Abstract
Functional specialization of areas along the anterior–posterior axis of the lateral prefrontal cortex has been speculated but little evidence exists about distinct neurophysiological properties between prefrontal sub-regions. To address this issue we divided the lateral prefrontal cortex into a posterior-dorsal, a mid-dorsal, an anterior-dorsal, a posterior-ventral, and an anterior ventral region. Selectivity for spatial locations, shapes, and colors was evaluated in six monkeys never trained in working memory tasks, while they viewed the stimuli passively. Recordings from over two thousand neurons revealed systematic differences between anterior and posterior regions. In the dorsal prefrontal cortex, anterior regions exhibited the largest receptive fields, longest response latencies, and lowest amount of information for stimuli. In the ventral prefrontal cortex, posterior regions were characterized by a low percentage of responsive neurons to any stimuli we used, consistent with high specialization for stimulus features. Additionally, spatial information was more prominent in the dorsal and color in ventral regions. Our results provide neurophysiological evidence for a rostral-caudal gradient of stimulus selectivity through the prefrontal cortex, suggesting that posterior areas are selective for stimuli even when these are not releant for execution of a task, and that anterior areas are likely engaged in more abstract operations.
Keywords: anatomical specialization, monkey, principal sulcus, visuospatial processing, working memory
Introduction
The primate prefrontal cortex (PFC) has long been associated with working memory, planning, decision making, and other properties of executive control (Miller and Cohen 2001; Baddeley 2003; Constantinidis and Procyk 2004; Riley and Constantinidis 2016). How stimulus information and cognitive functions are organized across the cortical surface of the prefrontal cortex has been a matter of debate. The pattern of anatomical connections (Petrides and Pandya 1984; Selemon and Goldman-Rakic 1988; Cavada and Goldman-Rakic 1989; Romanski et al. 1999) and some neurophysiological findings (Wilson et al. 1993; O'Scalaidhe et al. 1997) suggest a “domain-specific” organization along the dorso-ventral axis of the PFC, with the dorsal aspect being specialized for spatial information, and the ventral PFC for object information in working memory. Other studies favor a competing, “integrative” model, positing that prefrontal neurons capable of integrating different types of information are distributed throughout the prefrontal cortex, and neuronal responses are shaped by cognitive demands imposed by the task that subjects are trained to perform rather than selectivity for stimulus information (Rao et al. 1997; Rainer et al. 1998). Human imaging studies have also been unclear. Contrasting studies are either consistent with specialized processing in the dorsal and ventral subdivisions (Adcock et al. 2000; Leung et al. 2002; Sala and Courtney 2007; Volle et al. 2008), or favor an organization in terms of cognitive operations rather than type of information (Owen et al. 1996, 1998; Stern et al. 2000). No consensus about a dorso-ventral organization has been reached in the current literature (Qi and Constantinidis 2013).
Prefrontal specialization along an anterior–posterior axis has also been proposed (Badre and D'Esposito 2009). Human lesion and imaging studies suggest a hierarchy within the PFC in which posterior areas represent stimulus properties, whereas anterior areas are specialized for more abstract operations such as novelty detection, rule learning, temporal sequence, and metacognitive control (Strange et al. 2001; Koechlin et al. 2003; Ramnani and Owen 2004; Baird et al. 2013; Cole et al. 2016). Consistent with these findings, lesions of the anterior prefrontal cortex in monkeys result in impaired learning of abstract rules (Boschin et al. 2015). Nonetheless, neurophysiological evidence supporting an anterior–posterior specialization is lacking, with only sparse results in the literature about any type of specialization between anterior and posterior PFC regions, in the context of various tasks (Wallis et al. 2001; Katsuki and Constantinidis 2012; Donahue and Lee 2015; Markowitz et al. 2015).
To investigate a possible gradient of specialization along the anterior–posterior axis, we examined the response properties of neurons located at different regions of the prefrontal cortex. We performed recordings in monkeys prior to training in any type of working memory task, while they viewed the stimuli passively. This strategy allowed us to determine the response properties of prefrontal neurons without the influence of working memory task rules. We hypothesized that if an anterior–posterior hierarchy exists in the PFC, selectivity and information for stimuli would be maximal in posterior portions of PFC but would decline progressively in more anterior areas.
Materials and Methods
Six male rhesus monkeys (Macaca mulatta) weighing 5–12 kg were used in these experiments. None of the animals had any prior experimentation experience. Neural recordings were carried out in areas 8, 9, 9/46, 45, 46, and 47/12 of the lateral prefrontal cortex. Data from four of these monkeys were examined previously in a series of studies comparing the stimulus selectivity of dorsal and ventral PFC (Meyer et al. 2007, 2011; Qi et al. 2011). Newly acquired data from two additional monkeys are included in the analysis presented here. All experimental procedures followed guidelines by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals and the National Research Council's Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee.
Experimental Setup
Monkeys sat with their head fixed in a primate chair while viewing a monitor positioned 68 cm away from their eyes with dim ambient illumination. Animals were required to fixate a 0.2° white square appearing in the center of the screen. During each trial, animals had to maintain fixation on the square while visual stimuli were presented either at a peripheral location or over the fovea in order to receive a liquid reward. Any break of fixation immediately terminated the trial and no reward was given. Eye position was monitored throughout the trial using a non-invasive, infra-red eye position scanning system (model RK-716; ISCAN, Burlington, MA). The system achieved a < 0.3° resolution around the center of vision. Eye position was sampled at 240 Hz, digitized and recorded. Visual stimulus display, monitoring of eye position, and synchronization of stimuli with neurophysiological data were performed with in-house software (Meyer and Constantinidis 2005) implemented on the MATLAB environment (Mathworks, Natick, MA), and utilizing the psychophysics toolbox (Brainard 1997).
Task and Stimuli
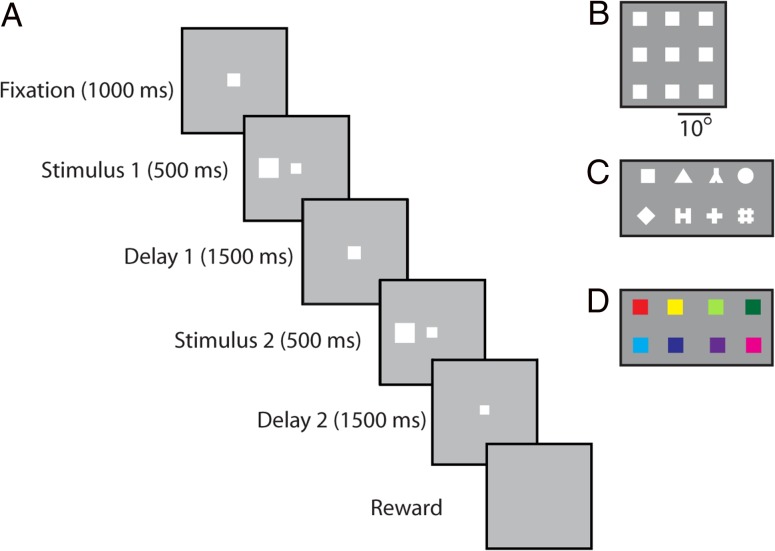
Monkeys were not trained to perform any working memory or other cognitive task. Instead, they received minimal training to maintain fixation while stimuli were displayed on the screen. Three different types of stimuli were used in these experiments: a spatial set, a shape set, and a color set (Fig. 1B–D). In the spatial set, a white 2° square stimulus was presented in one of nine possible locations arranged in a 3 × 3 grid with a 10° distance between adjacent stimuli. The shape set consisted of eight white geometric shapes calibrated for size and luminance (within 1% difference in number of pixels and fitting in a 2° outline). Similarly, the color set consisted of eight colored 2° squares equated for luminance.
Figure 1.
(A) The sequence of events during the passive viewing paradigm, using the spatial stimulus set. Monkeys were initially required to fixate for a period of 1 s after which a stimulus was shown for 0.5 s. After a delay period of 1.5 s, a second stimulus was shown for 0.5 s either matching the first in location/shape/color or of a different location/shape/color. Another delay period of 1.5 s followed after which the monkeys were rewarded for maintaining fixation throughout the entire trial. A break of fixation at any time aborted the current trial with no reward. (B) The spatial set involved presentation of a stimulus at one of the nine spatial locations arranged on a 3 × 3 grid of 10° dimensions. (C) Stimuli of the shape set. (D) Stimuli used in the color set.
The sequence of events during a trial was identical for all three sets. A fixation interval of 1 s where only the fixation point was displayed was followed by 500 ms of stimulus presentation, followed by a 1.5 s “delay” interval where, again, only the fixation point was displayed. A second stimulus was subsequently shown, either identical in location, shape, and color to the initial stimulus, or differing in one of these three dimensions. This second stimulus display was followed by another “delay” period of 1.5 s. The location and identity of stimuli in these experiments was of no behavioral relevance to the monkeys. We refer to delay periods intervening between the stimulus presentations in analogy to working memory tasks, though the animals were not required, or ever trained, to remember these stimuli.
Animals were conditioned to view stimuli passively in the following manner. Initially, animals were rewarded for moving their eyes to and maintaining fixation of the fixation point, which disappeared after 0.5 s. The duration of fixation period was progressively increased such that the monkeys were required to fixate for longer periods in order to achieve a reward. Once the monkeys could fixate for up to 5 s, the spatial stimuli were introduced. When first presented, the stimuli were of very low luminance and were barely perceptible. Over several weeks, the luminance was gradually increased while the monkey was rewarded for maintaining fixation and not saccading to the stimulus. The monkeys were exposed to the color and/or shape sets following exposure to the spatial sets at full luminance. During recordings, shape stimuli were positioned at one of the nine spatial locations used for the spatial grid. This was typically the spatial location that evoked the best response of the neurons being recorded or the location over the fixation point (at the fovea), if no clear response was observed for any location with the spatial set. Color stimuli were always presented over the fovea, where color sensitivity is greatest. Typically, 12–20 repetitions of each stimulus were recorded from each neuron.
Surgery and Neurophysiology
A 20 mm diameter craniotomy was performed over the lateral prefrontal cortex and a recording cylinder was implanted over the site. The location of the cylinder was visualized with anatomical MRI imaging (described below). In one monkey (MA), the recording cylinder was moved after an initial round of recordings so that an additional surface of the prefrontal cortex could be sampled. Extracellular recordings were performed with multiple microelectrodes. These were either glass- or epoxylite-coated tungsten electrodes with a 250 µm diameter and 1–4 MΩ impedance at 1 kHz (Alpha-Omega Engineering, Nazareth, Israel). Arrays of up to 8-microelectrodes spaced 0.5–1 mm apart were advanced into the cortex with a Microdrive system (EPS drive, Alpha-Omega Engineering) through the dura into the prefrontal cortex. The signal from each electrode was amplified and band-pass filtered between 500 Hz and 8 kHz while being recorded with a modular data acquisition system (APM system, FHC, Bowdoin, ME). Waveforms that exceeded a user-defined threshold were sampled at 25 µs resolution, digitized, and stored for off-line analysis. All neurons isolated from our electrodes were recorded, in an unbiased fashion, with no regard to response properties of a neuron being isolated.
Anatomical Segmentation
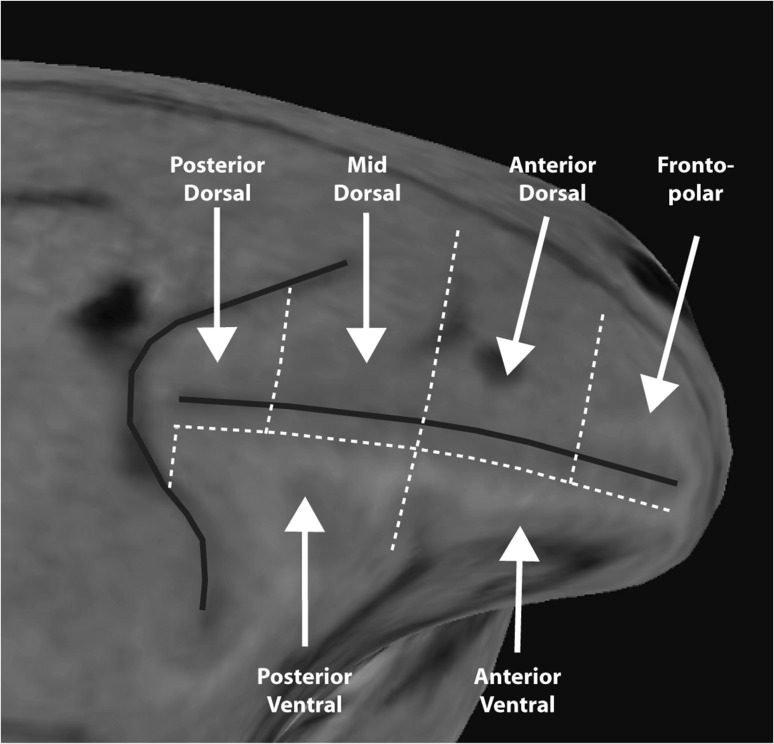
Each monkey underwent a magnetic resonance imaging scan prior to neurophysiological recordings. Electrode penetrations were mapped onto the cortical surface based on the MR image. We based our segmentation of the prefrontal cortex based on the Petrides and Pandya classification (Petrides and Pandya 1984, 1994, 2002). This segmentation identified 6 lateral prefrontal regions: a posterior dorsal region, including area 8A, a mid-dorsal region including area 8B and area 9/46, an anterior dorsal region, including area 9 and area 46, a posterior-ventral region, including area 45, an anterior-ventral region including area 47/12, and a fronto-polar region including area 10 (Fig. 2). The borders between these regions were obtained in each monkey in the following three steps: First, we determined the midpoint of the principal sulcus, along the anterior–posterior axis. A line vertical to the principal sulcus through this point defined the border between mid-dorsal and anterior-dorsal regions, as well between the posterior-ventral and anterior-ventral regions (Fig. 2). Second, borders between dorsal and ventral regions were set at 2 mm lateral to the center of the principal sulcus, so that the dorsal regions include both banks of the principal sulcus; the ventral regions extended along the entire length of the principal sulcus. Third, the dorsal prefrontal cortex was further segmented into a posterior-dorsal and a mid-dorsal region by a vertical line bisecting the distance between the genu of the arcuate and the midpoint of the principal sulcus; similarly the surface anterior to the midpoint was segmented into an anterior-dorsal and a fronto-polar region by a vertical line bisecting the surface between the frontal pole and the midpoint of the principal sulcus.
Figure 2.
Diagram of the PFC regions mapped over one monkey's brain. The PFC was divided into posterior-dorsal, mid-dorsal, anterior-dorsal, posterior-ventral, anterior-ventral, and fronto-polar regions.
Neural Data Analysis
All data analysis was implemented with the MATLAB computational environment (Mathworks, Natick, MA). Recorded spike waveforms were sorted into separate units using an automated cluster analysis relying on the KlustaKwik algorithm (Harris et al. 2000), which applied principal component analysis of the waveforms. Mean firing rate was then determined in each trial epoch. To ensure the stability of firing rate in the recordings analyzed, we identified recordings in which a significant effect of trial sequence was evident on the baseline firing rate (ANOVA, P < 0.05), e.g. due to a neuron disappearing or appearing during a run, as we were collecting data from multiple electrodes. Data from these sessions were truncated so that analysis was only performed on a range of trials with stable firing rate.
The first analysis performed tested whether responses from each neuron were higher during the first stimulus presentation compared to the 1 s fixation period preceding it. A neuron that had significantly greater responses during the presentation was identified as a visually responsive neuron (paired t-test; P < 0.05). Population discharge rates were evaluated by averaging activity from multiple neurons and constructing Peri-Stimulus Time Histograms (PSTHs). These were constructed using the best stimulus responses for each neuron. PSTHs averaged responses for each stimulus set and brain region.
Response latency was determined for each neuron using the Qsum algorithm, which identifies a rapid change from the baseline firing rate (Rowland et al. 2007; Katsuki and Constantinidis 2012). Cumulative distributions of neuronal latencies were constructed, and the distribution of latencies of different regions were compared using the Kolmogorov–Smirnov test at the α = 0.05 level.
An operational estimate of receptive field size was calculated for each neuron based on the responses to the nine stimuli of the spatial set, which covered an area of 20 × 20 degrees of visual angle. For each neuron, we determined the number of locations that elicited a response greater than the midpoint between the maximum and minimum firing rate to the nine locations. Additionally, we expressed the mean discharge rate during the stimulus presentation at each location as a percentage of the maximum discharge rate and plotted this response ratio as a function of distance from the location that elicited the maximum response.
Neuronal selectivity for different stimuli was evaluated by using a selectivity index defined as (Max − Min)/(Max + Min), where Max is the maximum response (during the stimulus presentation period, averaged across trials) and Min is the minimum response for the stimuli of the set. Selectivity indexes were calculated for each stimulus set presented to the neuron. The selectivity index values of each set were then averaged across neurons of a region, and means were compared between regions.
The discriminability between the best and worst stimulus of each set was determined by the use of a Receiver Operating Characteristic (ROC) analysis. The area under the ROC curve represents the probability that an ideal observer can discriminate between the best and worst stimulus based on their firing rate in each trial (Tolhurst et al. 1983). The analysis was performed separately for each neuron, in a time-resolved fashion, in a 100 ms window, computed in 1 ms steps and then averaged together. We calculated a baseline level of discriminability by calculating the area under the ROC curve in bins spanning the final 100 ms of the fixation period (prior to the onset of the stimulus) and averaging the ROC values in these bins. We also determined a peak level of discriminability, as the highest bin during the stimulus display. We then calculated the midpoint between these two values and identified the time point at which the area under the ROC curve value surpassed this midpoint as the average point of discrimination for each region.
An estimate of information represented in neuronal firing was obtained based on the bias-corrected, percentage of explained variance (PEV), also referred to as the ω2 statistic (Olejnik and Algina 2003). To obtain this, a 1-way ANOVA was performed for the firing rate of each neuron during the stimulus presentation period, separately for the spatial, feature, and color stimulus set. PEV is then defined as: ω2 = (SSBetween Groups−df × MSE) (SSTotal + MSE), where SSBetween Groups is the between groups sum of squares, df the degree of freedoms (stimuli in the set−1), MSE the mean squared error, and SSTotal the total sum of squares of the ANOVA table.
Results
Responses were recorded from 2054 prefrontal neurons in six monkeys, which were only required to view stimuli passively (Fig. 1), with no prior history of training in working memory tasks (monkey AD – 113 neurons, BE – 432, EL – 779, MA – 268, NI – 421, SCR – 41 neurons). To characterize the properties of prefrontal neurons along the anterior–posterior axis, we subdivided the lateral PFC into 6 regions prior to analysis (Fig. 2): posterior-dorsal region, mid-dorsal, anterior-dorsal, posterior-ventral, anterior-ventral, and fronto-polar. We analyzed neurophysiological recordings from five of these regions (posterior-dorsal: 182 neurons in 5 monkeys; mid-dorsal: 757 neurons in 6 monkeys; anterior-dorsal: 360 neurons in 5 monkeys; posterior-ventral 633 neurons in 5 monkeys; anterior-ventral 122 neurons in 3 monkeys). The fronto-polar region was not sampled sufficiently for this analysis and is depicted in Fig. 2 to illustrate the boundaries of the anterior-dorsal and anterior-ventral regions. A complete list of neurons in each area of each monkey can be found in Supplementary Table 1. In each region, we sampled neurons in an unbiased fashion, recording from all neurons encountered by our electrodes during each recording session, with no regard to their response properties. Stimuli of a spatial, a shape, and a color set were presented passively while the monkeys maintained fixation (Fig. 1). All stimulus sets were familiar to the animals prior to the onset of recordings, though the stimuli had no behavioral relevance.
Percentage of Responsive Neurons
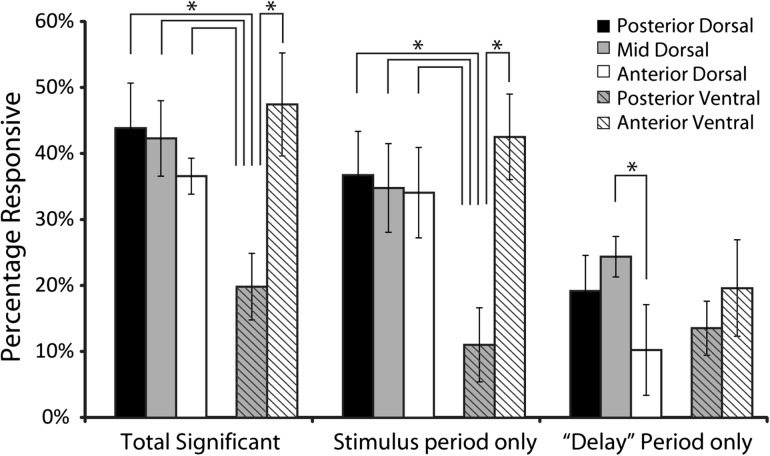
Our first order analysis was to examine if there were any differences between regions in terms of responsiveness, defined as the percentage of neurons with changes in firing rate during the presentation of stimuli or during the “delay” period following them, compared to the baseline fixation period. If anterior areas are generally more specialized for abstract operations, one might expect that responsiveness for stimuli presented passively would be highest for the most posterior areas. Contrary to this expectation, higher responsiveness did not prove to be a distinguishing property of more posterior areas. Overall, 717 (35%) of all PFC neurons exhibited significantly higher activity during the presentation of stimuli or during the delay period following the first stimulus presentation compared with baseline fixation (paired t-test; P < 0.05). When we compared responsiveness between regions during the stimulus presentation period (Fig. 3), we found that there was no significant difference between the three dorsal regions (posterior – 44%, mid – 42%, anterior – 37%; χ2-test, p > 0.5). On the other hand, responsiveness differed systematically between ventral regions; the percentage of neurons with elevated responses was much lower in the posterior-ventral region than the anterior-ventral region (posterior-ventral – 20%, anterior-ventral – 48%; χ2-test, p < 0.00001). The responsiveness of the posterior-ventral region was also significantly lower compared to each of the dorsal regions (χ2-test, p < 0.00001 in each comparison).
Figure 3.
Bar graphs represent the overall percentage of neurons that exhibited significant responses over the baseline period in either the cue or the delay period (left), in the cue period alone (middle) and in the delay period alone (right). Error bars represent standard error of the mean for percentages calculated separately in each monkey. Data are shown separately for each prefrontal region (N = 182 total neurons for posterior-dorsal, N = 757 for mid-dorsal, N = 360 for anterior-dorsal, N = 633 neurons for posterior-ventral, and N = 122 for anterior-ventral region). All line diagrams plotted represent significant differences between the corresponding bars, evaluated with a chi-square test (P < 0.05).
We refined our analysis by examining responsiveness during the stimulus presentation period alone. Again, we found no significant difference between the dorsal regions (posterior – 37%, mid – 35%, anterior – 34%; χ2-test, P > 0.8). Responsiveness of the posterior-ventral region was again significantly lower than the anterior-ventral region (posterior-ventral – 11%, anterior-ventral – 43%; χ2-test, p < 10−14). Similarly, the responsiveness of the posterior-ventral region was also lower than each of the three dorsal regions (χ2-test, p < 10−13 for each comparison).
Although the fixation task did not require the monkeys to remember and recall the stimuli, a proportion of neurons continued to be active during the delay period of the task, as reported previously (Meyer et al. 2007). We thus compared responsiveness between regions during the delay period following the presentation of the first stimulus. In this case, significantly fewer neurons in the anterior-dorsal PFC exhibited persistent discharges, compared to the posterior-dorsal and mid-dorsal regions (posterior-dorsal – 19%, mid-dorsal – 24%, anterior-dorsal – 10%; χ2-test, p < 0.0001). The difference in the percentage of neurons active during the “delay” period between the posterior-ventral and the anterior-ventral regions did not reach statistical significance (posterior-ventral – 14%, anterior-ventral – 20%; χ2-test, p > 0.1).
The lower responsiveness of the posterior-ventral region compared to all the other regions was highly reliable. A large sample from the posterior-ventral region was available for analysis (total 633 neurons) and the data were consistent within monkeys. Neurons recorded from both the posterior-ventral and posterior-dorsal regions were available in four monkeys. In each case, responsiveness of the posterior-ventral region was lower by at least 10% of the total number of neurons compared to the posterior-dorsal region (AD – 33% vs. 56%; BE – 19% vs. 29%; EL – 16% vs. 41%; MA – 38% vs. 68%). Also, data from both the posterior-ventral and anterior-ventral regions were available in two monkeys. In this case, too, the posterior-ventral region was systematically less responsive than the anterior-ventral region (MA – 38% vs. 49%; NI – 40% vs. 48%).
Responsiveness results indicate that the percentage of responsive neurons to the stimuli was not an obvious indicator of specialization. No significant differences were present between the dorsal regions whereas a higher percentage was observed in the anterior-ventral than the posterior ventral regions. These results may still be explained by the combined effects of a neuron's likelihood of activation by a passive visual stimulus, and selectivity for stimulus properties (discussed below).
Discharge Rate
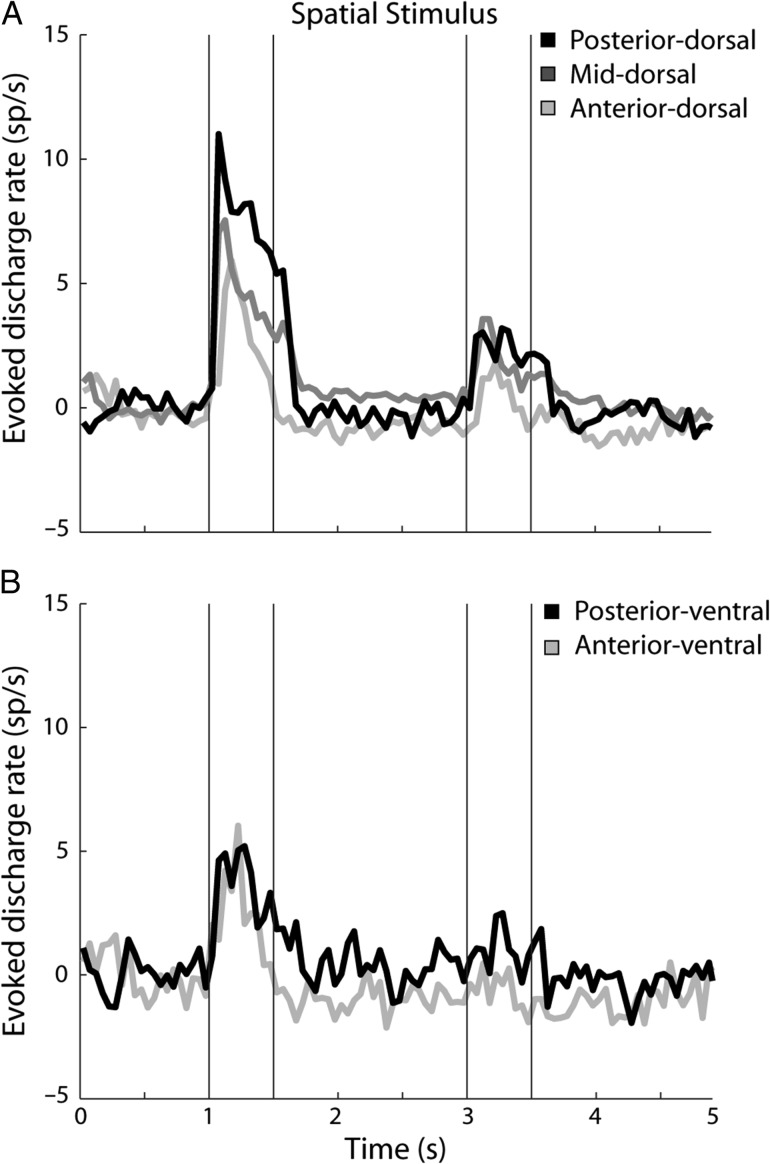
A difference between posterior and anterior regions may still be present in terms of the discharge rate elicited by the stimuli. To determine if there were inter-regional differences in firing rate, we examined the firing rate of the neurons in the spatial version of the task, in those neurons that responded significantly to the stimuli (Fig. 4). Among dorsal regions, we first observed that there were no differences in the firing rate during the fixation period (posterior – 7.1 spikes/s, mid – 5.1 spikes/s, anterior – 6.7 spikes/s; 1-way ANOVA, F2,419 = 1.84, P > 0.1). Most importantly, we observed a higher firing rate during the stimulus presentation for the posterior region, and lower firing rate for the mid-dorsal and anterior-dorsal regions. This analysis was based on the stimulus location that elicited the best response for each neuron. A difference was evident whether we compared the evoked firing rate during the stimulus presentation period after subtracting the baseline fixation rates (posterior 7.3 spikes/s, mid 4.5 spikes/s, anterior 2.9 spikes/s; ANOVA, F2,419 = 9.47, P < 0.0001; Fig. 4A), or the absolute firing rate of each region (posterior 14.4 spikes/s, mid 9.7 spikes/s, anterior 9.6 spikes/s; ANOVA, F2,419 = 3.63, P < 0.05). No significant differences in the firing rate between regions were present during the “delay” period following the stimulus presentation (absolute values: posterior 7.3 spikes/s, mid 5.9 spikes/s, anterior 5.9 spikes/s; ANOVA, F2,419 = 0.62, P > 0.5).
Figure 4.
Population PSTH for neurons with significant responses to the spatial stimuli (top: N = 65 neurons in posterior-dorsal, N = 253 in mid-dorsal, N = 104 in anterior-dorsal region; bottom: N = 66 in posterior-ventral, and N = 33 in anterior-ventral region). Vertical lines represent time of stimulus presentation. The best stimulus of the spatial set for each neuron was presented during the first stimulus interval. This was followed by either the same stimulus (match) or a stimulus at the diametric location (nonmatch), which have been averaged together in this PSTH.
As can be seen in Figure 4, the response during the second stimulus presentation was lower than that of the first. This occurred because trials have been selected in Figure 4 so that stimulus at the best location of each neuron appeared during the first presentation. That was followed by either a matching stimulus (involving presentation at the same, best location) or a nonmatching stimulus (involving presentation of the stimulus opposite to the best location in receptive field), which have been averaged together. Additionally, a matching stimulus at the best location elicited a weaker response in its second presentation for some neurons, akin to repetition suppression, as we have documented previously, even in untrained animals (Qi et al. 2012).
No systematic difference in the firing rate was evident between the ventral regions (Fig. 4B). There were no significant differences in the firing rate during the fixation period (posterior – 7.0 spikes/s, anterior – 9.0 spikes/s; ANOVA, F1,97 = 0.55, p > 0.4). There were also no differences in the stimulus rate, whether the baseline was subtracted (posterior 3.6 spikes/s, anterior 2.5 spikes/s; ANOVA, F1,97 = 1.1, P > 0.2), or not (posterior – 10.6 spikes/s, anterior – 11.5 spikes/s; ANOVA, F1,97 = 0.09, P > 0.7). No difference during the “delay” period was present, either (posterior – 7.5 spikes/s, anterior – 8.0 spikes/s; ANOVA, F1,97 = 0.05, P > 0.8).
Differences in the firing rate between regions were less pronounced when neurons were tested with the shape and color stimuli. There were no significant differences in evoked firing rate between dorsal regions in the stimulus period for either the shape stimuli (ANOVA, F2,308 = 0.2, P > 0.8) or the color stimuli (F2,131 = 0.32, P> 0.7). Similarly, no significant differences in evoked firing rate were observed for the ventral regions (ANOVA, F1,72 = 1.13, P > 0.3 for shape stimuli, F1,56 = 0.71, P > 0.4 for color stimuli). No comparisons in the delay period reached significance, either (ANOVA, F2,308 = 0.56, P > 0.5 for dorsal regions with shape stimuli; F2,131 = 0.11, P > 0.9 for dorsal regions with color stimuli; F1,76 = 1.73, P > 0.19 for ventral regions with shape stimuli; F1,56 = 1.4, P > 0.2 for ventral regions with color stimuli).
Overall, the discharge rate elicited by spatial stimuli was revealing of differences between dorsal regions, and consistent with stronger responses to stimuli by posterior regions. This was not evident in ventral regions. Stimulus sets that were less effective in driving neurons (shape and color stimuli in the dorsal regions) were also not differentiating between regions.
Response Latency
A hierarchical organization of the prefrontal cortex implies that the output of each region is transmitted to the next along the hierarchy. Response latency would thus be expected to increase for more anterior regions. We determined neuronal visual response latencies and compared them between regions. Only neurons with significant responses to visual stimuli were included in this analysis. Latency estimation was based on the best stimulus response of each neuron (across all stimulus sets).
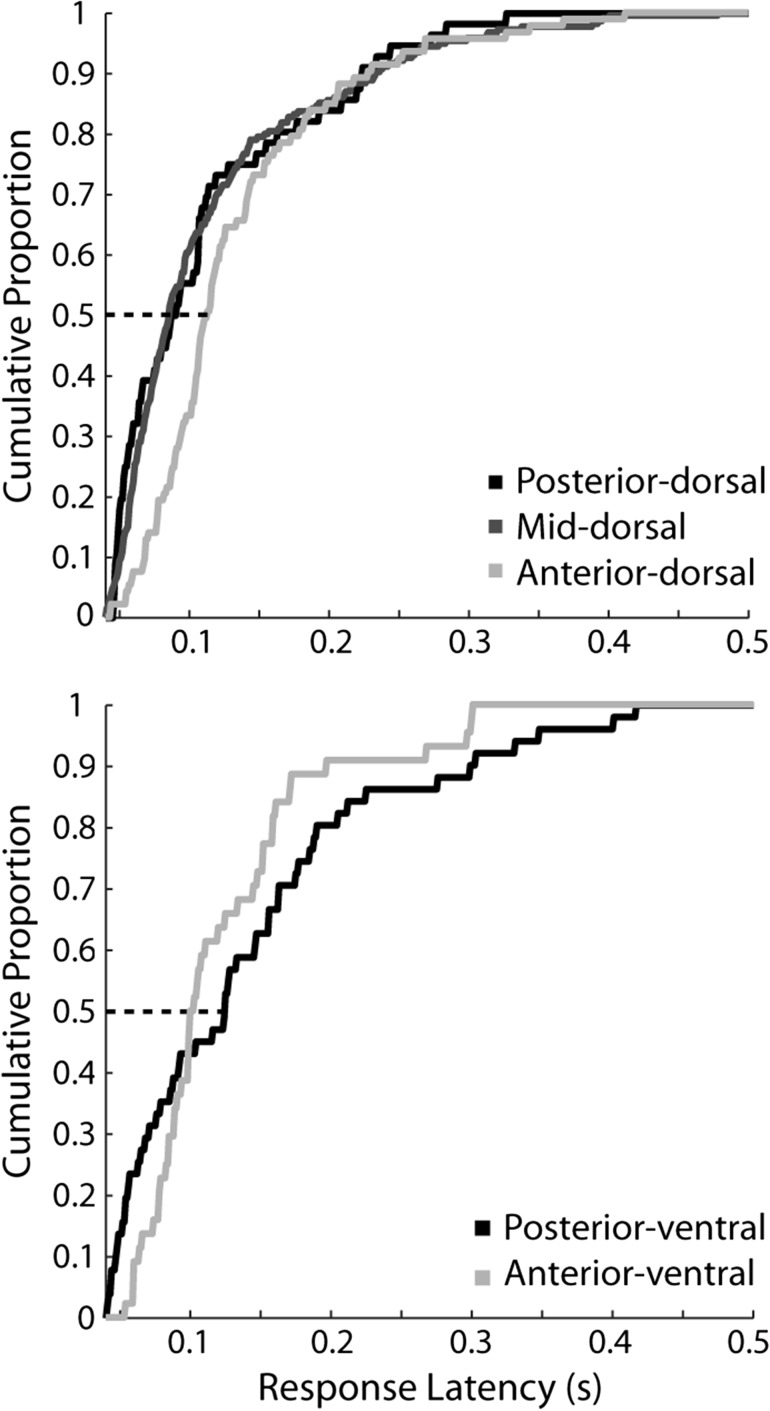
Our results indeed revealed a significant difference in the distribution of latencies between dorsal regions. The anterior-dorsal region had longer latencies overall, with a distribution that differed significantly from that of the posterior-dorsal and the mid-dorsal regions (Kolmogorov–Smirnov test, P < 0.05 in each case; median latencies: posterior-dorsal – 87 ms; mid-dorsal – 87 ms; anterior-dorsal – 113 ms).
No significant difference was present between ventral regions (median latencies: posterior-ventral – 125 ms; anterior-ventral – 100 ms; Kolmogorov–Smirnov test, P > 0.05). Comparing the two ventral regions revealed that the posterior-ventral region was characterized by the shortest overall latencies, though this difference reversed so that neurons with the longest latencies were also present in the posterior-ventral compared to the anterior-ventral region (Fig. 5). To ensure that the absence of a difference between the posterior and anterior ventral regions was not solely dependent on the precise border location between the two, we refined our analysis, and further bisected the posterior-ventral areas into a more posterior and a more anterior subdivision. In this case too, there was no significant difference between these ventral subdivisions (Kolmogorov–Smirnov test, P > 0.05), or even a trend towards shorter latencies in more posterior areas (median latency of posterior-most subdivision – 133 ms, of mid-ventral subdivision – 92 ms).
Figure 5.
Cumulative distribution of neuron latencies for the dorsal (top) and ventral (bottom) regions. Latencies were calculated using neurons that responded to any stimulus, tested with each neuron's preferred stimulus, across all sets tested (N = 67 posterior-dorsal, N = 264 mid-dorsal, N = 123 anterior-dorsal, N = 70 posterior-ventral, and N = 52 anterior-ventral). The dotted line represents the point at which half of the population of neurons has responded to the stimulus.
Receptive Field Size
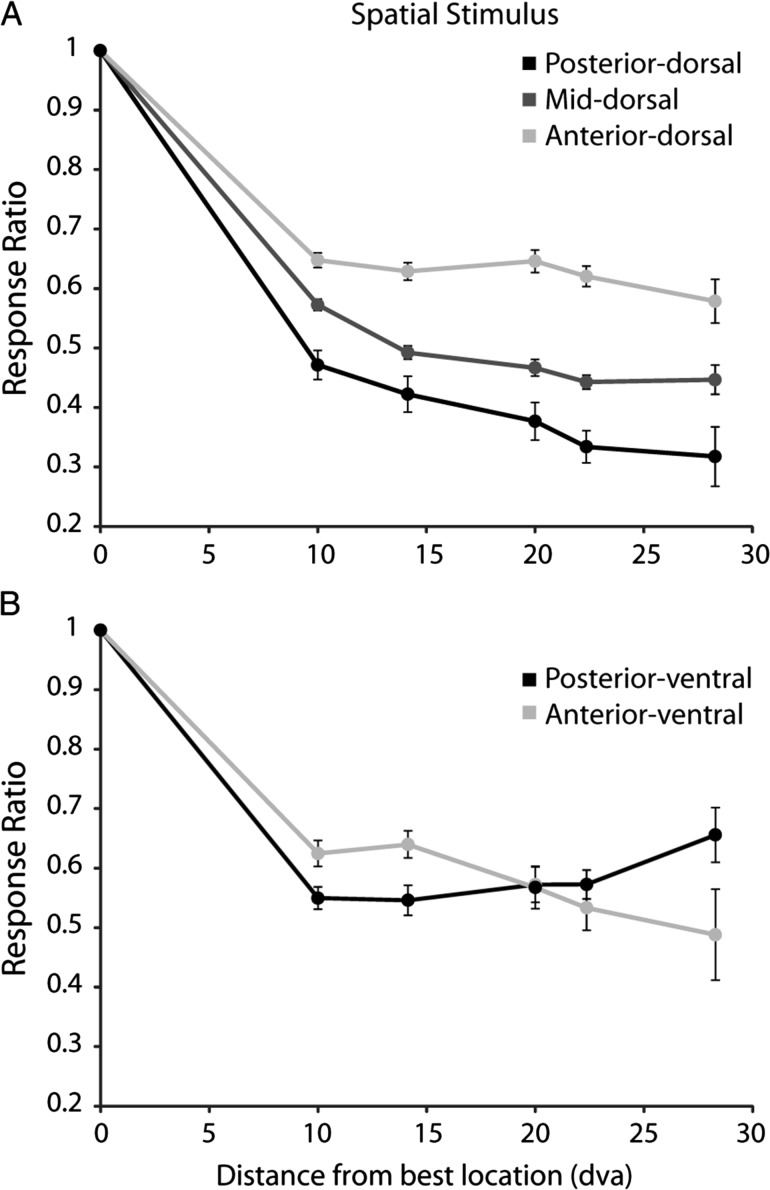
A hallmark of the functional organization of early visual cortical areas is the increase in the size of receptive fields along the visual hierarchy (Felleman and Van Essen 1991). We sought to determine whether a similar increase was present for prefrontal areas as well. Although we did not map receptive fields exhaustively, we obtained two operational estimates of receptive field size based on the number of stimuli neurons responded to in the context of the spatial set, and the percentage of the peak response as a function of distance. When determining the number of locations that elicited significant responses we indeed discovered a significant difference between regions. The posterior-dorsal exhibited the smallest receptive field size, whereas the anterior, the largest (posterior – 2.6 grid locations, mid – 2.9, anterior – 3.5; ANOVA, F2,419 = 9.27, P< 0.0005). We reached similar conclusions when we determined the mean percentage of peak response at different distances from the location that elicited the best response (Fig. 6). The posterior-dorsal region exhibited the sharpest decline of firing rate as a function of distance from the best location, as would be expected by the area with the smallest receptive fields. An intermediate slope was present in the mid-dorsal region and the least decline was observed in the anterior-dorsal region. These results were consistent with a hierarchical organization, with smaller receptive fields present at more posterior regions.
Figure 6.
Mean ratio of discharge rate for a stimulus at varying distances from the location that elicited the best response. (A) Dorsal regions. N = 65 neurons in posterior-dorsal, N = 253 in mid-dorsal, N = 104 in anterior-dorsal region. (B) Ventral regions; N = 66 in posterior-ventral and N = 33 in anterior-ventral. The ratio was calculated separately for each neuron, and averaged across neurons. Error bars represent the standard error of the mean calculated across neurons.
In the ventral PFC, neurons in the posterior-ventral region responded to more locations than the anterior-ventral region (posterior – 3.5 grid locations, anterior – 2.5; ANOVA, F1,97 = 7.83, p < 0.01). This difference between areas was mostly driven by neurons with large receptive fields, though. At short distances from the best location, posterior-ventral neurons exhibited more abrupt decrease in responses, suggesting that more neurons with small receptive fields were present in the posterior-ventral region (Fig. 6B). This relationship reversed at larger distances from the best location.
Stimulus Selectivity
We next considered the stimulus selectivity of neurons along the anterior–posterior axis of the prefrontal cortex. If posterior regions are more specialized for stimulus representation and anterior ones for more abstract operations that do not depend on the stimuli themselves, then higher levels of selectivity might be expected in the former. Our results largely confirmed this expectation. We evaluated stimulus selectivity for those neurons that exhibited significant responses to stimuli (33% of the total number of neurons), relying on a spatial, a shape, and a color stimulus set, and compared selectivity for different types of stimuli across regions.
Spatial Selectivity
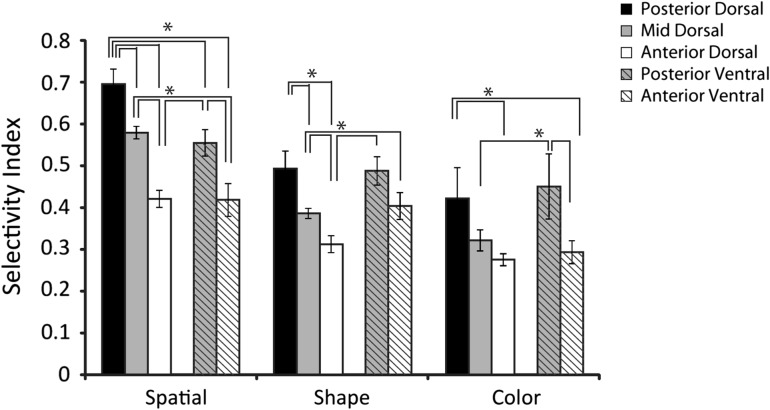
A total of 523 neurons with significant responsiveness during the stimulus period were tested with the spatial set in 6 monkeys. This set comprised identical white square stimuli that could appear at 9 locations in a 3 × 3 grid, with adjacent locations spaced 10° apart (Fig. 1B). We quantified the selectivity of each neuron with a selectivity index defined as (Max-Min)/(Max + Min). Mean values of the selectivity index were then compared across regions (Fig. 7). The three dorsal regions differed significantly in terms of spatial selectivity in this comparison (ANOVA, F2,419 = 28.74, P < 10−11). Spatial selectivity was highest in the posterior-dorsal region and declined for more anterior regions; the posterior-dorsal region exhibited a significantly higher selectivity index compared to either the mid- and anterior-dorsal regions of the PFC and the mid-dorsal region exhibited a significantly higher selectivity index to the anterior-dorsal region of the PFC (Tukey post-hoc test, P < 0.05). The posterior-ventral region had a significantly higher selectivity index compared to the anterior-ventral region (ANOVA, F1,97 = 6.74, P < 0.05). We also evaluated the relative gradient of spatial selectivity across the areas by performing a 2-way ANOVA with factors dorsal/ventral and anterior/posterior position (grouping the mid-dorsal and anterior-dorsal areas together). This test revealed a significant main effect of anterior/posterior position (F1,518 = 22.7, P < 10−5), as well as a main effect of dorsal/ventral position (F1,518 = 16.75, P < 10−4), but no interaction between the two factors (F1,518 = 0.16, P > 0.6).The result suggests that spatial selectivity declines for neurons located more anterior in both the dorsal and ventral PFC, and at approximately the same rate.
Figure 7.
Average selectivity index for each of the stimulus sets, among neurons with significant responses to stimuli (N = 67 posterior-dorsal, N = 264 mid-dorsal, N = 123 anterior-dorsal, N = 70 posterior-ventral, and N = 52 anterior-ventral). Error bars represent standard error of the mean computed across all neurons. All line diagrams plotted represent significant differences between the corresponding bars, evaluated with a 1-way ANOVA and Tukey post-hoc test (P < 0.05).
The differences in spatial selectivity were also consistent within subjects. Two monkeys had sufficient recordings for a comparison between regions. Monkeys EL and MA showed significant differences in the spatial indexes across dorsal regions (ANOVA, F2,186 = 3.54, P < 0.05 for monkey EL and F2,59 = 5.43, P < 0.01 for monkey MA), with the lower selectivity value observed in the anterior-dorsal region. Monkey NI showed significantly lower selectivity indexes for the anterior-ventral compared to the posterior-ventral regions (ANOVA, spatial F1,37 = 6.98, p < 0.05).
Comparing spatial selectivity between ventral and dorsal regions revealed that the posterior-dorsal region exhibited higher selectivity than either of the ventral regions (Tukey post-hoc test, P < 0.05). There was no differences between the mid-dorsal and posterior-ventral region as well as no difference between the anterior-dorsal and anterior-ventral regions (Tukey post-hoc test, P > 0.05 for either case).
Shape Selectivity
We evaluated selectivity for shapes in a similar manner. A total of 384 neurons with significant responsiveness during the stimulus period were tested with the shape set, in 5 monkeys. This set comprised 8 white geometric shapes of equal size (Fig. 1C). Similar to the results seen with the spatial selectivity index, we saw a significant difference between dorsal regions (ANOVA, F2,308 = 11.06, P < 0.0001). We again saw highest shape selectivity in the posterior-dorsal region and significantly lower values for the mid-dorsal and anterior dorsal regions, with the mid-dorsal having higher values than the anterior-dorsal region (Tukey post-hoc test, P < 0.05). Shape data from all dorsal regions were available in one monkey. Monkey EL showed a significant difference in the shape selectivity index along the dorsal regions (ANOVA; F2,189 = 4.42, P < 0.05) with lowest selectivity values in the anterior-dorsal region.
The difference between the posterior-ventral and anterior-ventral regions did not reach statistical significance in terms of the shape selectivity index (ANOVA, F1,72 = 2.02, P > 0.15). Comparing dorsal and ventral regions revealed higher values for the posterior-ventral region compared to both mid- and anterior-dorsal regions (Tukey post-hoc test, p < 0.05). There were no differences between posterior-dorsal and either of the ventral regions (Tukey post-hoc test, P > 0.05).
Color Selectivity
Color selectivity was tested in 192 neurons with significant responsiveness during the stimulus period, recorded in 2 monkeys. The color set comprised 8 different equiluminant colored squares (Fig. 1D). This set also revealed significant differences in stimulus selectivity between dorsal regions (ANOVA, F2,131 = 4.81, p < 0.01). Again, we observed that the posterior-dorsal region had a higher selectivity index value than the anterior-dorsal region (Tukey range test, p < 0.05). The result was consistent within one monkey (MA) that had sufficient recordings for a comparison between regions. A significant difference in color selectivity index values was present between dorsal regions in this monkey (ANOVA, F2,52 = 6.85, p < 0.005).
A significant difference in color selectivity was present between ventral regions. The posterior-ventral region exhibited higher selectivity index values than the anterior-ventral region (ANOVA, F1,56 = 5.76, p < 0.05). The result was consistent within one monkey (NI) that had sufficient recordings for a comparison between regions (ANOVA, F1,37 = 7.22, p < 0.05). Comparing color selectivity between dorsal and ventral regions, we observed higher posterior-ventral color SI values compared to the anterior-dorsal region (Tukey post-hoc, p < 0.05). Other comparisons did not reveal significant differences.
Joint Stimulus Selectivity
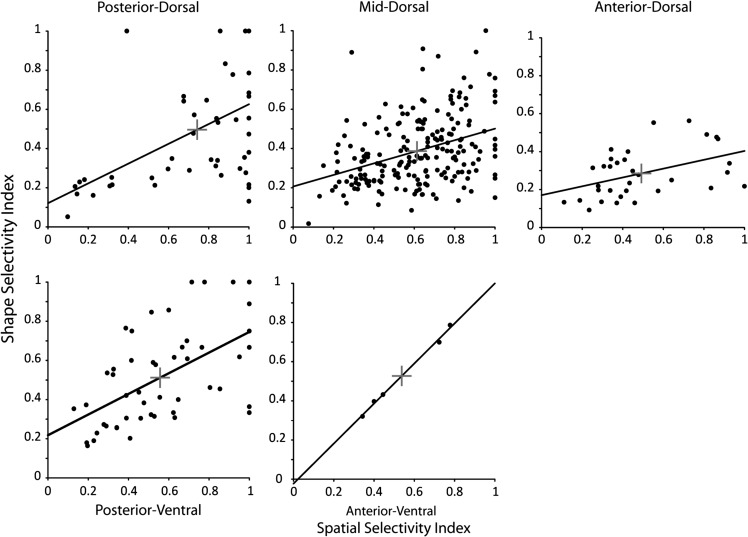
We considered next the selectivity of individual neurons for different types of stimuli. We thus plotted the shape selectivity index of each neuron as a function of its spatial selectivity index (Fig. 8). A predictive relationship was present between the two variables, for all regions examined (regression analysis, P < 0.0005 for the posterior-dorsal, P < 10−7 for the mid-dorsal, p < 0.05 for the anterior-dorsal, P < 0.00005 for the posterior ventral, and P < 0.0005 for the anterior ventral, respectively). Less data were available for the analysis of joint selectivity for spatial locations and color, nevertheless a significant relationship between the two variables was again present in the mid-dorsal, anterior-dorsal, and anterior-ventral regions (regression analysis, P < 0.00005, P < 0.005, and P < 0.005 respectively). We evaluated the joint selectivity of individual neurons for spatial locations and shapes by computing the geometric mean of their spatial and feature selectivity index (the square root of their product). A comparison of geometric means revealed a significant difference in joint selectivity across the dorsal regions (ANOVA, F2,280 = 14.9, p < 10−6), with the posterior-dorsal regions being significantly more selective than either the mid-dorsal or anterior-dorsal (Tukey post-hoc test, P < 0.05). No significant difference was seen in the geometric means for the ventral regions (ANOVA, F1,52 = 0.01, P > 0.9).
Figure 8.
Shape selectivity index is plotted as a function of spatial selectivity index in each prefrontal region. Each point represents a single neuron with significant responses to visual stimuli, tested with the spatial as well as the shape stimulus test (N = 49 posterior-dorsal, N = 201 mid-dorsal, N = 33 anterior-dorsal, N = 49 posterior-ventral, N = 5 anterior-ventral). Solid line represents regression line through the data. Cross indicates the mean in the shape selectivity and spatial selectivity dimensions in each region.
Overall, the stimulus selectivity analysis provided strong evidence of functional specialization. The gradient of stimulus selectivity declined systematically towards more anterior areas, and this was true across stimulus sets, and both for the dorsal and ventral prefrontal regions.
Time Course of Discrimination
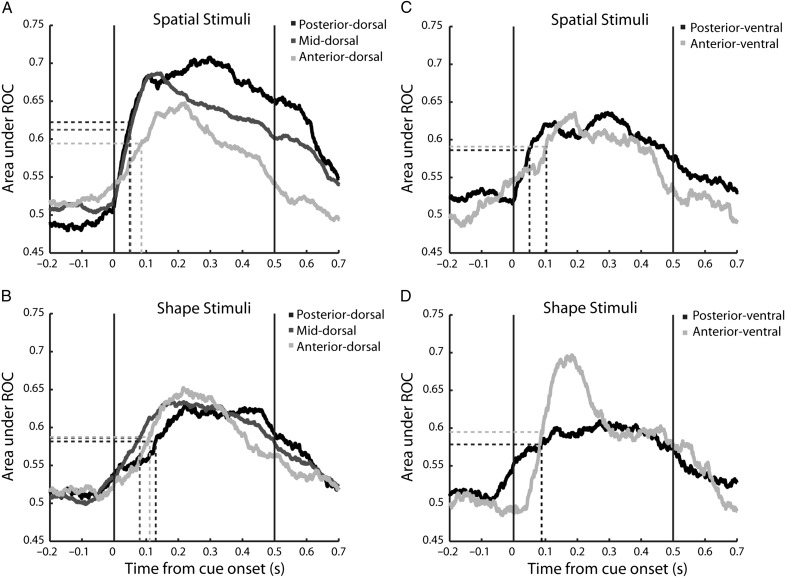
To obtain a measure on whether stimuli were more discriminable in posterior regions and whether this discrimination emerged earlier in time, we performed an ROC analysis, which does not only depend on the difference between the firing rate but also on the reliability of firing distributions (Fig. 9). We did this in a time resolved fashion and compared the time course of ROC by determining the time point at which a region reached the mid-point between its baseline and peak ROC value (Katsuki and Constantinidis 2012). Tested with the spatial stimuli, the half point of the peak ROC value was reached at earlier time points in the posterior-dorsal region (50 ms). The time course of ROC values followed an intermediate pattern for the mid-dorsal region (51 ms). Finally, the anterior-dorsal region reached the mid-point of ROC values at the latest time point (85 ms). We also determined the peak ROC value for each neuron for each stimulus presentation and saw that the posterior-dorsal and mid-dorsal had higher maximum ROC values than the anterior-dorsal region for spatial stimuli (posterior – 0.80, mid – 0.80, anterior – 0.75; ANOVA, F2,419 = 7.31, P < 0.01).
Figure 9.
Area under the ROC curve calculated in 100 ms time intervals stepped every 1 ms to determine the mid-point of discrimination between each neuron's best stimulus and worst stimulus for each stimulus set. (A,B) Discriminability for spatial and shape stimuli in dorsal regions. N = 67 posterior-dorsal, N = 264 mid-dorsal, N = 123 anterior-dorsal. (C,D) Discriminability for spatial and shape stimuli in ventral regions. N = 70 posterior-ventral, and N = 52 anterior-ventral. The mean point of discrimination was calculated by determining the midpoint between the baseline and peak ROC values during the stimulus presentation and determining the time-point where the population ROC trace crossed that point. Neuron populations are the same as Figure 7.
This pattern of earlier ROC values was not uniform across all stimulus sets, however (Fig. 9B). The mid-dorsal region and anterior-dorsal region exhibited faster discrimination of shapes than the posterior-dorsal region (F2,308 = 6.1, P< 0.01). The results suggest that although visual latencies were shorter overall and discrimination was higher for the posterior regions, not all types of information were first discriminated there.
In the ventral prefrontal cortex, we saw little difference between regions in the timing of discrimination when tested with the shape-based stimuli (Fig. 9D), but saw an earlier discrimination of spatial location (Fig. 9C) in the posterior-ventral region (50 ms) compared to anterior-ventral (103 ms). There were no differences in the maximum ROC value between regions for either stimulus set (spatial: posterior – 0.74, anterior – 0.73, ANOVA, F1,97 = 0.78, P > 0.35; shape: posterior – 0.73, anterior – 0.77, ANOVA, F1,72 = 3.0, P > 0.08).
The ROC analysis, largely confirmed the results of firing rate latency, and stimulus selectivity analysis. Although the respective measures are slightly different, a functional specialization of regions was present for stimuli that can be discriminated from the firing rate of neurons in each.
Stimulus Information
The selectivity index measures and ROC analysis presented above compare responses to only the best and worst location or feature. To more precisely quantify the ability of prefrontal neurons in different regions to represent the entirety of the stimulus sets, we estimated the amount of information that could be decoded for the stimulus features by calculating the percentage of explained variance (PEV) in the firing rates during the stimulus presentation period, for each stimulus set (Fig. 10).
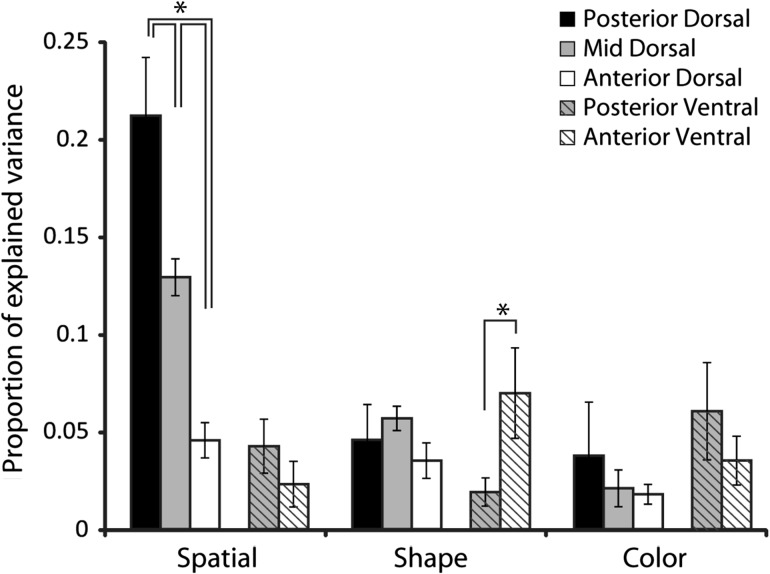
Figure 10.
Proportion of explained variance (PEV) for the stimuli sets during stimulus presentation (N = 67 posterior-dorsal, N = 264 mid-dorsal, N = 123 anterior-dorsal, N = 70 posterior-ventral, and N = 52 anterior-ventral). Error bars represent standard error of the mean PEV computed across all neurons. All line diagrams plotted represent significant differences between the corresponding bars, evaluated with a 1-way ANOVA and Tukey post-hoc test ( P < 0.05).
A significant difference between the dorsal regions was present in the mean amount of spatial information that could be decoded (ANOVA, F2,419 = 23.03, P < 10−9). The spatial information represented in the posterior-dorsal region was significantly higher than that of both the mid-dorsal and anterior-dorsal region (Tukey post-hoc text, P < 0.05). The result was consistent within one monkey (EL) with sufficient results for a comparison between dorsal regions; highest values of PEV were observed for the posterior-dorsal region and lowest for the anterior-dorsal, a difference that was significant (ANOVA, F2,186 = 13.01, P < 0.0001). The same pattern of PEV values across regions was also present in a second monkey (MA), though for this animal the difference between regions narrowly failed to reach statistical significance (ANOVA, F2,59 = 2.73, p = 0.07). Spatial information did not differ significantly between ventral regions (ANOVA, F1,97 = 0.84, P > 0.3). For the shape set, we also observed no differences in PEV for the dorsal regions (ANOVA, F2,308 = 1.21, p > 0.3). A significant difference was present between ventral regions in this case (ANOVA, F1,71 = 7.32, P < 0.02). Finally, using the color set, we observed no differences between the dorsal regions or between the ventral regions (ANOVA, F2,131 = 1.12, P > 0.3 for dorsal regions and F1,56 = 0.89, P > 0.3 for ventral regions).
Comparing the dorsal and ventral regions revealed differences in spatial and color information. Dorsal regions (pooled together) exhibited much greater spatial information than ventral regions (t-test, t174 = 5.29, P < 10−6). Ventral regions on the other hand were characterized by higher color information compared to dorsal regions, pooled together (t-test, t190 = 2.02, P < 0.05).
Overall, the analysis of information revealed a clear gradient of spatial information in dorsal regions, declining for more anterior areas. It also confirmed the greater representation of spatial information in the dorsal compared to ventral areas, and color for ventral than dorsal areas.
Discussion
The goal of these experiments was to determine if gradients of functional specialization exist along the anterior–posterior axis of the prefrontal cortex prior to any training in working memory tasks. We segmented the prefrontal cortex in three dorsolateral regions, the posterior-, mid-, and anterior-dorsal regions and two ventrolateral regions, the posterior- and anterior-ventral regions. Our results revealed a gradient of specialization along the anterior–posterior axis of the prefrontal cortex, with posterior areas being more selective for stimulus properties. In the dorsal PFC this specialization manifested itself as an increase in receptive field sizes, resulting in less selectivity for stimuli appearing at different locations, and less information about the location of the stimulus decoded from neuronal firing in more anterior areas. Results in the ventral prefrontal cortex were consistent with high selectivity for object identity of stimuli in the posterior regions, which would explain that many neurons would not respond at all to the limited set of stimuli we used. Our results also confirmed a functional specialization between dorsal and ventral regions in terms of the amount of spatial information represented in neural activity, which was greater overall for dorsal regions, and (to a lesser extent) color information, which was greater overall for the ventral regions. The combined effect of these two gradients meant that the relative difference in stimulus representation between dorsolateral and ventrolateral neurons depended on their position along the anterior–posterior axis, which may help explain conflicting results from prior studies about dorsal and ventral specialization.
Our findings have a number of caveats. Anatomical segmentation was based on well-defined landmarks but not on precise cyto-architectonic borders, which are not entirely agreed upon for these areas (Preuss and Goldman-Rakic 1991; Petrides and Pandya 1994). Multiple cortical areas were included in each region that we identified, for example Petrides' area 9 and area 46 in the anterior-dorsal region. This segmentation process was also necessitated by the fact that regular and dense sampling was not available from all six subjects, which made it impossible to consistently delineate precise borders between areas or abrupt transitions in properties along the cortical surface, which some studies have succeeded in identifying (Kaping et al. 2011). However, we did identify systematic differences between regions and any errors in identifying the borders between regions may only dilute the inter-areal differences we report here. Additionally, differences in properties between areas were consistent within individual monkeys.
A further caveat was that recordings were obtained from animals trained only to fixate, while stimuli were presented passively. This approach allowed us to reveal differences in response properties of neurons along a posterior-anterior axis absent the influence of task rules, which may alter the responsiveness of neurons, confounding stimulus with task effects. The choice of task has proven critical in human studies, with contrasting results depending on the task subjects have been required to do (Koechlin et al. 2003; Ramnani and Owen 2004; Baird et al. 2013; Crittenden and Duncan 2014). It is entirely possible that execution of different tasks will reveal additional differences between areas; representation of stimulus features is enhanced if they are relevant for the task (Rainer et al. 1998; Everling et al. 2002; Lennert and Martinez-Trujillo 2011, 2013). Finally, our stimulus sets were limited. Responses between areas may also be differentiated along other stimulus dimensions or based on more complex stimuli.
Response Properties along the Anterior–Posterior Axis
Our results in the dorsolateral prefrontal cortex were consistent with a hierarchical organization along the anterior–posterior axis. The anterior-dorsal region was generally characterized by the lowest firing rates, longest response latencies, largest receptive fields, and lowest stimulus selectivity, discriminability and information, particularly for spatial stimuli. Peak responses to stimuli in our sets were much weaker in anterior rather than posterior areas, even though there were no differences in baseline firing rates (Fig. 4). Stimulus selectivity decreased systematically from posterior to anterior areas. This gradient of selectivity was not the result of particularly strong visual responses to spatial stimuli in posterior-dorsal PFC. Shape and color stimuli also activated these neurons, and shape and color selectivity differed significantly between dorsal areas (Fig. 7), as did the joint selectivity for location and shape (Fig. 8). Posterior-dorsal neurons were most selective to the attributes of visual stimuli, in agreement with prior studies that showed robust stimulus selectivity in area 8a (Blatt et al. 1990; Bichot et al. 1996; Chafee and Goldman-Rakic 1998; Rainer et al. 1999). The population response latency and size of receptive field were also consistent with a functional differentiation between areas, with the longest latencies and largest receptive fields found in the anterior-dorsal region.
A seemingly unexpected finding was that no significant differences were present in the percentages of neurons that responded to stimuli in the posterior-, mid-, and anterior-dorsal regions (Fig. 3). The result suggests that even simple stimuli presented entirely passively are capable of recruiting similar proportions of neurons across the length of the dorsal prefrontal cortex. This finding is likely to be the result of the simultaneous, opposing effect of stimulus selectivity, which declines more anteriorly, making it more likely that a neuron will respond to any of the stimuli in our limited sets; and specialization for more abstract operations, which is speculated to increase more anteriorly (Badre and D'Esposito 2009), and which makes it less likely that a neuron will respond to a stimulus presented passively, for which no mental operation is required.
Systematic differences in receptive field size have also been reported within the dorsal prefrontal cortex, in a medial-to-lateral gradient (Suzuki and Azuma 1983). Larger peripheral receptive fields are found at more medial aspects and smaller, peri-foveal receptive fields closer to the principal sulcus. Our study did not address this second gradient, and we did not map receptive fields exhaustively. However, the overall size of receptive fields was larger in more anterior regions.
The parcellation of the dorsal prefrontal cortex in cyto-architectonic areas is not entirely agreed upon. Some studies (Petrides and Pandya 1984, 1994, 2002) divide the length of the principal sulcus into two sets of areas (area 9/46 and area 46, each with a medial and a lateral aspect, lining the two banks of the principal sulcus and rim cortex around them), which correspond roughly to the mid-dorsal and anterior-dorsal regions in our study. Others suggest a medial and a lateral area 46 running the entire length of the principal sulcus (Preuss and Goldman-Rakic 1991). In our analysis we find clear functional differences between the mid- and anterior-dorsal areas, supporting a functional specialization consistent with the first scheme. Anterior-dorsal neurons differed significantly from mid-dorsal neurons in terms of their response latency (Fig. 5), receptive field size (Fig. 6), selectivity for spatial and shape stimuli (Fig. 7), and amount of information that could be decoded from their activity (Fig. 10). Overall, dorsolateral PFC findings are consistent with a hierarchical organization with posterior regions more faithfully representing information about stimulus properties, which propagates into anterior prefrontal areas performing more abstract operations, as hypothesized based on human imaging studies (Koechlin et al. 2003; Badre and D'Esposito 2009).
Specialization of ventral regions was most evident in terms of the percentage of neurons that responded to any of the stimuli, which was much lower in posterior areas (Fig. 3). An important point for interpreting this finding is that we did not exhaustively probe neurons for their preferred stimulus object. Prior studies have revealed exquisite specialization of posterior ventral neurons for complex stimuli, such as faces or other objects (Wilson et al. 1993; O'Scalaidhe et al. 1997; Scalaidhe et al. 1999). A decrease in object specialization in more anterior areas would make it more likely that anterior-ventral neurons are activated by one of our stimuli, resulting in this profound difference in responsiveness. Among neurons that did respond to one of our stimuli, we also observed higher spatial selectivity in posterior- then the anterior-ventral region, mirroring the gradient of selectivity in the dorsal regions. On the other hand, mean firing rate and response latency did not differ between ventral regions, and higher shape-stimulus information was present in the anterior- than the posterior-ventral region. These results raise the possibility of some heterogeneity rather than a strictly serial organization of the ventral areas. The posterior-ventral region as defined here comprises area 45A, 45B and part of area 12, as defined by Preuss and Goldman-Rakic (1991). One possibility is that setting the border between our posterior and anterior-ventral regions based on the midpoint of the principal sulcus rather than determining the precise cyto-architectonic areas resulted in a region with a mixture of areas of different properties. However, in the case of response latency, further breaking down the posterior-ventral region in finer subdivisions did not indicate localization of the shortest latencies at the posterior-most aspect of the ventral prefrontal cortex. Instead, it seems possible that different ventral areas receive distinct inputs and are specialized for different functions.
Dorsal versus Ventral Prefrontal Specialization
The functional specialization between dorsal and ventral subdivisions of the prefrontal cortex has been another matter of debate. Early neurophysiological investigations attributed spatial information predominantly to the dorsal prefrontal cortex and shape information to the ventral prefrontal cortex (O'Scalaidhe et al. 1997, 1999; Wilson et al. 1993; Qi et al. 2011), two regions that receive major anatomic input from the posterior parietal and inferior temporal cortex, respectively (Petrides and Pandya 1984; Selemon and Goldman-Rakic 1988; Cavada and Goldman-Rakic 1989; Romanski et al. 1999). A number of other studies, while not specifically addressing areal specialization, have also documented at least a relative segregation of spatial and non-spatial properties between the dorsal and ventral regions (Hoshi et al. 1998; Ninokura et al. 2004). Other studies, however, have demonstrated that dorsal prefrontal neurons can exhibit significant shape and color selectivity and ventral neurons spatial selectivity, at least after monkeys have been trained in tasks that require these stimulus dimensions to be remembered (Rao et al. 1997; Rainer et al. 1998; Tsujimoto et al. 2012; Mante et al. 2013; Rigotti et al. 2013; Kadohisa et al. 2015). Our present findings, revealing specialization within the dorsal prefrontal cortex, can potentially reconcile some of these disparate findings.
Our results demonstrate that posterior-dorsal and posterior-ventral prefrontal cortexes are functionally discriminable in terms of responsiveness to stimuli presented passively. Over 40% of neurons in the posterior-dorsal region responded during or after the presentation of visual stimuli whereas only ~20% of posterior-ventral neurons did so (Fig. 3, middle). As discussed above, this difference is consistent with higher specialization for complex objects in the ventral than the dorsal PFC (or specific stimuli of other modalities). Previous studies have shown that this difference is reduced after training in a working memory task, when a greater proportion of ventral prefrontal neurons becomes responsive to the same stimuli, once they are incorporated in a cognitive task (Qi et al. 2011). Recent studies also suggest an important role for posterior-ventral cortex in feature attention (Bichot et al. 2015) and the absence of task execution may have been critical for the activation of this region in our experiment.
Spatial selectivity and information was considerably higher for the dorsal than the ventral regions with the spatial stimulus set we used, which involved presenting stimuli in a grid with stimulus eccentricity ranging up to 14°, for the diagonal stimuli in our grid. In contrast, shape selectivity and information failed to differentiate between dorsal and ventral areas altogether, and color difference was modest. In the case of shape stimuli, we should note that we used simple geometric shapes and did not exhaustively search for each neuron's preferred stimulus. Selectivity for highly complex stimuli, such as faces, has been shown to differentiate between dorsal and ventral prefrontal areas (O' Scalaidhe et al. 1997, 1999). Prior studies which have reported lack of functional specialization between dorsal and ventral areas have relied on an approach similar to ours, testing each neuron with a limited set of random stimuli (Rao et al. 1997). Thus, a key difference between prior studies may have been the degree to which they emphasized shape/color processing relative to spatial processing – with spatial studies finding strong evidence for dorsal-ventral functional segregation, and shape/color studies finding weak or no evidence for dorsal-ventral functional segregation. We should additionally point out that even spatial selectivity was sensitive to the position along the anterior–posterior axis, with difference between dorsal and ventral areas declining as electrode penetrations moved more anteriorly.
An additional important factor for the interpretation of our results is that we relied entirely on visual stimuli. Prior studies have illustrated anatomical specialization of prefrontal areas in terms of processing stimuli of other sensory modalities, including auditory (Romanski and Goldman-Rakic 2002; Sugihara et al. 2006) and somatosensory stimuli (Romo et al. 1999; Brody et al. 2003). The results of dorsal and ventral specialization we present are therefore not exhaustive.
Implications for Cognitive Specialization
The data we report here were recorded exclusively from monkeys that had not been trained to perform cognitive tasks, so the functional role of prefrontal areas along the anterior-posterior axis in cognitive functions may only be inferred indirectly. Hierarchical theories of PFC organization are based in part on the idea that most abstract cognitive operations are performed in anterior regions (Badre and D'Esposito 2009). The current data still support an important component of these theories, as they confirm that the precision of sensory representation falls off more anteriorly in PFC.
It is also important to point out that prefrontal neurons in naïve monkeys exhibit many properties that are commonly assumed to be present only during overt execution of cognitive operations. These include persistent activity in the delay period after the stimulus presentation (Scalaidhe et al. 1999; Meyer et al. 2007), which is often equated with willful maintenance of working memory (Frith and Dolan 1996; Postle 2006), and selectivity of individual neurons for more than one stimulus property (Meyer et al. 2011), which has been presumed to arise only after training in a task that requires integration of multiple stimulus features (Rao et al. 1997; Rainer et al. 1998).
Previous studies of the effects of training offer insights about the expected changes in functional properties of these neurons during the execution of cognitive tasks. Training in a working memory task has been shown to increase the firing rate of PFC neurons in non-human primates, particularly throughout the period of delay period of the task (Meyer et al. 2011; Qi et al. 2011). Importantly, average neuronal selectivity for stimulus properties did not increase even after extensive training so that decoding of stimulus information was not improved in trained animals (Qi et al. 2011; Meyers et al. 2012). This was so because the stimuli used in these experiments were highly discriminable from each other and the training did not involve learning to identify these any better, but rather incorporating them in a cognitive task. Considering the present results, it appears possible that anterior prefrontal neurons remain non-selective for the properties of simple stimuli such as those in the spatial set used here, even when the latter have been incorporated in a cognitive task (though this has not been tested explicitly). Neurons in anterior areas may still be more readily activated during task operations, regardless of the operant stimuli used. It is upon future studies to address directly the relative malleability of posterior and anterior areas and activation by abstract tasks and rules.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
Research reported in this paper was supported by the National Eye Institute of the National Institutes of Health under award number R01 EY017077 to C.C.; by NINDS training grant T32 NS073553; NIMH grant F31 MH104012 to M. R. R; and by the Tab Williams Family Endowment.
Supplementary Material
Notes
We wish to acknowledge the contribution of Travis Meyer in the collection of part of the data set analyzed here, Kathini Palaninathan for technical help, and Samson King for helpful comments on the manuscript. Conflict of interest: None declared.
REFERENCES
- Adcock RA, Constable RT, Gore JC, Goldman-Rakic PS. 2000. Functional neuroanatomy of executive processes involved in dual-task performance. Proc Natl Acad Sci USA. 97:3567–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A. 2003. Working memory: looking back and looking forward. Nat Rev Neurosci. 4:829–839. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. 2009. Is the rostro-caudal axis of the frontal lobe hierarchical. Nat Rev Neurosci. 10:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, Margulies DS.. 2013. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. J Neurosci. 33:16657–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Heard MT, DeGennaro EM, Desimone R. 2015. A source for feature-based attention in the prefrontal cortex. Neuron. 88:832–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bichot NP, Schall JD, Thompson KG.. 1996. Visual feature selectivity in frontal eye fields induced by experience in mature macaques. Nature. 381:697–699. [DOI] [PubMed] [Google Scholar]
- Blatt GJ, Andersen RA, Stoner GR.. 1990. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J Comp Neurol. 299:421–445. [DOI] [PubMed] [Google Scholar]
- Boschin EA, Piekema C, Buckley MJ.. 2015. Essential functions of primate frontopolar cortex in cognition. Proc Natl Acad Sci USA. 112:E1020–E1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. 1997. The psychophysics toolbox. Spat Vis. 10:433–436. [PubMed] [Google Scholar]
- Brody CD, Hernandez A, Zainos A, Romo R.. 2003. Timing and neural encoding of somatosensory parametric working memory in macaque prefrontal cortex. Cereb Cortex. 13:1196–1207. [DOI] [PubMed] [Google Scholar]
- Cavada C, Goldman-Rakic PS.. 1989. Posterior parietal cortex in rhesus monkey: II. Evidence for segregated corticocortical networks linking sensory and limbic areas with the frontal lobe. J Comp Neurol. 287:422–445. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS.. 1998. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 79:2919–2940. [DOI] [PubMed] [Google Scholar]
- Cole MW, Ito T, Braver TS.. 2016. The behavioral relevance of task information in human prefrontal Cortex. Cereb Cortex. 26:2497–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinidis C, Procyk E.. 2004. The primate working memory networks. Cogn Affect Behav Neurosci. 4:444–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden BM, Duncan J.. 2014. Task difficulty manipulation reveals multiple demand activity but no frontal lobe hierarchy. Cereb Cortex. 24:532–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue CH, Lee D.. 2015. Dynamic routing of task-relevant signals for decision making in dorsolateral prefrontal cortex. Nat Neurosci. 18:295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Tinsley CJ, Gaffan D, Duncan J.. 2002. Filtering of neural signals by focused attention in the monkey prefrontal cortex. Nat Neurosci. 5:671–676. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC.. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1:1–47. [DOI] [PubMed] [Google Scholar]
- Frith C, Dolan R.. 1996. The role of the prefrontal cortex in higher cognitive functions. Cogn Brain Res. 5:175–181. [DOI] [PubMed] [Google Scholar]
- Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsaki G.. 2000. Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol. 84:401–414. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Shima K, Tanji J.. 1998. Task-dependent selectivity of movement-related neuronal activity in the primate prefrontal cortex. J Neurophysiol. 80:3392–3397. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Kusunoki M, Petrov P, Sigala N, Buckley MJ, Gaffan D, Duncan J.. 2015. Spatial and temporal distribution of visual information coding in lateral prefrontal cortex. Eur J Neurosci. 41:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaping D, Vinck M, Hutchison RM, Everling S, Womelsdorf T.. 2011. Specific contributions of ventromedial, anterior cingulate, and lateral prefrontal cortex for attentional selection and stimulus valuation. PLoS Biol. 9:e1001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuki F, Constantinidis C.. 2012. Early involvement of prefrontal cortex in visual bottom-up attention. Nat Neurosci. 15:1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F.. 2003. The architecture of cognitive control in the human prefrontal cortex. Science. 302:1181–1185. [DOI] [PubMed] [Google Scholar]
- Lennert T, Martinez-Trujillo J.. 2011. Strength of response suppression to distracter stimuli determines attentional-filtering performance in primate prefrontal neurons. Neuron. 70:141–152. [DOI] [PubMed] [Google Scholar]
- Lennert T, Martinez-Trujillo JC.. 2013. Prefrontal neurons of opposite spatial preference display distinct target selection dynamics. J Neurosci. 33:9520–9529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HC, Gore JC, Goldman-Rakic PS.. 2002. Sustained mnemonic response in the human middle frontal gyrus during on-line storage of spatial memoranda. J Cogn Neurosci. 14:659–671. [DOI] [PubMed] [Google Scholar]
- Mante V, Sussillo D, Shenoy KV, Newsome WT.. 2013. Context-dependent computation by recurrent dynamics in prefrontal cortex. Nature. 503:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz DA, Curtis CE, Pesaran B.. 2015. Multiple component networks support working memory in prefrontal cortex. Proc Natl Acad Sci USA. 112:11084–11089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T, Constantinidis C.. 2005. A software solution for the control of visual behavioral experimentation. J Neurosci Methods. 142:27–34. [DOI] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Constantinidis C.. 2007. Persistent discharges in the prefrontal cortex of monkeys naive to working memory tasks. Cereb Cortex. 17 (Suppl 1):i70–i76. [DOI] [PubMed] [Google Scholar]
- Meyer T, Qi XL, Stanford TR, Constantinidis C.. 2011. Stimulus selectivity in dorsal and ventral prefrontal cortex after training in working memory tasks. J Neurosci. 31:6266–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers EM, Qi XL, Constantinidis C.. 2012. Incorporation of new information into prefrontal cortical activity after learning working memory tasks. Proc Natl Acad Sci USA. 109:4651–4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK, Cohen JD.. 2001. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 24:167–202. [DOI] [PubMed] [Google Scholar]
- Ninokura Y, Mushiake H, Tanji J.. 2004. Integration of temporal order and object information in the monkey lateral prefrontal cortex. J Neurophysiol. 91:555–560. [DOI] [PubMed] [Google Scholar]
- O'Scalaidhe S, Wilson FA, Goldman-Rakic PS.. 1997. Areal segregation of face-processing neurons in prefrontal cortex. Science. 278:1135–1138. [DOI] [PubMed] [Google Scholar]
- O'Scalaidhe SP, Wilson FA, Goldman-Rakic PS.. 1999. Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: evidence for intrinsic specialization of neuronal coding. Cereb Cortex. 9:459–475. [DOI] [PubMed] [Google Scholar]
- Olejnik S, Algina J.. 2003. Generalized eta and omega squared statistics: measures of effect size for some common research designs. Psychol Methods. 8:434–447. [DOI] [PubMed] [Google Scholar]
- Owen AM, Doyon J, Petrides M, Evans AC.. 1996. Planning and spatial working memory: a positron emission tomography study in humans. Eur J Neurosci. 8:353–364. [DOI] [PubMed] [Google Scholar]
- Owen AM, Stern CE, Look RB, Tracey I, Rosen BR, Petrides M.. 1998. Functional organization of spatial and nonspatial working memory processing within the human lateral frontal cortex. Proc Natl Acad Sci USA. 95:7721–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya D.. 1994. Comparative architectonic analysis of the human and the macaque frontal cortex In: Handbook of Neuropsychology. Amsterdam: Elsevier; p. 17–58. [Google Scholar]
- Petrides M, Pandya DN.. 1984. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 228:105–116. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN.. 2002. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 16:291–310. [DOI] [PubMed] [Google Scholar]
- Postle BR. 2006. Working memory as an emergent property of the mind and brain. Neuroscience. 139:23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Goldman-Rakic PS.. 1991. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 310:429–474. [DOI] [PubMed] [Google Scholar]
- Qi XL, Constantinidis C.. 2013. Neural changes after training to perform cognitive tasks. Behav Brain Res. 241:235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C.. 2011. Changes in prefrontal neuronal activity after learning to perform a spatial working memory task. Cereb Cortex. 21:2722–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XL, Meyer T, Stanford TR, Constantinidis C.. 2012. Neural correlates of a decision variable before learning to perform a match/nonmatch task. J Neurosci. 32:6161–6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainer G, Asaad WF, Miller EK.. 1998. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature. 393:577–579. [DOI] [PubMed] [Google Scholar]
- Rainer G, Rao SC, Miller EK.. 1999. Prospective coding for objects in primate prefrontal cortex. J Neurosci. 19:5493–5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM.. 2004. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 5:184–194. [DOI] [PubMed] [Google Scholar]
- Rao SC, Rainer G, Miller EK.. 1997. Integration of what and where in the primate prefrontal cortex. Science. 276:821–824. [DOI] [PubMed] [Google Scholar]
- Rigotti M, Barak O, Warden MR, Wang X-J, Daw ND, Miller EK, Fusi S.. 2013. The importance of mixed selectivity in complex cognitive tasks. Nature. 497:585–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MR, Constantinidis C.. 2016. Role of prefrontal persistent activity in working memory. Front Syst Neurosci. 9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Goldman-Rakic PS.. 2002. An auditory domain in primate prefrontal cortex. Nat Neurosci. 5:15–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM, Tian B, Fritz J, Mishkin M, Goldman-Rakic PS, Rauschecker JP.. 1999. Dual streams of auditory afferents target multiple domains in the primate prefrontal cortex. Nature Neurosci. 2:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo R, Brody CD, Hernandez A, Lemus L.. 1999. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 399:470–473. [DOI] [PubMed] [Google Scholar]
- Rowland BA, Quessy S, Stanford TR, Stein BE.. 2007. Multisensory integration shortens physiological response latencies. J Neurosci. 27:5879–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala JB, Courtney SM.. 2007. Binding of what and where during working memory maintenance. Cortex. 43:5–21. [DOI] [PubMed] [Google Scholar]
- Scalaidhe SP, Wilson FA, Goldman-Rakic PS.. 1999. Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: evidence for intrinsic specialization of neuronal coding. Cereb Cortex. 9:459–475. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS.. 1988. Common cortical and subcortical targets of the dorsolateral prefrontal and posterior parietal cortices in the rhesus monkey: evidence for a distributed neural network subserving spatially guided behavior. J Neurosci. 8:4049–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Owen AM, Tracey I, Look RB, Rosen BR, Petrides M.. 2000. Activity in ventrolateral and mid-dorsolateral prefrontal cortex during nonspatial visual working memory processing: evidence from functional magnetic resonance imaging. Neuroimage. 11:392–399. [DOI] [PubMed] [Google Scholar]
- Strange B, Henson R, Friston K, Dolan R.. 2001. Anterior prefrontal cortex mediates rule learning in humans. Cereb Cortex. 11:1040–1046. [DOI] [PubMed] [Google Scholar]
- Sugihara T, Diltz MD, Averbeck BB, Romanski LM.. 2006. Integration of auditory and visual communication information in the primate ventrolateral prefrontal cortex. J Neurosci. 26:11138–11147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Azuma M.. 1983. Topographic studies on visual neurons in the dorsolateral prefrontal cortex of the monkey. Exp Brain Res. 53:47–58. [DOI] [PubMed] [Google Scholar]
- Tolhurst DJ, Movshon JA, Dean AF.. 1983. The statistical reliability of signals in single neurons in cat and monkey visual cortex. Vision Res. 23:775–785. [DOI] [PubMed] [Google Scholar]
- Tsujimoto S, Genovesio A, Wise SP.. 2012. Neuronal activity during a cued strategy task: comparison of dorsolateral, orbital, and polar prefrontal cortex. J Neurosci. 32:11017–11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volle E, Kinkingnehun S, Pochon JB, Mondon K, Thiebaut de Schotten M, Seassau M, Duffau H, Samson Y, Dubois B, Levy R.. 2008. The functional architecture of the left posterior and lateral prefrontal cortex in humans. Cereb Cortex. 18:2460–2469. [DOI] [PubMed] [Google Scholar]
- Wallis JD, Anderson KC, Miller EK.. 2001. Single neurons in prefrontal cortex encode abstract rules. Nature. 411:953–956. [DOI] [PubMed] [Google Scholar]
- Wilson FA, Scalaidhe SP, Goldman-Rakic PS.. 1993. Dissociation of object and spatial processing domains in primate prefrontal cortex. Science. 260:1955–1958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.