ABSTRACT
The aim of the present study was to evaluate different biological activities of Trichoderma viride fungus (Family Hypocreaceae). Trichoderma viride isolated for the first time from the cucumber soil (rhizosphere). It was tested as antimicrobial, antioxidant and anticancer agent. Trichoderma viride from the cucumber soil (rhizosphere) caused inhibition of the mycelial growth of Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii. Also, the alcoholic extract of the fungal mycelia proved a potent antibacterial activity against Bacillus subtilis, Escherichia coli and Pseudomonas fluorescens. In addition, it exhibited a significant antifungal activity against Candida albicans, Fusarium solani, Fusarium oxysporium, Rhizoctonia solani and Pythium ultimum at 100 µg/disc. Study of the antimicrobial and antioxidant activities of the volatile constituents had been done. The in vitro antioxidant, anticancer and antiviral activities of the isolated proteins, and carbohydrates were determined. Furthermore, the volatile constituents were isolated from fresh mycelia of Trichoderma viride and subjected to GC/MS analysis. Total protein (10%), carbohydrate (19.57%), steroidal (13.95%) and triterpenoidal content (38.34%) were determined in the alcoholic extract of Trichoderma viride mycelia. In conclusion, this fungus showed antioxidant, anticancer, antiviral and antibacterial effects. Further studies must be done to identify the molecules responsible for its effect and to consider its application in the pharmacological and medicinal purposes.
KEYWORDS: Trichoderma viride, antioxidant, antimicrobial, anticancer, antiviral
1. Introduction
Fungus is the second largest kingdom after insects (Baron 1996). Fungi occur in Antarctic ice, tropical and temperate regions, surface of mountain rocks and seawater (Feofilova 2001). They represent an enormous source for natural products with diverse chemical structures and activities (Hawksworth 1991). Fungi produce a vast range of secondary metabolites and they are known for their capacity to secrete high levels of enzymes, antibiotics, vitamins, polysaccharides and organic acids (Meyer 2008).
Trichoderma viride is well known for its antagonistic ability towards plant pathogenic fungi and they have received considerable attention as biocontrol agent of soil-borne plant pathogens (Mannina and Segrc 1997). The production of secondary metabolites by Trichoderma strains also shows great variety and application potential in the medicinal field. Trichoderma viride seems to be an inexhaustible source of antibiotics by transformation of the acetaldehydes gliotoxin and viridin (Dennis and Webster 1971), to alpha-pyrones (Keszler et al. 2000).
The aim of this work was to evaluate Trichoderma viride fungus as antimicrobial and in vitro antioxidant agent. The study also had been extended to the isolation and identification of volatile constituents, proteins and carbohydrates. In addition, the determination of their biological activities such as anticancer and antiviral activities had been evaluated.
2. Materials and methods
2.1. Fungal isolation
Trichoderma viride (Family Hypocreaceae) was isolated from the cucumber soil (rhizosphere), Omar Makram Farm (Beheara Governorate, Egypt) during autumn and winter growing season of 2012/2013. The isolate was incubated on potato dextrose agar (PDA; Becton and Dickinson Co., MA, USA) for 2 weeks at 25 ± 3°C. Isolated fungus was identified at the Plant Pathology Department, National Research Centre, Egypt, and confirmed by Fungal Taxonomy Department, Plant Pathology Research Institute, Agricultural Research Centre, Giza, Egypt, according to the morphological and culture characters using the methods described by Barnett and Hunter (1972) and Ramirez (1982).
2.2. Production and extraction of Trichoderma viride
Erlenmayer flasks (2 L) containing 400 ml potato dextrose broth were autoclaved at 121°C for 15 min and then inoculated with mycelium plugs from a 5-day-old culture of Trichoderma viride on PDA. The flasks were incubated for 2 weeks at 25°C, the air-dried powder (in shade) of the mycelia of Trichoderma viride was extracted with 70% ethyl alcohol.
2.3. Investigation of the volatile constituents
Two hundred grams of fresh mycelia of Trichoderma viride have been subjected to hydro-distillation for 3 h in a modified Likens and Nickerson apparatus (Macleod and Cave 1975) which allowed the distillation and simultaneous extraction of the volatile constituents in an organic solvent (n-pentane). The n-pentane layer was collected, dried over anhydrous sodium sulphate. The pentane layer was evaporated under reduced pressure, and stored in a refrigerator at 4°C for bioactivity and chemical analysis. The volatile oil constituents were subjected to GC/MS analysis. Identification of the components had been performed by comparing the retention times and mass spectra with those of the available database libraries [Wiley (Wiley Int.) USA and NIST (Nat. Inst. St. Technol., USA)] and/or published data (Adams 1989). Quantitative determination was carried out based on peak area integration.
2.4. Determination of total protein content
Determination of total protein content was carried out in the crude 70% ethyl alcohol extract of Trichoderma viride by micro-Kjeldahl’s method using Markham distillation apparatus according to Pearson (1970) through determination of nitrogen content. The Kjeldahl’s method is based on the principle of digestion of the substance with concentrated sulphuric acid; a process in which the nitrogen is converted into ammonia.
Isolation of proteins from the alcoholic extract was performed according to El-Gengaihi et al. (1996), where 5 g extract was dissolved in 20 ml water, 45 ml ethanol and 5 ml concentrated sulphuric acid. It left for 20 min at 27°C and 50 ml water and 250 ml absolute ethanol were added then filtered. The PH was adjusted to 3 by 28% ammonium hydroxide. Acetone was added till precipitate was formed and precipitated by centrifugation at 3000 r.p.m then collected, weighted and dried. The HPLC analysis of amino acids was performed using a model Eppendorf-Germany LC 3000 amino acid analyser (Widner and Eggum 1966).
2.5. Quantitative estimation of total carbohydrate content
Total carbohydrate content of the crude (70% alcoholic) extract of Trichoderma viride mycelia was estimated as glucose by phenol sulphuric acid method according to Dubois et al. (1956).
2.6. Isolation of mucilage
Mucilage was isolated from the crude extract of Trichoderma viride according to Laidlow and Percival (1950), where 5 g of the crude extract was dissolved in least amount of acidified water at pH 4 using concentrated hydrochloric acid. Four volumes of absolute ethanol were added dropwise till complete precipitation occurred. The precipitate was separated by centrifugation, washed several times with ethanol and then dried by freeze dryer to obtain a crude polysaccharide. HPLC analysis of polysaccharide hydrolysate was performed.
2.7. Quantitative estimation of total steroidal and terpenoidal content
Spectrophotometric method was based on measuring the intensity of the colour developed when sterols and terpenes react with Lieberman–Burchard reagent and the percentage was calculated as β-sitosterol (sterols) and β-amyrin (terpenes) according to SwiftMary (1984).
2.8. Determination of the antifungal activity
Biculture test was done following the methods of Soytong and Quimio (1989). A virulent isolate of T. viride was used in biculture test with different antagonistic fungi. An agar disc taken from the edge of radial growth of F. solani, R. solani, or S. rolfsii separately in PDA plate was obtained using a sterile cork borer and placed in one side of a PDA plate about 2.0 cm from the centre. An agar disc of T. viride, the antagonistic fungus was placed on the other side of the plate. For control treatment, the agar plug of only pathogen was placed on PDA plates. The biculture plates were incubated at room temperature until colony of control grew to full plate. At this point, colony diameter was measured using ruler. Zone diameter was measured to the nearest whole millimetre at the point wherein there was a prominent reduction of 80% growth.
Percentage of the growth inhibition was calculated using the following formul:
% inhibition = A − B/A × 100
where: A = colony diameter of pathogen in control, B = colony diameter in biculture.
2.9. Strains of tested microorganisms
The antimicrobial activity of the crude mycelia extract of Trichoderma viride was performed using antibiotic assay method according to Thabrew et al. (1997). The antibacterial activity was tested against Bacillus subtilis (Gram-positive bacteria), Escherichia coli and Pseudomonas fluorescens (Gram-negative bacteria) on nutrient agar medium. The antifungal activity was tested against the yeast (Candida albicans) and the phytopathogenic fungi (Fusarium solani, Fusariumoxysporium, Rhizoctonia solani and Pythiumultimum) using Sabouraud dextrose agar medium.
2.10. Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of the crude mycelia extract of Trichoderma viride was determined by antibiotic assay method. Nutrient agar media was prepared and sterilised, then distributed in sterile petri dishes, each of 12 cm diameters. Each suspension of the test organisms was separately inoculated into the surface of a number of petri dishes. Each antibiotic assay disc (6 mm) was loaded with 50, 100, 200, 300 and 400 μg/disc of the crude mycelia extract of Trichoderma viridein dimethyl sulfoxide and firmly applied to the surface of the inoculated agar plates. The plates were observed for the growth of microorganisms. The lowest concentration of the extract inhibit the growth of the given bacteria/fungi was determined and considered as the MIC. The zone diameter is measured to the nearest whole millimetre at the point wherein there is a prominent reduction of 80% growth. Percentage of inhibition = A − B/A × 100, where A = colony diameter of control, B = colony diameter tested.
2.11. Antimicrobial activity of the alcoholic mycelia extract
The antimicrobial activity of the alcoholic mycelia extract was done using the disc diffusion method (Gnanamanickam and Mansfield 1981).The alcoholic extract at 100 μg/disc was screened in vitro for their antimicrobial activity against different pathogens (bacteria and fungi). Ampicillin (100 μg/disc) standard antibacterial and fluconazole (100 μg/disc) standard antifungal were used as reference drugs. This assay was replicated three times. All the experiments were done under aseptic conditions. Bacterial plates were incubated at 30°C for 24 h, while fungal plates were incubated at 28°C for 48 h. The diameter of the inhibition zone was recorded for each replicate and the average diameter was calculated.
2.12. Evaluation of antimicrobial activity of the volatile constituents
The volatile constituents isolated from the fresh fungus, Trichoderma viride, were subjected to evaluation of the antimicrobial activity using the disc diffusion method according to Thabrew et al. (1997). The antibacterial activity was tested against Bacillus subtilis (Gram-positive bacteria) and Escherichia coli (Gram-negative bacteria) on nutrient agar medium. The antifungal activity was tested against the yeast Candida albicans and the phytopathogenic fungi (Fusarium solani and Rhizoctonia solani) using Sabouraud dextrose agar medium.
2.13. Antioxidant activity of the isolated volatile constituents, proteins and carbohydrates
Volatile constituents, proteins and carbohydrates isolated from the fungus Trichoderma viride were in vitro evaluated as antioxidant agents. The antioxidant activities of serial concentrations of the proteins and carbohydrates (10, 50, 100 μg) were estimated by the method of Chen et al. (1999) using 2,2-diphenyl-1-picrylhydrazyl (DPPH-)-free radical. The decrease in optical density of DPPH- was calculated in relation to control as follows:
% inhibition percentages = (control − sample/control) × 100
2.14. Evaluation of cytotoxicity of the isolated proteins and carbohydrates
Cytotoxicity study (in vitro bioassay on human hepatocellular carcinoma cell line; HepG2) of the isolated proteins and carbohydrates was determined by the Bioassay-Cell Culture Laboratory, National Research Centre, Giza, Egypt. Cell viability was assessed by the mitochondrial dependent reduction of yellow MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide) to purple formazan according to Mosmann (1983). A positive control (adrinamycin) was used as a known cytotoxic natural agent (Thabrew et al. 1997). The experiment was performed in a sterile area using a laminar flow cabinet biosafety class II level (Baker, SG403INT and Sanford, ME, USA). The percentage of change in viability was calculated according to the following formula:
[(1 − (reading of extract))/(reading of negative control)] × 100.
A probit analysis was carried for IC50 and IC90 determination using SPSS 11 program.
2.15. Antiviral activity of the isolated proteins and carbohydrates
The assay was carried out according to the method of Hayden et al. (1980) in a six-well plate where MDCKM cells (105 cells/ml) were cultivated for 24 h at 37°C. A/CHICKEN/QALUBIA/1/2006 (H5N1) virus was diluted to give 104PFU/well and mixed with the concentration of the tested compounds; 1 µg/ml of L-1-(tosyl-amido-2-phenyl) ethyl chloromethyl ketone and incubated for 1 h at 37°C before being added to the cells. Growth medium was removed from the cell culture plates and virus-Cpd or virus-extract and Virus-Zanamivir mixtures were inoculated (100 µl/well). After 1 h contact time for virus adsorption, 3 ml of DMEM supplemented with 2% agarose was added onto the cell monolayer, plates were left to solidify and incubated at 37°C till formation of viral plaques (3–4 days). Formalin (10%) was added for 2 h and then plates were stained with 0.1% crystal violet in distilled water. Control wells were included where untreated virus was incubated with MDCK cells and finally plaques were counted and percentage reduction in plaque formation in comparison to control wells was recorded as follows:
% inhibition = viral count (untreated) – viral count (treated)/viral count (untreated) × 100.
2.16. Statistical analysis
Statistical analysis was carried out by independent student t-test using SPSS (Statistical Package for the Social Science; SPSS Inc., Chicago, IL, USA) Computer Program. Significance difference between groups was at p < 0.05.
3. Results
3.1. Fungal identification
The isolate was identified as Trichoderma viride based on the criteria of Kirk et al. (2011). The colony was woolly and become compact in time. Colonies of Trichoderma grow rapidly and mature in 5 days. At 25°C and on PDA, the colonies were woolly and become compact in time. From the front, the colour is white. As the conidia were formed, scattered bluish green or yellowish green patches become visible (Figure 1). These patches may sometimes form concentric rings (St-Germain and Summerbell 1996).
Figure 1.
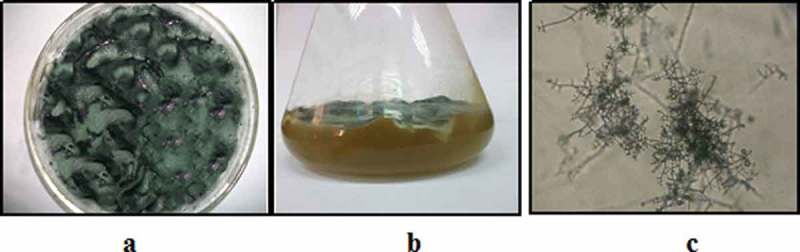
Petri dish (a), flask (b) and microscopic features (c) of Trichoderma viride.
The microscopic examination of T. viride revealed that the hyphae were septate, and conidiophores, phialides and the conidia were observed. Conidiophores were hyaline, branched and may occasionally display a pyramidal arrangement. Phialides were hyaline, flask-shaped and inflated at the base. They were attached to the conidiophores at right angles. The phialides may be solitary or arranged in clusters. Conidia (3 µm in diameter, average) were one-celled and round or ellipsoidal in shape. They were smooth-walled or rough-walled and grouped in sticky heads at the tips of the phialides. The colour of the conidia was mostly green (Sutton et al. 1998; Lieckfeldt et al. 1999).
The air-dried powder of the mycelia of Trichoderma viride was extracted with 70% ethyl alcohol, the yield was 75% (wt/wt % dried mycelia).
3.2. Volatile constituents
The yield of the volatile constituents was 0.19% of fresh mycelia. GC/MS analysis identified 30 compounds which represent 78.25% of the total volatile compounds. The volatile constituents were composed of hydrocarbons 3.61%, alcohols 18.76%, aldahyde 3.95%, ketones 2.47%, esters 3.56%, monoterpenes 11.83% and sesquiterpenes 34.07%. Cyclooctanol, caryophyllene oxide and α-bisabolol (8.48%, 5.12% and 5.04%, respectively) were represented as the major volatile constituents in Trichoderma viride mycelia as shown in Table 1.
Table 1.
GC/MS analysis of the volatile constituents from the mycelia of Trichoderma viride.
| Peak No. | Rt | Mol. formula | Mol. wt. | b.p. | Compound | % | Structure |
|---|---|---|---|---|---|---|---|
| 1 | 3.98 | C8H16O | 128 | 57 | Cyclooctanol | 8.48 |  |
| 2 | 4.59 | C6H10O3 | 130 | 41 | 6,8-Dioxabicyclo octan-4-ol | 2.45 | 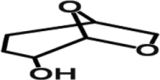 |
| 3 | 5.11 | C10H14 | 134 | 119 | Cymol | 3.66 | 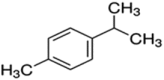 |
| 4 | 6.19 | C9H10O | 134 | 134 | Chavicol | 1.20 | 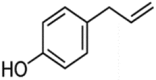 |
| 5 | 7.39 | C10H16 | 136 | 68 | Limonene | 2.70 | 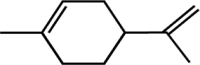 |
| 6 | 8.65 | C9H16O | 140 | 81 | 6-Methyl-bicyclooctan-7-ol | 1.66 | 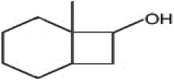 |
| 7 | 9.43 | C10H22 | 142 | 43 | n-Decane | 1.32 | |
| 8 | 10.21 | C10H18O | 154 | 43 | 1,8-Cineole | 1.48 | 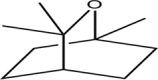 |
| 9 | 11.38 | C10H20O2 | 172 | 43 | 1,2-Dihydro-8-hydroxylinalool | 1.33 | 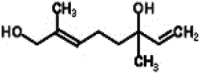 |
| 10 | 15.26 | C10H12O | 148 | 148 | Anethole | 1.43 | 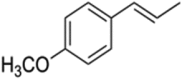 |
| 11 | 16.44 | C10H16O | 152 | 81 | 2,4-Decadienal | 2.50 | 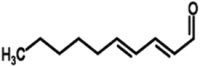 |
| 12 | 17.61 | C11H16O | 164 | 41 | Cis-jasmone | 2.47 | 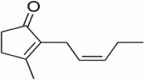 |
| 13 | 21.22 | C10H16O2 | 168 | 43 | Ascaridole | 1.23 | 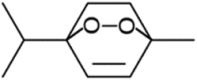 |
| 14 | 21.78 | C12H20O | 180 | 81 | 2,4-Dodecadienal | 1.45 |  |
| 15 | 22.86 | C12H24O | 184 | 71 | 1-Methyl cycloundecanol | 1.29 | 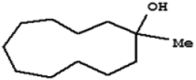 |
| 16 | 24.66 | C12H24O2 | 200 | 55 | 1,2-Cyclododecanediol | 1.46 | 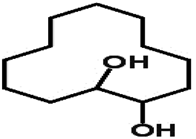 |
| 17 | 25.91 | C15H24 | 204 | 121 | α-Humulene | 2.45 | 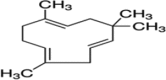 |
| 18 | 26.86 | C15H24 | 204 | 41 | Elemene | 3.30 | 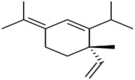 |
| 19 | 27.11 | C15H24 | 204 | 161 | α-Copaene | 2.23 |  |
| 20 | 34.12 | C15H24O | 220 | 41 | Caryophyllene oxide | 5.12 |  |
| 21 | 34.33 | C15H24O | 220 | 43 | Ledene oxide | 2.22 |  |
| 22 | 34.58 | C15H26O | 222 | 82 | α-Bisabolol | 5.04 |  |
| 23 | 35.04 | C15H26O | 222 | 161 | Guaiol | 1.82 | 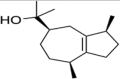 |
| 24 | 36.82 | C15H26O | 222 | 81 | 6-Epi-shyobunol | 2.22 | 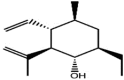 |
| 25 | 37.49 | C15H26O | 222 | 59 | Elemol | 2.11 | 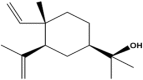 |
| 26 | 38.69 | C15H26O | 222 | 119 | α-Acorenol | 3.81 | 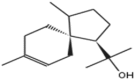 |
| 27 | 40.60 | C15H26O2 | 238 | 43 | Cedrane-8,13-diol | 3.75 | 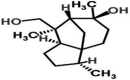 |
| 28 | 41.76 | C16H30O2 | 254 | 55 | 1,215,16-Diepoxyhexadecane | 2.29 | 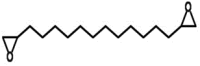 |
| 29 | 46.62 | C21H34O2 | 318 | 41 | Methyl arachidonate | 3.56 | 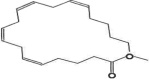 |
| 30 | 50.29 | C37H76O | 536 | 43 | 1-Heptatriacotanol | 2.22 | |
| Total identified compounds | 78.25% | ||||||
3.3. Total protein content
The crude extract of the fungus Trichoderma viride gave positive tests for proteins. The estimation of protein in the crude extract revealed that it contains 10% protein (wt/wt) of the dried crude extract.
Isolation of the proteins gave a percentage of 8.5% (wt/wt) of the dried crude extract. HPLC analysis led to identification of seven essential amino acids represented 38.39% (wt/wt) of the total amino acids and nine non-essential amino acids represented 61.61% (wt/wt) of the total amino acids. Lysine and methionine were detected as major essential amino acids and represented 7.23% and 11.88% (wt/wt), respectively,of the total amino acids content. However, glutamic acid was found as a major non-essential amino acid and represented 13.92% (wt/wt) of the total amino acid content as illustrated in Table 2.
Table 2.
Total amino acids of the crude extract of Trichoderma viride mycelia.
| Amino acids | RT (min.) | Relative percentage of total amino acids |
|---|---|---|
| Threonine | 13.08 | 3.34 |
| Valine | 30.73 | 3.88 |
| Methionine | 35.52 | 11.88 |
| Isoleucine | 38.15 | 2.88 |
| Leucine | 39.90 | 3.26 |
| Phenylalanine | 44.78 | 5.92 |
| Lysine | 53.20 | 7.23 |
| Total essential amino acids | 38.39 | |
| Aspartic | 10.13 | 7.83 |
| Serine | 13.93 | 5.26 |
| Glutamic acid | 15.52 | 13.92 |
| Glycine | 22.52 | 2.33 |
| Proline | 22.43 | 4.29 |
| Alanine | 24.13 | 7.57 |
| Tyrosine | 42.90 | 6.96 |
| Histidine | 50.20 | 7.48 |
| Arginine | 61.48 | 5.97 |
| Total nonessential amino acids | 61.61 | |
| Total identified amino acids | 100 | |
3.4. Total carbohydrates
The crude extract of T. viride mycelia gave positive test for carbohydrates. The estimation of the total carbohydrate content revealed that the alcoholic extract of T. viride mycelia contained 19.57% (wt/wt) of the dried extract.
3.5. Mucilage contents
Isolation of the mucilage gave a percentage of 17.5 (wt/wt) of the dried crude extract. HPLC analysis of the mucilage hydrolysate revealed the identification of five free sugars which represented 85.791% (wt/wt) of the total mucilage hydrolyzate. Glucose was presented as a major sugar (29.32% (wt/wt) of the total mucilage hydrolysate), followed by galacturonic acid, galactose, fructose and arabinose (Table 3).
Table 3.
HPLC analysis of mucilage hydrolysateof the 70% ethyl alcohol extract of Trichoderma viride mycelia.
| Authentic sugars | R.T. (min.) | Relative percentage (wt/wt%) of the total mucilage hydrolysate |
|---|---|---|
| Galacturonic acid | 6.601 | 16.901 |
| Glucose | 7.712 | 29.320 |
| Galactose | 8.033 | 8.447 |
| Fructose | 9.533 | 21.583 |
| Arabinose | 9.566 | 9.540 |
| Total identified sugars | 85.791 |
3.6. Total steroidal and terpenoidal contents
The absorbance of tested extract was 2.549 corresponding to 0.0005 g in 2 ml of the colour reagent. So, the total sterols and terpenes in the crude extract of T. viride mycelia were 13.95% and 38.34% (wt/wt), respectively, of the dried extract calculated as β-sitosterol and β-amyrin.
3.7. Antifungal activity
In the control plate, the pathogenic fungi grow faster and significantly formed larger colony diameter with a mean of 8.97 cm, while those in the biculture plate produced smaller colony with a mean diameter of 6.30 cm for Fusarium solani, 7.60 cm for Rhizoctonia solani and 7.20 cm for Sclerotium rolfsii.
Trichoderma viride, the fungal antagonist, caused 29.76, 15.27 and 19.73% inhibition of mycelial growth of Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii, respectively, as illustrated in Table 4 and Figure 2.
Table 4.
The colony diameters and the percentages of inhibition of Trichoderma viride against different pathogenic fungi.
| Treatment | Colony diameter (mm) mean ±S.D |
Inhibition % |
|---|---|---|
| Pathogens alone | 8.97 ± 0.21 | – |
| Fusarium solani in Biculture | 6.30٭± 0.75 | 29.76 |
| Rhizoctonia solani in Biculture | 7.60٭±0.55 | 15.27 |
| Sclerotium rolfsii in Biculture | 7.20٭± 0.74 | 19.73 |
Data are mean± SD of triplicate reading of the colony diameter.
٭Significantly different from the control at p ≤ 0.05 using one-way analysis of variance test.
Figure 2.
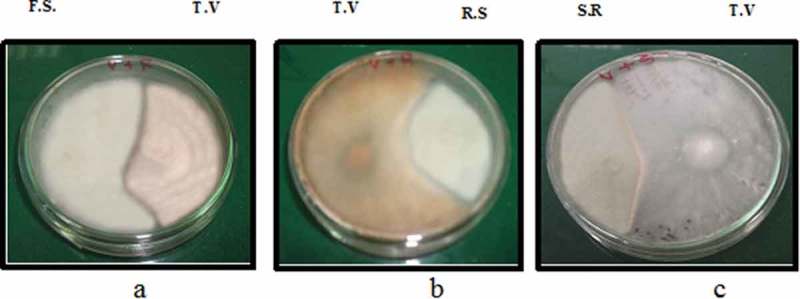
Biculture antagonistic test between Trichoderma viride (T.V.) and Fusarium solani (F.S.) (a), Rhizoctonia solani (R.S.) (b) and Sclerotium rolfsii (S.R.) (c).
3.8. Antimicrobial activity of the crude mycelia extract
The crude extract of Trichoderma viride was tested in 50, 100, 200, 300 and 400 µg/disc. Concentration of 50 µg/disc showed no significant inhibition zone on the tested pathogenic organisms (bacteria/fungi), while 100 µg/disc inhibited the growth of the given bacteria/fungi. The zone of inhibition was determined and this concentration was considered as the MIC. Concentration of 100 µg/disc of the alcoholic extract giving remarkable inhibition zone on Bacillus subtilis, Escherichia coli and Pseudomonas fluorescens. Maximum antifungal effect against Candida albicans, Rhizoctonia solani, Pythium ultimum, Fusarium solani and Fusarium oxysporium were recorded, the zones of the inhibition were increased as the concentration of the extract increase (Table 5).
Table 5.
Antimicrobial effects of the alcoholic mycelia extract of Trichoderma viride.
| Diameter of inhibition zone (mm) mean ± SD |
|||||
|---|---|---|---|---|---|
| Treatment | Concentration (µg/disc) | 100 µg/disc | 200 µg/disc | 300 µg/disc | 400 µg/disc |
| Bacteria | B. subtilis | 12.06 ± 0.115a | 13.10 ± 0.173a | 13.66 ± 0.577a | 14.33 ± 1.050a |
| E. coli | 12.06 ± 0.115a | 12.16 ± 0.152a | 13.13 ± 0.230a | 15.13 ± 0.230a | |
| P. fluorescens | 12.16 ± 0.288a | 12.33 ± 0.577a | 13.66 ± 0.577a | 15.00 ± 1.000a | |
| Fungi | C. albicans | 12.33 ± 0.577 | 12.93 ± 0.404 | 13.43 ± 0.513a | 14.66 ± 0.577a |
| F. solani | 11.93 ± 0.378a | 12.83 ± 0.568 | 14.13 ± 0.907a | 15.30 ± 0.264a | |
| F. oxysporium | 12.33 ± 0.577 | 14.26 ± 0.461a | 14.13 ± 0.230a | 15.33 ± 1.050a | |
| R. solani | 11.66 ± 0.577 | 12.26 ± 0.461a | 15.33 ± 0.577a | 16.16 ± 0.208a | |
| P. ultimum | 10.00 ± 1.00a | 11.66 ± 0.577a | 12.26 ± 0.251a | 14.33 ± 0.321a | |
Data are mean± SD of triplicate reading of the inhibition zone diameter.
aSignificantly different from the control at p ≤ 0.05 using one-way analysis of variance test.
3.9. Antimicrobial activity of the volatile constituents
Volatile constituents isolated from the fresh fungus Trichoderma viride was evaluated as antimicrobial agent (antibacterial and antifungal). The diameter of inhibition zone (mm) for each pathogen was determined exactly and the mean of the triplicates was calculated. The volatile constituents under study showed remarkable antibacterial and antifungal effects at concentration of 100 µl/disc as shown in Table 6.
Table 6.
Antimicrobial effects of the volatile constituents isolated from the fresh fungus Trichoderma viride.
| Treatment | Diameter of inhibition zone (mm) mean ±SD |
|
|---|---|---|
| Bacteria | B. subtilis | 13.16 ± 0.208 |
| E. coli | 11.33 ± 0.577 | |
| Fungi | C. albicans | 9.16 ± 0.763 |
| F. solani | 13.53 ± 5.774 | |
| R. solani | 12.66 ± 0.577 | |
Data are mean± SD of triplicate reading of the inhibition zone diameter.
3.10. Antioxidant activity of the isolated volatile constituents, proteins and carbohydrates
The antioxidant activity of the isolated volatiles, proteins and carbohydrates were determined as illustrated in Table 7. The isolated volatile constituents revealed high antioxidant effects by 29.62%, 63.12% and 70.37% at concentrations of 10, 50 and 100 µg, respectively. Moreover, proteins and carbohydrates recorded remarkable antioxidant effects by 3.70%, 14.81% and 33.00% for proteins and 3.00%, 18.00% and 23.00% for carbohydrates at concentrations of 10, 50 and 100 µg, respectively.
Table 7.
Percentages of the antioxidant activity of the isolated volatile constituents, proteins and carbohydrates.
| Inhibition percentages (%) at different concentrations | |||
|---|---|---|---|
| Concentration Treatment |
10 µg | 50 µg | 100 µg |
| Volatile constituents | 29.62 | 63.12 | 70.37 |
| Proteins | 3.70 | 14.81 | 33.00 |
| Carbohydrates | 3.00 | 18.00 | 23.00 |
Data are inhibition percentages (%) of DPPH--free radicals at different concentrations.
3.11. Cytotoxicity of the isolated proteins and carbohydrates
The isolated proteins and carbohydrates exhibited 42.6% and 16.7% killing of the HepG2 cell line, as compared to the standard reference natural drug adrinamycin.
3.12. Antiviral activity of the isolated proteins and carbohydrates
The antiviral activity of the isolated proteins and carbohydrates recorded 20% and 18%, respectively, at a concentration of 25 µg/µl (Table 8).
Table 8.
Antiviral activity of the isolated proteins and carbohydrates.
| Treatment | Initial viral count (X ± SE) | Viral count after treatment (X ± SE) | Inhibition % |
|---|---|---|---|
| Isolated proteins | 4.000 ± 0.0066 | 3.240 ± 0.0057 | 20 |
| Isolated carbohydrates | 3.036 ± 0.0033 | 2.480 ± 0.0057 | 18 |
Each value (X ± SE) represents the mean of the viral count ± SE.
4. Discussion
Trichoderma viride is the most promising and effective biocontrol agent to control the plant diseases as well as increase the plant growth. It has the antagonising ability to control a wide range of microorganisms for more than seven decades ago (Weinding 1934), but its use under field conditions came much later (Chet et al. 1997). In this study, Trichoderma viride fungus has exerted an inhibitory activity against the mycelial growth of Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii. So, it can be used clearly as a potential antagonist of various plant pathogens. The mechanism of mycoparasitism involves nutrient competition, hyperparasitism and antibiosis (Ajith and Lakshmidevi 2010). Recently, the role of extracellular enzymes has been well documented by several researchers such as proteolytic enzymes (Pozo et al. 2004; Kredics et al. 2005), β-1,3-glucanolytic system (Kubicek et al. 2001), β-1,6-glucanases (De La Cruz et al. 1995; De La Cruz and Llobell 1999), α-1,3-glucanases (Ait-Lahsen et al. 2001), chitinase (Zeilinger et al. 1999; Hoell et al. 2005) and proteases (Geremia et al. 1993), which are considered as key factors in the pathogen cell wall lysis during mycoparasitism (Brito-Vega et al. 2013).
Recently, Trichoderma viride fungus has an important role in the human health, where this fungus is capable of remediating the heavy metals, toxins such as cyanides and xenobiotics, and converts the anthracene to non-toxic naphthalene (Harman 2006). So, it can be used for bioremediation of pollutants. Moreover, the fungus Trichoderma viride produces several important secondary metabolites such as the viridian-analouge, trichosetin and peptaibiotics which were used as potential anticancer and antimicrobial drugs (Harman 2006).
The formation of the secondary metabolites is frequently limited to the external conditions, where the fungi employ unique biochemical pathway for synthesis of the bioactive metabolites from few precursors (Demain and Fang 2000). Fungal volatile constituents are synthesised from various precursors such as acetates, amino acids, fatty acids and keto acids. Monoterpenes and sesquiterpenes are biosynthesized from acetyl COA and converted to the final structure of the compound by the action of terpene synthase (Sivasithamparam and Ghisalberti 1998). Various other groups of the volatile constituents such as the alcohols, aldehydes and ketones are presented and derived from the fatty acids. The volatile constituents can influence the physiological processes of the fungi including nitrification, nitrogen mineralization and participation in the developmental processes of the fungi. It acts as a defence system and plays an important role in the communication of the fungus Trichoderma viride with the other microorganisms, insects and plants (Harman 2006). These observations confirmed our results through isolation and identification of the volatile constituents from the fresh fungus, Trichoderma viride as well as the determination of the total proteins, carbohydrates, steroidal and terpenoidal contents.
The antifungal activities of the fungus Trichoderma viride recorded antifungal effect against Fusarium solani, Rhizoctonia solani and Sclerotium rolfsii. Also, the antimicrobial activities of the crude mycelia extract recorded remarkable antibacterial effect on Bacillus subtilis, Escherichia coli and Pseudomonas fluorescens. It also recorded maximum antifungal effect against Candida albicans, Rhizoctonia solani, Pythium ultimum, Fusarium solani and Fusarium oxysporium.
The volatile constituents isolated from the fresh Trichoderma viride mycelia revealed promising antifungal, antibacterial and high antioxidant effects. Also, the isolated proteins and carbohydrates recorded remarkable cytotoxic effect on HepG2 cell line, moderate antiviral activity on H5N1 virus and antioxidant effect by inhibition of DPPH--free radical.
These observations are in line with the results of Gajera et al. (2016) who recorded antioxidant effect of Trichoderma viride against different pathogens. The anticancer activity of fungi has been verified to be connected with a variety of phytochemicals, such as polyphenols, flavonoids and catechins. Several recent studies have investigated the cytotoxicity of natural extracts against human cancer cell lines, where proliferation and viability of cancer cells were decreased after treatment with natural extracts. Cytotoxic activities of Trichoderma spp. against the liver cancer cell lines has not been studied enough. This is the first study that has used metabolites of the whole culture filtrate of Trichoderma viride as anticancer agents. Certain species of Trichoderma have been known as a productive producer of important secondary metabolites such as antibiotics, plant growth regulators and enzymes, which are mainly used to protect plants from pathogens (Respinis et al. 2010). The mechanism of action of Trichoderma as a biocontrol agent is a complex process mediated by the secretion of extracellular enzymes. Some enzymes produced by the Trichoderma species are known to have antitumor and antioxidant activity (Abd El-Rahman et al. 2014).
In conclusion, the cultural filtrates of Trichoderma viride are considered as the most promising and effective agents controlling wide range of microorganisms. Moreover, Trichoderma viride fungus proved to have various antimicrobial, antioxidant, anticancer and antiviral activities. Further studies are required to understand the mechanism(s) of action of these cultural filtrates on liver cancer cell line and against different pathogens. In addition, the study of Trichoderma spp. as a source of biologically active metabolites is especially significant and ensures interest on this subject for years to come.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abd El-Rahman AA, ElShafei SMA, Ivanova EV, Fattakhova AN, Pankova AV, El-Shafei MA, El- El-Morsi MA, Alimova FK.. 2014. Cytotoxicity of Trichoderma spp. cultural filtrate against human cervical and breast cancer cell lines. Asian Pac J Cancer Prev. 15:7229–7234. [DOI] [PubMed] [Google Scholar]
- Adams P.1989. Identification of essential oils by ion trap mass spectroscopy. New York: Academic Press, INC. [Google Scholar]
- Ait-Lahsen H, Soler A, Rey M, De La Cruz J, Monte E, Liobell A. 2001. An antifungal exo-ᾳ-1,3-glucanase (AGN13.1) from the biocontrol fungus Trichoderma harzianum. Appl Environ Microbiol. 67:5833–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajith PS, Lakshmidevi N. 2010. Effect of volatile and non-volatile compounds from Trichoderma spp. against Colletotrichum capsici incitant of anthracnose on bell peppers. Nat Sci. 8:265–269. [Google Scholar]
- Barnett HL, Hunter BB. 1972. Illustrated genera of imperfect fungi. Minneapolis: Burgess Publ. com; p. 241. [Google Scholar]
- Baron S. 1996. Introduction to Mycology. 4th ed. Galveston: University of Texas Medical Branch. [PubMed] [Google Scholar]
- Brito-Vega H, Espinosa-Victoria D, Salaya-Domínguez JM, Gómez-Méndez1 E. 2013. The soil biota: importance in agroforestry and agricultural systems. Tropical and Subtropical Agroecosystems. 16:445–453. [Google Scholar]
- Chen Y, Wang M, Rosen RT, Ho CT. 1999. 2,2-Diphenyl-2-picrylhydrazyl radical-scavenging active components from Polygonum multiflorum Thunb. J Agric Food Chem. 47:2226–2228. [DOI] [PubMed] [Google Scholar]
- Chet I, Inbar J, Hadar I. 1997. Fungal antagonists and mycoparasites In: Wicklow DT, Soderstorm B, eds.. Berlin: Springer, The Mycota IV: environmental and microbial relationships. p. 165–184. [Google Scholar]
- De La Cruz J, Llobell A. 1999. Purification and properties of a basic endo-b-1,6-glucanase (BGN16.1) from the antagonistic fungus Trichoderma harzianum. Eur J Biochem. 265:145–151. [DOI] [PubMed] [Google Scholar]
- De La Cruz J, Pintor-Toro JA, Benitez T, Llobell A. 1995. Purification and characterization of an endo-b-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol. 177:1864–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demain AL, Fang A. 2000. The natural function of secondary metabolites. Advanced in Biochemical engineering/Biochemistry. Heidelberg (Germany): Springer; p. 1–39. [DOI] [PubMed] [Google Scholar]
- Dennis C, Webster J. 1971. Antagonistic properties of species groups of Trichoderma: III. Hyphal interactions. Trans Br Mycol Soc. 57:363–369. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances. AnalChem. 28:350–356. [DOI] [PubMed] [Google Scholar]
- El-Gengaihi S, Karawya M, Selim M, Motawe H, Ibrahim N. 1996. Chemical and biological investigation of polypeptides of Monordica and Luffa Dpp. Fam. Cucurbitaceae. J Bull NRC. 21:269–276. [Google Scholar]
- Feofilova EP. 2001. The kingdom fungi: heterogeneity of physiological and biochemical properties and relationships with plants, animals, and prokaryotes. Appl Biochemistry Microbiol. 37:124–137. [PubMed] [Google Scholar]
- Gajera HP, Katakpara ZA, Patel SV, Golakiya BA. 2016. Antioxidant defense response induced by Trichoderma viride against Aspergillus niger Van Tieghem causing collar rot in groundnut (Arachis hypogaea L.). Microb Pathog. 91:26e–34. [DOI] [PubMed] [Google Scholar]
- Geremia RA, Goldman GH, Jacobs D, Ardiles W, Vila SB, Van Montagu M, Herreraestrella A. 1993. Molecular characterization of the proteinase-encoding gene, prb1, related to mycoparasitism by Trichoderma harzianum. Mol Microbiol. 8:603–613. [DOI] [PubMed] [Google Scholar]
- Gnanamanickam SS, Mansfield JW. 1981. Selective toxicity of wyerone and other phytoalexins to gram-positive bacteria. Phytochemistry. 20:997–1000. [Google Scholar]
- Harman GE. 2006. Overview of mechanisms and uses of Trichoderma spp. Phytopathology. 96:190–194. [DOI] [PubMed] [Google Scholar]
- Hawksworth DL. 1991. The fungal dimension of biodiversity: magnitude, significance, and conservation. Mycol Res. 95:641–655. [Google Scholar]
- Hayden FG, Cote KM, Douglas RG. 1980. Plaque inhibition assay for drug susceptibilitytesting of influenza viruses. Antimicrob Agents Chemother. 17:865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoell IA, Klemsdal SS, Vaaje-Kolstad G, Horn SJ, Eijsink VGH. 2005. Overexpression and characterization of a novel chitinase from Trichoderma atroviride strain P1. Biochim Biophys Acta. 1748:180–186. [DOI] [PubMed] [Google Scholar]
- Keszler A, Forgacs E, Kotal L, Vizcaino JA, Monte E, Garcia-Acha I. 2000. Separation and identification of volatile components in the fermentation broth of Trichoderma atroviride by solidphase extraction and gas chromatography-mass spectroscopy. J Chromatograph Sci. 38:421–424. [DOI] [PubMed] [Google Scholar]
- Kirk PM, Cannon PF, Minter DW, Stalpers JA. 2011. Dictionary of the fungi. 10th ed. UK: Wallingford; p. 131. [Google Scholar]
- Kredics L, Zsuzsanna A, Szekeres A, Hatvani L, Manczinger L, Cs V, Erzsebet N. 2005. Extracellular proteases of Trichoderma species-a review. Acta Microbiol Immunol Hung. 52:169–184. [DOI] [PubMed] [Google Scholar]
- Kubicek CP, Mach RL, Peterbauer CK, Lorito M. 2001. Trichoderma: from genes to biocontrol. J Plant Pathol. 83:11–23. [Google Scholar]
- Laidlow M, Percival V. 1950. The Chemistry of Gum and Mucilage. New York: Rembold Publishing Co. [Google Scholar]
- Lieckfeldt E, Samuels GJ, Nirenberg HI, Petrini O. 1999. A morphological and molecular perspective of Trichoderma viride: is it one or two species. Appl Environ Microbiol. 65:2418–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod AJ, Cave SJJ. 1975. Volatile flavour components of egg. Sci Food Agric. 26:351–358. [Google Scholar]
- Mannina L, Segrc AL. 1997. A new fungal growth inhibitor from Trichoderma viride. Tetrahedron. 53:3135–3144. [Google Scholar]
- Meyer V. 2008. Genetic engineering of filamentous fungi-Progress, obstacles and future trends. Biotechnol Adv. 26:177–185. [DOI] [PubMed] [Google Scholar]
- Mosmann T. 1983. Rapid colorimetric assays for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63. [DOI] [PubMed] [Google Scholar]
- Pearson D. 1970. The chemical analysis of foods. 6th ed. London: Churchill LTD; p. 9. [Google Scholar]
- Pozo MJ, Baek JM, Garcia JM, Kenerley CM. 2004. Functional analysis of tvsp1, a serine protease-encoding gene in the biocontrol agent Trichoderma virens. Fungal Genet Biol. 41:336–348. [DOI] [PubMed] [Google Scholar]
- Ramirez C. 1982. Manual and Atlas of the Penicilla. Amesterdam (New York, Oxford): Elserier Biomedical Press; p. 123. [Google Scholar]
- Respinis SD, Vogel G, Benagli C. 2010. MALDI-TOF MS of Trichoderma: model system for the identification of microfungi. Mycol Prog. 9:79–100. [Google Scholar]
- Sivasithamparam K, Ghisalberti EL. 1998. Secondary metabolism in Trichoderma and Gliocladium. Trichoderma and Gliocladium. Vol.1, Basic Biology, Taxonomy and Genetics. London: Taylor and Francis Ltd; p. 139–191. [Google Scholar]
- Soytong K, Quimio TH. 1989. Antagonism of Chaetomium globosum to the rice blast pathogen. Pyricularia Oryzae Kasetsart J Nat Sci. 23:198–203. [Google Scholar]
- St-Germain G, Summerbell R. 1996. Identifying filamentous fungi - a clinical laboratory handbook. 1st ed. Belmont (California): Star Publishing Company. [Google Scholar]
- Sutton DA, Fothergill AW, Rinaldi MG. 1998. Guide to clinically significant fungi. 1st ed. Baltimore: Williams & Wilkins. [Google Scholar]
- SwiftMary L. 1984. Analysis of molluscan sterols: colorimetric methods. Lipids. 19:625–630. [DOI] [PubMed] [Google Scholar]
- Thabrew MI, Hughes RD, McFarlane IG. 1997. Screening of hepatoprotective plant components using a HepG2 cell cytotoxicity assay. J Pharm Pharmacol. 49:1132–1135. [DOI] [PubMed] [Google Scholar]
- Weinding R. 1934. Studies on a lethal principle effective in the parasitic action of Trichoderma lignorum on Rhizoctonia solani and other soil fungi. Phytopath. 24:1153–1179. [Google Scholar]
- Widner K, Eggum OB. 1966. Protein analysis. A description of the method used at the of animal physiology in Copenhagen. Acta Agricultura Scandinavi. 16:15. [Google Scholar]
- Zeilinger S, Galhaup C, Payer K, Woo SL, Mach RL, Fekete C, Lorito M, Kubicek CP. 1999. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet Biol. 26:131–140. [DOI] [PubMed] [Google Scholar]


