Abstract
Background
Neurocognitive impairment in survivors of childhood cancer may be associated with direct neurotoxicity, as well as indirect effects of systemic health complications. We evaluated associations among treatment exposures, chronic health conditions, and neurocognitive outcomes in adult survivors of childhood cancer.
Methods
Participants included 5507 adult survivors of childhood cancer (47.1% male; mean [SD] age = 31.8 [7.6] years at evaluation; 23.1 [4.5] years postdiagnosis) in the Childhood Cancer Survivor Study who completed a self-report measure of neurocognitive function. Cardiac, pulmonary, and endocrine chronic health conditions were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Structural equation modeling was used to examine a priori hypothesized causal pathways among cancer treatment, subsequent chronic health conditions, and neurocognitive outcomes. Multivariable models were used to estimate relative risk for associations of treatments and chronic conditions on neurocognitive function. All statistical tests were two-sided.
Results
One-third of survivors with a grade 2 or higher chronic condition reported impairments in task efficiency and memory. In addition to direct effects of cranial radiation, path analyses and multivariable models demonstrated direct effects of cardiopulmonary (β = 0.10, P = .002; relative risk [RR] = 1.27, 95% confidence interval [CI] = 1.12 to 1.44) and endocrine (β = 0.07, P = .04; RR = 1.14, 95% CI = 1.02 to 1.28) conditions on impaired task efficiency. We identified similar effects of cardiopulmonary condition on memory (P = .01) and emotional regulation (P = .01). Thoracic radiation was associated with impaired task efficiency (P = .01) and emotional regulation (P = .01) through endocrine morbidity.
Conclusions
Non-neurotoxic exposures, such as thoracic radiation, can adversely impact survivors’ neurocognitive function through chronic conditions. Management of chronic diseases may mitigate neurocognitive outcomes among aging survivors of childhood cancer.
Contemporary treatment strategies have contributed to an observed decline in late mortality among five-year survivors of childhood cancer (1), though the prevalence of developing at least one chronic health condition is estimated to be as high as 93.5% 35 years after cancer diagnosis (2). Pulmonary, cardiac, endocrine, and nervous system morbidities are among the most prevalent conditions (2–9). Additionally, long-term survivors of childhood cancer are at risk for neurocognitive deficits in domains of attention, executive functions, processing speed, and memory (10–15), and these deficits have a negative impact on employment and occupational outcomes (16). Cranial radiation is consistently the strongest predictor of long-term neurocognitive deficits (17,18); however, survivors of childhood cancer who were not treated with central nervous system–directed therapies also demonstrate neurocognitive impairment at rates higher than the general population (19,20).
Within the general population, chronic health problems have been associated with neurocognitive impairment and psychosocial difficulties. Patients with congestive heart failure are at increased risk for deficits in executive function, memory, language, attention, and processing speed (21). Type II diabetes has been associated with impairments in executive functions and processing speed (22,23). Patients with chronic obstructive pulmonary disease demonstrate short- and long-term memory impairments and difficulties with executive functions and attention on neuropsychological assessment (24).
Although the impact of chronic health conditions on neurocognitive function is reported in the general population, neurocognitive outcomes in survivors of childhood cancer have largely been attributed to neurotoxic treatment exposures including cranial radiotherapy. However, as survivors age across the lifespan, they are at increased risk for cardiac, pulmonary, and endocrine late effects. Thus, the effect of chronic health conditions may contribute to poor long-term neurocognitive outcomes. The aim of this study was to examine associations between chronic morbidities and neurocognitive outcomes in adult survivors of childhood cancer while accounting for established associations with neurotoxic treatments. We hypothesized that beyond direct neurotoxic effects, treatment exposures may also impact neurocognitive outcomes through cardiovascular, pulmonary, and endocrine morbidities.
Methods
Population
The Childhood Cancer Survivor Study (CCSS) is a multi-institutional research cohort started in 1994 to investigate physical and behavioral outcomes in child and adolescent cancer survivors. The original cohort includes 14 357 survivors diagnosed with cancer younger than age 21 years (25). Survivors were diagnosed between January 1, 1970, and December 31, 1986, and had survived at least five years to be eligible (26,27). Eligible diagnoses included leukemia, central nervous system malignancies (all histologies), Hodgkin lymphoma, non-Hodgkin lymphoma, Wilms tumor, neuroblastoma, soft tissue sarcoma, and malignant bone tumor. Institutional review boards at the 26 participating institutions approved the CCSS study protocol, and participants provided informed consent.
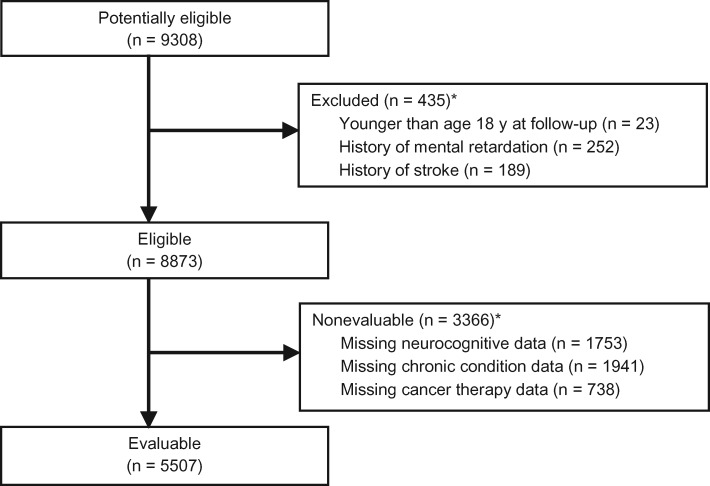
The study population for the current analyses included all cancer survivors who were age 18 years or older when they completed the Follow-up 2 survey (n = 9308), which included the Childhood Cancer Survivor Study Neurocognitive Questionnaire (CCSS-NCQ) (28,29). Survivors who reported mental retardation at baseline (n = 252) were excluded from analyses. Survivors who reported stroke events (n = 189) were excluded from analyses to ensure that the cardiac morbidities included in this study consisted of cardiovascular, structural, and conduction abnormalities, instead of overt neurologic injury. Survivors with missing treatment (n = 738), chronic disease (n = 1941), or neurocognitive (n = 1753) data were nonevaluable. Of the eligible 8873 survivors, 5507 were included in the current analyses, with a participation proportion of 62.1% (Figure 1). Other than a higher proportion of female survivors among participants as compared with nonparticipants (52.9% vs 43.1%, P < .001), we did not find clinically significant differences in characteristics between participants and nonparticipants (Supplementary Table 1, available online).
Figure 1.
Consort diagram. Selection of study participants from the Childhood Cancer Survivor Study (CCSS). *Overall numbers do not add up because there are survivors who met more than one exclusion criterion.
Outcomes Measures
Neurocognitive outcomes were measured using the CCSS-NCQ, a 25-item instrument that was developed and previously validated in the CCSS survivor and sibling sample (28,29). Four reliable factors are derived from this instrument: task efficiency, emotional regulation, organization, and memory. Raw scores for each factor were converted to T-scores based on sibling norms, with higher scores indicative of more neurocognitive problems. To identify clinically meaningful outcomes, neurocognitive impairment on these measures was defined as a T-score falling in the top 10th percentile of the sibling reference (ie, T-score ≥ 63), consistent with previous CCSS publications (17,30–34). A binary outcome (impaired vs nonimpaired) was applied for all analyses.
Chronic Conditions
The grading of chronic conditions was previously reported (3,9). At baseline and at subsequent follow-up evaluations, survivors completed a multi-item survey, which included the participant’s age at onset of organ-based health conditions. Conditions were graded for severity according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (CTCAE version 4.03) (35), which includes conditions with asymptomatic/mild symptoms (grade 1), moderate symptoms with minimal interventions (grade 2), severe/disabling symptoms with extensive interventions (grade 3), and life-threatening (grade 4) conditions. A clinically significant chronic morbidity is defined as a grade 2, 3, or 4 condition. For this study, the health conditions of interest were limited to cardiovascular, pulmonary, and endocrine systems, given their association with neurocognitive function in the general population (21–23,36–42). Only health conditions with a reported age of onset at or prior to completion of the CCSS-NCQ were included.
Clinical and Treatment Variables
Medical records from treating institutions were abstracted to obtain information on the original cancer diagnosis and treatment exposures. The maximum tumor dose to four body regions (brain, chest, abdomen, and pelvis) was determined based on detailed review of radiation therapy records.
Statistical Analysis
Descriptive statistics were calculated for the neurocognitive outcomes, chronic disease status, and demographic and treatment factors. Exploratory analysis was conducted to evaluate the association between risk of neurocognitive impairment (relative risk [RR] and 95% confidence interval [CI]) and specific types of chronic conditions using logistic regression.
Path analysis, a type of structural equation modeling, was used to examine the relationships between treatment exposures, presence of grade 2–4 (ie, moderate, severe/disabling, and life-threatening) chronic health conditions, and each of the four neurocognitive domains of task efficiency, emotional regulation, organization, and memory. Based on established long-term follow-up guidelines for childhood cancer and literature review, treatment variables that are commonly associated with both chronic conditions (cardiovascular, pulmonary, and endocrine) and neurocognitive outcomes were identified (20,43–46). An a priori hypothesized path model for each neurocognitive outcome was created (Supplementary Methods and Supplementary Figure 1, available online). The model was then expanded by adding clinically meaningful paths, one at a time, with a modification index of 3.6 or greater, beginning with the path that had the largest index value. The path model was then reduced by removing paths with statistically nonsignificant coefficients, beginning with the smallest. The probit model with a robust weighted least squares estimator approach was adopted. Details of the path analysis can be found in the Supplementary Methods (available online). This process was iterative until the best model-fitting criteria were achieved to include a comparative fit index (CFI) and Tucker Lewis Index (TLI) greater than 0.95, and a root mean square error of approximation (RMSEA) of less than 0.05 (47).
To examine the magnitude of associations between chronic health conditions and neurocognitive outcomes, log-binomial multivariable regression was used to estimate relative risks and 95% confidence intervals. Covariates included in multivariable models were statistically significant direct effects of treatment identified in the final path models. An exploratory log-binomial analysis was conducted to evaluate the association between the duration of the chronic condition (defined as years between the reported onset of the chronic conditions and the reported date of neurocognitive outcomes) and neurocognitive impairment.
The path analysis was conducted using Mplus version 7.11, and all other analyses were conducted using SAS version 9.4. A P value of less than .05 was considered statistically significant, and all statistical tests were two-sided.
Results
Chronic Conditions and Neurocognitive Impairment
On average, survivors were age 31.8 years at survey and 23.1 years from diagnosis (Table 1). The majority of the survivors were diagnosed with leukemia (34.0%), Hodgkin lymphoma (13.8%), or central nervous system (CNS) tumor (11.0%). At last follow-up, 21.2% of survivors reported at least one moderate (grade 2) chronic condition, 10.6% reported a severe/disabling (grade 3) condition, and 2.0% reported a life-threatening condition (grade 4) (Supplementary Table 2, available online). Grades 2–4 cardiovascular (16.5%) and endocrine (22.9%) conditions were prevalent among survivors, while pulmonary conditions were less prevalent (1.7%). As a result of these frequencies, pulmonary and cardiac conditions were combined (referred to as “cardiopulmonary” morbidity thereafter) in the path analysis due to the low prevalence (n = 92) of pulmonary conditions.
Table 1.
Demographic and clinical characteristics (n = 5507)*
| Characteristics | No. (%) | Mean (SD) | Range |
|---|---|---|---|
| Age at diagnosis, y | – | 8.2 (5.9) | 0–20 |
| Age at follow-up, y | – | 31.8 (7.6) | 18–54 |
| Years since diagnosis | – | 23.1 (4.5) | 16–34 |
| Sex | |||
| Male | 2592 (47.1) | – | – |
| Female | 2915 (52.9) | – | – |
| Health insurance | |||
| Yes | 4869 (89.5) | – | – |
| No | 572 (10.5) | – | – |
| Unknown | 66 | – | – |
| Diagnosis | |||
| Leukemia | 1875 (34.0) | – | – |
| CNS tumor | 607 (11.0) | – | – |
| Hodgkin lymphoma | 762 (13.8) | – | – |
| Non-Hodgkin lymphoma | 407 (7.4) | – | – |
| Wilms' | 351 (6.4) | – | – |
| Neuroblastoma | 510 (9.3) | – | – |
| Soft tissue sarcoma | 492 (8.9) | – | – |
| Osteosarcoma | 503 (9.1) | – | – |
| Chemotherapy† | |||
| No | 1127 (20.5) | – | – |
| Any, mg/m2 | 4366 (79.5) | – | – |
| Anthracyclines (IV) | 1934 (36.4) | 295.4 (237.4) | 10.0–8369.6 |
| Cytarbine (IV) | 796 (14.7) | 6201.9 (8714.1) | 5.0–73 650.7 |
| Alkylating agents (IV) | 2315 (45.7) | 8979.8 (9019.1) | 5.8–128 673.0 |
| Methotrexate (IV) | 1038 (19.2) | 34 558.9 (77 835.0) | 16.3–502 127.7 |
| Bleomycin (IV) | 275 (5.0) | 87.5 (47.6) | 0.1–332.1 |
| Corticosteroids (oral) | 2667 (48.4) | – | – |
| Platinum (IV) | 197 (3.6) | 559.4 (566.1) | 60.0–4668.3 |
| Intrathecal injections | 2049 (37.2) | 1.4 (0.6) | 1.0–5.0 |
| Radiation | |||
| No | 1865 (34.0) | – | – |
| Any, Gy | 3621 (66.0) | – | – |
| Cranial | 1708 (31.8) | 29.5 (14.1) | 0.5–106.0 |
| Chest | 1346 (25.1) | 30.3 (11.7) | 1.4–68.0 |
| Abdominal | 1299 (24.2) | 27.9 (10.9) | 1.4–72.0 |
| Pelvis | 999 (18.6) | 28.7 (11.8) | 0.6–78.0 |
| Surgery | |||
| No | 1331 (24.2) | – | – |
| Cardiovascular | 81 (1.5) | – | – |
| Pulmonary | 496 (9.0) | – | – |
| Central nervous system | 696 (12.6) | – | – |
Mean (SD) and range reported for treatment exposures if continuous dose was available from medical record abstraction. CNS = central nervous system; IV = intravenous.
Cumulative doses are only available for the indicated drugs and treatments.
Overall, 22.8% of survivors reported problems with task efficiency, 12.4% with memory, 12.7% with organization, and 12.3% emotional regulation (Table 2). Approximately 20% to 30% of survivors who had at least one moderate chronic condition reported impairments in task efficiency and memory. Exploratory analysis revealed that risk of impairment in task efficiency, memory, and emotional regulation was associated with growth hormone deficiency, gonadal dysfunction, and marginally for hypothyroidism (Supplementary Table 3, available online).
Table 2.
Neurocognitive function by grade of chronic health condition
| Chronic health condition (grade) | No. | Neurocognitive domain |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Task efficiency |
Memory |
Organization |
Emotional regulation |
||||||
| Mean (SD) | % impaired* | Mean (SD) | % impaired* | Mean (SD) | % impaired* | Mean (SD) | % impaired* | ||
| All survivors | 5507 | 53.3 (12.9)† | 22.8 | 51.7 (11.9)† | 12.4 | 49.7 (10.1)† | 12.7 | 51.8 (10.8)† | 12.3 |
| Cardiovascular | |||||||||
| None | 4167 | 53.0 (12.8) | 21.8 | 51.3 (11.7) | 12.7 | 49.5 (10.0) | 12.0 | 51.6 (10.8) | 11.8 |
| Mild (1) | 430 | 54.4 (13.5) | 26.0 | 53.0 (12.3) | 15.6 | 50.8 (10.5) | 15.6 | 52.8 (11.1) | 14.9 |
| Moderate (2) | 717 | 53.9 (13.3) | 25.0 | 52.7 (12.2) | 14.2 | 50.1 (10.1) | 14.4 | 52.4 (10.9) | 13.2 |
| Severe/life-threatening (3/4) | 193 | 55.5 (12.4) | 29.0 | 54.0 (12.2) | 19.7 | 51.5 (10.6) | 16.6 | 53.7 (10.3) | 13.0 |
| Endocrine | |||||||||
| None | 4017 | 52.4 (12.3) | 20.7 | 51.0 (11.5) | 11.9 | 49.5 (10.1) | 12.2 | 51.2 (10.7) | 11.1 |
| Mild (1) | 230 | 57.7 (15.3) | 35.2 | 55.5 (13.2) | 22.2 | 51.1 (10.5) | 17.8 | 53.9 (11.2) | 17.4 |
| Moderate (2) | 772 | 56.1 (14.2) | 29.0 | 53.4 (12.6) | 17.1 | 50.2 (9.9) | 12.7 | 53.0 (10.8) | 14.0 |
| Severe/life-threatening (3/4) | 488 | 54.0 (12.9) | 24.4 | 52.8 (12.6) | 15.8 | 50.3 (10.1) | 14.3 | 53.8 (11.1) | 16.8 |
| Pulmonary | |||||||||
| None | 4547 | 52.8 (12.6) | 21.8 | 51.3 (11.6) | 12.4 | 49.4 (10.0) | 11.8 | 51.5 (10.7) | 11.2 |
| Mild (1) | 868 | 55.3 (13.8) | 26.8 | 53.3 (12.7) | 17.6 | 51.1 (10.4) | 16.8 | 53.4 (11.3) | 16.5 |
| Moderate (2) | 32 | 52.9 (9.5) | 18.8 | 53.6 (11.6) | 12.5 | 52.7 (10.1) | 15.6 | 52.0 (11.9) | 15.6 |
| Severe/life-threatening (3/4) | 60 | 60.1 (15.1) | 38.3 | 57.9 (14.8) | 28.3 | 52.4 (9.9) | 18.3 | 57.0 (11.3) | 26.7 |
Percent impaired, defined as a score falling in the top 10th percentile of the sibling reference (ie, T score ≥ 63).
Group mean (standard deviation) standardized T-score (μ = 50, σ = 10) for each neurocognitive domain. A higher T-score is indicative of more neurocognitive problems.
Path Analysis of Treatment Exposures on Neurocognitive Outcomes
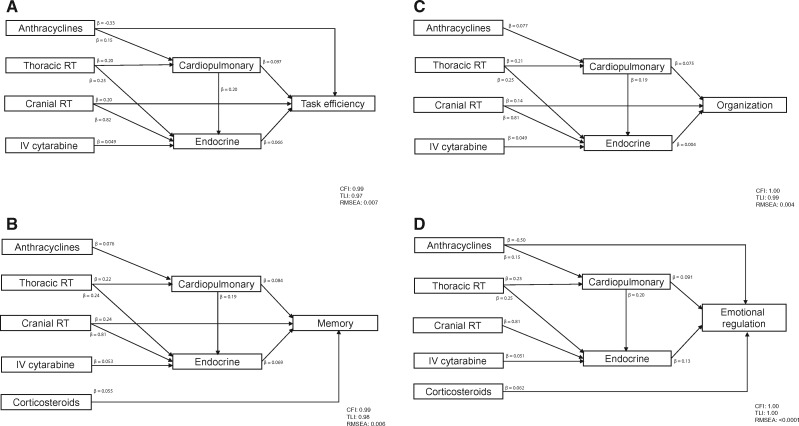
Figure 2 graphically represents predictors identified from the path analysis for each neurocognitive outcome. Details of the standardized and unstandardized estimates and statistical significance of the identified paths are summarized in Supplementary Table 4 (available online).
Figure 2.
Pathways of cancer therapy on neurocognitive outcomes. Final path models are presented for each neurocognitive measure. All models are adjusted for sex, time since diagnosis, and age at diagnosis. Model fit indices are represented by comparative fit index, Tucker Lewis Index, and root mean square error of approximation. A) The path model for task efficiency. The impact of cranial radiotherapy (RT) on task efficiency is modified by sex (P = .002) and age at diagnosis (P < .001). The impact of anthracyclines on task efficiency is modified by time since diagnosis (P = .03). The impact of thoracic RT on endocrine disease is modified by age at diagnosis (P = .001). The impact of cranial RT on endocrine disease is modified by sex (P = .01), time since diagnosis (P < .001), and age at diagnosis (P < .001). B) Figure 2B shows the path model for memory. The impact of thoracic RT on endocrine disease is modified by age at diagnosis (P = .001). The impact of cranial RT on endocrine disease is modified by sex (P = .003), time since diagnosis (P < .001), and age at diagnosis (P < .001). C) The path model for organization. The impact of thoracic RT on endocrine disease is modified by age at diagnosis (P = .001). The impact of cranial RT on endocrine disease is modified by sex (P = .002), time since diagnosis (P < .001), and age at diagnosis (P < .001). D) The path model for emotional regulation. The impact of anthracyclines on emotional regulation is modified by time since diagnosis (P = .002). The impact of thoracic RT on endocrine disease is modified by age at diagnosis (P = .001). The impact of cranial RT on endocrine disease is modified by sex (P = .004), time since diagnosis (P < .001), and age at diagnosis (P < .001). P values are derived from a probit model with a robust weighted least squares estimator (WLSMV). All P values are two-sided. CFI = comparative fit index; IV = intravenous; RMSEA = root mean square error of approximation; RT = radiation therapy; TLI = Tucker Lewis Index.
Cardiopulmonary (β = 0.097, P = .002) and endocrine (β = 0.066, P = .04) morbidities were associated with impaired task efficiency. Cranial radiation was associated with more impaired task efficiency in female survivors (P = .002), compared with male survivors, and in survivors diagnosed at younger ages (P < .001). Cardiopulmonary (P = .01) and endocrine (P = .001) conditions were directly associated with impaired emotional regulation. Cardiopulmonary morbidity was also associated with impaired memory (P = .01) and organization (P = .04). Treatment with corticosteroids was associated with impaired memory (P = .02) and emotional regulation (P = .009).
Thoracic radiation was associated with impairment in task efficiency (P = .01) and emotional regulation (P = .01) through endocrine morbidity. Substantial impact of thoracic radiation on all 4 neurocognitive domains through cardiopulmonary conditions was observed, even though the pathway from thoracic radiation to cardiopulmonary conditions did not reach statistical significance.
For all neurocognitive domains, in addition to a direct impact, cranial radiation was associated with poorer function through increased endocrine morbidity. This was particularly true for survivors who were male (all P = .002–.01), diagnosed at a younger age (all P < .001), and who had shorter time from diagnosis (all P < .001).
Risk Estimations
Multivariable models were generated to estimate relative risk for impairment on neurocognitive outcomes associated with variables that demonstrated statistically significant direct effects in the pathway analysis (Table 3). Cardiopulmonary morbidity increased risk for impaired memory (RR = 1.25, 95% CI = 1.06 to 1.48) and task efficiency (RR = 1.27, 95% CI = 1.12 to 1.44). Endocrine morbidity also increased risk for impaired task efficiency (RR = 1.14, 95% CI = 1.02 to 1.28) and memory (RR = 1.17, 95% CI = 1.01 to 1.36). A similar pattern was identified for emotion regulation impairment. Exploratory analysis revealed that every five-year increase from the reported date of onset of the condition is associated with a 3.0% to 8.0% higher risk of neurocognitive impairment (Supplementary Table 5, available online).
Table 3.
Multivariable models for neurocognitive outcomes*
| Predictors | Neurocognitive domain |
|||
|---|---|---|---|---|
| Task efficiency | Memory | Organization | Emotional regulation | |
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Chronic conditions | ||||
| Cardiopulmonary† | ||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 1.27 (1.12 to 1.44) | 1.25 (1.06 to 1.48) | 1.29 (1.08 to 1.54) | 1.34 (1.11 to 1.61) |
| Endocrine | ||||
| No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Yes | 1.14 (1.02 to 1.28) | 1.17 (1.01 to 1.36) | 1.02 (0.86 to 1.20) | 1.38 (1.17 to 1.62) |
| Treatment factors | ||||
| CRT, per 10 Gy | ‡ | 1.20 (1.16 to 1.24) | 1.07 (1.03 to 1.12) | to |
| Sex† | ||||
| Male | 1.23 (1.17 to 1.29) | – | – | – |
| Female | 1.29 (1.24 to 1.35) | – | – | – |
| Age at diagnosis†, per 10 Gy, y | ||||
| 1 | 1.26 (1.21 to 1.31) | – | – | – |
| 5 | 1.21 (1.18 to 1.24) | – | – | – |
| 10 | 1.16 (1.13 to 1.19) | – | – | – |
| 15 | 1.11 (1.06 to 1.17) | – | – | – |
| 20 | 1.07 (1.00 to 1.14) | – | – | – |
| Anthracyclines | ‡ | ‡ | ||
| Years from diagnosis†, per 100mg/m2 | ||||
| 15 | 0.92 (0.86 to 0.98) | – | – | 0.83 (0.76 to 0.91) |
| 20 | 0.95 (0.91 to 0.99) | – | – | 0.91 (0.86 to 0.96) |
| 25 | 0.98 (0.95 to 1.02) | – | – | 0.99 (0.95 to 1.04) |
| 30 | 1.01 (0.96 to 1.08) | – | – | 1.08 (1.01 to 1.17) |
| 35 | 1.05 (0.96 to 1.15) | – | – | 1.18 (1.05 to 1.33) |
| Corticosteroids | ||||
| No | – | 1.00 (reference) | – | 1.00 (reference) |
| Yes | – | 1.20 (1.05 to 1.38) | – | 1.27 (1.10 to 1.46) |
Only predictors that have direct effect on neurocognitive outcomes in path analysis are included in log-binomial multivariable regression. CI = confidence interval; CRT = cranial radiation therapy; RR = relative risk.
Pulmonary and cardiac conditions were combined in the path analysis due to the low prevalence (n = 92, 1.7 %) of grade 2 or higher pulmonary conditions in the cohort.
Main effects of the treatment factor are not presented due to the presence of interactions with clinical variables.
Discussion
With the large population and detailed annotation of treatment exposures and health outcomes in CCSS, this study was able to evaluate the impact of chronic health conditions on self-reported neurocognitive outcomes while controlling for demographic characteristics and primary treatment exposures. Path analysis was well suited for this purpose as it was able to identify determinants that may share a common pathway to impact outcomes. We found that while treatment exposures such as corticosteroids and cranial radiation have direct impact on neurocognitive impairment, chronic cardiopulmonary and endocrine morbidities that develop over time mediate and may exacerbate the impact of exposures on neurocognitive function. These findings have important clinical implications; although the effects of the primary treatment exposures cannot be reversed in these long-term survivors, treatment of cardiopulmonary and/or endocrine morbidities may be an avenue for improvement of neurocognitive function.
Cardiopulmonary morbidities appear to have a direct contribution to neurocognitive impairment in cancer survivors. Our previous study found that in survivors of Hodgkin lymphoma who were treated with thoracic radiation, anthracyclines, and bleomycin, the risk for neurocognitive impairment was associated with radiologic indices of reduced brain integrity and concurrent cardiopulmonary dysfunction (20). Cardiac and pulmonary morbidities may be related to cerebrovascular pathology through altered cerebral perfusion or reduced blood oxygenation. Inflammation and oxidative stress may be another mechanism that links cardiopulmonary pathology with neurocognitive impairment. Elevated homocysteine levels, which are associated with vascular injury, are observed in patients with atherosclerosis and cardiac conditions (48,49) and chronic obstructive pulmonary diseases (50). Homocysteine has also been found to be positively correlated with cerebral atrophy and worse global cognitive function (51,52). Our group previously reported that in adult survivors of childhood acute lymphoblastic leukemia, relative elevation of uric acid, a biomarker associated with chronic vascular injury and inflammation, during adolescence was predictive of cardiovascular health during adulthood, which was concurrently associated with poorer neurocognitive outcomes (39). Certain treatment exposures may initiate an ongoing process of inflammation and oxidative stress during survivorship, and this response may interact with cardiopulmonary or endocrine morbidity to exacerbate cerebrovascular injury. As comorbidities among cancer survivors are prevalent, future studies should explore how clinical and biological factors interact to impact functional outcomes.
Childhood cancer treatment exposures appear to have indirect contributions to neurocognitive impairment through chronic conditions. Our findings suggest that cardiopulmonary morbidity mediates the impact of thoracic radiation and anthracyclines on task efficiency and memory problems. Pulmonary health risk may result from cardiovascular morbidity induced by cardiotoxic agents such as anthracyclines. The dose-dependent impact of anthracyclines on task efficiency and emotional regulation through cardiopulmonary health was more pronounced as survivors aged. Studies have demonstrated that the lungs are one of the most radiation-sensitive structures in the body (3,8). Changes in brain perfusion due to hypoxemia in survivors with pulmonary conditions may increase cognitive impairment. Intermittent and continuous hypoxia resulting from poor lung function may lead to transient deficits in neurotransmitter metabolism in the central nervous system (37). Our previous work also demonstrated that factors that affect cardiovascular health and lung function, such as physical activity and smoking, may also impact neurocognitive function in survivors of childhood cancer. Recognizing the underlying association between cardiopulmonary health and functional outcomes will facilitate potential application of behavior interventions to improve neurocognitive function, such as aerobic activity and strength training, as well as smoking cessation.
Endocrine morbidities may mediate the impact of treatment exposures on neurocognitive impairment in survivors. For example, one common presentation of endocrine complications in survivors treated with thoracic radiation is primary hypothyroidism, which was associated with poorer task efficiency. Within the general population, memory and attention problems are common features of patients who suffer from inadequate hypothalamic-pituitary-adrenal (HPA) axis function and hypogonadism (36). As estrogen deficiency is known to be associated with neurocognitive impairment in postmenopausal women and breast cancer patients (53,54), future studies should also evaluate potential sex differences in the impact of treatment-induced gonadal dysfunction on neurocognitive impairment. Other than thyroid-related problems, long-term survivors may also develop other endocrine complications such as growth hormone deficiency, metabolic disorders, and precocious or delayed puberty from exposure to cyclophosphamide, cranial, and pelvic irradiation. Specific to survivors of childhood cancer, one study reported that growth hormone deficiency had a modest impact on long-term survivors’ verbal fluency and emotional dysregulation. Adolescent survivors of childhood acute lymphoblastic leukemia (ALL) who suffer from partial but persistent adrenal insufficiency, as evidenced by low-normal levels of dehydroepiandrosterone-sulfate, may be at risk for attention deficits (40). While prospective follow-up is needed to validate the above findings, this overall pattern of evidence reflects the importance of regular screening and appropriate treatments with hormonal replacement therapy during the early phase of cancer survivorship to ameliorate functional impairment in long-term survivors.
Consistent with previous findings (55,56), female survivors treated with cranial radiation reported more problems on task efficiency than male survivors. We also found that those who were treated with cranial radiation at a younger age had greater risk for endocrine morbidity, which appears to partially mediate the impact of cranial radiation on task efficiency and memory. Extensive literature supports the notion that females are more vulnerable to cranial radiation–related intellectual impairment (18,56). Other studies have also demonstrated that in long-term survivors of ALL treated with only chemotherapy, sex differences may have potential impact on the association among adrenal insufficiency, chronic inflammation, and neurocognitive outcomes (40,57). Although the underlying biological and physiological bases of these sex-specific risks are not well established, fundamental hormonal differences between the sexes may differentially impact development over the course of cancer survivorship. Female survivors may benefit from more rigorous monitoring of their health status and early preventive strategies to avoid poor long-term adverse outcomes.
Findings from this study should be interpreted in the light of several limitations. The temporal association between some chronic conditions and neurocognitive outcomes cannot be fully established. However, based on the pathophysiology of neurocognitive outcomes, it is logical to assume a casual pathway between chronic conditions and neurocognitive problems, and this clinical judgment was exercised when the path analysis was conducted. In addition to a relatively modest participation rate of 62%, chronic conditions were self-reported without external verification. Consequently, as previously identified by other CCSS reports (58), the actual rates and severity of conditions may be underestimated due to the high rates of undiagnosed disease and inconsistent screening of health conditions in a community setting. However, it is widely accepted that this approach is effective in facilitating collection of health outcomes data among a large, clinically heterogeneous pediatric cancer population, and important clinical characteristics were not different between participants and nonparticipants. While the treatment protocols represented in the CCSS cohort are now more than two decades old, the treatment agents remain the backbone of modern therapies for childhood cancer (59,60), and the cohort is representative of the growing population of aging cancer survivors in the current health care system. Although treatment current protocols are less intensive than those regimens represented by this cohort of survivors, recent studies have demonstrated that even contemporary treatment protocols carry risk for late effects and the profile of these adverse outcomes remains similar across different diagnoses (45). Therefore, findings from this study are still relevant because as the younger survivors age over the next decades, they may develop similar chronic conditions that impact functional outcomes.
Limitations notwithstanding, our study supports the mediating effect of chronic conditions between treatment exposures and neurocognitive impairment. In particular, survivors who develop cardiopulmonary and endocrine comorbidities from treatment exposures should be monitored more closely for neurocognitive deficits, and early interventions may be warranted. Tailored and personalized management on specific multimorbidity patterns will have implications for neurocognitive outcomes among aging survivors of childhood cancer.
Funding
This work was supported by the National Cancer Institute (CA55727, G. T. Armstrong). Support to St. Jude Children’s Research Hospital was also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts) and by ALSAC.
Notes
The funders had no role in design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. Armstrong GT, Chen Y, Yasui Y et al. , Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med. 2016;3749:833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hudson MM, Ness KK, Gurney JG et al. , Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;30922:2371–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oeffinger KC, Mertens AC, Sklar CA et al. , Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;35515:1572–1582. [DOI] [PubMed] [Google Scholar]
- 4. Hudson MM, Mertens AC, Yasui Y et al. , Health status of adult long-term survivors of childhood cancer: A report from the childhood cancer survivor study. JAMA. 2003;29012:1583–1592. [DOI] [PubMed] [Google Scholar]
- 5. Gurney JG, Kadan-Lottick NS, Packer RJ et al. , Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood cancer survivor study. Cancer. 2003;973:663–673. [DOI] [PubMed] [Google Scholar]
- 6. Oeffinger KC, Mertens AC, Sklar CA et al. , Obesity in adult survivors of childhood acute lymphoblastic leukemia: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;217:1359–1365. [DOI] [PubMed] [Google Scholar]
- 7. Oeffinger KC, Nathan PC, Kremer LCM.. Challenges after curative treatment for childhood cancer and long-term follow up of survivors. Hematol Oncol Clin North Am. 2008;551:251–273. [DOI] [PubMed] [Google Scholar]
- 8. Diller L, Chow EJ, Gurney JG et al. , Chronic disease in the Childhood Cancer Survivor Study cohort: A review of published findings. J Clin Oncol. 2009;2714:2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Armstrong GT, Kawashima T, Leisenring W et al. , Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;3212:1218–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jain N, Krull KR, Brouwers P et al. , Neuropsychological outcome following intensity-modulated radiation therapy for pediatric medulloblastoma. Pediatr Blood Cancer. 2008;512:275–279. [DOI] [PubMed] [Google Scholar]
- 11. Buizer AI, De Sonneville LMJ, Van Den Heuvel-Eibrink MM et al. , Behavioral and educational limitations after chemotherapy for childhood acute lymphoblastic leukemia or Wilms tumor. Cancer. 2006;1069:2067–2075. [DOI] [PubMed] [Google Scholar]
- 12. Campbell LK, Scaduto M, Sharp W et al. , A meta-analysis of the neurocognitive sequelae of treatment for childhood acute lymphocytic leukemia. Pediatr Blood Cancer. 2007;491:65–73. [DOI] [PubMed] [Google Scholar]
- 13. Waber DP, Pomeroy SL, Chiverton AM et al. , Everyday cognitive function after craniopharyngioma in childhood. Pediatr Neurol. 2006;341:13–19. [DOI] [PubMed] [Google Scholar]
- 14. von der Weid NX. Adult life after surviving lymphoma in childhood. Support Care Cancer. 2008;164:339–345. [DOI] [PubMed] [Google Scholar]
- 15. Bhatia S, Landier W.. Evaluating survivors of pediatric cancer. Cancer J. 2005;114:340–354. [DOI] [PubMed] [Google Scholar]
- 16. Kirchhoff AC, Krull KR, Ness KK et al. , Physical, mental, and neurocognitive status and employment outcomes in the childhood cancer survivor study cohort. Cancer Epidemiol Biomarkers Prev. 2011;209:1838–1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Clanton NR, Klosky JL, Li C et al. , Fatigue, vitality, sleep, and neurocognitive functioning in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2011;11711:2559–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krull KR, Brinkman TM, Li C et al. , Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: A report from the St jude lifetime cohort study. J Clin Oncol. 2013;3135:4407–4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mohrmann C, Henry J, Hauff M et al. , Neurocognitive outcomes and school performance in solid tumor cancer survivors lacking therapy to the central nervous system. J Pers Med. 2015;52:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krull KR, Sabin ND, Reddick WE et al. , Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. J Clin Oncol. 2012;3029:3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vogels RLC, Oosterman JM, Van Harten B et al. , Neuroimaging and correlates of cognitive function among patients with heart failure. Dement Geriatr Cogn Disord. 2007;246:418–423. [DOI] [PubMed] [Google Scholar]
- 22. Reaven GM, Thompson LW, Nahum D et al. , Relationship between hyperglycemia and cognitive function in older NIDDM patients. Diabetes Care. 1990;131:16–21. [DOI] [PubMed] [Google Scholar]
- 23. Lee JH, Choi Y, Jun C et al. , Neurocognitive changes and their neural correlates in patients with type 2 diabetes mellitus. Endocrinol Metab. 2014;292:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Crews WD, Jefferson AL, Bolduc T et al. , Neuropsychological dysfunction in patients suffering from end-stage chronic obstructive pulmonary disease. Arch Clin Neuropsychol. 2001;167:643–652. [PMC free article] [PubMed] [Google Scholar]
- 25. Leisenring WM, Mertens AC, Armstrong GT et al. , Pediatric cancer survivorship research: Experience of the childhood cancer survivor study. J Clin Oncol. 2009;2714:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robison LL, Mertens AC, Boice JD et al. , Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;384:229–239. [DOI] [PubMed] [Google Scholar]
- 27. Robison LL, Armstrong GT, Boice JD et al. , The childhood cancer survivor study: A national cancer institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;2714:2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krull KR, Gioia G, Ness KK et al. , Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;1138:2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kenzik KM, Huang IC, Brinkman TM et al. , The Childhood Cancer Survivor Study-Neurocognitive Questionnaire (CCSS-NCQ) Revised: Item response analysis and concurrent validity. Neuropsychology. 2015;291:31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong GT, Jain N, Liu W et al. , Region-specific radiotherapy and neuropsychological outcomes in adult survivors of childhood CNS malignancies. Neuro Oncol. 2010;1211:1173–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brackett J, Krull KR, Scheurer ME et al. , Antioxidant enzyme polymorphisms and neuropsychological outcomes in medulloblastoma survivors: A report from the Childhood Cancer Survivor Study. Neuro Oncol. 2012;148:1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brinkman TM, Zhang N, Ullrich NJ et al. , Psychoactive medication use and neurocognitive function in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor study. Pediatr Blood Cancer. 2013;603:486–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Blank PM, Fisher MJ, Lu L et al. , Impact of vision loss among survivors of childhood central nervous system astroglial tumors. Cancer. 2016;1225:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kadan-Lottick NS, Zeltzer LK, Liu Q et al. , Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. J Natl Cancer Inst. 2010;10212:881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Cancer Institute. Cancer Therapy Evalaution Program (CTEP). Common Terminology Crtieria for Adverse Evenets (CTCAE). Published May 28, 2009. (updated June 14, 2010). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed March 24, 2017.
- 36. Schultebraucks K, Wingenfeld K, Heimes J et al. , Cognitive function in patients with primary adrenal insufficiency (Addison's disease). Psychoneuroendocrinology. 2015;55:1–7. [DOI] [PubMed] [Google Scholar]
- 37. Roncero C, Campuzano AI, Quintano JA et al. , Cognitive status among patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;111:543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paul RH, Gunstad J, Poppas A et al. , Neuroimaging and cardiac correlates of cognitive function among patients with cardiac disease. Cerebrovasc Dis. 2005;202:129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung YT, Edelmann MN, Mulrooney DA et al. , Uric acid and neurocognitive function in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Cancer Epidemiol Biomarkers Prev. 2016;258:1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheung YT, Chemaitilly W, Mulrooney DA et al. , Association between dehydroepiandrosterone-sulfate and attention in long-term survivors of childhood acute lymphoblastic leukemia treated with only chemotherapy. Psychoneuroendocrinology. 2016;76:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Batty GD, Deary IJ, Zaninotto P.. Association of cognitive function with cause-specific mortality in middle and older age: Follow-up of participants in the english longitudinal study of ageing. Am J Epidemiol. 2016;1833:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferreira L, Tanaka K, Santosgalduróz RF et al. , Respiratory training as strategy to prevent cognitive decline in aging: A randomized controlled trial. Clin Interv Aging. 2015;10:593–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. American Academy of Pediatrics Section on Hematology/Oncology Children's Oncology Group: Long-term follow-up care for pediatric cancer survivors. Pediatrics. 2009;1233:906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheung YT, Krull KR.. Neurocognitive outcomes in long-term survivors of childhood acute lymphoblastic leukemia treated on contemporary treatment protocols: A systematic review. Neurosci Biobehav Rev. 2015;53:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armenian SH, Robison LL.. Childhood cancer survivorship: An update on evolving paradigms for understanding pathogenesis and screening for therapy-related late effects. Curr Opin Pediatr. 2013;251:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kadan-Lottick NS, Brouwers P, Breiger D et al. , Comparison of neurocognitive functioning in children previously randomly assigned to intrathecal methotrexate compared with triple intrathecal therapy for the treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 2009;2735:5986–5992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schreiber JB, Stage FK, King J et al. , Reporting structural equation modeling and confirmatory factor analysis results: A review. J Educ Res. 2006;996:323–337. [Google Scholar]
- 48. Petersen JF, Larsen BS, Sabbah M et al. , Long-term prognostic significance of homocysteine in middle-aged and elderly. Biomarkers. 2016;216:490–496. [DOI] [PubMed] [Google Scholar]
- 49. Ruan L, Chen W, Srinivasan SR et al. , Relation of plasma homocysteine to arterial stiffness in black and white young adults (from the Bogalusa Heart Study). Am J Cardiol. 2009;1037:985–988. [DOI] [PubMed] [Google Scholar]
- 50. Seemungal TAR, Lun JCF, Davis G et al. , Plasma homocysteine is elevated in COPD patients and is related to COPD severity. Int J Chron Obstruct Pulmon Dis. 2007;23:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ford AH, Garrido GJ, Beer C et al. , Homocysteine, grey matter and cognitive function in adults with cardiovascular disease. PLoS One. 2012;73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sachdev P. Homocysteine, cerebrovascular disease and brain atrophy. J Neurol Sci. 2004;226(1–2):25–29. [DOI] [PubMed] [Google Scholar]
- 53. Gervais NJ, Mong JA, Lacreuse A.. Ovarian hormones, sleep and cognition across the adult female lifespan: An integrated perspective. Front Neuroendocrinol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao C, Bernstein LJ, Rich JB.. Executive functioning impairment in women treated with chemotherapy for breast cancer: A systematic review. Breast Cancer Res Treat. In press. [DOI] [PubMed] [Google Scholar]
- 55. Jain N, Brouwers P, Okcu MF et al. , Sex-specific attention problems in long-term survivors of pediatric acute lymphoblastic leukemia. Cancer. 2009;11518:4238–4245. [DOI] [PubMed] [Google Scholar]
- 56. Armstrong GT, Sklar CA, Hudson MM et al. , Long-term health status among survivors of childhood cancer: Does sex matter? J Clin Oncol. 2007;2528:4477–4489. [DOI] [PubMed] [Google Scholar]
- 57. Cheung YT, Brinkman TM, Mulrooney DA et al. , Impact of sleep, fatigue and systemic inflammation on neurocognitive and behavioral outcomes in long-term survivors of childhood acute lymphoblastic leukemia. Cancer. 2017;12317:3410–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hudson MM, Oeffinger KC, Jones K et al. , Age-dependent changes in health status in the Childhood Cancer Survivor cohort. J Clin Oncol. 2015;335:479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hudson MM, Neglia JP, Woods WG et al. , Lessons from the past: Opportunities to improve childhood cancer survivor care through outcomes investigations of historical therapeutic approaches for pediatric hematological malignancies. Pediatr Blood Cancer. 2012;583:334–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Green DM, Kun LE, Matthay KK et al. , Relevance of historical therapeutic approaches to the contemporary treatment of pediatric solid tumors. Pediatr Blood Cancer. 2013;607:1083–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.