Abstract
The retrosplenial cortex (RSC) plays an important role in memory and spatial navigation. It shares functional similarities with the hippocampus, including the presence of place fields and lesion-induced impairments in spatial navigation, and the RSC is an important source of visual-spatial input to the hippocampus. Recently, the RSC has been the target of intense scrutiny among investigators of human memory and navigation. fMRI and lesion data suggest an RSC role in the ability to use landmarks to navigate to goal locations. However, no direct neurophysiological evidence of encoding navigational cues has been reported so the specific RSC contribution to spatial cognition has been uncertain. To examine this, we trained rats on a T-maze task in which the reward location was explicitly cued by a flashing light and we recorded RSC neurons as the rats learned. We found that RSC neurons rapidly encoded the light cue. Additionally, RSC neurons encoded the reward and its location, and they showed distinct firing patterns along the left and right trajectories to the goal. These responses may provide key information for goal-directed navigation, and the loss of these signals may underlie navigational impairments in subjects with RSC damage.
Keywords: landmark, learning and memory, visual cue, cingulate cortex
Introduction
The retrosplenial cortex (RSC) is a key component of the brain's memory and navigation systems (Vann et al. 2009; Miller et al. 2014). Effects of RSC lesions are strikingly similar to the well-known effects of hippocampal lesions, including impairments in spatial navigation (Sutherland et al. 1988; Takahashi et al. 1997; Harker and Whishaw 2002; Vann and Aggleton 2002), contextual memory (Keene and Bucci 2008a, 2008b, 2008c, 2009), and episodic memory (Valenstein et al. 1987; Bowers et al. 1988). Similar to the hippocampus, RSC neurons exhibit place fields (Cho and Sharp 2001; Smith et al. 2012, but see Alexander and Nitz 2015) and the firing of RSC neurons is sensitive to the spatial context (Smith et al. 2004). The RSC is an important hub for visual-spatial information from the dorsal stream into the hippocampus (Kravitz et al. 2011) and inactivation of the RSC disrupts hippocampal representations (Cooper and Mizumori 1999). The RSC also appears to be an important consolidation target for hippocampal-dependent memories (Katche et al. 2013), especially contextual memories (Keene and Bucci 2008a, 2008b, 2008c, 2009; Czajkowski et al. 2014; Tanaka et al. 2014; Sigwald et al. 2015). Consistent with findings from the hippocampus (Liu et al. 2012; Tonegawa et al. 2015), artificial reactivation of the same population of RSC neurons which was active during contextual learning can trigger contextual fear memories (Cowansage et al. 2014).
Despite this growing literature, the specific contribution of the RSC to spatial cognition is not known. One hypothesis is that the RSC plays a critical role in encoding important navigational cues (Auger et al. 2012; Smith et al. 2012; Miller et al. 2014). Human subjects with RSC lesions are frequently impaired in spatial navigation and a striking feature of this deficit is the inability to use landmarks to construct routes to goal locations (Takahashi et al. 1997; Maguire 2001; Ino et al. 2007; Kim et al. 2015). Many fMRI studies have suggested an RSC role in navigation (Sugiura et al. 2005; Epstein 2008; Sherrill et al. 2013; Epstein and Vass 2014), particularly in the use of navigational cues (Wolbers et al. 2004; Epstein et al. 2007). The RSC even appears to preferentially encode permanent features of the environment rather than temporary movable objects, which would be less useful for navigation (Auger et al. 2012; Auger et al. 2015). However, an unambiguous neurophysiological correlate of navigational cue processing has not been observed in RSC neurons.
A previous study from our laboratory provided tentative support for an RSC role in encoding navigational cues (Smith et al. 2012). We trained rats on a blocked alternation task, in which they approached one location for reward for the first half of the session and then they switched to a different location for the second half. In solving this task, rats invariably employed a “win-stay” strategy: they repeatedly went to the first location as long as rewards continued to be dispensed there and they only switched to the new location after failing to get a reward at the initial location. Consistent with the importance of the reward for this strategy, a large number of RSC neurons (~40%) selectively responded to either the left or right reward. It is possible that these neurons encoded the reward as a navigational cue (i.e. “return to this location on the next trial”). Alternatively, RSC neurons may have been sensitive to the conjunction of the reward and its spatial location. Similar conjunctive coding is a prominent feature of hippocampal responses (Komorowski et al. 2009; McKenzie et al. 2014) and recent findings indicate that RSC neurons are sensitive to conjunctions of movements (left and right turns) and the rat's location and trajectory within the environment (Alexander and Nitz 2015). In order to conclusively determine whether RSC neurons encode navigational cues, we trained rats on a T-maze task in which the reward location was explicitly cued by a flashing light which serves as a beacon that the rats must learn to approach. We chose to use a beacon, rather than one or more landmarks, because the light has an unambiguous onset time and we could be confident that the rats would attend to this highly salient cue. We recorded RSC neuronal responses to the light cue and other task events as well as spatial firing patterns throughout learning.
Materials and Methods
Subjects and Surgical Procedures
Subjects were 5 adult male Long Evans rats (Charles River Laboratories, Wilmington, MA) weighing 250–300 g upon arrival. Rats were placed on a 12 hr/12 hr light/dark cycle with lights on at 7 am and allowed to acclimate to the vivarium for at least one week prior to surgery. Rats were implanted with a custom-built electrode microdrive (Macdonald et al. 2011) containing 16 movable tetrodes made from twisting together four 17 μm platinum/iridium (90%/10%) wires, platinum plated to an impedance of 100–300 kΩ, and arranged in a linear 2 × 8 array that spanned approximately 4 mm along the rostrocaudal axis of the brain. Tetrodes were stereotaxically positioned unilaterally, just above the RSC at 2.5–6.5 mm posterior to Bregma, 0.5 mm lateral, and 0.84 mm ventral to the cortical surface. Rats were given 7 days to recover from surgery prior to lowering the tetrodes into the RSC over the course of several days (35–70 μm daily) until a depth of at least 1.0 mm was reached (granular area b of the RSC) and a sufficient population of neurons was acquired. All procedures complied with the guidelines of the Cornell University Animal Care and Use Committee.
Behavioral Training
The behavioral apparatus was a black PVC T-maze (112 cm long stem × 122 cm wide × 68 cm above the floor) that occupied a circular arena enclosed by black curtains. Visual cues of various shapes, sizes and colors were pinned to the curtains. Prior to training, rats were food restricted to 85–90% of free-feeding body weight and acclimated to the maze and chocolate milk rewards (200 μL, Nestle's Quik) until they consumed 20 rewards within 30 min, usually 2 sessions.
Prior to the regular training sessions, each rat was given a preliminary training session (pretraining) in order to collect baseline neural responses to the light cue before learning. During this session, a single light cue, identical to the lights that were later used to cue the reward locations (see below), was positioned at the choice point. During this session, the light was turned on for half of the 20 trials and both reward cups were always baited. Thus, the light did not signal the reward location and these recordings served as a measure of baseline responsivity to the light prior to associative learning.
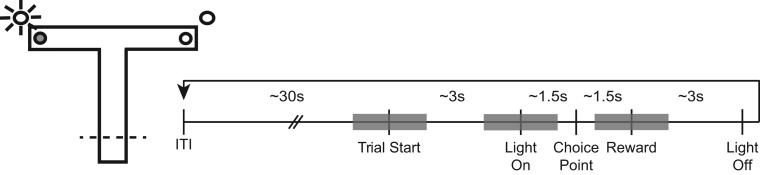
During regular training sessions, two light cues (each constructed from side by side LEDs, 5 cm apart that flashed alternatingly at 3 Hz) were positioned 9 cm above each of the reward locations. The timeline for the trials is indicated in Figure 1. At the start of each trial, the rat was placed on the stem of the maze facing away from the choice point. As soon as the rat turned around, the experimenter turned on one of the two light cues to indicate the reward location for that trial. The light remained on until the rat arrived at the reward location and consumed the reward, after which the rats were placed on a platform adjacent to the maze. During the intertrial interval (ITI, ~30 seconds), the experimenter baited the reward cup for the next trial. Each training session consisted of 40 trials with 20 left and 20 right rewards presented in an unpredictable sequence. A learning criterion of two consecutive days of greater than 80% correct choices was established. After achieving this criterion, the rats were given up to 10 additional training sessions in order to record neuronal activity during asymptotic performance. In order to record from a new population of neurons each day, the tetrodes were lowered 35–70 μm after each session.
Figure 1.
Maze diagram and schematic of the trials. The rats were trained on a T-maze task in which the reward locations (small circles) were indicated by flashing lights near the rewards (a left-rewarded example is indicated). The schematic timeline indicates key task events, including the trial start, light onset, arrival at the choice point and the reward. After a ~30 sec ITI, the rats were placed on the maze facing away from the choice point (Trial Start). One of the cue lights was illuminated ~3 sec later when the rat started down the stem of the maze (dashed line on the T-maze diagram). The light remained illuminated throughout the trial, until after the rat consumed the reward. Approximately 1.5 sec after light onset, the rats reached the choice point and after another ~1.5 sec the rat reached the reward location. Each of these times varied somewhat from one rat to another and across trials. Analysis of event-related firing of individual neurons compared firing during the 1 sec before and after the event (grey shaded regions).
Histology
After completion of the experiment, rats were transcardially perfused with 4% paraformaldehyde in phosphate-buffered saline. Brains were removed and stored overnight in 4% paraformaldehyde before being transferred to 30% sucrose in 4% paraformaldehyde for storage until slicing. Coronal sections (40 μm) were stained with 0.5% cresyl violet for visualization of tetrode tracks. The location of the tetrode tip was verified using The Rat Brain in Stereotaxic Coordinates (Paxinos and Watson 2013). Neuronal records from tetrodes with locations outside of the RSC were excluded from the data set. Our recordings targeted the granular b subregion of the RSC (commonly referred to as Rgb, sometimes also referred to as Brodmann's Area 29c) because that subregion is densely interconnected with the hippocampus and the anterior thalamic nuclei (van Groen and Wyss 2003), which are known to be important for similar maze tasks (Aggleton et al. 1986; Aggleton et al. 1995), and because previous recordings in the same region had identified the reward-location conjunctive responses of interest to the present study (Smith et al. 2012). Our recording locations spanned a distance of ~4 mm in the anterior-posterior dimension (2.01 mm to 6.04 mm posterior to bregma) and ~2 mm in the dorsal-ventral dimension (1.06–3.22 mm from the cortical surface). We compared the response properties of the neurons and found no apparent differences in the prevalence of the various response types described below in either the anterior-posterior or dorsal-ventral dimensions.
Data Acquisition and Analysis
We examined firing at the time of several key task invents, including the start of the trials (defined as the time when the rat arrived on the stem of the maze, facing away from the choice point), the onset of the light cue (which occurred ~3.2 sec later, on average, after the rat turned around and started down the stem of the maze), and the receipt of the reward (defined as the time the rat arrived at the reward location). These events were defined the same way for the pretraining and regular training sessions. Neuronal spike data were recorded throughout the training sessions (Digital Cheetah Data Acquisition System, Neuralynx Inc., Bozeman, MT), filtered at 600 Hz and 6 kHz, digitized, and stored to disc along with their timestamps for offline sorting (SpikeSort3D, Neuralynx Inc.). Video data were used to establish the rat's position, extract the onset times of the light cue, and establish the time of trial start, arrival at the reward and the rat's return to the ITI platform.
The goal of our analyses was to determine whether RSC neurons responded to the light cue and other task events (e.g. the reward) and to determine whether the responses developed with learning. We examined neuronal firing during several sessions that spanned learning, including the pretraining session, the first day of regular training, the middle training session, and the asymptotic performance sessions. The middle session was defined as the session that was mid-way between the first session and the criterial session, or the session after the mid-point in the case of an even number of sessions.
Individual neurons were classified as having a response to the light cue if their firing rate changed significantly at the time of the light onset. Go-left and go-right trials were also compared to determine whether responses distinguished these two trial types. To accomplish this, the firing rate data were submitted to a two-way repeated measures ANOVA (Komorowski et al. 2009), with time relative to light onset (1 sec before and after) and trial type (go-left or go-right) as factors. Any neuron with a significant main effect of time bin was classified as having a response to the light cue and any neuron with a significant interaction of the time bin and trial type was classified as having a trial type-specific light response. Other classification methods (e.g. Wilcoxon signed rank tests) produced a similar pattern of results. In order to determine whether responses to the light cue developed as a function of learning, we examined the prevalence of the responses across the four stages of training. This was done by subjecting the percentage of neurons with a significant response at each stage of training to Chi-square analysis.
The same analysis strategy was used to determine whether the neurons exhibited significant responses to the trial start, the reward, and arrival on the ITI platform after the completion of the trial. Neurons were classified as having a response whenever the firing rate changed significantly at the time of the event, regardless of whether firing increased or decreased from the pre-event baseline, and neurons could (and often did) exhibit responses to more than one task event (see results). Some events frequently happened in rapid succession (e.g. the trial start and the light onset, which typically occurred ~ 3.2 sec apart on average), so peri-event time histograms were examined to ensure that a single response was not erroneously classified twice (e.g. a trial start response was not also erroneously classified as a decrease in firing at the time of light cue onset).
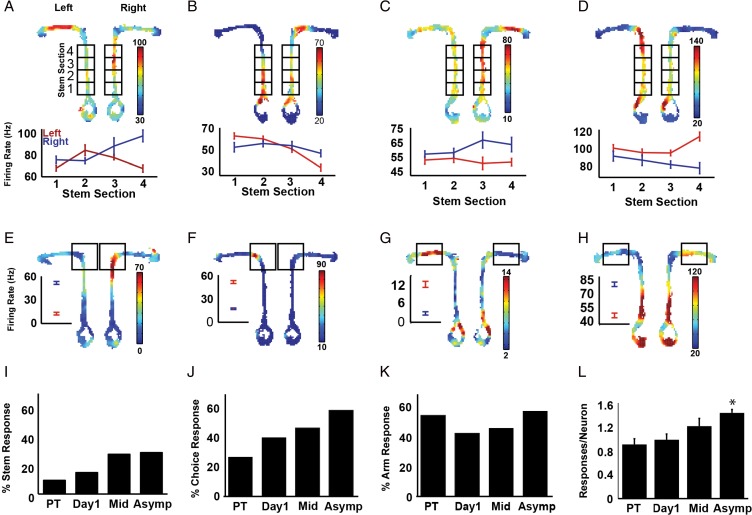
RSC neurons exhibit spatially localized firing (i.e. place fields, Cho and Sharp 2001; Smith et al. 2012) and the RSC may be important for translating spatial information provided by navigational cues into specific routes and trajectories to the goal. If so, then RSC neurons might be expected to discriminate the routes to the left and right reward locations. To examine this, the maze was divided into several sectors (see Fig. 6) and we computed the firing rate within each sector for each trial and then compared left and right trials. In order to examine differential firing on the stem of the maze before left or right trials, we divided the stem of the T-maze into 4 sectors and submitted the firing rate data to a two-way repeated measures ANOVA with sector (4 levels) and trial type (go-left or go-right reward location) as factors, following procedures previously used in the hippocampus (Wood et al. 2000). Atypical trajectories were identified by uncommonly long dwell periods in any of the four maze sectors, or uncommonly long total latency to reach the reward (>2SD from the mean), and were excluded from the analysis. We also examined spatial firing at the choice point and on the approach to the reward location using t-tests. These measures of spatial firing were examined at each stage of training.
Figure 6.
Spatial firing during cued T-maze performance. Examples of neurons that exhibited differential firing on the stem of the maze for go-left and go-right trials (plots A–D). For each neuron separate firing rate maps are shown for left and right trials, with the sectors of the maze that were analyzed indicated. The average firing rates in each sector are shown in the line graphs below each plot. RSC neurons also frequently exhibited differential firing on left and right trials at the choice point (E–F) or on the reward arm as the rat approached the reward (G–H). Mean firing rates for the analyzed maze sections are shown to the left of the firing rate maps. Bar plots illustrating the percentage of neurons that exhibited differential firing on the stem (I), at the choice point (J) and reward arm (K) are shown for each stage of training. Plot L illustrates the average number of differential spatial responses per neuron for each stage of training (*P < 0.05 compared to the pretraining session). A color version of this figure is available in the online version of this article.
Results
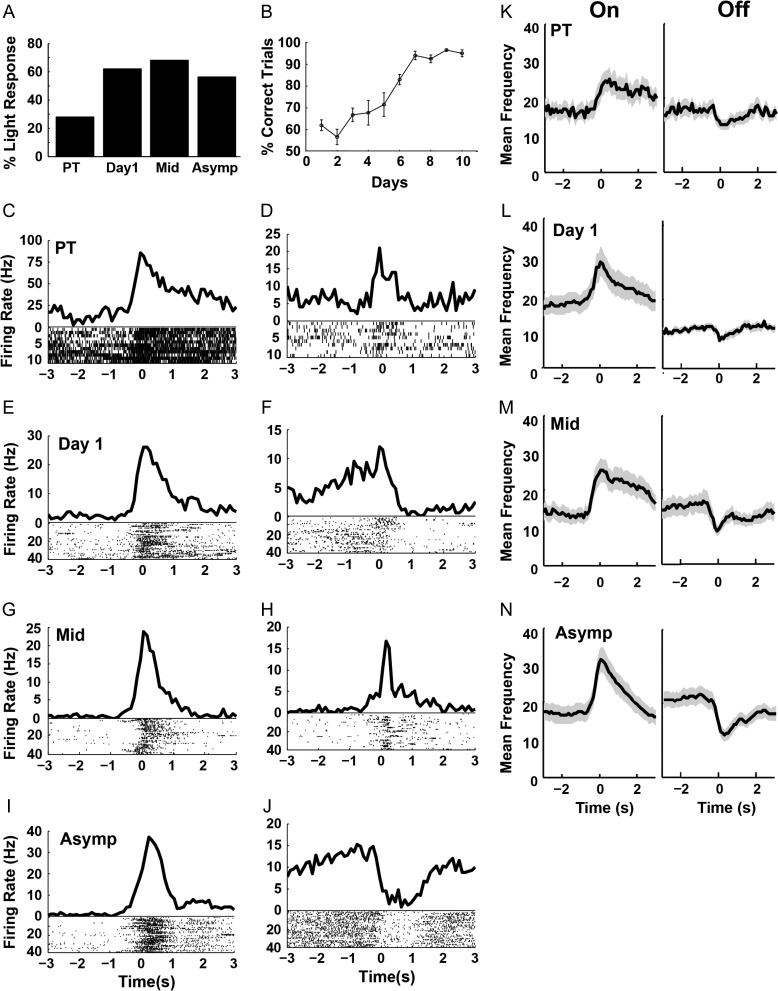
For this study, we recorded a total of 424 RSC neurons (64 during pretraining, 67 on the first day, 47 on the middle training day, and 246 during asymptotic performance which we recorded for several sessions). Rats readily learned to associate the light cues with the location of the chocolate milk reward. On average, rats reached the criterion in 6.4 ± 0.6 (mean ± SEM) sessions, with asymptotic performance at 94.0% ± 5.4% correct (Fig. 2B). Many RSC neurons responded to the light cue. These light responses were present in a substantial proportion of neurons (N = 18/64 cells) during the pretraining session, when the light was a salient visual stimulus but did not signal the reward location (see Methods). Nevertheless, the prevalence of light responses increased significantly on the first day of training (Fig. 2A, chi-square comparing the percentage of neurons during pretraining and the first day, χ2 = 15.15, P < 0.0001) and remained increased thereafter (χ2 = 22.55, P < 0.0001). The percentage of neurons with light responses increased significantly as soon as the light became a reliable indicator of the reward location and, interestingly, long before the rats began to perform the task reliably. At asymptote, more than half of the recorded neurons (142/246) showed significant responses to the light cue. Thus, the RSC exhibits large scale encoding of this navigational cue.
Figure 2.
Responses to light cue during cued T-maze performance. Plot A illustrates percentage of neurons with a significant response to the light cue at each stage of training, including the pretraining session (PT), the first day, the middle training session (Mid) and during asymptotic performance (Asymp). Plot B illustrates the average percentage of correct choices across training stages. Plots C–J illustrates peri-event time histograms of example light responses from individual neurons (light onset occurs at time zero on the x-axis), with examples from each stage of training. Plots K–N illustrate the average firing rate of all neurons with significant light responses, shown for each stage of training and separately plotted for neurons with “On” and “Off” responses (shading indicates SEM).
Light responses took the form of increased firing (“On” responses, 62.6% of light responses, Fig. 2K–N, left) or decreased firing (“Off” responses, 37.3% of light responses, Fig. 2K–N, right) at the time of light onset. These “responses” sometimes began slightly before the onset of the light cue, which is not surprising since the rats presumably learned to anticipate that the cue would be given as soon as they turned around and started down the stem of the maze. Anticipatory firing before a predictable cue has been reported previously in the RSC (Smith et al. 2002). Our previous study indicated that RSC reward responses were remarkably selective for different spatial locations (Smith et al. 2012). In the present study, the two light cues were in different locations, so the responses might have been specific to one location or the other. However, only a small minority of the neurons exhibited significantly different responses to the right and left light cues (5.9% over all training stages) and this percentage did not change significantly with training (χ2 = 0.93, P = 0.81). Although the light cue was strongly represented in the RSC, the representation was not sensitive to the location of the light.
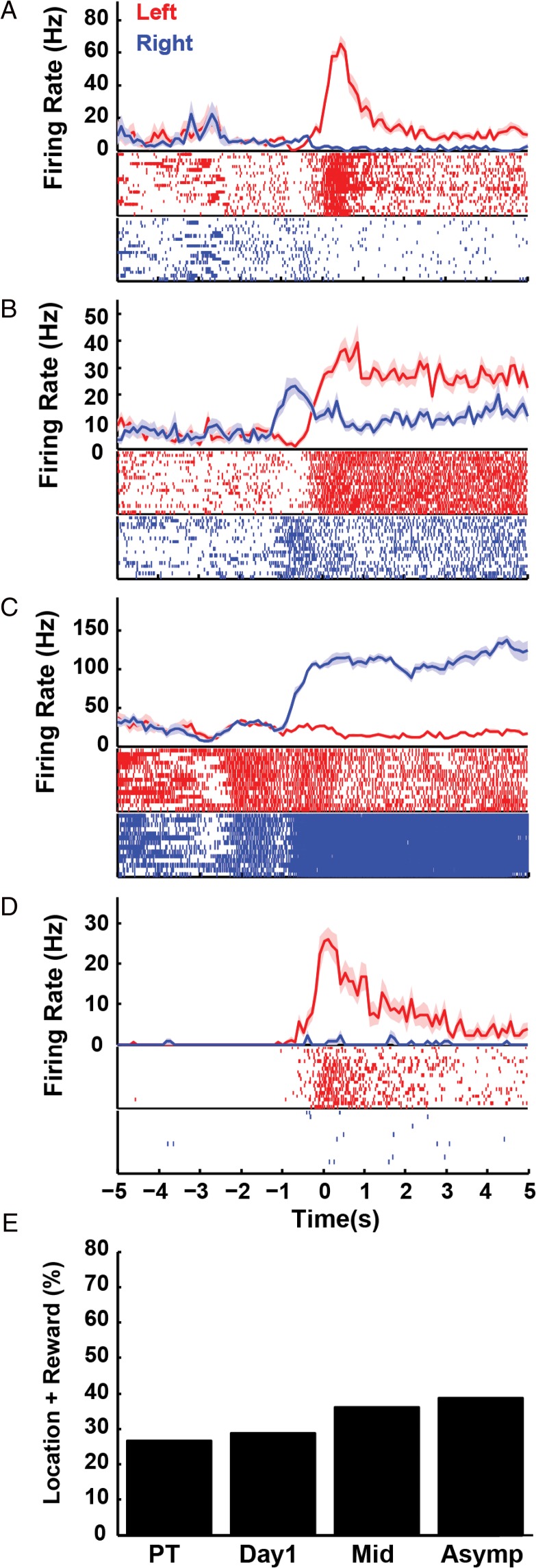
Previously, we found that RSC neurons encode reward-location conjunctions in a blocked alternation task that did not have an explicit cue indicating the current reward location (Smith et al. 2012). In the present study, with the addition of a reliable navigational cue, reward-location conjunctive coding remained a prominent characteristic of RSC activity (Fig. 3). We refer to these firing patterns as “reward-location responses” rather than as place fields that happen to coincide with the reward locations because they appear to be time locked to the receipt of the reward and because our previous study demonstrated that similar firing did not occur at these locations when a reward was not expected (Smith et al. 2012). At asymptote, 32.54% of RSC neurons exhibited location-specific reward responses. However, unlike our previous study (Smith et al. 2012), the prevalence of these responses did not change significantly with training (Fig. 3E, χ2 = 3.53, P = 0.32), a discrepancy that was likely caused by differences between protocols that we revisit in the discussion.
Figure 3.
Location-specific reward responses in the RSC. Peri-event time histograms illustrating reward responses that differentiated the left and right reward locations for four different example neurons are shown in plots A–D (reward occurs at time zero on the x-axis, shading indicates SEM). Left and right rewarded trials are plotted separately in red and blue in the histogram and sorted in the raster display (for illustration, the order of left and right trials was randomized). Plot E illustrates the percentage of RSC neurons with location-specific reward responses. A color version of this figure is available in the online version of this article.
Our primary interest was in determining whether RSC neurons respond to navigational cues and whether reward-location conjunctive responses remain prevalent when an explicit cue for the reward location was provided, as described above. However, we noted that a surprisingly large percentage of the recorded population responded to the cues in our task and many neurons responded to several task relevant events or cues (Fig. 4). For example, among the neurons that responded significantly to the light cue, 84% also responded to at least one other task event (reward, trial start or the start of the ITI), including 35% that exhibited location-reward conjunctive responses (e.g. Fig. 4B,D). On average, each neuron responded to 1.46 ± 0.13 events during pretraining and the number of events that elicited a significant response increased significantly to 2.10 ± 0.22 on the first day of training (Fig. 4E, F(3,428) = 7.66, P < 0.0001).
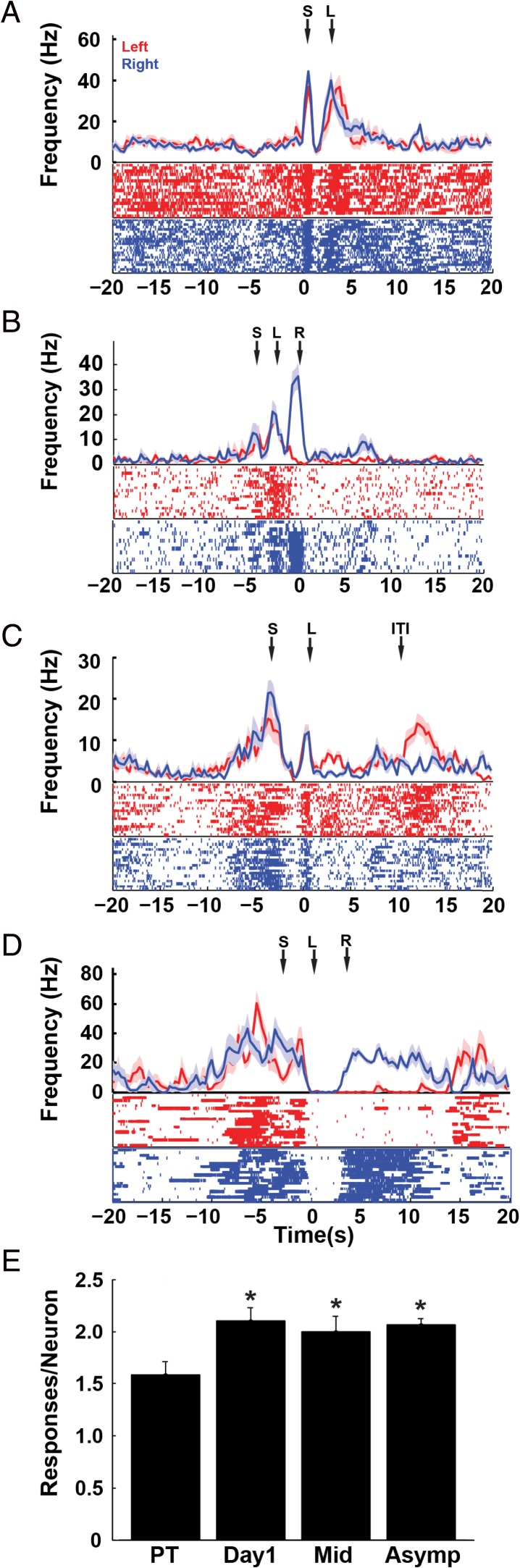
Figure 4.
Multiple event responses in RSC neurons. Many neurons exhibited significant responses to two or more task events (A–D). Plots A–D are aligned to various events at time zero (A is aligned to Trial Start, B is aligned to Reward, C and D are aligned to Light Onset). Additional events are indicated by arrows showing the average time of the events above the plots (S = Trial Start, L = Light, R = Reward, ITI = arrival on ITI platform). Plot E illustrates the average number of event responses per neuron at each stage of training (*P < 0.05 compared to the pretraining session). A color version of this figure is available in the online version of this article.
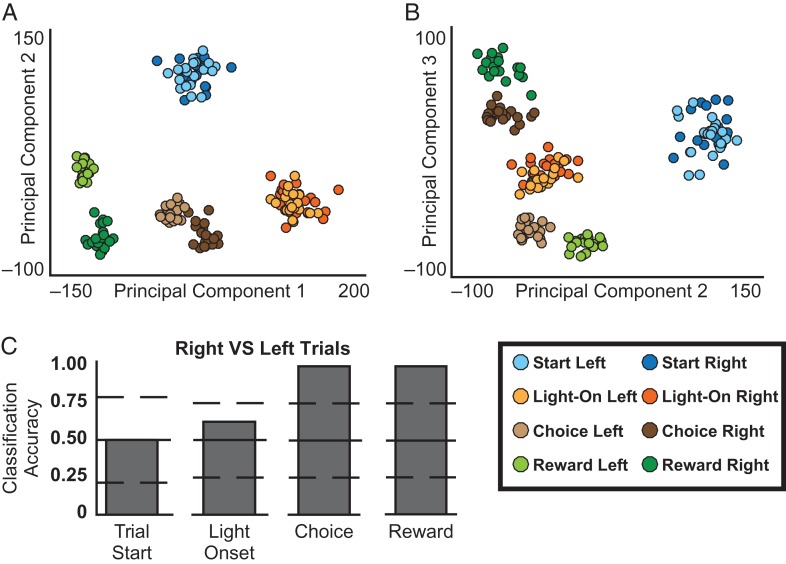
The observation that many neurons responded to multiple trial events suggests that highly specific representations might rely on coding at the population level. To examine this possibility, we selected four salient events (trial start, light-onset, arrival at the choice point [see choice region defined in Fig. 6E,F], and reward), and we asked whether we could use ensemble firing patterns to correctly identify each event using linear discriminant analysis (LDA). We combined the 246 neurons recorded from all rats during the asymptotic performance sessions into a single ensemble and computed population vectors containing the firing rates during 1 sec following each event. Computationally, LDA requires more observations (trials and events) than variables (neurons) in order to adequately estimate covariance. Therefore, we preprocessed the data with principle components analysis (PCA) to reduce dimensionality using previously validated procedures (Nicolelis et al. 1998; Turner et al. 2003). Importantly, PCA simply finds orthogonal axes that explain the greatest proportion of the variance and is agnostic to the to-be-classified events. We then performed LDA on the first 10 principal components, using the first half of the session for training and the second half for testing the classifier. The same pattern of results was obtained using a different dimensional reduction approach, by repeatedly and exhaustively subsampling from the ensemble of 246 neurons.
The population activity was highly distinct between the four trial events. This can be readily seen in the PCA plots (Fig. 5A,B). For example, in plot 5A the activity patterns form distinct clusters for each event (with separate sub-clusters for right and left choice and reward events, discussed below). Consistent with this, the linear classifier was able to sort the test sample of population vectors as to the four events with perfect accuracy (20 out of 20 trials classified correctly for each event, P < 0.001 compared to a distribution of 10,000 shuffles of the mean firing rates of each cell between the trial events). At the single-neuron level, some responses distinguished left and right trials (e.g. reward responses) while others did not (e.g. light responses). To determine whether the population activity distinguished between left and right trials, we repeated the same analysis for each event, but with trial type (go-left and go-right) as the variable of interest (Fig. 5C). Population activity at the time of the trial start did not distinguish between left and right trials. This was expected and serves as a useful check on our methods: since the light was not illuminated until 2–3 sec after the rat was first place on the maze (i.e. trial start, see Fig. 1), the rat could not know whether it was going to be a left or right trial. Consistent with the single-neuron data, population responses to the light onset also did not distinguish left and right trials beyond chance levels. However, population activity at the choice point and at the time of the reward classified left and right trials with perfect accuracy. This can also be seen in the PCA plots, particularly in plot 5B where the left and right trials are widely separated for the choice point and the reward, but highly overlapping for the trial start and light onset. Overall, these results indicate that most of the variance in ensemble activity patterns, as reflected in the principle components, coincides with the occurrence of key task events. Thus, RSC ensembles provide remarkably specific representations of these events.
Figure 5.
Ensemble coding of task events. Population vectors containing firing rates of 246 neurons (all rats and sessions of asymptotic performance) were subjected to principal components analysis. Plots of the principal components 1–3 are shown in A and B, with each dot representing data from a single trial. Dots are color coded for the events (Trial Start, Light Onset, Choice and Reward) with light and dark colors indicating left and right trials. Distinct clusters are seen for the trial start and the light onset, which did not differ with regard to left and right trials. Separate clusters are seen for left and right trials at the time of the reward and arrival at the choice point. Linear discriminant analysis was able to correctly classify each of the four events for every test trial (not shown, see text). Plot C illustrates classifier accuracy for distinguishing left and right trials for each event. The classifier performed at chance levels at the trial start (before the cue light was illuminated) and at light onset (mean and 95% confidence intervals of shuffled data are indicated by the horizontal solid and dashed lines, respectively). The classifier perfectly distinguished left and right trials at the choice point and the reward. A color version of this figure is available in the online version of this article.
Hippocampal firing during T-maze tasks has been extensively documented, and hippocampal neurons have been shown to fire differentially on the stem of the T-maze depending on the trial type (left or right, Wood et al. 2000), commonly referred to as “splitters” (Dudchenko and Wood 2014). To date, RSC neurons have not been shown to exhibit splitter firing. To assess this, we divided the stem into four sectors and compared firing on go-left and go-right trials, using procedures similar to those of Wood et al. (Wood et al. 2000, see Methods). As in the hippocampus, many RSC neurons showed differential firing on the stem of the maze (Fig. 6A–D) and these splitter responses developed as a function of training (Fig. 6I, χ2 = 10.6, P < 0.05). These left-right differences in firing could not be attributed to differences in running speed or lateral position on the stem during go-left and go-right trials since neither factor was correlated with the proportion of neurons classified as splitters (velocity: r = 0.30, P = 0.13; position: r = −0.24, P = 0.13). Interestingly, these differential spatial responses were common (28.29% of RSC neurons at asymptote), whereas differential responses to the left and right light cues were not (7.17% at asymptote) even though these analyses both involved firing on the stem of the maze. Analysis of the light responses was restricted to one second after light onset, whereas the spatial analysis included the full length of the stem. The substantially different outcomes of these two analyses suggests that firing triggered by light onset is at least partially separable from firing associated with spatial location.
We also compared the firing at the choice point (Fig. 6J) and on the approach to the reward location (i.e. on the reward arm but excluding the reward location, Fig. 6K) for left and right trials. Unlike the stem of the maze, where the rat's trajectory is similar on left and right trials, these sectors involve different spatial locations, turning behaviors and direction of travel so differential firing may be entirely attributable to those factors. Nevertheless, a relatively large percentage of neurons exhibited differential firing at these locations during asymptotic performance (choice point: 58.0%, reward approach: 57.4%), suggesting that these locations and the behaviors that occur there are heavily represented in the RSC. The differential choice point responses are consistent with the population analyses described above and these responses developed significantly with training (χ2 = 12.0, P < 0.01). Differential reward approach firing was prevalent even during the pretraining session and remained prevalent throughout training (χ2 = 4.77, P = 0.19).
As with the responses to the cues and events described above, many RSC neurons exhibited spatial responses at more than one location on the maze. For example, among the neurons that exhibited splitter firing on the stem of the maze, 60% also responded differentially at the choice point or during the approach to the reward (Fig. 6A–D). The number of differential spatial responses exhibited by each neuron (stem, choice point, and reward approach arm) increased significantly with training (F(3, 428) = 2.63, P < 0.00005, Fig. 6L).
Discussion
Our results indicate that RSC neurons encode a number of variables that are important for goal directed spatial tasks. First, RSC neurons do, in fact, respond to important navigational cues. After learning the task, more than half of the recorded neurons exhibited a significant response to the light cue. Although many neurons responded to the light during pretraining, before it became an explicit navigational cue, the percentage of neurons that encoded the cue doubled as soon as the light became a reliable indicator of the reward location on the first day of training. These large-scale responses could provide a critical signal for navigation tasks by encoding landmarks, beacons, and other spatial cues, and they may provide important input to the hippocampus and the broader circuitry that mediates spatial cognition. The loss of these signals may underlie the topographical disorientation syndrome seen in humans with RSC damage (Takahashi et al. 1997; Maguire 2001; Kim et al. 2015) and the spatial impairments seen in rodents with RSC lesions (Sutherland et al. 1988; Vann and Aggleton 2005; Nelson et al. 2015).
RSC neurons also encoded important spatial conjunctions. For example, many neurons exhibited responses that were tightly time-locked to the reward, but only when the reward was presented at one of the two goal locations (left or right). Similar conjunctive coding is a well-documented characteristic of hippocampal neurons, which also respond to reward-location conjunctions (Smith and Mizumori 2006) and conjunctions of other task relevant cues, such as odor cues and locations (Komorowski et al. 2009; McKenzie et al. 2014). Previously, we reported reward-location conjunctive responses in the RSC (Smith et al. 2012). However, because the rats used a win-stay strategy, continually returning to the previous reward location, the reward location was, itself, an important predictive cue (i.e. it signaled the reward location for the next trial) and we suggested that the RSC responses may have reflected cue encoding processes. However, the present results demonstrate that RSC neurons exhibit conjunctive responses even when the reward location cannot serve as a predictive cue, as in the present study where the reward locations were randomized from one trial to the next. This result suggests that conjunctive coding of important events and the locations where they occur is a general characteristic of RSC coding, as it is in the hippocampus.
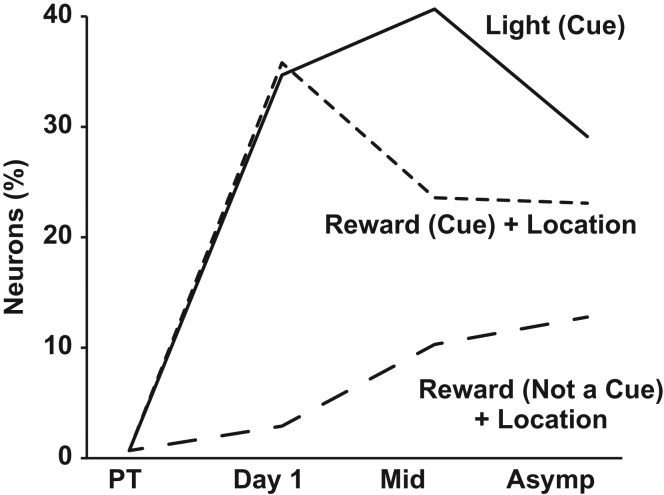
Because we found reward-location conjunctive responses in the present study, we can compare the development of these responses under conditions of differing information value (Fig. 7). In our previous study (Smith et al. 2012), when the reward location served as an important navigational cue, responses developed immediately, just as the light responses developed in the present study. However, when the reward location could not be used as a navigational cue, the responses did not suddenly appear early in learning. This difference is particularly striking since the stimuli that evoked the response, two drops of chocolate milk delivered to a metal cup at the end of the maze arm, were the same in both experiments. This comparison suggests that whenever a particular stimulus can serve as a navigational cue, regardless of whether it is an explicit cue such as the light or a more abstract cue such as the reward location in a win-stay task, it evokes a large and rapid response in the RSC. When the same stimuli are not useful as navigational cues, the RSC may still encode them but not as rapidly or robustly.
Figure 7.
Rapid encoding of navigationally important cues. The percentage of RSC neurons that encode an explicit navigational cue, such as the light cue in the present study, increases dramatically on the first day of training (solid line). In our previous study where rats used a win-stay strategy (Smith et al. 2012), the reward and its location served as an important navigational cue and similarly rapid encoding was seen (fine dashed line). In contrast, the reward location could not have served as a cue in the present study because it did not indicate the reward location for the next trial. Under these conditions, responses to the same stimuli (chocolate milk reward and its location at the end of a specific maze arm) did not show a sudden increase early in training (coarse dashed line). Data are expressed as the increase in the percentage of neurons at each stage of training, relative to the pre-training baseline, and are intended to illustrate the qualitative differences in how the neuronal responses developed in different experiments.
Goal locations and trajectories to the goal also appear to be particularly important for RSC encoding. At asymptote, about 40% of RSC neurons selectively responded to the reward at either the left or right location (Fig 3E). In addition to spatial input from the anterior thalamus and hippocampus, the RSC receives dopaminergic input (Berger et al. 1985) and is anatomically connected with the striatum (van Groen and Wyss 2003). Thus, the RSC is well positioned to integrate spatial information with goal directed behaviors and rewards. The location-selective reward responses may reflect this integration and provide a prominent signal indicating the goal locations of the task.
In addition to reward locations, the analysis of spatial firing patterns showed that RSC neurons encode the trajectories to the goal. RSC neurons are known to exhibit spatially localized firing (Cho and Sharp 2001; Smith et al. 2012; Alexander and Nitz 2015). Here, we show for the first time that RSC neurons exhibit so-called splitter responses, where they fire differentially on the stem of the maze, depending on the trial type (go-left or go-right, Fig. 6A–D). Importantly, the left-right differences in firing could not be attributed to slight differences in the rat's spatial position or running speed as they traversed the stem and they developed with training, suggesting that they are related to the associative learning requirements of the task. Splitters have been studied extensively in the hippocampus (for review see Dudchenko and Wood 2014), and are thought to be important because the neural firing reflects the overall trajectory of the rat to the future goal locations rather than simply encoding the rats current position. Our results suggest that similar trajectory coding occurs in the RSC.
RSC neurons also fired differentially on left and right trials as the rats traversed the choice point and approached the reward on the goal arms (Fig. 6E–H). Firing was particularly prominent at the choice point, with 58% of RSC neurons firing differentially on left and right trials at asymptote (Fig. 6J). RSC firing is known to be sensitive to egocentric turning behavior and spatial location (Cho and Sharp 2001; Alexander and Nitz 2015), so we cannot determine whether firing at the choice point was driven by one of these factors or some other aspect of the rat's decision. Regardless of the specific factors that drove firing, RSC neuronal activity strongly differentiated right and left trajectories throughout the trials.
The light cues reliably elicited responses from many RSC neurons, but is the light really a navigational cue? The light-cued T-maze task used here is similar to conditional discrimination tasks which require subjects to make one response (e.g. press the right lever) in response to one cue and a different response (e.g. press the left lever) in response to another cue, and the rats could treat the task as a discrimination problem. The RSC has a well-documented role in differentially encoding the auditory cues used in an instrumental discrimination task (e.g. Gabriel 1993) and similarly, RSC neurons differentially encoded the two reward locations of the present study. However, very few neurons differentially encoded the left and right light cues, suggesting that the neurons did not treat the lights as discriminative cues but instead as a navigational beacon (a distant cue associated with a goal location). Moreover, the spatial firing patterns we observed suggest that RSC neurons encoded some of the task relevant information as part of a navigational coding scheme.
Interestingly, although the RSC is clearly involved in navigation, its specific contribution is not known. The RSC might generate an allocentric cognitive map similar to that seen in the hippocampus, but because the necessary experimental manipulations have not been done (e.g. cue rotation and cue scrambling) this has not been clearly established. The fact that RSC spatial responses tend to be less specific and reliable than hippocampal responses casts some doubt on that hypothesis (Smith et al. 2012; Alexander and Nitz 2015). Beacon navigation does not require an allocentric cognitive map, so the present study cannot resolve this question. However, our data suggest that one key contribution of the RSC to spatial cognition is the encoding of navigational cues, trajectories, and goal locations.
Although responses to the light cue were particularly prevalent, individual RSC neurons showed a remarkable tendency to respond to many task relevant cues, events, and spatial features. Often, this “multi-responsivity” took the form of several discrete responses to different task events, such as the start of the trial, the onset of the light cue and the reward (see Fig. 4). The average number of distinct responses exhibited by the neurons increased with training, suggesting that learning to use the cues to navigate to the reward location drives this multi-responsivity. This is notably different from the firing characteristics of hippocampal neurons. Within a particular experimental context, most hippocampal neurons are silent (Thompson and Best 1989) and the neurons that are responsive are typically highly specific to a particular location, cue or conjunction of stimuli. In contrast, nearly all RSC neurons (94.21%) exhibited at least one identifiable response and, as mentioned above, most neurons (67.36%) responded to more than one thing. Thus, although the RSC and hippocampus are known to be involved in many of the same cognitive functions (contextual memory, navigation, etc.) and have similar responses (e.g. spatial firing and conjunctive responses), the basic coding scheme seems to be quite different in the two structures. The functional consequences of these differences are not known. However, the combination of highly specific responses in the hippocampus and multi-responsivity in the RSC may allow downstream brain regions to extract representations at varying levels of specificity, from highly specific information about a particular event to a very general signal that something important has happened.
Another apparent advantage of the multi-responsivity of RSC neurons is that they produce a remarkably accurate ensemble code for task events. Our principle components analysis revealed that population activity states during a given event (e.g. light onset) were highly consistent from one trial to the next, but quite distinct from one event to another (e.g. light onset compared to reward, Fig. 5A). As a result, a given activity state could easily be classified as to whether it indicated trial start, light onset, choice behavior or the reward. Consistent with the data of individual neurons, the principle components and classifier did not distinguish between left and right trials for the trial start and light onset but easily distinguished them for the choice point and reward locations. Thus, RSC output could readily inform other brain regions, such as the hippocampus, anterior thalamus, striatum, anterior cingulate cortex, and prelimbic cortex (van Groen and Wyss 2003) about the occurrence of salient events.
Our results provide a basis for understanding the fMRI activation seen in human subjects performing spatial tasks (Sugiura et al. 2005; Epstein et al. 2007; Epstein 2008; Sherrill et al. 2013; Epstein and Vass 2014) and the well-documented spatial deficits seen in patients with RSC damage (Takahashi et al. 1997; Maguire 2001; Kim et al. 2015). However, there is ample evidence that the RSC role is not limited to spatial coding (for review see Miller et al. 2014). The RSC is critical for a number of learning tasks that do not have an obvious navigational component (Keene and Bucci 2008a, 2008b, 2008c, 2009; Nelson et al. 2014; Robinson et al. 2014) and RSC neurons respond to cues such as auditory tones used in non-spatial discrimination tasks (Gabriel 1993). Consistent with its interconnections with frontal regions such as the anterior cingulate and prelimbic cortex, the RSC has recently been implicated in cognitive control using a rodent analogue of the Stroop task (Nelson et al. 2014). Additionally, the RSC is critically involved in contextual memory processes (Keene and Bucci 2008a, 2008b, 2008c, 2009), suggesting that the RSC may encode these non-spatial items and events in conjunction with the context where they occur (Bar and Aminoff 2003). Consistent with this idea, RSC neurons show reversed responses to preferred and non-preferred auditory discrimination cues depending on the context where they were presented (Smith et al. 2002) and artificial reactivation of RSC neurons triggers the retrieval of a previously learned contextual fear memory (Cowansage et al. 2014). Thus, although the present results suggest a specific role of the RSC in encoding navigationally significant cues, this may be one facet of a broader RSC role in encoding the conjunction of important cues and events and the context in which they occur.
Funding
This work was supported by the National Institute of Mental Health grant number MH083809 awarded to D. Smith.
Notes
Thank you to William Mau for assisting in the collection of these data. Conflict of Interest: None declared.
References
- Aggleton JP, Hunt PR, Rawlins JN.. 1986. The effects of hippocampal lesions upon spatial and non-spatial tests of working memory. Behav Brain Res. 19:133–146. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Neave N, Nagle S, Hunt PR.. 1995. A comparison of the effects of anterior thalamic, mamillary body and fornix lesions on reinforced spatial alternation. Behav Brain Res. 68:91–101. [DOI] [PubMed] [Google Scholar]
- Alexander AS, Nitz DA.. 2015. Retrosplenial cortex maps the conjunction of internal and external spaces. Nat Neurosci. 18:1143–1151. [DOI] [PubMed] [Google Scholar]
- Auger SD, Mullally SL, Maguire EA.. 2012. Retrosplenial cortex codes for permanent landmarks. PloS One. 7:e43620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger SD, Zeidman P, Maguire EA.. 2015. A central role for the retrosplenial cortex in de novo environmental learning. Elife. 4:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M, Aminoff E.. 2003. Cortical analysis of visual context. Neuron. 38:347–358. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Alvarez C, Vigny A, Helle KB.. 1985. New dopaminergic terminal fields in the motor, visual (area 18b) and retrosplenial cortex in the young and adult rat. Immunocytochemical and catecholamine histochemical analyses. Neuroscience. 15:983–998. [DOI] [PubMed] [Google Scholar]
- Bowers D, Verfaellie M, Valenstein E, Heilman KM.. 1988. Impaired acquisition of temporal information in retrosplenial amnesia. Brain Cogn. 8:47–66. [DOI] [PubMed] [Google Scholar]
- Cho J, Sharp PE.. 2001. Head direction, place, and movement correlates for cells in the rat retrosplenial cortex. Behav Neurosci. 115:3–25. [DOI] [PubMed] [Google Scholar]
- Cooper BG, Mizumori SJ.. 1999. Retrosplenial cortex inactivation selectively impairs navigation in darkness. Neuroreport. 10:625–630. [DOI] [PubMed] [Google Scholar]
- Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M.. 2014. Direct reactivation of a coherent neocortical memory of context. Neuron. 84:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski R, Jayaprakash B, Wiltgen B, Rogerson T, Guzman-Karlsson MC, Barth AL, Trachtenberg JT, Silva AJ.. 2014. Encoding and storage of spatial information in the retrosplenial cortex. Proc Natl Acad Sci. 111:8661–8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudchenko PA, Wood ER.. 2014. Splitter cells: hippocampal place cells whose firing is modulated by where the animal is going or where it has been In: Derdikman D, Knierim JJ, editors. Space,time and memory in the hippocampal formation. Vienna: Springer Vienna; p. 253–272. [Google Scholar]
- Epstein RA. 2008. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci. 12:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Parker WE, Feiler AM.. 2007. Where am I now? Distinct roles for parahippocampal and retrosplenial cortices in place recognition. J Neurosci. 27:6141–6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Vass LK.. 2014. Neural systems for landmark-based wayfinding in humans. Philos Trans R Soc Lond B Biol Sci. 369:20120533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M. 1993. Discriminative avoidance learning: A model system In: Vogt BA, Gabriel M, editors. Neurobiology of cingulate cortex and limbic thalamus. Boston: Birkhauser; p. 478–523. [Google Scholar]
- Harker KT, Whishaw IQ.. 2002. Impaired spatial performance in rats with retrosplenial lesions: importance of the spatial problem and the rat strain in identifying lesion effects in a swimming pool. J Neurosci. 22:1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ino T, Doi T, Hirose S, Kimura T, Ito J, Fukuyama H.. 2007. Directional disorientation following left retrosplenial hemorrhage: a case report with fmri studies. Cortex. 43:248–254. [DOI] [PubMed] [Google Scholar]
- Katche C, Dorman G, Gonzalez C, Kramar CP, Slipczuk L, Rossato JI, Cammarota M, Medina JH.. 2013. On the role of retrosplenial cortex in long-lasting memory storage. Hippocampus. 23:295–302. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ.. 2008. a. Contributions of the retrosplenial and posterior parietal cortices to cue-specific and contextual fear conditioning. Behav Neurosci. 122:89–97. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ.. 2008. b. Involvement of the retrosplenial cortex in processing multiple conditioned stimuli. Behav Neurosci. 122:651–658. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ.. 2008. c. Neurotoxic lesions of retrosplenial cortex disrupt signaled and unsignaled contextual fear conditioning. Behav Neurosci. 122:1070–1077. [DOI] [PubMed] [Google Scholar]
- Keene CS, Bucci DJ.. 2009. Damage to the retrosplenial cortex produces specific impairments in spatial working memory. Neurobiol Learn Mem. 91:408–414. [DOI] [PubMed] [Google Scholar]
- Kim JG, Aminoff EM, Kastner S, Behrmann M.. 2015. A neural basis for developmental topographic disorientation. J Neurosci. 35:12954–12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komorowski RW, Manns JR, Eichenbaum H.. 2009. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 29:9918–9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M.. 2011. A new neural framework for visuospatial processing. Nat Rev Neurosci 12:217–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ramirez S, Pang PT, Puryear CB, Govindarajan A, Deisseroth K, Tonegawa S.. 2012. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature. 484:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald CJ, Lepage KQ, Eden UT, Eichenbaum H.. 2011. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 71:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA. 2001. The retrosplenial contribution to human navigation: a review of lesion and neuroimaging findings. Scand J Psychol. 42:225–238. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Frank AJ, Kinsky NR, Porter B, Riviere PD, Eichenbaum H.. 2014. Hippocampal representation of related and opposing memories develop within distinct, hierarchically organized neural schemas. Neuron. 83:202–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AMP, Vedder LC, Law LM, Smith DM.. 2014. Cues, context, and long-term memory: the role of the retrosplenial cortex in spatial cognition. Front Hum Neurosci. 8:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Hindley EL, Haddon JE, Vann SD, Aggleton JP.. 2014. A novel role for the rat retrosplenial cortex in cognitive control. Learn Mem. 21:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson AJ, Powell AL, Holmes JD, Vann SD, Aggleton JP.. 2015. What does spatial alternation tell us about retrosplenial cortex function. Front Behav Neurosci. 9:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis MA, Ghazanfar AA, Stambaugh CR, Oliveira LM, Laubach M, Chapin JK, Nelson RJ, Kaas JH.. 1998. Simultaneous encoding of tactile information by three primate cortical areas. Nat Neurosci. 1(7):621–630. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.. 2013. The rat brain in stereotaxic coordinates. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Robinson S, Todd TP, Pasternak AR, Luikart BW, Skelton PD, Urban DJ, Bucci DJ.. 2014. Chemogenetic silencing of neurons in retrosplenial cortex disrupts sensory preconditioning. J Neurosci. 34:10982–10988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill KR, Erdem UM, Ross RS, Brown TI, Hasselmo ME, Stern CE.. 2013. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J Neurosci. 33:19304–19313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigwald E, Genoud M, Giachero M, de Olmos S, Molina V, Lorenzo A.. 2015. Selective neuronal degeneration in the retrosplenial cortex impairs the recall of contextual fear memory. Brain Struct Funct. 221:1861–1875. [DOI] [PubMed] [Google Scholar]
- Smith DM, Barredo J, Mizumori SJ.. 2012. Complimentary roles of the hippocampus and retrosplenial cortex in behavioral context discrimination. Hippocampus. 22:1121–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Freeman JH Jr, Nicholson D, Gabriel M.. 2002. Limbic thalamic lesions, appetitively-motivated discrimination learning and training-induced neuronal activity in rabbits. J Neurosci. 22(18):8212–8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Mizumori SJY.. 2006. Learning-related development of context-specific neuronal responses to places and events: the hippocampal role in context processing. J Neurosci. 26:3154–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DM, Wakeman D, Patel J, Gabriel M.. 2004. Fornix lesions impair context-related cingulothalamic neuronal patterns and concurrent discrimination learning. Behav Neurosci. 118:1225–1239. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Shah NJ, Zilles K, Fink GR.. 2005. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. J Cogn Neurosci. 17:183–198. [DOI] [PubMed] [Google Scholar]
- Sutherland RJ, Whishaw IQ, Kolb B.. 1988. Contributions of cingulate cortex to two forms of spatial learning and memory. J Neurosci. 8:1863–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K.. 1997. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 49:464–469. [DOI] [PubMed] [Google Scholar]
- Tanaka KZ, Pevzner A, Hamidi AB, Nakazawa Y, Graham J, Wiltgen BJ.. 2014. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 84:347–354. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Best PJ.. 1989. Place cells and silent cells in the hippocampus of freely-behaving rats. J Neurosci. 9:2382–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S, Liu X, Ramirez S, Redondo R.. 2015. Memory engram cells have come of age. Neuron. 87:918–931. [DOI] [PubMed] [Google Scholar]
- Turner JA, Anderson KC, Siegel RM.. 2003. Cell responses in macaque superior temporal polysensory area measured by temporal discriminants. Neural Comput. 15:2067–2090. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT.. 1987. Retrosplenial amnesia. Brain. 110:1631–1646. [DOI] [PubMed] [Google Scholar]
- van Groen T, Wyss JM.. 2003. Connections of the retrosplenial granular b cortex in the rat. J Comp Neurol. 463:249–263. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP.. 2002. Extensive cytotoxic lesions of the rat retrosplenial cortex reveal consistent deficits on tasks that tax allocentric spatial memory. Behav Neurosci. 116:85–94. [PubMed] [Google Scholar]
- Vann SD, Aggleton JP.. 2005. Selective dysgranular retrosplenial cortex lesions in rats disrupt allocentric performance of the radial-arm maze task. Behav Neurosci. 119:1682–1686. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP, Maguire EA.. 2009. What does the retrosplenial cortex do. Nat Rev Neurosci. 10:792–802. [DOI] [PubMed] [Google Scholar]
- Wolbers T, Weiller C, Buchel C.. 2004. Neural foundations of emerging route knowledge in complex spatial environments. Brain Res Cogn Brain Res. 21:401–411. [DOI] [PubMed] [Google Scholar]
- Wood ER, Dudchenko PA, Robitsek RJ, Eichenbaum H.. 2000. Hippocampal neurons encode information about different types of memory episodes occurring in the same location. Neuron. 27:623–633. [DOI] [PubMed] [Google Scholar]