Abstract
Converging preclinical and human evidence indicates that the decline in ovarian estradiol production during the menopausal transition may play a mechanistic role in the neuronal changes that occur early in the aging process. Here, we present findings from a population-based fMRI study characterizing regional and network-level differences in working memory (WM) circuitry in midlife men and women (N = 142; age range 46–53), as a function of sex and reproductive stage. Reproductive histories and hormonal evaluations were used to determine menopausal status. Participants performed a verbal WM task during fMRI scanning. Results revealed robust differences in task-evoked responses in dorsolateral prefrontal cortex and hippocampus as a function of women's reproductive stage, despite minimal variance in chronological age. Sex differences in regional activity and functional connectivity that were pronounced between men and premenopausal women were diminished for postmenopausal women. Critically, analyzing data without regard to sex or reproductive status obscured group differences in the circuit-level neural strategies associated with successful working memory performance. These findings underscore the importance of reproductive age and hormonal status, over and above chronological age, for understanding sex differences in the aging of memory circuitry. Further, these findings suggest that early changes in working memory circuitry are evident decades before the age range typically targeted in cognitive aging studies.
Keywords: cognitive aging, estradiol, fMRI, prefrontal cortex, sex steroid hormones
Introduction
Age-related deficits in cognitive function, in the absence of overt neurodegenerative disease, are well-established. Normal aging is accompanied by a decline in working memory (Verhaeghen and Salthouse 1997; Park et al. 2002), the ability to maintain and manipulate information over short timescales (Baddeley and Hitch 1974; Baddeley 1986). Preclinical and human studies have characterized the cellular, synaptic, and network-level changes in working memory (WM) circuitry that occur with aging, with an emphasis on alterations within prefrontal cortex (Rajah and D'Esposito 2005; Morrison and Baxter 2012). Since cognitive neuroscience of aging studies typically target individuals aged 65 and older, less is known about changes in WM circuitry in early midlife (e.g., 45–55). Studying adults early in the aging process, as women transition into menopause, may reveal sex-dependent characteristics underlying the aging of working memory circuitry. Further, substantial evidence from preclinical studies has established 17β-estradiol's influence on synaptic organization within the dorsolateral prefrontal cortex (DLPFC) and hippocampus (Bailey et al. 2011; Boulware et al. 2012). Despite significant implications for human health, less is known about the role of sex steroids in the aging of working memory circuitry at a human cognitive neuroscience level.
Two decades of experimental evidence in rodents has demonstrated estradiol's role in hippocampal structural plasticity and hippocampal-dependent cognitive function (Woolley and McEwen 1994; Woolley et al. 1990; Woolley and McEwen 1993; McEwen 2002; Liu et al. 2008; Brinton 2009; Dimitriu et al. 2010; Frick 2012; Hara et al. 2012). More recently, quantitative morphometric studies in nonhuman primates have established estradiol's influence on synaptic organization in DLPFC (Hao et al. 2006; Morrison et al. 2006). For example, in female monkeys estrogen receptor (ER)-α is present in approximately 50% of axospinous synapses in PFC, and the abundance of ER-α is postively correlated with their performance on a delayed-response task (Wang et al. 2010). In a series of studies, Morrison and colleagues showed that cyclic estradiol administration in ovariectomized females leads to increased spine density in DLPFC neurons (Hao et al. 2006) and improved working memory performance relative to estradiol-depleted animals (Rapp et al. 2003). The changes observed as a consequence of reproductive senescence mirror the morphological changes that occur over the course of chronological aging (Morrison and Baxter 2012). By decoupling reproductive and chronological aging with ovariectomization paradigms, Morrison and colleagues demonstrated that ovarian estradiol depletion exerts a strong effect on DLPFC structure and function. This body of work has made significant progress towards characterizing the synaptic basis of menopause-related working memory decline in nonhuman primates.
In parallel, there is now mounting evidence from human neuroimaging studies implicating sex steroids in the regulation of memory circuitry (Duff and Hampson 2000; Sherwin 2003; Grigorova et al. 2006; Dumas et al. 2010; Jacobs and D'Esposito 2011; Epperson et al. 2012; Hampson and Morley 2013; Shanmugan and Epperson 2014). This research builds on the pioneering work of Berman et al. (1997) and Shaywitz et al. (1999), who used pharmacological blockade and hormone replacement techniques to illustrate estradiol and progesterone's influence on regional activity in memory circuitry. These studies provide further evidence that functional changes in ER-rich regions of memory circuitry are tied to ovarian status. Thus, the depletion of ovarian hormones during menopause may impact specific neural circuits early in the aging process (Adams et al. 2002; Morrison et al. 2006; Epperson et al. 2013). In contrast, the largest randomized clinical trial of hormone therapy (HT) in postmenopausal women found no benefit of HT for slowing the rate of cognitive decline and found an increased risk of dementia (Shumaker et al. 2003), although exogenous hormone administration differs from endogenous physiologic hormone production. Reconciling long-standing discrepancies between basic animal studies and large-scale clinical trials on estradiol's neural and cognitive effects is critical for advancing women's health (Hogervorst et al. 2000; Morrison et al. 2006; Sherwin 2006; Brinton 2009; Greendale et al. 2011; Boulware et al. 2012; Frick 2012; Maki and Henderson 2012). A human cognitive neuroscience approach bridges preclinical and clinical perspectives by interrogating the role of sex steroid hormones in specific neural circuits.
To that end, in this large-scale, population-based fMRI study of midlife men and women, we characterized the regional and network-level changes in working memory circuitry that occur early in the aging process, as a function of sex and women's reproductive stage. Verbal working memory tasks with a maintenance and manipulation component (such as the well-validated n-back task used here) rely on the coordinated activity of multiple brain regions, including DLPFC (Brodmann Area (BA) 9/46) and posterior parietal cortices (BA40/7) (D'Esposito et al. 1998; Lorenz and Sylvester 2005). Medial temporal regions were traditionally thought to play an exclusive role in the encoding and maintenance of long-term episodic memories, but their role in working memory is gaining recognition. Recent neuroimaging evidence demonstrates hippocampal modulation during WM maintenance and retrieval processes (Ranganath and D'Esposito 2001; Axmacher et al. 2007, 2009; Öztekin et al. 2009; Schon et al. 2009; Van Vugt et al. 2010; Faraco et al. 2011). Given the abundant population of sex steroid receptors, including ER-α and ER-β in the hippocampus (MacLusky et al. 1987; Österlund et al. 2000; Österlund et al. 2001; Perlman et al. 2005), and the emerging recognition of this region's role in WM, our investigation into the hormonal regulation of WM circuitry included the hippocampus and functional interactions with the frontal lobe (Meyer-Lindenberg et al. 2005; Axmacher et al. 2009). We predicted that reproductive aging, independent of chronological aging, would be associated with functional changes in regional activity and inter-regional connectivity within working memory circuitry, particularly the ER-rich regions DLPFC and hippocampus.
Materials and Methods
Subjects
Participants were selected from 17 741 pregnancies that constitute the New England Family Study (NEFS), a Boston-Providence subsidiary of the national Collaborative Perinatal Project (CPP). The NEFS is a prospective study initiated over 50 years ago to investigate prenatal and familial antecedents of pediatric, neurological, and psychological disorders of childhood (Niswander and Gordon 1972). Pregnant women, recruited between 1959 and 66, were representative of patients receiving prenatal care in the Boston-Providence area. In a series of studies over the last 20 years, we have followed a subset of NEFS offspring to investigate the fetal programming of adult phenotypes and sex differences therein (NIMH R01 MH090291). In the current follow-up study, offspring were re-recruited as midlife adults, 46–53 years of age, and completed clinical, cognitive, and neuropsychological assessments in addition to functional and structural magnetic resonance imaging (fMRI/sMRI). Exclusionary criteria included any history of neurologic disease, CNS damage, head injury with loss of consciousness, endocrine disorders, heart disease, alcohol-related diseases, current or history of psychosis, current use of hormonal contraceptives, other medical illnesses that may significantly alter CNS function, or any MRI contraindication. The set of analyses reported here focused on fMRI and behavioral evaluations of the first 142 participants (63 males) enrolled. One subject (male) was not able to complete the functional runs due to claustrophobia, producing a sample of 141. Three subjects experienced button-box failure and were not included in behavioral analyses. After enrollment, 7 women reported current use of a hormone replacement regimen and were excluded from all analyses related to reproductive stage. Their data were included in the “supergroup” analyses of fMRI data used to generate generic task-evoked functional regions of interest (ROIs). Human subjects' approval was granted by Partners Healthcare and Brown University. All volunteers gave written informed consent and were paid for their participation.
Study Design and Procedures
Subjects were seen at Brigham and Women's Hospital Outpatient Clinical Research Center. Women who were still menstruating were scheduled in the early follicular phase (Day 3–5) of their menstrual cycle. Subjects fasted for ≥8 h prior to a morning baseline blood draw. Subjects were offered a light standardized breakfast (excluding caffeine) followed by a 1 h MRI scanning session. Following the scan, subjects completed a structured clinical interview, neuropsychological testing, family medical history, and a reproductive/menstural cycle history administered by an experienced clinical interviewer/clinician.
Neuropsychological Assessments
Participants completed a neuropsychological battery including digit span backwards (Wechsler 1997), Controlled Oral Word Fluency “verbal fluency” to the letters F-A-S and Categories (Benton 1968), and the American Adult Reading Test (AMNART), used as an estimate of verbal IQ (Nelson 1982). Participants also completed the Spielberger State-Trait Anxiety Index (STAI), Profile of Moods Questionnaire (POMS), and 2 sleep measures (Pittsburg Sleep Quality Index and Insomnia Severity Index), the latter of which were not the focus of this report. Buschke Selective Reminding Test (Grober et al. 2000; Lemos et al. 2015), and the Face-Name Associative Memory Task (Sperling et al. 2003; Rentz et al. 2011) were collected in a subset of participants and were not the focus of this report.
Endocrine Assessments
Trained nurses inserted a saline lock IV line in the nondominant forearm and acquired a fasting morning blood at approximately 0800 h to evaluate hypothalamic–pituitary–gonadal axis hormones, including serum levels of sex steroids (estradiol, progesterone, and testosterone) and gonadotropins (leutinizing hormone [LH] and follicle-stimulating hormone [FSH]). Approximately 10 cc of blood were collected at Brigham and Women's Hospital Center for Clinical Investigation. Samples were allowed to clot for 30–45 min, after which blood was centrifuged (1500 × g for 10 min), and serum was aliquoted into 2 mL microtubes. Serum aliquots were stored at −20 °C for neuroendocrine evaluations and archiving. 17β-Estradiol, progesterone, and testosterone concentrations were determined via LC-mass spectrometry at the Brigham and Women's Hospital Research Assay Core (BRAC). Assay sensitivities, dynamic range, and intra-assay coefficients of variation were as follows, respectively: Estradiol (1 pg/mL, 1–500 pg/mL, <5% RSD); Progesterone (0.05 ng/mL, 0.05–10 ng/mL, 5.75% RSD); Testosterone (1.0 ng/dL, 1–2000 ng/dL, <2% RSD). FSH levels were determined via chemoluminescent assay (Beckman Coulter). The assay sensitivity was 0.2 mIU/mL; the dynamic range was 0.2–200 mIU/mL; and the intra-assay coefficient of variation was 3.1–4.3%. Leutenizing hormone (LH) levels were also determined via chemoluminescent assay (Beckman Coulter), with an assay sensitivity of 0.2 mIU/mL, dynamic range of 0.2–250 mIU/mL, and intra-assay coefficient of variation of 4.3–6.4%.
Menopausal Staging
The timing of menopausal transition between the first clinical appearance of decreased ovarian function (i.e., shorter intermenstrual time periods) to menstrual irregularity and final amenorrhea is highly variable and can occur over several years. Women in this sample were between the ages of 46 and 53 years and were in various states of ovarian decline. Some women were already in menopause with permanent amenorrhea, low estradiol levels, and elevated gonadotropins; some exhibited signs of follicular failure (elevated FSH and oligo-amenorrhea); and some showed normal cycling. Reproductive histories and hormonal evaluations were used to determine the reproductive stage of women in our sample following the Stages of Reproductive Aging Workshop (STRAW)-10 guidelines (Harlow et al. 2012). Women were categorized as late reproductive (“premenopause,” n = 26), menopausal transition (“perimenopause,” n = 25), or early postmenopausal (“postmenopause,” n = 20).
Working Memory fMRI Paradigm
Participants performed a verbal working memory N-back task during fMRI scanning. The task consisted of 2 conditions, 0-back and 2-back. In each condition, subjects were presented with a sequence of white upper case letters on a black background presented centrally (200 ms duration, 1800 ms inter-stimulus interval) in a pseudo-random order. Subjects performed 2 experimental runs of the task, with each run lasting 5.6 min. Each run contained six 32 s blocks. Each block was preceded by a 20-s fixation period and a 4 s instruction screen. During 0-back blocks, subjects responded to every letter using one of 2 buttons to indicate whether or not the target letter (X) appeared. During 2-back blocks, subjects responded to every letter using one of 2 buttons to indicate whether it matched or did not match the letter seen 2 previously. Response times and accuracy were recorded. Response time (RT) values <100 ms were considered null and not included in the computation of subjects' average RT.
fMRI Data Acquisition
MRI data were acquired with a Siemens 3 T Tim Trio scanner (Siemens, Erlangen, Germany), equipped with a 12-channel head coil. Functional data were obtained using a T2*-weighted echo-planar imaging sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (repetition time, 2000 ms; echo time, 30 ms; field of view, 200 mm; flip angle, 90°; voxel size, 3.1 × 3.1 × 3.0). Each functional volume consisted of 33 (3 mm) oblique axial slices. A T1-weighted image was collected using a high-resolution 3D Multi-Echo (ME) MPRAGE sagittal sequence with an isotropic resolution of 1 mm3. Following acquisition, MRI data were converted to Nifti format and preprocessed in SPM8 (Wellcome Department of Cognitive Neurology, London, UK). Preprocessing included realignment and geometric unwarping of echo-planar imaging images using magnetic field maps, correction for head motion, nonlinear volume-based spatial normalization (Montreal Neurological Institute template MNI-152), and spatial smoothing with a Gaussian filter (6 mm [full width at half maximum]). Additional software (http://web.mit.edu/swg/software.htm) was used to identify and exclude outliers in the global mean image time series (threshold 3.0 SD from the mean) and movement (threshold 1.0 mm; measured as scan-to-scan movement, separately for translation and rotation) parameters. Statistical parametric maps of BOLD activation were calculated in SPM8 using the general linear model approach (Worsley and Friston 1995).
fMRI Data Analyses
Hemodynamic responses were modeled using a gamma function and convolved with onset times of 2-back and 0-back blocks to form the general linear model (GLM) at the single subject level. Outlier time points and the 6 rigid-body movement parameters were included in the GLM as covariates of no interest. To test a priori hypotheses targeting ROIs within working memory circuitry, we conducted ROI analyses on functionally defined masks of bilateral DLPFC (BA9/46; MNI coordinates, left: −48,11,34; right: 39,26,40) and inferior parietal cortex (iPAR, BA40; left: −42,−49,49; right: 45,−43,46) (10 mm spheres around peak loci), and anatomically defined masks of the hippocampus. Functional ROIs were defined from “supergroup” whole-brain analyses in the larger sample (N = 141; Fig. 2B) based on peak task-evoked activity generated at P < 10−10, T = 8.88, df = 140. The left and right hippocampal ROI was anatomically defined using a manually segmented MNI-152 brain (based on methods previously published by the Center for Morphometric Analysis at Massachusetts General Hospital and Harvard Medical School; see Makris et al. 2013). ROIs were created with the Wake Forest University PickAtlas ROI toolbox for SPM (Maldjian et al. 2003). Mean β weights from the ROIs were extracted for each participant as a function of WM load (2-back > 0-back) using the REX toolbox (Whitfield-Gabrieli 2009). For each participant and ROI, β estimates were entered into an analysis of variance (ANOVA) with reproductive status (premenopausal, perimenopausal, postmenopausal) as a between-subject factor and age as a covariate. In light of the NIH and Office of Research on Women's Health (ORWH)'s recent advocacy around the importance of understanding sex differences, we wanted our analyses to reflect our prediction that to understand sex differences in midlife, one must account for women's menopausal/hormone status. Thus, parallel analyses were run to test for overall sex differences (male vs. female) or sex differences as a function of women's menopausal status.
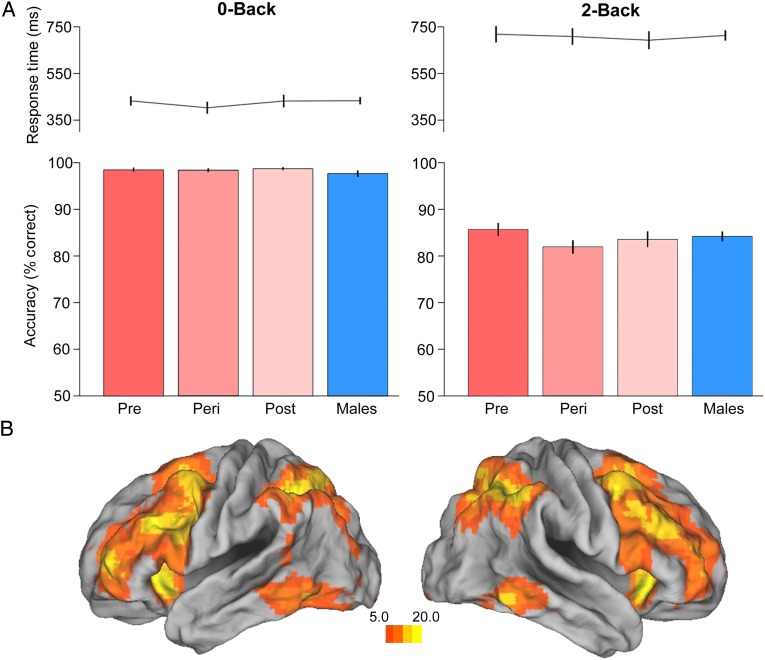
Figure 2.
Working memory performance and circuitry. (A) N-back reaction time and accuracy by group (Pre, n = 26; Peri, n = 23, Post, n = 20, Men, n = 62). (B) Task-evoked BOLD responses throughout working memory circuitry, 2-back > 0-back, displayed at P < 10−7, N = 141. Error bars represent ±1 SEM.
To investigate WM-dependent alterations in functional connectivity by reproductive status and sex, we performed a psychophysiological (PPI) analysis with a seed region in DLPFC. Given the strong left-hemispheric dominance in verbal working memory tasks (Reuter-Lorenz and Sylvester 2005; Owen et al. 2005), time courses from left DLPFC seed region were extracted for PPI analyses. For each participant, subject-level GLMs were constructed as described earlier, with the addition of the left DLPFC seed time course as a regressor and 2 additional PPI regressors (the interaction of the seed time course with the regressors for 2-back and 0-back condition). These interaction regressors are orthogonal to the task and seed regressors and describe the contribution of the interaction above and beyond the main effects of the task and seed time course (McLaren et al. 2012). DLPFC connectivity was measured at the single subject level by estimating the difference between the interaction of the seed time course with the regressor for 2-back versus 0-back blocks. Single subject activation maps were entered into second-level random effects analysis to probe group differences in WM-dependent DLPFC connectivity. Given extensive evidence for the involvement of iPAR (BA40) in working memory (Owen et al. 2005) and sex differences in PFC-iPAR structural covariance (Abbs et al. 2011), DLPFC connectivity with iPAR was examined by applying small volume correction (SVC) to bilateral iPAR masks. Similarly, given accumulating evidence for the role of hippocampus in WM (Ranganath and D'Esposito 2001; Axmacher et al. 2007, 2009; Öztekin et al. 2009; Schon et al. 2009; Van Vugt et al. 2010; Faraco et al. 2011; Cousijn et al. 2012) and hippocampal connectivity with frontal regions (Meyer-Lindenberg et al. 2005; Axmacher et al. 2009), DLPFC-hippocampal connectivity was examined by applying SVC of the anatomically defined left and right hippocampus. For clusters showing load-dependent group differences in connectivity, mean β values were extracted and plotted separately by condition (0-back and 2-back) to better understand the nature of the group difference. Finally, exploratory whole-brain analyses probed group differences in activation and connectivity outside a priori ROIs at uncorrected P < 0.005 in >10 contiguous voxels. These parameters were chosen to confirm the absence of additional group differences even at a very liberal threshold.
Behavioral data were analyzed with SPSS version 20.0 (SPSS Inc., Chicago, IL, USA) using ANOVAs for demographic and clinical characteristics with continuous variables where equal variance was assumed. Pearson correlations and linear regression models were conducted to determine the relationship between ROI β values and PPI connectivity β values with WM performance (with or without adjustement for estimated verbal IQ, to distinguish working memory ability from general verbal skills). A value of P < 0.05 was designated for statistical significance.
Results
Demographic and Clinical Characteristics
The community-based sample was 93% Caucasian, 7% African American. Participants were in early midlife (mean age 49.5) with an average of 2 years of college and an average verbal IQ >115. Table 1 reports demographic characteristics of the sample in men and by reproductive stage in women. Groups were comparable on age, body mass index, education, parental socioeconomic status, estimated verbal IQ, and ethnicity. Nine-one percent of the sample was right-handed (9% left-handed). Neuropsychological and mood assessments indicated that groups did not differ on verbal fluency (composite of FAS and Categories, F3,133 = 0.27, P = 0.85), digit span (backwards, F3,133 = 1.4, P = 0.25), State Anxiety (F3,133 = 1.1, P = 0.33), Trait Anxiety (F3,133 = 0.97, P = 0.41), or mood scores from the POMS (subscores for Tension-Anxiety, Depression-Dejection, Vigor-Activity, Fatigue-Inertia, Confusion-Bewilderment, all F3,133 < 1.6, and Ps > 0.2). The POMS subscore for Anger-Hostility showed a group difference (F3,133 = 3.27, P = 0.023, partial η2 = 0.07) and post hoc tests revealed that men reported overall greater Anger-Hostility than women (P < 0.05). Parallel analyses by sex, irrespective of women's menopausal status, established that neuropsychogical and mood scores did not differ between men and women except as noted (all t < 2, P > 0.15; see Table 1). Men showed marginally better performance on digit span backwards compared with women overall (t(131) = 1.8, P = 0.070, r = 0.15). Verbal fluency was not different between sexes when the composite score was used (t(131) = 0.29, P = 0.77); however, analysis of the 2 subscores (“FAS” and “Categories”) revealed a female advantage in the Categories condition (t(131)=−2.11, P = 0.036, r = 0.18). Systematic structured diagnoses (SCID) were conducted by a clinical interviewer with >25 years of experience, and final consensus diagnoses were made on all subjects by the PI and clinical team. There were no significant differences in diagnoses by group. In addition, psychotropic medication use was uncommon and similarly distributed across groups: benzodiazapines (3 premenopausal, 2 perimenopausal, 3 postmenopausal women, and 7 men); antidepressants (SSRI; 5 premenopausal, 3 perimenopausal, 4 postmenopausal women, 3 men). Non-psychotropic medications included those used for cardiac-related conditions: (9 premenopausal, 9 perimenopausal, 6 postmenopausal women, 29 men).
Table 1.
Demographic characteristics of the sample
| Characteristics | Pre (n = 26) |
Peri (n = 23) |
Post (n = 20) |
Men (n = 62) |
F | P value* | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| Age (years) | 49.3 | 1.6 | 49.6 | 1.7 | 50.1 | 2.0 | 49.3 | 2.1 | 0.98 | 0.41 |
| BMI | 29.0 | 6.4 | 27.2 | 5.0 | 28.5 | 5.9 | 29.4 | 6.2 | 0.88 | 0.45 |
| Parental SESa | 5.9 | 1.7 | 5.5 | 1.9 | 5.9 | 1.7 | 5.7 | 2.1 | 0.63 | 0.60 |
| Education (years) | 14.7 | 1.9 | 15.0 | 1.7 | 14.9 | 1.6 | 14.1 | 2.7 | 0.25 | 0.87 |
| Verbal IQb | 118.1 | 8.2 | 115.0 | 11.3 | 115.0 | 10.7 | 116.9 | 12.2 | 0.53 | 0.67 |
| n | % | n | % | n | % | n | % | |||
| Ethnicity (% Caucasian) | 24 | 92 | 24 | 96 | 18 | 90 | 59 | 94 | ||
Note: Pre, Premenopausal; Peri, Perimenopausal; Post, Postmenopausal; BMI, body mass index.
aParental socioeconomic status (SES) was a composite index of family income, education, and occupation and ranged from 1.0 (low) to 9.3 (high).
bVerbal IQ was estimated from the American Adult Reading Test (AMNART) (Nelson 1982).
*In addition to comparing groups broken down by women's menopausal status, no overall sex differences were observed (Age, P = 0.35; BMI, P = 0.26; Parental SES, P = 0.97; Education, P = 0.34; Verbal IQ, P = 0.69).
Hormonal Evaluations
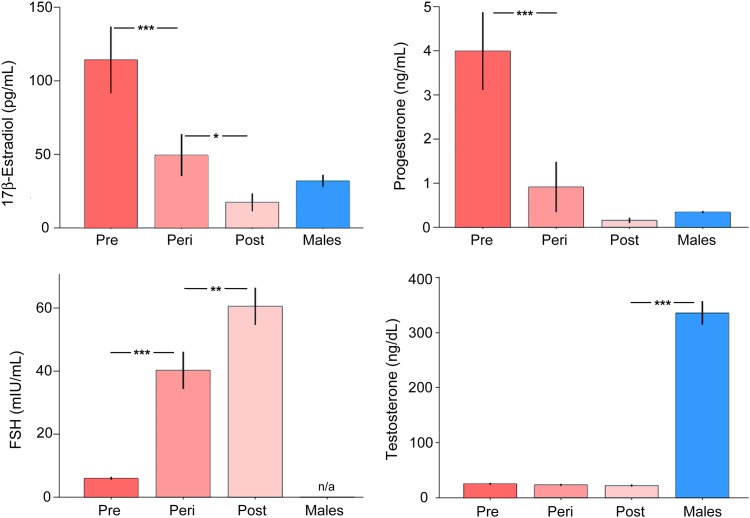
Analysis of HPG-axis steroid hormones (assessed in women and in men) and gonadotropins (assessed in women only) confirmed that serum estradiol (F3,129 = 13.34, P < 0.001, r = 0.49) and progesterone (F3,129 = 15.85, P < 0.001, r = 0.52) levels differed by group, declining significantly as a function of advancing reproductive age in women, while FSH levels rose (F2,68 = 35.03, P < 0.001, r = 0.71) (Fig. 1). Post-hoc tests confirmed that premenopausal women had higher estradiol and progesterone levels relative to perimenopausal (P < 0.001) and postmenopausal women (P < 0.001) and men (P < 0.001). In turn, perimenopausal women had lower sex steroid hormone concentrations relative to postmenopausal women (estradiol, P = 0.08; progesterone, P < 0.001). Relative to men, estradiol and progesterone levels were substantially higher in premenopausal women, as expected, (estradiol, P < 0.001; progesterone, P < 0.001) but did not differ relative to perimenopausal women (estradiol, P = 0.15; progesterone, P = 0.32) or postmenopausal women (estradiol, P = 0.51; progesterone, P = 0.76). Testosterone levels were uniformly higher in men, as expected (F3,127 = 85.84, P < 0.001, r = 0.82; all post hoc group comparisons were significant at P < 0.001; Fig. 1). For gonadotropins, post hoc tests confirmed that FSH was lower in premenopausal relative to perimenopausal women (P < 0.001) and, in turn, was lower in perimenopausal relative to postmenopausal women (P < 0.001). Finally, as with FSH, LH levels rose as a function of advanced reproductive stage (F2,68 = 18.93, P < 0.001, r = 0.60). Values by group were as follows (mean, SD): premenopausal women (5.86 mIU/mL, 6.19 mIU/mL), perimenopausal women (23.21 mIU/mL, 17.27 mIU/mL), and postmenopausal women (28.48 mIU/mL, 14.33 mIU/mL). Post-hoc comparisons showed that mean LH values were significantly lower in premenopausal women relative to perimenopausal (P < 0.001) and postmenopausal (P < 0.001) women.
Figure 1.
Sex steroid and gonadotropin hormone concentrations by menopausal stage. Serum estradiol (F3,129 = 13.34, P < 0.001) and progesterone (F3,129 = 15.85, P < 0.001) levels declined while FSH (F2,68 = 35.03, P < 0.001) levels rose as a function of advancing reproductive age in women. Testosterone levels were substantially higher in men, as expected (F3,127 = 85.84, P < 0.001). Error bars represent ±1 SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Working Memory Behavioral Performance
Behavioral performance on the Nback task was comparable across groups, with no significant differences observed for accuracy (0-back: F3,127 = 0.48, P = 0.69); 2-back: F3,127 = 0.95, P = 0.42) or response time (0-back: F3,127 = 0.39, P = 0.76; 2-back: F3,127 = 0.09, P = 0.96) (Fig. 2A). Similarly, parallel analyses by sex confirmed that no difference in performance was evident between men and women, independent of menopausal stage (all nonsignificant, Ps > 0.25).
Regional BOLD Response in WM Circuitry
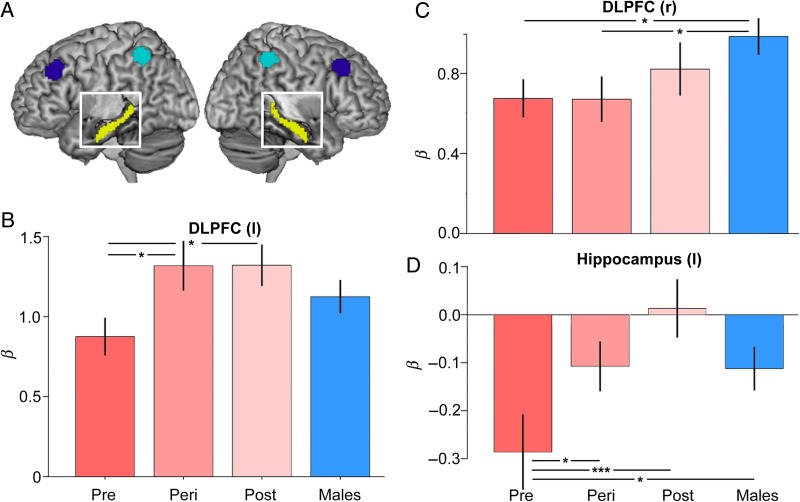
The N-back paradigm evoked robust responses throughout working memory circuitry (Fig. 2B). Region of interest analyses examined group differences in task-evoked activity within bilateral DLPFC, inferior parietal cortices, and hippocampus (Fig. 3A). We began by analyzing the data by sex, irrespective of women's reproductive status. No significant differences between men and women were observed for task-evoked responses in left DLPFC, bilateral parietal, or bilateral hippocampus (all t(132) < 0.80, P > 0.40). The only exception was in right DLPFC, which men activated more strongly than women (t(132) = 2.4, P = 0.01, r = 0.20). Between-sex and within-women differences emerged after taking into account the reproductive status of women. First, significant differences in DLPFC and hippocampal responses were observed as a function of women's reproductive stage, despite minimal variance in chronological age. Task-evoked activity in left DLPFC increased over the menopausal transition (F = 4.06, P = 0.022, r = 0.33), with postmenopausal (t = 2.31, P = 0.024, r = 0.28) and perimenopausal (P = 0.013) women exhibiting greater activity relative to premenopausal women (Fig. 3B). Task-evoked responses in left DLPFC did not differ between perimenopausal and postmenopausal women (t = 0.16, P > 0.88). Left hippocampus was systematically deactivated during n-back. The most pronounced deactivation was observed in premenopausal women, with increasingly attenuated deactivation over the menopausal transition in women and in men (F3,129 = 3.12, P = 0.028, r = 0.26; Pre vs. Post, P = 0.004; Pre vs. Peri, P = 0.05; Pre vs. Men, P = 0.03) (Fig. 3D). Linear regression analyses indicated that across all women, this decrease in hippocampal deactivation was strongly related to a decline in endogenous estradiol (Overall model, F3,65 = 5.6, P = 0.002, r = 0.45; estradiol covariate, P = 0.012, β= −0.30) and progesterone (F3,65 = 6.7, P = 0.001, r = 0.49; progesterone covariate, P = 0.003, β= −0.34) concentrations, adjusting for age and task performance. Finally, although men showed greater activity than women, overall, in contralateral right DLPFC (as reported above), post hoc tests revealed that this sex difference was driven by premenopausal (P = 0.039) and perimenopausal (P = 0.046) women, and was diminished for postmenopausal women (P = 0.32) (Fig. 3C). Univariate activity in left and right iPAR did not differ by sex or reproductive status (nonsignificant, with all F3,134 < 0.40, all Ps > 0.7). Also note that in no region was there an appreciable difference in task-evoked activity between right-handed (91% of sample) and left-handed participants.
Figure 3.
Modulation of working memory-related PFC and hippocampal activity by menopausal status. (A) Surface location of functionally defined masks of DLPFC (BA 9; dark blue) and inferior parietal cortex (BA 40; cyan) on a rendered brain. The white box shows a cut out of anatomically defined hippocampus (yellow). Functional ROIs were generated from a supergroup activity map in a larger sample (2-back > 0-back; N = 141). (B) Task-evoked activity in left DLPFC increased over the menopausal transition. Postmenopausal and perimenopausal women exhibited significantly greater activity relative to premenopausal women. (C) Men showed greater activity in right DLPFC relative to Pre and Peri women, a sex difference that no longer reached significance for Post women. (D) Task-related hippocampal deactivations diminished over the menopausal transition. Parameter estimates are adjusted for age and performance. Error bars represent ±1 SEM. *P < 0.05, ***P < 0.005.
DLPFC Connectivity During WM
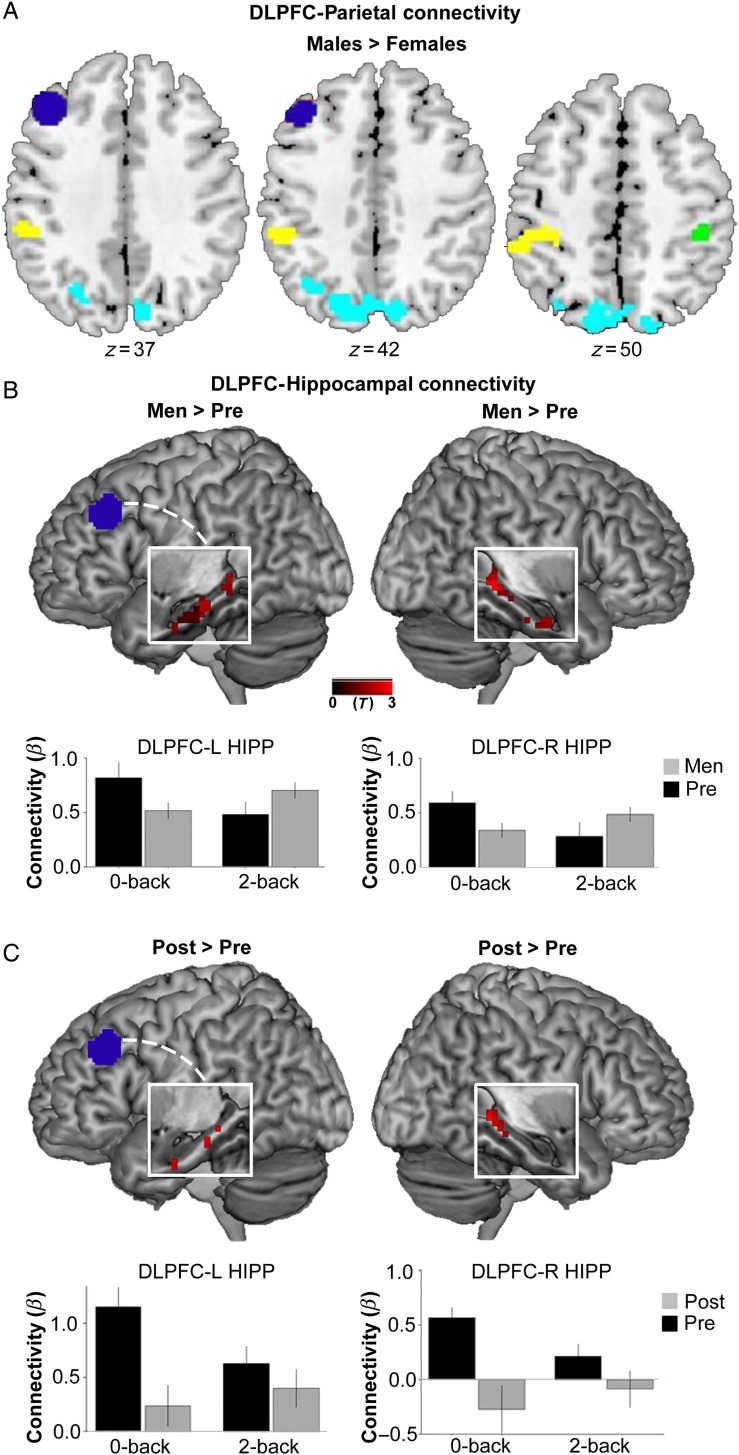
Psychophysiological interaction analyses examined group differences in WM-dependent DLPFC connectivity. Analyses by sex revealed 2 clusters, 1 in left iPAR (BA40) and 1 in right iPAR (BA40), that displayed greater functional connectivity with left DLPFC for men relative to women (PFWE-corrected <0.05) (Fig. 4A). Exploratory analysis of the greater parietal lobe revealed several additional clusters throughout posterior parietal cortex that were more strongly connected to DLPFC in men relative to women (cyan clusters, Fig. 4A). Even at the liberal threshold of uncorrected P < 0.005, no clusters in iPAR showed stronger DLPFC connectivity in women (as a whole) compared with men. Distinguishing groups only by sex, no differences between men and women were observed for DLPFC-hippocampal connectivity. However, analyzing data with respect to women's reproductive age revealed that postmenopausal women displayed heightened connectivity between the left DLPFC seed and multiple bilateral hippocampal clusters relative to premenopausal women. Plotting PPI β values separately for each condition revealed that premenopausal women had reduced DLPFC-hippocampal connectivity during 2-back relative to 0-back. In contrast, postmenopausal women had greater DLPFC-hippocampal connectivity during 2-back relative to 0-back (in left hippocampus) and a reduced negative PPI β value (in right hippocampus) (Fig. 4C). A similar pattern was observed for men, who exhibited greater load-related DLPFC-hippocampal connectivity relative to premenopausal women (Fig. 4B). Plotting PPI β values separately by condition showed that premenopausal women had reduced DLPFC-hippocampal connectivity during 2-back relative to 0-back. In contrast, men showed relatively greater DLPFC-hippocampal connectivity during 2-back relative to 0-back (see Fig. 4B). Perimenopausal women showed a more consistent, low level of DLPFC-hippocampal connectivity across conditions (see Supplementary Results). Functional connectivty plots depicted by load and across all groups are shown in Supplementary Figures 1 and 2. In sum, DLPFC-hippocampal functional connectivity differed between men and premenopausal women, a sex difference that was abolished for postmenopausal women.
Figure 4.
Working memory load-dependent functional connectivity by sex and menopausal status. (A) Relative to all women, men showed greater WM load-related connectivity between DLPFC (dark blue seed) and several clusters throughout inferior and posterior parietal cortex (yellow, green, and cyan clusters, displayed at P < 0.005, uncorrected). Clusters in left BA 40 (−42,−49,58) and right BA 40 (42,−34,49) were significant at P < 0.05, FWE-corrected. SVC was applied using the bilateral parietal ROI (see Fig 3A). (B,C) White boxes display a cut out of the left or right hippocampus on a rendered brain. Men and Postmenopausal women showed greater WM load-sensitive (2-back > 0-back) DLPFC-hippocampal connectivity relative to Premenopausal women (to illustrate full spatial extent, findings are displayed at P < 0.05, uncorrected). For Men > Pre, clusters in L Hipp (−33,−16,−20) and R Hipp (27,−37,−2) were significant at P < 0.05, FWE-corrected. SVC was applied using the bilateral hippocampal ROI (see Fig. 3A). For Post > Pre, clusters in L Hipp (−9,−40,1) and R Hipp (27, −40, −2) were significant at P < 0.005, uncorrected, but did not meet FWE-level significance. Bar graphs display mean β values representing the strength of functional connectivity (from the clusters shown above) separately by condition.
Brain–Behavior Relationships
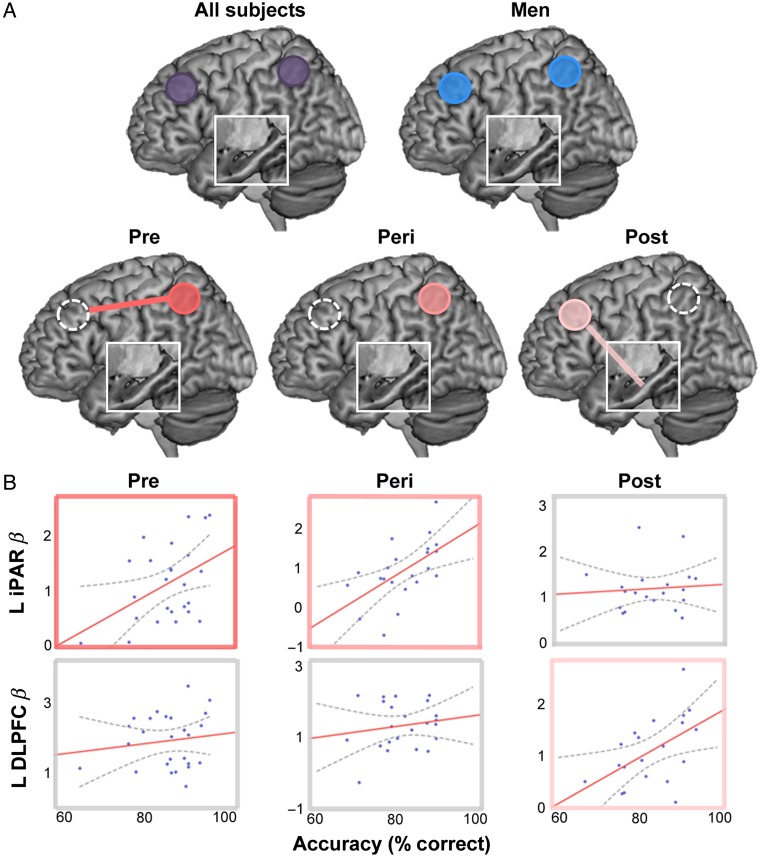
The magnitude of regional activity and connectivity within WM circuitry was strongly related to WM performance. Across all subjects, regional activity in bilateral iPAR (left, r = 0.33, P [2-tailed] <0.001; right, r = 0.26, P [2-tailed] = 0.003) and DLPFC (left, r = 0.24, P [2-tailed] = 0.006; right, r = 0.22, P [2-tailed] = 0.01) was related to accuracy on the WM task. However, analyzing the data without regard to participants' sex or, further, women's reproductive stage obscured notable differences between groups (Fig. 5). For premenopausal women, better WM performance was related to heightened iPAR activity (r = 0.43, P [2-tailed] =0.03) and the strength of DLPFC-iPAR connectivity (r = 0.37, trend P [2-tailed] = 0.07). Perimenopausal women showed a comparable pattern within iPAR (r = 0.57, P [2-tailed] = 0.005). Neither group showed a relationship between performance and DLPFC (Pre, P = 0.35; Peri, P = 0.22) or DLPFC-hippocampal connectivity (Pre, r= −0.07, P = 0.75; Peri, r= −0.006, P = 0.98). Conversely, for postmenopausal women, performance was strongly related to the magnitude of DLPFC activity (r = 0.51, P [2-tailed] = 0.021) and DLPFC-hippocampal connectivity (r = 0.55, P [2-tailed] = 0.017), but not iPAR (r = 0.07,6 P > 0.7) or DLPFC-iPAR (r= −0.008, P = 0.98). A Fisher transformation was applied to the correlation coefficients to further test the significance of these differences between the 2 extreme groups (Pre, Post). Formal comparisons of the connectivity-performance correlations indicated that the relationship between DLPFC-HIPP connectivity and WM performance was stronger for Postmenopausal relative to Premenopausal women (P = 0.01). Conversely, the relationship between DLPFC-iPAR connectivity and performance was nominally stronger for Premenopausal relative to Postmenopausal women (trend significance, P = 0.10). This double dissociation-like pattern suggests that with advancing reproductive age there may be a shift in reliance from PFC-parietal to PFC-hippocampal pathways for successful WM performance (Fig. 5B). All findings in women were left lateralized. In contrast, for men, WM accuracy was related to bilateral DLPFC (left, r = 0.26, P [2-tailed] = 0.039; right, r = 0.25, P [2-tailed] = 0.046) and bilateral iPAR activity (left, r = 0.31, P [2-tailed] = 0.017; right, r = 0.28, P [2-tailed] = 0.028).
Figure 5.
The relationship between regional activity/connectivity and WM performance differs by menopausal status and sex. (A) The magnitude of regional activity (solid circles: significant ROIs) and connectivity (solid lines: significant connectivity) within WM circuitry was strongly related to WM performance. Across all subjects, regional activity in bilateral iPAR and DLPFC was related to accuracy on the WM task. However, analyzing subjects' data as a singular group obscured underlying between-sex and within-women differences. For premenopausal women, better WM performance was associated with increased iPAR activity and DLFPC-iPAR connectivity. Perimenopausal women showed a comparable pattern within iPAR. In contrast, for postmenopausal women, performance was strongly related to DLPFC activity and DLPFC-Hipp connectivity (and, notably, not iPAR or DLPFC-iPAR connectivity). For all women, findings were left lateralized. For men, better WM performance was related to bilateral DLPFC and bilateral iPAR activity. Univariate activity within an ROI is represented by a circle; connectivity between regions is represented by a line. Brain–behavior models are depicted for Premenopausal women (dark pink), Perimenopausal women (intermediate pink), Postmenopausal women (light pink), Men (blue), and all subjects (purple). The white box displays a cut out of the hippocampus. (B) Brain–behavior scatterplots are shown as a function of menopausal status in women and correspond to the models depicted in A. Significant associations are outlined in pink, nonsignificant associations are outlined in gray. Gray dotted lines represent 95% CI.
The only association between response time (RT) and BOLD measures was for postmenopausal women, for whom a faster RT was associated with a stronger left DLPFC BOLD response (r= −0.512, P [2-tailed] = 0.021). Thus, for this group, “better” performance on the verbal WM task, whether indexed by a faster RT or greater accuracy, was associated with a more robust left DLPFC BOLD response. Adjusting for estimated verbal IQ did not alter the direction of these associations. Notably, analyzing subjects' data as a singular group produced a pattern of results driven by men, obscuring between-sex and within-women differences.
Discussion
Cognitive aging studies typically target participants aged 65 and older (Spreng et al. 2010; Grady et al. 2006; Grady 2012). In this population-based fMRI study, we stepped back by over a decade to characterize the differences in working memory circuitry in early midlife (aged ∼45–55) as a function of sex and menopausal status in women. We observed pronounced effects of reproductive age on task-evoked responses in WM circuitry, despite minimal variance in chronological age between groups. Postmenopausal women recruited left DLPFC and hippocampus more strongly than premenopausal women while performing the verbal WM task. Men showed greater activity than women in contralateral right DLPFC, a sex difference that was strongest relative to pre- and perimenopausal women and diminished relative to postmenopausal women. Next, using gPPI, we explored the impact of sex and reproductive stage on integrated activity across task-related brain regions. Men showed greater WM-dependent DLPFC-iPAR functional connectivity compared with women. Analyses within women revealed a reorganization of functional networks across the menopausal transition. Postmenopausal women showed evidence of enhanced DLFPC-hippocampal connectivity relative to premenopausal women. Finally, the magnitude of regional activity and functional connectivity within WM circuitry was related to WM performance. Critically, analyzing these data without regard to sex or reproductive status obscured group differences in the circuit-level neural strategies associated with successful working memory performance. These findings underscore the importance of considering the role of reproductive age, independent of chronologic age, to strengthen our understanding of sex differences in the aging of memory circuitry. Taken together, these findings suggest that early changes in working memory circuitry are evident decades before the age range typically targeted by cognitive neuroscience of aging studies.
Given that chronological age was comparable between groups (differing, on average, by <11 months), the observed differences in task-evoked BOLD responses are unlikely to be attributable to age-related differences in cerebral vasculature (D'Esposito et al. 2003). Further, the impact of reproductive status and sex steroid concentrations on BOLD were region specific and thus not likely driven by global differences in blood oxygenation.
In our study, working memory performance did not differ as a function of menopausal stage or sex steroid hormone concentrations (although differences were evident for neuropsychological assessments of verbal and associative memory, which is the focus of a separate report; Rentz et al. under review). This is not suprising given that our fMRI paradigm assessed working memory performance at moderate levels of load. Preclinical, clinical, and epidemiological evidence has produced long-standing discrepancies regarding the precise nature and extent of menopause-related cognitive changes (for a broader discussion of this topic, see Hogervorst et al. 2000; Morrison et al. 2006, 2011; Sherwin 2006; Brinton 2009; Greendale et al. 2011; Hara et al. 2011; Boulware et al. 2012; Maki and Henderson 2012; Shanmugan and Epperson 2014). In humans, one of the most consistently observed cognitive changes is within the domain of verbal learning and memory (Berent-Spillson et al. 2012; Epperson et al. 2013; Rentz et al. in review), although changes in frontally mediated executive functions (including working memory and attention) are also observed (Shanmugan and Epperson 2014). In nonhuman primate studies, decrements in working memory performance have been associated with low estradiol concentrations (Rapp et al. 2003) and DLPFC estradiol receptor expression levels (Wang et al. 2010) in aging female monkeys. Our study adds to an emerging body of evidence demonstrating that even in the absence of differences at the behavioral level, reproductive aging has a discernable impact at the neuronal level, as measured through in vivo brain imaging. Collectively, a human cognitive neuroscience approach has begun to bridge preclinical and clinical perspectives by interrogating the role of sex steroid hormones in specific neural circuits and specific neurochemical systems (Dumas et al. 2008; Jacobs and D'Esposito 2011; Epperson et al. 2012; Jacobs et al. 2015).
Although speculative, our finding that postmenopausal women recruited DLPFC more strongly than premenopausal women and showed less deactivation of the hippocampus, may represent a compensatory response since the magnitude of activity in these regions, and the strength of functional connectivity between them was associated with better WM performance. Further, controlling for chronological age, as estradiol and progesterone levels declined across women the magnitude of hippocampal disengagement/deactivation decreased, strongly implicating sex steroids in the regulation of this circuitry. This putative “compensatory” hypothesis of reproductive aging mirrors the compensatory responses commonly observed in chronological aging studies, whereby increased recruitment of task-relevant regions (typically PFC) in older adults helps maintain their performance at a level comparable to younger adults (assuming subjects are performing within working memory capacity) (Reuter-Lorenz et al. 2000; Cabeza et al. 2002; Davis et al. 2008; Reuter-Lorenz and Cappell 2008; Spreng et al. 2010; Cabeza and Dennis 2012; Grady 2012). In contrast to postmenopausal women's reliance on DLPFC-hippocampal connectivity for successful WM performance, premenopausal women showed a strong reliance on fronto-parietal connectivity. This pattern is reminiscent of previous findings showing stronger PFC-parietal connectivity in younger versus older adults during an executive control task (Madden et al. 2010). Here, we observed a diminished reliance on PFC-parietal connectivity in postmenopausal women, in favor of PFC-hippocampal pathways.
It is important to note that these data cannot formally disambiguate whether neural responses reflect dedifferentiation or compensatory activity, nor are we able to probe progressive load-dependent effects (Schneider-Garces et al. 2010; Cabeza and Dennis 2012). Further, it would be of interest for future studies to identify subgroups of low- and high-performing postmenopausal women to see whether the pattern of neural activity in high performers resembles that of pre- or perimenopausal women. Accumulating evidence suggests that the hippocampus plays a role in WM processes (Ranganath and D'Esposito, 2001; Karlsgodt et al. 2005; Meyer-Lindenberg et al. 2005; Axmacher et al. 2007, 2009; Öztekin et al. 2009; Schon et al. 2009; Van Vugt et al. 2010; Faraco et al. 2011; Cousijn et al. 2012), although task-induced activations and deactivations have been observed. In our study, we found strong hippocampal disengagement (i.e., a task-induced decrease in blood flow, observed as a negative signal change) during the working memory task in premenopausal women. The degree of hippocampal disengagement was reduced for perimenopausal women and reduced further still for postmenopausal women (for whom task-induced hippocampal activity was positive, on average). A previous n-back working memory study found progressive hippocampal deactivation with increasing task demands (Cousign et al. 2012). Thus, instead of being compensatory, our finding that the hippocampus fails to deactivate and shows more synchronous activity with the DLPFC in postmenopausal women could reflect an inability of the hippocampus to effectively disengage under high task demands. The loss of hippocampal deactivation could be evidence for a deficit in control processes or failure of inhibitory function. Alternative evidence that may bear on our interpretation of the findings comes from Axmacher and colleagues, who found that the maintenance of individual items in WM is related to hippocampal deactivation (“hippocampus-independent WM”), and that this deactivation is reduced during WM for multiple items (“hippocampus-dependent WM”) (Axmacher et al. 2007, 2009). In the present study, PPI analyses revealed that postmenopausal women had greater DLPFC-hippocampal connectivity during the high load condition (2-back) relative to control condition (0-back). This is in contrast to premenopausal women, for whom the opposite pattern was observed (stronger PFC-hippocampal connectivity under low versus high load). Since the degree of hippocampal engagement and DLPFC-hippocampal functional connectivity was positively related to working memory performance for postmenopausal women, we speculate that this neural pattern reflects a compensatory response. However, additional studies are neccesary to test the neuronal compensation hypothesis (e.g., by using functional lesion methods to determine whether disrupting the putative compensatory region impacts performance) (Cabeza and Dennis 2012).
One of the most consistent patterns of age-related changes in brain activity is increased bilateral recruitment of frontal activity (Davis et al. 2008). In our midlife sample, we observed a sex difference in whether this bilateral PFC response was related to WM performance. For women, all brain–behavior relationships were left-lateralized. However, for midlife men, WM performance was related to bilateral responses in DLPFC and inferior parietal cortex. Notably, when subjects were analyzed as a single group, the bilateral brain–behavior relationship remained significant, obscuring the underlying sex-dependent nature of the finding. This raises the possibility that existing cognitive models describing the loss of hemispheric asymmetry with age (e.g., Hemispheric Asymmetry Reduction in Older Adults (HAROLD)) contain unexplained sex differences (Cabeza 2002). Alternatively, these models may reliably represent the data observed in men and women aged 65+, while leaving open the possibility that sex differences are evident earlier in the aging process. For example, the timing of when age-related bihemispheric effects emerge may differ between men and women and only be revealed in studies targeting the early midlife window (i.e., when women transition to menopause). In fact, sex differences in hemispheric assymetry begin in fetal development (Geshwind and Galaburda 1985). Some of these same processes impact sex differences in verbal IQ. Thus, a potential explanation for the sex-dependent lateralization findings is that women differ in verbal IQ or in their reliance on verbal strategies to complete the N-back task. However, the persistence of brain–behavior associations after adjusting for estimated verbal IQ suggests that differences in verbal ability do not fully account for these findings.
These results extend our previous study examining the impact of estradiol on working memory circuitry in young, cycling women. Using a within-woman, repeated-measures approach that capitalized on the natural fluctuations in estradiol over the menstrual cycle, we found that sustained (block-level) PFC activity was reduced under high versus low estradiol conditions despite comparable performance, a putative marker of neural efficiency (Jacobs and D'Esposito 2011). Here, we see a similar “efficient” DLPFC response in midlife women, prior to the decline in ovarian estradiol. Together, these fMRI findings suggest that estradiol may help generate a neurally efficient PFC response, a pattern that is observed in other populations using the same task (Gray et al. 2003). More broadly, the data demonstrate that a complete understanding of PFC function—be it in the young healthy brain or in the aging brain—must include a role for sex steroids.
Working memory is critically dependent on prefrontal dopamine signaling (Brozoski et al. 1979; Sawaguchi and Goldman-Rakic 1991; Williams and Goldman-Rakic 1995). Strong experimental evidence in animals and indirect evidence in humans indicates that estradiol impacts WM in part by modulating activity within the dopaminergic system (Chiodo and Caggiula 1980; Becker 1990, 2000; Pasqualini et al 1995; Jacobs and D'Esposito 2011). Additional studies will be critical for clarifying the complex relationship between the midlife ovarian decline in neuroactive gonadal hormones, dopaminergic signaling, and working memory. For example, subpopulations of women may be more resilient to the midlife decline in estradiol, based on common genetic variability in dopaminergic pathways (e.g., COMT val158met). Taking into account gene–hormone interactions could reveal individual differences in the impact of reproductive aging on working memory circuitry.
Limitations of the current study should be considered when interpreting the data. First, we used the MNI-152 standard template brain to define our hippocampal ROIs. Since the MNI-152 template was constructed based on data from healthy younger adults on average, it may not reflect the exact hippocampal architecture of our early midlife sample and could be overly inclusive. Importantly, Freesurfer-defined hippocampal volumes of our participants did not differ by menopausal status or by age within this fairly small age-range sample, suggesting that spurious findings in the fMRI data as a result of using the MNI template are unlikely. Moving forward, using a study-specific template or age-specific reference data generated from a large sample would be preferable. Second, it will be of interest for future studies to probe the pattern of functional connectivity from alternative seed regions, including right DLPFC, as these regions and their functional connections may provide additional sources of neuronal compensation.
Taken together, these results contribute to our broader understanding of the impact of sex and reproductive status on the aging of memory circuitry. In keeping with preclinical studies, our findings suggest that the loss of ovarian estradiol during menopause plays a significant role in shaping PFC and hippocampal function. Moving forward, using convergent techniques from systems and cognitive neuroscience and fostering collaborations between basic and clinical scientists will be critical for identifying sex-dependent therapeutic targets that can be applied prior to overt cognitive decline.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
This work was supported by the National Institutes of Health (grant number MH090291 to J.M.G.) with additional support from the National Institutes of Health Office of Research on Women's Health (ORWH-NICHD BIRCWH K12 HD051959 to E.G.J.), and the Harvard Clinical and Translational Science Center (NIH UL1 RR025758).
Supplementary Material
Notes
The authors thank Harlyn Aizley, MEd, for administering structured clinical interviews; Anne Remington, MA and Barbara Vincenty for recruitment and project management (AR); Katie Lancaster, MS, and Julia Longenecker, MA, for help with data collection; and Shalendar Bhasin, MD, for mass spectrometry. Conflict of Interest: None declared.
References
- Abbs B, Liang L, Makris N, Tsuang M, Seidman LJ, Goldstein JM. 2011. Covariance modeling of MRI brain volumes in memory circuitry in schizophrenia: sex differences are critical. Neuroimage. 56:1865–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WGM, Hayashi S, Milner TA, McEwen BS, Morrison JH. 2002. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 22:3608–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Elger CE, Fell J. 2009. Working memory-related hippocampal deactivation interferes with long-term memory formation. J Neurosci. 29:1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axmacher N, Mormann F, Fernández G, Cohen MX, Elger CE, Fell J. 2007. Sustained neural activity patterns during working memory in the human medial temporal lobe. J Neurosci. 27:7807–7816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. 1986. Working memory. Oxford: Oxford University Press. [Google Scholar]
- Baddeley AD, Hitch G. 1974. Working memory. In: Bower GH, editor. The psychology of learning and motivation. Vol. 8 New York: Academic Press; p. 47–89. [Google Scholar]
- Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. 2011. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience. 191:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB. 1990. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci Lett. 118:169–171. [DOI] [PubMed] [Google Scholar]
- Becker JB. 2000. In: Chadwick D, Goode J, editors. Neuronal and Cognitive Effects of Oestrogens. West Sussex: Wiley; p. 134–145. [Google Scholar]
- Benton AL. 1968. Differential behavioral effects in frontal lobe disease. Neuropsychologia. 6:53–60. [Google Scholar]
- Berent-Spillson A, Persad CC, Love T, Sowers M, Randolph JF, Zubieta JK, Smith YR. 2012. Hormonal environment affects cognition independent of age during the menopause transition. J Clin Endocrinol Metab. 97:E1686–E1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. 1997. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci USA. 94:8836–8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulware MI, Kent BA, Frick KM. 2012. The impact of age-related ovarian hormone loss on cognitive and neural function. Curr Top Behav Neurosci. 10:165–184. [DOI] [PubMed] [Google Scholar]
- Brinton RD. 2009. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 30:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. 1979. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science. 205:929. [DOI] [PubMed] [Google Scholar]
- Cabeza R. 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 17:85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. 2002. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 17:1394–1402. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Dennis N. 2012. Frontal lobes and aging: deterioration and compensation. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. 2nd edition New York: Oxford UP; p. 628–652. [Google Scholar]
- Chiodo LA, Caggiula AR. 1980. Alterations in basal firing rate and autoreceptor sensitivity of dopamine neurons in the substantia nigra following acute and extended exposure to estrogen. Eur J Pharmacol. 67:165–166. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. 2008. Que PASA? The posterior–anterior shift in aging. Cereb Cortex. 18:1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Esposito M, Aguirre GK, Zarahn E, Ballard D, Shin RK, Lease J. 1998. Functional MRI studies of spatial and nonspatial working memory. Cogn Brain Res. 7:1–3. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Deouell LY, Gazzaley A. 2003. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 4:863–872. [DOI] [PubMed] [Google Scholar]
- Duff SJ, Hampson E. 2000. A beneficial effect of estrogen on working memory in postmenopausal women taking hormone replacement therapy. Horm Behav. 38:262–276. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. 2008. Estradiol interacts with the cholinergic system to affect verbal memory in postmenopausal women: evidence for the critical period hypothesis. Horm Behav. 53:159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas JA, Kutz AM, Naylor MR, Johnson JV, Newhouse PA. 2010. Increased memory load-related frontal activation after estradiol treatment in postmenopausal women. Horm Behav. 58:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. 2010. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 1204:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Amin Z, Ruparel K, Gur R, Loughead J. 2012. Interactive effects of estrogen and serotonin on brain activation during working memory and affective processing in menopausal women. Psychoneuroendocrinology. 37:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epperson CN, Sammel MD, Freeman EW. 2013. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 98:3829–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraco CC, Unsworth N, Langley J, Terry D, Li K, Zhang D, Liu T, Miller LS. 2011. Complex span tasks and hippocampal recruitment during working memory. Neuroimage. 55:773–787. [DOI] [PubMed] [Google Scholar]
- Frick KM. 2012. Building a better hormone therapy? How understanding the rapid effects of sex steroid hormones could lead to new therapeutics for age-related memory decline. Behav Neurosci. 126:29–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM. 1985. Cerebral lateralization: biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch Neurol. 42:428. [DOI] [PubMed] [Google Scholar]
- Grady C. 2012. The cognitive neuroscience of ageing. Nat Rev Neurosci. 13:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. 2006. Age-related changes in brain activity across the adult lifespan. J Cogn Neurosci. 18:227–241. [DOI] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. 2003. Neural mechanisms of general fluid intelligence. Nat Neurosci. 6:316–322. [DOI] [PubMed] [Google Scholar]
- Greendale GA, Derby CA, Maki PM. 2011. Perimenopause and cognition. Obstet Gynecol Clin North Am. 38:519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorova M, Sherwin BB, Tulandi T. 2006. Effects of treatment with leuprolide acetate depot on working memory and executive functions in young premenopausal women. Psychoneuroendocrinology. 31:935–947. [DOI] [PubMed] [Google Scholar]
- Grober E, Lipton RB, Hall C, Crystal H. 2000. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 54:827–832. [DOI] [PubMed] [Google Scholar]
- Hampson E, Morley EE. 2013. Estradiol concentrations and working memory performance in women of reproductive age. Psychoneuroendocrinology. 38:2897–2904. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR et al. . 2006. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 26:2571–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Punsoni M, Rapp PR, Morrison JH. 2011. Synaptic characteristics of dentate gyrus axonal boutons and their relationships with aging, menopause, and memory in female rhesus monkeys. J Neurosci. 31:7737–7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR. 2012. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. 33:421.e17-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ. 2012. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Climacteric. 15:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Riedel W, Jolles J. 2000. The nature of the effect of female gonadal hormone replacement therapy on cognitive function in post- menopausal women: a meta-analysis. Neuroscience. 101:485–512. [DOI] [PubMed] [Google Scholar]
- Jacobs E, D'Esposito M. 2011. Estrogen shapes dopamine-dependent cognitive processes: implications for women's health. J Neurosci. 31:5286–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs EG, Holsen LM, Lancaster K, Makris N, Whitfield-Gabrieli S, Remington A, Weiss B, Buka S, Klibanski A, Goldstein JM. 2015. 17β-Estradiol differentially regulates stress circuitry activity in healthy and depressed women. Neuropsychopharmacology. 40:566–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon D, Maillet D, Pasvanis S, Ankudowich E, Grady CL, Rajah MN. 2015. Context memory decline in middle aged adults is related to changes in prefrontal cortex function. Cereb Cortex. 10.1093/cercor/bhv068. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos R, Simões MR, Santiago B, Santana I. 2015. The free and cued selective reminding test: validation for mild cognitive impairment and Alzheimer's disease. J Neuropsychol. 9:242–257. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muñiz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V et al. . 2008. Activation of estrogen receptor-β regulates hippocampal synaptic plasticity and improves memory. Nat Neurosci. 11:334–343. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Clark AS, Naftolin F, Goldman-Rakic PS. 1987. Estrogen formation in the mammalian brain: possible role of aromatase in sexual differentiation of the hippocampus and neocortex. Steroids. 50:459–474. [DOI] [PubMed] [Google Scholar]
- Madden DJ, Costello MC, Dennis NA, Davis SW, Shepler AM, Spaniol J, Bucur B, Cabeza R. 2010. Adult age differences in functional connectivity during executive control. Neuroimage. 52:643–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Henderson VW. 2012. Hormone therapy, dementia, and cognition: the Women's Health Initiative 10 years on. Climacteric. 15:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. 2003. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 19:1233–1239. [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2002. Sex, stress and the hippocampus: allostasis, allostatic load and the aging process. Neurobiol Aging. 23:921–939. [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, Johnson SC. 2012. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 61:1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg AS, Olsen RK, Kohn PD, Brown T, Egan MF, Weinberger DR, Berman KF. 2005. Regionally specific disturbance of dorsolateral prefrontal–hippocampal functional connectivity in schizophrenia. Arch Gen Psychiatry. 62:379–386. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. 2012. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 13:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Briton RD, Schmidt PJ, Gore AC. 2006. Estrogen, menopause, and the aging brain: how basic neuroscience can inform hormone therapy in women. J Neurosci. 26:10332–10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HE. 1982. National Adult Reading Test (NART) test manual. Windsor: (UK: ): NFER-Nelson. [Google Scholar]
- Niswander KR, Gordon MJ. 1972. The women and their pregnancies: the Collaborative Perinatal Study of the National Institute of Neurological Diseases and Stroke. National Institute of Health; for sale by the Superintendent of Documents, U.S. Government Publishing Office.
- Österlund MK, Hurd YL. 2001. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 64:251–267. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. 2005. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 25:46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öztekin I, McElree B, Staresina BP, Davachi L. 2009. Working memory retrieval: contributions of the left prefrontal cortex, the left posterior parietal cortex, and the hippocampus. J Cogn Neurosci. 21:581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. 2002. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 172:299. [PubMed] [Google Scholar]
- Pasqualini C, et al. 1995. Acute stimulatory effect of estradiol on striatal dopamine synthesis. Neurochem. 65:1651–1657. [DOI] [PubMed] [Google Scholar]
- Perlman WR, Tomaskovic-Crook E, Montague DM, Webster MJ, Rubinow DR, Kleinman JE, Weickert CS. 2005. Alteration in estrogen receptor α mRNA levels in frontal cortex and hippocampus of patients with major mental illness. Biol Psychiatry. 58:812–824. [DOI] [PubMed] [Google Scholar]
- Rajah MN, D'Esposito M. 2005. Region-specific changes in prefrontal function with age: a review of PET and fMRI studies on working and episodic memory. Brain. 128:1964–1983. [DOI] [PubMed] [Google Scholar]
- Ranganath C, D'Esposito M. 2001. Medial temporal lobe activity associated with active maintenance of novel information. Neuron. 31:865–873. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. 2003. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 23:5708–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Carmasin J, Maye JE, Johnson KA, Sperling RA. 2011. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 49:2776–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA. 2008. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 17:177–182. [Google Scholar]
- Reuter-Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, Koeppe RA. 2000. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci. 12:174–187. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Sylvester CY. 2005. The cognitive neuroscience of working memory and aging. J Cognitive Neurosci. 186–217. [Google Scholar]
- Sawaguchi T, Goldman-Rakic PS. 1991. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 251:947–950. [DOI] [PubMed] [Google Scholar]
- Schneider-Garces NJ, Gordon BA, Brumback-Peltz CR, Shin E, Lee Y, Sutton BP, Maclin EL, Gratton G, Fabiani M. 2010. Span, CRUNCH, and beyond: working memory capacity and the aging brain. J Cogn Neurosci. 22:655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon K, Quiroz YT, Hasselmo ME, Stern CE. 2009. Greater working memory load results in greater medial temporal activity at retrieval. Cereb Cortex. 19:2561–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN. 2014. Estrogen and the prefrontal cortex: towards a new understanding of estrogen's effects on executive functions in the menopause transition. Hum Brain Mapp. 35:847–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, Constable RT, Naftolin F, Palter SF, Marchione KE et al. . 1999. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 281:1197–1202. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. 2006. Estrogen and cognitive aging in women. Neuroscience. 138:1021–1026. [DOI] [PubMed] [Google Scholar]
- Sherwin BB. 2003. Estrogen and cognitive functioning in women. Endocr Rev. 24:133–151. [DOI] [PubMed] [Google Scholar]
- Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD et al. . 2003. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA. 289:2651–2662. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. 2003. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer's disease. J Neurol Neurosurg Psychiatry. 74:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Wojtowicz M, Grady CL. 2010. Reliable differences in brain activity between young and old adults: a quantitative meta-analysis across multiple cognitive domains. Neurosci Biobehav Rev. 34:1178–1194. [DOI] [PubMed] [Google Scholar]
- van Vugt MK, Schulze-Bonhage A, Litt B, Brandt A, Kahana MJ. 2010. Hippocampal gamma oscillations increase with memory load. J Neurosci. 30:2694–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P, Salthouse TA. 1997. Meta-analyses of age—cognition relations in adulthood: Estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1223:231. [DOI] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. 2010. Synaptic estrogen receptor-a levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 30:12770–12776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. 1997. WAIS-III, Wechsler Adult Intelligence Scale- third edition, administration and scoring manual. New York: (NY: ): The Psychological Corporation. [Google Scholar]
- Whitfield-Gabrieli S. 2009. Region of interest extraction (REX) toolbox. Boston: (MA: ). p. 497. [Google Scholar]
- Williams GV, Goldman-Rakic PS. 1995. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 376:572–575. [DOI] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Frankfurt M, McEwen BS. 1990. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 10:4035–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1994. Estradiol regulates hippocampal dendritic spine density via an N- methyl-D-aspartate receptor-dependent mechanism. J Neurosci. 14:7680–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. 1993. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J Comp Neurol. 336:293–306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.