot rule out the possibility that systematic differences in thought content or reaction to the scanning environment are responsible for the findings presented here. Indeed, some work has suggested that BDNF genotype affects predisposition to ruminative thoughts in response to life stressors (Clasen et al. 2011). Lastly, depending on the magnitude of the effect size of the BDNF × HA interaction on rCBF, which has been heretofore unknown, it is possible that the present dataset may be underpowered. Suboptimal statistical power increases the risk of a false-negative result but may also inflate false-discovery rates. This is particularly the case where the variance of the predictor is restricted or the prior probability of finding a significant effect is low (Duncan and Keller 2011). In the present study, however, these concerns are relatively diminished because of the sizeable Met allele carrier group, the fair distribution of the HA scores, and the consistent prior biological evidence for convergence of BDNF and anxiety effects in the examined circuitry. Along with the robustness of our findings, which achieve P-values well below corrected thresholds, these considerations suggest the most likely interpretation of our findings is that they are driven by true biological signal rather than statistical error. In addition, our study adopts a hypothesis-driven approach involving a single polymorphism, already well known to be functional in the studied circuitry at both the cellular and systems level, as well as an incisive PET assay of basal rCBF.
In conclusion, while correlation does not infer causality, our data suggest that trait anxiety-limbic system neurophysiology relationships are genetically influenced. Further studies including examining the observed genotype-by-personality-trait interaction on resting rCBF in a separate larger cohort and investigating the effects of sex on the present findings are necessary to understand how BDNF-related characteristics of these reward systems-level relationships might contribute to susceptibility to stress-related neuropsychiatric illness.
Funding
This work was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
Notes
We thank the Positron Emission Tomography Department of the National Institutes of Health for their help in data collection. Conflict of Interest: None declared.
References
- Bouchard TJ Jr, Loehlin JC. 2001. Genes, evolution, and personality. Behav Genet. 31(3):243–273. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. 2001. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry. 50(4):260–265. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Jing DQ, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS et al. 2006. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 314(5796):140–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS. 2004. Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci. 24(18):4401–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasen PC, Wells TT, Knopik VS, McGeary JE, Beevers CG. 2011. 5-HTTLPR and BDNF Val66Met polymorphisms moderate effects of stress on rumination. Genes Brain Behav. 10(7):740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR. 1986. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 4(3):167–166. [PubMed] [Google Scholar]
- Cloninger CR, Przybeck TR, Svrakic DM. 1991. The tridimensional personality questionnaire—United-States normative data. Psychol Rep. 69(3):1047–1057. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. 1997. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 17(7):2295–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore DE, Tracy BA, Parikh V. 2013. Exogenous BDNF facilitates strategy set-shifting by modulating glutamate dynamics in the dorsal striatum. Neuropharmacology. 75:312–323. [DOI] [PubMed] [Google Scholar]
- Drake CT, Milner TA, Patterson SL. 1999. Ultrastructural localization of full-length trkB immunoreactivity in rat hippocampus suggests multiple roles in modulating activity-dependent synaptic plasticity. J Neurosci. 19(18):8009–8026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Heninger GR, Nestler EJ. 1997. A molecular and cellular theory of depression. Arch Gen Psychiatry. 54(7):597–606. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. 2011. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. Am J Psychiatry. 168(10):1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M et al. 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 112(2):257–269. [DOI] [PubMed] [Google Scholar]
- Feingold A. 1994. Gender differences in personality—a meta-analysis. Psychol Bull. 116(3):429–456. [DOI] [PubMed] [Google Scholar]
- Floderus-Myrhed B, Pederson N, Rasmuson I. 1980. Assessment of heritability for personality, based on a short-form of the Eysenck personality inventory: a study of 12,898 twin pairs. Behav Genet. 10:153–162. [DOI] [PubMed] [Google Scholar]
- Forbes CE, Poore JC, Barbey AK, Krueger F, Solomon J, Lipsky RH, Hodgkinson CA, Goldman D, Grafman J. 2011. BDNF polymorphism-dependent OFC and DLPFC plasticity differentially moderates implicit and explicit bias. Cereb Cortex. 2211:2602–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Schule C, Schmitt G, Born C, Baghai T, Zill P, Bottlender R, Rupprecht R, Bondy B, Reiser M et al. 2007. Association of the brain-derived neurotrophic factor Val66Met polymorphism with reduced hippocampal volumes in major depression. Arch Gen Psychiatry. 64(4):410–416. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Pozzi G, Gianfagna F, Manzoli L, Boccia S. 2008. Meta-analysis of the brain-derived neurotrophic factor gene (BDNF) Val66Met polymorphism in anxiety disorders and anxiety-related personality traits. Neuropsychobiology. 58(3-4):163–170. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. 2002. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 15:870–878. [DOI] [PubMed] [Google Scholar]
- Gerritsen L, Tendolkar L, Franke B, Vasquez AA, Kooijman S, Buitelaar J, Fernández G, Rijpkema M. 2012. BDNF Val66Met genotype modulates the effect of childhood adversity on subgenual anterior cingulate cortex volume in healthy subjects. Mol Psychiatry. 17(6):597–603. [DOI] [PubMed] [Google Scholar]
- Haldane JB. 1954. An exact test for randomness of mating. J Genet. 52:631–635. [Google Scholar]
- Hansenne MJ, Reggers J, Pinto E, Kjiri K, Ajamier A, Ansseau M. 1999. Temperament and character inventory (TCI) and depression. J Psychiatr Res. 33(1):31–36. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR. 2003. Brain-derived neurotrophic factor val(66)met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 23(17):6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer M, Pagliusi SR, Hohn A, Leibrock J, Barde YA. 1990. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 9(8):2459–2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huntley GW, Benson DL, Jones EG, Isackson PJ. 1992. Developmental expression of brain derived neurotrophic factor messenger-RNA by neurons of fetal and adult monkey prefrontal cortex. Dev Brain Res. 70(1):53–63. [DOI] [PubMed] [Google Scholar]
- Jiang XY, Xu K, Hoberman J, Tian F, Marko AJ, Waheed JF, Harris CR, Marini AM, Enoch MA, Lipsky RH. 2005. BDNF variation and mood disorders: a novel functional promoter polymorphism and Val66Met are associated with anxiety but have opposing effects. Neuropsychopharmacology. 30(7):1353–1361. [DOI] [PubMed] [Google Scholar]
- Jylha P, Isometsa E. 2006. Temperament, character and symptoms of anxiety and depression in the general population. Eur Psychiatry. 21(6):389–395. [DOI] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Hodgkinson C, Leibenluft E, Nelson E, Sankin L, Pine DS, Ernst M. 2009. BDNF gene polymorphism (Val66Met) predicts amygdala and anterior hippocampus responses to emotional faces in anxious and depressed adolescents. Neuroimage. 53(3):952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotfipour S, Ferguson E, Leonard G, Perron M, Pike B, Richer L, Séguin JR, Toro R, Veillette S, Pausova Z et al. 2009. Orbitofrontal cortex and drug use during adolescence: role of prenatal exposure to maternal smoking and BDNF genotype. Arch Gen Psychiatry. 66(11):1244–1252. [DOI] [PubMed] [Google Scholar]
- Lu B. 2003. BDNF and activity-dependent synaptic modulation. Learn Mem. 10(2):86–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EI, Ressler KJ, Binder E, Nemeroff CB. 2009. The neurobiology of anxiety disorders: brain imaging, genetics, and psychoneuroendocrinology. Clin Lab Med. 30(4):865. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, Schwalb JM, Kennedy SH. 2005. Deep brain stimulation for treatment-resistant depression. Neuron. 45(5):651–660. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Bardsen K, Srebro B, Bramham CR. 1998. Acute intrahippocampal infusion of BDNF induces lasting potentiation of synaptic transmission in the rat dentate gyrus. J Neurophysiol. 79(1):496–499. [DOI] [PubMed] [Google Scholar]
- Montag C, Basten U, Stelzel C, Fiebach CJ, Reuter M. 2010. The BDNF Val66Met polymorphism and anxiety: support for animal knock-in studies from a genetic association study in humans. Psychiatry Res. 179(1):86–90. [DOI] [PubMed] [Google Scholar]
- Nery FG, Hatch JP, Glahn DC, Nicoletti MA, Monkul ES, Najt P, Fonseca M, Bowden CL, Cloninger CR, Soares JC. 2008. Temperament and character traits in patients with bipolar disorder and associations with comorbid alcoholism or anxiety disorders. J Psychiatr Res. 42:569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG, Hatch JP, Nicoletti MA, Monkul ES, Najt P, Matsuo K, Cloninger CR, Soares JC. 2009. Temperament and character traits in major depressive disorder: influence of mood state and recurrence of episodes. Depress Anxiety. 26(4):382–388. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. 2002. Neurobiology of depression. Neuron. 34(1):13–25. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. 2010. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 466(7308):864–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongur D, Farabaugh A, Iosifescu DV, Perlis R, Fava M. 2005. Tridimensional personality questionnaire factors in major depressive disorder: relationship to anxiety disorder comorbidity and age of onset. Psychother Psychosom. 74:173–178. [DOI] [PubMed] [Google Scholar]
- Papagni SA, Benetti S, Arulanantham S, McCrory E, McGuire P, Mechelli A. 2011. Effects of stressful life events on human brain structure: a longitudinal voxel-based morphometry study. Stress. 14(2):227–232. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, Sklar P. 2010. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry. 15(8):810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Verchinski BA, Mattay VS, Callicott JH, Kolachana BS, Straub RE, Egan MF, Meyer-Lindenberg A, Weinberger DR. 2004. The brain-derived neurotrophic factor val66met polymorphism and variation in human cortical morphology. J Neurosci. 24(45):10099–10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi B, Cordero MI, Sandi C. 2010. Learning under stress: the inverted-U-shape function revisited. Learn Mem. 17:522–530. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Duman RS. 2010. Peripheral BDNF produces antidepressant-like effects in cellular and behavioral models. Neuropsychopharmacology. 35(12):2378–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M. 2004. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. Am J Med Genet B Neuropsychiatr Genet. 126B(1):122–123. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I. 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 35(1):169–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. 2002. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 22(8):3251–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. 1995. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 15(3):1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH et al. 2010. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 327(5967):863–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. 1996. Structured Clinical Interview for DSM-IV Axis I Disorders Research Version (SCID-I). New York: New York State Psychiatric Institute, Biometrics Research. [Google Scholar]
- Warner-Schmidt JL, Duman RS. 2006. Hippocampal neurogenesis: opposing effects of stress and antidepressant treatment. Hippocampus. 16(3):239–249. [DOI] [PubMed] [Google Scholar]
- Wei SM, Eisenberg DP, Kohn PD, Kippenhan JS, Kolachana BS, Weinberger DR, Berman KF. 2012. Brain-derived neurotrophic factor Val66Met polymorphism affects resting regional cerebral blood flow and functional connectivity differentially in women versus men. J Neurosci. 32(20):7074–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. 2008. The global burden of disease: 2004 update, Table A2: Burden of disease in DALYs by cause, sex and income group in WHO regions, estimates for 2004 Geneva, Switzerland: World Health Organization; http://www.who.int/healthinfo/global_burden_disease/GBD_report_2004update_full.pdf. [Google Scholar]
- Ye Y, Wang G, Wang H, Wang X. 2011. Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett. 503(1):15–19. [DOI] [PubMed] [Google Scholar]
- Yu H, Wang DD, Wang Y, Liu T, Lee FS, Chen ZY. 2012. Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci. 32(12):4092–4101. [DOI] [PMC free article] [PubMed] [Google Scholar]