Abstract
Background: Merkel cell carcinoma (MCC) has a high risk of recurrence after initial surgical therapy. Adjuvant radiation therapy (RT) and chemotherapy may be used to reduce the risk of locoregional and systemic recurrence, respectively, but there are conflicting data regarding their impact on survival. We performed a retrospective analysis of MCC cases from the National Cancer Data Base (NCDB) to assess whether adjuvant therapy was associated with differences in survival.
Methods: Six thousand nine hundred and eight MCC patients with staging, treatment, and survival data were included. Multivariable analyses were conducted for overall survival (OS) with various treatment modalities while adjusting for prognostic variables including age, sex, comorbidities (Charlson/Deyo score), margin status, primary tumor site and size, and lymph node status. All statistical tests were two-sided.
Results: For localized MCC (stage I: n = 3369, stage II: n = 1474 ), surgery plus adjuvant RT was associated with statistically significantly better OS than with surgery alone in multivariable analyses (stage I: hazard ratio [HR] = 0.71, 95% confidence interval [CI] = 0.64 to 0.80, P < .001; stage II: HR = 0.77, 95% CI = 0.66 to 0.89, P < .001). In patients with regional nodal metastases (stage III: n = 2065 ), neither adjuvant RT nor chemotherapy was associated with statistically significantly improved or worsened OS.
Conclusions: In this study of the largest MCC cohort reported to date, adjuvant RT was associated with improved OS in stages I-II MCC. Neither adjuvant RT nor chemotherapy was associated with improved OS in stage III MCC. These results, with the limitations of retrospective analyses, are consistent with earlier studies suggesting benefit with adjuvant RT but do not support the routine use of adjuvant chemotherapy in MCC.
Merkel cell carcinoma (MCC) is a rare and aggressive skin cancer with an estimated 1600 cases per year in the United States ( 1–3 ). The reported incidence has tripled over the past 20 years, and the health impact of MCC is growing rapidly ( 1 , 2 , 4 ). The increasing incidence could in part be because of improved diagnosis (eg, adoption of the cytokeratin-20 antibody [ 5 ]) but likely also reflects the higher prevalence of known risk factors for MCC: T-cell immune suppression and Caucasian ethnicity in individuals older than age 50 years with extensive prior sun exposure ( 6 ).
The prognosis for MCC patients is closely linked to stage at presentation. Per the American Joint Committee on Cancer (AJCC) classification system, stages I and II represent lower-risk and higher-risk localized primary disease, respectively, while stages III and IV represent the presence of nodal and distant metastases, respectively. The reported five-year relative survival for patients with localized, nodal, or distant metastatic disease at presentation is 64%, 39%, or 18%, respectively ( 3 ). Although surgery is curative for some patients with stages I-III, relapses are common ( 7 ). Because MCC tumors are generally considered to be sensitive to radiation therapy (RT) and/or chemotherapy, these therapies are commonly considered for adjuvant therapy after surgery in patients at high risk of loco-regional and systemic recurrence, respectively. Nonetheless, there are limited and sometimes conflicting data regarding the impact of adjuvant RT and chemotherapy on survival of MCC patients.
Previously reported studies of adjuvant RT and/or chemotherapy for MCC patients are mostly retrospective and have several limitations, which include small sample sizes and minimal information on potential confounders to address selection and treatment biases ( 8–14 ). The largest published studies evaluating the effects of adjuvant RT and chemotherapy in MCC analyzed 1166 and 102 patients, respectively ( 8 , 14 ). In our study, we assessed the survival impact of adjuvant RT and/or chemotherapy in 6908 MCC cases in the National Cancer Data Base (NCDB), a national tumor registry that captures approximately 70% of all cancer diagnoses in the United States. This retrospective study represents the largest known cohort of MCC patients (total n = 12 301) analyzed to date and also includes data on chemotherapy and comorbidity status, neither of which were available in prior studies using the Surveillance, Epidemiology, and End Results (SEER) database ( 8 , 9 ).
Methods
NCDB Cohort
The NCDB is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The data used in the study were derived from a de-identified NCDB file. The American Cancer Society (ACS) and the CoC have not verified the data files and are not responsible for analytic or statistical methodology employed, or the conclusions in this report.
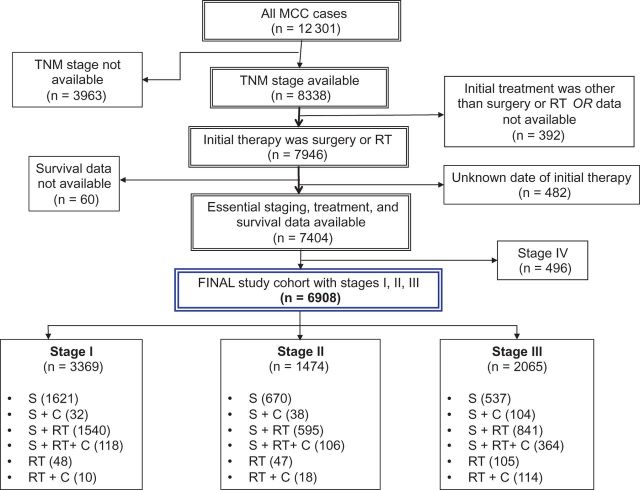
We identified 12 301 MCC cases diagnosed between January 1, 1996, and December 31, 2008, from the NCDB. Cases that had data available on TNM staging, survival status (with 5-year or longer follow-up), and treatment details (including the type of therapy [surgery, RT, chemotherapy] and the time of administration) were included in the analyses ( Figure 1 ). The final study cohort consisted of 6908 MCC patients who presented with stages I, II, or III and had received surgery and/or RT as the primary treatment modality. The primary RT group was included in this analysis because of the literature supporting the use of definitive RT ( 15–20 ). (Note: This group constitutes less than 5% of the study cohort, and its inclusion did not have a statistically significant impact on the surgery group analyses).
Figure 1.
Flow diagram for the selection of the National Cancer Data Base (NCDB) cohort that consists of 6908 Merkel cell carcinoma patients included in the final analyses of this study. C = chemotherapy; MCC = Merkel cell carcinoma; RT = radiation therapy; S = surgery; TNM = Tumor-Node-Metastases staging classification (per the American Joint Committee on Cancer).
In this retrospective and nonrandomized study, we included several potential confounding variables that can influence the natural history of MCC or the selection of treatment modalities. These variables include age, sex, primary MCC site, primary tumor size, comorbidity status, margin status, lymph node evaluation status, extent of lymph node resection, and the number of positive lymph nodes. Comorbidity scores are captured in the NCDB using the Charlson/Deyo index ( 21 ). Because the vast majority of the patients had Charlson/Deyo scores of either 0 or 1 (very few had a score > 1; see Table 1 ), we categorized the comorbidity status as absent (score = 0), present (score ≥1), or unknown (score not available). Margin status was categorized as positive (for residual tumor identified at either gross or microscopic examination of resected specimen), negative (no residual tumor identified), or unknown. Regional lymph node examination status (to capture the prognostic impact of sentinel lymph node biopsy) was included in analyses of MCC patients with stages I and II. The number of positive lymph nodes in stage III patients was also included. We have also attempted to capture the impact of regional lymphadenectomy in stage III patients by arbitrarily defining this procedure as being done if five or more lymph nodes were removed (assuming that a smaller number might have been removed as part of a staging procedure). Detailed information on some important variables was not available in the NCDB, and hence we could not include details regarding some aspects of primary surgery (wide excision or another type) or the site(s) of adjuvant radiation therapy (primary site and/or nodal) or chemotherapy specifics (dose, regimen, etc.) in the multivariable analyses.
Table 1.
Characteristics of the 6908 MCC patients in the NCDB study cohort
| Characteristic | Stage, No. (%) |
||
|---|---|---|---|
| Stage I (n = 3369) | Stage II (n = 1474) | Stage III (n = 2065) | |
| Treatment | |||
| Surgery | 1621 (48) | 670 (45) | 537 (26) |
| Surgery + RT | 1540 (46) | 595 (40) | 841 (41) |
| Surgery + chemo | 32 | 38 (3) | 104 (5) |
| Surgery + RT + chemo | 118 (4) | 106 (7) | 364 (18) |
| RT | 48 | 47 (3) | 105 (5) |
| RT + chemo | 10 (<1) | 18 | 114 (6) |
| Sex | |||
| Male | 1974 (59) | 881 (60) | 1356 (66) |
| Female | 1395 (41) | 593 (40) | 709 (34) |
| Age, y * | |||
| ≤64 | 634 (19) | 249 (17) | 436 (21) |
| 65–74 | 820 (24) | 339 (23) | 514 (25) |
| 75–84 | 1310 (39) | 526 (36) | 800 (39) |
| ≥85 | 605 (18) | 360 (24) | 315 (15) |
| Charlson/Deyo comorbidity score | |||
| 0 | 2102 (62) | 861 (58) | 1273 (62) |
| 1 | 389 (12) | 198 (13) | 277 (13) |
| 2+ | 86 (3) | 54 (4) | 79 (4) |
| Unknown | 792 (24) | 361 (24) | 436 (21) |
| Site of primary tumor | |||
| Head & neck | 1707 (51) | 522 (35) | 825 (40) |
| Trunk | 243 (7) | 223 (15) | 263 (13) |
| Limbs (and others) | 1419 (42) | 729 (49) | 977 (47) |
| Primary tumor size (diameter) † , cm | |||
| ≤1 | 1368 (41) | – | – |
| >1–≤2 | 1370 (41) | – | – |
| >2–≤3 | – | 713 (48) | – |
| >3–≤4 | – | 253 (17) | – |
| >4 | – | 333 (23) | – |
| Unknown | 631 (19) | 175 (12) | – |
| Margin status ‡ , § | |||
| Negative | 2905 (88) | 1095 (78) | 1437 (78) |
| Positive | 266 (8) | 245 (17) | 296 (16) |
| Unknown | 140 (4) | 69 (5) | 113 (6) |
| Lymph nodes examined? (stage I, II only) † , § | |||
| No | 1650 (50) | 832 (59) | – |
| Yes | 1621 (49) | 551 (39) | – |
| Unknown | 40 | 26 (2) | – |
| Regional lymphadenectomy (stage III only) § | |||
| No (<5 removed) | – | – | 777 (42) |
| Yes (≥5 removed) | – | – | 965 (52) |
| Unknown | – | – | 104 (6) |
| Number of metastatic nodes (stage III only) ‖ | |||
| 1 | – | – | 806 (39) |
| 2 | – | – | 289 (14) |
| 3–6 | – | – | 301 (15) |
| 7–12 | – | – | 122 (6) |
| 13–59 | – | – | 71 (3) |
| Unknown | – | – | 476 (23) |
*Median age at diagnosis for entire cohort was 76 years (range = 20–90 years). chemo = chemotherapy; MCC = Merkel cell carcinoma; NCDB = National Cancer Database; RT = radiation therapy.
†Listed only for stages I and II here, as were included in multivariable analyses for these stages.
‡Positive margin status indicates residual tumor identified, grossly or microscopically, after surgery.
§Includes surgery patients only (ie, excludes patients who got RT alone).
‖Regional lymphadenectomy was arbitrarily defined as done only if five or more lymph nodes were removed (as lesser number could have been done as part of the sentinel lymph node biopsy procedure). Also, this variable is listed for stage III only, as included in multivariable analyses for this stage.
Statistical Analyses
Multivariable Cox regression analyses were conducted for overall survival outcomes. The underlying time axis in the analyses is the time from diagnosis. Patients enter the analysis and become ‘at risk’ at the time of initial therapy (left truncation). Treatment groups were defined in a time-dependent manner to minimize potential bias that arises when early deaths prevent patients from receiving additional modalities; that is, delayed treatment modalities are only evaluated among those who are actually ‘at risk’ at the time that the modality is initiated. For example, patients were included in the surgery plus radiation group at the time that radiation was started; before that, they were included in the surgery group. Similarly, patients were included in the chemotherapy group when chemotherapy was started; before that, they were included in the no chemotherapy group.
Hazard ratios presented in this study are mortality hazard ratios. These summarize the relative mortality rates across time among patients at risk in the defined covariate groups. Patients with missing data on confounding variables were retained as a separate category to permit use of the full dataset. Analyses were carried out using SAS software (SAS Institute Inc., Cary, NC). All P values are two-sided and derived from Wald statistics. A P value of less than .05 was considered statistically significant.
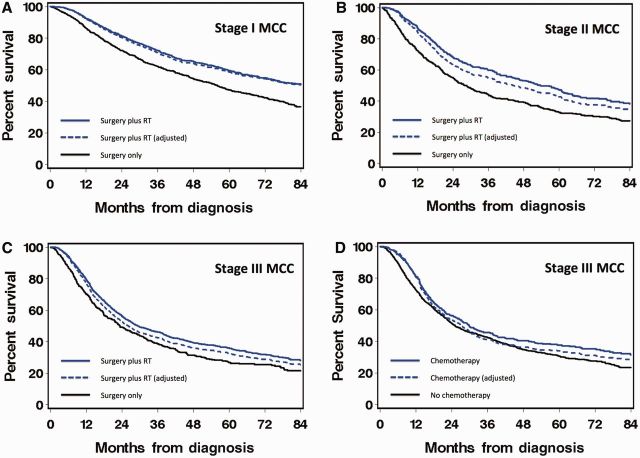
For purposes of graphical illustration only, survival curves ( Figure 2 ) are presented as a function of time since diagnosis and do not include the time-dependencies noted above. Solid lines represent actual survival as estimated by Kaplan-Meier. Dashed lines represent adjusted survival and use model-based adjustment for covariate imbalances to estimate the survival curves that would have arisen had both groups had the same distribution of covariates ( 22 ).
Figure 2.
Overall survival of patients with stage I (A) , II (B) , and III (C, D) Merkel cell carcinoma (MCC) by the type of adjuvant therapy. Adjuvant radiation therapy (RT) is represented in panels (A–C), and adjuvant chemotherapy is represented in (D) . Solid lines represent actual survival as estimated by Kaplan-Meier. Dashed lines represent adjusted survival and use model-based adjustment for covariate imbalances to estimate the survival curves that would have arisen had both groups had the same distribution of covariates (22). These curves are presented for purposes of graphical illustration only and ignore the time dependencies included in the multivariable analyses of mortality hazard ratios (see Methods). MCC = Merkel cell carcinoma.
Results
The study cohort includes 6908 MCC patients who presented as stage I, II, or III and had received surgery and/or RT as the primary treatment modality ( Figure 1 ). The baseline characteristics of the study cohort are summarized in Table 1 . The proportion of patients by stage at diagnosis was 49% (stage I), 21% (stage II), and 30% (stage III). The median age at diagnosis was 76 years (range: 20–90 years), and the male to female ratio was 1.6:1. The Charlson/Deyo comorbidity score was available for 77% of patients, and the majority (80%) of these patients had a score of ‘0.’ The distribution of the selected variables in different treatment groups is summarized in Tables 2 and 3 . Multivariable analyses for overall survival are shown in Tables 4 and 5 . As expected, increasing age, male sex, the presence of comorbidities, positive margin status, and increasing tumor burden (reflected by primary tumor size in stages I and II and by the number of positive lymph nodes in stage III) were associated with poorer survival across all stages.
Table 2.
Patient characteristics according to stage and surgery/radiation
| Characteristic | Stage I (n = 3369), % |
Stage II (n = 1474), % |
Stage III (n = 2065), % |
||||||
|---|---|---|---|---|---|---|---|---|---|
| S (n = 1653) | S + RT (n = 1658) | RT (n = 58) | S (n = 708) | S + RT (n = 701) | RT (n = 65) | S (n = 641) | S + RT (n = 1205) | RT (n = 219) | |
| Received adjuvant chemotherapy | |||||||||
| No | 98 | 93 | 83 | 95 | 85 | 72 | 84 | 70 | 48 |
| Yes | 2 | 7 | 17 | 5 | 15 | 28 | 16 | 30 | 52 |
| Age at diagnosis, y | |||||||||
| ≤64 | 18 | 20 | 9 | 14 | 20 | 12 | 17 | 23 | 23 |
| 65–74 | 24 | 25 | 17 | 21 | 26 | 11 | 21 | 26 | 27 |
| 75–84 | 38 | 40 | 38 | 34 | 36 | 48 | 42 | 38 | 33 |
| ≥85 | 20 | 15 | 36 | 31 | 17 | 29 | 20 | 12 | 18 |
| Sex | |||||||||
| Male | 58 | 59 | 59 | 56 | 63 | 63 | 63 | 66 | 73 |
| Female | 42 | 41 | 41 | 44 | 37 | 37 | 37 | 34 | 27 |
| Comorbidity | |||||||||
| Absent | 61 | 63 | 66 | 56 | 61 | 65 | 60 | 61 | 73 |
| Present | 16 | 13 | 5 | 19 | 15 | 12 | 18 | 18 | 10 |
| Unknown | 23 | 24 | 29 | 25 | 24 | 23 | 22 | 21 | 17 |
| Margin status * | |||||||||
| Negative | 89 | 87 | – | 78 | 77 | – | 77 | 78 | – |
| Positive | 6 | 10 | – | 17 | 18 | – | 17 | 16 | – |
| Unknown | 5 | 4 | – | 6 | 4 | – | 6 | 6 | – |
| Lymph nodes examined? (stages I, II only) * , † | |||||||||
| No | 49 | 50 | – | 64 | 54 | – | – | – | – |
| Yes | 49 | 49 | – | 34 | 44 | – | – | – | – |
| Unknown | 1 | 1 | – | 2 | 1 | – | – | – | – |
| Lymphadenectomy (stage III only) * , ‡ | |||||||||
| No (<5 removed) | – | – | – | – | – | – | 45 | 41 | – |
| Yes (≥5 removed) | – | – | – | – | – | – | 50 | 53 | – |
| Unknown | – | – | – | – | – | – | 5 | 6 | – |
| Site of primary tumor | |||||||||
| Head & neck | 48 | 53 | 67 | 32 | 37 | 63 | 39 | 41 | 36 |
| Trunk | 8 | 7 | 5 | 15 | 16 | 5 | 14 | 13 | 8 |
| Limbs (and others) | 44 | 41 | 28 | 53 | 47 | 32 | 48 | 45 | 57 |
| Primary tumor size (stages I, II only) † , cm | |||||||||
| ≤1 | 40 | 42 | 26 | – | – | – | – | – | – |
| >1–≤2 | 41 | 40 | 47 | – | – | – | – | – | – |
| >2–≤3 | – | – | – | 47 | 51 | 26 | – | – | – |
| >3–≤4 | – | – | – | 20 | 14 | 20 | – | – | – |
| >4 | – | – | – | 22 | 22 | 34 | – | – | – |
| Unknown | 19 | 18 | 28 | 11 | 12 | 20 | – | – | – |
| Number of involved nodes (stage III only) | |||||||||
| 1 | – | – | – | – | – | – | 42 | 40 | 25 |
| 2 | – | – | – | – | – | – | 14 | 16 | 5 |
| 3-6 | – | – | – | – | – | – | 13 | 17 | 5 |
| 7–12 | – | – | – | – | – | – | 7 | 6 | 2 |
| 13–59 | – | – | – | – | – | – | 4 | 3 | 2 |
| Unknown | – | – | – | – | – | – | 20 | 18 | 60 |
*Includes surgery patients (ie, excludes patients who got RT alone). RT = radiation therapy; S = surgery.
†Listed for stages I, II only as included in multivariable analyses for these stages.
‡Listed for stage III only as included in multivariable analyses for this stage.
Table 3.
Patient characteristics according to stage and chemotherapy
| Characteristic | Stage I (n = 3369), % |
Stage II (n = 1474), % |
Stage III (n = 2065), % |
|||
|---|---|---|---|---|---|---|
| No chemo (n = 3209) | Chemo (n = 160) | No chemo (n = 1312) | Chemo (n = 162) | No chemo (n = 1483) | Chemo (n = 582) | |
| Surgery/RT administration | ||||||
| Surgery only | 51 | 20 | 51 | 23 | 36 | 18 |
| Surgery plus RT | 48 | 74 | 45 | 65 | 57 | 63 |
| RT only | 2 | 6 | 4 | 11 | 7 | 20 |
| Age at diagnosis, y | ||||||
| ≤64 | 18 | 39 | 15 | 35 | 15 | 37 |
| 65–74 | 24 | 33 | 22 | 32 | 23 | 30 |
| 75–84 | 40 | 25 | 37 | 25 | 43 | 28 |
| ≥85 | 19 | 3 | 27 | 7 | 20 | 6 |
| Sex | ||||||
| Male | 58 | 63 | 59 | 66 | 65 | 68 |
| Female | 42 | 38 | 41 | 34 | 35 | 32 |
| Comorbidity | ||||||
| Absent | 63 | 55 | 59 | 54 | 61 | 63 |
| Present | 14 | 12 | 17 | 16 | 17 | 17 |
| Unknown | 23 | 33 | 24 | 30 | 22 | 20 |
| Margin status * | ||||||
| Negative | 88 | 81 | 78 | 74 | 80 | 72 |
| Positive | 8 | 16 | 17 | 19 | 14 | 21 |
| Unknown | 4 | 3 | 5 | 7 | 6 | 7 |
| Lymph nodes examined? (stages I, II only) * , † | ||||||
| No | 50 | 54 | 60 | 53 | – | – |
| Yes | 49 | 43 | 39 | 42 | – | – |
| Unknown | 1 | 3 | 2 | 4 | – | – |
| Lymphadenectomy? (stage III only) * , ‡ | ||||||
| No (<5 removed) | – | – | – | – | 43 | 39 |
| Yes (≥5 removed) | – | – | – | – | 52 | 53 |
| Unknown | – | – | – | – | 5 | 8 |
| Site of primary tumor | ||||||
| Head & neck | 51 | 44 | 36 | 29 | 44 | 29 |
| Trunk | 7 | 9 | 14 | 22 | 11 | 17 |
| Limbs (and others) | 42 | 46 | 49 | 49 | 45 | 54 |
| Primary tumor size † , cm | ||||||
| ≤1 | 41 | 33 | – | – | – | – |
| >1 | 41 | 43 | – | – | – | – |
| Unknown | 18 | 25 | – | – | – | – |
| ≤3 | – | – | 50 | 34 | – | – |
| >3–≤4 | – | – | 17 | 17 | – | – |
| >4 | – | – | 21 | 35 | – | – |
| Unknown | – | – | 12 | 14 | – | – |
| Number of involved nodes (stage III only) | ||||||
| 1 | – | – | – | – | 42 | 32 |
| 2 | – | – | – | – | 15 | 13 |
| 3-6 | – | – | – | – | 13 | 18 |
| 7–12 | – | – | – | – | 6 | 6 |
| 13–59 | – | – | – | – | 4 | 3 |
| Unknown | – | – | – | – | 21 | 28 |
*Includes surgery patients (ie, excludes patients who got RT alone). chemo = chemotherapy; RT = radiation therapy.
†Listed for stages I, II only as included in multivariable analyses for these stages.
‡Listed for stage III only as included in multivariable analyses for this stage.
Table 4.
Multivariable analysis of risk factors for mortality for localized MCC (stages I and II)
| Risk factor | Stage I(n = 3369) (1341 deaths) |
Stage II(n = 1474) (800 deaths) |
||
|---|---|---|---|---|
| HR (95% CI) | P * | HR (95% CI) | P * | |
| Surgery/radiation | ||||
| Surgery only | 1.0 | – | 1.0 | – |
| Surgery + radiation | 0.71 (0.64 to 0.80) | <.001 | 0.77 0.66 to 0.89) | <.001 |
| Radiation only | 0.92 (0.65 to 1.30) | .62 | 1.03 (0.73 to 1.46) | .85 |
| Chemotherapy | ||||
| No | 1.0 | – | 1.0 | – |
| Yes | 0.79 (0.60 to 1.05) | .11 | 1.14 (0.89 to 1.45) | .30 |
| Age at diagnosis, y | ||||
| ≤64 | 1.0 | – | 1.0 | – |
| 65–74 | 1.58 (1.26 to 1.98) | <.001 | 1.71 (1.25 to 2.33) | <.001 |
| 75–84 | 2.62 (2.13 to 3.23) | <.001 | 3.75 (2.80 to 5.02) | <.001 |
| ≥85 | 4.68 (3.75 to 5.85) | <.001 | 6.17 (4.54 to 8.39) | <.001 |
| Sex | ||||
| Male | 1.0 | – | 1.0 | – |
| Female | 0.56 (0.50 to 0.63) | <.001 | 0.66 (0.57 to 0.77) | <.001 |
| Comorbidity | ||||
| Absent | 1.0 | 1.0 | ||
| Present | 1.35 (1.14 to 1.60) | <.001 | 1.64 (1.34 to 2.00) | <.001 |
| Unknown | 1.10 (0.97 to 1.25) | .13 | 1.10 (0.93 to 1.29) | .27 |
| Margin status | ||||
| Negative | 1.0 | – | 1.0 | – |
| Positive | 1.23 (1.03 to 1.48) | .02 | 1.49 (1.24 to 1.79) | <.001 |
| Unknown | 0.97 (0.76 to 1.24) | .82 | 1.34 (1.00 to 1.79) | .05 |
| Lymph nodes examined? | ||||
| No | 1.0 | – | 1.0 | – |
| Yes | 0.57 (0.51 to 0.65) | <.001 | 0.65 (0.55 to 0.78) | <.001 |
| Unknown | 1.08 (0.72 to 1.61) | 0.72 | 1.30 (0.77 to 2.20) | .33 |
| Primary tumor site | ||||
| Limbs (and others) | 1.0 | – | 1.0 | – |
| Trunk | 1.48 (1.20 to 1.82) | <.001 | 1.46 (1.19 to 1.79) | <.001 |
| Head & neck | 1.25 (1.10 to 1.41) | .0004 | 1.05 (0.90 to 1.23) | .55 |
| Primary tumor size, cm | ||||
| ≤1 | 1.0 | – | – | – |
| >1 | 1.58 (1.39 to 1.79) | <.001 | – | – |
| Unknown | 1.22 (1.04 to 1.41) | .01 | – | – |
| ≤3 | – | – | 1.0 | – |
| >3–≤4 | – | – | 1.26 (1.03 to 1.53) | .02 |
| >4 | – | – | 1.24 (1.03 to 1.48) | .02 |
| Unknown | – | – | 0.95 (0.75 to 1.20) | .65 |
*All P values are two-sided and derived from Wald statistics. CI = confidence interval; HR = hazard ratio (for overall survival); MCC = Merkel cell carcinoma.
Table 5.
Multivariable analysis of risk factors for mortality for nodal MCC (stage III)
| Risk factor | Stage III (n = 2065) (1195 deaths) |
|
|---|---|---|
| HR (95% CI) | P * | |
| Surgery/radiation | ||
| Surgery only | 1.0 | |
| Surgery + radiation | 0.98 (0.86 to 1.12) | .80 |
| Radiation only | 1.19 (0.95 to 1.50) | .13 |
| Chemotherapy | ||
| No | 1.0 | |
| Yes | 0.97 (0.85 to 1.12) | .71 |
| Age at diagnosis, y | ||
| ≤64 | 1.0 | |
| 65–74 | 1.26 (1.04 to 1.53) | .02 |
| 75–84 | 2.01 (1.68 to 2.39) | <.001 |
| ≥85 | 3.24 (2.63 to 3.98) | <.001 |
| Sex | ||
| Male | 1.0 | |
| Female | 0.79 (0.69 to 0.89) | <.001 |
| Comorbidity | ||
| Absent | 1.0 | |
| Present | 1.31 (1.12 to 1.54) | <.001 |
| Unknown | 1.14 (0.99 to 1.31) | .07 |
| Margin status | ||
| Negative | 1.0 | |
| Positive | 1.46 (1.25 to 1.71) | <.001 |
| Unknown | 1.01 (0.79 to 1.30) | .95 |
| Lymphadenectomy? | ||
| No (<5 nodes removed) | 1.0 | |
| Yes (≥5 nodes removed) | 0.70 (0.60 to 0.82) | <.001 |
| Unknown | 1.10 (0.84 to 1.45) | .47 |
| Primary tumor site | ||
| Limbs (and others) | 1.0 | |
| Trunk | 1.34 (1.12 to 1.61) | .001 |
| Head & neck | 1.16 (1.01 to 1.32) | .03 |
| Number of involved nodes | ||
| 1 | 1.0 | |
| 2 | 1.36 (1.13 to 1.65) | .001 |
| 3–6 | 1.75 (1.45 to 2.12) | <.001 |
| 7+ | 2.69 (2.17 to 3.33) | <.001 |
| Unknown | 1.34 (113 to 1.59) | <.001 |
*All P values are two-sided and derived from Wald statistics. CI = confidence interval; HR = hazard ratio (for overall survival); MCC = Merkel cell carcinoma.
Adjuvant Radiation
Comparison of patient characteristics in different treatment groups (surgery alone, surgery plus RT, RT alone) for stages I, II, and III is shown in Table 2 . Nearly half of patients in each stage received surgery plus RT (50%, 47%, 59%, for stages I, II, and III, respectively). Multivariable analyses of overall survival were performed in all three stages. The survival of patients who had received surgery alone was compared with those who had received surgery plus RT. After adjustment for the other variables, patients who had surgery plus RT had statistically significantly improved overall survival compared with patients who had surgery alone among those with localized MCC (stage I: HR = 0.71, 95% CI = 0.64 to 0.80, P < .001; stage II: HR = 0.77, 95% CI = 0.66 to 0.89, P < .001) ( Table 4 , Figure 2, A and B ). In patients with nodal involvement, there was no statistically significant difference in overall survival between patients who had surgery plus RT vs surgery alone (stage III: HR = 0.98, 95% CI = 0.86 to 1.12, P = .80) ( Table 5 , Figure 2C ).
Adjuvant Chemotherapy
As expected, patients with localized disease rarely received chemotherapy ( Table 1 ). In contrast, 29% of stage III patients received adjuvant chemotherapy ( Table 1 ). Compared with patients who did not receive chemotherapy, those who received adjuvant chemotherapy were more likely to be younger and have more than one involved lymph node but otherwise had a similar distribution of sex and comorbidity scores ( Table 3 ). In multivariable analyses, adjuvant chemotherapy was not associated with statistically significantly different overall survival in stage I (HR = 0.79, 95% CI = 0.60 to 1.05, P = .11), stage II (HR = 1.14, 95% CI = 0.89 to 1.45, P = .30) ( Table 4 ), or stage III (HR = 0.97, 95% CI = 0.85 to 1.12, P = .71) ( Table 5 , Figure 2D ).
Discussion
Our retrospective analysis of 6908 MCC patients suggests that adjuvant radiation therapy is associated with improved survival in patients with localized (stages I and II) MCC. However, neither adjuvant RT nor adjuvant chemotherapy was associated with improved survival for patients with stage III MCC. This study represents the largest cohort analyzed to date for outcomes with adjuvant therapy in this rare skin cancer. In particular, it represents the first large dataset that examines the association of adjuvant chemotherapy with survival in MCC. It also includes a much greater number of patients included in multivariable analyses (6908 as compared with 747 patients in the SEER cohort [ 9 ]) and includes several additional prognostic variables in the multivariable analyses (discussed below) that were not included in prior studies, including those using the SEER database ( 8 , 9 ).
There are several important limitations of this retrospective, nonrandomized analysis of a population database analysis. First, the NCDB does not capture disease-specific mortality or relapse data, hence compromising the ability to assess the impact of adjuvant therapies on the natural history of the disease. Second, the comparison groups are not randomized and unknown confounders, which could influence the natural history of disease and/or the selection/administration of treatment modalities, may be affecting the results. Third, the available NCDB data on many variables may not fully capture the impact of potential confounders. For example, the Charlson/Deyo score may not accurately represent the impact of comorbidities on the performance status of patients, which in turn correlates strongly with outcomes in most cancers. It does not include immune-suppressive comorbidities that are usually associated with poor outcomes in MCC patients ( 23 ). Additionally, a sizable proportion of the stage I (50%) or stage II (60%) patients in the study cohort did not undergo pathologic lymph node evaluation (not surprising given the relatively recent incorporation of sentinel lymph node mapping in the routine management of localized MCC). Finally, several important details relevant to the administration of various treatment modalities, including the extent, type, or site(s) of treatment and specific doses and drug regimens used are not available in the NCDB. Despite these important limitations, results from this study may be useful in considering the impact of adjuvant therapies for MCC, a rare disease for which there are no data from prospective randomized controlled trials.
The finding of improved overall survival associated with adjuvant RT in patients with localized (stage I or II) MCC in our study mirrors similar observations reported previously. The largest published study to date on adjuvant RT was comprised of 1166 patients (including stages I, II, and III) in the SEER registry and reported a positive association between adjuvant RT and survival in a univariate analysis ( 8 ). This association remained statistically significant in a multivariable analysis of 603 patients that included sex, age, size and location of the primary tumor, stage, and the extent of resection ( 8 ). Another SEER study conducted a multivariable analysis of 747 patients (including stages I, II, and III) and reported adjuvant RT to be associated with statistically significantly improved OS but not with MCC-specific survival ( 9 ), suggesting patient selection bias rather than treatment effect. Several other studies have also associated adjuvant RT with decreased risk of loco-regional recurrences and/or improved overall survival ( 7 , 10–12 , 24 ). However, these studies were usually missing data on several important variables such as the comorbidity/performance status, the margin status of resections, the status of lymph node evaluation (for localized MCC), the number of involved regional lymph nodes, and the use of chemotherapy. The prognostic importance of many of these variables was confirmed in our study. Specifically, we confirmed previously reported ( 2 ) worsened survival associated with increasing age, male sex, presence of comorbidities, positive surgical margins, primary tumor location in trunk or head/neck, absence of sentinel lymph node biopsy (in stages I and II), increasing primary tumor size (in stages I and II), and increasing number of tumor-positive lymph nodes (in stage III). However, our data do not allow us to analyze whether some subsets of stage I/II MCC defined by these or other prognostic factors benefit more or less than others from adjuvant RT. A subset of patients with early-stage MCC may have good outcomes with surgery alone without additional benefit from the use of adjuvant RT, as has previously been proposed by some institutions ( 7 , 25 ). Incorporation of alternative predictors of recurrence, such as primary tumor lympho-vascular invasion ( 7 ) or intratumoral CD8+ T-cell infiltration status ( 26 ), may be important to identification of this subset.
We did not find a statistically significant association between adjuvant RT and OS in stage III MCC. It is plausible that the survival in stage III MCC is mostly driven by the presence of subclinical distant metastases that are often present in these patients ( 7 ). Unfortunately, the available NCDB data did not contain important details regarding the site of adjuvant radiation (primary and/or nodal) and was also insufficient to study the effects of adjuvant RT in microscopic (stage IIIA) vs macroscopic (stage IIIB) nodal disease. Based on our findings, we suggest that the use of adjuvant RT in a patient with nodal metastases should carefully consider the risks of RT (scar/fibrosis, lymphedema, etc.) vs its well-documented benefit for improving loco-regional control ( 11 ), rather than improving survival. Loco-regional control from adjuvant RT may not be unimportant as loco-regional relapses can be quite challenging to manage and are detrimental to patients’ quality of life.
The currently available data regarding adjuvant chemotherapy in MCC are quite limited ( 7 , 13 , 14 , 24 ). A single-institution retrospective study reported worsened disease-specific survival with adjuvant chemotherapy in multivariable analysis of 237 patients with stages I-III MCC; the disease-specific survival in the subgroup of stage III MCC patients who received adjuvant chemotherapy (n = 23) as compared with those who did not (n = 53) ( 13 ) was not statistically significantly different. Another study of 102 patients with high-risk localized or nodal MCC included 40 patients who received adjuvant chemotherapy and reported no statistically significant survival improvement associated with its use ( 14 ). A recent meta-analysis of 34 studies reported no additional survival benefit with adjuvant chemo-radiotherapy (n = 301 total; 132 with nodal involvement) over adjuvant RT ( 24 ). The present study includes the largest number of patients analyzed to date regarding outcomes with adjuvant chemotherapy. Approximately 30% (n = 583) of all stage III patients (n = 2065) in our study cohort received adjuvant chemotherapy. After adjustment for the known prognostic parameters in multivariable analysis, adjuvant chemotherapy was not associated with improved survival in stage III patients. This result may be considered somewhat surprising by some, especially given the fairly high response rate (greater than 50%) seen with initial cytotoxic chemotherapy in patients with metastatic MCC ( 27 ). However, despite the high initial response rates, the responses are seldom durable (the median progression-free survival in a recent report was a modest 93 days ( 28 ]), reflecting the rapid emergence of resistance to chemotherapy in MCC tumor cells. It is also plausible that the unintended adverse effects of chemotherapy on the host immune system may negate any potential benefit derived from its cytotoxic effects on the MCC cells. Indeed, several studies have suggested a strong association of host immunity to survival in MCC patients ( 23 , 26 , 29 ), an association that has been greatly bolstered by the recent discovery of the Merkel cell polyomavirus and its role in MCC pathogenesis ( 30–32 ). Fueled by the recent advances in our understanding of immune evasion mechanisms in this cancer ( 32–34 ), several immunotherapy clinical trials have recently been launched for patients with advanced MCC. Pembrolizumab (anti-PD-1 antibody) is being investigated in treatment-naive metastatic MCC ( www.clinicaltrials.gov ; NCT02267603) and was recently reported to have a high response rate (70%) in this setting ( 35 ). Avelumab (anti-PDL-1 antibody) is being investigated in metastatic MCC previously treated with chemotherapy (NCT02155647). The results of these ongoing trials may help to identify candidate systemic therapies worthy of prospective investigation in the adjuvant setting. A randomized trial of adjuvant ipilimumab vs observation in patients with resected MCC has recently opened to accrual in Europe (NCT02196961).
In conclusion, the results of our study suggest improved survival associated with the use of adjuvant RT in stage I and II MCC. However, neither adjuvant RT nor chemotherapy was associated with improved survival in stage III MCC. These survival-associated findings should not impact the use of adjuvant RT in node-positive disease as RT in this setting is indicated for improving loco-regional control, which is supported with extensive existing data. In contrast, the lack of an observed survival benefit with adjuvant chemotherapy in node-positive MCC has clinically important implications for management as improved survival via elimination of microscopic disease is in fact the goal of adjuvant chemotherapy. Prospective multicenter evaluations of adjuvant therapies for MCC are warranted, and it is hoped that newer therapeutic modalities, including immune therapies, will offer improved options in both the adjuvant and metastatic settings.
Funding
This work was supported by: National Center for Advancing Translational Sciences (NCATS) grants TL1 TR000422, P30-CA015704-39, K24-CA139052, and R01-CA162522 from the National Institutes of Health (NIH); Michael Piepkorn endowment; and Merkel patient gift funds.
Notes
The results of this study were presented in a Poster discussion session at the 2014 Annual ASCO meeting in Chicago on June 2, 2014.
The authors declare no conflicts of interest.
The study funders had no role the design of the study; the collection, analysis, or interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication. The data used for the study analyses was procured from a de-identified file from the National Cancer Data Base (NCDB), a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The ACS and the CoC have not verified the data files and are not responsible for analytic or statistical methodology employed or the conclusions in this report.
References
- 1. Lemos B, Nghiem P. Merkel cell carcinoma: more deaths but still no pathway to blame . J Invest Dermatol . 2007. ; 127 ( 9 ): 2100 – 2103 [DOI] [PubMed] [Google Scholar]
- 2. Albores-Saavedra J, Batich K, Chable-Montero F , et al. . Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: a population based study . J Cutan Pathol. 2010. ; 37 ( 1 ): 20 – 27 . [DOI] [PubMed] [Google Scholar]
- 3. Lemos BD, Storer BE, Iyer JG , et al. . Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system . J Am Acad Dermatol. 2010. ; 63 ( 5 ): 751 – 761 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodgson NC. Merkel cell carcinoma: changing incidence trends . J Surg Oncol. 2005. ; 89 ( 1 ): 1 – 4 . [DOI] [PubMed] [Google Scholar]
- 5. Moll R, Löwe A, Laufer J , et al. . Cytokeratin 20 in human carcinomas. A new histodiagnostic marker detected by monoclonal antibodies . Am J Pathol. 1992. ; 140 ( 2 ): 427 – 447 . [PMC free article] [PubMed] [Google Scholar]
- 6. Heath M, Jaimes N, Lemos B , et al. . Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features . J Am Acad Dermatol. 2008. ; 58 ( 3 ): 375 – 381 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fields RC, Busam KJ, Chou JF , et al. . Recurrence after complete resection and selective use of adjuvant therapy for stage I through III Merkel cell carcinoma . Cancer. 2012. ; 118 ( 13 ): 3311 – 3320 . [DOI] [PubMed] [Google Scholar]
- 8. Mojica P, Smith D, Ellenhorn JD. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin . J Clin Oncol. 2007. ; 25 ( 9 ): 1043 – 1047 . [DOI] [PubMed] [Google Scholar]
- 9. Kim JA, Choi AH. Effect of radiation therapy on survival in patients with resected Merkel cell carcinoma: a propensity score surveillance, epidemiology, and end results database analysis . JAMA Dermatol. 2013. ; 149 ( 7 ): 831 – 838 . [DOI] [PubMed] [Google Scholar]
- 10. Jouary T, Leyral C, Dreno B , et al. . Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: a multicentric prospective randomized study . Ann Oncol. 2012. ; 23 ( 4 ): 1074 – 1080 . [DOI] [PubMed] [Google Scholar]
- 11. Lewis KG, Weinstock MA, Weaver AL , et al. . Adjuvant local irradiation for Merkel cell carcinoma . Arch Dermatol. 2006. ; 142 ( 6 ): 693 – 700 . [DOI] [PubMed] [Google Scholar]
- 12. Jabbour J, Cumming R, Scolyer RA , et al. . Merkel cell carcinoma: assessing the effect of wide local excision, lymph node dissection, and radiotherapy on recurrence and survival in early-stage disease--results from a review of 82 consecutive cases diagnosed between 1992 and 2004 . Ann Surg Oncol. 2007. ; 14 ( 6 ): 1943 – 1952 . [DOI] [PubMed] [Google Scholar]
- 13. Allen PJ, Bowne WB, Jaques DP , et al. . Merkel cell carcinoma: prognosis and treatment of patients from a single institution . J Clin Oncol. 2005. ; 23 ( 10 ): 2300 – 2309 . [DOI] [PubMed] [Google Scholar]
- 14. Poulsen MG, Rischin D, Porter I , et al. . Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int J Radiat Oncol Biol Phys. 2006. ; 64 ( 1 ): 114 – 119 . [DOI] [PubMed] [Google Scholar]
- 15. Mortier L, Mirabel X, Fournier C , et al. . Radiotherapy alone for primary Merkel cell carcinoma . Arch Dermatol. 2003. ; 139 ( 12 ): 1587 – 1590 . [DOI] [PubMed] [Google Scholar]
- 16. Fang LC, Lemos B, Douglas J , et al. . Radiation monotherapy as regional treatment for lymph node-positive Merkel cell carcinoma . Cancer. 2010. ; 116 ( 7 ): 1783 – 1790 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pacella J, Ashby M, Ainslie J , et al. . The role of radiotherapy in the management of primary cutaneous neuroendocrine tumors (Merkel cell or trabecular carcinoma): experience at the Peter MacCallum Cancer Institute (Melbourne, Australia) . Int J Radiat Oncol Biol Phys. 1988. ; 14 ( 6 ): 1077 – 1084 . [DOI] [PubMed] [Google Scholar]
- 18. Sundaresan P, Hruby G, Hamilton A , et al. . Definitive radiotherapy or chemoradiotherapy in the treatment of Merkel cell carcinoma . Clin Oncol (R Coll Radiol). 2012. ; 24 ( 9 ): e131 – e136 . [DOI] [PubMed] [Google Scholar]
- 19. Suntharalingam M, Rudoltz MS, Mendenhall WM , et al. . Radiotherapy for Merkel cell carcinoma of the skin of the head and neck . Head Neck. 1995. ; 17 ( 2 ): 96 – 101 . [DOI] [PubMed] [Google Scholar]
- 20. Veness M, Foote M, Gebski V , et al. . The role of radiotherapy alone in patients with Merkel cell carcinoma: reporting the Australian experience of 43 patients . Int J Radiat Oncol Biol Phys. 2010. ; 78 ( 3 ): 703 – 709 . [DOI] [PubMed] [Google Scholar]
- 21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases . J Clin Epidemiol. 1992. ; 45 ( 6 ): 613 – 619 . [DOI] [PubMed] [Google Scholar]
- 22. Storer BE, Gooley TA, Jones MP. Adjusted estimates for time-to-event endpoints . Lifetime Data Anal. 2008. ; 14 ( 4 ): 484 – 495 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Paulson KG, Iyer JG, Blom A , et al. . Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage . J Invest Dermatol. 2013. ; 133 ( 3 ): 642 – 646 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hasan S, Liu L, Triplet J , et al. . The role of postoperative radiation and chemoradiation in Merkel cell carcinoma: a systematic review of the literature . Front Oncol. 2013. ; 3 : 276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bajetta E, Celio L, Platania M , et al. . Single-institution series of early-stage Merkel cell carcinoma: long-term outcomes in 95 patients managed with surgery alone . Ann Surg Oncol. 2009. ; 16 ( 11 ): 2985 – 2993 . [DOI] [PubMed] [Google Scholar]
- 26. Paulson KG, Iyer JG, Simonson WT , et al. . CD8+ lymphocyte intratumoral infiltration as a stage-independent predictor of Merkel cell carcinoma survival: a population-based study . Am J Clin Pathol. 2014. ; 142 ( 4 ): 452 – 458 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tai PT, Yu E, Winquist E , et al. . Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases . J Clin Oncol. 2000. ; 18 ( 12 ): 2493 – 2499 . [DOI] [PubMed] [Google Scholar]
- 28. Iyer J, Blom A, Doumani R. et al. . Response rate and durability of chemotherapy for metastatic Merkel cell carcinoma among 62 patients . J Clin Oncol . 2014. ; 32:5s(Suppl) ;abstr 9091. [Google Scholar]
- 29. Paulson KG, Iyer JG, Tegeder AR , et al. . Transcriptome-wide studies of merkel cell carcinoma and validation of intratumoral CD8+ lymphocyte invasion as an independent predictor of survival . J Clin Oncol. 2011. ; 29 ( 12 ): 1539 – 1546 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feng H, Shuda M, Chang Y , et al. . Clonal integration of a polyomavirus in human Merkel cell carcinoma . Science. 2008. ; 319 ( 5866 ): 1096 – 1100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shuda M, Feng H, Kwun HJ , et al. . T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus . Proc Natl Acad Sci U S A. 2008. ; 105 ( 42 ): 16272 – 16277 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhatia S, Afanasiev O, Nghiem P. Immunobiology of Merkel cell carcinoma: implications for immunotherapy of a polyomavirus-associated cancer . Curr Oncol Rep. 2011. ; 13 ( 6 ): 488 – 497 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iyer JG, Afanasiev OK, McClurkan C , et al. . Merkel cell polyomavirus-specific CD8(+) and CD4(+) T-cell responses identified in Merkel cell carcinomas and blood . Clin Cancer Res. 2011. ; 17 ( 21 ): 6671 – 6680 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paulson KG, Tegeder A, Willmes C , et al. . Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma . Cancer Immunol Res. 2014. ; 2 ( 11 ): 1071 – 1079 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nghiem P, Bhatia S, Daud A , et al. . Activity of PD-1 blockade with pembrolizumab as first systemic therapy in patients with advanced Merkel cell carcinoma. ESMO Annual Meeting . 2015:22LBA.