Abstract
Background: Use of magnetic resonance (MR) imaging to improve prostate biopsy efficiency is rapidly gaining in popularity. The aim of this study was to assess the biopsy efficiency of MR/ultrasound (MR/US) fusion-guided (“targeted”) biopsies vs extended-sextant 12-core (“standard”) biopsies for overall and high-grade prostate cancer detection.
Methods: From August 2007 to February 2014, 1003 men were enrolled in a prospective trial comparing the diagnostic yield of targeted and standard prostate biopsies performed during the same session. A total of 17 619 biopsy cores were reviewed. Biopsy efficiency was determined by dividing the total number of cores by the number of positive cores obtained. All statistical tests were two-sided.
Results: A mean of 12.3 (95% confidence interval [CI] = 12.2 to 12.3) standard and 5.3 (95% CI = 5.1 to 5.5) targeted biopsy cores were obtained from each patient. Targeted biopsy detected 461 cases of prostate cancer, of which 173 (37.5%) were high grade (Gleason score ≥ 4 + 3), while standard biopsy detected 469 cases of prostate cancer, of which 122 (26.5%) were high grade. The percentage of biopsy cores positive for prostate cancer, irrespective of grade, was statistically significantly higher for targeted than for standard biopsies (27.9% vs 13.5%, respectively, P < .001), with 11.5 targeted cores vs 26.2 standard cores utilized per diagnosis of prostate cancer. For detection of high-grade cancer, 30.7 targeted vs 100.8 standard cores were utilized per diagnosis.
Conclusion: In men with MR-visible prostate lesions, targeted biopsy is more efficient than standard biopsy, diagnosing a similar number of cancer cases and more high-grade cases while sampling 56.1% fewer biopsy cores.
The roles of multiparametric magnetic resonance imaging (MP-MRI) have been recently evaluated for the diagnostic evaluation of men at risk for prostate cancer ( 1 , 2 ). As the role of imaging has expanded, MP-MRI/ultrasound fusion–guided biopsy (eg, “targeted biopsy”) has emerged as a method to take advantage of imaging information to guide the prostate biopsy. Accordingly, this approach is potentially a more efficient diagnostic tool than standard extended-sextant 12-core biopsy (eg, “standard biopsy”), with a 77% sensitivity and 68% specificity for targeted biopsy vs 53% and 66% for standard biopsy( 3 ). Compared with the standard biopsy, targeted biopsy has been shown to upgrade the Gleason score in as many as 32% of men ( 4 ). Targeted biopsy is capable of diagnosing cancer that was missed even after multiple attempts by the standard approach ( 5 ). Furthermore, targeted biopsy has been shown to improve the diagnostic rate of capturing high-grade prostate cancer while decreasing the diagnosis of clinically insignificant low-grade disease ( 3 , 6 , 7 ).
Targeted biopsy accomplishes these outcomes while requiring fewer biopsy cores ( 8 ). This has the potential to improve patient acceptance and decrease the morbidity associated with prostate biopsy such as sepsis, bleeding, and erectile dysfunction ( 9 ). Although numerous studies have demonstrated an improved accuracy of targeted biopsy over standard biopsy on a per-patient basis, studies focused on efficiency as reflected on number of cores taken through each approach are lacking. As the claim that targeted biopsies require fewer biopsy cores is frequently touted as an advantage for patients, a dedicated analysis of this topic is of great utility. Such information also would be of great utility to clinicians and policy makers trying to quantify positive and negative costs associated with targeted vs standard biopsy. The aim of this study was to compare the efficiency (on a per-core basis) of targeted and standard prostate biopsies for the detection of overall and high-grade prostate cancer.
Methods
Study Population
Patients were prospectively enrolled in an institutional review board–approved clinical trial to study the use of electromagnetic tracking devices to locate disease using multimodality navigated procedures. Enrollment occurred between August 2007 and February 2014. Written informed consent was obtained from all patients. Inclusion criteria for this analysis included a clinical suspicion for prostate cancer (elevated PSA or abnormal digital rectal exam) and a diagnostic 3 Tesla MP-MRI of the prostate with at least one targetable lesion present. Exclusion criteria included a history of prostate cancer treatment or a contraindication for the MP-MRI exam. A total of 1215 men were assessed for eligibility, of which 181 were excluded because of negative MP-MRI findings, and a further 31 were excluded because of prior treatment history. The trial was registered on clinicaltrials.gov with the identifier NCT00102544.
Imaging and Biopsy Protocols
MP-MRI was performed with a 3.0 Tesla MRI (Achieva, Philips Healthcare) using a 16-channel surface coil (SENSE cardiac surface coil, Philips Healthcare) and, in most cases, (all but 4 patients) an endorectal coil (BPX-30, Medrad). T2-weighted, diffusion-weighted imaging, dynamic contrast–enhanced, and MR spectroscopy pulse sequences were obtained in all patients. The images were reviewed by two radiologists with eight and 14 years of experience interpreting prostate MP-MRI studies, and a consensus read was established in each case. Lesions were identified and assigned suspicion scores of low, moderate, or high based on previously described criteria ( 10 ). It is important to note that recently an international standard, known as Prostate Imaging Radiology Assessment and Diagnostic System (PIRADS v2), for assessing prostate cancer suspicion on MP-MRI was adopted ( 11 ). Under this system, low suspicion in the current study would correspond to PIRADS scores of 1 to 3, moderate suspicion to PIRADS 4, and high suspicion to PIRADS 5. Lesions were then electronically annotated using DynaCAD (InVivo), and the prostate gland was electronically segmented in preparation for targeted biopsy.
In an outpatient setting, at a time separate from that of the acquisition of the MP-MRI, a targeted MR/US fusion–guided biopsy using the UroNav device (InVivo) was performed to sample lesions identified as suspicious for prostate cancer by this imaging technique. In the same setting, standard 12-core biopsies were performed with standard TRUS guidance sequentially after the targeted biopsy. Each lesion was sampled in the axial and sagittal planes with an end-fire transrectal ultrasound probe (IT-22, Philips). Two biopsies were performed in this manner per MRI lesion. Abnormalities detected on ultrasound were also biopsied as part of the standard biopsy portion of the procedure. One genitourinary pathologist reviewed all pathologic specimens and was blinded to the source at the time of the pathology review.
Data Analysis
The data were collected prospectively into a pretrial designed database. High-grade prostate cancer was defined as Gleason score 4 + 3 or higher. Biopsy efficiency was characterized in multiple ways to capture the various dimensions to this concept. The percent positive biopsy cores was calculated by dividing the total number of biopsies performed by the number of events of interest for each given subgroup analyzed. Number of biopsy cores necessary per diagnosis was calculated by the number of biopsy cores divided by the number of diagnosis in each subgroup analyzed. Number of men needed to biopsy (NNB) for each diagnosis was determined by dividing the number of men in each subgroup by the number of cancer diagnosis of interest within that subgroup. The number of additional men needed to biopsy was calculated by taking the inverse of the differences of the inverses of NNB by the target and standard approaches. For example, in this study the NNB for diagnosis of one high-grade tumor by standard is biopsy was 8.2 and targeted biopsy was 5.8. In the total cohort of 1003 men, this meant that 122 (1003/8.2) tumors were diagnosed by standard and 173 (1003/5.8) by targeted approaches. Overall target diagnosed 51 more tumors than standard in this group of 1003 men, and the NNB for each additional diagnosis of cancer was 1003 per 51 or 19.7. Through rearrangement of terms, this is calculated through the formula 1/[[1/5.8] – [1/8.2]] = 19.7. Statistical significance levels were all two-sided, with a threshold P value of less than .05 for statistical significance. Values for NNB were presented as descriptive results. The Wilcoxon paired value signed rank test was used to compare standard and targeted frequencies of core positivity for cancer. The Spearman rank correlation test was used to assess the correlation between MRI suspicion score and frequency of targeted core positivity for cancer. McNemar’s test was used to compare the biopsy methods with regard to the number of cancers detected and number of high-risk cancers detected.
Results
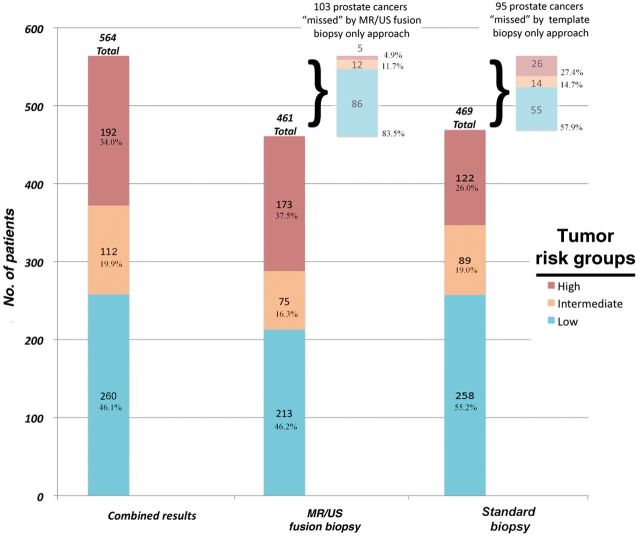
A total of 1003 men who underwent prostate biopsy were reviewed. Patient demographics are listed in Table 1 . A total of 17 619 biopsy cores were reviewed. The mean number of standard biopsy cores per patient was 12.3 (95% confidence interval [CI] = 12.2 to 12.3) cores while the mean number of targeted cores was 5.3 (95% CI = 5.1 to 5.5). This resulted in 12 303 standard biopsy cores and 5316 targeted biopsy cores analyzed. A total of 564 men (56.2%) were diagnosed with prostate cancer either on standard biopsy, targeted biopsy, or both. The number of men detected to have any prostate cancer was slightly higher with the standard biopsy only vs targeted biopsy only (103 vs 95); however, only 19 of the 103 men detected by standard biopsy had high-grade cancer, as compared with 70 of the 95 men diagnosed by targeted biopsy only Table 1 . Figure 1 demonstrates the distribution of cancer diagnosis between the two modalities. Targeted MR/US fusion biopsy diagnosed a similar number of total cancer patients (461 men) compared with the standard biopsy approach (469 men). However, more men with high-grade disease were identified by the targeted than by the standard biopsy (173 vs 122 men, 41.8% increase, P < .001).
Table 1.
Patient demographics
| Demographic | No. of men |
|---|---|
| Men, No. (%) | 1003 (100) |
| Number of biopsy cores analyzed | |
| Standard | 12 303 |
| Targeted | 5316 |
| Mean age (95% CI), y | 62.0 (61.5 to 62.5) |
| Mean PSA (95% CI), ng/mL | 10.0 (9.1 to 10.9) |
| Mean prostate volume (95% CI), cc | 59.5 (58.4 to 62.8) |
| Cancer Suspicion Score on MP-MRI, No. % | |
| Low | 176 (17.5) |
| Moderate | 718 (71.6) |
| High | 109 (10.9) |
| Patients with anterior lesions, No. (%) | 446 (44.5) |
| Total men with prostate cancer, No. (%) | 564 (56.2) |
| On both standard and targeted biopsy | 366 (36.5) |
| On standard biopsy only | 103 (10.3) |
| On targeted biopsy only | 95 (9.5) |
| Total men with high-grade * prostate cancer, No. (%) | 192 (19.1) |
| On both standard and targeted biopsy | 103 (10.3) |
| On standard biopsy only | 19 (1.9) |
| On targeted biopsy only | 70 (7.0) |
| Prior negative biopsies, No. (%) | 429 (42.8) |
| Mean No. of standard biopsies per patient (95%CI) | 12.3 (12.2 to 12.3) |
| Mean No. of targeted biopsies per patient (95% CI) | 5.3 (5.1 to 5.5) |
*Gleason score ≥ 4 + 3. CI = confidence interval; MP-MRI = multiparametric magnetic resonance imaging; PSA = prostate-pecific antigen.
Figure 1.
Distribution of prostate cancer diagnosis by biopsy approach. The rate of prostate cancer diagnosis by the targeted magnetic resonance/ultrasound, standard extended-sextant biopsy, and the two modalities combined. Risk groups are listed as low grade (Gleason score 6 and low-volume Gleason score 3 + 4), intermediate grade (high-volume Gleason 3 + 4), and high grade (Gleason score ≥ 4 + 3). MR/US = magnetic resonance/ultrasound.
The efficiency with which the standard and targeted biopsy approaches diagnosed men with prostate cancer in general, and high-grade prostate cancer in particular, were further examined. Table 2 demonstrates that out of the total cohort of 1003 men the rate at which targeted biopsy cores were positive for cancer was 27.9% (or 2.6 negative for every positive core) compared with 13.5% for standard biopsy cores (or 6.4 negative biopsy cores for every positive core, P < .001). Within the sample of 564 men who were diagnosed with prostate cancer, the core cancer positivity rate was 24.0% for standard and 44.5% for targeted biopsy (negative/positive core ratio 3.2 vs 1.3, P < .001). Amongst the 192 men with high-grade disease, the core positive rate was 34.1% for standard and 63.9% for targeted biopsy (negative/positive core ratio 1.9 vs 0.6, P < .001). In all scenarios, targeted biopsy resulted in fewer negative cores compared with standard biopsy. Table 3 demonstrates the influence of MP-MRI suspicion level on biopsy positivity rate. The percent of targeted cores that were positive for cancer went from 12.1% in cases where the MP-MRI lesion was considered to be “low” suspicion to 55.1% when the MP-MRI lesion was deemed “high” suspicion ( P < .001). Similarly, in Table 4 comparison of the men with a history of prior negative prostate biopsy vs those without such a history demonstrated that targeted biopsy improved the cancer-positive rate in both cohorts although the biopsy core cancer–positive rate was overall lower in men with a history of prior negative biopsy.
Table 2.
Biopsy core–based analysis for all patients and subsets of patients with prostate cancer diagnosis
| Biopsy core subset | All biopsies (n = 1003) |
All prostate cancer patients (n = 564) |
High-grade (n = 192) |
|||
|---|---|---|---|---|---|---|
| Standard | Targeted | Standard | Targeted | Standard | Targeted | |
| Total cores | 12 303 | 5316 | 6911 | 3331 | 2364 | 1233 |
| Prostate cancer–positive cores | 1657 | 1483 | 1657 | 1483 | 807 | 788 |
| Prostate cancer–negative cores | 10 646 | 3833 | 5255 | 1848 | 1557 | 445 |
| % of total prostate cancer–positive | 13.5 | 27.9 * | 24.0 | 44.5 * | 34.1 | 63.9 * |
| Negative/positive ratio | 6.42 | 2.58 | 3.17 | 1.25 | 1.93 | 0.56 |
* P < .001 for difference in cancer-positive core rate, McNemar’s test, two-sided.
Table 3.
Biopsy core–based analysis by maximal MP-MRI lesion suspicion
| Biopsy core subset | Targeted biopsy by MP-MRI suspicion |
||
|---|---|---|---|
| Low | Moderate | High | |
| Total cores | 882 | 3737 | 697 |
| Prostate cancer–positive cores | 107 | 992 | 384 |
| Prostate cancer–negative cores | 775 | 2745 | 313 |
| % of total prostate cancer–positive | 12.1 | 26.5 | 55.1 * |
| Negative/positive ratio | 7.24 | 2.77 | 0.82 |
* P < .001 for increasing trend, Spearman’s rank correlation test. MP-MRI = multiparametric magnetic resonance imaging.
Table 4.
Biopsy-core based analysis by history of prior negative biopsy
| Biopsy core subset | Prior negative biopsy (n = 429) |
No prior negative biopsy (n = 574) |
||
|---|---|---|---|---|
| Standard | Targeted | Standard | Targeted | |
| Total cores | 5258 | 2077 | 7045 | 3239 |
| Prostate cancer–positive cores | 346 | 433 | 1311 | 1050 |
| Prostate cancer–negative cores | 4912 | 1644 | 5734 | 2189 |
| % of total prostate cancer–positive | 6.6 | 20.8 * | 18.6 | 32.4 * |
| Negative/positive ratio | 14.2 | 3.8 | 4.4 | 2.1 |
* P < .001 for difference in cancer-positive core rate, McNemar’s test, two-sided.
Targeted biopsy demonstrated a higher efficiency in the diagnosis of prostate cancer compared with standard biopsy. Targeted biopsy was able to diagnose all prostate cancer with 56.1% fewer cores per diagnosis compared with standard biopsy (11.5 vs 26.2 cores per diagnosis of prostate cancer, 5316 cores for 461 prostate cancer diagnoses by targeted vs 12 303 cores for 469 prostate cancer diagnoses by standard biopsy) ( Tables 1 and 2 ). For high-grade disease, this difference was even greater with targeted biopsy diagnosing high-grade prostate cancer, with 69.6 % fewer cores than standard biopsy (30.7 vs 100.8 cores, 5316 cores for 173 high-grade prostate cancer diagnoses by targeted vs 12 303 for 122 high-grade diagnoses by standard biopsy) ( Tables 1 and 2 ). The number of patients needed to biopsy (NNB) for one cancer diagnosis was also examined ( Table 5 ). The NNB to diagnose one case of prostate cancer was similar between the standard and targeted approach (2.1 vs 2.2 men); however, the NNB to diagnose one case of high-grade prostate cancer was 41% greater for standard vs targeted biopsy (8.2 vs 5.8 men). This reflects the finding in Figure 1 that the total number of prostate cancer diagnoses was similar for the two approaches, but more high-grade and fewer low-grade tumors were diagnosed by the targeted than the standard approach. Furthermore, the efficiency of the targeted biopsy approach was greatly improved by incorporation of MRI suspicion level, particularly for detection of high-grade disease. The NNB to detect one high-grade prostate cancer by the targeted approach in men with low-suspicion MP-MRI was 88 men; by contrast, the NNB in men with high-suspicion MP-MRI findings was 1.8 men. Targeted biopsy utilized lower numbers to biopsy for each additional high-grade prostate cancer diagnosis for men with higher PSA, smaller prostates, and older age as well.
Table 5.
Analysis of number of patients needed to biopsy for one high-grade prostate cancer diagnosis
| Demographic | Standard biopsy, No. | Targeted biopsy, No. | NNB for one more high-grade prostate cancer diagnosis by targeted vs standard biopsy |
|---|---|---|---|
| All men | 8.2 | 5.8 | 19.7 |
| MRI suspicion | |||
| Low | 35.2 | 88.0 | -58.7 |
| Moderate | 10.3 | 6.4 | 17.1 |
| High | 2.3 | 1.8 | 9.1 |
| PSA quartiles, ng/mL | |||
| <4.4 | 22.8 | 22.8 | No difference |
| 4.4–6.6 | 10.8 | 8.9 | 49.8 |
| 6.6–10.7 | 10.0 | 6.5 | 18.0 |
| ≥10.7 | 4.0 | 2.6 | 7.8 |
| Prostate volume quartiles, cc | |||
| <38 | 6.3 | 4.0 | 10.5 |
| 38–51 | 7.8 | 5.4 | 17.9 |
| 51–72 | 8.4 | 6.8 | 35.0 |
| ≥72 | 12.1 | 9.4 | 42.5 |
| Age quartiles, y | |||
| <57 | 13.3 | 11.9 | 113 |
| 57–62 | 11.0 | 7.7 | 25.6 |
| 62–67 | 9.9 | 4.9 | 9.9 |
| ≥67 | 5.0 | 4.0 | 20.7 |
| Prior negative biopsy | |||
| Yes | 11.3 | 6.2 | 13.8 |
| No | 6.8 | 5.5 | 28.7 |
*MRI = magnetic resonance imaging; NNB = number of men who need to be biopsied; PSA = prostate-specific antigen.
We examined the incremental benefit of using MR-MRI-guided fusion biopsy vs the standard approach. For high-grade cancer, 19.7 men had to be biopsied by the targeted approach rather than the standard approach to diagnose one additional high-grade cancer ( Table 5 ). When stratified by PSA quartiles, little benefit was seen by targeted biopsy over standard approach for low PSA, but benefit was seen increasingly for higher PSA. Conversely, when stratified by ultrasound prostate volume, the number of men needed to undergo targeted biopsy for each additional diagnosis of high-grade disease was lower for small prostates. There was no discernable association of differential benefit of targeted biopsy over the standard approach by age. A prior history of negative biopsy also seemed to increase the benefit of utilizing targeted biopsy over the standard approach ( Table 5 ).
Lastly, we examined the question of how many men with low-grade Gleason score 6 prostate cancers are missed by targeted vs standard biopsy. Particularly in the current environment, where overtreatment of low-grade, clinically insignificant prostate cancer is of major concern, decreased diagnosis of these tumors holds potential benefit and should be considered a complimentary question of biopsy efficiency. Table 6 demonstrates the NNB for the diagnosis of Gleason 6 prostate cancer. Of note, for every 17 men biopsied by targeted rather than standard biopsy, one fewer person is diagnosed with Gleason 6 prostate cancer. This value is improved for low MRI suspicion (NNB by targeted = 12.6 for one fewer Gleason 6 tumor diagnosis) PSAs greater than 10.6 ng/mL (NNB = 11.4), large prostate volumes greater than 72 cc (NNB = 12.8), and men older than age 67 years (NNB = 12.6). While a history of prior negative biopsy improved diagnosis of high-risk disease, such a history did not seem to change the diagnostic rate of Gleason 6 cancer (NNB = 17.2 in men with prior negative prostate biopsy history, NNB = 16.9 with no such history).
Table 6.
Analysis of number of patients needed to biopsy for one Gleason 6 cancer diagnosis
| Demographic | Standard biopsy, No. | Targeted biopsy, No. | NNB by target vs standard biopsy to diagnose one fewer Gleason 6 cancer |
|---|---|---|---|
| All men | 4.9 | 6.8 | 17.0 |
| MRI suspicion | |||
| Low | 3.7 | 5.2 | 12.6 |
| Moderate | 4.9 | 6.7 | 18.0 |
| High | 9.9 | 18.2 | 21.8 |
| PSA quartiles, ng/mL | |||
| <4.4 | 3.7 | 4.4 | 25.1 |
| 4.4–6.6 | 4.6 | 5.8 | 22.6 |
| 6.6–10.7 | 5.5 | 8.4 | 15.8 |
| ≥10.7 | 6.4 | 14.8 | 11.4 |
| Prostate volume quartiles, cc | |||
| <38 | 4.4 | 6.3 | 14.9 |
| 38–51 | 4.2 | 6.0 | 14.7 |
| 51–72 | 6.0 | 6.8 | 49.0 |
| ≥72 | 5.2 | 8.8 | 12.8 |
| Age quartiles, y | |||
| <57 | 4.7 | 6.3 | 18.8 |
| 57–62 | 4.2 | 5.8 | 15.3 |
| 62–67 | 5.0 | 6.1 | 28.6 |
| ≥67 | 5.6 | 10.0 | 12.6 |
| Prior negative biopsy | |||
| Yes | 7.3 | 12.6 | 17.2 |
| No | 3.9 | 5.1 | 16.9 |
*MRI = magnetic resonance imaging; NNB = number of men who need to be biopsied; PSA = prostate-specific antigen.
Discussion
In this study, targeted biopsy was more efficient on a per-core basis than standard prostate biopsy in the diagnosis of prostate cancer in general and in high-grade prostate cancer in particular. Biopsy efficiency was examined from a variety of perspectives, and in each case the targeted approach demonstrated advantages. On a per-individual basis, standard biopsy utilized 2.3 times more biopsy cores (12.3 vs 5.3 cores per patient) for the workup of prostate cancer. Targeted biopsy had a higher rate of cores positive for cancer and, accordingly, fewer negative cores. Globally, targeted biopsy utilized fewer cores per diagnosis of cancer both in the case of all prostate cancer and high-grade disease. Lastly, in cases of clinically significant high-grade disease, fewer men had to be biopsied by targeted biopsy vs by standard biopsy per diagnosis of cancer. Overall, a benefit of one additional high-grade prostate cancer diagnosis was seen for every 20 men biopsied by targeted vs standard biopsy approach.
This study also demonstrated that various factors such as PSA, ultrasound prostate volume, and MP-MRI lesion suspicion levels could be utilized to further optimize the efficiency of the biopsy approach. When men were stratified by MP-MRI suspicion level, there was a 49-fold improvement in the NNB to diagnose each case of high-grade prostate cancer from 88 to 1.8. Not as dramatically, comparison of the highest and lowest quintiles of PSA, prostate volume, and age decreased the NNB for each high-grade prostate cancer by 8.8-, 2.4-, and 3-fold, respectively.
An important question that this study gave some insight into is the question of how to manage men who undergo MR/US fusion–targeted biopsy for a suspicious MRI lesion but no disease is found. Based on the findings of this study, in a patient who had a negative targeted biopsy of his MRI lesions, there would be an approximately 19.0% chance (103 out of 542 men) of finding prostate cancer if a standard biopsy was then performed but only a 3.5% likelihood (19 out of 542 men) of finding Gleason 4 + 3 or higher disease. Although the likelihood is not zero, the 3.5% chance is certainly improved from the 19.1% prevalence of high-grade disease that existed in the entire cohort prior to targeted biopsy. As demonstrated in this study, based on factors such as age, prostate volume, and MRI suspicion, the likelihood of high-grade prostate cancer can be further stratified. Future studies may utilize this information to further refine which patients who have a negative targeted biopsy may warrant further biopsy workup vs careful observation alone.
As utilization of MP-MRI and targeted biopsy increase for the workup of men with suspected prostate cancer, the importance of this study is that it provides further quantitative guidance with regard to the difference between the traditional standard biopsy approach and the newer targeted approach. In agreement with a systemic review of 15 studies that the number of biopsy cores needed for fusion biopsy is less than for standard biopsy ( 8 ), this study expounds on those findings by demonstrating pre-MRI variables that further enhance the biopsy efficiency in diagnosis of clinically significant prostate cancer. The more detailed biopsy core level of information presented here may be of clinically significant benefit to help shape data-driven guidelines as to when the benefit of a targeted biopsy over a standard biopsy can justify the extra cost and resources and in which patients perhaps such a biopsy technique is unnecessary. The ability of pre-MRI variables to identify those men most likely to benefit from targeted biopsy may optimize the use of targeted MR/ultrasound fusion prostate biopsy ( 12 ). Given the added costs of MR/ultrasound fusion prostate biopsy, a more detailed understanding of the comparative performance of this technique over the standard approach will allow the quantification of any clinically relevant improvement the targeted biopsy offers and in which subsets of men the benefit is greatest (and least). Such information may facilitate further research into questions such as the cost-effectiveness of the targeted and standard biopsy approaches ( 13 ).
This study has a few limitations. First, the cohort of men studied is composed of only men with visible lesions on MP-MRI. As the study design was dependent on comparing targeted and standard biopsy within the same patient, it was not possible to incorporate men with no findings on MP-MRI into this study. Because a proportion of these 180 men may ultimately have been diagnosed with cancer on standard biopsy, exclusion of this group may bias the result in favor of targeted biopsy. Other studies, however, that have examined men with no lesions on MP-MRI suggest that such a bias is unlikely to be the case as the negative predictive value of a negative MRI has been described as 83% for any cancer and 98% for Gleason 7 or greater cancer ( 14 ). Second, this study was in a cohort of men of whom 43% of had a prior negative prostate biopsy. Such a cohort may be enriched for cancers difficult to detect by standard biopsy but able to be captured on targeted biopsy. The efficiency values in this case could thus be greater than would be encountered in a purely biopsy-naive cohort although some studies suggest this may not be the case ( 15 ). A subcohort analysis in this study demonstrated that while a history of prior negative biopsy may augment the efficacy of targeted biopsy, the benefit of targeted biopsy persisted nonetheless in men without a history of prior negative prostate biopsy. Third, by design the targeted biopsies were performed before standard biopsies. This is in contrast to normal practice of standard biopsy only, where men will not have had a biopsy before their standard biopsy, and it is not clear in cases with very small components of high-grade disease if a tandem biopsy will affect the outcome of the standard biopsy. As the prostate biopsy core diameter is typically approximately one millimeter, however, this should not be a limitation that alters the overall study results. Lastly, while we attempt to stratify the effects of the technology away from the effects of the number of biopsies performed, the two aspects of the study are heavily confounded as standard biopsy by definition utilizes more cores than the targeted approach. A more rigorous trial design would utilize the same number of cores for the two approaches; however, it is unlikely such a trial will ever be feasible clinically.
In conclusion, in men with MR-visible prostate lesions, targeted biopsy is more efficient than standard biopsy, diagnosing a similar number of cancer cases and more high-grade cases while sampling 57% fewer cores. Higher-suspicion MRI lesions, men with higher PSA, and smaller prostates have even greater yield per biopsy session. These findings have implications for future studies aimed at studying decreases in prostate biopsy morbidity and improved cost-effectiveness associated with targeted biopsy and may help define specific patient populations where targeted biopsy is of greatest value.
Funding
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute, Center for Cancer Research, and Center for Interventional Oncology. The NIH, Philips Healthcare, and Invivo have a cooperative research and development agreement. The NIH, Philips Healthcare, and Invivo share intellectual property in the field.
Notes
The funding source for this study had no role in the study design, data collection, analysis, interpretation of data, writing of the report, or the decision to submit the manuscript for publication.
Drs Choyke and Pinto report holding a patent related to the MR/ultrasound fusion biopsy platform. Dr Wood reports holding multiple related patents in the field, including a method and system for performing biopsies, a system and method for fusing real-time ultrasound images with pre-acquired medical images, and others, and contributing to three invention reports related to prostate disease diagnosis and treatment.
References
- 1. Rais-Bahrami S, Siddiqui MM, Turkbey B , et al. . Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer . J Urol . 2013. ; 190 ( 5 ): 1721 – 1727 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosenkrantz AB, Kim S, Lim RP , et al. . Prostate cancer localization using multiparametric MR imaging: comparison of Prostate Imaging Reporting and Data System (PI-RADS) and Likert scales . Radiology. 2013. ; 269 ( 2 ): 482 – 492 . [DOI] [PubMed] [Google Scholar]
- 3. Siddiqui MM, Rais-Bahrami S, Turkbey B , et al. . Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer . JAMA. 2015. ; 313 ( 4 ): 390 – 397 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siddiqui MM, Rais-Bahrami S, Truong H , et al. . Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy . Eur Urol. 2013. ; 64 ( 5 ): 713 – 719 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sonn GA, Chang E, Natarajan S , et al. . Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen . Eur Urol. 2014. ; 65 ( 4 ): 809 – 815 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pokorny MR, de Rooij M, Duncan E , et al. . Prospective study of diagnostic accuracy comparing prostate cancer detection by transrectal ultrasound-guided biopsy versus magnetic resonance (MR) imaging with subsequent MR-guided biopsy in men without previous prostate biopsies . Eur Urol. 2014. ; 66 ( 1 ): 22 – 29 . [DOI] [PubMed] [Google Scholar]
- 7. Salami SS, Vira MA, Turkbey B , et al. . Multiparametric magnetic resonance imaging outperforms the Prostate Cancer Prevention Trial risk calculator in predicting clinically significant prostate cancer . Cancer . 2014. ; 120 ( 18 ): 2876 – 2882 . [DOI] [PubMed] [Google Scholar]
- 8. Valerio M, Donaldson I, Emberton M , et al. . Detection of Clinically Significant Prostate Cancer Using Magnetic Resonance Imaging-Ultrasound Fusion Targeted Biopsy: A Systematic Review . Eur Urol. 2014. ; 68 ( 1 ): 8 – 19 . [DOI] [PubMed] [Google Scholar]
- 9. Loeb S, Vellekoop A, Ahmed HU , et al. . Systematic review of complications of prostate biopsy . Eur Urol. 2013. ; 64 ( 6 ): 876 – 892 . [DOI] [PubMed] [Google Scholar]
- 10. Yerram NK, Volkin D, Turkbey B , et al. . Low suspicion lesions on multiparametric magnetic resonance imaging predict for the absence of high-risk prostate cancer . BJU Int. 2012. ; 110(11 Pt B) : E783 – E788 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barentsz JO, Richenberg J, Clements R , et al. . ESUR prostate MR guidelines 2012 . Eur Radiol. 2012. ; 22 ( 4 ): 746 – 757 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shakir NA, George AK, Siddiqui MM , et al. . Identification of threshold prostate specific antigen levels to optimize the detection of clinically significant prostate cancer by magnetic resonance imaging/ultrasound fusion guided biopsy . J Urol. 2014. ; 192 ( 6 ): 1642 – 1648 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. de Rooij M, Crienen S, Witjes JA , et al. . Cost-effectiveness of Magnetic Resonance (MR) Imaging and MR-guided Targeted Biopsy Versus Systematic Transrectal Ultrasound-Guided Biopsy in Diagnosing Prostate Cancer: A Modelling Study from a Health Care Perspective . Eur Urol. 2014. ; 66 ( 3 ): 430 – 436 . [DOI] [PubMed] [Google Scholar]
- 14. Wysock JS, Rosenkrantz AB, Meng X , et al. . Predictive value of negative 3T multiparametric prostate MRI on 12 core biopsy results . In. American Urologic Association Annual Meeting . Orlando FL: ; 2014. . [Google Scholar]
- 15. Habchi H, Bratan F, Paye A , et al. . Value of prostate multiparametric magnetic resonance imaging for predicting biopsy results in first or repeat biopsy . Clin Radiol. 2014. ; 69 ( 3 ): e120 – e128 . [DOI] [PubMed] [Google Scholar]