Delayed treatment with recombinant tissue plasminogen activator (tPA) for ischaemic stroke may exacerbate blood-brain barrier breakdown and lead to lethal haemorrhagic transformation. Mao, Li et al. report that regulatory T cell adoptive transfer reduces tPA-mediated blood-brain barrier damage and brain haemorrhage after stroke by two inhibitory mechanisms involving CCL2 and MMP9.
Keywords: regulatory T cell, blood–brain barrier, haemorrhagic transformation, CCL2, matrix metalloproteinase 9
Abstract
Delayed thrombolytic treatment with recombinant tissue plasminogen activator (tPA) may exacerbate blood–brain barrier breakdown after ischaemic stroke and lead to lethal haemorrhagic transformation. The immune system is a dynamic modulator of stroke response, and excessive immune cell accumulation in the cerebral vasculature is associated with compromised integrity of the blood–brain barrier. We previously reported that regulatory T cells, which function to suppress excessive immune responses, ameliorated blood–brain barrier damage after cerebral ischaemia. This study assessed the impact of regulatory T cells in the context of tPA-induced brain haemorrhage and investigated the underlying mechanisms of action. The number of circulating regulatory T cells in stroke patients was dramatically reduced soon after stroke onset (84 acute ischaemic stroke patients with or without intravenous tPA treatment, compared to 115 age and gender-matched healthy controls). Although stroke patients without tPA treatment gradually repopulated the numbers of circulating regulatory T cells within the first 7 days after stroke, post-ischaemic tPA treatment led to sustained suppression of regulatory T cells in the blood. We then used the murine suture and embolic middle cerebral artery occlusion models of stroke to investigate the therapeutic potential of adoptive regulatory T cell transfer against tPA-induced haemorrhagic transformation. Delayed administration of tPA (10 mg/kg) resulted in haemorrhagic transformation in the ischaemic territory 1 day after ischaemia. When regulatory T cells (2 × 106/mouse) were intravenously administered immediately after delayed tPA treatment in ischaemic mice, haemorrhagic transformation was significantly decreased, and this was associated with improved sensorimotor functions. Blood–brain barrier disruption and tight junction damages were observed in the presence of delayed tPA after stroke, but were mitigated by regulatory T cell transfer. Mechanistic studies demonstrated that regulatory T cells completely abolished the tPA-induced elevation of MMP9 and CCL2 after stroke. Using MMP9 and CCL2 knockout mice, we discovered that both molecules partially contributed to the protective actions of regulatory T cells. In an in vitro endothelial cell-based model of the blood–brain barrier, we confirmed that regulatory T cells inhibited tPA-induced endothelial expression of CCL2 and preserved blood–brain barrier integrity after an ischaemic challenge. Lentivirus-mediated CCL2 knockdown in endothelial cells completely abolished the blood–brain barrier protective effect of regulatory T cells in vitro. Altogether, our studies suggest that regulatory T cell adoptive transfer may alleviate thrombolytic treatment-induced haemorrhage in stroke victims. Furthermore, regulatory T cell-afforded protection in the tPA-treated stroke model is mediated by two inhibitory mechanisms involving CCL2 and MMP9. Thus, regulatory T cell adoptive transfer may be useful as a cell-based therapy to improve the efficacy and safety of thrombolytic treatment for ischaemic stroke.
Introduction
Recombinant tissue plasminogen activator (tPA) is the only FDA-approved thrombolytic treatment for acute ischaemic stroke. Unfortunately, the use of tPA increases the risk of haemorrhagic transformation, especially when delayed beyond 4.5 h after the onset of ischaemia (Dijkhuizen et al., 2002; Yepes et al., 2003; Su et al., 2008). Haemorrhagic transformation is associated with poor clinical outcomes in stroke patients (Dijkhuizen et al., 2002; Lansberg et al., 2007). Evidence from preclinical and clinical studies suggests that loss of integrity of the blood–brain barrier (BBB) is a major risk factor for haemorrhagic transformation (Jiang et al., 2002; Ding et al., 2005; Zhang et al., 2014b). The normal structure and function of the BBB are maintained by a number of components, including junctional protein-sealed capillary endothelial cells, astrocyte endfeet, pericytes, and the basement membrane (Hu et al., 2012). Disruption of BBB integrity occurs quite early after stroke (Shi et al., 2016). Delayed tPA treatment after stroke further exacerbates BBB damage and increases the risk of lethal haemorrhagic transformation (Potrovita et al., 2004; Wang et al., 2015). Therefore, therapeutic strategies that fortify the BBB may reduce the incidence of haemorrhagic transformation and improve the safety of tPA treatment.
Inflammation in cerebral vessels mainly involves interactions between injured endothelial cells and activated immune cells, and plays a critical role in BBB damage after ischaemia/reperfusion. The initial ischaemic challenge triggers the expression and release of chemokines from endothelial cells, which facilitate the infiltration of peripheral immune cells into the brain during reperfusion (del Zoppo, 2009). For example, chemokine (C-C motif) ligand 2 (CCL2), also called monocyte chemotactic protein 1, is released from endothelial cells and mediates BBB disruption and the recruitment of immune cells after cerebral ischaemia (Dimitrijevic et al., 2006). The infiltrating neutrophils, monocytes, and other peripheral immune cells also carry with them a variety of inflammatory mediators and proteases, including matrix metalloproteinases (MMPs). The microvascular accumulation of these immune cells and their secreted factors disrupts the adhesions between endothelial cells and degrades the extracellular matrix, resulting in irreversible BBB disruption. tPA treatment has been shown to exacerbate post-stroke inflammation in the microvasculature through multiple mechanisms, including the induction of MMP activity and the enhancement of leucocyte infiltration (Lapchak et al., 2000; Montaner et al., 2003; Wang et al., 2003, 2004, 2014; Cheng et al., 2006; Copin et al., 2011). These mechanisms all contribute to the rupture of the BBB and lead to lethal haemorrhages in the ischaemic brain. Thus, restriction of inflammatory processes in brain microvasculature may serve to reinforce BBB integrity and enhance the safety of tPA treatment for stroke.
Regulatory T cells (Tregs) are a subpopulation of T cells that represents 5–10% of circulating CD4+ T cells (Battaglia et al., 2006). They are important for the maintenance of immune homeostasis and the suppression of excessive immune responses. In stroke patients, the number of circulating Tregs decreases dramatically soon after stroke. This transient drop is followed by a significant and persistent increase in Treg numbers for several weeks (Ishibashi et al., 2009; Urra et al., 2009; Yan et al., 2009). Animal studies reveal that depletion of Tregs from the circulation profoundly increases brain damage and exacerbates functional outcomes at 7 days after middle cerebral artery occlusion (MCAO), suggesting a protective role for Tregs in experimental stroke (Liesz et al., 2009). Our recent studies have shown that delayed adoptive transfer of Tregs raised the number of Tregs in the periphery and protected the brain against ischaemia/reperfusion injury by attenuating neutrophil-released MMP9 and maintaining BBB integrity (Li et al., 2013a, b). This protection of the BBB prompted us to further explore whether Treg treatment could ameliorate the risk of haemorrhagic transformation following tPA treatment and thus enhance the efficacy and safety of thrombolytic therapies.
In the current study, our clinical data demonstrate a prolonged loss of circulating Tregs in tPA-treated stroke patients. Using both the suture and embolic MCAO models of stroke, we show for the first time that intravenous administration of Tregs immediately after delayed tPA treatment significantly reduces tPA-induced cerebral haemorrhage and improves long-term functional outcomes in stroke mice. Furthermore, Tregs ameliorate BBB damage in tPA-treated stroke mice by attenuating MMP9 production after stroke and reducing tPA-induced CCL2 production from injured endothelial cells.
Materials and methods
Experimental animals
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Male C57BL/6 J, Mmp9−/− and Ccl2−/− mice were purchased from the Jackson Laboratory and used at 8–10 weeks of age. Animals were assigned randomly to different treatment groups through the use of a lottery drawing box. All assessments were performed by investigators who were blinded to experimental group assignments.
Antibodies for western blot and immunofluorescent staining
Primary antibodies for immunohistochemical staining include rat anti-CD31 (1:200, BD Biosciences), goat anti-MMP9 (1:100, R&D systems), and rabbit anti-CCL2 (1:100, Abcam). Biotin-conjugated anti-mouse IgG (Vector Laboratories) was used to detect extravascular IgG molecules. Secondary antibodies for immunostaining were purchased from Jackson ImmunoResearch Laboratories. The primary antibodies for western blot include goat anti-CCL2 (1:1000, R&D systems), rabbit anti-VE-Cadherin (1:2000, Cell Signaling), and rabbit anti-Claudin5 (1:500, Abcam). IRDye secondary antibodies (1:10 000, LiCor) were used for western blotting.
Murine suture model of transient focal ischaemia
Focal cerebral ischaemia was produced by intraluminal occlusion of the left MCA with silicone-coated suture (Doccol Corporation) as described (Li et al., 2013a). The brain was reperfused 2 h after ischaemia. For MMP9 inhibition, SB-3CT (25 mg/kg, Sigma-Aldrich) was injected intraperitoneally at 2 h after MCAO, immediately after tPA.
Murine embolic model of transient focal ischaemia
The left common carotid artery, the external carotid artery and its superior thyroid artery branch, and the internal carotid artery and its pterygopalatine artery branch were exposed through a midline cervical skin incision. The superior thyroid and distal external carotid arteries were permanently coagulated, while the other arteries were temporarily ligated. A 5-mm blood clot in a modified PE-10 catheter was introduced into the lumen of the external carotid artery. After releasing the ligation of the internal carotid artery, the catheter was advanced beyond the pterygopalatine artery branch and the clot was quickly injected into the internal carotid artery with a short but gentle burst. Next, the catheter was removed, the external carotid artery was ligated, the ligation of the common carotid artery was released, and the skin incision was sutured shut.
Reperfusion with tPA and adoptive transfer of regulatory T cells
Tregs were prepared using the regulatory T cell isolation kit (Miltenyi Biotec) as described (Li et al., 2013a). At 2 h after suture occlusion or 3 h after clot occlusion, the right femoral vein was cannulated for drug administration. Human recombinant tPA [Activase® (alteplase), Genetech] was diluted in saline and injected at a dose of 10 mg/kg (Tsuji et al., 2005). Recipient mice received a femoral vein injection of freshly isolated CD4+CD25+ Tregs (2 × 106 cells/animal) in 0.2 ml Dulbecco’s phosphate-buffered saline (PBS) immediately after tPA infusion (2 h after the onset of suture occlusion or 3 h after the onset of clot occlusion). PBS-treated animals were used as controls, because mice with splenocyte (2 × 106/mouse) or PBS treatment develop similar cerebral haemorrhages after MCAO followed by tPA infusion (not shown).
Two-dimensional laser speckle imaging techniques
Cortical blood flow was monitored using the laser speckle technique as described previously (Li et al., 2013a). Laser speckle perfusion images were obtained before MCAO, during ischaemia, and after reperfusion. Cerebral blood flow changes were recorded over time and expressed as a percentage of pre-MCAO baselines.
Spectrophotometric assay of haemoglobin concentrations
To obtain a standard curve, normal mice were subjected to complete transcardial perfusion with PBS to remove intravascular blood. Hemispheric brain tissue was collected and homogenized. Incremental volumes of homologous blood (0, 2, 4, 8, 16, 32 μl) were added to each hemispheric sample and the total volume was adjusted to 3 ml with PBS. For sample measurements, brain samples from experimental animals were collected and homogenized in PBS. Drabkin’s reagent (1.6 ml, Sigma-Aldrich) was added to 0.4 ml aliquots of standard samples or experimental samples. Optical density was measure at 540 nm with a spectrophotometer. Measurements from perfused brains subjected to ischaemia and/or tPA reperfusion were compared with this standard curve to obtain haemorrhage volumes in μl (Asahi et al., 2000).
Evans blue administration and quantification
Evans blue (4% in PBS, 4 ml/kg, Sigma-Aldrich) was intravenously injected, followed by 3 h of circulation before sacrifice. N,N-dimethylformamide was added to the brain tissue and the tissue was sonicated on ice for 1 min. Evans blue levels in each hemisphere were determined with the formula: {A620nm – [(A500nm + A740nm) / 2]} / mg wet weight. Background Evans blue levels in the non-ischaemic hemisphere were subtracted from the ischaemic hemisphere ipsilateral to the MCAO (Su et al., 2008).
Measurements of infarct volume
Staining with 2,3,5-triphenyltetrazolium chloride (TTC, Sigma-Aldrich) was performed and quantified as described (Li et al., 2013a). For MAP2 staining, free-floating sections were prepared and stained with MAP2 antibodies (1:200, Santa Cruz Biotechnology). For both TTC and MAP2 staining, infarct area was determined with NIH ImageJ. Infarct volumes (with correction for brain oedema) were calculated as the volume of the contralateral hemisphere minus the non-infarcted volume of the ipsilateral hemisphere.
Behavioural tests
Behavioural tests were performed by an individual blinded to experimental groups. The corner test was performed as described previously to measure asymmetries in turning behaviour (Li et al., 2013a). Corner test performance was expressed as the number of left turns out of 10 turn trials per day. The Rotarod test (IITC Life Science) was also performed to determine motor coordination. Latency to fall off the rotating rod was recorded as a measure of loss of balance and sensorimotor coordination. Data were expressed as mean values from three trials.
Intravenous injection, quantification, and detection of tracers
Cadaverine conjugated to Alexa Fluor®555 (950 Da, 200 µg/mouse, Thermo Fisher Scientific) was injected into the tail vein after reperfusion. NIH ImageJ analysis was used for blinded quantification of leakage volume (Li et al., 2013a).
Transmission electron microscopy
Samples for transmission electron microscopy were prepared as described (Li et al., 2013a), and examined with a CM120 electron microscope at 80 kV. Four images in the peri-infarct areas of each brain were evaluated. The tight junction length, tight junction number, and basement thickness were analysed using ImageJ as described (Jackman et al., 2013). The tight junction tortuosity factor, which indicates tight junction complexity, was calculated as tight junction length divided by the diagonal of the rectangle that contains the length and height of the complete tight junction.
Oxygen–glucose deprivation
Two-thirds of the culture medium was replaced four times with serum- and glucose-free medium, resulting in a final glucose concentration of <1 mm (Cao et al., 2007). The glucose-deprived cultures were then placed in a Billups-Rothenberg modular incubator chamber, which was flushed for 5 min with 95% argon and 5% CO2, and then sealed. The chamber was maintained at 37°C for 4 h and then returned to 95% air, 5% CO2, and glucose-containing medium for the period of time indicated in each experiment. Control cultures were incubated for the same period of time at 37°C in humidified 95% air and 5% CO2.
In vitro blood–brain barrier model and permeability assay
Primary mouse endothelial cells were purchased from Cell Biologics Inc. The in vitro BBB model was established in cell culture inserts. The trans-well PET membranes (0.4 μm pore, 11-mm diameter; Corning) were coated with collagen (15 μg/ml) and fibronectin (30 μg/ml). Cells were seeded onto the membrane at a density of 2.5 × 105 per membrane and maintained for 4 days to reach confluence. Cultures were subjected to 4 h of oxygen–glucose deprivation followed by tPA (500 ng/ml) or PBS treatment. Preactivated (with CD3/CD28) Tregs or same numbers of splenocytes were added into the luminal chamber. FITC-dextran (40 kDa; Sigma-Aldrich) was then added into the luminal chamber at a concentration of 2 mg/ml in 250 μl media. Fluorescence intensity was measured with a fluorescence reader at 30-min intervals for 1–6 h by removing 30 μl media from the lower (abluminal) chamber. The concentrations of tracers in samples were calculated from a standard curve using known concentrations of tracers.
Regulatory T cell suppression assay
CD4+CD25− T effector cells (Teffs) were plated at 2 × 105 per well in a U bottom 96-well plate in the presence of anti-CD3/CD28 activation beads (Miltenyi) to stimulate their proliferation. Tregs were added at a ratio of 1:1, 1:2, 1:4, 1:8, or 1:16 to the number of Teffs. Cells were incubated for 2 days and then bromodeoxyuridine (BrdU) was added for 24 h. BrdU incorporation into proliferating cells was measured by an ELISA quantification kit (Roche).
Lentiviral transfection of endothelial cells
Ccl2 knockdown was achieved by transecting Ccl2-specific short hairpin RNAs (shRNA) into the endothelial cells 72 h before experiments. Lentiviral vectors expressing shRNA against murine Ccl2 or a scrambled sequence were purchased from OriGene.
Patients
The clinical study was approved by the ethical review board of Beijing Luhe Hospital and the First Affiliated Hospital of Soochow University. From May 2014 to February 2015, 84 consecutive patients with a clinical diagnosis of acute ischaemic stroke between the ages of 35 and 85 years and whose symptoms had started within 6 h before admission were included. All the tPA-treated patients received tPA intravenous infusion within 4.5 h after stroke onset according to AHA-ASA guidelines. Symptomatic brain haemorrhage was not observed in any patients of this cohort who received tPA treatment. Patients with brain haemorrhages, transient ischaemic attacks, neurological deficits due to trauma or neoplasms, or acute infections after stroke were excluded. Informed consent was obtained from patients or their legal representatives. Blood samples were drawn upon hospital admission (0–1 h after admission, before tPA treatment). Blood was also obtained from 115 age- and gender-matched healthy volunteers without a history of stroke or other vascular diseases. The changes in Treg populations were measured in 65 stroke patients (42 non-tPA treated and 23 tPA treated) at 1 and 7 days after admission. In addition, for the correlation analysis between CD4+CD25+CD127− and CD4+CD25+Foxp3+ populations, 15 stroke patients and 20 age and gender-matched healthy volunteers were included. Blood cells were collected by centrifugation at 300g for 10 min at 4°C. The pelleted cells were used for fluorescence-activated cell sorting (FACS) analysis after red blood cell lysis. The number of Tregs was deduced from the lymphocyte number in 1 µl blood multiplied by the percentage of Tregs in total lymphocytes.
Statistical analyses
Results are presented as means ± standard error of the mean (SEM) for continuous data. Sample sizes for animal studies were determined by power calculations for the primary parameter with mean differences and standard deviations based on pilot studies or the literature (power 80%, α 0.05). GraphPad Prism software (version 6.0) was used for statistical analyses. The difference in means between two groups was assessed by the two-tailed Student’s t-test. Differences in means across multiple groups were analysed using one-way ANOVAs. Differences in means across multiple groups with multiple measurements over time were analysed using repeated measures ANOVA. When the ANOVA revealed significant differences, the post hoc Bonferroni test was used for pairwise comparisons between means. The difference between each time point in different groups was analysed using two-way ANOVAs followed by the Bonferroni post hoc test. Changes in Tregs at stroke onset and Day 1 and Day 7 after stroke were analysed by ANCOVA with covariates of age, gender, and tPA treatment. In all analyses, P ≤ 0.05 was deemed statistically significant.
Results
Thrombolytic treatment inhibits the repopulation of circulating regulatory T cells after stroke
In the first human cohort, 84 acute ischaemic stroke patients (age: 63.45 ± 12.23; male:female = 2.23:1) and 115 age and gender-matched healthy controls (age: 62.91 ± 12.31; male:female = 2.03:1) without any history of stroke or other vascular diseases were enrolled. Leucocytes were stained with anti-CD3, anti-CD4, anti-CD25, and anti-CD127 monoclonal antibodies (mAbs) to identify CD3+CD4+CD25+CD127− Tregs in peripheral blood (Fig. 1A). The number of circulating Tregs was significantly reduced soon after stroke onset upon hospital admission (Fig. 1B). Further analyses indicated that Tregs were significantly reduced in both male and female stroke patients (Fig. 1C and D). The percentage of Tregs among CD3+CD4+ lymphocytes also dropped significantly after stroke onset in both genders (Fig. 1E–G). In a separate cohort of blood samples from stroke patients (n = 15) and age and gender-matched healthy volunteers (n = 20), both CD4+CD25+CD127− and CD4+CD25+Foxp3+ were used for flow cytometry as selective markers for Tregs. More than 92% of CD3+CD4+CD25+CD127− cells were Foxp3+ (Supplementary Fig. 1A). Both sets of markers confirmed significant decreases in the number of Tregs after the onset of ischaemic stroke, compared to healthy controls (Supplementary Fig. 1B and C). There was a highly significant correlation between the percentages of CD25+CD127− and CD25+FoxP3+ cells within CD3+CD4+ populations in both stroke patients (r2 = 0.93; P ≤ 0.001) and controls (r2 = 0.93; P ≤ 0.001) (Supplementary Fig. 1D), consistent with many other studies comparing CD127 and Foxp3 expression in human Tregs under healthy and disease conditions, including ischaemic stroke (Liu et al., 2006; Yan et al., 2012).
Figure 1.
Thrombolytic treatment inhibits the repopulation of circulating Tregs in stroke patients. (A) Representative gating strategy for the FACS analysis of CD3+CD4+CD25+CD127− Tregs in human blood cells. (B–D) The number of Tregs (per µl blood) decreased after stroke onset (upon admission) in both male (C) and female (D) patients (n = 84 for stroke patients; n = 115 for healthy controls). (E–G) The percentages of Tregs (in CD3+CD4+ T cells) decreased after stroke onset (upon admission) in both male (F) and female (G) patients. (H–J) tPA treatment inhibited the repopulation of Tregs at Day 1 and Day 7 after stroke. (H) The number of Tregs at Day 0, Day 1, and Day 7 after stroke in tPA-treated and non-tPA-treated patients. (I–J) Changes in Treg numbers at Day 1 (I) and Day 7 (J) after stroke with or without tPA-treatment (n = 42 for non-tPA-treated; n = 23 for tPA-treated). Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Next, we compared the changes in Treg numbers between tPA-treated and non-tPA treated stroke patients at 1 and 7 days after tPA. Forty-two non-tPA treated and 23 tPA-treated patients were included in this analysis. The basic clinical characteristics of these patients are summarized in Supplementary Table 1. There were no significant differences in age, gender, and the percentages of patients with diabetes, hypertension, or hyperlipidaemia between the two groups. Notably, there was a significant increase in the number of Tregs in non-tPA-treated patients at 1 and 7 days after stroke, suggesting a repopulation of Tregs over time (Fig. 1H). tPA treatment, however, decelerated the repopulation of Tregs after stroke. Covariance analyses (ANCOVA) with the covariates of age and gender showed that the increase in Treg number on Day 1 after stroke was significantly inhibited in tPA-treated patients [95% confidence interval (CI): −5.42 to 5.684] compared to non-treated patients (95% CI: 3.996 to 12.03) (Fig. 1I). The increase in Treg percentages at Day 7 after stroke was slightly inhibited in tPA-treated patients (95% CI: −7.08 to 11.92) but showed no significant difference compared to non-treated patients (95% CI: 3.749 to 16.54) (Fig. 1J). Age and gender were not significant factors in this model. The inhibitory effect of tPA on the number of Tregs was not due to the difference in post-stroke lymphopenia (Urra et al., 2009), as tPA-treated and non-tPA-treated patients had similar numbers of blood lymphocytes at Day 0 (1.98 ± 0.12 × 103/µl versus 1.96 ± 0.12 × 103/µl; P = 0.92), Day 1 (1.84 ± 0.12 × 103/µl versus 1.93 ± 0.10 × 103/µl; P = 0.57) and Day 7 (1.99 ± 0.13 × 103/µl versus 2.00 ± 0.10 × 103/µl; P = 0.87) after stroke.
Collectively, these clinical data show that the number of circulating Tregs was markedly reduced after stroke onset, followed by a gradual repopulation; however, tPA thrombolytic treatment inhibited the repopulation of Tregs.
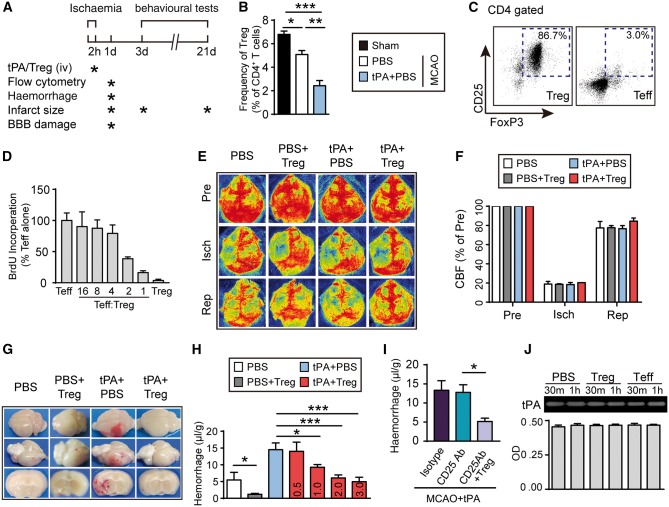
Regulatory T cells ameliorate delayed tPA-induced intracerebral haemorrhage in a suture model of stroke
C57/BL6 mice were subjected to 2 h suture occlusion of the MCA, followed by tPA (Activase®, 10 mg/kg, intravenously) infusion into the femoral vein (Fig. 2A). This delayed administration of tPA reproducibly induced haemorrhagic transformation in the ischaemic territory. Non-tPA-treated control mice received the same volume of PBS. Consistent with our clinical data, flow cytometry analyses demonstrated reduced frequency of Tregs in the blood 1 day after MCAO compared to sham controls. Delayed treatment with haemorrhage-inducing dose of tPA further lowered Treg frequency after ischaemia compared to non-tPA-treated ischaemic mice (Fig. 2B). Animals were then randomly assigned to Treg (designated as PBS+Treg and tPA+Treg group) or PBS (designated as PBS and tPA+PBS group) treatment groups. The purity and quality of isolated CD4+CD25+ Tregs were confirmed by their high expression of the Treg immunophenotypic marker Foxp3 (86.7% were Foxp3+, Fig. 2C) and their dose-dependent suppression of the proliferation of CD4+CD25− T effective cells (Fig. 2D and Supplementary Fig. 2). Regional cerebral blood flow was monitored before, during, and after MCAO by laser speckle 2D imaging (Fig. 2E). There was no statistical difference in cerebral blood flow during and after MCAO among the experimental groups, verifying that all animals were subjected to the same degree of ischaemia and achieved the same extent of reperfusion (Fig. 2F). Massive haemorrhages were observed in the ischaemic area at 1 day after ischaemia/reperfusion in tPA-infused mice and this effect was dramatically suppressed when Tregs were infused immediately after tPA (Fig. 2G). The severity of the intracerebral haemorrhage was quantified by the spectrophotometric haemoglobin assay (Fig. 2H). As expected, delayed tPA infusions exacerbated intracerebral haemorrhages, as indicated by increased haemoglobin content in brain homogenates collected from tPA-treated stroke mice. Treg transfer dose-dependently reduced tPA-induced intracerebral haemorrhage 1 day after stroke (Fig. 2H). The lowest dose of Tregs (2 × 106/mouse) that conferred the maximal protection against haemorrhagic transformation was used as the optimal dose in subsequent experiments. The optimal dose of exogenous Tregs significantly reduced brain haemorrhage after MCAO+tPA, even in the absence of endogenous Tregs (Fig. 2I). It is noted that depletion of endogenous Tregs with the CD25 antibody did not exacerbate brain haemorrhages induced by delayed tPA treatment in stroke mice (Fig. 2I). This result was not surprising because delayed tPA treatment alone robustly decreased the number of Tregs in the circulation by ∼70% compared to normal levels (Fig. 2B). Such a dramatic Treg reduction may contribute to maximal induction of brain haemorrhages, such that CD25 antibody-mediated Treg depletion was not able to induce more haemorrhage. These results suggest that the 30% remaining Tregs present in stroke mice with delayed tPA treatment are below the threshold levels required to protect against brain haemorrhage. The results of zymography and tPA chromogenic activity assay (Fig. 2J) confirmed that this optimal dose of Tregs did not inhibit the thrombolytic activity of tPA per se, suggesting that Tregs protect against tPA-induced haemorrhagic transformation after stroke without a direct inhibitory effect on tPA.
Figure 2.
Administration of Tregs dramatically reduces tPA-induced intracerebral haemorrhage in a murine suture model of stroke. (A) Scheme for experimental design in a suture model of MCAO. tPA (10 mg/kg) was continuously infused into the femoral vein over 20 min at 2 h after MCAO. Tregs were isolated from pooled spleens and lymph nodes of donors and transferred (2 × 106 cells/animal) to ischaemic recipients immediately after tPA delivery through the femoral vein. The timeline for outcome measurements is also illustrated. (B) The percentages of Tregs in CD3+CD4+ T cells in the blood collected from sham, MCAO, and MCAO+tPA groups. (C) Representative flow cytometry plots of CD25+Foxp3+ cells in CD4+CD25+ Tregs and CD4+CD25− T effective cells (Teff) after double selection. (D) T cell suppression test. Graded numbers of Tregs were added to the Teff cells in the presence of anti-CD3 (10 µg/ml) and anti-CD28 (5 µg/ml) stimulation. The proliferation of Teff was measured 24 h later by BrdU incorporation test. (E and F) Regional cerebral blood flow (CBF) was monitored using 2D laser speckle imaging. (E) Representative images of cerebral blood flow before MCAO, during MCAO, and at 10 min after reperfusion for each group. (F) Quantification of cerebral blood flow. Results are expressed as percent change from baseline (pre-MCAO). n = 4–5 per group. (G) Representative images of the dorsal (top) and ventral (middle) surfaces of the brain and a coronal section (bottom) showing the location of the intracerebral haemorrhage 1 day after stroke in mice treated with PBS, PBS+Treg, tPA+PBS, or tPA+Treg. (H) Quantification of cerebral haemorrhage after different doses of Treg treatment by spectrophotometric haemoglobin assay 1 day after stroke. (I) Exogenous Tregs reduced tPA-induced brain haemorrhage in the absence of endogenous Tregs. Mice were injected intraperitoneally with either PBS (control) or 300 mg of CD25-specific antibody (CD25 Ab) 2 days prior to MCAO. tPA (10 mg/kg) was given at 2 h after MCAO. Brain haemorrhage was measured 1 day after MCAO. (n = 4–6/group). (J) Tregs did not directly inhibit tPA activity. 20 µg tPA was incubated with or without 1.6 × 105 Tregs in 160 µl media for 30 min or 1 h. The activity of tPA was determined by zymography (top) and tPA chromogenic activity assay (bottom). Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01.
Regulatory T cell treatment confers long-term protection against neurological dysfunction in tPA-infused stroke mice
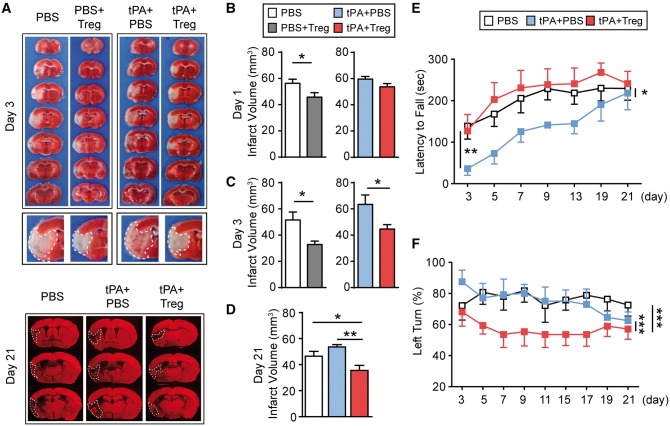
Next, we quantified the effects of Treg treatment on brain infarct size in tPA-treated stroke mice. Treg treatment significantly attenuated brain infarct volume at 1 and 3 days after 2 h MCAO without tPA treatment (Fig. 3A–C). In the delayed tPA-treated stroke mice, we did not observe a significant difference in infarct volume between PBS-treated and Treg-treated ischaemic mice at 1 day following MCAO. However, when measured at 3 days following MCAO, a significant reduction in infarct volume in tPA+Treg-treated ischaemic mice compared to tPA+PBS-treated ischaemic mice was noted, and persisted at least until 21 day after MCAO (Fig. 3A and D). Sensorimotor deficits were significantly reduced in Treg-treated ischaemic mice with tPA infusion compared to ischaemic mice treated with tPA alone, as demonstrated by performance on the Rotarod test for balance and sensorimotor coordination (Fig. 3E) and the corner test for turning asymmetry (Fig. 3F). Taken together, these results suggest that Treg therapy provided robust protection at both the structural and functional levels in vivo.
Figure 3.
Treg treatment confers long-term protection against neurological dysfunction after cerebral ischaemia and tPA treatment. (A) Representative TTC-stained (top: 3 days after stroke) and MAP2-stained (bottom: 21 day after stroke) coronal sections in PBS, PBS+Treg, tPA+PBS, or tPA+Treg.-treated MCAO mice. Middle: The enlarged fourth sections of all groups are presented. The infarct areas are highlighted. Note that the tPA+PBS post-stroke brain shows massive parenchyma breeding within the infarct, whereas the breeding is less obvious in the tPA+Treg brain. (B–D) Quantification of infarct volume by TTC staining (3 days after stroke) and MAP2 staining (1 and 21 days after stroke) (n = 4–6/group). (E and F) Sensorimotor dysfunction after ischaemia was significantly improved in tPA+Treg-treated mice as assessed by the Rotarod test (E) and corner test (F) (n = 10–15/group). Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Regulatory T cell treatment reduces tPA-induced intracerebral haemorrhage in the embolic model of stroke
We further evaluated the effect of Tregs on haemorrhagic transformation using the thromboembolic stroke model followed by delayed tPA treatment (Activase®, 10 mg/kg intravenously). This is a commonly used rodent model for haemorrhagic transformation with some clinical relevance, wherein delayed tPA treatment (3 h after the onset of thromboembolic stroke in mice; equivalent to >4.5 h in clinic) induces brain haemorrhage in the ischaemic territory but fails to reduce brain infarct size (Henninger et al., 2009; Garcia-Yebenes et al., 2011; Campos et al., 2013; Zuo et al., 2014; Simao et al., 2017). Adult male C57/BL6 mice were randomly assigned to one of the following three stroke groups: (i) 3 h MCAO with blood clot followed by PBS infusion (n = 10); (ii) 3 h MCAO with blood clot followed by tPA (10 mg/kg) infusion via the femoral vein (n = 10); or (iii) 3 h MCAO with blood clot followed by tPA (10 mg/kg) infusion and then Tregs (2 × 106 cells/animal) adoptive transfer via the femoral vein (n = 8). Laser speckle 2D images revealed that both tPA-treated stroke groups were adequately reperfused at 1 day after stroke (Fig. 4A and B). Compared to clot MCAO without thrombolysis, tPA infusion 3 h after clot formation remarkably increased intracerebral haemorrhage at 1 day after stroke (Fig. 4C and D); and failed to reduce infarct volume (Fig. 4E and F). In contrast, Treg adoptive transfer immediately after post-stroke tPA infusion significantly attenuated tPA-induced haemorrhagic transformation 1 day after ischaemic onset (Fig. 4C and D). Infarct volume in tPA+Treg-treated mice was also significantly reduced compared to control stroke mice (PBS treatment without tPA infusion) at 1 day after clot MCAO (Fig. 4E and F). These results demonstrate that the combination of Treg and tPA treatment can achieve neuroprotective effect (reduction in brain infarct) in the thromboembolic stroke model when the tPA-induced intracerebral haemorrhage is effectively controlled.
Figure 4.
Treg treatment protects against tPA thrombolysis-associated haemorrhagic transformation in the embolic mouse model of stroke. Mice were subjected to 3 h MCAO with blood clot. tPA (10 mg/kg) was continuously infused into the femoral vein over 20 min at 3 h after MCAO. Tregs (2 × 106 cells/animal) were transferred to ischaemic recipients immediately after tPA delivery through the femoral vein. PBS served as the control. Sham animals underwent the same anaesthesia and surgical exposure of the arteries without MCAO induction. (A) Representative images of laser speckle cerebral blood flow (CBF) at 5 min, 3 h, 3.5 h, and 24 h after clot MCAO. (B) Quantification of cerebral blood flow. Results are expressed as percentage change from baseline (pre-MCAO). (C) Representative images of the dorsal and ventral surfaces showing intracerebral haemorrhage 1 day after embolic MCAO in mice treated with PBS, tPA+PBS, or tPA+Treg. (D) Quantification of cerebral haemorrhage by spectrophotometric hemoglobin assay 1 day after embolic tMCAO (n = 4/group). (E) Representative images of TTC-stained brain slices showing infarct areas in 6 consecutive coronal sections (1 mm apart) at 1 day after clot stroke. (F) Quantification of infarct volume by TTC staining at 1 day after clot stroke in mice treated with PBS (n = 10), tPA+PBS (n = 10), or tPA+Treg (n = 8). Data are mean ± SE. *P ≤ 0.05.
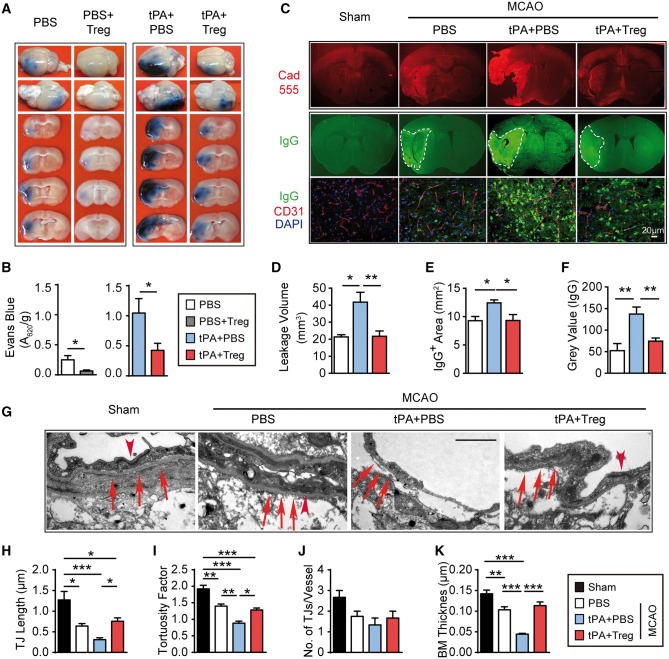
Regulatory T cell treatment blocks tPA-induced blood–brain barrier disruption after cerebral ischaemia
We then explored whether Tregs reduce intracerebral haemorrhage after tPA by prevention of BBB breakdown. First, the extravasation of Evans blue or fluorescent molecule cadaverine was used to quantify BBB damage. Treg treatment significantly reduced the extravasation of Evans blue in stroke mice with or without tPA infusion (Fig. 5A and B). Leakage of cadaverine after tPA treatment was also attenuated by Treg treatment (Fig. 5C and D). Similarly, the intracranial leakage of plasma-derived IgG after tPA was mitigated in Treg-treated stroke mice (Fig. 5C, E and F) compared to PBS-treated controls. Using transmission electron microscopy, delayed tPA treatment dramatically exacerbated BBB ultrastructural damage after stroke (Fig. 5G). Tight junctions were shorter (Fig. 5H) and less complex (Fig. 5I), but minimal changes in the total number of tight junctions per vessel were observed (Fig. 5J). The basement membrane was thinner (Fig. 5K) and poorly defined after tPA treatment. However, combinatorial treatment with Treg and delayed tPA preserved the ultrastructure of tight junctions and basement membranes in the ischaemic brain. These results indicate a potent BBB protective effect of adoptively transferred Tregs in tPA-treated stroke mice.
Figure 5.
Treg treatment blocks tPA-induced BBB disruption after cerebral ischaemia. (A) Representative images of Evans blue extravasation 1 day after stroke in mice treated with PBS, PBS+Treg, tPA+PBS, or tPA+Treg. (B) Quantification of Evans blue fluorescence intensity (n = 5–6/group). (C) Representative images showed the intracerebral penetration of intravenously injected tracer (cadaverine–Alexa-555) and endogenous IgG extravasation into the brain at 1 day after MCAO. (D) Quantification of leakage volumes of cadaverine–Alexa-555 at 1 day after MCAO (n = 4–5/group). (E and F) Quantification of endogenous IgG-positive area (E) and grey values of IgG immunostaining (F) 1 day after stroke (n = 4–5/group). (G–K) Transmission electron microscopy performed at 1 day after stroke in mice treated with PBS, tPA+PBS, or tPA+Treg. (G) Representative electron microscopy images. Arrowheads = tight junctions (TJ); arrows = basement membrane. Treg treatment preserved BBB ultrastructure in tPA-treated stroke mice. Images are representative of brain sections from four animals per group. Scale bar = 1 µM. (H) Quantification of tight junction length (n = 4/group). (I) Quantification of tight junction tortuosity. (n = 4/group). (J) Quantification of the number of tight junctions per vessel (n = 4 animals/group, four images were quantified per mouse). (K) Quantification of basement membrane (BM) thickness. (n = 4/group). Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01.
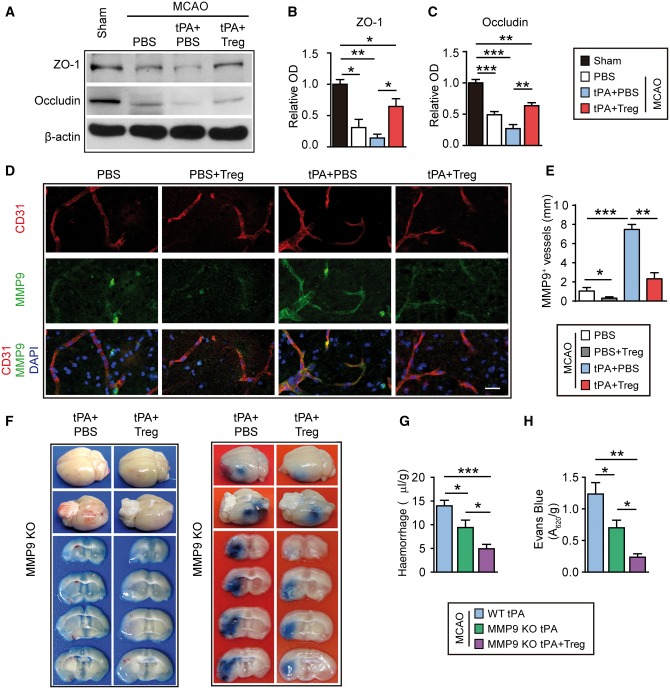
Regulatory T cell treatment inhibits the elevation of MMP9 associated with delayed tPA treatment after stroke
Tight junction complexes involve associations between the tight junction proteins occludin and claudin, junctional adhesion molecules, and the tight junction accessory/anchoring proteins zonula occludens-1 (ZO-1), ZO-2, and ZO-3. Previous studies suggest that tPA may increase the breakdown of tight junction proteins after stroke (Hiu et al., 2008). Here, we confirmed that the enhancement of BBB breakdown related to delayed tPA administration was accompanied by the degradation of tight junction proteins ZO-1 and occludin. Treg treatment increased the expression of these junctional proteins in tPA-infused stroke mice (Fig. 6A–C), suggesting that Tregs counteract the destructive effects of tPA and maintain tight junction structure in the ischaemic brain.
Figure 6.
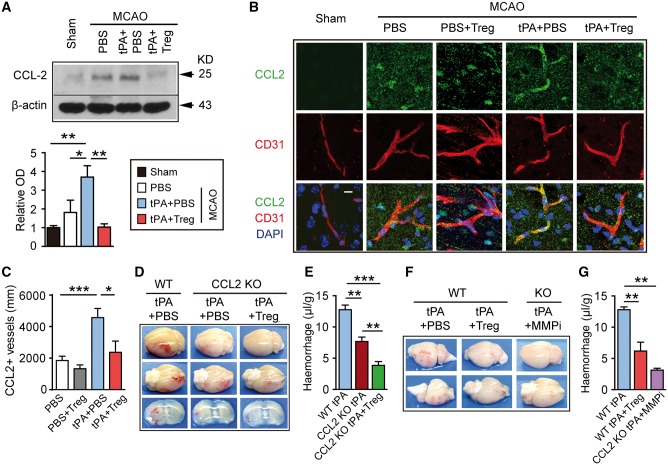
Treg treatment inhibits the tPA-induced elevation of MMP9 after stroke, which may partially contribute to its protective actions. (A) Representative western blot images of ZO-1 and occludin 1 day after stroke in mice treated with PBS, tPA+PBS, or tPA+Treg. β-Actin was used as the loading control. (B and C) Quantification of western immunoblotting for ZO-1 (B) and occludin (C) in each group (n = 3–4/group). Data are normalized to sham. (D) Representative confocal images of MMP9 and CD31 double immunostaining in the brains obtained from MCAO mice treated with PBS, PBS+Treg, tPA+PBS, or tPA+Treg. Scale bar = 10 µm. (E) Quantification of the length of MMP9+/CD31+ blood vessels in the brain (n = 4/group). (F) Representative images of the dorsal and ventral surfaces of the brain and coronal sections showing cerebral haemorrhages (left) and Evans blue extravasation (right) 1 day after stroke in MMP9−/− mice treated with tPA+PBS or tPA+Treg. (G) Quantification of cerebral haemorrhage by spectrophotometric haemoglobin assay for each group in F (n = 8–12/group). (H) Quantification of Evans blue fluorescence intensity for each group in F (n = 4–5 /group). Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
MMP9 is a critical molecule in tPA-enhanced BBB disruption (Wang et al., 2003). Treg treatment reduced MMP9 expression in the brain of tPA-infused stroke mice (Fig. 6D and E). One day after MCAO, MMP9 immunostaining was observed in the ischaemic area surrounding CD31+ blood vessels. Delayed tPA infusion significantly increased the intensity of MMP9 staining around CD31+ blood vessels following stroke, but the addition of Treg co-treatment dramatically reduced the perivascular expression of MMP9 (Fig. 6D). Consistent with this, the length of MMP9-positive blood vessels, as defined by dual labelling of MMP9 with CD31, was significantly decreased in Treg-treated stroke mice with or without tPA treatment (Fig. 6E).
Consistent with previous studies employing MMP inhibitors, we observed that MMP9 knockout mice exhibited smaller cerebral haemorrhages (Fig. 6F and G) and less Evans blue leakage (Fig. 6F and H) after stroke and delayed tPA infusion. Notably, Treg treatment further lessened BBB damage and haemorrhage in MMP9 knockout mice, suggesting that one or more MMP9 independent mechanism may contribute to the protective effect of Tregs. Taken together, these results suggest that Treg treatment suppresses tPA-induced MMP9 expression, but this only partially contributes to its protective effects on BBB integrity and cerebral haemorrhage.
CCL2 also plays a role in regulatory T cell-afforded protection of the blood–brain barrier
In an effort to elucidate additional targets of Treg-afforded protection, we found that Treg treatment significantly inhibited the tPA-induced expression of CCL2 in the ischaemic brain (Fig. 7A). The length of CCL2+ blood vessels—as defined by dual labelling of CCL2 with CD31—remained at relatively low levels after 2 h MCAO without tPA treatment; and co-administration of Tregs showed minimal effect on CCL2 expression in this model. The length of CCL2+ blood vessels was increased by tPA infusion after stroke, and this effect was significantly attenuated by Treg treatment (Fig. 7B and C). These results suggest that endothelial CCL2 is another viable target for Treg-afforded BBB protection.
Figure 7.
CCL2 is another target for Treg-afforded BBB protection in tPA-treated stroke mice. (A) Western blot analysis of CCL2 1 day after stroke in mice treated with PBS, tPA+PBS, or tPA+Treg. β-actin was used as the loading control (n = 3/group). Data are normalized to sham. (B) Representative confocal image of CCL2 and CD31 double immunostaining in the brains obtained from MCAO mice treated with PBS, PBS+Treg, tPA+PBS, or tPA+Treg. Scale bar = 10 µm. (C) Quantification of the length of CCL2+/CD31+ blood vessels in the brain (n = 4/group). (D) Representative images of the dorsal and ventral surfaces of the brain and coronal sections showing cerebral haemorrhages 1 day after stroke in wild-type mice treated with tPA or CCL2 −/− mice treated with tPA+PBS or tPA+Treg. (E) Quantification of cerebral haemorrhage by spectrophotometric haemoglobin assay for each group in E (n = 4–6/group). (F) Representative images of the dorsal and ventral surfaces of the brain showing cerebral haemorrhages 1 day after stroke in wild-type mice treated with tPA+PBS or tPA+Treg, and in CCL2−/− mice treated with tPA+MMP9 inhibitor (SB-3CT, 25 mg/kg). (G) Quantification of cerebral haemorrhages by spectrophotometric haemoglobin assay for each group in F (n = 4–5/group). Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Next, we subjected CCL2 knockout mice to 2 h MCAO followed by tPA infusion with either Treg or PBS treatment. As shown in Fig. 7D and E, tPA-induced haemorrhagic transformation after stroke was significantly reduced in CCL2 knockout mice. Similar to the observations in MMP9 knockout mice, Tregs further reduced the post-tPA haemorrhagic transformation in CCL2 knockout mice. Notably, when we injected a selective MMP9 inhibitor (SB-3CT, 25 mg/kg) (Hadass et al., 2013) into the CCL2 knockout mice after tPA, we observed potent protection against tPA-induced haemorrhagic transformation—comparable to the degree of protection achieved by Treg treatment (Fig. 7F and G). These results are consistent with the view that Treg treatment protects against tPA-induced BBB leakage and haemorrhagic transformation after stroke by targeting the post-stroke elevations in both MMP9 and CCL2, and that these two pathways work as distinct processes in post-ischaemic pathology.
Regulatory T cells inhibit tPA-induced endothelial expression of CCL2 and preserve blood–brain barrier integrity in vitro
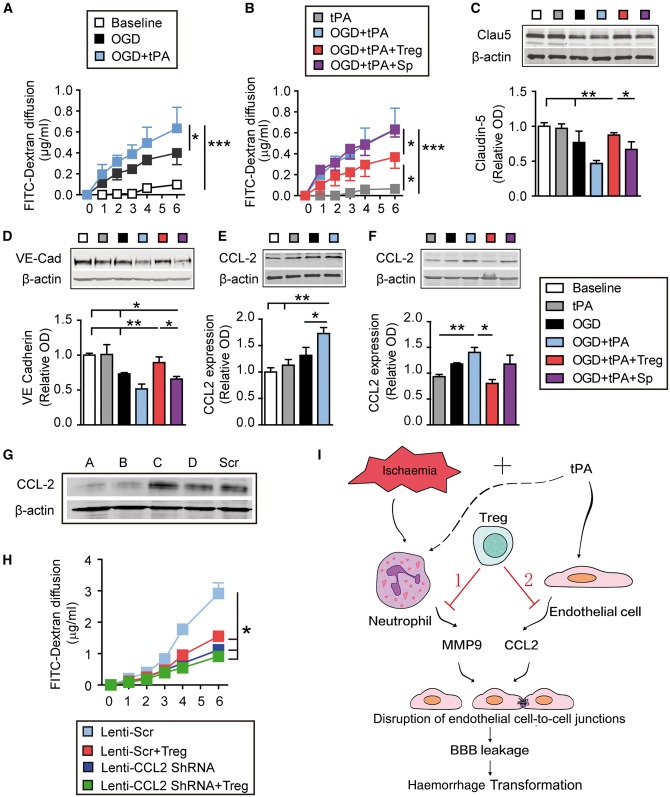
To verify the effects of Tregs on endothelial CCL2 and extend them at a mechanistic level, we used an in vitro model of the BBB—cell culture inserts with a monolayer of mouse endothelial cells. The in vitro BBB was exposed to 4 h of oxygen–glucose deprivation followed by tPA (500 ng/ml). As shown in Fig. 8A, FITC-dextran readily diffused across the in vitro BBB at 1 h after oxygen–glucose deprivation and thereafter. Consistent with our in vivo results, addition of tPA after oxygen–glucose deprivation significantly exacerbated the oxygen–glucose deprivation-induced endothelial hyperpermeability. We then co-cultured oxygen–glucose deprivation+tPA-treated endothelial BBB with Tregs or non-selected splenocytes, at a ratio of endothelial cell:Treg/splenocyte = 2:1. Co-culture with Tregs, but not splenocytes, significantly prevented BBB disruption after oxygen–glucose deprivation+tPA exposure (Fig. 8B). Tregs inhibited oxygen–glucose deprivation+tPA-induced degradation of the tight junction protein claudin-5 (Fig. 8C) and adherens junctional protein VE-cadherin (Fig. 8D and Supplementary Fig. 3) in mouse endothelial cells.
Figure 8.
Tregs protect the in vitro BBB against oxygen-glucose deprivation by inhibiting tPA-induced endothelial expression of CCL2. Primary mouse endothelial cells in cell culture inserts or plates were exposed to 4 h of oxygen–glucose deprivation followed by tPA (500 ng/ml). For cell treatments, endothelial cells exposed to oxygen–glucose deprivation (OGD) and/or tPA were co-cultured with Tregs or non-selected splenocytes at a 2:1 ratio. (A and B) The diffusion of FITC-dextran from the luminal to abluminal chamber was measured over time. (C–F) Cell lysates were collected 4 h after oxygen–glucose deprivation and subjected to western blot analyses. (C and D) Representative western blot images and quantification of VE-Cadherin (C) and Claudin-5 (D) (n = 4/group). (E and F) Representative western blot images and quantification of CCL2 (n = 4/group). β-Actin was used as the loading control. Data are normalized to baseline. (G and H) CCL2 is critical for Treg-afforded BBB protection in vitro. Endothelial cells in cell culture inserts were transfected with lenti-scramble or lenti-Ccl2 shRNA for 72 h. Endothelial cells were then exposed to oxygen–glucose deprivation (4 h) + tPA (500 ng/ml) treatment, followed by co-culture with Tregs or non-selected splenocytes. The diffusion of FITC-dextran from the luminal to abluminal chamber was measured over time. (G) Four shRNA constructs (A–D) were used to knock down CCL2 expression in endothelial cells. Western blots demonstrate knockdown of CCL2 with shRNA constructs A and B. Construct A was then used for subsequent experiments. (H) Quantification of FITC-dextran leakage into the luminal chamber over time (n = 6/group) Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. (I) Schematic illustrating dual mechanisms underlying Treg-mediated mitigation of BBB rupture and haemorrhagic transformation (HT) after ischaemic stroke and delayed tPA treatment. Adoptive transferred Tregs may protect against haemorrhagic transformation induced by delayed tPA treatment after ischaemic stroke by two mechanisms. (1) Ischaemic injury can induce the release of MMP9 from peripheral neutrophils, which cause BBB disruption. Tregs inhibit neutrophil-derived MMP9 (Li et al., 2013a). (2) tPA enhances the expression of CCL2 on endothelial cells. CCL2 can further break down the BBB. Treg treatment inhibits the expression of CCL2 on endothelial cells and provides BBB protection.
Next, we investigated whether CCL2 is involved in Treg-afforded BBB protection against oxygen–glucose deprivation and tPA. Four-hour oxygen–glucose deprivation induced a slight increase in CCL2 in endothelial cells, and the addition of tPA further enhanced CCL2 expression in this group (Fig. 8E). The endothelial induction of CCL2 after oxygen–glucose deprivation+tPA treatment was greatly reduced in the presence of Tregs, but not in the presence of splenocytes (Fig. 8F). These results confirm our in vivo observation that Tregs inhibit tPA-enhanced endothelial CCL2 expression after ischaemic injury. Lentiviral-mediated knockdown of CCL2 in endothelial cells (Fig. 8G) protected the BBB against oxygen–glucose deprivation+tPA treatment (Supplementary Fig. 4), and the scrambled sequence did not significantly influence the protective effect of Tregs on BBB integrity (Supplementary Fig. 5). Importantly, Tregs were unable to protect the in vitro BBB in CCL2 knockdown cultures any more than CCL2 knockdown by itself (Fig. 8H and Supplementary Fig. 4). These findings suggest that a reduction in CCL2 expression is critical for the protective impact of Tregs against BBB permeability upon oxygen–glucose deprivation+tPA treatment.
Discussion
Although tPA is an FDA-approved medicine for thrombolytic treatment in acute ischaemic stroke, the delayed application of tPA beyond the 4.5 h therapeutic window leads to an increased risk of lethal haemorrhagic transformation. Recent reports suggest that endogenous Tregs are neuroprotective and that boosting the number of circulating Tregs robustly protects against ischaemic brain injury (Li et al., 2013a; Liesz et al., 2013; Brea et al., 2014). The present study adds to this body of work by demonstrating that the administration of Tregs after tPA infusion dramatically reduces tPA-induced cerebral haemorrhages in both the suture and embolic models. The potential clinical significance of these findings is that tPA, when given in conjunction with Treg treatment, may be delivered to many more stroke patients who would otherwise miss the short time window for safe use of tPA thrombolysis. This protective effect of Tregs was long-lasting and resulted in sustained improvements in long-term neurological outcomes.
We have shown previously that Tregs have a potent BBB protective effect in models of cerebral ischaemia/reperfusion without tPA (Li et al., 2013a). In this study, we observed that Tregs also lessened tPA-mediated BBB damage in stroke mice. In line with our earlier report (Li et al., 2013a), we demonstrated that Treg treatment mitigated MMP9 induction in tPA-treated stroke mice. In the ischaemia/reperfusion model without tPA, MMP9 deficiency fully replicated the protective effect of Tregs on the BBB. In the present study, the adoptive transfer of Tregs was able to provide additional protection of the BBB in MMP9 knockout mice subjected to tPA+MCAO, suggesting that one or more MMP9-independent mechanism(s) contributes to the protection of the BBB by Tregs in the thrombolytic model of ischaemia/reperfusion. Subsequent studies identified endothelial cell-derived CCL2 as another potential target for Tregs in tPA-infused stroke mice.
CCL2 is a highly expressed chemokine after stroke and is known to propagate ischaemic brain injury. Depletion of CCL2 or its receptor CCR2 decreases cerebral infarct size in models of stroke (Dimitrijevic et al., 2006, 2007; Strecker et al., 2013). The CCL2–CCR2 axis plays manifold roles in cerebral ischaemia, including BBB disruption and the recruitment of peripheral leucocytes. Furthermore, plasmin-cleaved CCL2 has been shown to be more potent than full-length CCL2 in compromising BBB integrity in vitro (Yao and Tsirka, 2011). Our data clearly show that tPA treatment further elevates CCL2 expression in the ischaemic brain and in the in vitro BBB model after oxygen–glucose deprivation exposure. This CCL2 induction was dramatically inhibited by Treg treatment. Inhibition of CCL2 is expected to mitigate the infiltration of peripheral inflammatory cells, such as monocytes and neutrophils (Schilling et al., 2009), thereby reducing the accumulation of BBB destructive factors such as MMP9. However, our results demonstrate that MMP9 or CCL2 alone only partially contribute to Treg-afforded BBB protection against tPA. Application of the MMP9 inhibitor in CCL2 knockout mice almost completely replicated the effect of Tregs on BBB integrity. These results are consistent with the view that the dual inhibitory effects of Tregs on CCL2 and MMP9 work in concert to achieve potent BBB protection in tPA-infused stroke mice.
CCL2 can be released from various types of CNS cells, such as endothelial cells, neurons, astrocytes, and microglia (Che et al., 2001; Dimitrijevic et al., 2006). We found that the endothelial expression of CCL2 was greatly reduced in Treg-treated ischaemic brains 1 day after stroke and tPA infusion. Adoptively transferred Tregs cannot infiltrate the ischaemic brain at 1 day after stroke (Li et al., 2013a), and therefore may not directly inhibit CCL2 in other CNS cells at this early time point. Furthermore, Treg treatment showed minimal effects on plasma levels of CCL2 in stroke mice (data not shown). In vitro studies confirmed that Tregs can inhibit endothelial production of CCL2 after ischaemic and tPA challenges, and that this was essential for Treg-afforded BBB protection. These data suggest that endothelial cells are the direct target for the inhibitory effect of Tregs on CCL2. Notably, Treg treatment was able to exert additional protective effects in CCL2 knockout mice but not in BBB cultures with CCL2 knockdown. These data support our conclusion that Tregs may also protect the brain via a non-endothelial factor (e.g. neutrophil-derived MMP9) that is absent in the in vitro model (Fig. 8I).
Direct interactions between Tregs and endothelial cells have been reported previously using co-cultures of human umbilical vein endothelial cells and Tregs. Tregs inhibited pro-inflammatory responses in endothelial cells exposed to lipopolysaccharide, oxidized low-density lipoprotein or particulate matter, by downregulating adhesion molecules and reducing inflammatory cytokines (Li et al., 2011; Zhang et al., 2014a). These studies suggest that both direct cell-cell contacts and soluble factors from Tregs (IL-10 and TGF-β1) may mediate Treg-endothelial cell interactions. However, we found that IL-10 deficiency did not abolish the BBB protective effect of Tregs (Supplementary Fig. 6), suggesting that IL-10 may not be responsible for Treg-afforded BBB protection after oxygen–glucose deprivation and tPA treatment. Other mechanisms or cumulative effects of multiple mechanisms may contribute to Treg-mediated inhibition of tPA-enhanced BBB damage.
In addition to the reports of protective effects of Tregs in models of stroke, there are a few studies reporting detrimental outcomes of Treg augmentation (Kleinschnitz et al., 2013; Schuhmann et al., 2015). In the latter two studies, the adoptive transfer of Tregs or in vivo expansion of Tregs worsened functional outcomes and promoted thrombo-inflammatory lesion growth during the acute stages of ischaemic stroke. It is known that thrombo-inflammation is prominent in suture models of transient ischaemia mainly due to the prompt revascularization, but not generally observed in rodents and non-human primates with gradual reperfusion (Gauberti et al., 2014). Nevertheless, to address the potential concern of thrombo-inflammation, the effect of Tregs in tPA-induced haemorrhagic transformation was tested in both suture model (abrupt reperfusion) and embolic model (gradual reperfusion) of stroke, and proven to be protective in both models.
In conclusion, our study demonstrates that adoptive transfer of Tregs preserves BBB integrity and reduces the risk of tPA-induced haemorrhagic transformation after stroke. This BBB protection may be achieved through dual inhibitory effects of Tregs on MMP9 and endothelial CCL2 after ischaemia and tPA treatment (Fig. 8I). Our clinical study found that the number of circulating Tregs was significantly reduced after stroke onset, and that this was followed by a gradual Treg repopulation. These observations are consistent with previous studies (Ishibashi et al., 2009; Urra et al., 2009; Yan et al., 2009, 2012). Notably, even when delivered within the therapeutic window (4.5 h in the clinical study) that did not cause brain haemorrhage, tPA treatment significantly inhibited the repopulation of circulating Tregs. We also demonstrated in the MCAO model that the haemorrhage-inducing dose of tPA suppressed the Treg population in comparison to non-tPA treated stroke mice. These data suggest a potential need for Treg supplementation, especially in tPA-treated patients with blunted repopulation of Tregs. Given their capacity to reduce the risk of haemorrhagic transformation in experimental models, Tregs may extend the therapeutic window of tPA and reduce the risk of thrombolysis in the clinic.
Funding
This work was supported by the NIH/National Institute of neurological disorders and stroke (NINDS) grants NS094573 and NS092618 (to X.H.), NS095671 and NS089534 (to J.C). J.C. is a recipient of the VA Senior Research Career Scientist Award. X.H. is also supported by a Chinese Natural Science Foundation (NCSF) grant 81571152. J. C is also supported by a NCSF grant 81529002. L.M. is supported by the Natural Science Foundation of Shandong of China (ZR2014HQ027). P.L. is supported by the Shanghai Rising-Star Program (16QA1402600), the Shanghai Natural Science Foundation (13ZR1452200) and NCSF (81400956). Z.L. is supported by the Beijing Natural Science Foundation (7163219). W.Y. is supported by NCSF (81370513). Y.G. is supported by NCSF (81371306 and 81571285) and the Shanghai Committee of Science and Technology Support Program (14431907002). G.C. is supported by NCSF (81422013 and 81471196).
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Glossary
Abbreviations
- BBB
blood–brain barrier
- MCAO
middle cerebral artery occlusion
- tPA
tissue plasminogen activator
- Treg
regulatory T cell
References
- Asahi M, Asahi K, Wang X, Lo EH. Reduction of tissue plasminogen activator-induced hemorrhage and brain injury by free radical spin trapping after embolic focal cerebral ischemia in rats. J Cereb Blood Flow Metab 2000; 20: 452–7. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol 2006; 177: 8338–47. [DOI] [PubMed] [Google Scholar]
- Brea D, Agulla J, Rodriguez-Yanez M, Barral D, Ramos-Cabrer P, Campos F. et al. Regulatory T cells modulate inflammation and reduce infarct volume in experimental brain ischaemia. J Cell Mol Med 2014; 18: 1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos F, Qin T, Castillo J, Seo JH, Arai K, Lo EH. et al. Fingolimod reduces hemorrhagic transformation associated with delayed tissue plasminogen activator treatment in a mouse thromboembolic model. Stroke 2013; 44: 505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Xing J, Xiao X, Liou AK, Gao Y, Yin XM. et al. Critical role of calpain I in mitochondrial release of apoptosis-inducing factor in ischemic neuronal injury. J Neurosci 2007; 27: 9278–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Che X, Ye W, Panga L, Wu DC, Yang GY. Monocyte chemoattractant protein-1 expressed in neurons and astrocytes during focal ischemia in mice. Brain Res 2001; 902: 171–7. [DOI] [PubMed] [Google Scholar]
- Cheng T, Petraglia AL, Li Z, Thiyagarajan M, Zhong Z, Wu Z. et al. Activated protein C inhibits tissue plasminogen activator-induced brain hemorrhage. Nat Med 2006; 12: 1278–85. [DOI] [PubMed] [Google Scholar]
- Copin JC, Bengualid DJ, Da Silva RF, Kargiotis O, Schaller K, Gasche Y. Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse. Eur J Neurosci 2011; 34: 1085–92. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience 2009; 158: 972–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Asahi M, Wu O, Rosen BR, Lo EH. Rapid breakdown of microvascular barriers and subsequent hemorrhagic transformation after delayed recombinant tissue plasminogen activator treatment in a rat embolic stroke model. Stroke 2002; 33: 2100–4. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Effects of the chemokine CCL2 on blood-brain barrier permeability during ischemia-reperfusion injury. J Cereb Blood Flow Metab 2006; 26: 797–810. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke 2007; 38: 1345–53. [DOI] [PubMed] [Google Scholar]
- Ding G, Nagesh V, Jiang Q, Zhang L, Zhang ZG, Li L. et al. Early prediction of gross hemorrhagic transformation by noncontrast agent MRI cluster analysis after embolic stroke in rat. Stroke 2005; 36: 1247–52. [DOI] [PubMed] [Google Scholar]
- Garcia-Yebenes I, Sobrado M, Zarruk JG, Castellanos M, Perez de la Ossa N, Davalos A. et al. A mouse model of hemorrhagic transformation by delayed tissue plasminogen activator administration after in situ thromboembolic stroke. Stroke 2011; 42: 196–203. [DOI] [PubMed] [Google Scholar]
- Gauberti M, Martinez de Lizarrondo S, Orset C, Vivien D. Lack of secondary microthrombosis after thrombin-induced stroke in mice and non-human primates. J Thromb Haemost 2014; 12: 409–14. [DOI] [PubMed] [Google Scholar]
- Hadass O, Tomlinson BN, Gooyit M, Chen S, Purdy JJ, Walker JM. et al. Selective inhibition of matrix metalloproteinase-9 attenuates secondary damage resulting from severe traumatic brain injury. PLoS One 2013; 8: e76904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger N, Bratane BT, Bastan B, Bouley J, Fisher M. Normobaric hyperoxia and delayed tPA treatment in a rat embolic stroke model. J Cereb Blood Flow Metab 2009; 29: 119–29. [DOI] [PubMed] [Google Scholar]
- Hiu T, Nakagawa S, Hayashi K, Kitagawa N, Tsutsumi K, Kawakubo J. et al. Tissue plasminogen activator enhances the hypoxia/reoxygenation-induced impairment of the blood-brain barrier in a primary culture of rat brain endothelial cells. Cell Mol Neurobiol 2008; 28: 1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Zhang M, Leak RK, Gan Y, Li P, Gao Y. et al. Delivery of neurotherapeutics across the blood brain barrier in stroke. Curr Pharm Des 2012; 18: 3704–20. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Maric D, Mou Y, Ohtani R, Ruetzler C, Hallenbeck JM. Mucosal tolerance to E-selectin promotes the survival of newly generated neuroblasts via regulatory T-cell induction after stroke in spontaneously hypertensive rats. J Cereb Blood Flow Metab 2009; 29: 606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman K, Kahles T, Lane D, Garcia-Bonilla L, Abe T, Capone C. et al. Progranulin deficiency promotes post-ischemic blood-brain barrier disruption. J Neurosci 2013; 33: 19579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang RL, Zhang ZG, Knight RA, Ewing JR, Ding G. et al. Magnetic resonance imaging characterization of hemorrhagic transformation of embolic stroke in the rat. J Cereb Blood Flow Metab 2002; 22: 559–68. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Kraft P, Dreykluft A, Hagedorn I, Gobel K, Schuhmann MK. et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood 2013; 121: 679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansberg MG, Albers GW, Wijman CA. Symptomatic intracerebral hemorrhage following thrombolytic therapy for acute ischemic stroke: a review of the risk factors. Cerebrovasc Dis 2007; 24: 1–10. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Chapman DF, Zivin JA. Metalloproteinase inhibition reduces thrombolytic (tissue plasminogen activator)-induced hemorrhage after thromboembolic stroke. Stroke 2000; 31: 3034–40. [DOI] [PubMed] [Google Scholar]
- Li M, Wang X, Fu W, He S, Li D, Ke Q. CD4+CD25+Foxp3+ regulatory T cells protect endothelial function impaired by oxidized low density lipoprotein via the KLF-2 transcription factor. Cell Physiol Biochem 2011; 28: 639–48. [DOI] [PubMed] [Google Scholar]
- Li P, Gan Y, Sun BL, Zhang F, Lu B, Gao Y. et al. Adoptive regulatory T-cell therapy protects against cerebral ischemia. Ann Neurol 2013a; 74: 458–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Mao L, Zhou G, Leak RK, Sun BL, Chen J. et al. Adoptive regulatory T-cell therapy preserves systemic immune homeostasis after cerebral ischemia. Stroke 2013b; 44: 3509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S. et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med 2009; 15: 192–9. [DOI] [PubMed] [Google Scholar]
- Liesz A, Zhou W, Na SY, Hammerling GJ, Garbi N, Karcher S. et al. Boosting regulatory T cells limits neuroinflammation in permanent cortical stroke. J Neurosci 2013; 33: 17350–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S. et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 2006; 203: 1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaner J, Fernandez-Cadenas I, Molina CA, Monasterio J, Arenillas JF, Ribo M. et al. Safety profile of tissue plasminogen activator treatment among stroke patients carrying a common polymorphism (C-1562T) in the promoter region of the matrix metalloproteinase-9 gene. Stroke 2003; 34: 2851–5. [DOI] [PubMed] [Google Scholar]
- Potrovita I, Zhang W, Burkly L, Hahm K, Lincecum J, Wang MZ. et al. Tumor necrosis factor-like weak inducer of apoptosis-induced neurodegeneration. J Neurosci 2004; 24: 8237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling M, Strecker JK, Ringelstein EB, Schabitz WR, Kiefer R. The role of CC chemokine receptor 2 on microglia activation and blood-borne cell recruitment after transient focal cerebral ischemia in mice. Brain Res 2009; 1289: 79–84. [DOI] [PubMed] [Google Scholar]
- Schuhmann MK, Kraft P, Stoll G, Lorenz K, Meuth SG, Wiendl H. et al. CD28 superagonist-mediated boost of regulatory T cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J Cereb Blood Flow Metab 2015; 35: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X. et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat Commun 2016; 7: 10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simao F, Ustunkaya T, Clermont AC, Feener EP. Plasma kallikrein mediates brain hemorrhage and edema caused by tissue plasminogen activator therapy in mice after stroke. Blood 2017; 129: 2280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker JK, Minnerup J, Schutte-Nutgen K, Gess B, Schabitz WR, Schilling M. Monocyte chemoattractant protein-1-deficiency results in altered blood-brain barrier breakdown after experimental stroke. Stroke 2013; 44: 2536–44. [DOI] [PubMed] [Google Scholar]
- Su EJ, Fredriksson L, Geyer M, Folestad E, Cale J, Andrae J. et al. Activation of PDGF-CC by tissue plasminogen activator impairs blood-brain barrier integrity during ischemic stroke. Nat Med 2008; 14: 731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuji K, Aoki T, Tejima E, Arai K, Lee SR, Atochin DN. et al. Tissue plasminogen activator promotes matrix metalloproteinase-9 upregulation after focal cerebral ischemia. Stroke 2005; 36: 1954–9. [DOI] [PubMed] [Google Scholar]
- Urra X, Cervera A, Villamor N, Planas AM, Chamorro A. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience 2009; 158: 1174–83. [DOI] [PubMed] [Google Scholar]
- Wang W, Li M, Chen Q, Wang J. Hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for Ischemic stroke: mechanisms, models, and biomarkers. Mol Neurobiol 2015; 52: 1572–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 2003; 9: 1313–17. [DOI] [PubMed] [Google Scholar]
- Wang X, Tsuji K, Lee SR, Ning M, Furie KL, Buchan AM. et al. Mechanisms of hemorrhagic transformation after tissue plasminogen activator reperfusion therapy for ischemic stroke. Stroke 2004; 35 (11 Suppl 1): 2726–30. [DOI] [PubMed] [Google Scholar]
- Yan J, Greer JM, Etherington K, Cadigan GP, Cavanagh H, Henderson RD. et al. Immune activation in the peripheral blood of patients with acute ischemic stroke. J Neuroimmunol 2009; 206: 112–17. [DOI] [PubMed] [Google Scholar]
- Yan J, Read SJ, Henderson RD, Hull R, O’Sullivan JD, McCombe PA. et al. Frequency and function of regulatory T cells after ischaemic stroke in humans. J Neuroimmunol 2012; 243: 89–94. [DOI] [PubMed] [Google Scholar]
- Yao Y, Tsirka SE. Truncation of monocyte chemoattractant protein 1 by plasmin promotes blood-brain barrier disruption. J Cell Sci 2011; 124 (Pt 9): 1486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yepes M, Sandkvist M, Moore EG, Bugge TH, Strickland DK, Lawrence DA. Tissue-type plasminogen activator induces opening of the blood-brain barrier via the LDL receptor-related protein. J Clin Invest 2003; 112: 1533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WC, Wang YG, Zhu ZF, Wu FQ, Peng YD, Chen ZY. et al. Regulatory T cells protect fine particulate matter-induced inflammatory responses in human umbilical vein endothelial cells. Mediators Inflamm 2014a; 2014: 869148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Y, Zuo Z, Wang Z, Roy J, Hou Q. et al. Effects of tissue plasminogen activator timing on blood-brain barrier permeability and hemorrhagic transformation in rats with transient ischemic stroke. J Neurol Sci 2014b; 347: 148–54. [DOI] [PubMed] [Google Scholar]
- Zuo W, Chen J, Zhang S, Tang J, Liu H, Zhang D. et al. IMM-H004 prevents toxicity induced by delayed treatment of tPA in a rat model of focal cerebral ischemia involving PKA-and PI3K-dependent Akt activation. Eur J Neurosci 2014; 39: 2107–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.