Abstract
We described an extensive network of cortical pyramidal neurons in the human brain with abundant acetylcholinesterase (AChE) activity. Emergence of these neurons during childhood/adolescence, attainment of highest density in early adulthood, and virtual absence in other species led us to hypothesize involvement of AChE within these neurons in higher cortical functions. The current study quantified the density and staining intensity of these neurons using histochemical procedures. Few faintly stained AChE-positive cortical pyramidal neurons were observed in children/adolescents. These neurons attained their highest density and staining intensity in young adulthood. Compared with the young adult group, brains of cognitively normal elderly displayed no significant change in numerical density but a significant decrease in staining intensity of AChE-positive cortical pyramidal neurons. Brains of elderly above age 80 with unusually preserved memory performance (SuperAgers) showed significantly lower staining intensity and density of these neurons when compared with same-age peers. Conceivably, low levels of AChE activity could enhance the impact of acetylcholine on pyramidal neurons to counterbalance other involutional factors that mediate the decline of memory capacity during average aging. We cannot yet tell if elderly with superior memory capacity have constitutively low neuronal AChE levels or if this feature reflects adaptive neuroplasticity.
Keywords: acetylcholinesterase, aging, cognitive performance, cortical pyramidal neurons
Introduction
Acetylcholinesterase (AChE) is one of the most ubiquitous enzymes present in central cholinergic pathways (Geula and Mesulam 1999). At cholinergic synapses, it hydrolyzes and thus terminates the synaptic action of acetylcholine (Soreq and Seidman 2001). Roles for AChE have also been described in neurotransmitter recycling, proteolysis, neurogenesis, morphogenesis, neural differentiation, and even amyloid fibril assembly (Silver 1974; Robertson et al. 1985; Small et al. 1991; Layer et al. 1993; Inestrosa et al. 1996; Koenigsberger et al. 1997). Histochemical procedures reveal high AChE activity in perikarya of cholinergic and cholinoceptive neurons. Only cholinergic neurons, defined by the presence of the synthetic enzyme choline acetyltransferase (ChAT), also have AChE-rich axons. These axons display selective laminar and areal distributions in the central nervous system (Silver 1974; Mesulam and Geula 1994; Geula et al. 1995; Geula and Mesulam 1999).
In the cerebral cortex, high AChE enzymatic activity is present in 4 groups of cortical neurons: 1) Cajal–Retzius cells in layer 1, which are particularly prominent during development; 2) heteromorphic neurons in layer 6 and the underlying white matter; 3) polymorphic intracortical neurons of layers 2 through 6; and 4) intracortical pyramidal neurons of layers 3 and 5 (Mesulam and Geula 1991; Geula et al. 1993, 1995); the last group appears to be a relatively specific feature of the human cerebral cortex. None of these neurons contain ChAT and are most likely cholinoceptive but not cholinergic.
In prior reports, we described an extensive network of cortical pyramidal neurons in the human brain that contain a high intensity of histochemically identified AChE reaction product, and designated them “AChE-rich” (Mesulam and Geula 1988a, 1988b, 1991). Qualitatively, the density of these AChE-rich neurons was low in paralimbic cortical areas, primary visual cortex, and primary auditory cortex, intermediate in posterior parietal, prefrontal, and lateral temporal association cortices, and high in premotor and motor cortex. In general, the density of AChE-rich cortical pyramidal neurons was higher in frontoparietal and peristriate cortices than cortical areas in the temporal lobe. This neuronal class was distributed in both layers 3 and 5, but its density in each layer varied among different cortical areas. The appearance of specific cytoarchitectonic and laminar patterns suggests that the AChE enzyme activity in neurons is selectively regulated.
We have also shown an age-dependent pattern in the emergence and maintenance of AChE-rich cortical pyramidal neurons (Mesulam and Geula 1991). Prominent AChE enzyme activity is virtually absent in cortical pyramidal neurons at birth and during childhood. It emerges during adolescence and encompasses a greater number of neurons during early adulthood. The emergence of AChE-rich pyramidal neurons in young adulthood at a time of significant cognitive maturation and the known importance of cholinergic transmission for memory and attention led to the speculation that these neurons may participate in the process of cognitive development.
Not all cortical neurons with a positive histochemical reaction fit the AChE-rich designation. A more inclusive quantitative analysis of heavily as well as lightly stained AChE-positive cortical neurons suggested a potential decrease in the overall number of these neurons in the course of normal aging (Bu et al. 2003). In order to further pursue the fate of neuronal AChE through the human lifespan, we quantitatively analyzed both the number of AChE-positive cortical pyramidal neurons and their densitometric staining intensity in a cross-sectional study that extended from childhood to late-adult life using unbiased stereological techniques. We found that the density and staining intensity of AChE-positive cortical pyramidal neurons is higher in young-adult brains when compared with brains of children and adolescents, consistent with prior findings (Mesulam and Geula 1991). Compared with young-adult brains, there was no significant decrease in the quantitative density of AChE-positive neurons in the elderly with average memory capacity, a finding that is also in keeping with our earlier reports on AChE-rich neurons. However, this group of cognitively average elderly showed a significant decline in the AChE staining intensity of pyramidal neurons when compared with younger adults. In addition to elderly subjects with average memory capacity, we also included a unique group of elderly individuals 80 years of age or older with unusually well-preserved memory performance (cognitive SuperAgers) (Rogalski et al. 2013). We found that SuperAgers had a significant decrease in both the staining intensity and regional density of these neurons when compared with age-matched subjects with average memory capacity.
Materials and Methods
Case Characterization and Tissue Preparation
Brain tissue was obtained from a number of neuropathologists across the US and from the Northwestern University Alzheimer's Disease Center Brain Bank (see Table 1 for characteristics of participants). The cohort consisted of 32 normal cases with no indication of antemortem neurologic or psychiatric disorders, many of whom were extensively characterized through neuropsychological testing (Table 1). Clinical records were also available for each case. For those cases in which neuropsychological test performance were unavailable, careful chart review was undertaken and information was obtained from the next of kin whenever necessary. No cases had a history of prior AChE inhibitor use.
Table 1.
Characteristics of participants
| Case number | Group | Age (years) | Gender | PMIa (h) | Braak stageb | Neuropsychologicalc testing | Memory z-scores | Memory test scores |
|---|---|---|---|---|---|---|---|---|
| 1 | Child | 2.5 | F | 5 | — | No | — | — |
| 2 | Child | 10 | M | 19 | — | No | — | — |
| 3 | Adolescent | 13 | M | 18 | — | No | — | — |
| 4 | Adolescent | 19 | M | 9 | — | No | — | — |
| 5 | Young | 22 | F | 5 | — | No | — | — |
| 6 | Young | 26 | M | 8 | — | No | — | — |
| 7 | Young | 35 | F | 18 | — | No | — | — |
| 8 | Young | 43 | F | 11 | — | No | — | — |
| 9 | Young | 45 | M | 13 | — | No | — | — |
| 10 | Middle-Aged | 50 | F | 48 | — | No | — | — |
| 11 | Middle-Aged | 52 | M | 23 | — | No | — | — |
| 12 | Middle-Aged | 57 | F | 8 | — | No | — | — |
| 13 | Normal Old | 72 | M | 17 | II | No | — | — |
| 14 | Normal Old | 73 | M | 16 | III–IV | No | — | — |
| 15 | Normal Old | 74 | M | 25 | II | No | — | — |
| 16 | Normal Old | 76 | M | 17 | II | No | — | — |
| 17 | Normal Old | 77 | M | 15 | — | No | — | — |
| 18 | Normal Old | 77 | F | 15 | III | No | — | — |
| 19 | Normal Old | 82 | M | 24 | III | No | — | — |
| 20 | Normal Old | 83 | F | 33 | III–IV | No | — | — |
| 21 | Normal Old | 88 | M | 12 | III–IV | Yes | −0.89 | 5/10d |
| 22 | Normal Old | 89 | F | 6 | III–IV | Yes | 0.68 | 8/10e |
| 23 | Normal Old | 89 | F | 9 | II | Yes | 0.68 | 8/10d |
| 24 | Normal Old | 90 | M | 3 | III | Yes | −0.36 | 6/10d |
| 25 | Normal Old | 91 | M | 2 | III | No | — | — |
| 26 | Normal Old | 93 | F | 22 | IV | No | — | — |
| 27 | Normal Old | 96 | F | 5 | IV | Yes | 0.68 | 8/10d |
| 28 | SuperAger | 95 | F | 5 | 0 | Yesf | 1.21 | 9/10f |
| 29 | SuperAger | 87 | F | 11 | III | Yes | 2.27 | 13/15e |
| 30 | SuperAger | 90 | F | 5 | II | Yes | 1.66 | 11/15e |
| 31 | SuperAger | 89 | F | 10 | 0–I | Yes | 1.21 | 9/10d |
| 32 | SuperAger | 90 | F | 4 | III | Yes | 1.97 | 12/15e |
aPMI, postmortem interval.
bBraak stage 0 denotes absence of neurofibrillary tangles, and Braak state VI represents the highest density and most widespread distribution commonly observed in Alzheimer's disease.
cCognitive measures were not identical for all participants. Cognitive scores are provided as actual/possible score.
dDelayed recall score from the CERAD word list (Morris et al. 1989).
eDelayed recall score from the RAVLT (Schmidt 2004).
fTotal score from the telephone interview for cognitive status (Brandt and Folstein 1988) was 49/50, which included a delayed recall score of 9/10 on a 10-word list recall test. CERAD norms were used to establish z-score in this case.
This cohort included cases aged 19 years or younger (N = 4), designated as “child/adolescent,” cases aged 22–57 years (N = 8) designated as “normal young,” and cases aged 72–95 years (N = 15), designated as “normal elderly.” In some analyses, the normal young group was further subdivided into normal “younger” adults, individuals aged 22–45 years (N = 5), and normal “middle-aged” adults, aged 50–57 years (N = 3). An additional 5 brain specimens were obtained from elderly participants known as cognitive SuperAgers, defined as individuals 80-years-old and over, who possess episodic memory functions that are at least at the same level as, or better than, average performance for individuals 20–30 years younger and whose performance in other domains is at least average for age based on a comprehensive battery of neuropsychological tests (Rogalski et al. 2013). Information on the total 32 cases is displayed in Table 1. The study was approved by the Northwestern University Institutional Review Board and conducted in accordance with the Helsinki Declaration (http://www.wma.net/e/policy/17-c_e.html).
Criteria for Cognitively Superior Elderly (Cognitive SuperAgers)
Cognitive SuperAgers represent individuals whose performance on tests of episodic memory is at least as good as individuals 20–30 years their junior. Thus, SuperAgers have either resisted the downward trend of cognitive decline that is considered “typical” with advancing age or had such high baseline memory capacity that it remained above average despite age-related involutional changes. Five SuperAgers were identified based on their age (at least 80-years-old at the time of enrollment) and were required to meet strict psychometric criteria on a battery of neuropsychological tests, which were chosen for their relevance for cognitive aging and their sensitivity to detect clinical symptoms associated with Alzheimer's disease (see Gefen et al. 2014). Briefly, the delayed recall score of the Rey Auditory Verbal Learning Test (RAVLT) (Schmidt 2004) was used as a measure of episodic memory and SuperAgers were required to perform at or above average normative values for individuals in their 50 and 60 s (midpoint age = 61; RAVLT delayed-recall raw score ≥9; RAVLT delayed-recall scaled score ≥10). A 30-item version of the Boston Naming Test (Weintraub et al. 2009), Trail-Making Test Part B (Reitan 1958), and Category Fluency test (“Animals,” Morris et al. 1989) were used to measure cognitive function in non-memory domains. On the BNT-30, TMT Part B, and Category Fluency, SuperAgers were required to perform within or above one standard deviation of the average range according to published normative values based on age and demographic factors (Saxton et al. 2000; Shirk et al. 2011). In one participant, neuropsychological data were available from the telephone interview for cognitive status (Brandt and Folstein 1988), which includes immediate and delayed recall of a 10-word list; on this task, subjects were required to recall at least 9/10 words on the delayed recall portion. One other SuperAging participant was included based on the delayed recall memory performance from the Consortium to Establish a Registry for Alzheimer's Disease (CERAD) word list (Morris et al. 1989).
Criteria for Normal Elderly
A total of 15 cases were identified as cognitively normal elderly; these individuals were 65 years of age or older at time of death. Normal elderly were identified as cognitively “average” compared with their same-age peers. In 5 cases, this was based on neuropsychological norms for the same tests administered to SuperAgers, with scores falling within one standard deviation of the average range for their age and education according to published normative values (Saxton et al. 2000; Shirk et al. 2011) (Table 1). The rest were elderly who, according to clinical records, did not suffer from cognitive abnormalities prior to death. In SuperAging and normal elderly participants with neuropsychological test scores available, z-scores were calculated based on age-appropriate normative data (Morris et al. 1989; Schmidt 2004 and Morris et al. 1989); these scores were used to establish relationships between memory performances and measures of AChE reactivity.
Tissue Preparation
All brains were examined macroscopically and microscopically by a neuropathologist. There was no major vascular disease and no evidence of major neurodegeneration other than Alzheimer-type neurofibrillary tangles and amyloid deposits, which were attributed to age. Nonetheless, it is important to note that more than half of the elderly subjects had neurofibrillary degeneration at Braak stages of III or IV (see Table 1). Amyloid distribution was not quantitated. One hemisphere of each brain (left in most cases) was cut into 2–3 cm thick blocks and immersed in 4% paraformaldehyde at 4 °C for 30–36 h, followed by sucrose gradients (10–40% in 0.1 M sodium phosphate buffer, pH 7.4) for cryoprotection. Blocks were then cut on a freezing microtome into whole hemisphere 40 μm sections and stored in 0.1 M sodium phosphate buffer at 4 °C.
AChE Histochemistry
The enzymatic activity of AChE was detected through a modified Koelle–Friendenwald procedure (Koelle and Freidenwald 1949; Geula et al. 1993). Briefly, tissue sections were mounted on glass slides, and air-dried at room temperature overnight. Sections were rinsed twice for 4 min in 0.1 M sodium acetate buffer at pH 5.5 (titrated with glacial acetic acid), and incubated in a solution containing 0.072 g ethopropazine (inhibitor of nonspecific cholinesterases), 2.312 g acetylthiocholine iodide, 0.75 g glycine, 0.5 g cupric sulfate, and 6.8 g sodium acetate in 1000 mL of distilled water. This solution was titrated to pH 5.5 using glacial acetic acid. Incubation times were based on trials for optimum staining, defined as clearly identifiable AChE reaction product of highest intensity in perikarya without background staining (4.5–6 h). Following incubation, sections were rinsed 6 times for 30 s each in the acetate buffer and incubated in a solution consisting of 19.2 g sodium sulfide in 450 mL 0.1 N HCl at pH 7.8. The tissue was rinsed again (6 × 30 s) in acetate buffer, and then incubated in a 1% silver nitrate solution in distilled water for 1 min. The sections were rinsed once more in acetate buffer (4 times for 30 s), dehydrated through graded ethanols, cleared in xylene and coverslipped under Permount.
Unbiased Stereological Counting Method
Numerical density of AChE-positive cortical pyramidal neurons was determined via modified stereological methods according to procedures previously described in detail (Geula et al. 2003; Gefen et al. 2015). All stained pyramidal neurons in layers 3 and 5 of cortex, in which reaction product was detected throughout the cytoplasm, were counted regardless of the apparent staining intensity, using the fractionator method of the StereoInvestigator software (MBF Biosciences, MicroBrightField, Inc.). Two to three representative sections were used for each of 5 cortical areas, consisting of the supplementary motor cortex (SMC, Brodmann area [BA] 6), middle frontal gyrus (MFG, BA 9), middle temporal gyrus (MTG, BA 21), inferior parietal lobule (IPL, BA 39–40), and the anterior cingulate cortex (AC, BA 24). In the child/adolescent cases, only BA 21 was consistently available. Pyramidal neurons were identified by an observer blind to age and cognitive status, based on their distinct pyramidal shape, their location within cortical layers 3 and 5, and the granular AChE reaction product that permeated the cytoplasm. A 200 × 200 μm counting frame was used and the top and bottom 2 μm of each section were used as guard height. The section thickness was measured by the microcator on the microscope. Cortical regions were traced at ×4 magnification and counting was carried out at ×20. Sections were treated as adjacent. The density of AChE-positive cortical pyramidal neurons was expressed as counts per cubic millimeter. Stereologic parameters used resulted in coefficient of error <0.1.
Measurement of Optical Density
The intensity of the AChE reaction product was determined using optical density measures according to a previously described procedure (Baker-Nigh et al. 2015). Two to three representative sections were used for analysis in each of the 5 cortical areas and layers (layers 3 and 5). Analyses were completed only on BA 21 for child/adolescent cases. Photomicrographs of random fields selected by an observer were taken at ×20 magnification. Images were analyzed using the National Institutes of Health image analysis software ImageJ (version 1.451; http://imagej.nih.gov/ij/). Each AChE-positive cortical pyramidal neuron was manually outlined and the optical density of reaction product in each cell was determined. The system was calibrated for maximum and minimum light transmissions through a glass slide as the light was on and off on the microscope, respectively. Based on this calibration, high optical density values correspond with high AChE enzymatic activity and low light transmission. The optical density of individual AChE-positive pyramidal neuron, regardless of staining intensity, was quantified. On average, the optical density of 100 pyramidal neurons was determined in each cortical area. Measures reported represent mean optical density values of pyramidal neurons in each area.
Staging of Neurofibrillary Tangle Density and Distribution
To determine the potential relationship between formation of neurofibrillary tangles, which are commonly found in the brains of the normal elderly in relatively low density and restricted distribution (Price et al. 1991; Arriagada et al. 1992), and AChE activity in cortical pyramidal neurons in the elderly, distribution of neurofibrillary tangles (Price et al. 1991; Arriagada et al. 1992) was staged in each normal elderly and SuperAger case according to the Braak criteria (Braak and Braak 1991, 1995). For this purpose, sections stained with Thioflavin S, or immunostained using PHF-1 (generous gift of Dr Peter Davies, Albert Einstein College of Medicine, NY, 1/1000), a mouse monoclonal antibody that recognizes an epitope of Tau phosphorylated at Ser396/404 (Augustinack et al. 2002; Lauckner et al. 2003) or the monoclonal Alz50 antibody (Wolozin et al. 1986; Wolozin and Davies 1987; Hyman et al. 1988), employing the Vectastain Elite ABC Kit (Vector Laboratories), were used.
Statistical Analysis
Two-way, non-repeated measures ANOVAs were used to compare measures between cortical areas and across groups for numerical density and optical density of cortical AChE-positive pyramidal neurons. Bonferroni post hoc tests were used for pairwise comparisons. Percentages of increase/decrease were calculated between all groups. Relationships were determined using Spearman correlations. The probability for statistical significance was set at P < 0.05.
Results
AChE Histochemistry in the Cerebral Cortex
The histochemical method used resulted in deposition of granular dark brown/black reaction product throughout the cerebral cortex. As expected, reactivity was visualized in Cajal–Retzius cells in layer 1, heteromorphic neurons in layer 6 and underlying white matter, polymorphic, intracortical neurons of layers 2 through 6, and intracortical pyramidal neurons of layers 3 and 5. At low magnification, cortical pyramidal neurons were the most conspicuous AChE-positive elements. Within these cells, the reaction product filled the cytoplasm and proximal dendrites, clearly distinguishing the neurons from background (Fig. 1). Staining intensity was variable across age and area, but all groups of brains displayed positive intracellular staining in the 5 cortical areas of interest. Cortical pyramidal neurons were qualitatively similar in staining intensity in layer 3 as compared with layer 5 within a given cortical area.
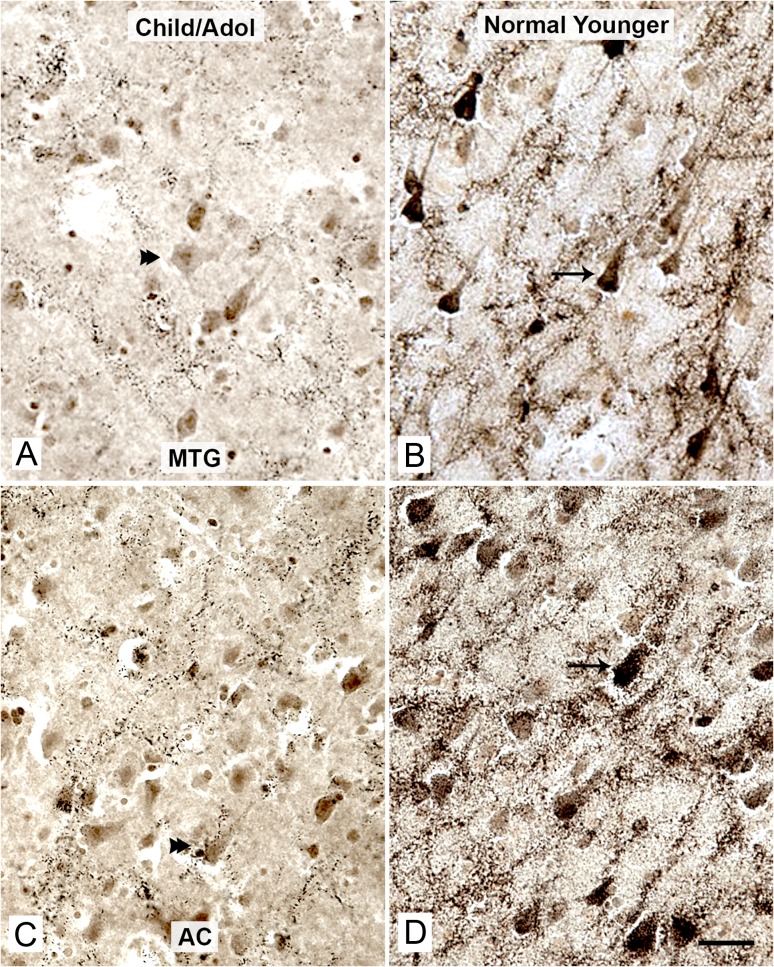
Figure 1.
Strong AChE activity in cortical pyramidal neurons emerges in young adulthood. AChE-positive pyramidal neurons in the MTG (A and B) and the AC cortex (C and D), in children (A, Case 1, 2.5 years old, and C, Case 2, 10 years old) and a normal younger adult (B and D, Case 9, 45 years old). Note the very faint AChE reaction product in some pyramidal neurons in the children (A and C, double arrowheads), and intense AChE reaction product in many pyramidal neurons in the normal younger adult (B and D, arrows). Scale bars represent 100 µm.
As expected, across all groups (with the exception of the child/adolescent group in which tissue was unavailable), the SMC showed the highest density and staining intensity of AChE-positive pyramidal neurons compared with other cortical regions examined (Figs 2 and 3).
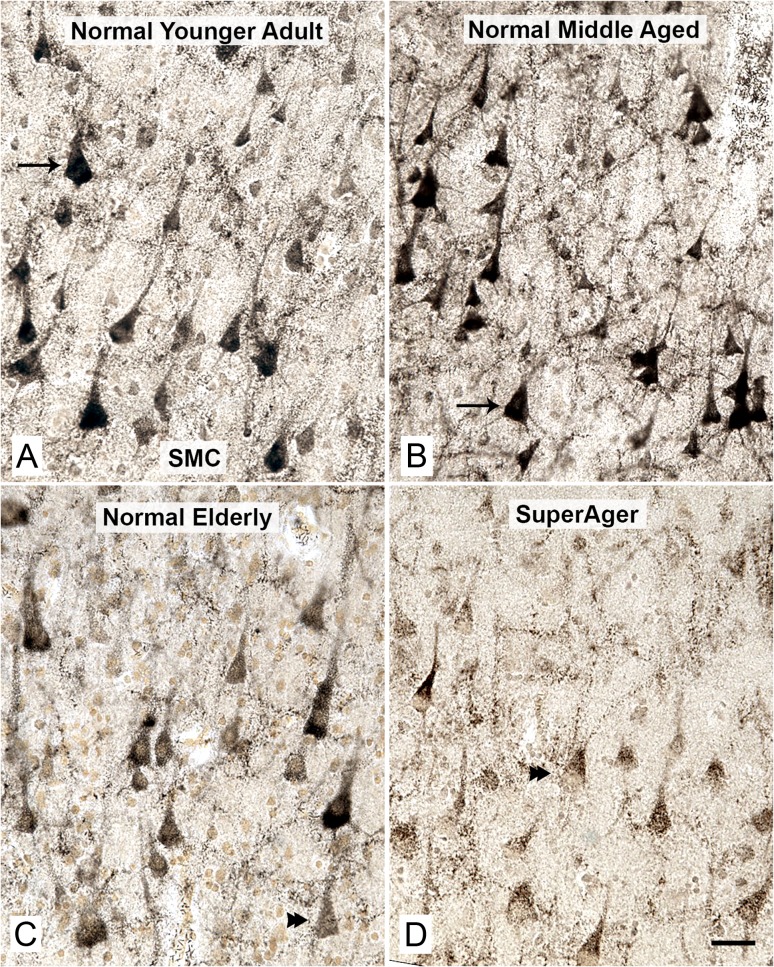
Figure 2.
Reduced AChE activity in pyramidal neurons in the SMC of normal elderly and SuperAgers. The density of AChE-positive pyramidal neurons in SMC and the intensity of AChE reaction product remained relatively constant when younger adult cases (A, Case 9, 45 years old, arrow) were compared with middle-aged adults (B, Case 11, 52 years old, arrow). This region contained the highest density of AChE-positive cortical pyramidal neurons when compared with all other regions studied. The density and particularly the staining intensity of these neurons displayed a consistent decrease in the normal elderly (C, Case 24, 90 years old, double arrowhead), and a further decrease in elderly with superior cognitive performance/SuperAgers (D, Case 28, 95 years old, double arrowhead) when compared with the other groups. Scale bars represent 100 µm.
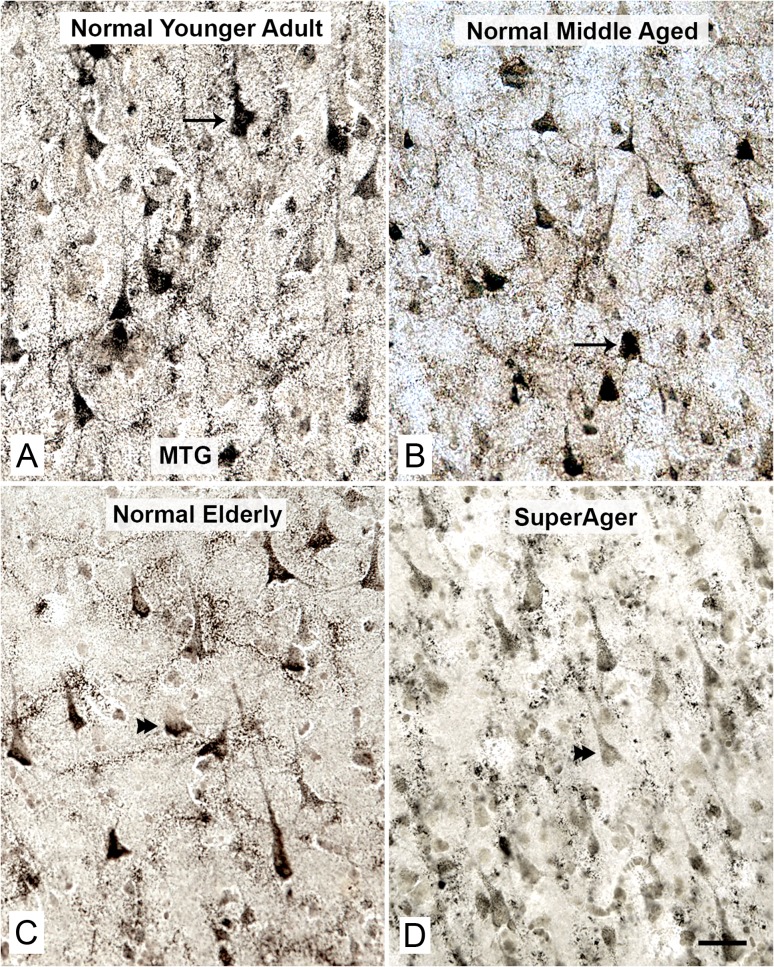
Figure 3.
Reduced AChE activity in pyramidal neurons in the MTG of normal elderly and SuperAgers. The density of AChE-positive pyramidal neurons in MTG and the intensity of AChE reaction product remained relatively constant when younger adult cases (A, Case 6, 26 years old, arrow) were compared with middle-aged adults (B, Case 11, 52 years old, arrow). The MTG had lower density and staining intensity of AChE-positive cortical pyramidal neurons when compared with SMC (Fig. 2). The density and particularly the staining intensity of these neurons displayed a consistent decrease in the normal elderly (C, Case 20, 83 years old, double arrowhead), and a further decrease in elderly with superior cognitive performance/SuperAgers (D, Case 30, 90 years old, double arrowhead) when compared with the other groups. Scale bars represent 100 µm.
Children/Adolescents (Age < 19) Versus Younger Adults (Age 20–45)
The apparent density of AChE-positive cortical pyramidal neurons, as well as quantitative information in MTG, revealed the lowest numerical density of these neurons in the children (2.5 and 10 years), followed by adolescents (13 and 19 years). The highest numerical and optical densities were observed in the younger adult group (Figs 2 and 3). The numerical density of AChE-positive cortical pyramidal neurons displayed an increase in younger adults (N = 5) when compared with children/adolescents (N = 4) in MTG (34.5%; approaching significance, P = 0.085). Optical density of AChE reaction product displayed a similar increase in younger adults in MTG (32.6%; approaching significance, P = 0.055).
Younger Adults (Age 20–45) Versus Middle-Aged Adults (Age 50–57)
Cases that comprised the normal young group were alternatively sub-grouped into younger adult (N = 5) and middle-aged cases (N = 3) to analyze more specific age-related differences in cortical AChE-positive pyramidal neurons (Figs 2 and 3). In the SMC, although not statistically significant, there was a small quantitative increase in numerical (7.17%) and optical (8.52%) density in the middle-aged group compared with the young adult group. This small difference was not seen in other cortical areas. Therefore, these groups were combined into a normal young group (22–57 years) for the remainder of the analyses.
Age-Dependent Differences Across Cortical Regions
ANOVA with Bonferroni correction showed significant differences in numerical density across the 5 cortical areas (P < 0.05) and across groups (P < 0.0001). Similar significant differences were detected in optical density measures across cortical areas (P < 0.001) and groups (P < 0.0001).
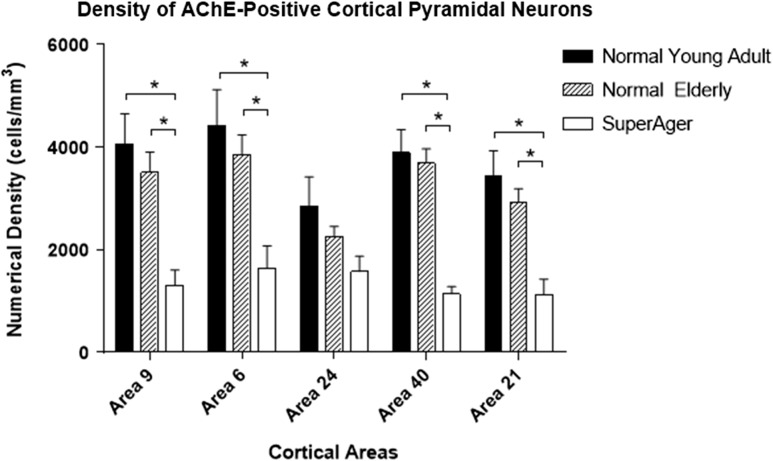
Across all cortical regions, there was a consistent decrease in the density of AChE-positive cortical pyramidal neurons where the normal young group (ages 22–57) showed greatest numerical density, followed by a consistent but statistically non-significant decrease in the normal elderly (5.1–20.8% decrease; P > 0.05), with a further significant decrease in cognitive SuperAgers compared with normal elderly (30.6–69.2% decrease; significant in SMC, MFG, MTG, and IPL; P < 0.05) (Fig. 4). When SuperAgers were compared with the 5 elderly controls who had undergone neuropsychological testing, significantly smaller densities of AChE-positive cortical pyramidal neurons were found in SMC and IPL in SuperAgers (57% and 71% decrease, P < 0.03 and P < 0.003, respectively). In a direct comparison between the normal young control group and cognitive SuperAgers, a significantly larger decline (43.3–70.8%) was found in all areas (P < 0.05) except the AC (P > 0.05; Fig. 4). When the elderly control and SuperAger groups were combined, the density of AChE-positive cortical pyramidal neurons in IPL displayed a negative correlation with z-scores on tests of memory, which approached significance (r = −0.56, P = 0.08); no other significant correlations were observed.
Figure 4.
Stereologically determined density of AChE-positive cortical pyramidal neurons displayed reductions in the normal elderly and particularly in SuperAgers. The density of AChE-positive cortical pyramidal neurons displayed a small and statistically non-significant (P > 0.05) but consistent decrease in the normal elderly when compared with normal young adults in all BA studied. A further decrease was observed in SuperAgers when compared with the normal elderly (statistically significant in areas 9, 6, 40, and 21; P < 0.05), or the normal young groups (statistically significant in areas 9, 6, 40, and 21; P < 0.05). Area 9: MFG; area 6: SMC; area 39–40: IPL; and area 21: MTG. *Significant at P < 0.05; **Significant at P < 0.01.
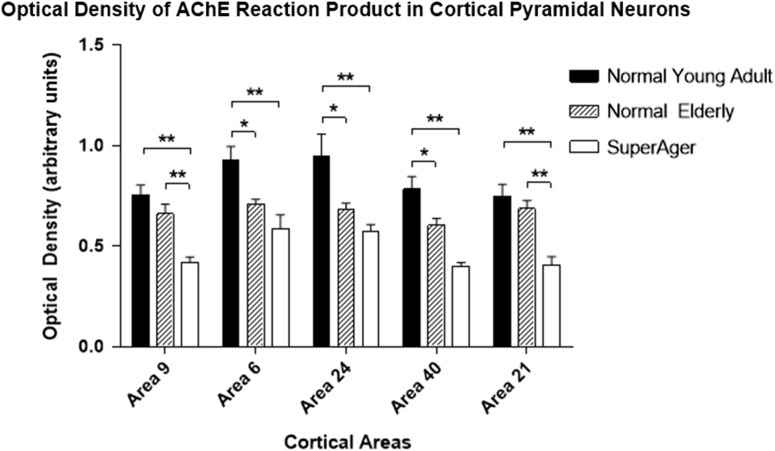
A similar pattern was observed for optical density values (high values represent high AChE reactivity) where the normal young group showed greatest optical density, followed by the normal elderly group (9.15–28.6% decrease; significant in SMC, IPL and AC; P < 0.05), with further decline in SuperAgers (15.7–40.7% decrease compared with normal elderly; significant in MFG and MTG; P < 0.01, Fig. 5). When SuperAgers were compared with the 5 elderly controls who had undergone neuropsychological testing, significantly lower optical density of AChE reaction product in cortical pyramidal neurons was found in MFG in SuperAgers (37% decrease, P < 0.03). Again, in a direct comparison between cognitive SuperAgers and the normal young group, there was a significant decrease in optical density values (37.3–48.9%) across all cortical areas (P < 0.01, Fig. 5). When the elderly control and SuperAger groups were combined, the optical density of AChE reaction product in MFG displayed a significant negative correlation with z-scores on tests of memory (r = −0.79, P < 0.008), and a negative correlation was found in SMC which approached significance (r = −0.61, P = 0.07). No other significant correlations were observed. When the normal young and normal elderly groups were combined, optical density of AChE activity displayed a negative correlation with age (significant in SMC, IPL, and AC; r = 0.58–0.66, P = 0.002–0.008).
Figure 5.
Optical density (i.e., staining intensity) of AChE reaction product in cortical pyramidal neurons displayed reductions in the normal elderly and SuperAgers. The optical density of AChE reaction product in cortical pyramidal neurons displayed a robust decrease in normal elderly when compared with the normal young group (statistically significant in areas 6, 24, and 39–40; P < 0.05). A further decrease in optical density was observed in SuperAgers when compared with the normal elderly (statistically significant in areas 9 and 21; P < 0.01), or the normal young groups (statistically significant across all cortical areas, P < 0.01). Area 9: MFG; area 6: SMC; area 39–40: IPL; and area 21: MTG. *Significant at P < 0.05; **Significant at P < 0.01.
The SMC also showed a particularly impressive decline in both numerical (10.3%) and optical density (22.6%) when the normal elderly were compared with the middle-aged group (Figs 2 and 3); and an even larger decline in SuperAgers (56.4%, numerical density; 28.8% optical density) was observed in this particular area when compared with the middle-aged group. A similar trend was seen in the MTG (Figs 2 and 3).
According to selection criteria, the SuperAger group consisted of individuals above age 80. We did not find a significant difference between age in the normal elderly group when compared with the SuperAger group (P > 0.05), validating the statistical comparisons of neuronal and optical densities in these 2 groups. Furthermore, large decreases in the numerical density of AChE-positive cortical pyramidal neurons (27–65%) and the optical density of AChE reaction product (5–40%) were noted in the areas investigated when the 5 SuperAgers were compared with the 5 oldest normal cases in the cohort (Table 1, Cases 23–27, age range 89–96 years).
Braak stages of neurofibrillary tangle distribution in the normal elderly and SuperAgers are presented in Table 1. In the elderly control and SuperAger groups combined, there was no significant correlation between Braak stage and the numerical density or staining intensity of AChE in cortical pyramidal neurons in any of the regions investigated (P > 0.05). However, we did not quantify markers of neurofibrillary or amyloid pathology in each cortical area.
Discussion
The results of this study confirm the existence of an extensive network of AChE-positive cortical pyramidal neurons in the human cerebral cortex. Our histochemical procedure elicited reaction product within AChE-positive neurons that was dark and coarsely granular. Some of these neurons fit the designation of AChE-rich, but others did not. The remaining cortical neurons were not necessarily devoid of AChE enzyme activity, but lacked detectable deposits of reaction product within the constraints of the histochemical procedure used. While the density and staining intensity of AChE-positive cortical pyramidal neurons varied in different cortical areas, all areas of the cerebral cortex examined contained such neurons. The density and staining intensity of these neurons were higher in the frontal cortical regions, particularly in the SMC, when compared with parietal and temporal regions. These findings are consistent with our earlier qualitative observations on AChE-rich neurons (Mesulam and Geula 1988a, 1988b, 1991).
The density and staining intensity of AChE-positive cortical pyramidal neurons displayed substantial changes across the lifespan. A low density of lightly stained cortical pyramidal neurons was observed in children and adolescents. These neurons attained their highest number and staining intensity during young adulthood. The number of these neurons did not show a significant age-related change in comparisons confined to cognitively average elderly, in keeping with our earlier qualitative observations (Mesulam and Geula 1988a, 1988b, 1991). However, normal aging was associated with a significant decrease in the staining intensity of AChE-positive cortical pyramidal neurons. Compared with their peers with average memory capacity, the elderly with exceptional memory performance (SuperAgers) had significantly fewer AChE-positive cortical pyramidal neurons and lighter intensity of the neuronal AChE reaction product. These findings show that AChE enzyme activity in cortical neurons changes according to chronological age and also according to memory capacity. Whether the lower AChE activity in SuperAgers reflects a life-long or adaptive phenomenon remains to be determined. It is also important to note that the “average” and SuperAging elderly brains contained neurofibrillary pathology (Braak III or IV in 12 of the 19 cases where the information was available) as well as amyloid deposits. The Braak staging is unlikely to account for the observed changes since it tended to be higher in the average elderly. In the future, correlations of AChE activity, markers of tau pathology, and amyloid deposits within the same cortical area may help to address this question.
Function(s) of AChE in Cortical Pyramidal Neurons
Immunohistochemical and autoradiographic studies have consistently demonstrated the presence of high densities of nicotinic and muscarinic cholinergic receptors in human cortical pyramidal neurons (Schroder et al. 1989; Zilles et al. 1989; Rubboli et al. 1994; Graham et al. 2003). These cholinoceptive pyramidal neurons require perikaryal AChE in order to hydrolyze the acetylcholine released by presynaptic cholinergic terminals (Mesulam and Geula 1991). The AChE-positive neurons investigated in this report are therefore likely to represent one element of such cholinoceptive neurons. These AChE-positive cortical neurons are not cholinergic since they lack perikaryal ChAT (Mesulam and Geula 1988a, 1988b; Geula and Mesulam 1999). The cholinergic innervation of cortical cholinoceptive neurons in the human brain comes exclusively from the basal forebrain Ch1–Ch4 cell groups (Mesulam and Geula 1988a, 1988b; Mesulam et al. 1992).
In addition to their cholinergic function, cholinesterases in general, and AChE in particular, are associated with a number of other functions, including neurotransmitter recycling, proteolysis, neurogenesis, morphogenesis, neural differentiation, and amyloid fibril assembly (Silver 1974; Robertson et al. 1985; Small et al. 1991; Layer et al. 1993; Inestrosa et al. 1996; Koenigsberger et al. 1997). While it is possible that AChE in cortical pyramidal neurons is involved in one or more non-cholinergic functions, these additional functions have not been investigated in relation to AChE in cortical pyramidal neurons. The evidence supporting such functions is derived primarily from in vitro models or developing brains. Therefore, future studies will need to address non-cholinergic functions of AChE in vivo and in the adult brain to elucidate such potential functions of AChE in cortical pyramidal neurons.
AChE in Cortical Pyramidal Neurons and Cognitive Function
An age-related decline of memory capacity is a characteristic of normal aging (Rowe and Kahn 1987; Salthouse et al. 2003; Salthouse 2009; Yaffe et al. 2009). This is why average (i.e., normal) scores for an 80-year-old on neuropsychological tests are considerably lower than average scores for a 50-year-old, necessitating use of age-adjusted values for interpreting cognitive test performance. The SuperAger group used in the present study either had an unusually high baseline memory capacity or had the ability to resist the typical age-related erosion of this faculty. A large body of evidence, including information derived from nonhuman primates, indicates that the cholinergic innervation of the cerebral cortex is intimately involved in the cognitive processes related to memory and attention (Flicker et al. 1983; Aigner et al. 1987; Irle and Markowitsch 1987; Muir et al. 1992; Fine et al. 1997). In fact, a number of studies indicate that inhibition of AChE in normal individuals may improve cognitive performance (Yesavage et al. 2002; Mumenthaler et al. 2003; Ashare et al. 2012). Of note, we observed a significant negative correlation between the optical density of AChE reaction product in cortical pyramidal neurons in MFG and memory performance in the normal elderly and SuperAger groups combined. We speculate that the decrease of AChE activity in the SuperAgers, and perhaps also in the normal elderly, may reflect a phenomenon of neuroplasticity, triggered through a yet unidentified mechanism, that optimizes memory capacity by enhancing the impact of presynaptically released acetylcholine. Naturally, we cannot dismiss the alternate possibility that this phenomenon is part of an overall age-related involutional process that has no functional impact on memory capacity.
Notes
Conflict of Interest: None declared.
Funding
The National Institute on Aging (AG045571; T32 AG20506); the Northwestern University Alzheimer's Disease Center (AG13854); the Davee Foundation, and the Florane and Jerome Rosenstone Fellowship.
References
- Aigner TG, Mitchell SJ, Aggleton JP, Delong MR, Struble RG, Price DL, Wenk GL, Mishkin M. 1987. Effects of scopolamine and physostigmine on recognition memory in monkeys with ibotenic-acid lesions of the nucleus basalis of Meynert. Psychopharmacol. 92:292–300. [DOI] [PubMed] [Google Scholar]
- Arriagada PV, Marzloff K, Hyman BT. 1992. Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer's disease. Neurology. 42:1681–1688. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Ray R, Lerman C, Strasser AA. 2012. Cognitive effects of the acetylcholinesterase inhibitor, donepezil, in healthy, non-treatment seeking smokers: a pilot feasibility study. Drug Alcohol Depend. 126:263–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. 2002. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer's disease. Acta Neuropathol (Berl). 103:26–35. [DOI] [PubMed] [Google Scholar]
- Baker-Nigh A, Vahedi S, Davis EG, Weintraub S, Bigio EH, Klein WL, Geula C. 2015. Neuronal amyloid-beta accumulation within cholinergic basal forebrain in ageing and Alzheimer's disease. Brain. 138:1722–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. 1991. Neuropathological staging of Alzheimer's disease. Acta Neuropathol. 82:239–259. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. 1995. Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol Aging. 16:271–278. [DOI] [PubMed] [Google Scholar]
- Brandt JSM, Folstein M. 1988. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1:111–117. [Google Scholar]
- Bu J, Sathyendra V, Nagykery N, Geula C. 2003. Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Exp Neurol. 182:220–231. [DOI] [PubMed] [Google Scholar]
- Fine A, Hoyle C, Maclean CJ, Levatte TL, Baker HF, Ridley RM. 1997. Learning impairments following injection of a selective cholinergic immunotoxin, ME20.4 IgG-saporin, into the basal nucleus of Meynert in monkeys. Neuroscience. 81:331–343. [DOI] [PubMed] [Google Scholar]
- Flicker C, Dean RL, Watkins DL, Fisher SK, Bartus RT. 1983. Behavioral and neurochemical effects following neurotoxic lesions of a major cholinergic input to the cerebral cortex in the rat. Pharmacol Biochem Behav. 18:973–981. [DOI] [PubMed] [Google Scholar]
- Gefen T, Peterson M, Papastefan ST, Martersteck A, Whitney K, Rademaker A, Bigio EH, Weintraub S, Rogalski E, Mesulam MM, et al. . 2015. Morphometric and histologic substrates of cingulate integrity in elders with exceptional memory capacity. J Neurosci. 35:1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen T, Shaw E, Whitney K, Martersteck A, Stratton J, Rademaker A, Weintraub S, Mesulam MM, Rogalski E. 2014. Longitudinal neuropsychological performance of cognitive SuperAgers. J Am Geriatr Soc. 62:1598–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, Parker R, Wu CK. 2003. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 455:249–259. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM. 1999. Cholinergic systems in Alzheimer disease In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. Philadelphia: Lippincott Williams and Wilkins; p. 269–292. [Google Scholar]
- Geula C, Mesulam MM, Kuo CC, Tokuno H. 1995. Postnatal development of cortical acetylcholinesterase-rich neurons in the rat brain: permanent and transient patterns. Exp Neurol. 134:157–178. [DOI] [PubMed] [Google Scholar]
- Geula C, Mesulam MM, Tokuno H, Kuo CC. 1993. Developmentally transient expression of acetylcholinesterase within cortical pyramidal neurons. Dev Brain Res. 76:23–31. [DOI] [PubMed] [Google Scholar]
- Graham AJ, Ray MA, Perry EK, Jaros E, Perry RH, Volsen SG, Bose S, Evans N, Lindstrom J, Court JA. 2003. Differential nicotinic acetylcholine receptor subunit expression in the human hippocampus. J Chem Neuroanat. 25:97–113. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Wolozin BL, Davies P, Kromer LJ, Damasio AR. 1988. Alz-50 antibody recognizes Alzheimer-related neuronal changes. Ann Neurol. 23:371–379. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Alvarez A, Perez CA, Moreno RD, Vicente M, Linker C, Casanueva OI, Soto C, Garrido J. 1996. Acetylcholinesterase accelerates assembly of amyloid-beta- peptides into Alzheimer's fibrils: possible role of the peripheral site of the enzyme. Neuron. 16:881–891. [DOI] [PubMed] [Google Scholar]
- Irle E, Markowitsch HJ. 1987. Basal forebrain-lesioned monkeys are severely impaired in tasks of association and recognition memory. Ann Neurol. 22:735–743. [DOI] [PubMed] [Google Scholar]
- Koelle BG, Freidenwald JS. 1949. A histochemical method for localizing cholinesterase activity. Proc Soc Exp Biol Med. 70:617–622. [DOI] [PubMed] [Google Scholar]
- Koenigsberger C, Chiappa S, Brimijoin S. 1997. Neurite differentiation is modulated in neuroblastoma cells engineered for altered acetylcholinesterase expression. J Neurochem. 69:1389–1397. [DOI] [PubMed] [Google Scholar]
- Lauckner J, Frey P, Geula C. 2003. Comparative distribution of tau phosphorylated at Ser262 in pre-tangles and tangles. Neurobiol Aging. 24:767–776. [DOI] [PubMed] [Google Scholar]
- Layer PG, Weikert T, Alber R. 1993. Cholinesterases regulate neurite growth of chick nerve cells in vitro by means of a non-enzymatic mechanism. Cell Tissue Res. 273:219–226. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. 1988. a. Acetylcholinesterase-rich pyramidal neurons in the human neocortex and hippocampus: absence at birth, development during the life span, and dissolution in Alzheimer's disease. Ann Neurol. 24:765–773. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. 1988. b. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J Comp Neurol. 275:216–240. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. 1991. Acetylcholinesterase-rich neurons of the human cerebral cortex: cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol. 306:193–220. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Geula C. 1994. Chemoarchitectonics of axonal and perikaryal acetylcholinesterase along information processing systems of the human cerebral cortex. Brain Res Bull. 33:137–153. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Hersh LB, Mash DC, Geula C. 1992. Differential cholinergic innervation within functional subdivisions of the human cerebral cortex: a choline acetyltransferase study. J Comp Neurol. 318:316–328. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C, Investigators C. 1989. The consortium to establish a registry for Alzheimer's disease. Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 39:1159–1165. [DOI] [PubMed] [Google Scholar]
- Muir JL, Dunnett SB, Robbins TW, Everitt BJ. 1992. Attentional functions of the forebrain cholinergic systems: effects of intraventricular hemicholinium, physostigmine, basal forebrain lesions and intracortical grafts on a multiple-choice serial reaction time task. Exp Brain Res. 89:611–622. [DOI] [PubMed] [Google Scholar]
- Mumenthaler MS, Yesavage JA, Taylor JL, O'Hara R, Friedman L, Lee H, Kraemer HC. 2003. Psychoactive drugs and pilot performance: a comparison of nicotine, donepezil, and alcohol effects. Neuropsychopharmacology. 28:1366–1373. [DOI] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. 1991. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 12:295–312. [DOI] [PubMed] [Google Scholar]
- Reitan RM. 1958. Validity of the Trail Making Test as an indicator of organic brain damage. Percep Mot Skills. 8:271–276. [Google Scholar]
- Robertson RT, Tijerina AA, Gallivan ME. 1985. Transient patterns of acetylcholinesterase activity in visual cortex of the rat: normal development and the effects of neonatal monocular enucleation. Dev Brain Res. 21:203–214. [DOI] [PubMed] [Google Scholar]
- Rogalski EJ, Gefen T, Shi J, Samimi M, Bigio E, Weintraub S, Geula C, Mesulam MM. 2013. Youthful memory capacity in old brains: anatomic and genetic clues from the Northwestern SuperAging Project. J Cog Neurosci. 25:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe JW, Kahn RL. 1987. Human aging: usual and successful. Science. 237:143–149. [DOI] [PubMed] [Google Scholar]
- Rubboli F, Court JA, Sala C, Morris C, Chini B, Perry E, Clementi F. 1994. Distribution of nicotinic receptors in the human hippocampus and thalamus. Eur J Neurosci. 6:1596–1604. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. 2009. When does age-related cognitive decline begin? Neurobiol Aging. 30:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. 2003. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J Exp Psychol Gen. 132:566–594. [DOI] [PubMed] [Google Scholar]
- Saxton J, Ratcliff G, Munro CA, Coffey EC, Becker JT, Fried L, Kuller L. 2000. Normative data on the Boston Naming Test and two equivalent 30-item short forms. Clin Neuropsychol. 14:526–534. [DOI] [PubMed] [Google Scholar]
- Schmidt M. 2004. Rey Auditory Verbal Learning Test: A Handbook. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schroder H, Zilles K, Luiten PGM, Strosberg AD, Aghchi A. 1989. Human cortical neurons contain both nicotinic and muscarinic acetylcholine receptors: an immunocytochemical double-labeling study. Synapse. 4:319–326. [DOI] [PubMed] [Google Scholar]
- Shirk SD, Mitchell MB, Shaughnessy LW, Sherman JC, Locascio JJ, Weintraub S, Atri A. 2011. A web-based normative calculator for the uniform data set (UDS) neuropsychological test battery. Alzheimers Res Ther. 3:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver A. 1974. The Biology of Cholinesterases. Amsterdam: Elsevier. [Google Scholar]
- Small DH, Moir RD, Fuller SJ, Michaelson S, Bush AI, Li QX, Milward E, Hilbich C, Weidemann A, Beyreuter K, et al. . 1991. A protease activity associated with acetylcholinesterase releases the membrane-bound form of the amyloid protein precursor of Alzheimer's disease. Biochemistry. 30:10795–10799. [DOI] [PubMed] [Google Scholar]
- Soreq H, Seidman S. 2001. Acetylcholinesterase--new roles for an old actor. Nat Rev Neurosci. 2:294–302. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Salmon D, Mercaldo N, Ferris S, Graff-Radford NR, Chui H, Cummings J, DeCarli C, Foster NL, Galasko D, et al. . 2009. The Alzheimer's Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B, Davies P. 1987. Alzheimer-related neuronal protein A68: specificity and distribution. Ann Neurol. 22:521–526. [DOI] [PubMed] [Google Scholar]
- Wolozin BL, Pruchnicki A, Dickson DW, Davies P. 1986. A neuronal antigen in the brains of Alzheimer patients. Science. 232:648–650. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Fiocco AJ, Lindquist K, Vittinghoff E, Simonsick EM, Newman AB, Satterfield S, Rosano C, Rubin SM, Ayonayon HN, et al. , Health ABCS . 2009. Predictors of maintaining cognitive function in older adults: the Health ABC study. Neurology. 72:2029–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Mumenthaler MS, Taylor JL, Friedman L, O'Hara R, Sheikh J, Tinklenberg J, Whitehouse PJ. 2002. Donepezil and flight simulator performance: effects on retention of complex skills. Neurology. 59:123–125. [DOI] [PubMed] [Google Scholar]
- Zilles K, Schroder H, Schroder U, Horvath E, Werner L, Luiten PG, Maelicke A, Strosberg AD. 1989. Distribution of cholinergic receptors in the rat and human neocortex. EXS. 57:212–228. [DOI] [PubMed] [Google Scholar]