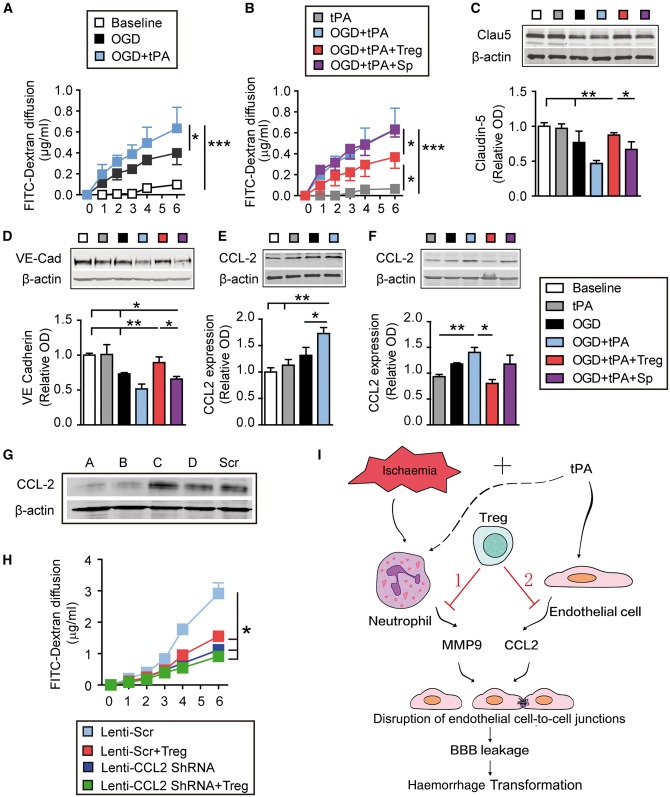
Figure 8.
Tregs protect the in vitro BBB against oxygen-glucose deprivation by inhibiting tPA-induced endothelial expression of CCL2. Primary mouse endothelial cells in cell culture inserts or plates were exposed to 4 h of oxygen–glucose deprivation followed by tPA (500 ng/ml). For cell treatments, endothelial cells exposed to oxygen–glucose deprivation (OGD) and/or tPA were co-cultured with Tregs or non-selected splenocytes at a 2:1 ratio. (A and B) The diffusion of FITC-dextran from the luminal to abluminal chamber was measured over time. (C–F) Cell lysates were collected 4 h after oxygen–glucose deprivation and subjected to western blot analyses. (C and D) Representative western blot images and quantification of VE-Cadherin (C) and Claudin-5 (D) (n = 4/group). (E and F) Representative western blot images and quantification of CCL2 (n = 4/group). β-Actin was used as the loading control. Data are normalized to baseline. (G and H) CCL2 is critical for Treg-afforded BBB protection in vitro. Endothelial cells in cell culture inserts were transfected with lenti-scramble or lenti-Ccl2 shRNA for 72 h. Endothelial cells were then exposed to oxygen–glucose deprivation (4 h) + tPA (500 ng/ml) treatment, followed by co-culture with Tregs or non-selected splenocytes. The diffusion of FITC-dextran from the luminal to abluminal chamber was measured over time. (G) Four shRNA constructs (A–D) were used to knock down CCL2 expression in endothelial cells. Western blots demonstrate knockdown of CCL2 with shRNA constructs A and B. Construct A was then used for subsequent experiments. (H) Quantification of FITC-dextran leakage into the luminal chamber over time (n = 6/group) Data are mean ± SE. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. (I) Schematic illustrating dual mechanisms underlying Treg-mediated mitigation of BBB rupture and haemorrhagic transformation (HT) after ischaemic stroke and delayed tPA treatment. Adoptive transferred Tregs may protect against haemorrhagic transformation induced by delayed tPA treatment after ischaemic stroke by two mechanisms. (1) Ischaemic injury can induce the release of MMP9 from peripheral neutrophils, which cause BBB disruption. Tregs inhibit neutrophil-derived MMP9 (Li et al., 2013a). (2) tPA enhances the expression of CCL2 on endothelial cells. CCL2 can further break down the BBB. Treg treatment inhibits the expression of CCL2 on endothelial cells and provides BBB protection.