Abstract
In mammals, subplate neurons (SPNs) are among the first generated cortical neurons. While most SPNs exist only transiently during development, a number of SPNs persist among adult Layer 6b (L6b). During development, SPNs receive thalamic and intra-cortical input, and primarily project to Layer 4 (L4). SPNs are critical for the anatomical and functional development of thalamocortical connections and also pioneer corticothalamic projections. Since SPNs are heterogeneous, SPN subpopulations might serve different roles. Here, we investigate the connectivity of one subpopulation, complexin-3 (Cplx3)–positive SPNs (Cplx3-SPNs), in mouse whisker somatosensory (barrel) cortex (S1). We find that many Cplx3-SPNs survive into adulthood and become a subpopulation of L6b. Cplx3-SPNs axons project to thalamorecipient layers, that is, L4, 5a, and 1. The L4 projections are biased towards the septal regions between barrels in the second postnatal week. Thus, S1 Cplx3-SPN targets co-localize with the eventual projections of the medial posterior thalamic nucleus (POm). In addition to their cortical targets, Cplx3-SPNs also extend long-range axons to several thalamic nuclei, including POm. Thus, Cplx3-SPN/L6b neurons are associated with paralemniscal pathways and can potentially directly link thalamocortical and corticothalamic circuits. This suggests an additional key role for SPNs in the establishment and maintenance of thalamocortical processing.
Keywords: complexin-3, cortical development, Cplx3, subplate, thalamocortical projections
Introduction
In mammals, subplate neurons (SPNs) are a heterogeneous population of neurons that are among the first generated neuronal populations in the cerebral cortex (Kostovic and Rakic 1980; Kanold and Luhmann 2010) (Rodents and primates differ in many important respects of subplate connectivity and function, which we compare and contrast below. Sauropsids, i.e., reptiles and birds, also have a subplate zone (Wang et al. 2011), but the relation to the mammalian subplate is as yet incompletely understood and we do not discuss them further). SPNs play an instrumental role in the establishment and refinement of early cortical circuits (Kanold and Luhmann 2010) and pioneer the corticofugal (McConnell et al. 1989, 1994) and corticopetal (Ghosh et al. 1990) pathways. They play an essential role in the establishment and functional maturation of thalamocortical connections, including whisker barrels in Rodent S1, as well as intra-cortical inhibitory connections (Ghosh and Shatz 1992; Kanold et al. 2003; Kanold and Shatz 2006; Tolner et al. 2012). Many of these roles of SPNs are consistent with SPNs receiving thalamic inputs and providing early excitation to ipsilateral Layer 4 (Friauf et al. 1990; Hanganu et al. 2002; Higashi et al. 2002; Molnár et al. 2003; Zhao et al. 2009). Over development, many SPNs undergo programmed cell death (apoptosis), while some survive into adulthood to form 2 closely related populations: the subgriseal neurons in Layer 6b (L6b) and the interstitial neurons in the nearby white matter (Clancy et al. 2001; Kanold and Luhmann 2010; Mortazavi et al. 2016).
The subplate zone and its relation to cortical development have been well studied in both primates and rodents (and to a lesser extent other mammals). In primates, the subplate at one point constitutes the single largest structure in the telencephalon, whereas in rodents, the expansion during development is less profound (Hoerder-Suabedissen and Molnár 2015). The precise delineation of the subplate is very clear in primates; distinct demarcation of the boundary with the cortical plate (above) and the intermediate zone (below) are clear even with simple Nissl staining. In rodents, both boundaries are difficult to distinguish from cytological staining alone but are visible with antibody or RNA probes against specific molecular markers. Another important difference between the classes is that primate SPNs project more broadly than do their rodent equivalents, for instance, to contralateral cortex and the superior colliculus (Hoerder-Suabedissen and Molnár 2015). Furthermore, in addition to the persistent Layer 6b population, which is present in both classes, primates show many more remnant SPNs, that is, interstitial neurons in the white matter (Kostovic and Rakic 1980; Judaš et al. 2010; Mortazavi et al. 2016). It should also be noted that in all species, there appear to be wide differences in SPN properties across different cortical regions.
The development of thalamocortical interactions in rodents has been studied to a reasonable extent (Molnár and Blakemore 1995). Thalamocortical afferents begin their journey to the cortex early in development (~E11); SPN axons undergo concomitant migration towards the thalamus. As these 2 populations of afferents come into contact (E13), it appears that some combination of molecular signals from the afferents, and from the local milieu (typically the intermediate zone), causes the thalamocortical afferents to follow the paths left by the SPN axons (the “handshake hypothesis”) (Molnár and Blakemore 1995) to reach the subplate, which the thalamic afferents begin to invade (E15). By E18, the afferents have entered the cortical plate (which has greatly expanded since E15 but lacks clear lamination), and by the first postnatal days (e.g., P0 in V1) (Molnár and Blakemore 1995), they have reached Layer 4 (of the fully laminated cortical plate) and begun arborizing. Over time, these afferents broadly arborize in Layer 4 and send some axon branches into Layer 6. Less is known about the tracts followed by the SPN axons during these time frames, and this is the subject we sought to better illuminate in this study.
Recent molecular profiling revealed subsets of SPNs with differential expression patterns (Hoerder-Suabedissen et al. 2009; Hoerder-Suabedissen and Molnár 2013), raising the possibility that there are diverse SPN cell types. As with other brain regions, it is possible that these cell types form distinct sub-circuits and serve different functions. A prominent marker of SPNs is complexin-3 (Cplx3) (Hoerder-Suabedissen et al. 2009; Hoerder-Suabedissen and Molnár 2013), a soluble component of the SNARE machinery for vesicle release (Hu et al. 2002; Xue et al. 2008; Vaithianathan et al. 2015). Recent studies have demonstrated that Cplx3 is highly localized within the subplate, across the entire anterior–posterior extent of the cortex (Hoerder-Suabedissen et al. 2009; Viswanathan et al. 2012; Hoerder-Suabedissen and Molnár 2013). Cplx3-SPNs arise early in the development of the subplate (Hoerder-Suabedissen and Molnár 2013), with some arising in and migrating from extra-cortical areas (Pedraza et al. 2014). Since Cplx3 is present in presynaptic terminals but not in the dendritic compartment, it is ideally suited to reveal the axonal targets of SPNs. We find that many Cplx3-SPNs (Cplx3-SPNs) in barrel cortex survive into adulthood and become part of Layer 6b. Cplx3-SPNs project to thalamocortical recipient layers, in particular Layer 5a and the septal region of Layer 4, which are targets of projections from POm in adults. We also find that Cplx3-SPNs directly target regions in the POm. These results show that Cplx3-SPNs are uniquely positioned to integrate ascending and descending thalamocortical and corticothalamic circuits and might be associated with higher order nuclei such as POm. This projection pattern suggests an additional key role for Cplx3-SPNs in the development and later function of the cortex, in addition to their well-established path-finding roles in organizing thalamocortical afferents.
Materials and Methods
All procedures were approved by the Animal Use and Care Committees of University of Maryland College Park and HHMI Janelia Research Campus. We used mice (C57BL/6J) of either sex from The Jackson Laboratory (jax.org). Cplx3-/- mice were a generous gift of Dr Josh Singer (UMd). Ages are provided for each experiment.
Perfusion and sectioning
Brains were perfused trans-cardially with 4% paraformaldehyde (PFA), post-fixed for 2 h in 4% PFA at room temperature or overnight at 4°C. Fixed brains were rinsed in cold phosphate buffered saline (PBS) and cut coronally at 50 µm thickness and collected in cold PBS and stored at 4°C. Horizontal sections were made as described previously (Tolner, Sheikh et al. 2012).
Immunofluorescence
Free floating sections were blocked in 3% bovine serum albumin (BSA) and 0.3% Triton X-100 (Pierce) for 1 h at room temperature followed by incubation in primary antibody overnight at 4°C. Sections were rinsed in 0.3% Triton 3 times (15’ each) to remove nonspecifically bound primary antibody followed by incubation in secondary antibody. Sections were finally rinsed in 0.3% Triton to remove nonspecific secondary antibody and mounted onto glass slides. Sections were air dried at room temperature, rehydrated in PBS and coverslipped with Vectashield (Vector Labs). Antibodies and concentrations are given below (Table 1).
Table 1.
Antibodies and concentrations used
| Name | Vendor | Catalog # | Dilution | Species |
|---|---|---|---|---|
| Cplx3 | Synaptic Systems | 122 302 | 1:1000 | Rb |
| Vglut2 | Synaptic Systems | 135 404 | 1:5000–10 000 | Gp |
| NeuN | Chemicon | MAB377 | 1:100–1:500 | Ms |
| Name | Vendor | Catalog # | Dilution | Conjugate |
| Gt anti Rb |
Invitrogen | A-11008 | 1:500 | AF 488 |
| Gt anti Gp |
Invitrogen | A-11075 | 1:500 | AF 568 |
| Gt anti Ms |
Invitrogen | A-21235 | 1:500 | AF 647 |
Rb, rabbit; Gt, goat; Ms, mouse; Gp, guinea pig; AF, Alexa Fluor.
Imaging
Fluorescent sections were imaged with a Pannoramic 250 Flash Whole Slide Digital Scanner to analyze cell and projection distribution. Quantification of cells and projections was done on images taken on a confocal microscope (Zeiss 510 or 710 Meta). Filter sets were chosen to minimize any bleed-through between fluorophores.
Analysis
Images acquired as above were adjusted for contrast and brightness in ImageJ. Projection analysis was done in ImageJ and plotted using custom MATLAB scripts.
Results
To study the projection patterns of Cplx3-expressing SPNs (Cplx3-SPNs), we used immunofluorescence against Cplx3 in wild-type C57/BL6J mice (either sex; Jackson Labs) (Viswanathan et al. 2012). The specificity of the Cplx3 antibody was first verified through labeling of sections from Cplx3−/− mice (Supplementary Fig. S1). Very little background labeling was seen on the KO sections, even at high laser power and with contrast enhancement, showing that the cortical and subplate staining seen in WT animals is specific to Cplx3 protein.
Cplx3 Specifically Labels a Subpopulation of SPNs in Multiple Cortical Areas
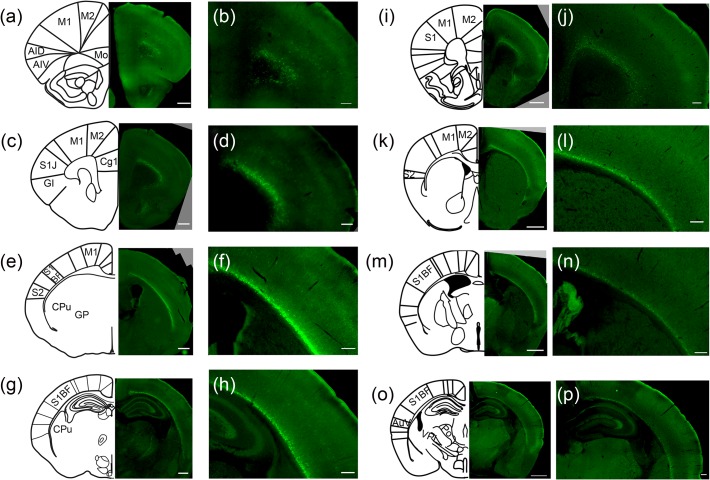
We first assessed the specificity of Cplx3 as a marker of SPNs. In situ hybridizations have identified that Cplx3 is highly expressed in the subplate (Hoerder-Suabedissen et al. 2009; Hoerder-Suabedissen and Molnár 2013); however, expression is also present in a subset of Layer 5 neurons. Immunohistochemistry shows that Cplx3 protein is expressed fairly specifically in the subplate of all cortical areas in both young (P7) and adult (P21) ages (Fig. 1), with only a few neurons labeled in the cortical plate. Thus, while many SPNs undergo apoptosis at the end of the critical period (Kanold and Luhmann 2010), other Cplx3-SPNs survive and integrate into L6b, where they might represent a specialized population. At P7, some cells in the cortical plate are also labeled, especially in Layer 5 (Fig. 1a–h), but this population disappears by P21 (Fig. 1i–p). These results indicate that either a small subpopulation of cortical neurons in rostral areas is Cplx3+ or that a subpopulation of Cplx3-SPNs is heterotopic and is later eliminated. Regardless, the majority of Cplx3+ cells at any age reside within the subplate.
Figure 1.
Cplx3+ neuron distribution at P7 and P21 (a–h) Cplx3+ neuron distribution at P7. Cplx3 immunofluorescence of coronal sections of P7 mouse along rostral (a,b) to caudal (g, h) gradient. Left (a,c,e,g): Low-power images and matching atlas cartoons. Scale bars 500 μm. Right (b,d,f,h): High-power images. Note that some Cplx3+ cell bodies appear in the cortical plate especially in Layer 5. Scale bars 200 µm. (i–p) Cplx3+ neuron distribution at P21. Cplx3 immunofluorescence on coronal sections of P21 mouse along rostral (i, j) to caudal (o, p) gradient. Left (i,k,m,o): Low-power images and matching atlas cartoons. Scale bars 1 mm. Right (j,k,n,p): High-power images. Scale bars 200 µm. M1, primary motor cortex; M2, secondary motor cortex; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; Mo, medial orbital cortex; AID, dorsal agranular insular area; AIV, ventral agranular insular area; Cg1, cingulate cortex, area 1; S1J; somatosensory cortex, jaw region; GI, granular insular cortex; CPu, striatum (caudoputamen); S1BF, somatosensory cortex, barrel field; GP, globus pallidus; AuV; ventral secondary auditory cortex; PO, posterior thalamic nucleus; VPL, ventral posterolateral thalamic nucleus.
Since SPNs play an essential role in the establishment and refinement of thalamocortical circuitry during the critical period (Kanold and Luhmann 2010) and because Cplx3 is not robustly expressed at embryonic and early postnatal ages (Hoerder-Suabedissen et al. 2009), we focused on ages P7–P9, corresponding to the S1 critical period (Erzurumlu and Gaspar 2012).
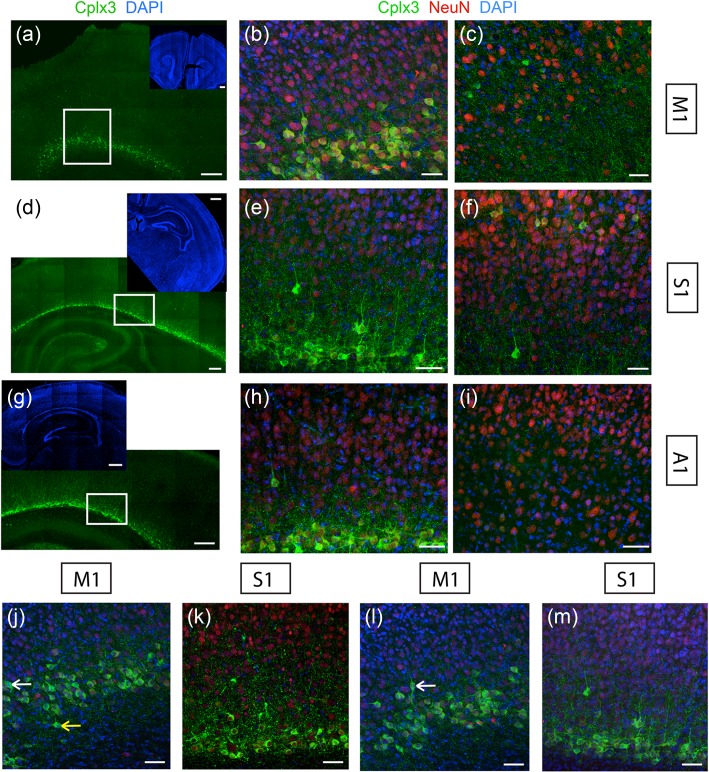
To investigate if Cplx3 labels similar populations of SPNs in different cortical areas, we first compared somatic Cplx3 label in S1, primary vibrissal motor (M1), and primary auditory (A1) cortex (Fig. 2a,d,g). A dense band of Cplx3-SPN somata was present in all cortical areas above the white matter, corresponding to the subplate (Hoerder-Suabedissen and Molnár 2013). Co-labeling with the neuronal nuclear antigen NeuN showed that in all areas, somatic Cplx3 expression was consistently restricted to a large subset of SPNs but not all (Fig. 2b,e,h). Labeling of the cortical plate (Fig. 2c,f,i) showed Cplx3 expression in M1 and S1 but not A1.
Figure 2.
Cplx3 labeling in diverse cortical areas. (a–i) Expression of Cplx3 (green), NeuN (red), and DAPI label (blue) in P7 coronal sections. Sections cover M1 (top, a–c), S1 (middle, d–f) and A1 (bottom, g–i). Left: Low-power images. Insets in these panels show representative images of the entire section. The white box indicates the subplate area imaged. Middle: High-power images of the subplate. Right: High-power images of the cortical plate, in areas directly above those shown in middle column. Scale bars: (a,d,g): 200 µm, inset 500 µm; others: 50 µm. (j–m) Cplx3-SPN morphology across cortical areas. Coronal sections showing Cplx3 (green) and NeuN (red) expression and DAPI label (blue). (j) Examples of Cplx3-SPNs occupying different SP laminae in M1. White arrow shows a neuron in upper SP; yellow arrow shows a neuron in lower SP. (k) Diverse somatic morphologies of Cplx3-SPNs in S1. (l) Cplx3+ neurons in M1. White arrow shows a bipolar neuron. (m) Cplx3+ neurons in S1. Less morphological variation was seen in S1 than in M1; in particular no bipolar cells were seen. Scale bars: 50 µm.
Cplx3+ neurons in the subplate exhibited a variety of morphologies including horizontal and pyramidal, and these morphologies tended to vary between upper and lower subplate laminae. As Cplx3 is a presynaptic protein, immunofluorescence did not fill entire neurons to allow tracing, and hence we restricted the study to a qualitative classification. We also observed qualitative differences in morphologies between different cortical areas. While Cplx3+ neurons in rostral areas such as M1 exhibited a range of morphologies such as pyramidal, horizontal, and to a lesser extent bipolar, Cplx3+ neurons in caudal areas like S1 had rather uniform horizontal and pyramidal morphology with no bipolar cells (Fig. 2j–m). While further systematic morphometric studies will be required to validate this, these observations point to an areal difference in Cplx3-SPN morphology, which might correlate with differences in SPN function.
Cplx3-SPNs project to non-lemniscal thalamorecipient layers in S1
Since SPNs play an instructive role during the critical period of thalamocortical development (Kanold and Luhmann 2010), SPN projections could guide or associate with thalamic axons and thereby sculpt thalamocortical connectivity. Moreover, neurites of SPNs in the golli-tau-eGFP mouse (which expresses GFP in SPNs and some L6 neurons) associate with the developing barrels and septa in an age-dependent fashion (Pinon et al. 2009). However, since eGFP labeled both axons and dendrites, the association of SPN outputs with patterned thalamocortical projections could not be assessed. As mentioned above, the presynaptic terminal localization of Cplx3 makes it an ideal tool for the study of axonal projection fields. Within L4 of S1, thalamic afferents segregate into barrels (Agmon et al. 1995), which are separated by inter-barrel spaces called septa. In order to determine the layers and regions to which Cplx3-SPNs project, we localized SPN projections with respect to thalamic afferents. S1 receives thalamic projections via at least 3 distinct pathways (Koralek et al. 1988; Lu and Lin 1993; Bureau et al. 2006; Urbain and Deschenes 2007; Diamond et al. 2008; Meyer et al. 2010; Wimmer et al. 2010). These pathways originate at the whisker follicle, are relayed by the trigeminal ganglion, and are thought to encode different stimulus properties (Diamond et al. 2008). The lemniscal pathway, after being relayed in the trigeminal ganglion, passes through the dorsomedial section of the thalamic ventral posterior medial nucleus (VPMdm) and projects to L4 barrels. In contrast, the extra-lemniscal pathway passes through ventrolateral VPM (VPMvl) and projects to L4 septa and secondary somatosensory cortex (S2), while the paralemniscal pathway passes through POm and projects to S1 Layer 5a (L5a) as well as S2. We thus investigated with which thalamocortical signaling system Cplx3-SPNs are associated by investigating the laminar and tangential distribution of Cplx3+ terminals in S1.
In barrel cortex, thalamic afferents utilize Vesicular Glutamate Transporter 2 (Vglut2) as the primary vesicular neurotransmitter transporter for thalamocortical signaling (Nahmani and Erisir 2005). As such, Vglut2 serves as a reliable marker to identify thalamic afferents and hence the different cortical layers.
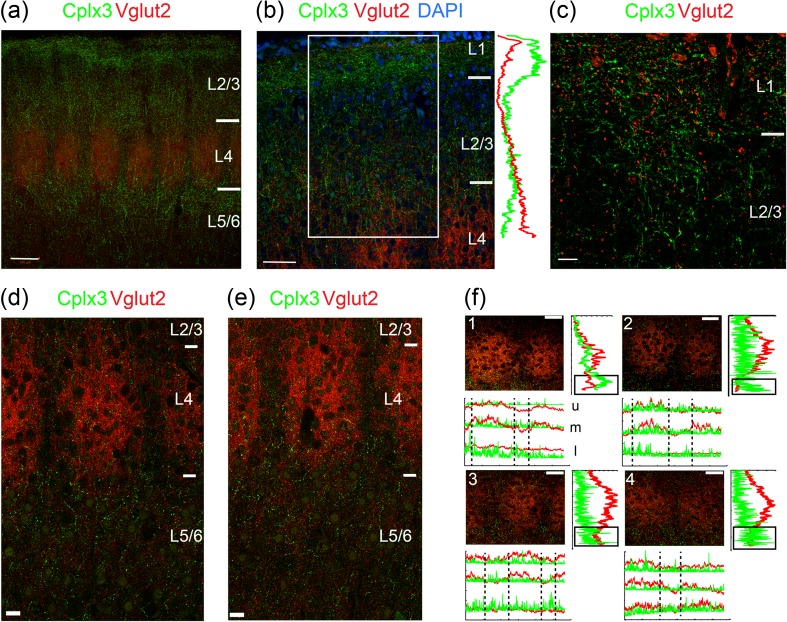
In S1, punctate Cplx3 labeling indicating presynaptic terminals from SPNs was present in thalamorecipient Layers L1 and L4 and could also be detected in L5a (Fig. 3), reinforcing the notion that these are paralemniscal projections (Diamond et al. 2008). High-magnification images revealed punctate Cplx3+ axon terminals and varicosities along with Vglut2 terminals in L1 (Fig. 3c), consistent with prior studies (Clancy and Cauller 1999). Coronal sections from P8-9 animals showed that most projections from Cplx3-SPNs were concentrated not within, but rather above and below, the Vglut2+ barrels in L4, as well as sparse punctate labeling in L4 of the septa (Fig. 3d), although the diffuse nature of the label made quantification difficult.
Figure 3.
Cplx3-SPN projections to the cortical plate. Coronal sections of wild-type mice (P8-9), stained for Vglut2 (red) and Cplx3 (green). (a) Tiled view of the barrel field with barrels (red) and Cplx3+ projections from SPNs (green). Note the projections in the cortical plate around the barrel field (i.e., septa). Scale bar 100 µm. (b) Zoomed-in view of the area above the barrels showing Cplx3 (green), Vglut2 (red), and DAPI (blue). Profiles on the right shows integrated intensities of Cplx3 (green) and Vglut2 (red) in the boxed region. Note the Cplx3+ terminals in the pial and barrel boundaries. Scale bar 50 µm. (c) High-power image of the region around L1. Scale bar 10 µm. (d–e) Two examples of tiled images at and below the barrels. Note the punctate green Cplx3-SPN signal below the barrel field. Scale bars 20 µm. (e) Images from coronal brain sections from P8 mice stained for Cplx3 (SPN terminals) and Vglut2 (thalamic afferents). Scale bars 20 µm. (f) Graphs quantifying labeling intensity in the 2 channels along the horizontal and vertical axes. In vertical graphs Cplx3 intensity shows an increase below the peak of Vglut2 label in the barrel. In horizontal graphs, Cplx3 intensity peaks in the septa could be observed in some cases, mostly in the lower septal region. Scale bars 50 µm. u, upper; m, middle; l, lower.
To validate this observation, we quantified the mean pixel intensity from Cplx3+ terminals across the laminar extent and observed an increase in intensity in Cplx3 at laminar locations just below high Vglut2 label (Fig. 3e). We observed this laminar enrichment for Cplx3-SPN terminals in L5a in 6 barrels from 6 animals.
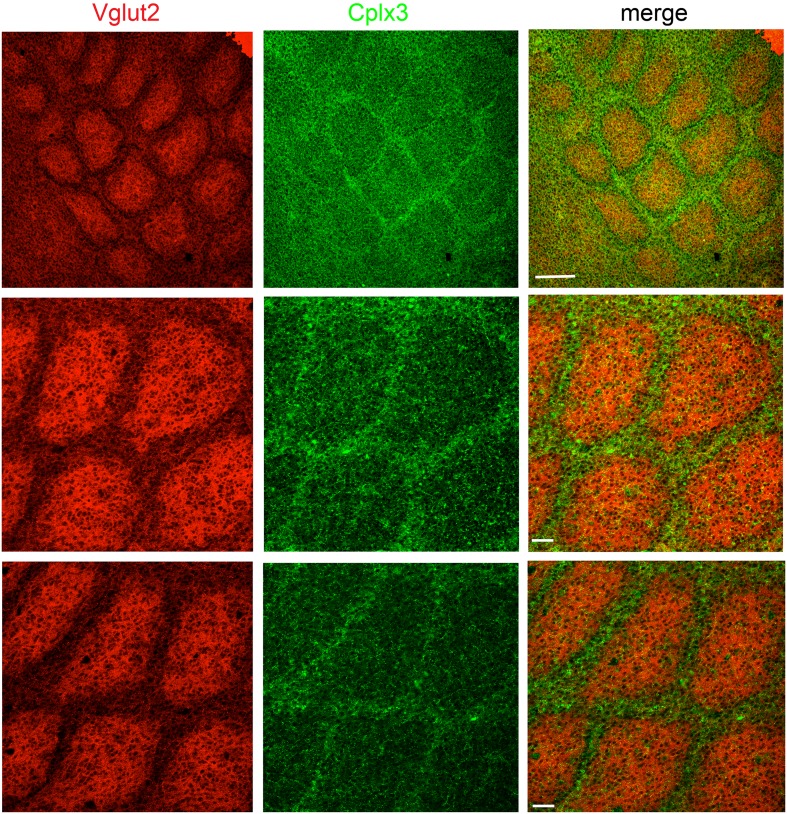
Because a subpopulation of SPNs labeled by the golli-promoter (Campagnoni et al. 1993) has been shown to preferentially extend neurites towards septa at the ages used here (Pinon et al. 2009), we tested if projections from Cplx3-SPNs showed a similar septal bias. Individual coronal sections showed slight barrel/septal preferences in L5a (e.g., barrel: Fig. 3f2; septal: 3f3; none: 3f1,4), but as a population we found no clear preference for either the barrel or septum in these sections, likely due to the sparse nature of the signal. However, we did observe a septal preference in flattened P9 horizontal sections (Fig. 4), the preparation of which integrates the septal signal across multiple planes of the section.
Figure 4.
Cplx3-SPNs projections in S1. Images from flattened horizontal brain sections at P9 stained for Cplx3 (SPN terminals) and Vglut2 (thalamic afferents). Label is densest in barrel septa. Scale bars: 200 µm (upper row) and 50 µm (middle and lower rows). The three rows are flattened sections from three representative fields of view.
The laminar enrichment of Cplx3-SPN terminals in L5a and the septal projections in L4 were reminiscent of projections of POm to S1 (Wimmer et al. 2010), again consistent with Cplx3-SPNs being associated with the paralemniscal pathway (Diamond et al. 2008).
Cplx3-SPNs project back to the thalamic PO nucleus
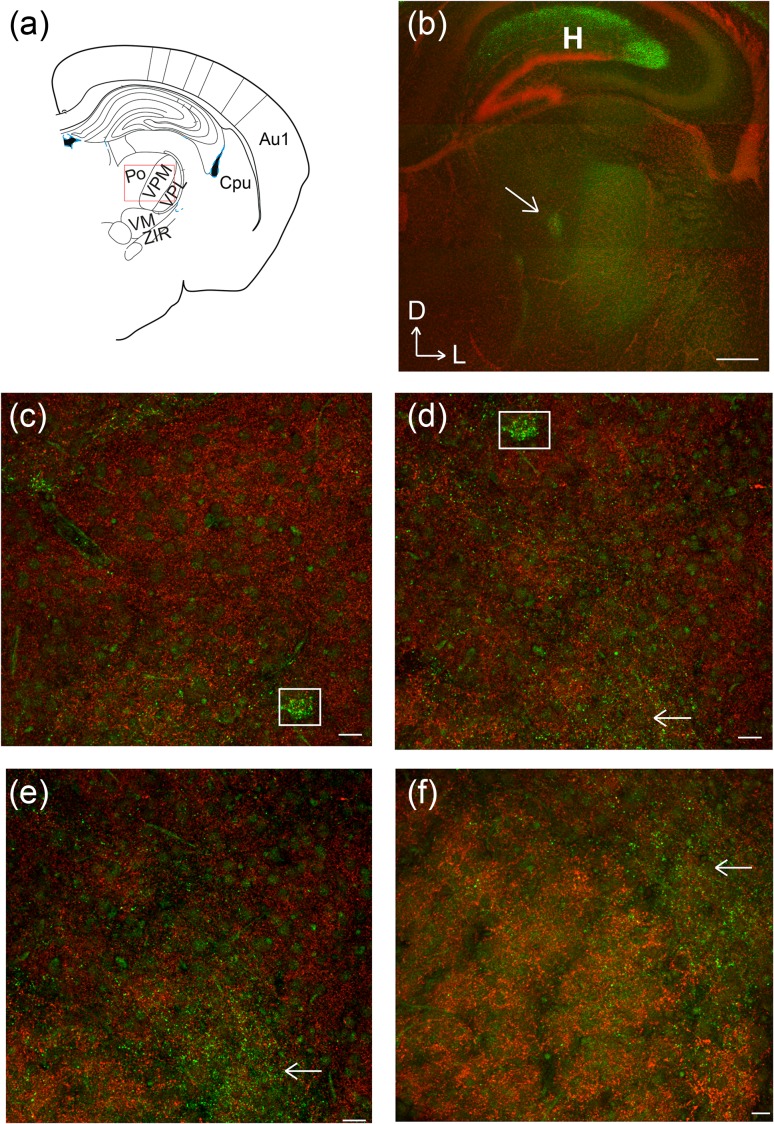
SPNs have been implicated in pioneering the corticothalamic pathway (McConnell et al. 1989, 1994; Grant et al. 2012; Hoerder-Suabedissen and Molnár 2012). We thus investigated if Cplx3-SPNs also project back to the thalamus. We observed extensive terminal fields throughout the internal capsule and punctate Cplx3+ terminals in the thalamus (Fig. 5a,b), broadly corresponding to POm. High-power images showed that while some diffuse punctae were also observed in the space between the barreloids, a cluster of punctate Cplx3 signal (white boxes in Fig. 5c–f) localized in regions adjacent to the barreloids, consistent with POm labeling, instead of VPM (Van Der Loos 1976).
Figure 5.
Cplx3-SPN projections to thalamus. Coronal brain slices at P9, showing Cplx3 (green) and Vglut2 (red). (a) Atlas cartoon of the region imaged. (b) Low-power image showing the thalamic region with dense terminals highlighted (arrow). Strongest labeling is seen in POm. Diffuse labeling is also observed in other thalamic nuclei. Scale bar 500 µm.(c–f) High-power images showing that Cplx3 label (boxes and arrows) does not co-localize with VPM barreloids. Scale bars: 20 µm. H, hippocampus.
Discussion
SPNs are among the first-born cortical neurons and play a key role in cortical development (Kanold and Luhmann 2010). SPNs comprise a heterogeneous class of neurons. Neuronal cell types are perhaps most commonly classified according to their epigenetic/transcriptomic identity, morphology, or by functional classification based on their connectivity. Here, we investigated the projections of a particular molecular class of SPNs, Cplx3-SPNs. We find that Cplx3-SPNs in whisker barrel cortex project to layers and regions (septa) in barrel cortex receiving paralemniscal inputs at later postnatal ages (>P8). Moreover, this population of SPNs also projects back to the thalamus. Thus, a single molecularly defined population of SPNs is ideally positioned to potentially provide both feed-forward and feedback connectivity to link together the thalamic POm nucleus and its cortical projections.
Cplx3 is a strong SPN marker and partially co-localizes with connective tissue growth factor (CTGF), another molecular SP marker in rodents (Hoerder-Suabedissen et al. 2009; Viswanathan et al. 2012; Hoerder-Suabedissen and Molnár 2013) and other species (Wang et al. 2011). Cplx3 has high spatiotemporal specificity to SPNs across development. Cplx3 belongs to a family of 4 closely related proteins involved in vesicle release (Hu et al. 2002; Xue et al. 2008; Vaithianathan et al. 2015); its localization to axon terminals allowed us to study long-range projections of this subset of SPNs versus nonspecific neurite labeling with GFP in the golli-tau-eGFP mouse (Pinon et al. 2009). It should be noted that Cplx3 expression is upregulated in the first postnatal week and reaches a peak at P8 (Hoerder-Suabedissen et al. 2009), and thus an analysis of early Cplx3-SPN birth, migration, and connectivity would be desirable (but technically challenging). One potential caveat of using Cplx3 as an axonal marker is that it is unclear if Cplx3 is present in all axonal terminals of Cplx3+ neurons. As such, this study might underreport axonal connections.
Cplx3 transcripts (which are mostly retained in the nucleus/cell body), as seen by in situ hybridization, not only show subplate-specific enrichment in all sensory areas (Hoerder-Suabedissen et al. 2009; AllenBrainAtlas 2015) but also low levels of expression in Layer 5. In contrast, Cplx3 protein (which is actively transported to axon terminals) only labels neurons in the cortical plate during development (Hoerder-Suabedissen et al. 2009) (Fig. 1), indicating that Cplx3 is post-transcriptionally regulated to be predominantly subplate-specific. Since Cplx3 functions in the axon terminal, immature protein in the endoplasmic reticulum likely explains the cell body labeling during the ages studied here. It is likely that some SPNs get displaced during cortical migration, becoming heterotopic in the upper cortical layers but later eliminated by apoptosis. It is also possible that some migratory neurons express Cplx3 in their soma, and at later stages in development, either Cplx3 expression is down-regulated or these cells are eliminated. The somatic expression in upper cortical layers undergoes a dramatic reduction at stages past the critical period. Moreover, the number of neurons expressing somatic Cplx3 in the upper cortical layers shows inter-areal differences. At P7, for instance, Cplx3+ neurons in the upper cortical layers are more numerous in S1 than in A1. This could represent functional differences or simply differential rates of maturation. Our results show that the particular subset of SPNs labeled depends on the cortical area, suggesting functional specialization of SPNs.
Many Cplx3-SPNs survive to older ages, integrating into the populations of subgriseal and interstitial neurons. Cplx3-SPN circuits show many similarities to L6 circuits overall. Both Cplx3-SPNs and L6 cells project to thalamus and innervate L5a (Briggs 2010; Kim et al. 2014). Moreover, SPNs are the first target of thalamic fibers (Friauf et al. 1990; Hanganu et al. 2002; Higashi et al. 2002; Molnár et al. 2003; Zhao et al. 2009), which in the adult project extensively to L6 (Meyer et al. 2010; Wimmer et al. 2010). Since paralemniscal inputs to S1 can play a circuit-modulatory role (Viaene et al. 2011), Cplx3-SPNs and Cplx3+ Layer 6b neurons might similarly control the gain of both thalamocortical and corticothalamic transmission. The fact that these neurons project both to thalamocortical target layers as well as to the thalamus itself makes them uniquely suited to control thalamocortical synchrony. Specifically, the activity in Cplx3-SPNs neurons could contribute to the synchronization of neural activity in the thalamus and cortex, which might be required for the maturation of both thalamocortical and corticothalamic circuits. This is consistent with the observation that cortical oscillatory spindle activity, which is thought to rely on thalamocortical activity, is abolished when SPNs are ablated (Blumenfeld and McCormick 2000; Khazipov and Luhmann 2006; Tolner et al. 2012).
It is well established that SPNs receive functional thalamic inputs, which are then relayed to the developing cortex (Friauf et al. 1990; Hanganu et al. 2002; Higashi et al. 2002; Molnár et al. 2003; Zhao et al. 2009; Kanold and Luhmann 2010). Here, we show that a particular molecular class of SPNs has axonal projections to thalamorecipient layers and can thus fulfill this functional feed-forward role.
Most Cplx3-SPNs project to the main thalamorecipient layers. Consistent with prior studies (Clancy and Cauller 1999), we find Cplx3+ terminals in L1, a cell-sparse, neuropil-rich zone (Hestrin and Armstrong 1996; Ma et al. 2014), where connections between cortical and subcortical regions converge. In addition to apical dendrites from different cortical layers, for example, L2/3 and L5/6, L1 receives intra-areal corticocortical projections (Cauller et al. 1998) and inputs from subcortical areas like thalamus (Herkenham 1986). Thus, Cplx3-SPNs can influence the integration of subcortical and cortical inputs.
VPM and POm are distinct thalamic nuclei that subserve parallel pathways to S1 and S2 (Koralek et al. 1988; Lu and Lin 1993; Bureau et al. 2006; Diamond et al. 2008; Meyer et al. 2010; Wimmer et al. 2010). In S1, lemniscal fibers from VPM target L4, while paralemniscal fibers from POm target L5a (Koralek et al. 1988; Chmielowska et al. 1989; Lu and Lin 1993). While VPM transmits tactile information, POm transmits dynamic, nociceptive, and modulatory signals (Gauriau and Bernard 2002; Yu et al. 2006; Diamond et al. 2008; Haidarliu et al. 2008). Our data show that after P8, Cplx3-SPNs target cortical areas associated with POm inputs (as well as POm itself). Within the primary somatosensory cortex, we observe that Cplx3 label is enriched at the L4/L5a boundary, at the bottom of the barrel formed by thalamic afferents from VPM and POm. In L4, we observe a septal preference of Cplx3 labeling. Since Cplx3-SPNs target S1 L4, L5a, and L1, as well as S2, they are likely associated with the paralemniscal pathway (Diamond et al. 2008) and connections from POm.
Cplx3, as a member of the complexin family, regulates neurotransmitter vesicle release by the SNARE complex. Cplx1 and Cplx2 are the best-studied family members and have been shown to regulate vesicle priming, such that knockdown and knockout mice exhibited weaker release synchronization and more spontaneous release in cortex (Reim et al. 2005; Xue et al. 2008; Südhof 2012; Yang et al. 2013). Cplx3 has been functionally characterized primarily in the retina, where it is involved with ribbon bipolar synapses; there, knockout desynchronized vesicle release led to profound malformation of the ribbon synapses and dramatically decreased the ability of the mice to see (Reim et al. 2005; Reim et al. 2009). In cortex, the presence of Cplx3 in the paralemniscal pathway, which is thought to encode dynamic whisker information (Diamond et al. 2008), is consistent with a synaptic specialization for temporal processing. Thus, it seems enticing to speculate that the early presence of Cplx3 in this developing circuit is functionally relevant and might help synchronize inputs required for corticothalamic and thalamocortical maturation.
We observed a septal preference in flattened sections cut in the horizontal plane, but this preference was less clear in coronal sections. This difference is likely due to the angle of sectioning. Since the terminals have a laminar preference, sectioning in the horizontal plane and imaging at different layers might represent the laminar localization of the terminals differently from the coronal sections. Thus, the septal label in horizontal sections could originate from terminals present in L4, L5a, or both. Alternatively, septal L4 signal could be boosted in flattened sections by averaging due to tissue compression. As Cplx3+ terminals are also observed in L1, Cplx3-SPNs would appear to form a scaffold around the barrel hollow after P8, similar to the “barrel nets” formed by L2/3 axons (Sehara et al. 2010). This is similar to the pattern of SPN neurites in the golli-tau-EGFP mouse (which expresses GFP in SPNs and some L6 neurons) (Pinon et al. 2009). Moreover, SPN neurites in L4 in the golli-tau-eGFP mouse seem to associate with thalamic projections in the barrel hollow and remodel between P6 and P10 (Pinon et al. 2009). Moreover, the neurite pattern in the golli-tau-EGFP mouse could be altered by sensory experience, in this case whisker plucking (Pinon et al. 2009), suggesting that the integration of SPN neurites into the thalamocortical circuitry is dynamic, consistent with a role of SPNs in critical period plasticity (Kanold and Shatz 2006). The similarity of our results using an axon-specific label with the golli-tau-EGFP mouse suggests that many neurites seen in the golli-tau-EGFP mouse are axons. Thus, our data suggest that golli-tau and Cplx3 might represent overlapping populations of SPNs, with the golli-tau-EGFP population being broader.
SPNs are thought to pioneer corticothalamic projections (McConnell et al. 1989, 1994; Grant et al. 2012; Hoerder-Suabedissen and Molnár 2012) and corticothalamic SPN axons might interact, or “handshake”, with ingrowing thalamic axons (Molnár and Blakemore 1995). Our data show that by P9, Cplx3-SPNs extend axons through the internal capsule and project to different thalamic nuclei. In contrast, tracing studies with the hydrophobic dye DiI from the ventrobasal thalamus and corpus callosum have shown that another class of SPNs, CTGF+ SPNs, do not project to the thalamus (Grant et al. 2012). Some SPNs co-express CTGF and Cplx3 (Viswanathan et al. 2012) and thus at least with respect to corticothalamic projections, these molecular subtypes do not fully segregate into functional cell types.
Here, we describe the projection targets of one molecular subclass of SPNs, Cplx3-SPNs. The functional role of these neurons will of course depend on their inputs. While it has been shown that SPNs receive thalamic inputs (Friauf et al. 1990; Hanganu et al. 2002; Higashi et al. 2002; Molnár et al. 2003; Zhao et al. 2009), it is unknown what thalamic nucleus innervates Cplx3-SPNs. If Cplx3-SPNs receive input from VPM, then they already provide lemniscal input to L5a. Given that SPN removal prevents the formation of barrels (Tolner et al. 2012), we speculate that under such a scenario, Cplx3-SPNs are required for the topographical alignment of VPM and POm projections. If Cplx3-SPNs receive POm input, then they would provide both feedback to the POm as well as feed-forward input to POm targets. Such a circuit might be involved in the generation of oscillations consistent with the observed role of SPNs in the generation of spindle bursts (Tolner et al. 2012). Further experiments are required to precisely define the molecular details of the interacting thalamic and SP neurons. Importantly, conclusively identifying whether single Cplx3+ SPNs simultaneously innervate both thalamus and cortex will require techniques such as viral tracing and whole-brain imaging (Economo et al. 2016), and perhaps even electron microscopy or super-resolution imaging.
Here, we provide anatomical evidence for the integration of a particular type of persistent SPN into the thalamocortical and corticothalamic circuitry and the maintenance of this circuit with age. Since SPNs play a key role in the establishment of thalamocortical connectivity, this is consistent with an important role of a particular class of SPNs in the establishment and/or maintenance of this important circuit.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/.
Funding
National Institutes of Health (grant R01DC009607 to P.O.K.).
Supplementary Material
Notes
We thank Yohannes Ghenbot and Justin Penzenstadler (UM) for technical assistance and Karel Svoboda (Janelia Research Campus), Eike Budinger (Leibniz Institute for Neurobiology), and Zoltán Molnár (Oxford) for helpful comments. S.V., L.L.L., and P.O.K. designed research. S.V. and A.S. performed experiments. S.V., L.L.L., P.O.K. wrote the paper. Conflict of Interest: None declared.
References
- Agmon A, Yang LT, Jones EG, O'Dowd DK. 1995. Topological precision in the thalamic projection to neonatal mouse barrel cortex. J Neurosci. 15:549–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AllenBrainAtlas 2015. © 2015 Allen Institute for Brain Science. Allen Developing Mouse Brain Atlas [Internet]. Available from: http://developingmouse.brain-map.org.
- Blumenfeld H, McCormick DA. 2000. Corticothalamic inputs control the pattern of activity generated in thalamocortical networks. J Neurosci. 20:5153–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs F. 2010. Organizing principles of cortical layer 6. Front Neural Circuits. 4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bureau I, von Saint Paul F, Svoboda K. 2006. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 4:e382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnoni AT, Pribyl TM, Campagnoni CW, Kampf K, Amur-Umarjee S, Landry CF, Handley VW, Newman SL, Garbay B, Kitamura K. 1993. Structure and developmental regulation of Golli-mbp, a 105-kilobase gene that encompasses the myelin basic protein gene and is expressed in cells in the oligodendrocyte lineage in the brain. J Biol Chem. 268:4930–4938. [PubMed] [Google Scholar]
- Cauller LJ, Clancy B, Connors BW. 1998. Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J Comp Neurol. 390:297–310. [PubMed] [Google Scholar]
- Chmielowska J, Carvell GE, Simons DJ. 1989. Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol. 285:325–338. [DOI] [PubMed] [Google Scholar]
- Clancy B, Cauller LJ. 1999. Widespread projections from subgriseal neurons (layer VII) to layer I in adult rat cortex. J Comp Neurol. 407:275–286. [PubMed] [Google Scholar]
- Clancy B, Silva-Filho M, Friedlander MJ. 2001. Structure and projections of white matter neurons in the postnatal rat visual cortex. J Comp Neurol. 434:233–252. [DOI] [PubMed] [Google Scholar]
- Diamond ME, von Heimendahl M, Knutsen PM, Kleinfeld D, Ahissar E. 2008. ‘Where’ and ‘what’ in the whisker sensorimotor system. Nat Rev Neurosci. 9:601–612. [DOI] [PubMed] [Google Scholar]
- Economo MN, Clack NG, Lavis LD, Gerfen CR, Svoboda K, Myers EW, Chandrashekar J. 2016. A platform for brain-wide imaging and reconstruction of individual neurons. Elife. 5:e10566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzurumlu RS, Gaspar P. 2012. Development and critical period plasticity of the barrel cortex. Eur J Neurosci. 35:1540–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friauf E, McConnell SK, Shatz CJ. 1990. Functional synaptic circuits in the subplate during fetal and early postnatal development of cat visual cortex. J Neurosci. 10:2601–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, Bernard JF. 2002. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 87:251–258. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Antonini A, McConnell SK, Shatz CJ. 1990. Requirement for subplate neurons in the formation of thalamocortical connections. Nature. 347:179–181. [DOI] [PubMed] [Google Scholar]
- Ghosh A, Shatz CJ. 1992. Involvement of subplate neurons in the formation of ocular dominance columns. Science. 255:1441–1443. [DOI] [PubMed] [Google Scholar]
- Grant E, Hoerder-Suabedissen A, Molnár Z. 2012. Development of the corticothalamic projections. Front Neurosci. 6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidarliu S, Yu C, Rubin N, Ahissar E. 2008. Lemniscal and extralemniscal compartments in the VPM of the rat. Front Neuroanat. 2:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanganu IL, Kilb W, Luhmann HJ. 2002. Functional synaptic projections onto subplate neurons in neonatal rat somatosensory cortex. J Neurosci. 22:7165–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M. 1986. New perspectives on the organization and evolution of nonspecific thalamocortical projections In: Jones EG, Peters A, editors.Cerebral cortex: sensory-motor areas and aspects of cortical connectivity. New York: Plenum; pp. 403–445. [Google Scholar]
- Hestrin S, Armstrong WE. 1996. Morphology and physiology of cortical neurons in layer I. J Neurosci. 16:5290–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi S, Molnár Z, Kurotani T, Toyama K. 2002. Prenatal development of neural excitation in rat thalamocortical projections studied by optical recording. Neuroscience. 115:1231–1246. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnár Z. 2012. Morphology of mouse subplate cells with identified projection targets changes with age. J Comp Neurol. 520:174–185. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnár Z. 2013. Molecular diversity of early-born subplate neurons. Cereb Cortex. 23:1473–1483. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Molnár Z. 2015. Development, evolution and pathology of neocortical subplate neurons. Nat Rev Neurosci. 16:133–146. [DOI] [PubMed] [Google Scholar]
- Hoerder-Suabedissen A, Wang WZ, Lee S, Davies KE, Goffinet AM, Rakic S, Parnavelas J, Reim K, Nicolic M, Paulsen O, et al. 2009. Novel markers reveal subpopulations of subplate neurons in the murine cerebral cortex. Cereb Cortex. 19:1738–1750. [DOI] [PubMed] [Google Scholar]
- Hu K, Carroll J, Rickman C, Davletov B. 2002. Action of complexin on SNARE complex. J Biol Chem. 277:41652–41656. [DOI] [PubMed] [Google Scholar]
- Judaš M, Sedmak G, Pletikos M, Jovanov-Milošević N. 2010. Populations of subplate and interstitial neurons in fetal and adult human telencephalon. J Anat. 217::381–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanold PO, Kara P, Reid RC, Shatz CJ. 2003. Role of subplate neurons in functional maturation of visual cortical columns. Science. 301:521–525. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Luhmann HJ. 2010. The subplate and early cortical circuits. Annu Rev Neurosci. 33:23–48. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Shatz CJ. 2006. Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron. 51:627–638. [DOI] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. 2006. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 29:414–418. [DOI] [PubMed] [Google Scholar]
- Kim J, Matney CJ, Blankenship A, Hestrin S, Brown SP. 2014. Layer 6 corticothalamic neurons activate a cortical output layer, layer 5a. J Neurosci. 34:9656–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koralek KA, Jensen KF, Killackey HP. 1988. Evidence for two complementary patterns of thalamic input to the rat somatosensory cortex. Brain Res. 463:346–351. [DOI] [PubMed] [Google Scholar]
- Kostovic I, Rakic P. 1980. Cytology and time of origin of interstitial neurons in the white matter in infant and adult human and monkey telencephalon. J Neurocytol. 9:219–242. [DOI] [PubMed] [Google Scholar]
- Lu SM, Lin RC. 1993. Thalamic afferents of the rat barrel cortex: a light- and electron-microscopic study using Phaseolus vulgaris leucoagglutinin as an anterograde tracer. Somatosens Mot Res. 10:1–16. [DOI] [PubMed] [Google Scholar]
- Ma J, Yao XH, Fu Y, Yu YC. 2014. Development of layer 1 neurons in the mouse neocortex. Cereb Cortex. 24:2604–2618. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. 1989. Subplate neurons pioneer the first axon pathway from the cerebral cortex. Science. 245:978–982. [DOI] [PubMed] [Google Scholar]
- McConnell SK, Ghosh A, Shatz CJ. 1994. Subplate pioneers and the formation of descending connections from cerebral cortex. J Neurosci . 14:1892–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer HS, Wimmer VC, Hemberger M, Bruno RM, de Kock CP, Frick A, Sakmann B, Helmstaedter M. 2010. Cell type-specific thalamic innervation in a column of rat vibrissal cortex. Cereb Cortex. 20:2287–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Blakemore C. 1995. How do thalamic axons find their way to the cortex. Trends Neurosci. 18:389–397. [DOI] [PubMed] [Google Scholar]
- Molnár Z, Kurotani T, Higashi S, Yamamoto N, Toyama K. 2003. Development of functional thalamocortical synapses studied with current source-density analysis in whole forebrain slices in the rat. Brain Res Bull. 60:355–371. [DOI] [PubMed] [Google Scholar]
- Mortazavi F, Wang X, Rosene DL, Rockland KS. 2016. White matter neurons in young adult and aged rhesus monkey. Front Neuroanat. 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahmani M, Erisir A. 2005. VGluT2 immunochemistry identifies thalamocortical terminals in layer 4 of adult and developing visual cortex. J Comp Neurol. 484:458–473. [DOI] [PubMed] [Google Scholar]
- Pedraza M, Hoerder-Suabedissen A, Albert-Maestro MA, Molnár Z, De Carlos JA. 2014. Extracortical origin of some murine subplate cell populations. Proc Natl Acad Sci USA. 111:8613–8618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinon MC, Jethwa A, Jacobs E, Campagnoni A, Molnár Z. 2009. Dynamic integration of subplate neurons into the cortical barrel field circuitry during postnatal development in the Golli-tau-eGFP (GTE) mouse. J Physiol. 587:1903–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Regus-Leidig H, Ammermüller J, El-Kordi A, Radyushkin K, Ehrenreich H, Brandstätter JH, Brose N. 2009. Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4. J Cell Sci. 122:1352–1361. [DOI] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstatter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, Brose N. 2005. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 169:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehara K, Toda T, Iwai L, Wakimoto M, Tanno K, Matsubayashi Y, Kawasaki H. 2010. Whisker-related axonal patterns and plasticity of layer 2/3 neurons in the mouse barrel cortex. J Neurosci. 30:3082–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC. 2012. Calcium control of neurotransmitter release. Cold Spring Harb Perspect Biol. 4:a011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolner EA, Sheikh A, Yukin AY, Kaila K, Kanold PO. 2012. Subplate neurons promote spindle bursts and thalamocortical patterning in the neonatal rat somatosensory cortex. J Neurosci. 32:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Deschenes M. 2007. A new thalamic pathway of vibrissal information modulated by the motor cortex. J Neurosci. 27:12407–12412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaithianathan T, Henry D, Akmentin W, Matthews G. 2015. Functional roles of complexin in neurotransmitter release at ribbon synapses of mouse retinal bipolar neurons. J Neurosci. 35:4065–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Loos H. 1976. Barreloids in mouse somatosensory thalamus. Neurosci Lett. 2:1–6. [DOI] [PubMed] [Google Scholar]
- Viaene AN, Petrof I, Sherman SM. 2011. Properties of the thalamic projection from the posterior medial nucleus to primary and secondary somatosensory cortices in the mouse. Proc Natl Acad Sci USA. 108:18156–18161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan S, Bandyopadhyay S, Kao JP, Kanold PO. 2012. Changing microcircuits in the subplate of the developing cortex. J Neurosci. 32:1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WZ, Oeschger FM, Montiel JF, Garcia-Moreno F, Hoerder-Suabedissen A, Krubitzer L, Ek CJ, Saunders NR, Reim K, Villalon A, et al. 2011. Comparative aspects of subplate zone studied with gene expression in sauropsids and mammals. Cereb Cortex. 21:2187–2203. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Bruno RM, de Kock CP, Kuner T, Sakmann B. 2010. Dimensions of a projection column and architecture of VPM and POm axons in rat vibrissal cortex. Cereb Cortex. 20:2265–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M, Stradomska A, Chen H, Brose N, Zhang W, Rosenmund C, Reim K. 2008. Complexins facilitate neurotransmitter release at excitatory and inhibitory synapses in mammalian central nervous system. Proc Natl Acad Sci USA. 105:7875–7880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Cao P, Südhof TC. 2013. Deconstructing complexin function in activating and clamping Ca2+-triggered exocytosis by comparing knockout and knockdown phenotypes. Proc Natl Acad Sci USA. 110:20777–20782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C, Derdikman D, Haidarliu S, Ahissar E. 2006. Parallel thalamic pathways for whisking and touch signals in the rat. PLoS Biol. 4:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Kao JP, Kanold PO. 2009. Functional excitatory microcircuits in neonatal cortex connect thalamus and layer 4. J Neurosci. 29:15479–15488. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.