Abstract
Background: Our objective was to evaluate progression-free survival (PFS) and distant metastasis–free survival (DMFS) as surrogate end points for overall survival (OS) in randomized trials of chemotherapy in loco-regionally advanced nasopharyngeal carcinomas (NPCs).
Methods: Individual patient data were obtained from 19 trials of the updated Meta-Analysis of Chemotherapy in Nasopharyngeal Carcinoma (MAC-NPC) plus one additional trial (total = 5144 patients). Surrogacy was evaluated at the individual level using a rank correlation coefficient ρ and at the trial level using a correlation coefficient R2 between treatment effects on the surrogate end point and OS. A sensitivity analysis was performed with two-year PFS/DMFS and five-year OS.
Results: PFS was strongly correlated with OS at the individual level (ρ = 0.93, 95% confidence interval [CI] = 0.93 to 0.94) and at the trial level (R2 = 0.95, 95% CI = 0.47 to 1.00). For DMFS, too, the individual-level correlation with OS was strong (ρ = 0.98, 95% CI = 0.98 to 0.98); at trial level, the correlation was high but the regression adjusted for measurement error could not be computed (unadjusted R2 = 0.96, 95% CI = 0.94 to 0.99). In the sensitivity analysis, two-year PFS was highly correlated with five-year OS at the individual level (ρ = 0.89, 95% CI = 0.88 to 0.90) and at the trial level (R2 = 0.85, 95% CI = 0.46 to 1.00); two-year DMFS was highly correlated with five-year OS at the individual level (ρ = 0.95, 95% CI = 0.94 to 0.95) and at the trial level (R2 = 0.78, 95% CI = 0.33 to 1.00).
Conclusions: PFS and DMFS are valid surrogate end points for OS to assess treatment effect of chemotherapy in loco-regionally advanced NPC, while PFS can be measured earlier.
Nasopharyngeal carcinoma (NPC) is a rather uncommon cancer and bears a unique pattern of geographical distribution. The International Agency for Research on Cancer (1) estimated 85 500 new cases in 2012, over 60 000 of those in Eastern and South-Eastern Asia. Given these relatively low and geographically heterogeneous incidence rates compared with other cancers and the rapid pace of technical and pharmaceutical developments, conducting research in this disease is challenging. One of the ways to speed up clinical research is through the use of end points that could be obtained earlier than overall survival (OS). Such end points, to be clinically relevant, should be consistently defined and measured across trials, correlated to the gold-standard end point both at the trial and patient levels, and available earlier than this latter end point.
The individual patient data meta-analysis of chemotherapy in nasopharyngeal carcinoma (MAC-NPC) included most randomized trials conducted up to 2010 evaluating the role of adding chemotherapy to radiotherapy in nonmetastatic NPC patients (2). Given its exhaustiveness and the availability of individual patient data, it is a unique database to validate surrogate end points in NPC, as was previously performed by our team for squamous cell head and neck (H&N) carcinoma (3) and lung cancer (4). The primary objective of the present study was to evaluate progression-free survival (PFS) as surrogate end point for OS in randomized trials evaluating cytotoxic chemotherapy in NPC. Given the progressive shift from local to distant progressions observed with the use of intensity modulated radiotherapy and concomitant chemotherapy (5), our secondary objective was to assess the validity of distant metastasis–free survival (DMFS) as a surrogate end point for OS in this setting.
Methods
Patients and Study Objectives
Individual patient data (n = 5144) were available from 19 randomized trials and included the updated MAC-NPC meta-analysis (2,6) plus one trial (7). All trials in the MAC-NPC meta-analysis compared radiotherapy (RT) with RT plus chemotherapy (CT), or a treatment strategy (RT plus concomitant or induction or adjuvant CT) with the same strategy plus CT at another timing. Both published and unpublished trials were included. Patients were recruited between 1988 and 2010. The overall median follow-up time was 7.7 years. One more trial that compared two timings of CT (7) was included in the present analysis. One 2x2 trial (8) that randomized the use of CT and RT fractionation was counted as two separate comparisons, one for each RT fractionation regimen. Another 2x2 trial (9) that randomized adjuvant CT and concomitant CT was duplicated and counted as four comparisons. In total, there were 24 comparisons in the analyzed data set. Six patients were excluded because of missing progression data (7). Seventy-four further patients from the HeCOG (10) and INT-0099 (11), having missing information about the type of progression, were excluded from the analyses for DMFS. In total, 5360 patients were analyzed, with a median of 175.5 patients per trial (range = 65–509). Supplementary Table 1 (available online) describes the comparisons. All patients were analyzed according to the intention-to-treat principle.
The preplanned primary objective was the assessment of PFS as a surrogate end point for OS in patients with loco-regionally advanced NPC. The secondary objective was the assessment of DMFS as a surrogate end point for OS in the same population. This retrospective analysis of clinical trial data was part of the meta-analysis protocol, which was approved by the Gustave Roussy Institutional Review Board, and the statistical methodology was preplanned (http://goo.gl/4Vitzs).
Sensitivity Analyses
In order to enhance interpretation from the clinician’s point of view, we contrasted in a preplanned sensitivity analysis the two-year surrogate end point vs the five-year OS, which reflect typical trial conditions. Preplanned sensitivity analyses were also performed by calendar period: the nine oldest trials (9,11–17) were reanalyzed separately from the 11 most recent ones (7,8,10,18–25).
End Point Definitions
Overall survival (OS) was defined as the time from random assignment until death from any cause. Progression-free survival (PFS) was the time from random assignment until first progression (loco-regional or distant) or death from any cause. Distant metastasis–free survival (DMFS) was the time from random assignment until distant progression or death from any cause. Because in some trials only the first event was recorded, patients with a loco-regional progression as first event were censored for DMFS. If both a loco-regional progression and a distant failure were recorded at the same time, patients were considered as having an event for DMFS. Patients alive and free from events at the end of the study were censored at the date of last follow-up. Of note, the previous study in H&N (3) included both trials of patients with resectable tumors and trials of patients with nonresectable tumors. Event-free survival (EFS) was used to denote disease- and progression-free survival, respectively, for resectable and nonresectable tumors. Thus, considering here PFS for trials including only patients with nonresectable tumors coincides with the definition of EFS in the H&N study. To describe the potential benefit of employing each surrogate end point as compared with OS, we computed the number of patients for which the surrogate was observed before death and the ratio of the median surrogate time to the median OS time (Kaplan-Meier estimates). The 95% confidence interval (CI) of the ratio between the median times was based on the delta method.
Statistical Methods
A correlation approach was used to assess the validity of each end point as surrogate for OS (26), as already used by Sargent et al. (27) and Buyse et al. (28) in colon cancer, by Burzykowski et al. (29) in breast cancer, by Michiels et al. (3) in locally advanced H&N cancer, by Oba et al. (30) and Paoletti et al. (31) in gastric cancer, and by Mauguen et al. (4) in lung cancer. This approach investigates the correlation at a trial level and at an individual level.
The association between distributions of OS and the candidate surrogate end point was evaluated by a bivariate survival model (32,33) (copula). Clayton and Plackett copula models were fitted (34); the Plackett copula was chosen because of better convergence. Based on the copula dependence parameter, we computed the Spearman rank correlation coefficient ρ; a Spearman coefficient close to 1 indicates a strong correlation.
Treatment effects were estimated by log hazard ratios in bivariate survival models. The correlation between treatment effect on the surrogate and treatment effect on OS was quantified through a linear regression model accounting via an error-in-variables model (35) for the uncertainty about the effects estimated by copulas. Whenever such a model failed to converge, we approximated it using the simpler model with estimated effects taken as fixed, weighted by the trial size. If the estimated R2 was close to 1, then the risk reduction for OS was considered strongly correlated with the risk reduction for the candidate surrogate. As done previously (4), the R2 was considered excellent if higher than 0.9, very good if higher than 0.75, good if higher than 0.5, moderate if higher than 0.25, and poor otherwise.
One objective of a surrogate end point is to predict the treatment effect on OS, based on the treatment effect on the surrogate end point. The surrogate threshold effect (STE) (36) was computed in order to estimate this prediction threshold: The STE is defined as the minimum treatment effect that is necessary on the surrogate to be able to predict a nonzero effect on OS. In other words, the STE corresponds to the smallest estimated treatment effect on the surrogate, which is predictive of a statistically significant effect of the treatment on OS, irrespectively of its clinical significance, which has to be considered case by case. Its calculation was based on the linear regression used for the determination of trial-level surrogacy.
A leave-one-out cross-validation was used to evaluate model predictions (3). For each trial, the bivariate model for OS and the surrogate end point was refitted on the remaining trials to predict the treatment effect on OS in the left-out trial, based on the treatment effect on the surrogate end point. For each trial, the direct estimate of the hazard ratio was compared with the predicted hazard ratio and 95% prediction interval. The analyses were performed using SAS (version 9.3, SAS Institute, Cary, NC) and R (version 3.2.2, R foundation for Statistical Computing, Vienna, Austria). All statistical tests were two-sided.
Results
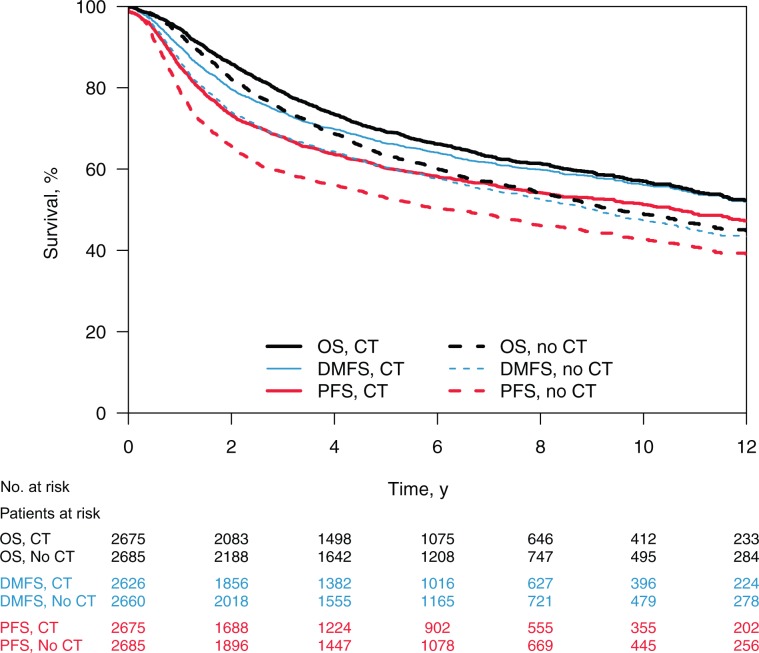
Overall 2162 patients (40.3%) died, among which 1690 (78.2%) had a progression recorded before death. This progression (first event) was a loco-regional progression in 684 patients (31.6%) and a distant metastasis in 1006 (46.5%). The estimated median time was 8.2 years for PFS and 11.3 years for OS, with a ratio of 0.726 (95% CI = 0.723 to 0.730), suggesting that a trial based on PFS could be roughly 27% faster than a trial based on OS. The median DMFS time was 10.9 years; the DFMS-to-OS ratio was 0.964 (95% CI = 0.960 to 0.968). Using DMFS instead of OS could speed up a clinical trial only by 4%. Figure 1 shows the OS, PFS, and DMFS curves by treatment group, and Table 1 gives the number of events at two, five, and 10 years. Supplementary Figure 1 (available online) shows the cumulative probability of deaths, metastases, and loco-regional progressions. Most of events occur in the first five years, 10% to 20% after five years, and 5% after 10 years. Of note, the number of PFS events at two years (n = 1613) is similar to the number of OS events at five years (n = 1658).
Figure 1.
Survival curves for overall survival, progression-free survival, and distant metastasis–free survival in chemotherapy and control arms. Overall survival, progression-free survival, and distant metastasis–free survival in the chemotherapy and control arms. CT = chemotherapy; DMFS = distant metastasis–free survival; no CT = control; PFS = progression-free survival; OS = overall survival.
Table 1.
Cumulative number of events for overall, progression-free, and distant metastasis-free survival at 2, 5, and 10 years
| End point | 2 years No. (%) | 5 years No. (%) | 10 years No. (%) | Total No. (%) |
|---|---|---|---|---|
| Overall survival | 837 (38.7) | 1658 (76.7) | 2039 (94.3) | 2162 (100.0) |
| Control arm | 468 (40.6) | 897 (77.7) | 1099 (95.2) | 1154 (100.0) |
| Chemotherapy arm | 369 (36.6) | 761 (75.5) | 940 (93.3) | 1008 (100.0) |
| Progression-free survival | 1613 (62.0) | 2224 (85.5) | 2501 (96.2) | 2600 (100.0) |
| Control arm | 907 (65.2) | 1206 (86.6) | 1350 (97.0) | 1392 (100.0) |
| Chemotherapy arm | 706 (58.4) | 1018 (84.3) | 1151 (95.3) | 1208 (100.0) |
| Distant metastasis–free survival | 1270 (54.4) | 1891 (81.1) | 2218 (95.1) | 2333 (100.0) |
| Control arm | 716 (57.0) | 1026 (81.7) | 1205 (95.9) | 1256 (100.0) |
| Chemotherapy arm | 554 (51.4) | 865 (80.3) | 1013 (94.1) | 1077 (100.0) |
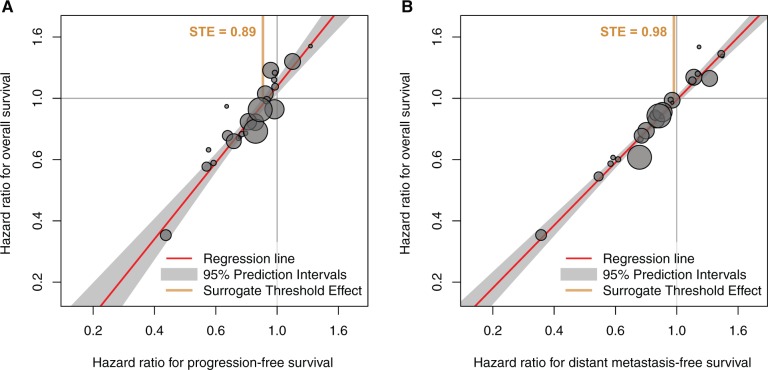
The results of the surrogacy assessment are summarized in Table 2 and detailed in Supplementary Tables 2 and 3 (available online). Supplementary Table 4 (available online) shows good consistency between results obtained with Clayton and Plackett copulas. The individual-level association between PFS and OS was strong, with rank correlation coefficient ρ = 0.93 (95% CI = 0.93 to 0.94) (Table 2). The squared linear correlation coefficient R2—adjusted for measurement error—between treatment effects on PFS and OS at the trial level was 0.95 (95% CI = 0.47 to 1.00) (Figure 2A). The surrogate threshold effect for PFS was 0.89 (Table 2). The individual-level association between DMFS and OS was strong, with a rank correlation coefficient ρ of 0.98 (95% CI = 0.98 to 0.98). The regression model adjusted for measurement error failed to converge for DMFS, thus trial-level surrogacy was assessed without adjustment. The squared linear correlation coefficient R2 between treatment effects on DMFS and OS was 0.96 (95% CI = 0.94 to 0.99) (Figure 2B). The STE for DMFS was 0.98 (Table 2).
Table 2.
Surrogate end points in nonmetastatic nasopharyngeal carcinoma: summary of the results*
| Surrogacy measures | All trials, all follow-up | By subgroup of trials |
Surrogate at 2 y vs OS at 5 y | |
|---|---|---|---|---|
| Old trials† | Recent trials‡ | |||
| Events, no. | ||||
| OS | 2162 | 1208 | 954 | 1658 |
| PFS | 2600 | 1466 | 1134 | 1613 |
| DMFS | 2333 | 1285 | 1048 | 1270 |
| PFS vs OS | ||||
| Individual level | ||||
| ρ (95% CI) | 0.93 (0.93 to 0.94) | 0.93 (0.92 to 0.94) | 0.94 (0.93 to 0.95) | 0.89 (0.88 to 0.90) |
| Trial level | ||||
| R2 (95% CI) | 0.95 (0.47 to 1.00) | 0.81§ (0.62 to 1.00) | 0.92§ (0.84 to 1.00) | 0.85 (0.46 to 1.00) |
| STE | 0.89 | 0.84§ | 0.91§ | 0.83 |
| DMFS vs OS | ||||
| Individual level | ||||
| ρ (95% CI) | 0.98 (0.98 to 0.98) | 0.99 (0.99 to 0.99) | 0.97 (0.97 to 0.98) | 0.95 (0.94 to 0.95) |
| Trial level | ||||
| R2 (95% CI) | 0.96§ (0.94 to 0.99) | 0.98§ (0.95 to 1.00) | 0.97§ (0.94 to 1.00) | 0.78 (0.33 to 1.00) |
| STE | 0.98§ | 0.97§ | 0.99§ | 0.90 |
Individual- and trial-level surrogacy for progression-free survival and distant metastasis–free survival vs overall survival in the main analysis (20 trials, 5360 patients) and in sensitivity analyses. CI = confidence interval; DMFS = distant metastasis–free survival; OS = oversall survival; PFS = progression-free survival; STE = surrogate threshold effect.
Nine trials (12 comparisons, 2484 patients) = PWH-88, AOCOA, VUMCA-89, INT-0099, Japan-91, TCOG-94, PWHQEH-94, QMH-95, VUMCA-95.
Eleven trials (12 comparisons, 2876 patients) = SQNP01, NPC-9901, NPC-9902, Guangzhou 2001, NPC008, Guangzhou 2002-01, Guangzhou 2002-02, Guangzhou 2003, HeCOG, Shanghai 2004, Guangzhou 2006.
Results obtained without adjustment for estimation error because of a lack of convergence of the adjusted model.
Figure 2.
Correlation between treatment effects on the surrogate and overall survival in loco-regionally advanced nasopharyngeal carcinomas. A) Progression-free survival. B) Distant metastasis–free survival. Each circle is a trial, and its size is proportional to the number of patients. STE = surrogate threshold effect.
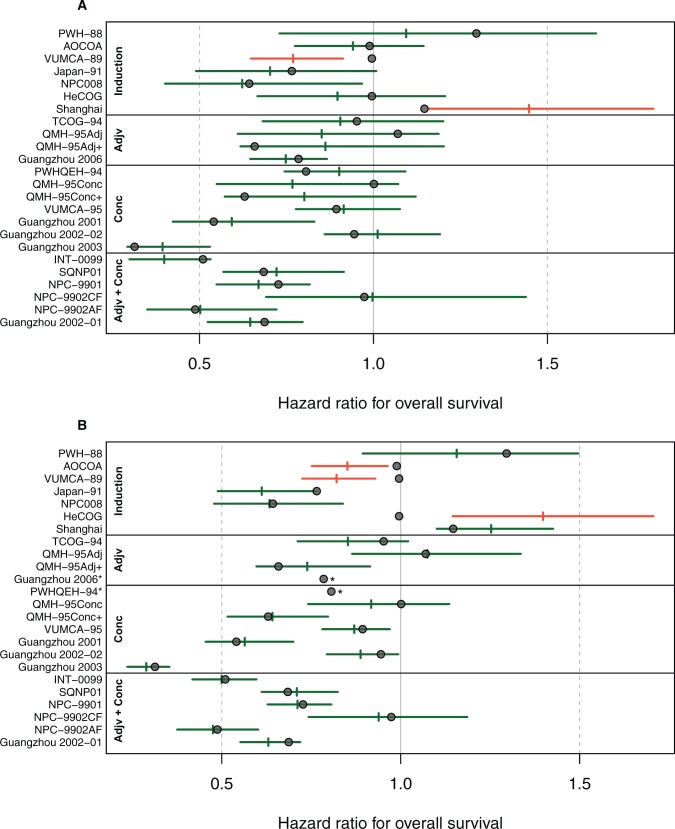
Figure 3 shows the results from leave-one-trial-out cross-validation. The 95% prediction intervals for the treatment effect on OS contained the observed effect in 22 of 24 comparisons when prediction was based on the observed treatment effect on PFS. When prediction was based on the observed treatment effect on DMFS, the surrogate model failed to converge in two comparisons. The prediction intervals for the treatment effect on OS contained the observed effect in 19 of the 22 remaining comparisons.
Figure 3.
Leave-one-out cross-validation analysis for the model predicting the treatment effect on overall survival based on progression-free survival (A) and distant metastasis-free survival (B) effects. Circles are the observed hazard ratios for the effect on overall survival. Horizontal segments correspond to 95% prediction intervals (PI). The vertical segments are the predicted effects on overall survival using the observed hazard ratio on progression or distant metastasis-free survival of each trial and using the surrogate model fitted on the other trials. *The surrogate model could not be fitted due to convergence issues. Individual trials are grouped according to the timing of chemotherapy and are named the same way as in our initial publication(2), while references can be found in the Supplementary Table 1. Adjv = adjuvant; Conc = Concomitant.
In a sensitivity analysis, we censored the surrogate end points at two years and OS at five years to reflect clinical trial conditions. Under this constraint, PFS was slightly less correlated to OS both at the individual level (ρ = 0.89, 95% CI = 0.88 to 0.90) and at the trial level (R2 = 0.85, 95% CI = 0.46 to 1.00), but correlations remained very good. DMFS, too, was slightly less correlated with OS with restrained follow-up, with excellent individual-level correlation (ρ = 0.95, 95% CI = 0.94 to 0.95) and very good trial-level correlation (R2 = 0.78, 95% CI = 0.33 to 1.00) (Table 2). As the incidence of events increased up to three years, we also performed an unplanned analysis with three-year PFS vs five-year OS, which showed similar individual correlation (ρ = 0.90, 95% CI = 0.90 to 0.91) and lower trial correlation (unadjusted R2 = 0.74, 95% CI = 0.56 to 0.92). The same analysis for DMFS gave no results because of convergence issues. PFS showed slightly smaller trial-level correlation in the group of old trials (R2 = 0.81, 95% CI = 0.62 to 1.00) than within the recent trials (R2 = 0.92, 95% CI = 0.84 to 1.00), but confidence intervals largely overlapped (Table 2). Of note, for both subgroups we were able to fit only nonadjusted regression. Accordingly, the STE was lower for old trials (0.84) than for recent ones (0.91), and both STEs were lower with restrained follow-up: 0.74 and 0.87, respectively. The results for DMFS were similar in the two subgroups.
Discussion
The present analysis shows that, for trials investigating the role of chemotherapy in nasopharyngeal carcinoma, PFS and DMFS were strongly correlated with OS, both at the trial and the individual level. Leave-one-out cross-validations confirmed the internal validity of the results. The surrogate threshold effect was 0.89 for PFS and 0.98 for DMFS. This suggests that if in a new trial the confidence interval of the hazard ratio on PFS has an upper limit below 0.89, then the benefit on OS is likely to be statistically significant, whereas for DMFS the upper limit of the confidence interval should be below 0.98 to predict a benefit on OS.
Rigorous validation of plausible surrogate end points is important because of unintended, unanticipated, and unrecognized mechanisms of action (37). The theoretical bases of the statistical validation of surrogate end points have been widely discussed since the publication of the Prentice criteria (38). As previously done for H&N squamous cell carcinoma (3) and in a number of other disease sites (4,27–31), we adopted a correlation approach, which deems a surrogate end point acceptable if both the individual-level and the trial-level correlations ρ and R2 are close to 1. Although only superiority trials were included, all the results but the surrogate threshold effects are valid also for noninferiority trials.
The clinical usefulness of a surrogate end point depends mostly on its reliability and generalizability to future clinical trials. To be reliable, a surrogate must accurately reflect the final end point. It needs to be validated on high-quality data using a standardized definition and robust statistical methods. We used individual patient data for all trials included in this analysis, we checked the quality of each trial, we recomputed end points consistently using updated follow-up when available, and we validated surrogacy both at the trial and individual levels. In comparison, a recent analysis used published data only (39) and PFS could only be evaluated in nine trials where the end point was defined consistently. Besides, as always in published data meta-analyses (40), some input data—not clearly mentioned in the text—were estimated based on survival curves. Although this method (41) is frequently used, its limitations are well known (42). This translates into a lower R2 for PFS than the one presented here, although the STEs are very close. Furthermore, in that paper DMFS could not be evaluated because of the lack of information on distant progressions in the articles, and no patient-level analysis could be performed for any surrogate end point. To conclude, while a published data meta-analysis on surrogacy is certainly a first step, the surrogacy should be confirmed by an individual patient data analysis.
In the present analysis, two surrogate end points were evaluated, and the question arises as to whether one is preferable. While the correlation coefficients for DMFS are higher than for PFS when considering unrestrained follow-up, this statistical superiority of DMFS is less obvious when considering a “real life” clinical trial situation, which is the prediction of five-year OS knowing the efficacy on the two-year surrogate. The trial-level correlation, the one that matters in this situation, is superior for PFS. In general, PFS is measured earlier and provides a higher power, as more events are taken into account, than DMFS. Furthermore, the surrogate-to-OS ratio of median times is lower for PFS, implying that the trial analysis would be more accelerated by using PFS than DMFS. Nevertheless, one can expect that such difference will be smaller in new trials, in which mandatory 3D-conformal/IMRT techniques will likely make DMFS and PFS more closely associated. Lastly, PFS was slightly more robust in cross-validation, with a wrong prediction of treatment effect on OS based on the effect on PFS for two trials, as compared with three for DMFS. Overall, we believe that the use of PFS as a surrogate is more reliable and should be encouraged for future trials evaluating chemotherapy in NPC.
The major limitations of the current study relate to its relevance with regards to the future of clinical research in NPC. Modern imaging techniques could allow the detection of recurrences earlier and the triggering of potentially curative salvage treatments. Then the treatment effect on the surrogate end point in a new trial could be diluted and potentially not detectable on OS. However, at present, salvage loco-regional treatments are seldom curative and can be applied to a highly selective patient population (43). Systemic treatment remains palliative in this setting, as shown by the OS durations in the recurrent and metastatic setting (43). The validity of PFS or DMFS remains strong in our data despite this limitation. Another important issue is the validity in the context of new systemic treatments, although most recent trials in NPC patients still investigate cytotoxic chemotherapies: A trial search performed in the summer of 2015 found that 10 out of 12 identified ongoing or recently completed trials investigated cytotoxic agents, while only two investigated nimotuzumab, a monoclonal antibody directed against the epidermal growth factor. Furthermore, and as mentioned earlier, even if a targeted agent is used for primary treatment in combination with radiotherapy, the relationship between progression and death might unfortunately remain valid because of the absence of broadly applicable effective salvage therapies. When cure is the goal, the switch from “progression-free” to “recurrent/metastatic” will likely remain a major determinant of survival in the context of targeted therapies or immunotherapies should be investigated, especially in future trials that incorporate maintenance systemic therapy. Last, even if an effect is demonstrated on the early surrogate end point, patients should still be followed up, as early end points will not capture long-term treatment-related toxicities and deaths, which are of major relevance in this disease given its high survival rates.
In conclusion, this individual patient data validation of surrogate end points in localized nasopharyngeal carcinoma treated with radiotherapy and chemotherapy demonstrates that PFS and DMFS are strong surrogates for OS. Two-year PFS could be used as the primary end point in the design of future randomized trials to speed up the research and lower the associated costs. Whatever the surrogate end point chosen, OS should still be measured and reported at a meaningful time point, such as five years, to detect possible long-term detrimental effects.
Funding
This work was supported by grants from the French Ministry of Health (Programme d’actions integrees de recherche VADS), Ligue Nationale Contre le Cancer, and the French National Cancer Institute (SHS 2014-141). The INT0099 (SWOG 8892) trial was supported by the National Cancer Institute, National Institutes of Health (CA180888 and CA180819). The HeCOG trial was supported by the Hellenic Cooperative Oncology Group (HE R_5G).
Notes
The sponsors did not participate in the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or in the decision to submit the manuscript for publication. We thank Francoise Delassus for her secretarial assistance.
Investigators of the MAC-NPC Collaborative Group: Muhyi Al-Sarraf (Wm. Beaumont Hospital, Royal Oak, MI), Bertrand Baujat (Hopital Tenon, Paris, France), Pierre Blanchard (Gustave Roussy, Villejuif, France), Ellen Benhamou (Gustave Roussy, Villejuif, France), Jean Bourhis (CHUV, Lausanne, Switzerland), Anthony T. C. Chan (Partner State Key Laboratory of Oncology in South China, Sir YK Pao Centre For Cancer, The Chinese University of Hong Kong, China), Lai-Kwan Chan (Pamela Youde Nethersole Eastern Hospital, Hong Kong, China), Shie Lee Cheah (National Cancer Centre Singapore, Singapore), Lei Chen (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Qiu-Yan Chen (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Yong Chen (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Kwan-Hwa Chi (Shin Kong Wu Ho-Su Memorial Hospital, Tapei, Taiwan), Richard J. Chappell (University of Wisconsin, Madison, WI), Daniel T. T. Chua (Hong Kong Sanatorium and Hospital, Hong Kong, China), George Fountzilas (Aristotle University of Thessaloniki School of Medicine, Thessaloniki, Greece), Georgia Gourgioti (Hellenic Cooperative Oncology Group, Athens, Greece), Masuto Hareyama (Sapporo Medical University, Sapporo, Japan), Ming-Huang Hong (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Pei-Yu Huang (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Edwin Pun Hui (Partner State Key Laboratory of Oncology in South China, Sir Y. K. Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong, China), Michael Kam (Partner State Key Laboratory of Oncology in South China, Sir Y. K. Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong, China), Dora L. W. Kwong (Queen Mary Hospital, Hong Kong, China), Ka On Lam (Queen Mary Hospital, Hong Kong, China), Julie Leclercq (Gustave Roussy, Villejuif, France), Anne Lee (Pamela Youde Nethersole Eastern Hospital, Hong Kong, China), Ho Fun Victor Lee (Li Ka Shing Faculty of Medicine, Hong Kong, China), Tai-Xiang Lu (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Béranger Lueza (Gustave Roussy, Villejuif, France), Brigette Ma (Partner State Key Laboratory of Oncology in South China, Sir Y. K. Pao Centre For Cancer, The Chinese University of Hong Kong, China), Jun Ma (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Hai-Qiang Mai (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Sophie Marguet (Gustave Roussy, Villejuif, France), Stefan Michiels (Gustave Roussy, Villejuif, France), Frankie Mo (Partner State Key Laboratory of Oncology in South China, Sir Y. K. Pao Centre For Cancer, The Chinese University of Hong Kong, China), James Moon (SWOG Statistical Center, Seattle, WA), Wai Tong Ng (Pamela Youde Nethersole Eastern Hospital, Hong Kong, China), Roger Ngan (Queen Elizabeth Hospital, Hong Kong, China), Brian O’Sullivan (Princess Margaret Hospital, Toronto, Canada), Jean Pierre Pignon (Gustave Roussy, Villejuif, France), Jonathan Sham (The University of Hong Kong, Hong Kong, China), Yoke Lim Soong (National Cancer Centre, Singapore, Singapore), Yuk Tung (Tuen Mun Hospital, Hong Kong, China), Joseph Wee (National Cancer Centre, Singapore, Singapore), Xuang Wu (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Tingting Xu (Fudan University Shanghai Cancer Center, Shanghai, P. R. China), Li Zhang (Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China), Guopei Zhu (Fudan University Shanghai Cancer Center, Shanghai, P. R. China).
Authors: Federico Rotolo, Jean-Pierre Pignon, Jean Bourhis, Sophie Marguet, Julie Leclercq, Wai Tong Ng, Jun Ma, Anthony T. C. Chan, Pei-Yu Huang, Guopei Zhu, Daniel T. T. Chua, Yong Chen, Hai-Qiang Mai, Dora L. W. Kwong, Yoke Lim Soong, James Moon, Yuk Tung, Kwan-Hwa Chi, George Fountzilas, Li Zhang, Edwin Pun Hui, Anne W. M. Lee, Pierre Blanchard*, Stefan Michiels*; on behalf of the MAC-NPC Collaborative Group
*Authors contributed equally to this work.
Affiliations of authors: Service de Biostatistique et d’Epidémiologie, Gustave Roussy Cancer Campus, Villejuif, France (FR, JPP, SM, JL, PB, SM); INSERM U1018, CESP, Université Paris-Sud, Université Paris-Saclay, Villejuif, France (FR, JPP, SM, PB, SM); Ligue Nationale Contre le Cancer Meta-Analysis Platform, Gustave Roussy Cancer Campus, Villejuif, France (FR, JPP, JL, SM); Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland (JB); Pamela Youde Nethersole Eastern Hospital, Hong Kong, China (WTN, AWML); State Key Laboratory of Oncology in South China, Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China (JM, PYH, YC); Sir YK Pao Centre for Cancer, The Chinese University of Hong Kong, Hong Kong, China (ATCC, EPH); Shanghai Ninth People's Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, P. R. China (GZ); Hong Kong Sanatorium and Hospital, Hong Kong, China (DTTC); Sun Yat-Sen University Cancer Center, Guangzhou, P. R. China (HQM, LZ); Queen Mary Hospital, Hong Kong, China (DLWK); National Cancer Centre, Singapore, Singapore (YLS); SWOG Statistical Center, Seattle, WA (JM); Tuen Mun Hospital Hong Kong, China (YT); Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan (KHC); Aristotle University of Thessaloniki School of Medicine, Thessaloniki, Greece (GF); Department of Radiation Therapy, Gustave Roussy, Villejuif, France (PB).
Supplementary Material
References
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Accessed August 11, 2016.
- 2. Blanchard P, Lee A, Marguet S, et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: An update of the MAC-NPC meta-analysis. Lancet Oncol. 2015;166:645–655. [DOI] [PubMed] [Google Scholar]
- 3. Michiels S, Le Maître A, Buyse M, et al. Surrogate endpoints for overall survival in locally advanced head and neck cancer: Meta-analyses of individual patient data. Lancet Oncol. 2009;104:341–350. [DOI] [PubMed] [Google Scholar]
- 4. Mauguen A, Pignon JP, Burdett S, et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re-analysis of meta-analyses of individual patients’ data. Lancet Oncol. 2013;147:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chua MLK, Wee JTS, Hui EP, Chan ATC.. Nasopharyngeal carcinoma. Lancet. 2016;38710022:1012–1024. [DOI] [PubMed] [Google Scholar]
- 6. Baujat B, Audry H, Bourhis J, et al. Chemotherapy in locally advanced nasopharyngeal carcinoma: An individual patient data meta-analysis of eight randomized trials and 1753 patients. Int J Radiat Oncol. 2006;641:47–56. [DOI] [PubMed] [Google Scholar]
- 7. Xu T, Zhu G, He X, Ying H, Hu C.. A phase III randomized study comparing neoadjuvant chemotherapy with concurrent chemotherapy combined with radiotherapy for locoregionally advanced nasopharyngeal carcinoma: Updated long-term survival outcomes. Oral Oncol. 2014;502:71–76. [DOI] [PubMed] [Google Scholar]
- 8. Lee AWM, Tung SY, Chan ATC, et al. A randomized trial on addition of concurrent-adjuvant chemotherapy and/or accelerated fractionation for locally-advanced nasopharyngeal carcinoma. Radiother Oncol. 2011;981:15–22. [DOI] [PubMed] [Google Scholar]
- 9. Kwong DLW. Concurrent and adjuvant chemotherapy for nasopharyngeal carcinoma: A factorial study. J Clin Oncol. 2004;2213:2643–2653. [DOI] [PubMed] [Google Scholar]
- 10. Fountzilas G, Ciuleanu E, Bobos M, et al. Induction chemotherapy followed by concomitant radiotherapy and weekly cisplatin versus the same concomitant chemoradiotherapy in patients with nasopharyngeal carcinoma: A randomized phase II study conducted by the Hellenic Cooperative Oncology Group. Ann Oncol. 2012;232:427–435. [DOI] [PubMed] [Google Scholar]
- 11. Al-Sarraf M, LeBlanc M, Giri PG, et al. Chemoradiotherapy versus radiotherapy in patients with advanced nasopharyngeal cancer: Phase III randomized Intergroup study 0099. J Clin Oncol. 1998;164:1310–1317. [DOI] [PubMed] [Google Scholar]
- 12. Chan ATC, Teo PML, Leung TWT, et al. A prospective randomized study of chemotherapy adjunctive to definitive radiotherapy in advanced nasopharyngeal carcinoma. Int J Radiat Oncol. 1995;333:569–577. [DOI] [PubMed] [Google Scholar]
- 13. Chua DTT, Sham JST, Choy D, et al. Preliminary report of the asian-oceanian clinical oncology association randomized trial comparing cisplatin and epirubicin followed by radiotherapy versus radiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma. Cancer. 1998;8311:2270–2283. [PubMed] [Google Scholar]
- 14. International Nasopharynx Cancer Study Group: VUMCA I Trial. Preliminary results of a randomized trial comparing neoadjuvant chemotherapy (cisplatin, epirubicin, bleomycin) plus radiotherapy vs radiotherapy alone in stage IV (≥N2, M0) undifferentiated nasopharyngeal carcinoma. Int J Radiat Oncol. 1996;353:463–469. [DOI] [PubMed] [Google Scholar]
- 15. Hareyama M, Sakata K, Shirato H, et al. A prospective, randomized trial comparing neoadjuvant chemotherapy with radiotherapy alone in patients with advanced nasopharyngeal carcinoma. Cancer. 2002;948:2217–2223. [DOI] [PubMed] [Google Scholar]
- 16. Chi KH, Chang YC, Guo WY, et al. A phase III study of adjuvant chemotherapy in advanced nasopharyngeal carcinoma patients. Int J Radiat Oncol. 2002;525:1238–1244. [DOI] [PubMed] [Google Scholar]
- 17. Chan ATC, Leung SF, Ngan RKC, et al. Overall survival after concurrent cisplatin-radiotherapy compared with radiotherapy alone in locoregionally advanced nasopharyngeal carcinoma. Journal Natl Cancer Inst. 2005;977:536–539. [DOI] [PubMed] [Google Scholar]
- 18. Wee J. Randomized trial of radiotherapy versus concurrent chemoradiotherapy followed by adjuvant chemotherapy in patients with American Joint Committee on Cancer/International Union Against Cancer Stage III and IV nasopharyngeal cancer of the endemic variety. J Clin Oncol. 2005;2327:6730–6738. [DOI] [PubMed] [Google Scholar]
- 19. Lee AWM, Tung SY, Chua DTT, et al. Randomized trial of radiotherapy plus concurrent-adjuvant chemotherapy vs radiotherapy alone for regionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst. 2010;10215:1188–1198. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Huang PY, Peng PJ, et al. Long-term follow-up of a phase III study comparing radiotherapy with or without weekly oxaliplatin for locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2013;248:2131–2136. [DOI] [PubMed] [Google Scholar]
- 21. Hui EP, Ma BB, Leung SF, et al. Randomized phase II trial of concurrent cisplatin-radiotherapy with or without neoadjuvant docetaxel and cisplatin in advanced nasopharyngeal carcinoma. J Clin Oncol. 2009;272:242–249. [DOI] [PubMed] [Google Scholar]
- 22. Huang PY, Cao KJ, Guo X, et al. A randomized trial of induction chemotherapy plus concurrent chemoradiotherapy versus induction chemotherapy plus radiotherapy for locoregionally advanced nasopharyngeal carcinoma. Oral Oncol. 2012;4810:1038–1044. [DOI] [PubMed] [Google Scholar]
- 23. Chen Y, Sun Y, Liang SB, et al. Progress report of a randomized trial comparing long-term survival and late toxicity of concurrent chemoradiotherapy with adjuvant chemotherapy versus radiotherapy alone in patients with stage III to IVB nasopharyngeal carcinoma. Cancer. 2013;11912:2230–2238. [DOI] [PubMed] [Google Scholar]
- 24. Chen QY, Wen YF, Guo L, et al. Concurrent chemoradiotherapy vs radiotherapy alone in stage II nasopharyngeal carcinoma: Phase III randomized trial. J Natl Cancer Inst. 2011;10323:1761–1770. [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Hu CS, Chen XZ, et al. Concurrent chemoradiotherapy plus adjuvant chemotherapy versus concurrent chemoradiotherapy alone in patients with locoregionally advanced nasopharyngeal carcinoma: A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2012;132:163–171. [DOI] [PubMed] [Google Scholar]
- 26. Buyse M. The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics. 2000;11:49–67. [DOI] [PubMed] [Google Scholar]
- 27. Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: Individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;2334:8664–8670. [DOI] [PubMed] [Google Scholar]
- 28. Buyse M, Burzykowski T, Carroll K, et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;2533:5218–5224. [DOI] [PubMed] [Google Scholar]
- 29. Burzykowski T, Buyse M, Piccart-Gebhart MJ, et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol. 2008;2612:1987–1992. [DOI] [PubMed] [Google Scholar]
- 30. Oba K, Paoletti X, Alberts S, et al. Disease-free survival as a surrogate for overall survival in adjuvant trials of gastric cancer: A meta-analysis. J Natl Cancer Inst. 2013;10521:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paoletti X, Oba K, Bang YJ, et al. Progression-free survival as a surrogate for overall survival in advanced/recurrent gastric cancer trials: A meta-analysis. J Natl Cancer Inst. 2013;10521:1667–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burzykowski T, Molenberghs G, Buyse M.. The Evaluation of Surrogate Endpoints. New York: Springer; 2005. [Google Scholar]
- 33. Burzykowski T, Molenberghs G, Buyse M, Geys H, Renard D.. Validation of surrogate end points in multiple randomized clinical trials with failure time end points. J R Stat Soc C. 2001;504:405–422. [Google Scholar]
- 34. Hougaard P. Analysis of Multivariate Survival Data. New York: Springer; 2000. [Google Scholar]
- 35. van Houwelingen HC, Arends LR, Stijnen T.. Advanced methods in meta-analysis: Multivariate approach and meta-regression. Stat Med. 2002;214:589–624. [DOI] [PubMed] [Google Scholar]
- 36. Burzykowski T, Buyse M.. Surrogate threshold effect: An alternative measure for meta-analytic surrogate endpoint validation. Pharm Stat. 2006;53:173–186. [DOI] [PubMed] [Google Scholar]
- 37. Fleming TR. Surrogate end points in clinical trials: Are we being misled? Ann Intern Med. 1996;1257:605. [DOI] [PubMed] [Google Scholar]
- 38. Prentice RL. Surrogate endpoints in clinical trials: Definition and operational criteria. Stat Med. 1989;84:431–440. [DOI] [PubMed] [Google Scholar]
- 39. Chen YP, Sun Y, Chen L, et al. Surrogate endpoints for overall survival in combined chemotherapy and radiotherapy trials in nasopharyngeal carcinoma: Meta-analysis of randomised controlled trials. Radiother Oncol. 2015;1162:157–166. [DOI] [PubMed] [Google Scholar]
- 40. Blanchard P, Ribassin-Majed L, Lee A, Pignon JP.. Chemoradiotherapy regimens for locoregionally advanced nasopharyngeal carcinoma: A Bayesian network meta-analysis. Eur J Cancer. 2015;51:1570–1579, comment: 2016;56:183–5. [DOI] [PubMed] [Google Scholar]
- 41. Parmar MKB, Torri V, Stewart L.. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;1724:2815–2834. [DOI] [PubMed] [Google Scholar]
- 42. Guyot P, Ades A, Ouwens MJ, Welton NJ.. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;121:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan OSH, Ngan RKC.. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol. 2014;509:791–797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.