ABSTRACT
Taiwanofungus camphoratus is a precious medicinal fungus endemic to Taiwan and has been used as traditional medicine for a long time. Many pharmacological studies have revealed that T. camphoratus possessed various biological activities, such as immunomodulatory effects, anticancer activity and liver protective function. The aim of this study is to investigate the non-specific and antigen (ovalbumin [OVA])-specific immunomodulation effects of solid-state cultivated powder of T. camphoratus (Leader Antrodia cinnamomea [LAC]) in BABL/c male mice. In non-specific and antigen-specific immune function studies, 8-week-old mice were orally administered with LAC for 6 and 8 weeks, respectively. The results have shown that the proliferation of splenic immune cells, phagocytic activity of macrophages and cytolytic activity of natural killer cells were enhanced by LAC. Additionally, LAC increased the levels of IL-2, TNF-α, INF-γ, GM-CSF and serum OVA-IgG and OVA-IgM. These findings provided evidences that LAC had the immunomodulation effects on both antigen-specific and non-specific immune responses in mice.
KEYWORDS: Taiwanofungus camphoratus, phagocytic activity, natural killer cells, specific immune response, non-specific immune response
1. Introduction
Taiwanofungus camphoratus (syn. Antrodia cinnamomea, Antrodia camphorata) is an edible medicinal fungus native to Taiwan. It grows on the inner cavity of Cinnamomum kanehirae Hayata (Lauraceae) and has been used to promote health, treat liver disease, drug and food intoxication, hangover, exhaustion and cancers by the aboriginals in Taiwan for a long time (Wu SHR and Chang 1997; Geethangili and Tzeng 2011; Yue et al. 2012). Several bioactive compounds of T. camphoratus have been identified and the biological functions in immunomodulatory (Song et al. 2014; Kuo et al. 2008; Sheu et al. 2009), anticancer (Huang et al. 2015; Kumar et al. 2015; Lin et al. 2015), antiinflammation (Chen YF et al. 2014; Hsieh et al. 2010), antioxidant (Wu MD et al. 2011; Hseu et al. 2008) and hepatoprotective (Huang CH et al. 2010; Gokila Vani et al. 2014; Chang et al. 2016) effects were widely studied.
The regulation of immune system is important for human to fight with microorganism invasion and tumour cells, prevent autoimmune disease and ease allergy symptoms. Various studies have revealed mushrooms and mushroom polysaccharides possessed potent immunomodulatory effects and could induce different types of immune response. Ganoderma lucidum is able to trigger Th1 and Th2 cytokines expression and activate macrophages and T lymphocytes (Wasser 2002; Chan et al. 2005). Agaricus bisporus showed in vivo immunomodulatory effects on reducing the clinical signs of autoimmune encephalomyelitis in rats by regulation of the innate and adaptive responses (Ditamo et al. 2016). Oral administration of the extract of Agaricus blazei in mice increased the IgG level in serum, promoted phagocytic activity of peritoneal macrophages and natural killer (NK) cells activity (Ni et al. 2013). T. camphoratus also showed the effects on regulation of immune system and stimulating Th1 pathway in mice (Chen YJ et al. 2008; Cheng et al. 2008; Kuo et al. 2008). Besides, methyl antcinate K, the triterpenoids of T. camphoratus was demonstrated to activate dendritic cells and prime Th2 differentiation (Yu et al. 2009).
T. camphoratus has been widely used as food supplements and health food products in Taiwan. The purpose of this study is to evaluate the effects of solid-state cultivated powder of T. camphoratus (Leader Antrodia cinnamomea [LAC]) on non-specific and specific immune response and provide further insight of immunomodulatory effects of T. camphoratus. The results showed (198, 593, 988 mg/kg) oral administration of LAC in mice markedly increased the immune cell proliferation and enhanced phagocytic cell and NK cell activity. LAC also increased Th1 type cytokines secretion and the level of serum ovalbumin (OVA)-IgG and OVA-IgM.
2. Materials and method
2.1. LAC preparation
LAC capsule was manufactured by Taiwan Leader Biotech Corp. (Taipei, Taiwan), which contained 99% solid-state cultivated powder of T. camphoratus and 1% magnesium stearate. LAC was dissolved in sterile water to obtain dosing solutions of low (198 mg/kg bw), medium (593 mg/kg bw) and high (988 mg/kg bw) doses that were equivalent to one-, three- and fivefold recommended human daily intake, respectively.
2.2. Animals and treatments
BABL/c male mice were purchased from BioLASCO Taiwan Co., Ltd. (Taipei, Taiwan). Animals were housed at the laboratory animal centre of the MedGaea Life Sciences Institute under 12-h light/12-h dark cycle with free access to food and water. Animals were weighed once per week during the experimental period. For the non-specific immune tests, 8-week-old mice were divided into five groups of 10 mice each. Control group was treated with sterile water, positive control group was treated with the health food with immunomodulation effects on the market (260 mg/kg bw) and three treatment groups were treated with low, medium and high dose of LAC by oral gavage daily for 6 weeks. For the antigen-specific immune tests, animals were divided into five groups of 10 mice each and orally administration with LAC daily for 8 weeks. After 4 weeks of LAC treatment, each mouse was first immunised OVA by intraperitoneal injection of 50 μg/100 μl OVA (Sigma-Aldrich, St. Louis, MO, USA) emulsified in the Complete Freund’s Adjuvant (Sigma-Aldrich). Two weeks later, each mouse was intraperitoneally injected with OVA 100 μg/100 μl emulsified with Incomplete Freund’s Adjuvant (Sigma-Aldrich) to further to enhance the OVA-specific immune responses. Blood samples and spleen were collected at indicated time for further analysis.
2.3. Cell proliferation assay
The spleens were removed, and single-cell suspensions were cultured in RPMI 1640 medium. Splenocytes at a final density of 2.0 × 105 cells/well were placed in 96-well plates. Cells were treated with 5 μg/mL concanavalin A (Con A), 10 μg/mL lipopolysaccharide (LPS) or 50 μg/mL OVA for 44 h. Cell proliferation assay was measured by using CellTiter 96® AQueous One Solution Cell Proliferation Assay Kit (Promega, Madison, WI, USA). In brief, after incubation, reagent containing a tetrazolium compound [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] and an electron coupling reagent (phenazine ethosulphate; PES) was added to each well. After 4 h incubation, 10% sodium dodecyl sulfate (SDS) was added to each well to stop the reaction, and the absorbance at 490 nm (OD490) was measured. The quantity of formazan product as measured by the amount of 490 nm absorbance is directly proportional to the number of living cells in culture. The stimulation index was calculated by the OD490 of mitogen-stimulated cells/OD490 of non-stimulated cells.
2.4. Cytokine analysis
The supernatants of Con A-, LPS- or OVA-stimulated cultured splenic cells were harvested after 24–48 h. The concentration of IL-2, IL-4, TNF-α, IFN-γ and GM-CSF cytokines in the culture supernatants was determined by ELISA (eBioscience, San Diego, CA, USA).
2.5. Phagocytic activity
To determine the phagocytes activity of mice treated with LAC, the granulocytes at a cell density of 1 × 106 cells were cultured with FITC-labelled E. coli by using Phagocytosis analysis kit (Gene Research Lab. Co. Ltd., Taipei, Taiwan). Granulocytes were incubated with FITC-labelled E. coli in duplicate tubes (one at 4°C and the other at 37°C) for 15 min and analysed by flow cytometry. The proportion of phagocytosis (%) = E. coliFITC37°C –E. coliFITC4°C.
2.6. NK cell activity
Splenocytes (effector cells) were cultured with PHK67 green fluorescent labelled YAC-1 cells (target cells, 2 × 105 cells) at effector: target ratios (E/T ratios) of 6.25:1 and 12.5:1, respectively. After 4 h incubation, cells were stained with propidium iodide and proportion of non-viable target cell (YAC-1 cell) was determined by flow cytometry.
2.7. Determination of serum OVA-IgG and OVA-IgM levels
Blood samples were collected from retro-orbital sinus of mice at the beginning of the study and in week 4, 5, 6, 7 and 8. After centrifuged at 3600 rpm for 10 min, the serum was collected for detection of anti-OVA-IgG and anti-OVA-IgM by ELISA. The level of anti-OVA-IgG and anti-OVA-IgM antibodies was calculated using the following formula:
ELISA unit (E.U.) = (ODsample – ODblank)/(ODpositive serum – ODblank)
2.8. Statistical analysis
Results were presented as mean ± standard deviation. Statistical analysis was performed by one-way ANOVA followed by Duncan’s multiple range test using SPSS 13.0 software (SPSS Inc., Chicago IL). A value of p < 0.05 was considered as statistical significance.
3. Results
3.1. Proliferative activity of immune cells
In non-specific immune response tests, LAC at low, medium and high dose significantly increased the proliferation activity of splenocytes stimulated by Con A. However, no effect on LPS-stimulated immune cell proliferation (Table 1) was observed. In specific immune response tests, LAC had no effects on OVA-induced splenocytes proliferation.
Table 1.
Effect of LAC on immune cell proliferation.
| Stimulation index |
||||
|---|---|---|---|---|
| Group | Dose (mg/kg) | Con A | LPS | OVA |
| NC | – | 1.289 ± 0.121 | 1.521 ± 0.107 | 1.047 ± 0.057 |
| LAC | 198 | 1.486 ± 0.193* | 1.572 ± 0.229 | 0.995 ± 0.075 |
| LAC | 593 | 1.595 ± 0.149* | 1.622 ± 0.135 | 1.002 ± 0.069 |
| LAC | 988 | 1.438 ± 0.129* | 1.568 ± 0.208 | 1.007 ± 0.040 |
| PC = | 260 | 1.450 ± 0.157* | 1.523 ± 0.198 | 1.037 ± 0.056 |
All data were presented as mean ± SD.
NC: normal control; PC: positive control.
LAC: solid-state cultivated powder of T. camphoratus.
*p < 0.05 compared to normal control.
3.2. Effect of LAC on NK cell activity
LAC at low, medium and high dose obviously increased the proportion of YAC-1 cell death at both E/T ratios of 6.25:1 and 12.5:1 in a dose-dependent manner (Figure 1). The results suggested that LAC effectively enhanced NK cell activity.
Figure 1.
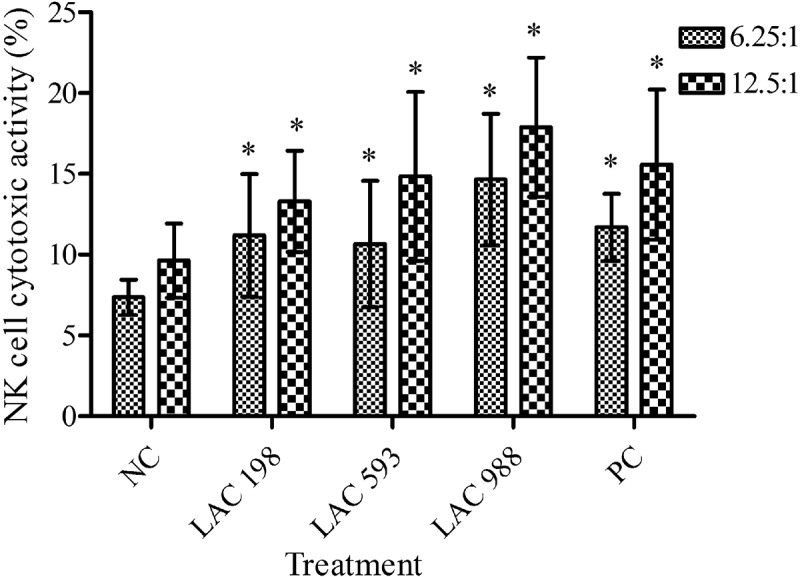
Effect of LAC on natural killer (NK) cell activity. The mice were administered with LAC (198, 593, 988 mg/kg) and positive control (PC, 260 mg/kg) for 6 weeks. The spleen cells were isolated from mice and NK cell activity was analysed by cytotoxic assay. The effector-to-target ratios (E/T ratios) were 6.25:1 and 12.5:1. All data were presented as mean ± SD. *p < 0.05 compared to normal control (NC).
3.3. Effect of LAC on phagocytes activity
The percent of phagocytosis of LAC groups at low (18.42 ± 3.45), medium (22.23 ± 5.84) and high dose (23.61 ± 2.71) was significantly increased compared to normal control (13.67 ± 3.59) and with the dose-dependent effect. The results of positive control (14.49 ± 2.45) showed no difference versus normal control (Figure 2).
Figure 2.
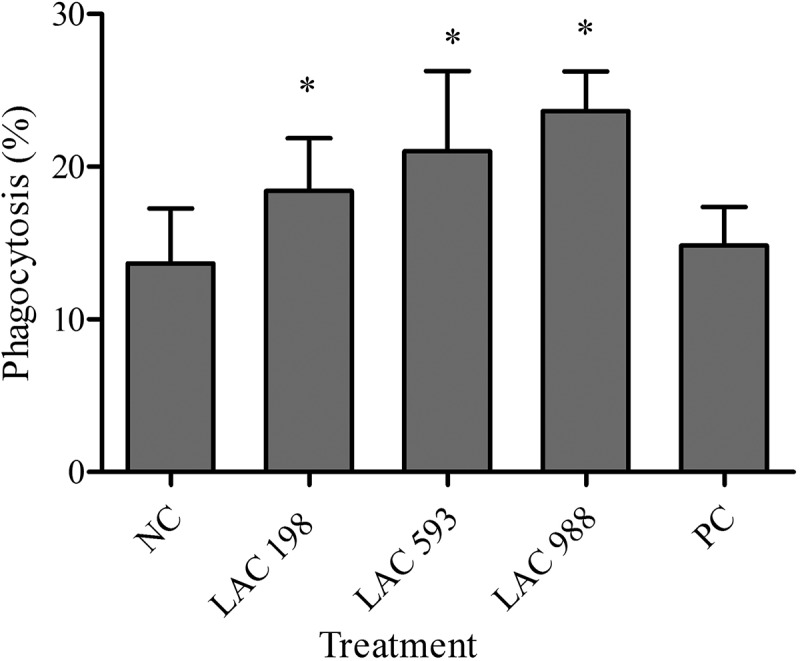
Effect of LAC on phagocytic cell activity. The mice were administered with LAC (198, 593, 988 mg/kg) and positive control (PC, 260 mg/kg) for 6 weeks. The spleen cells were isolated from mice and phagocytosis of E. coli by leukocytes was analysed by flow cytometry. All data were presented as mean ± SD. *p < 0.05 compared to normal control (NC).
3.4. Effect of LAC on cytokines level
In non-specific immune study model, the splenocytes were isolated and stimulated by Con A and LPS. The level of IL-2, IL-4, TNF-α, IFN-γ and GM-CSF was analysed. As shown in Table 2, there was no significance change without mitogen stimulation among all groups. The level of IL-2, TNF-α, IFN-γ and GM-CSF in all LAC-treated groups showed markedly increased while stimulated by Con A and LPS. In specific immune study model, the splenocytes were isolated and stimulated by Con A and OVA and the results showed LAC markedly increased the level of IL-2, TNF-α, IFN-γ and GM-CSF in splenocytes stimulated by Con A. LAC only significantly increased IL-2 and IFN-γ in splenocytes stimulated by OVA. IL-4 showed no significance change among all groups (Table 3).
Table 2.
Effect of LAC on cytokines secretion in non-specific immune response.
| Group | Dose (mg/kg) | Con A (5 μg/mL) | LPS (10 μg/mL) |
|---|---|---|---|
| IL-2 (pg/mL) | |||
| NC | – | 221.44 ± 56.47 | 53.37 ± 9.86 |
| LAC | 198 | 293.43 ± 67.66* | 86.92 ± 17.35* |
| LAC | 593 | 291.99 ± 61.01* | 85.73 ± 15.81* |
| LAC | 988 | 307.87 ± 45.74* | 86.35 ± 23.85* |
| PC | 260 | 262.87 ± 51.48 | 69.70 ± 21.97 |
| IL-4 (pg/mL) | |||
| NC | – | 21.25 ± 9.80 | 4.92 ± 1.34 |
| LAC | 198 | 21.28 ± 7.19 | 7.28 ± 3.36 |
| LAC | 593 | 19.10 ± 5.90 | 7.25 ± 3.70 |
| LAC | 988 | 17.72 ± 4.30 | 7.53 ± 2.89 |
| PC | 260 | 14.86 ± 7.50 | 5.09 ± 1.80 |
| TNF-α (pg/mL) | |||
| NC | – | 313.93 ± 107.64 | 221.79 ± 90.35 |
| LAC | 198 | 410.36 ± 82.17* | 331.79 ± 125.30 |
| LAC | 593 | 410.24 ± 106.57* | 433.81 ± 176.07* |
| LAC | 988 | 417.19 ± 77.95* | 410.00 ± 138.37* |
| PC | 260 | 442.19 ± 90.03* | 293.08 ± 77.07 |
| IFN-γ (pg/mL) | |||
| NC | – | 1329.82 ± 345.51 | 186.23 ± 58.04 |
| LAC | 198 | 1698.73 ± 323.08* | 204.77 ± 67.81 |
| LAC | 593 | 1690.90 ± 360.44* | 276.86 ± 97.37* |
| LAC | 988 | 1765.42 ± 207.41* | 270.92 ± 109.60* |
| PC | 260 | 1737.08 ± 210.89* | 199.36 ± 76.81 |
| GM-CSF (pg/mL) | |||
| NC | – | 226.52 ± 62.19 | 21.04 ± 4.13 |
| LAC | 198 | 290.70 ± 42.21* | 25.33 ± 6.32 |
| LAC | 593 | 317.65 ± 95.27* | 31.47 ± 7.95* |
| LAC | 988 | 316.36 ± 68.19* | 29.57 ± 9.55* |
| PC | 260 | 214.17 ± 70.38 | 21.96 ± 7.85 |
All data were presented as mean ± SD.
NC: normal control; PC: positive control.
LAC: solid-state cultivated powder of T. camphoratus.
*p < 0.05 compared to normal control.
Table 3.
Effect of LAC on cytokines secretion in specific immune response.
| Group | Dose (mg/kg) | Con A (5 μg/ml) | OVA (50μg/ml) |
|---|---|---|---|
| IL-2 (pg/ml) | |||
| NC | – | 125.4 ± 41.6 | 9.5 ± 3.8 |
| LAC | 198 | 241.3 ± 81.5* | 14.4 ± 3.9* |
| LAC | 593 | 234.9 ± 85.8* | 15.1 ± 3.7* |
| LAC | 988 | 239.7 ± 66.3* | 15.7 ± 5.5* |
| PC | 260 | 144.9 ± 57.4 | 10.2 ± 4.2 |
| IL-4 (pg/ml) | |||
| NC | – | 17.1 ± 4.5 | 11.5 ± 3.1 |
| LAC | 198 | 18.9 ± 7.2 | 12.0 ± 2.5 |
| LAC | 593 | 16.0 ± 6.9 | 11.2 ± 4.8 |
| LAC | 988 | 16.1 ± 5.9 | 11.0 ± 3.9 |
| PC | 260 | 14.4 ± 5.1 | 11.0 ± 4.5 |
| TNF-α (pg/ml) | |||
| NC | – | 145.5 ± 27.7 | 26.3 ± 8.2 |
| LAC | 198 | 462.5 ± 151.8* | 37.5 ± 4.3 |
| LAC | 593 | 449.2 ± 163.4* | 35.0 ± 20.4 |
| LAC | 988 | 456.8 ± 183.8* | 31.9 ± 12.9 |
| PC | 260 | 196.7 ± 75.1 | 20.7 ± 7.9 |
| IFN-γ (pg/ml) | |||
| NC | – | 688.2 ± 140.3 | 22.1 ± 7.0 |
| LAC | 198 | 1124.4 ± 529.8* | 34.0 ± 7.4* |
| LAC | 593 | 1118.2 ± 534.2* | 36.2 ± 7.9* |
| LAC | 988 | 1287.4 ± 583.6* | 33.3 ± 7.8* |
| PC | 260 | 709.2 ± 219.2 | 20.9 ± 4.8 |
| GM-CSF (pg/ml) | |||
| NC | – | 52.8 ± 13.3 | 3.2 ± 3.5 |
| LAC | 198 | 81.9 ± 15.5* | 2.3 ± 2.3 |
| LAC | 593 | 81.1 ± 17.8* | 3.2 ± 3.0 |
| LAC | 988 | 81.5 ± 20.7* | 3.5 ± 2.6 |
| PC | 260 | 60.3 ± 13.2 | 4.4 ± 2.6 |
All data were presented as mean ± SD.
NC: normal control; PC: positive control.
LAC: solid-state cultivated powder of T. camphoratus.
*p < 0.05 compared to normal control.
3.5. Effect of LAC on serum antibody secretion
The serum OVA-IgG and OVA-IgM were determined after mice were immunised by OVA in week 4. The results showed both OVA-IgG and OVA-IgM were increased as time increased in each group after OVA stimulation compared to non-stimulation. There was a markedly increased after the second OVA immunisation (week 6). LAC significantly increased the level of serum OVA-IgG and OVA-IgM in week 4–8 and week 7–8, respectively and with the dose-dependent effect (Figure 3).
Figure 3.
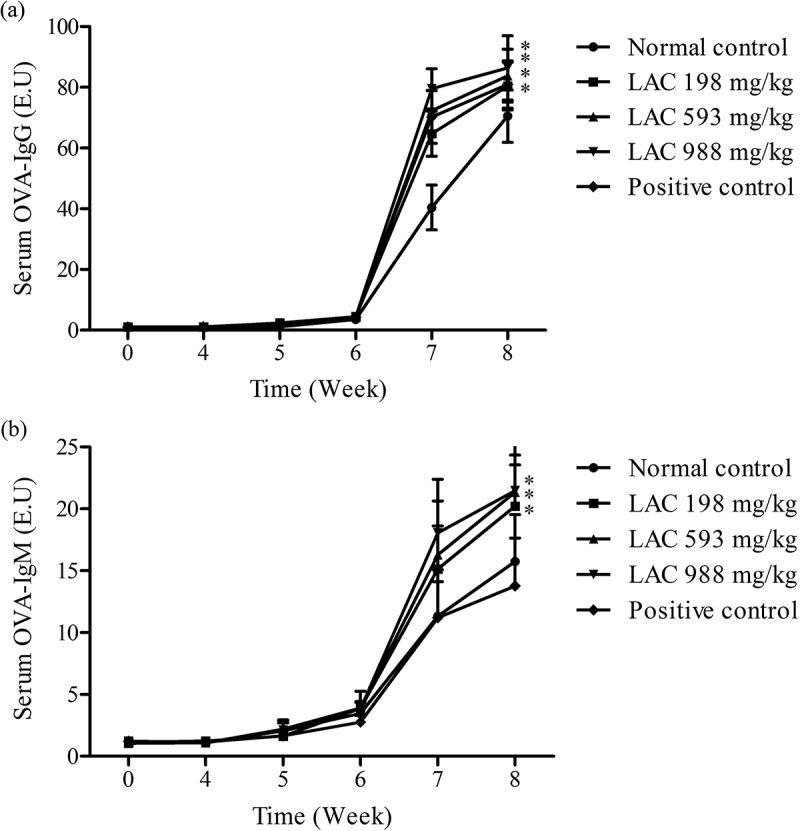
Effect of LAC on serum OVA-IgG and OVA-IgM secretion. The mice were administered with LAC (198, 593, 988 mg/kg) and positive control (PC, 260 mg/kg) for 8 weeks. (a) The serum OVA-IgG and (b) OVA-IgM were analysed using ELISA assays. All data were presented as mean ± SD. *p < 0.05 compared to normal control (NC).
4. Discussion
T. camphoratus has demonstrated the immunomodulatory effects in various studies. Most of these findings indicated polysaccharides from T. camphoratus mycelium had the great potential to develop as immunomodulatory agent to treat infection disease or as a vaccine adjuvant for cancer therapy (Chen et al. 2008; Cheng et al. 2008; Kuo et al. 2008; Lin et al. 2015). In this study, we found LAC, composed of solid-state cultivated powder of T. camphoratus, had the ability to regulate non-specific and specific immune response. There was no effect on body weight, organ weight and no obvious clinical signs observed in mice administered with LAC (data not shown). These results suggested orally gavage of LAC at dose up to 988 mg/kg bw showed no toxic in mice.
For non-specific immune study, mice fed with LAC showed increasing immune cell proliferation under stimulation by Con A. Con A was a stimulator that selectively induced T cell proliferation and LPS is a potent mitogen of B cells. The results suggested that LAC had the ability on enhancing T cell proliferation but no effect on stimulating B cell proliferation. Besides, LAC enhanced NK cell activity and phagocytic cell activity in a dose-dependent manner. NK cell and phagocytes play important roles in innate immunity. Kuo et al. have indicated that T. camphoratus could increase phagocytic activity of leucocytes, monocytes and human polymorphonuclear neutrophils. Polysaccharides and adenosine might be the main components of T. camphoratus that contribute to immunomodulatory effects (Kuo et al. 2008). Our finding was consistent with this study. LAC was the solid-state cultivated powder of T. camphoratus, which contains many bioactive components such as polysaccharides and adenosine. This suggested LAC had the potential to increase innate immune responses to protect body from microbial infection. However, which components were the major active factors need to be clarified in further studies.
Various researches have indicated polysaccharides isolated from T. camphoratus-induced Th1 type cytokines, IL-2, TNF-α and IFN-γ, and promote T cell towards Th1 pathway. Besides, T. camphoratus has been found to prevent asthma and inhibit Schistosoma mansoni infection by enhancing Th1 responses (Wasser 2002; Chen et al. 2008; Cheng et al. 2008; Lu et al. 2009). In this study, it was found that LAC increased IL-2, TNF-α, IFN-γ and GM-CSF in spleen cells under stimulation by Con A and LPS. Moreover, the function of these cytokines could promote T cell proliferation and activate macrophages and NK cell (Gaffen and Liu 2004; Broere et al. 2011). These findings suggested that LAC increased T cell proliferation and NK cell activity through inducing Th1 type cytokines and appeared to trigger immune system developing to Th1 response. The present study also found LAC regulated specific immune response. The results showed LAC increased serum IgG, IgM levels in mice after OVA immunisation. Additionally, LAC increased OVA-stimulated IL-2 and IFN-γ secretion, but had no effect on immune cell proliferation after OVA immunisation. According to these observations, LAC showed the ability to modulate cellular immunity and humoral immunity.
In conclusion, LAC exhibited the effects on increasing immune cell proliferation, enhancing phagocytes and NK cell activity. However, the exact mechanism needs to be explored in the future studies. This study provided evidences that LAC, solid-state cultivated powder of T. camphoratus, had immunomodulatory effects and is safe for further human use.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Broere F, Apasov SG, Sitkovsky MV, Van Eden W.. 2011. A2 T cell subsets and T cell-mediated immunity In: Nijkamp FP, Parnham MJ, editors. Principles of Immunopharmacology: 3rd revised and extended edition. Basel: Birkhäuser Basel; p. 15–27. [Google Scholar]
- Chan WK, Lam DT, Law HK, Wong WT, Koo MW, Lau AS, Lau YL, Chan GC. 2005. Ganoderma lucidum mycelium and spore extracts as natural adjuvants for immunotherapy. J Altern Complement Med. 11(6):1047–1057. [DOI] [PubMed] [Google Scholar]
- Chang CW, Chen YS, Chen CC, Chan IO, Chen CC, Sheu SJ, Lin TW, Chou SH, Liu CJ, Lee TC, et al. 2016. Targeting cancer initiating cells by promoting cell differentiation and restoring chemosensitivity via dual inactivation of STAT3 and src activity using an active component of Antrodia cinnamomea mycelia. Oncotarget. 7(45):73016–73031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Shiau AL, Wang SH, Yang JS, Chang SJ, Wu CL, Ts W. 2014. Zhankuic acid A isolated from Taiwanofungus camphoratus is a novel selective TLR4/MD-2 antagonist with anti-inflammatory properties. J Immunol. 192(6):2778–2786. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YJ, Cheng PC, Lin CN, Liao HF, Chen YY, Chen CC, Lee KM. 2008. Polysaccharides from Antrodia camphorata mycelia extracts possess immunomodulatory activity and inhibits infection of Schistosoma mansoni. Int Immunopharmacol. 8(3):458–467. eng. [DOI] [PubMed] [Google Scholar]
- Cheng PC, Hsu CY, Chen CC, Lee KM. 2008. In vivo immunomodulatory effects of Antrodia camphorata polysaccharides in a T1/T2 doubly transgenic mouse model for inhibiting infection of Schistosoma mansoni. Toxicol Appl Pharmacol. 227(2):291–298. eng. [DOI] [PubMed] [Google Scholar]
- Ditamo Y, Rupil LL, Sendra VG, Nores GA, Roth GA, Irazoqui FJ. 2016. In vivo immunomodulatory effect of the lectin from edible mushroom Agaricus bisporus. Food Funct. 7(1):262–269. [DOI] [PubMed] [Google Scholar]
- Gaffen SL, Liu KD. 2004. Overview of interleukin-2 function, production and clinical applications. Cytokine. 28(3):109–123. [DOI] [PubMed] [Google Scholar]
- Geethangili M, Tzeng YM. 2011. Review of pharmacological effects of Antrodia camphorata and its bioactive compounds EvidBased Complementary and Alternat Med. 2011:212641 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokila Vani M, Kumar KJ, Liao JW, Chien SC, Mau JL, Chiang SS, Lin CC, Kuo YH, Wang SY. 2014. Antcin C from Antrodia cinnamomea protects liver cells against free radical-induced oxidative stress and apoptosis in vitro and in vivo through Nrf2-dependent mechanism. Evid Based Complement Alternat Med. 2013:296082 eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hseu YC, Chen SC, Yech YJ, Wang L, Yang HL. 2008. Antioxidant activity of Antrodia camphorata on free radical-induced endothelial cell damage. J Ethnopharmacol. 118(2):237–245. eng. [DOI] [PubMed] [Google Scholar]
- Hsieh YH, Chu FH, Wang YS, Chien SC, Chang ST, Shaw JF, Chen CY, Hsiao WW, Kuo YH, Wang SY. 2010. Antrocamphin A, an anti-inflammatory principal from the fruiting body of Taiwanofungus camphoratus, and its mechanisms. J Agric Food Chem. 58(5):3153–3158. eng. [DOI] [PubMed] [Google Scholar]
- Huang CH, Chang YY, Liu CW, Kang WY, Lin YL, Chang HC, Chen YC. 2010. Fruiting body of Niuchangchih (Antrodia camphorata) protects livers against chronic alcohol consumption damage. J Agric Food Chem. 58(6):3859–3866. eng. [DOI] [PubMed] [Google Scholar]
- Huang YL, Chu YL, Ho CT, Chung JG, Lai CI, Su YC, Kuo YH, Sheen LY. 2015. Antcin K, an active triterpenoid from the fruiting bodies of basswood-cultivated Antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J Agric Food Chem. 63(18):4561–4569. [DOI] [PubMed] [Google Scholar]
- Kumar KJ, Vani MG, Chueh PJ, Mau JL, Wang SY. 2015. Antrodin C inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via suppression of Smad2/3 and beta-catenin signaling pathways. PloS One. 10(2):e0117111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MC, Chang CY, Cheng TL, Wu MJ. 2008. Immunomodulatory effect of Antrodia camphorata mycelia and culture filtrate. J Ethnopharmacol. 120(2):196–203. eng. [DOI] [PubMed] [Google Scholar]
- Lin CC, Chen CC, Kuo YH, Kuo JT, Senthil Kumar KJ, Wang SY 2015. 2,3,5-Trimethoxy-4-cresol, an anti-metastatic constituent from the solid-state cultured mycelium of Antrodia cinnamomea and its mechanism. J Nat Med. 69(4):513–521. eng. [DOI] [PubMed] [Google Scholar]
- Lin CC, Pan IH, Li YR, Pan YG, Lin MK, Lu YH, Wu HC, Chu CL. 2015. The adjuvant effects of high-molecule-weight polysaccharides purified from Antrodia cinnamomea on dendritic cell function and DNA vaccines. PloS One. 10(2):e0116191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M-C, Hwang S-L, Chang F-R, Chen Y-H, Chang -T-T, Hung C-S, Wang C-L, Chu Y-H, Pan S-H, Wu Y-C. 2009. Immunostimulatory effect of Antrodia camphorata extract on functional maturation of dendritic cells. Food Chem. 113(4):1049–1057. [Google Scholar]
- Ni WY, Wu MF, Liao NC, Yeh MY, Lu HF, Hsueh SC, Liu JY, Huang YP, Chang CH, Chung JG. 2013. Extract of medicinal mushroom Agaricus blazei Murill enhances the non-specific and adaptive immune activities in BALB/c mice. In Vivo. 27(6):779–786. [PubMed] [Google Scholar]
- Sheu F, Chien PJ, Hsieh KY, Chin KL, Huang WT, Tsao CY, Chen YF, Cheng HC, Chang HH. 2009. Purification, cloning, and functional characterization of a novel immunomodulatory protein from Antrodia camphorata (bitter mushroom) that exhibits TLR2-dependent NF-kappaB activation and M1 polarization within murine macrophages. J Agric Food Chem. 57(10):4130–4141. eng. [DOI] [PubMed] [Google Scholar]
- Song AR, Qin D, Zhao C, Sun XL, Huang F, Kong C, Yang S 2014. Immunomodulatory effect of polysaccharides extracted from the medicinal mushroom Antrodia camphorata (higher Basidiomycetes) in specific pathogen-free chickens. Int J Med Mushrooms. 16(1):95–103. eng. [DOI] [PubMed] [Google Scholar]
- Wasser SP. 2002. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl Microbiol Biotechnol. 60(3):258–274. [DOI] [PubMed] [Google Scholar]
- Wu MD, Cheng MJ, Wang WY, Huang HC, Yuan GF, Chen JJ, Chen IS, Wang BC. Antioxidant activities of extracts and metabolites isolated from the fungus Antrodia cinnamomea. Nat Prod Res. 25(16):1488–1496. eng. [DOI] [PubMed] [Google Scholar]
- Wu SHR L, Chang TT. 1997. Antrodia camphorata (‘niu-chang-chih’), new combination of a medicinal fungus in Taiwan. Bot Bull Academia Sinica. 38(4):273–275. [Google Scholar]
- Yu YL, Chen IH, Shen KY, Huang RY, Wang WR, Chou CJ, Chang TT, Chu CL. 2009. A triterpenoid methyl antcinate K isolated from Antrodia cinnamomea promotes dendritic cell activation and Th2 differentiation. Eur J Immunol. 39(9):2482–2491. eng. [DOI] [PubMed] [Google Scholar]
- Yue PY, Wong YY, Chan TY, Law CK, Tsoi YK, Leung KS. 2012. Review of biological and pharmacological activities of the endemic Taiwanese bitter medicinal mushroom, Antrodia camphorata (M. Zang et C. H. Su) Sh. H. Wu et al. (higher Basidiomycetes). Int J Med Mushrooms. 14(3):241–256. eng. [DOI] [PubMed] [Google Scholar]


