Abstract
Embodied theories of cognition emphasize the central role of sensorimotor transformations in the representation of others’ actions. Support for these theories is derived from the discovery of the mirror neuron system (MNS) in primates, from noninvasive techniques in humans, and from a limited number of intracranial studies. To understand the neural dynamics of the human MNS, more studies with precise spatial and temporal resolutions are essential. We used electrocorticography to define activation patterns in sensorimotor, parietal and/or frontal neuronal populations, during a viewing and grasping task. Our results show robust high gamma activation for both conditions in classic MNS sites. Furthermore, we provide novel evidence for 2 different populations of neurons: sites that were only active for viewing and grasping (“pure mirroring”) and sites that were also active between viewing and grasping, and perhaps serve a more general attentional role. Lastly, a subgroup of parietal electrodes showed earlier peaks than all other regions. These results highlight the complexity of spatial-temporal patterns within the MNS and provide a critical link between single-unit research in monkeys and noninvasive techniques in human.
Keywords: ECoG, imitation, mirror neurons, motor simulation
Introduction
Embodied theories of cognition emphasize the central role of “sensorimotor transformations,” a correspondence between sensory and motor domains, in the representation and understanding of actions and emotions of others. These theories claim that automatic simulation is required to understand others’ actions. Support for these theories has been provided by reports of mirror neurons in nonhuman primates. These neurons are modulated both when an individual executes a specific motor action and when they observe the same or similar action performed by another individual. Their discovery in the macaque monkey 2 decades ago prompted the notion that action execution and observation are closely related processes, and indeed that our ability to interpret the actions of others requires the involvement of our own “mirroring” motor system (Rizzolatti and Fabbri-Destro 2008; Kilner and Lemon 2013).
Since the discovery of the monkey MNS, there has been much speculation over the existence of mirror neurons in humans and what possible functional role they might play. For instance, the human mirror neuron system (hMNS) has been linked to numerous aspects of human cognition, including motor learning (Stefan et al. 2008), speech perception (Rizzolatti and Arbib 1998), language development (Arbib 2005), and formation of key social skill (Iacoboni et al. 2005; Gallese 2007). Additionally, dysfunction to this network has been suggested to be the basis of different social deficits, such as those seen in Autism Spectrum Disorder (Oberman et al. 2005; Rizzolatti et al. 2009; Rizzolatti and Fabbri-Destro 2010).
Studies using noninvasive recording techniques support the existence of a putative MNS in humans, using functional brain imaging (for reviews see Fabbri-Destro and Rizzolatti 2008; Morin and Grèzes 2008), transcranial magnetic stimulation (Fadiga et al. 1995), magnetoencephalography (MEG) (for reviews see Nishitani and Hari 2000; Hari 2006), and electroencephalography (EEG) (Cochin et al. 1999; Muthukumaraswamy et al. 2004; Perry and Bentin 2009; for a review see Pineda 2005). It was originally suggested that the putative hMNS is a frontal-parietal network (Rizzolatti and Craighero 2004; Filimon et al. 2007) with later studies adding somatosensory and motor cortices as an extended part of the network for action understanding and imitation (Pineda 2008; Keysers and Gazzola 2009; Keysers et al. 2010).
However, while noninvasive techniques enable defining general brain regions with “mirror” properties active for both viewing and performing a motor action, to fully understand the neural dynamics of the hMNS, methods with more precise spatial and temporal information are required. Direct intracranial cortical recordings (electrocorticography [ECoG]) offer a unique opportunity to acquire neural signals with an unprecedented combination of temporal and spatial resolution. To our knowledge, there is one single-unit recording study which provided clear evidence for the existence of mirror neurons in humans (Mukamel et al. 2010). However, this study recorded from medial areas, and could not provide information about the existence and/or the neural dynamics of a lateral system, similar to the one found in monkeys. Two recent ECoG studies focused on properties of low-frequency rhythms in MNS regions (Halje et al. 2015; Babiloni et al. 2016), but did not fully analyze the high gamma (HG) signal that indexes task-related activity of neural populations directly underneath the ECoG electrodes, enabling a more accurate picture of the spatial and temporal distributions of the hMNS (Crone et al. 1998, 2006; Miller et al. 2007; Schalk et al. 2007). An additional ECoG study that examined HG and compared it with suppression in lower frequencies, focused solely on the premotor cortex during viewing, with no grasping condition or analyses of other regions in this network (Caruana et al. 2014). A recent study looked at local field potentials (LFPs) recorded in the premotor and primary motor cortex of 2 macaque monkeys (Waldert et al. 2015). The authors showed that the low-frequency LFP can be modulated during action observation in both cortical areas and that temporal modulations in LFP are correlated for execution versus observation. Lastly, Alegre et al. (2010) used deep brain stimulation electrodes to directly record basal ganglia subthalamic nucleus (STN) oscillations in patients with Parkinson's. They report bilateral beta reduction in subthalamic power and cortico-STN coherence during both movement and movement observation, suggesting subcortical hMNS activation (Alegre et al. 2010; see also Marceglia et al. 2009). These studies both in humans and in monkeys are crucial for bridging the gap between monkey single-unit recordings and noninvasive studies in humans.
In the present study, we utilized the spatial and temporal advantages of ECoG (with a high sampling rate of 1000 Hz or above) and its high quality of signal, to study activation patterns of neuronal populations in mirror neuron regions. We tested 7 participants with intractable epilepsy, who had been implanted with electrode grids over the right or left sensorimotor, parietal and/or frontal cortices for preoperative monitoring. Patients viewed grasping actions toward different objects, and following a waiting period of 2 s, had to perform the same actions (see Methods). Our results show robust HG activation in the expected mirror neuron regions: parietal, inferior frontal gyrus (IFG), motor, and somatosensory cortices. Within each of the 4 regions, complex activation patterns emerged. We found mirroring sites that were adjacent to pure grasping or viewing sites. We also differentiated between 2 reoccurring patterns: sites that were active only for viewing and grasping and were silent in between these task components; and sites that were active in between viewing and grasping providing a bridge between them. Lastly, a subgroup of parietal electrodes showed reliable earlier peaks than all other regions, while all other regions peaked together, around the viewing of the grasp. We confirm the existence of a human mirror neuron network with high temporal and spatial resolution focused on local cortical activation manifested in the HG frequency band. These results help bridge the gap between monkey single cell recordings and human noninvasive studies of the MNS, and highlight the complexity of the spatial and temporal patterns within this network.
Materials and Methods
Participants
ECoG signals were recorded from a total of 7 subjects (S1–S7); this consisted of 2 male and 5 female participants who were undergoing intraoperative neurosurgical treatment for refractory epilepsy—3 at University of California Irvine Hospital (S4, age 35; S5, age 48; S6, age 52), 3 at the University of California San Francisco Hospital (S1, age 23; S2 age 29; S3, age 36), and 1 at Stanford University Hospital (S7, age 23). All medical treatment decisions, including the size and location of the craniotomy site and electrode positioning, were dictated solely by the clinical needs of the patient. Patients were informed that administered tasks were for research purposes only. Written informed consented to participate in the study was obtained from each patient by the medical staff prior to testing, and was verbally confirmed throughout the experiment. All testing was performed at bedside in the postoperative monitoring rooms. The experimental protocol was approved by each site's Institutional Review Boards and Committees on Human Research, and the experiment was performed in accordance to the sites’ guidelines and regulations.
Electrode Locations
Recordings were obtained from patients with refractory epilepsy who had electrode grids or strips implanted for 4–10 days to localize seizure foci and perform cortical stimulation mapping. Electrodes were located over prefrontal, motor, temporal, or parietal areas of either the right or left hemisphere. One participant was implanted with a 16 × 16 electrode grid (4 mm spacing) over the right fronto-parietal cortex, 4 subjects has 8 × 8 grids (1 cm spacing) over the right fronto-parietal cortex as well as electrode strips covering other regions, and 2 subjects has 8 × 8 grids (1 cm spacing) over the left fronto-parietal cortex in addition to several strips. All grids included the sensorimotor cortex (see Supplementary Table S1 for patient information, Figure S1 for all coverage collapsed on a Montreal Neurological Institute (MNI) brain, and Figure S2 for specific coverage information for each patient). Electrodes were classified by a neurologist according to anatomical location (IFG, parietal lobe, motor and premotor cortex, sensorimotor cortex) within each subject's anatomical space. Electrodes were excluded from the data if they showed 60 Hz line noise, epileptic activity, or other artifacts such as excessive noise due to poor contact. All epochs with spread of epileptic activity from the primary epileptic site were excluded from analysis. Each participant's preoperative magnetic resonance imaging and postimplantation computed tomography (CT) were used to reconstruct electrode location. Affine point-based registration was applied with BioImage Suite to transform CT coordinates to magnetic resonance (MR) space to localize electrodes on the MR. Final coregistered images were assessed for anatomical accuracy. While analysis was done separately for each subject, brains and electrodes were transformed into MNI space across subjects for presentation purposes.
Experimental Design
Task
Participants were seated ~60 cm from a computer screen, with 3 objects (a cup, bottle, and pencil) placed on a tray near them. On each trial, the participant heard an auditory signal (200 ms) after which a background image appeared for 1200 ms from which the baseline was taken from, followed by a 2000 ms clip of a hand grasping 1 of the 3 objects. This followed by a 2400 ms waiting period, followed by another 200 ms auditory signal, signaling the patient to imitate the same action toward the same object as accurately as possible (see Supplementary Figure S3 for a visualization of the design). The next trial began between 7000 and 7500 ms after the auditory signal, enabling enough time to perform the motor act and return to rest. The hand (right/left) that the participant viewed, and used for imitation, was always the hand contralateral to the grid (i.e., if the grid was on the right hemisphere, the participant saw a left hand and acted with their left hand throughout the experiment). There were 40 repetitions of View-Wait-Grasp trials in each block, and the block was repeated between 1 and 3 times, depending on participant's willingness to continue the testing. Five of the 7 participants completed 2 blocks, 1 completed 1 block (S2) and 1 completed 3 blocks (S6).
Stimuli
The experimental stimuli consisted of 2000 ms long video clips presenting a right or left hand of a male or a female reaching toward an object and grasping it (adapted from Perry and Bentin 2009). A small white square appeared in the bottom left corner of the screen simultaneously with any change in stimuli. This change in luminosity was detected by the photodiode and was synchronized and recorded along with ECoG signals. E-Prime2 was used for data presentation and response recording.
Control Tasks
Three additional patients were run with the imitation task and additional control tasks. Since one of these patients (S10) had a frontal lesion, we focused on parietal sites in this patient.
Nonbiological Motion Control Task
Two patients were run with an additional nonbiological motion task, to examine whether mirror sites were specific for biological motion stimuli. Participants were seated ~60 cm from a computer screen, with 3 objects (a cup, bottle, and pencil) placed on a tray near them (intended to make this an identical setting to the original study, but not used for this task). On each trial, the participant heard an auditory signal (200 ms) after which a background image appeared for 1200 ms from which the baseline was taken, followed by a 2000 ms clip of a tennis ball rolling from the left or from the right. The background was identical to the one used in the imitation clips. This was followed by a 2400 ms waiting period, and another auditory signal, directing the patient to indicate (using the keyboard) whether the ball had rolled left or right. We analyzed the viewing portion, in an identical manner to the analysis of the imitation study (see below).
Passive Viewing (Nonimitation) Control Task
Two patients participated in a passive viewing task, in which they watched the original viewing and grasping task, and were instructed to simply pay attention to the stimuli on the screen. This allowed to differentiate between mirror sites that are active for viewing goal-directed actions in general versus those specific for later imitation. These patients did the passive viewing task first, in order to avoid being influenced by the imitation instructions. We analyzed the viewing portion, in an identical manner to the analysis of the imitation study (see below).
Results for these 3 additional patients can be found in the Supplementary Figure S7.
ECoG Recordings
Cortical and peripheral signals were acquired either using a Tucker Davis Technologies recording system, containing a 256-channel amplifier and Z-series digital signal processing board (UCSF and Stanford) or a Nihon Kohden recording system, with a JE-120A amplifier, 128 channels (UC Irvine). ECoG and photodiode channels were sampled at 24 414.1 and 3051.8 Hz at the UCSF site, 1525.88 and 24 414 Hz at the Stanford University site, and were both 5000 Hz at the UC Irvine site.
Data Analysis
Behavioral
Five subjects had deidentified video or motion tracking information (used for a different task) that allowed us to examine whether and when the subject was performing the task correctly, and to remove any incorrect trials, or trials in which the participant moved during the view or wait periods. The participants that were not filmed were nonetheless closely monitored, and viewing or resting trials in which the participant was seen moving were dismissed from further analysis. Onset times for each event were identified at stimulus presentation using the photodiode channel. Events (View, Wait, or Grasp) were excluded from analysis if any time from 100 ms before onset to offset overlapped with any artifact (see below), or if the subject moved. The total number of trials used for each patient for data analysis was as follows: S1, 74 view, 72 wait, 72 grasp; S2, 32 view, 31 wait, 29 grasp; S3, 68 view, 67 wait, 76 grasp; S4, 48 view, 77 wait, 49 grasp; S5, 73 view, 73 wait, 73 grasp; S6, 117 view, 117 wait, 115 grasp; S7, 62 view, 58 wait, 68 grasp.
ECoG
All ECoG data were first resampled to 1000 Hz, low-pass filtered at 180 Hz, high-pass filtered at 0.5 Hz, and notch-filtered at 60 Hz and its harmonics. ECoG data were then examined by a neurologist to identify channels with ictal or peri-ictal epileptiform activity and other artifacts. Channels and epochs contaminated by epileptiform activity or abnormal signal (e.g., poor contact, excess drift, high-frequency noise) and those located over tissue that was later resected were removed from analyses. Data processing used custom functions written in MATLAB and the EEGLAB toolbox (Delorme and Makeig 2004). Continuous ECoG data were rereferenced to a common average reference (i.e., mean of artifact-free channels).
Cortical activation was indexed by quantifying changes in analytic amplitude within the HG frequency range (70–150 Hz) using the filtfilt function in Matlab and a subsequent Hilbert transform; 8–13 and 15–25 Hz were used for the alpha and beta bands, respectively. HG signal (as well as alpha and beta suppression) was calculated relative to (% change) a 400-ms prestimulus baseline (−600 to −200 from View onset) for each trial per electrode in a given block. HG signals were analyzed as averages across trials as well as on a single-trial basis.
In order to identify periods of significant HG activation for each channel, one-sample t-tests were performed on 1 ms windows for each electrode per condition, comparing the mean baseline corrected HG signal to 0. The false discovery rate (FDR) correction (with alpha levels set at 0.05) was applied to each channel in order to account for multiple comparisons, and only those channels that showed at least 100 ms of consecutive significant HG signal that was greater than a 10% difference from baseline were classified as active for any given condition. Manual visual inspection of single trial and average traces was used to ensure that the identified activation was not spurious. Electrodes without significant increases in HG power were considered inactive and were not analyzed further (an identical method was used for finding significant alpha/mu or beta suppression, which are not the main focus of the paper, see Supplementary Figure S4).
Defining “Pure Mirror Sites” and “Mnemonic Mirror Sites”
Within sites that were active both for viewing and for grasping, we further distinguished between 2 HG mirroring patterns. The first pattern (pure mirroring sites) consisted of sites that had significant activation during the viewing period that decreased to baseline during the waiting period, and then showed significant activation again during grasping. The second pattern (mnemonic mirroring sites) consisted of sites that were active for all 3 conditions: viewing, waiting, and grasping.
Latency Analysis
In order to compare the timing of activation between regions, we ran the following analyses: onset and offset of activation, average peak latency, and single trial peak latency. Onset and offset of activation: onsets and offsets of HG activity were computed by taking the first and last time sample that passed significance. Next, we functionally separated all electrodes into “early” (onset <818 ms) and “late” (onset >818 ms). The cutoff, 818 ms, was determined by taking the average time where the hand in the different videos began the grasping motion. Peak latency: average peak activation was defined for the viewing and grasping conditions as the maximum amplitude in the HG range during that condition for each electrode. An analysis of variance was conducted on the latencies of peak neural activity for each functional group (early, late) comparing latencies between parietal, IFG, motor, and somatosensory regions (for a similar analysis, see Flinker et al. 2015).
Peak Latency Single Trials Analysis for View and Grasp
To verify the temporal-spatial patterns that we observed in averaged cortical responses, we ran an ANOVA separately within each of the 2 subjects with full coverage of all 4 regions “focusing on the single-trial level” (S1 and S5). Having 2 patients with coverage of all ROIs enabled us to run a similar analysis for the Grasping condition, to examine if the temporal pattern for Grasping matched that of the Viewing condition. Note that the patients grasping times were not identical, and so running an analysis that compares timing between regions with all patients together would not provide a reliable account of temporal patterns between regions. Hence, a comparison between regions was done only for the 2 patients that enabled such analysis, focusing on single trials within subjects. We estimated single trial peaks for each electrode in each trial (maximum amplitude in the predefined window of the relevant condition, 2000 ms for view and 3000 ms for grasp). As stated earlier, some of the grasping electrodes showed a peak of activity at time 1, and inspection of these electrodes showed a clear peak for the auditory onset. In these cases, the second peak was chosen as depicting the grasping. We then averaged the single trial peaks over early or late electrodes from each region and conducted a repeated-measure ANOVA using the average peak of the single trials, with region as the within subject variable.
Event-Related Spectral Perturbations (Figs 1 and S5)
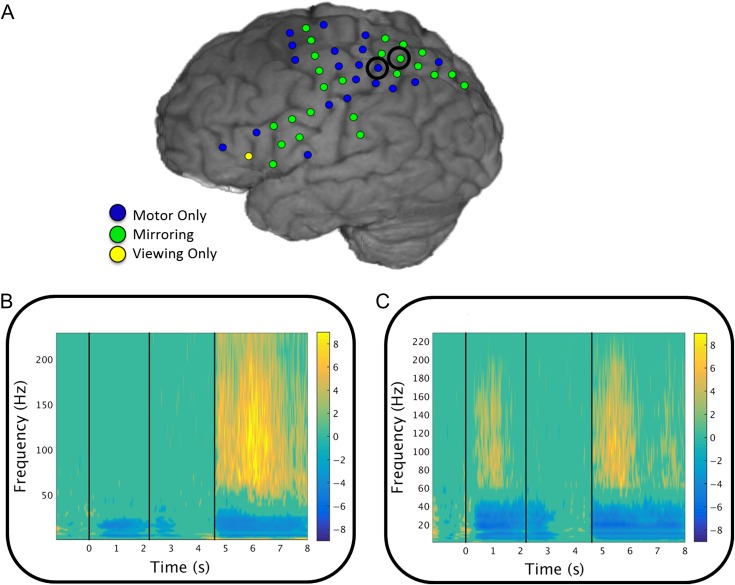
Figure 1.
Significant HG activation. (A) Activation in the 4 regions of interest in a representative subject (S5) with “mirror” electrodes showing significant activation for both viewing and grasping (green), next to electrodes showing significant activation only for grasping (blue) or only for viewing (one frontal in yellow). Electrodes plotted in (B) and (C) are marked with a black circle. (B) ERSPs, averaged across trials in a grasping electrode (vertical lines mark the viewing, waiting, and grasping onsets). (C) ERSPs, averaged across trials in a viewing + grasping (“mirror”) electrode (vertical lines mark the viewing, waiting, and grasping onsets).
Event-related spectral perturbations (ERSPs) were calculated for each electrode. Forty-four logarithmically equally spaced frequency bands were created from 1 to 250 Hz. The instantaneous power time series was calculated for each frequency band and ERSPs were created for each time series by averaging the power time series across all trials from that electrode. A bootstrapped distribution of baseline values was created by randomly choosing N (= number trials/condition) baseline values and averaging across the time bins (for 1000 iterations). All time and frequency significance values were corrected for multiple comparisons using an FDR correction (q = 0.05). The yellow and blue portions of each graph are at least 2 standard deviations from the mean of the baseline.
Results
We used increases in the HG band (70–150 Hz) to measure task-related neural activation in parietal, IFG, motor, and somatosensory regions. There was significant HG activity in hMNS regions across all subjects. All regions of interest showed a complex pattern of activations in which significant HG activity for both viewing and grasping (i.e., “mirror” sites) was interleaved with sites that were active just for grasping or just for viewing (see Fig. 1A and Supplementary Table S2 and Figure S2). As expected, the 2 auditory cues, signaling the viewing and grasping conditions, elicited strong and consistent brief HG activations in auditory cortical sites (see Supplementary Figure S5 for a map of auditory activations, and an example of the robust HG signal elicited by the cue). For the grasping condition, HG activation that was found in the first 100 ms following the cue was marked as auditory and not motor.
Furthermore, although not the main focus of the current paper, we also compared the HG activation with suppression in lower alpha/mu (8–13 Hz) and beta (15–25) frequencies, responses typically seen in EEG/MEG. Both bands showed strong suppression that was focused around somatosensory and motor regions in accord with EEG/MEG studies and with Babiloni et al. (2016). For a descriptive measures of the lower frequencies and their locations, see Supplementary Figure S4.
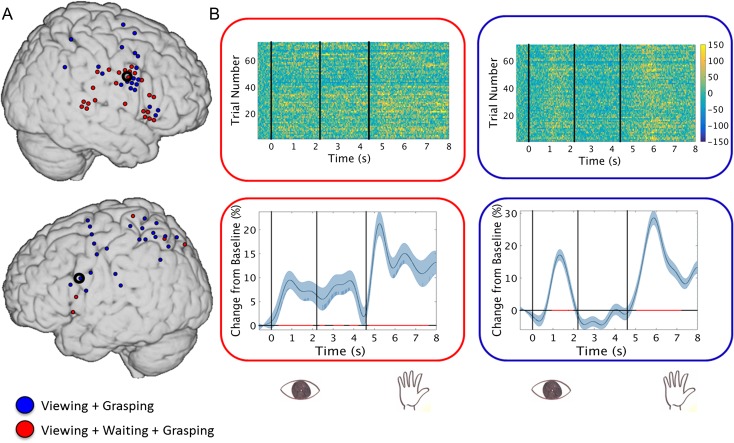
We observed 2 distinct HG mirroring patterns. The first pattern consisted of sites that had significant activation during the viewing period that decreased to baseline during the waiting period, and then showed significant activation again during grasping. We also found sites that were active for all 3 conditions: viewing, waiting, and grasping. The distribution of the 2 patterns is shown in Figure 2A separately for each hemisphere, and representative examples of the 2 patterns are shown in Figure 2B (see Supplementary Figure S6 for more examples from all regions). While all regions had sites showing both patterns, the majority of responsive mirror sites in the motor cortex showed “pure mirroring” (76% [19/25] showed the “pure mirroring” pattern; χ2 = 6.76, P = 0.009) while there was no significant difference in distribution in the other 3 sites (“pure mirroring” sites consisted of 62.5% [10/16] of somatosensory mirror sites, 53% [9/17] in parietal sites and 47% [7/16] in IFG; all P > 0.250). We observed a greater percentage of active “mnemonic” mirroring electrodes in the right hemisphere (24/46; 52% of all mirroring electrodes), that is, when the subjects had to watch, and perform the task with, their nondominant hand, than in the left hemisphere (5/29; 17%; Fisher's exact test, P < 0.005).
Figure 2.
(A) The distribution of mirror electrodes from the 4 regions of interest, divided into “pure mirroring” electrodes, which showed significant activations only for viewing and grasping (blue) and “mnemonic” electrodes that showed significant activation during viewing, waiting, and grasping (red), on an MNI brain collapsed across all participants. (B) Vertically stacked single trials in a “mnemonic mirroring” electrode (top left) and in a “pure mirroring” electrode (top right); and the corresponding HG trace and SE for the electrode seen above it (bottom left and right, smoothed at 2 Hz for presentation purposes). Red line on X-axis depicts significant intervals. See Supplementary Figure S4 for more examples from all regions of interest.
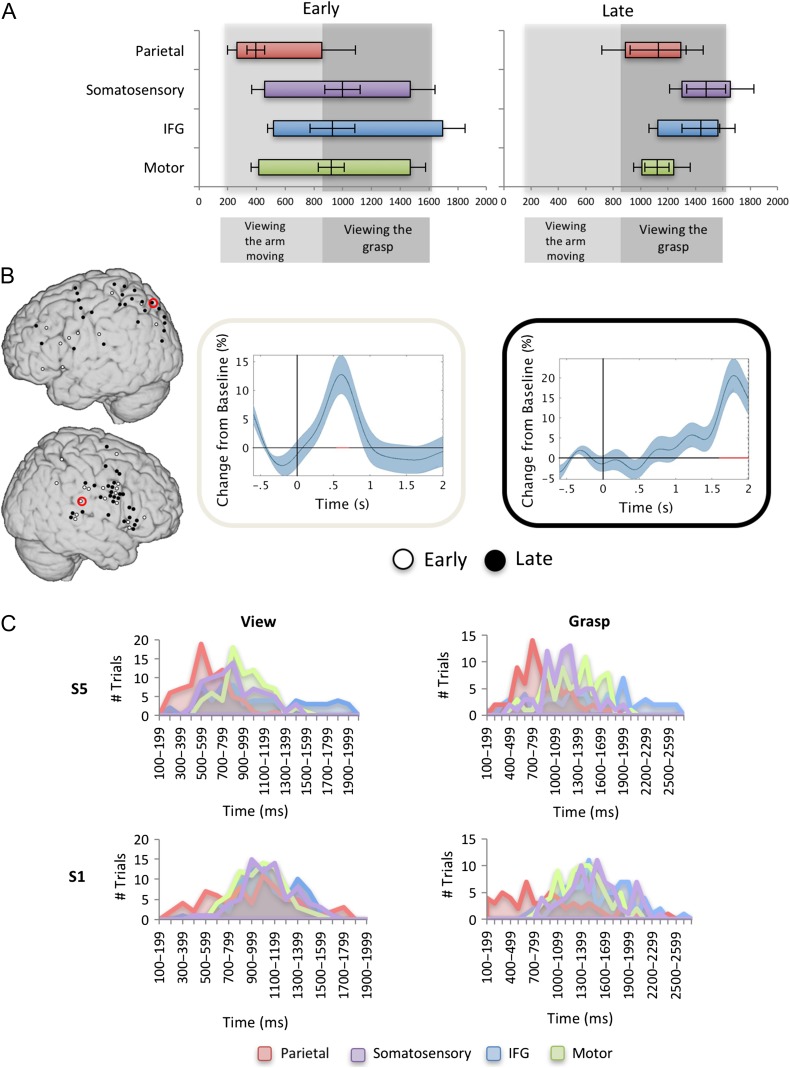
We next focused on our main condition of interest—the viewing condition. Timing of activation for this condition was measured both as the average onset time and as the peak HG activation time for each patient at each electrode. Since each 2 s video depicted 2 distinct movements—hand and arm movement toward the object, followed by a grasping action, electrodes were first divided by those whose onset was before the grasping (“early”; onset <818 ms from stimulus onset) and those whose onset was after the grasping began (“late”; onset >818 ms). Each of the 4 regions of interest had both kinds of activations, distributed equally between the hemispheres (both hemispheres had 66% “early” electrodes, Fig. 3A,B). We next averaged the temporal information (onset, offset, peak) for “early” and “late” electrodes separately for each region and compared the temporal differences between the 4 regions, separately for the “early” and “late” sites using a one-way ANOVA (see Flinker et al. 2015 for a similar analysis). Only “early” sites exhibited a systematic temporal difference in peak activity between parietal and all other regions. Activation in these early parietal sites (7 electrodes) onset on average at 265 ms, ended by 856 ms and peaked at 396 ms; while activation in motor (19 electrodes), somatosensory (11 electrodes), and IFG (9 electrodes) onset, peaked and ended later (onset average at 414, 456, and 518 ms, respectively, offset 1471, 1469, and 1693 ms and peaked at 919, 998, and 930 ms). Note that only these early parietal sites peaked during viewing of the arm movement, and before the viewing of the grasping began; while all other sites peaked around the timing of the grasp, with their offset around the end of the movement (see Fig. 3A for average onsets, peak and offset of all electrodes; and Fig. 3B for electrode locations and representative parietal electrodes). An analysis of variance was conducted on the latencies of peak neural activity relative to stimulus onset, and yielded a significant effect for region (F(3,42) = 3.988, P = 0.014). Posthoc Bonferroni-corrected comparisons revealed that early parietal electrodes peaked earlier than all other regions (differences between parietal and motor: P = 0.025; somatosensory P = 0.016, IFG P = 0.058), with no significant differences between other regions. Onset latencies were not different across regions (one-way ANOVA, F(3,42) = 1.882, P = 0.147), while offset latencies, similarly to peaks, were different across regions (one-way ANOVA, F(3,42) = 3.802, P = 0.017). Posthoc Bonferroni-corrected comparisons revealed that parietal offset was earlier than IFG (P = 0.014) with no other significant differences observed. There were no significant differences between regions for the “late” electrodes in onset, offset or peak activation (Onset: F(3,22) = 1.626, P = 0.210; Offset: F(3,24) = 0.838, P = 0.486; Peak: F(3,24) = 1.172, P = 0.341).
Figure 3.
(A) A representation of the onsets and offsets (colored bars with standard errors) and peaks (vertical lines crossing them, and their standard errors, respectively) for each region, in the early and late electrodes. (B) Left: Mirror electrodes from the 4 regions of interest, divided into early electrodes (white; average onset <818 ms), and late electrodes (black; average onset was after 818 ms) on an MNI brain collapsed across all participants; Right: The HG trace and SE for representative early and late electrodes from parietal cortex (smoothed at 2 Hz for presentation purposes). Red line on X-axis depicts significant intervals (C). The distribution of peaks in the early electrodes, for viewing (left) and grasping (right) in 2 subjects that had coverage of all 4 regions of interest. The Y-axis denotes number of trials and the X-axis denotes time (ms) from the onset of the event.
To verify the temporal-spatial patterns that we observed in averaged cortical responses, we ran the same analyses separately within each of the 2 subjects with full coverage of all 4 regions focusing on the single-trial level (S1 and S5). These single subject analyses were consistent with the previous group results. In S1 (74 trials), comparing regions in early onset electrodes revealed a significant effect for region (F(3,219) = 4.241, P = 0.011) with parietal sites peaking earlier than all other sites (mean peaks: parietal: 961.59 ms; motor: 1025.22 ms; somatosensory: 1093.16 ms; IFG: 1097.11 ms; significant differences after Bonferroni-corrected comparisons were seen between parietal and somatosensory cortex, P = 0.034), with no significant differences between other sites. In S5 (73 trials), the same analysis revealed a significant effect for region (F(3,216) = 23.47, P < 0.001) with parietal sites peaking earlier than all other sites (mean peaks: parietal: 608.79 ms; motor: 955 ms; somatosensory: 867.35 ms; IFG: 1006.09 ms; significant differences after Bonferroni-corrected comparisons were seen between parietal and all regions, all P’s < 0.001), with no significant differences between other sites (Fig. 3A).
We predicted a similar temporal pattern in the grasping condition, that is, the same “early parietal” sites in viewing, would show earlier peaks in the actual grasping trials. To address this hypothesis, we analyzed the grasping data in a similar way, comparing the 4 regions in a one-way ANOVA, within the 2 subjects that had full coverage of the regions of interest (note that grasping time was different for each subject). The timing was relative to the auditory cue. In S1, comparing regions in the “early” sites revealed a significant effect for region (72 trials; F(3,213) = 58.67, P < 0.001) with parietal sites peaking earlier than all other sites (mean peaks from auditory onset signaling to start grasping: parietal: 734.32 ms; motor: 1366.73 ms; somatosensory: 1517.59 ms; IFG: 1543.32 ms; significant differences after Bonferroni-corrected comparisons were seen between parietal and all other regions, P < 0.001), and an additional significant difference was found between motor cortex and IFG (P = 0.024). In S5, the same analysis revealed a significant effect for region (73 trials; F(3,216) = 21.57, P < 0.001) with parietal peaking earlier than all other regions (mean peaks: parietal: 873.42 ms; motor: 1269.77 ms; somatosensory: 1182.88 ms; IFG: 1504.37 ms; significant differences after Bonferroni-corrected comparisons were seen between parietal regions and all other regions, all P’s < 0.001), with an additional significant difference between IFG and somatosensory cortex (P = 0.007). Figure 3C depicts the distribution of single trial peaks in each region for viewing and for grasping, in the 2 subjects that had coverage over all sites.
Discussion
Abundant noninvasive research has focused on mirror regions in humans but questions remain regarding the neural underpinnings of the hMNS (Mahon and Caramazza 2008; Goldman and de Vignemont 2009; Hickok 2009; Rizzolatti and Sinigaglia 2010). Our results provide a comprehensive account of hMNS activations with the temporal and spatial resolution enabled by direct cortical recordings, over similar regions to those previously described both in the monkey literature and using noninvasive techniques in humans. We confirm the presence of a parietal, frontal, and sensorimotor hMNS, which is active both for viewing and for performing goal-directed actions. Critically, we delineate new functional properties of the hMNS, and shed light on the complex patterns of activations within mirror neuron regions.
First, while mirror activations were found primarily in parietal, IFG, motor, and somatosensory cortices, they were also found in other regions of the brain (see Fig. 1A and Supplementary Figure S2). This result fits well with Mukamel's single-unit study that showed that multiple regions in humans, outside the classic proposed MNS, are endowed with neural mechanisms of mirroring (Mukamel et al. 2010) and may serve different purposes (e.g., storing these actions in memory). Moreover, the mirror electrodes found in the classic mirror network were found adjacent to electrodes in the same regions that showed only motor (grasping) properties, and a minority of electrodes that showed only viewing properties (Fig. 1A and Supplementary Figure S2). This pattern helps answer a recurrent question in mirror neuron research—if motor regions, including primary motor cortex, are active for both execution and perception, how does one differentiate between one's own actions and another's. Put differently, why does not one imitate all the time? The current results show that populations of neurons within the hMNS have different properties, and their activation together with other populations in the network determines whether a motor action is being viewed, imagined, planned, or performed. Monkey research has shown similar complex patterns within motor regions, where only a subpopulation of motor neurons fire both for viewing and for grasping (e.g., Rizzolatti et al. 1996). Moreover, recent single-unit recordings in monkeys (Vigneswaran et al. 2013) strengthen this point by showing, in addition to “mirroring” sites in M1, neurons that are suppressed during perception but active during action (see similar findings in single-unit recordings in humans, in different brain regions in Mukamel et al. 2010). These results provide evidence that “mirroring” of others actions can be a function of motor regions without confusion between self and other, as not all neuronal populations in these regions are activated in the same way at the same time. Note that although the graspable objects were present in front of the patient throughout the task, the activation we found cannot be attributed to canonical neurons, neurons that were shown to discharge when the monkey observes graspable objects (Rizzolatti and Umiltà 2013), as they were also present throughout the baseline to which all conditions were compared.
Importantly, our results differentiate between 2 unique patterns of activation, both appearing in all 4 regions of interest: electrodes that were active only for viewing and grasping, and electrodes that were also active during the wait period (between viewing and grasping). The majority of motor and somatosensory electrodes displayed the first pattern, that is, showing “pure” mirror properties, perhaps creating online motor representations of the actions viewed for further analysis and planning in parietal and frontal regions (Pineda 2008). The second pattern of “mnemonic” mirror neurons may be involved in rehearsing, planning, or imagining the subsequent motor response. This type of electrodes was more prominent in the right hemisphere, when subjects had to view the left hand and perform the task with their left, nondominant, hand. These results raise the possibility that when the action to be imitated does not come as naturally to the participant (both viewing a left hand grasping, and using your nondominant hand), the action needs to be further rehearsed, imagined, or planned in the relevant brain areas when waiting to perform the action. These sites might in fact not play a specific role in “mirroring,” but serve as attentional or working memory sites, which “rehearse” the intended action from when it is seen until it is completed. Further research is needed in order to differentiate between general cognitive control/working memory sites and mnemonic mirror sites, dedicated to rehearsing the specific action seen. It should be noted that this pattern was more prominent in the parietal and frontal regions, fitting well with their more general proposed roles for attention, planning, decision-making, and coordination of motor movements (Andersen and Cui 2009), but nevertheless appeared over the sensorimotor cortex as well.
Estimation of the timing of activation revealed a mostly parallel pattern, where apart from a clear differentiation between early parietal electrodes and all other sites, most hMNS sites showed peaks distributed throughout the movement period, with an average around the viewing of the grasping action. The early parietal activity may reflect simple perceptual properties of the viewing scene—the arm moving, the direction of movement, or recognition of the goal (similar to reaching parietal neurons found for actual grasping, see Rizzolatti and Sinigaglia 2010; Konen et al. 2013) or serving as a “salience detector” of the external world, binding visuospatial, motor, and cognitive relevant information (Gottlieb 2007); while late parietal activity might reflect the larger intention of the motor action (Rizzolatti and Sinigaglia 2010). All other regions, whether starting their activation before the grasp begins or after, peak around the grasp, which is, for this task, the most informative part of the video differentiating between the different optional movements for future imitation. Note that although these results suggest parallel activations between regions, we do not claim that there is no sequential order in which information is transferred between hMNS regions when viewing goal-directed actions. In fact, the vast connections between these areas enable fast parallel transferring of information in both directions, which may account for the lack of differences in timing when examining population of neurons. Future studies, perhaps with single-unit recordings over MNS regions, or animal studies, might be able to better define the flow of information between MNS regions when viewing goal-directed actions.
Lastly, since invasive recordings in humans are rare, comparing HG activations to suppression in lower (alpha and beta) frequencies is valuable for further EEG and MEG research. Caruana et al. (2014) recently revealed that beta suppression co-occurred with HG activation over the premotor cortex, when viewing biological actions. Similarly, our data revealed robust beta suppression (15–25 Hz), and alpha/mu suppression (8–13 Hz) in sites where HG activation was present. Note that both alpha and beta suppression were most prominent over sensorimotor regions and less so in frontal and parietal sites, fitting with the EEG/MEG literature (Pineda 2005, 2008).
There are a few limitations to this study. Since there are time constraints in conducting intracranial studies in humans (taking into consideration the patients’ span of attention, wellness, stress levels, and energy while they are awaiting surgery), we had to limit the number of trials and the different conditions shown. For this reason, we did not have enough power to differentiate between viewing the grasping of the different objects. Furthermore, future studies would benefit from adding a nonbiological motion trial, enabling the differentiation between sites that are active for viewing movement in general, to those that are specific to biological motion. Our data from 3 control patients suggest that while other regions might be generally sensitive to vision-, motion-, or task-related stimuli, the regions of interest in this study are mostly specific to grasping and to viewing biological motion (Supplementary Figure S7). An additional control task of passive viewing of the same experiment confirmed that these mirroring sites are not specific for viewing for imitation; instead, most of the electrodes in our regions of interest that were active for viewing and grasping were also active for passive viewing of these goal-directed actions.
To conclude, ECoG results focused on HG activations confirm noninvasive hMNS findings, but also reveal complex and interleaved patterns of functional and temporal activations. These results provide a critical link between single-unit research in monkeys and noninvasive techniques in human. We identify 2 types of mirror sites, one showing activation only for viewing and grasping, and one also showing activation in between the viewing and grasping stages. We also found mirroring sites adjacent to both pure grasping and pure viewing sites. Finally, both for viewing and for grasping, a subgroup of parietal electrodes showed reliable earlier peaks than all other regions, while all other regions of interest peaked together, stressing parallel rather than hierarchical processing.
Supplementary Material
Notes
Conflict of Interest: None declared.
Supplementary Material
Supplementary material are available at Cerebral Cortex online.
Funding
EU Marie Curie Global Fellowship (to A.P.); NINDS R37 NS21135 and the Nielsen Corporation (to R.T.K.); the NIH NINDS NS060993 K23 and UC Irvine School of Medicine Bridge Fund (to J.J.L.); the R01NS078396 and NSF grant BCS1358907 (to J.P.).
References
- Alegre M, Rodriguez-Oroz MC, Valencia M, Perez-Alcazar M, Guridi M, Iriarte J, JObeso JA, Artieda J. 2010. Changes in subthalamic activity during movement observation in Parkinson's disease: is the mirror system mirrored in the basal ganglia? Clin Neurophys. 121(3):414–425. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Cui H. 2009. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 63(5):568–583. [DOI] [PubMed] [Google Scholar]
- Arbib MA. 2005. From monkey-like action recognition to human language: an evolutionary framework for neurolinguistics. Behav Brain Sci. 28(02):105–124. [DOI] [PubMed] [Google Scholar]
- Babiloni C, Del Percio C, Vecchio F, Sebastiano F, Di Gennaro G, Quarato PP, Morace R, Pavone L, Soricelli A, Noce G, Esposito V. 2016. Alpha, beta and gamma electrocorticographic rhythms in somatosensory, motor, premotor and prefrontal cortical areas differ in movement execution and observation in humans. Clin Neurophys. 127(1):641–654. [DOI] [PubMed] [Google Scholar]
- Caruana F, Sartori I, Russo GL, Avanzini P. 2014. Sequencing biological and physical events affects specific frequency bands within the human premotor cortex: an intracerebral EEG study. PLoS One. 9(1):e86384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochin S, Barthelemy C, Roux S, Martineau J. 1999. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur J Neurosci. 11(5):1839–1842. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP, Crone N. 1998. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 121:2301–2315. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. 2006. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 159:275–295. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. 2004. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 134(1):9–21. [DOI] [PubMed] [Google Scholar]
- Fabbri-Destro M, Rizzolatti G. 2008. Mirror neurons and mirror systems in monkeys and humans. Physiology (Bethesda). 23(3):171–179. [DOI] [PubMed] [Google Scholar]
- Fadiga L, Fogassi L, Pavesi G, Rizzolatti G. 1995. Motor facilitation during action observation: a magnetic stimulation study. J Neurophysiol. 73(6):2608–2611. [DOI] [PubMed] [Google Scholar]
- Filimon F, Nelson JD, Hagler DJ, Sereno MI. 2007. Human cortical representations for reaching: mirror neurons for execution, observation, and imagery. NeuroImage. 37(4):1315–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE. 2015. Redefining the role of Broca's area in speech. Proc Natl Acad Sci USA. 112(9):2871–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallese V. 2007. Before and below “theory of mind”: embodied simulation and the neural correlates of social cognition. Philos Trans R Soc Lond B Biol Sci. 362(1480):659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman A, de Vignemont F. 2009. Is social cognition embodied? Trends Cogn Sci. 13(4):154–159. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. 2007. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 53(1):9–16. [DOI] [PubMed] [Google Scholar]
- Halje P, Seeck M, Blanke O. 2015. Inferior frontal oscillations reveal visuo-motor matching for actions and speech: evidence from human intracranial recordings. Neuropsychologia. 79:206–214. [DOI] [PubMed] [Google Scholar]
- Hari R. 2006. Action–perception connection and the cortical mu rhythm. Prog Brain Res. 159:253–260. [DOI] [PubMed] [Google Scholar]
- Hickok G. 2009. Eight problems for the mirror neuron theory of action understanding in monkeys and humans. J Cogn Neurosci. 21(7):1229–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC, Rizzolatti G. 2005. Grasping the intentions of others with one's own mirror neuron system. PLoS Biol. 3(3):e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. 2009. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 19(6):666–671. [DOI] [PubMed] [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. 2010. Somatosensation in social perception. Nat Rev Neurosci. 11(6):417–428. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Lemon RN. 2013. What we know currently about mirror neurons. Curr Biol. 23(23):R1057–R1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konen CS, Mruczek RE, Montoya JL, Kastner S. 2013. Functional organization of human posterior parietal cortex: grasping- and reaching-related activations relative to topographically organized cortex. J Neurophysiol. 109(12):2897–2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahon BZ, Caramazza A. 2008. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 102(1):59–70. [DOI] [PubMed] [Google Scholar]
- Marceglia S, Fiorio M, Foffani G, Mrakic-Sposta S, Tiriticco M, Locatelli M, Caputo E, Tinazzi M, Priori A. 2009. Modulation of beta oscillations in the subthalamic area during action observation in Parkinson's disease. Neuroscience. 161(4):1027–1036. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. 2007. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 27(9):2424–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin O, Grèzes J. 2008. What is “mirror” in the premotor cortex? A review. Clin Neurophys. 38(3):189–195. [DOI] [PubMed] [Google Scholar]
- Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. 2010. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 20(8):750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Johnson BW, McNair NA. 2004. Mu rhythm modulation during observation of an object-directed grasp. Cogn Brain Res. 19(2):195–201. [DOI] [PubMed] [Google Scholar]
- Nishitani N, Hari R. 2000. Temporal dynamics of cortical representation for action. Proc Natl Acad Sci USA. 97(2):913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Hubbard EM, McCleery JP, Altschuler EL, Ramachandran VS, Pineda JA. 2005. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn Brain Res. 24(2):190–198. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S. 2009. Mirror activity in the human brain while observing hand movements: a comparison between EEG desynchronization in the μ-range and previous fMRI results. Brain Res. 1282:126–132. [DOI] [PubMed] [Google Scholar]
- Pineda JA. 2008. Sensorimotor cortex as a critical component of an “extended” mirror neuron system: does it solve the development, correspondence, and control problems in mirroring. Behav Brain Funct. 4(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda JA. 2005. The functional significance of mu rhythms: translating “seeing” and “hearing” into “doing”. Brain Res Rev. 50(1):57–68. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Arbib MA. 1998. Language within our grasp. Trends Neurosci. 21(5):188–194. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu Rev Neurosci. 27:169–192. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. 2008. The mirror system and its role in social cognition. Curr Opin Neurobiol. 18(2):179–184. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M. 2010. Mirror neurons: from discovery to autism. Exp Brain Res. 200(3–4):223–237. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fabbri-Destro M, Cattaneo L. 2009. Mirror neurons and their clinical relevance. Nat Clin Pract Neuro. 5(1):24–34. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L. 1996. Premotor cortex and the recognition of motor actions. Cogn Brain Res. 3 (2):131–141. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. 2010. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 11(4):264–274. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Umiltà MA. 2013. Canonical Neurons. In: Runehov ALC, Oviedo L, editors. Encyclopedia of Sciences and Religions. Netherlands: Springer. p. 305. [Google Scholar]
- Schalk G, Kubánek J, Miller KJ, Anderson NR, Leuthardt EC, Ojemann JG, et al. 2007. Decoding two-dimensional movement trajectories using electrocorticographic signals in humans. J Neural Eng. 4(3):264–275. [DOI] [PubMed] [Google Scholar]
- Stefan K, Classen J, Celnik P, Cohen LG. 2008. Concurrent action observation modulates practice-induced motor memory formation. Eur J Neurosci. 27(3):730–738. [DOI] [PubMed] [Google Scholar]
- Vigneswaran G, Philipp R, Lemon RN, Kraskov A. 2013. M1 corticospinal mirror neurons and their role in movement suppression during action observation. Curr Biol. 23(3):236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldert S, Vigneswaran G, Philipp R, Lemon RN, Kraskov A. 2015. Modulation of the intracortical LFP during action execution and observation. J Neurosci. 35(22):8451–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.