Abstract
Patients with schizophrenia show specific abnormalities in visual perception, and patients with bipolar disorder may have related perceptual deficits. During tasks that highlight perceptual dysfunction, patients with schizophrenia show abnormal activity in visual brain areas, including the lateral occipital complex (LOC) and early retinotopic cortex. It is unclear whether the anatomical structure of those visual areas is atypical in schizophrenia and bipolar disorder. In members of those two patient groups and healthy controls, we localized LOC and early retinotopic cortex individually for each participant using functional magnetic resonance imaging (MRI), then measured the thickness of those regions of interest using structural MRI scans. In both regions, patients with schizophrenia had the thinnest cortex, controls had the thickest cortex, and bipolar patients had intermediate cortical thickness. A control region, motor cortex, did not show this pattern of group differences. The thickness of each visual region of interest was significantly correlated with performance on a visual object masking task, but only in schizophrenia patients. These findings suggest an anatomical substrate for visual processing abnormalities that have been found with both neural and behavioral measures in schizophrenia and other severe mental illnesses.
Keywords: mental illness, MRI, neuroanatomy, perception, visual cortex
Introduction
Specific abnormalities in visual perception have been well characterized in schizophrenia (Butler et al. 2008; Green, Butler, et al. 2009; Javitt 2009; Green et al. 2012; Javitt and Freedman 2015). Emerging evidence suggests that similar types of perceptual dysfunction might exist in other mental illnesses that share genetic risk factors and clinical characteristics with schizophrenia, such as bipolar disorder (Chen et al. 2005; Chkonia et al. 2012; Jahshan et al. 2014). Yet the neural bases of perceptual dysfunction in major mental illnesses such as schizophrenia remain poorly understood.
The human visual system comprises an interlinked hierarchy of discrete visual areas with distinct functional specializations (Felleman and Van Essen 1991). Studies using functional magnetic resonance imaging (fMRI) have linked some specific visual processing deficits in schizophrenia patients to abnormal activity in particular visual areas. One area that has been directly linked to visual dysfunction in schizophrenia is the lateral occipital complex (LOC), a mid-level visual processing area in the lateral occipital lobe that is preferentially responsive to images of objects (Malach et al. 1995). In schizophrenia patients, abnormal LOC activity has been associated with specific abnormalities in visual masking and contour integration tasks (Green, Lee, et al. 2009; Green et al. 2011; Harvey et al. 2011; Silverstein et al. 2015).
There is also growing evidence that early visual areas (e.g., early retinotopic visual cortex, including V1, V2, V3, and other low-level visual areas) might also function abnormally in schizophrenia and bipolar disorder (Butler et al. 2001, 2005; Javitt 2009; Kantrowitz et al. 2009; Javitt and Freedman 2015). One focus of considerable recent research has been on impaired surround suppression and related phenomena, which are thought to depend upon lateral interconnections in early visual cortex, in schizophrenia, and bipolar disorder (e.g., Must et al. 2004; Dakin et al. 2005; Yoon et al. 2009, 2010; Yang et al. 2012a, 2012b; Schallmo et al. 2013, 2015).
The reasons why LOC and early retinotopic cortex might function abnormally in schizophrenia remain unclear. One possibility that has not been systematically investigated is that patient populations could have specific abnormalities in the neural architecture of those areas. Structural MRI provides a way to assess such potential structural abnormalities noninvasively in vivo. In particular, modern analytical techniques allow the thickness of cortex to be measured with submillimeter resolution in individual participants from standard structural scans (Fischl and Dale 2000; Dickerson et al. 2008).
Numerous studies have examined cortical thickness throughout the entire brain between samples of schizophrenia patients and healthy controls (Kuperberg et al. 2003; Narr et al. 2005; Schultz et al. 2010; Sprooten et al. 2013), bipolar patients and controls (Lyoo et al. 2006), or members of both psychiatric groups versus controls (Rimol et al. 2010, 2012; Pol et al. 2012; Janssen et al. 2014; Padmanabhan et al. 2014). Many such studies have noted group thickness differences in frontal and temporal areas of the brain, with patients showing thinner cortex in those areas than controls. However, group differences in thickness are not commonly reported in the occipital lobe, although they have been noted in some cases (e.g., Sprooten et al. 2013).
The approach used in previous studies is poorly suited to identifying cortical thickness differences in specific functional areas. LOC and other visual areas can only be reliably identified using functional localizers, because they vary in size and anatomical position across individuals, even in healthy populations (Larsson and Heeger 2006). Therefore, traditional anatomical co-registration techniques like those used in previous cortical thickness comparisons do not allow accurate comparison of functional visual areas across individuals or groups. Thus, the apparent absence of cortical thickness abnormalities in occipital regions could reflect a true lack of group differences between these psychiatric populations and controls, or it could reflect a limitation of the methods used in previous studies.
In the present study, we use a novel approach to overcome this limitation, employing fMRI to map the location of LOC and early retinotopic visual cortex in individual participants, then comparing the cortical thickness of those areas between groups of schizophrenia patients, bipolar patients, and matched healthy controls. To our knowledge, this is the first study to use functionally defined regions of interest (ROIs) to investigate cortical thickness in these psychiatric populations, although this approach has been used successfully in healthy populations (Frank et al. 2016). In an exploratory analysis, we also investigated possible correlations between cortical thickness and performance on a visual object masking task in each of the three groups.
For our primary analysis, we had separate predictions for the two visual ROIs. Based on known functional deficits in LOC in schizophrenia, we hypothesized that schizophrenia patients would have thinner cortex in LOC than controls. Because bipolar disorder is thought to share some but not all phenotypic features with schizophrenia, and because evidence for dysfunction of visual perception in bipolar disorder is equivocal, we hypothesized that the cortical thickness of LOC would be intermediate in bipolar patients, compared with the other two groups. By contrast, given the lack of strong neuroimaging findings directly implicating early visual processing areas as functionally abnormal in schizophrenia or bipolar disorder, we did not predict a significant difference in the thickness of early retinotopic cortex between patient group and controls.
Materials and Methods
Participants
All recruitment methods and experimental procedures were approved by the Institutional Review Boards of the VA Greater Los Angeles Healthcare System (GLA) and the University of California, Los Angeles (UCLA). All participants provided written informed consent prior to participation. The participants were part of a larger, ongoing, NIMH-sponsored study of visual processing in major mental illness.
Patients were recruited from outpatient treatment facilities in the Los Angeles area, UCLA outpatient clinics, and mental health clinics at the GLA. Healthy controls were recruited with Internet ads. Selection criteria for all subjects included: (1) age 18–65 years, (2) understood spoken English sufficiently to comprehend testing procedures, (3) no evidence of IQ <70 or developmental disability based on chart review, (4) no clinically significant neurological disease determined by medical history (e.g., epilepsy), (5) no history of serious head injury (i.e., loss of consciousness >1 h, neuropsychological sequelae, cognitive rehabilitation post-head injury), (6) no sedatives or benzodiazepines within 12 h of testing, (7) vision/corrected vision of at least 20/30, (8) no positive urine toxicology screening on day of assessment, (9) no known contraindications for MRI scanning, and (10) no history of a mood episode in the past two months. The exclusion of participants with a history of mood episode in the previous two months was done to minimize the chances that participants' scores on performance-based and self-report measures would be influenced by abnormal mood state (e.g., mania), in keeping with previous studies comparing schizophrenia and bipolar samples (Lee et al. 2013).
Selection criteria for patient participants included: (1) a diagnosis of schizophrenia or bipolar disorder based on the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (First et al. 1997), (2) no substance or alcohol dependence in the past three months; no evidence of substance or alcohol abuse in past month, and (3) clinically stable (i.e., no inpatient hospitalizations for 3 months prior to enrollment, no changes in psychoactive medication in the 4 weeks prior to enrollment). Additional selection criteria for healthy controls included: (1) no history of psychotic disorder, bipolar spectrum disorder, or other major mood disorder based on SCID-I interview (First et al. 1997) or of avoidant, paranoid, schizotypal, schizoid, or borderline personality disorders based on the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (First et al. 1996), (2) no family history of a psychotic disorder or bipolar disorder in first-degree relatives, based on participant report, and (3) no history of substance or alcohol dependence or abuse in the past month.
All SCID interviewers were trained through the Treatment Unit of the Department of Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC) to a minimum k of 0.75 for key psychotic and mood items (Ventura et al. 1998). When available, medical records and reports from treating clinicians were used to corroborate retrospective self-reported information for patient diagnoses.
The groups did not differ significantly on age, gender, handedness, or parental education, but they differed on personal education (Table 1). The two patient groups did not differ in number of years since their diagnosis (Table 1). Patients' clinical symptoms were characterized using several scales: the Brief Psychiatric Rating Scale (BPRS), Young Mania Rating Scale (YMRS), and Hamilton Depression rating scale (HAMD) (Table 1) (Hamilton 1960; Young et al. 1978; Ventura et al. 1993). Patient participants were on clinically determined doses of medication (Table 1).
Table 1.
Characterization of participants
| SZ patients (n = 33) | BD patients (n = 31) | Controls (n = 30) | Group comparison |
||
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Statistic | P-value | |
| Age | 45.15 (12.57) | 43.74 (13.15) | 48.00 (6.54) | F(2,91) = 1.14 | 0.33 |
| Illness duration | 23.91 (13.51) | 22.50 (12.61) | t(58) = 0.42 | 0.68 | |
| Education | 12.97 (2.11) | 13.94 (2.34) | 14.33 (1.83) | F(2,91) = 3.53 | 0.03 |
| Parental education | 12.87 (2.51) | 13.83 (2.74) | 13.43 (3.01) | F(2,83) = 0.90 | 0.41 |
| Gender (M/F) | 23/10 | 13/18 | 15/15 | χ2(2) = 5.29 | 0.07 |
| Handedness (R/L) | 28/5 | 27/4 | 26/4 | χ2(2) = 0.77 | 0.96 |
| BD type: I/II | 20/11 | ||||
| BPRS (total) | 40.70 (12.11) | 32.77 (9.09) | t(62) = 2.95 | 0.01 | |
| HAM-D (total) | 7.82 (6.67) | 7.26 (4.61) | t(62) = 0.39 | 0.70 | |
| YMRS (total) | 5.15 (3.91) | 4.06 (5.02) | t(62) = 0.97 | 0.34 | |
| Antipsychotic medication (Y/N) | 29/4 | 20/11 | χ2(1) = 4.86 | 0.03 | |
| CPZ-equivalent dosage (mg/day) | 516.15 (407.08) | 257.60 (160.03) | t(33) = 2.32 | 0.03 | |
| Mood stabilizer medication (Y/N) | 10/23 | 23/8 | χ2(1) = 12.33 | 0.01 | |
Bolded rows differ significantly between groups.
MRI Data Collection
All MRI data were collected at the UCLA Staglin Center for Cognitive Neuroscience on a 3 T Siemens Tim Trio scanner equipped with a 12-channel head coil (Siemens Medical Solutions, Erlangen, Germany). T1-weighted structural scans were collected using a Magnetization-Prepared Rapid Gradient Echo (MPRAGE) sequence (1.9 s TR, 3.4 ms TE, 9° flip angle, 1 mm isotropic voxels, 256 × 256 × 160 voxel field of view). Localizer scans used a standard Echo-Planar Imaging (EPI) sequence (2.5 s TR, 35 ms TE, 75° flip angle, 3 × 3 mm voxels with a 3.3 mm center-to-center inter-slice distance, 64 × 64 voxel field of view with 38 slices).
Participants completed two functional localizer scans (2.5 min each): a retinotopic mapping scan, and a localizer scan for LOC (Malach et al. 1995; Engel et al. 1997). In the retinotopic mapping scan, participants fixated on a central point, while a contrast-reversing checkerboard wedge subtending ∼20° of visual angle slowly rotated around the central point (5 cycles at 2 cycles/min; 8 Hz contrast-reversal). To ensure their eyes remained fixed on the central point, participants pressed a button when the contrast-polarity of the fixation dot inverted, which occurred periodically. In the LOC localizer task, participants saw 12.5 s blocks of objects (abstract sculptures) which alternated with 12.5 s blocks of spatially scrambled images of the objects. They were instructed to press a button every time the type of images changed between intact and scrambled. All functional localizer stimuli were presented via MR-compatible VisuaStim goggles (Resonance Technology, Inc., Los Angeles, CA, USA). Participant responses to the localizer stimuli were logged using an MR-compatible response box.
MRI Data Analysis
Overview
All analyses of both structural and functional MRI data were performed in FreeSurfer 5.3.0 (Martinos Center for Biomedical Imaging, Charlestown, MA, USA). The study used an ROI-based approach to investigate cortical thickness differences in visual areas across the participant groups. Individualized functional ROIs for early retinotopic cortex and LOC were created for each participant using the localizer data. Within each of these three ROIs, the mean cortical thickness was computed by measuring the distance between the white matter/gray matter boundary and the gray matter/pia mater boundary. We compared the cortical thickness of the two functionally defined visual ROIs across groups using a 2 × 3 ANOVA with within-subjects factor “ROI” and between-subjects factor “group.” Based on our findings in the planned analysis of the functionally defined visual ROIs, we added a post hoc nonvisual control ROI, primary motor cortex. Motor cortex was defined for each participant using anatomical landmarks (FreeSurfer parcellation), because the study did not include a functional localizer for motor cortex. Thickness of anatomically defined motor cortex was compared across groups with a one-way ANOVA.
Functional Analyses
The fMRI data were pre-processed using a standard pipeline (preprocsess): each scan was motion-corrected to the first TR of the run and spatially smoothed (5 mm full-width at half-maximum). Those scans were then processed in GLM analyses constrained to the cortical surface (i.e., masking out subcortical areas) with selxavg3-sess. For the retinotopic mapping scan, the GLM was performed using a standard polar-mapping analysis that identifies voxels whose activity varies in relation to the phase of the rotating checkerboard wedge (DeYoe et al. 1996; Engel et al. 1997). For the LOC localizer, a standard block-design GLM analysis was performed, contrasting intact >scrambled objects.
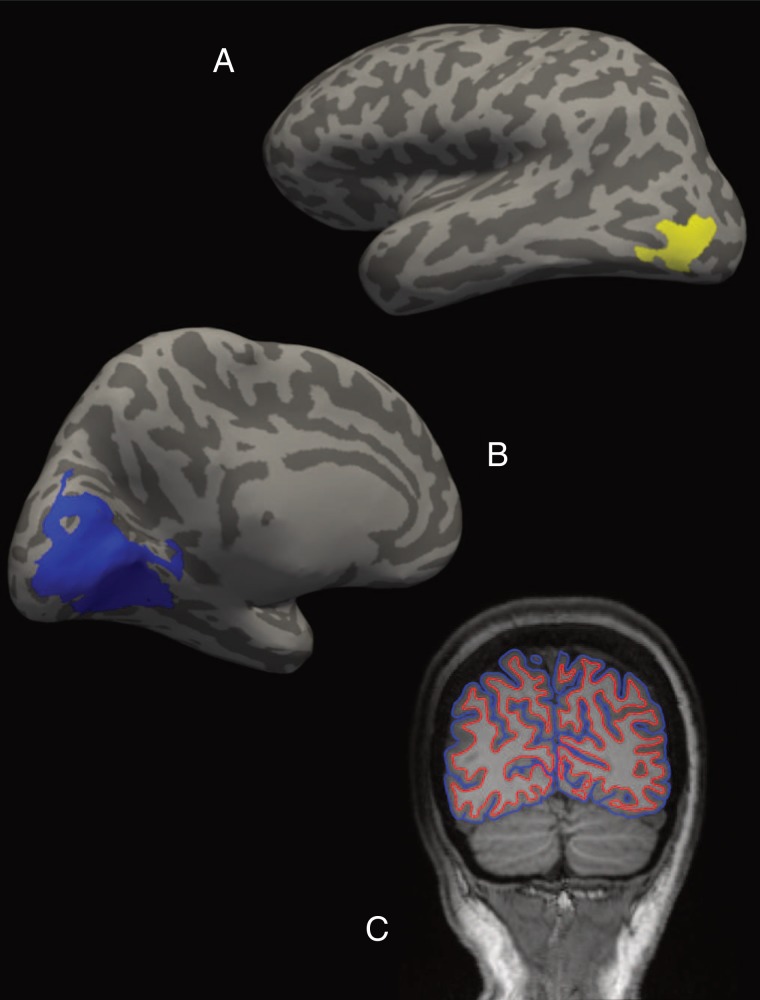
For each individual participant, each ROI was defined as the largest cluster in each hemisphere at a false discovery rate threshold of 0.05 within the expected anatomical neighborhood (constrained using automated anatomical parcellations from the structural analysis stream; see the Structural analyses section). Thus, retinotopic cortex was defined as the largest cluster on the medial surface of the occipital lobe (within the combined cuneus, pericalcarine, and lingual gyrus FreeSurfer labels) (Fig. 1A). For LOC, it was the largest cluster within the anatomically defined lateral occipital cortex label (Fig. 1B).
Figure 1.
Individualized visual ROIs and cortical boundaries for an example participant (healthy control). (A) LOC (in yellow) on an inflated lateral view of the left hemisphere. (B) Location of early retinotopic visual cortex (in blue) on an inflated medial view of the left hemisphere. (C) Coronal slice transecting the two visual ROIs from the participant's structural scan (radiological coordinates). White matter/gray matter and gray matter/pia mater boundaries are depicted in red and blue, respectively. ROI cortical thickness was calculated as the mean distance between these two boundaries within that area of the cortical surface.
Structural Analyses
Structural scans were processed using the standard FreeSurfer reconstruction pipeline (recon-all). The pipeline automatically processes each participant's structural MRI scan individually, using a permutation-based analysis of its unique anatomical features (Fischl et al. 2004; Desikan et al. 2006). The reconstruction process segments the cortical surface of each cerebral hemisphere into 34 gyral regions using anatomical landmarks (Desikan et al. 2006). These gyral labels were used to constrain the functional ROIs (see above). The post hoc motor cortex ROI was defined exclusively on the basis of this anatomical segmentation: we selected each participant's anatomically defined precentral gyrus.
Cortical thickness measurements were also derived from the FreeSurfer reconstruction pipeline. For each individual participant, across the entire cortex, the reconstruction process automatically identifies the anatomical boundaries between white matter and gray matter, and between gray matter and pia mater (see Fig. 1C for an example) (Dale and Sereno 1993; Dale et al. 1999; Fischl and Dale 2000). The distance between those two boundaries at any point on the cortical surface is equal to the cortical thickness at that point, with submillimeter resolution (Fischl and Dale 2000; Dickerson et al. 2008). The mean cortical thickness of each ROI was computed from these boundaries using mris_anatomical_stats and averaged across hemispheres within each participant.
Quality Assurance
Except for visual inspection of the data to ensure quality, described below, all processing of the MRI data was performed using automated scripts that applied the same predetermined analytical steps and thresholds to every participant to ensure equal treatment of data across the three groups.
Several precautions were taken to ensure high data quality. Stringent exclusion criteria were applied before statistical comparisons of cortical thickness between groups were performed. Participants were excluded from all further analyses if they had more than 3 mm motion displacement in either functional run or if problems with excessive participant movement during any part of the MRI session were noted in testing records. This very conservative motion exclusion threshold was adopted because cortical thickness estimates can be influenced by participant movement (Reuter et al. 2015). The criterion resulted in a total of 29 exclusions (14 schizophrenia, 6 bipolar disorder, 9 healthy controls). Each structural scan from the remaining participants was visually inspected for evidence of subject movement (e.g., ringing artifacts) or poor image contrast. This step resulted in two additional exclusions (1 schizophrenia and 1 bipolar disorder). Three more participants were excluded due to technical problems with a localizer scan (2 bipolar disorder, 1 healthy control). Hence, the final sample post-exclusions contained 33 schizophrenia, 31 bipolar disorder, and 30 healthy control participants. We compared the age, gender, handedness, education level, parental education level, and symptom rating scores (BPRS, YMRS, and HAM-D) for the included versus excluded participants within each of the three participant groups using independent-samples t-tests (χ2 tests for categorical variables). There were no significant differences between included and excluded participants in any of the three groups for any of those variables.
Additional steps were taken to evaluate data quality for the included participants. Accuracy of the automatic alignment of functional scans to the structural scan was visually inspected for each participant; no alignment errors were found. Accuracy of the automated demarcation of the boundaries of the cortical ribbon used to measure cortical thickness from the structural scans was also visually inspected and found to be acceptable for all included participants.
Masking Task
A backward-masking task using common household objects as targets was administered in a separate session on a 120 Hz monitor. Briefly, we presented one of six different objects, followed by a mask (overlapping black and white curved lines) at 8 time-varying inter-stimulus intervals (ISIs) ranging from 8.33–125 ms in 16.67 ms increments. The target was presented for 8.33 ms and the mask for 75 ms. There were 12 trials per ISI, as well as 12 unmasked trials. At the end of each trial, a list of the six object categories was presented and participants verbally responded which they thought had been presented.
Calculation of participant masking thresholds was performed in MATLAB (MathWorks, Natick, MA, USA) by fitting psychometric functions to each participant's data using standard techniques with the Palamedes Toolbox (Prins and Kingdom 2009). A Weibull function was fitted to each individual's masking data, across all ISIs, using a maximum likelihood criterion and fixed parameters for γ and λ, which were based on the task chance performance level and all participants' overall mean performance in the unmasked condition, respectively (γ = 0.166, λ= 0.1). Thresholds were calculated for 50% performance (i.e., threshold ISI for each participant was defined as the point at which the fitted Weibull function crossed the 50% accuracy point). Estimated thresholds that exceeded the ISI range the experiment was designed to measure (i.e., thresholds estimated to be >108.33 ms) were excluded as invalid (4 schizophrenia, 5 bipolar participants).
Results
Primary Analysis: Cortical Thickness by ROI and Group
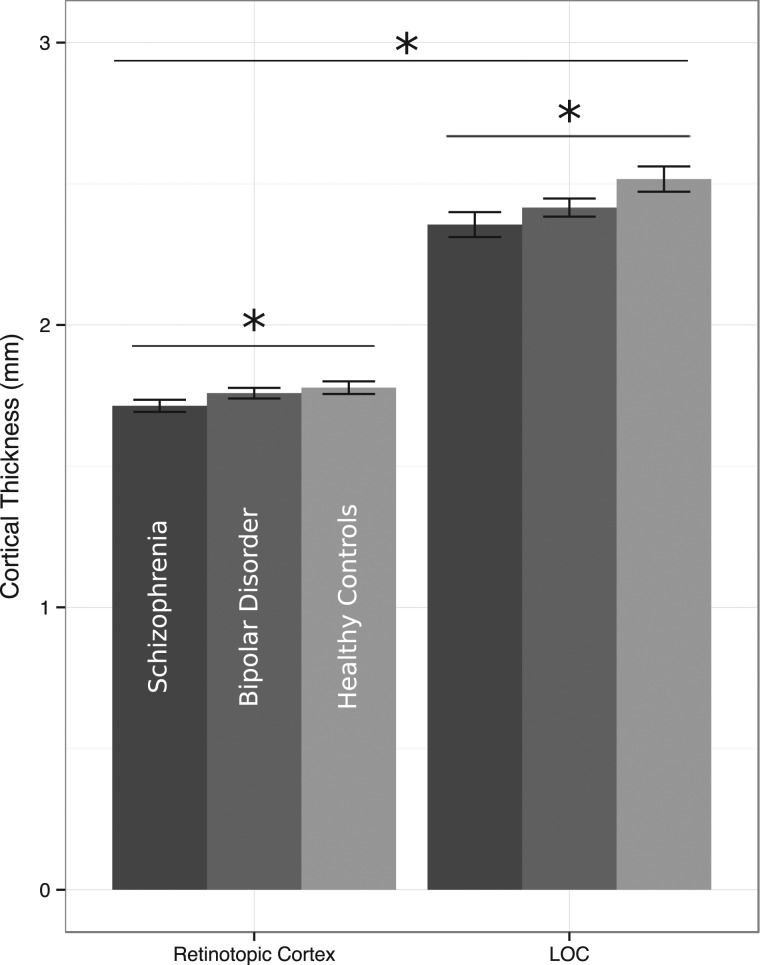
The primary analysis comparing the cortical thickness of early retinotopic cortex and LOC across groups of schizophrenia, bipolar disorder, and healthy control participants yielded significant main effects of group [F(2,91) = 4.82, P= 0.01, partial η2 = 0.10] and ROI [F(1,91) = 882.38, P< 0.01, partial η2 = 0.91], but no significant interaction [F(2,91) = 1.70, P= 0.19, partial η2 = 0.04] (Fig. 2). Across both ROIs, the cortex was thickest in the healthy control group (M = 2.15 mm), thinnest in the schizophrenia group (M = 2.04 mm), and intermediate in the bipolar disorder group (M = 2.09 mm). The post hoc t-tests show a significant difference in cortical thickness across ROIs between the healthy control and schizophrenia groups [ROIs together: t(61) = 2.88, P= 0.01; visual cortex alone: t(61) = 2.08, P= 0.04; LOC alone: t(61) = 2.56, P= 0.01] but not between healthy controls and bipolar disorder [ROIs together: t(59) = 1.71, P= 0.09; visual cortex alone: t(59) = 0.67, P= 0.50; LOC alone: t(59) = 1.84, P= 0.07] or bipolar disorder and schizophrenia [ROIs together: t(62) = 1.52, P= 0.13; visual cortex alone: t(62) = 1.56, P= 0.12; LOC alone: t(62) = 1.09, P= 0.28]. Across groups, the mean thickness of LOC (M = 2.43 mm) was greater than retinotopic cortex (M = 1.75 mm). This is consistent with classic neuroanatomical findings of thinner cortex in primary sensory cortices and more recent studies in healthy humans and other primates showing graded increases in cortical thickness relative to brain areas' position in sensory hierarchies (Wagstyl et al. 2015).
Figure 2.
Comparison of visual ROI cortical thickness by group and region. Across groups, retinotopic cortex was significantly thinner than LOC. Across ROIs, cortical thickness was significantly different between the participant groups: schizophrenia patients had the thinnest cortex in both ROIs, followed by bipolar patients, and healthy controls had the thickest cortex in both areas. In a direct comparison, schizophrenia patients had significantly thinner cortex than healthy controls across both ROIs, but comparisons between bipolar patients and the other two groups showed only trend-level differences. No significant group-by-ROI interaction was present in the data.
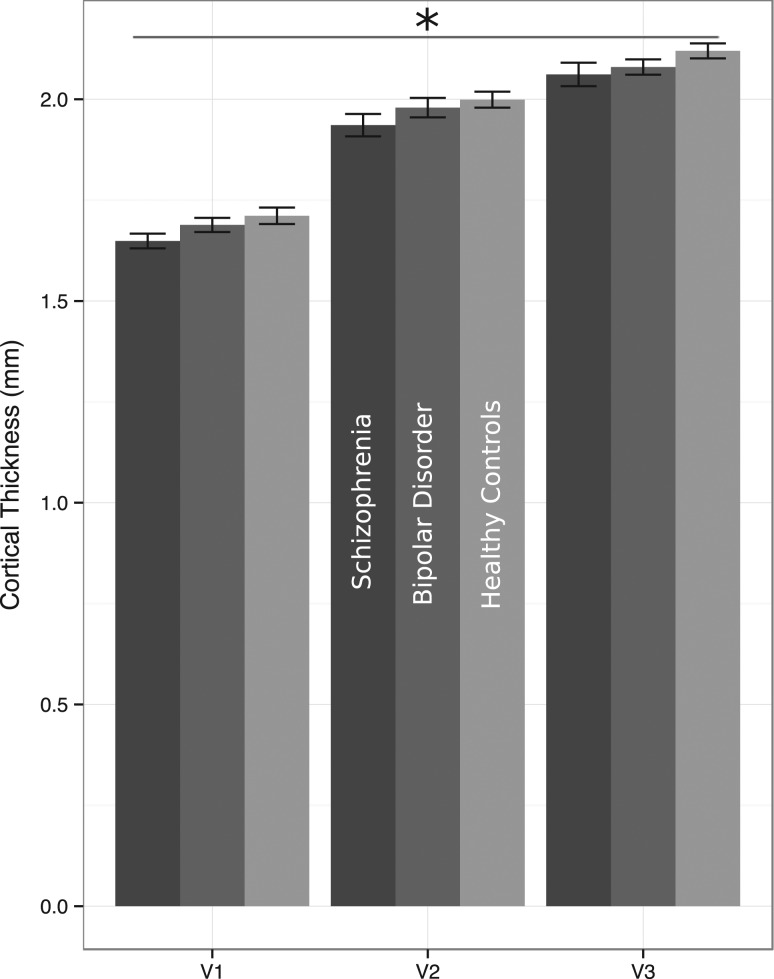
Follow-up Analysis: Subregions of Retinotopic Cortex
The presence of a group difference in the thickness of early retinotopic cortex raised the question of which specific regions within early retinotopic cortex (e.g., V1, V2, V3) differed between groups. V1, V2, and V3 were identified with a recent method based on individuals' cortical topography (Benson et al. 2012, 2014). Cortical thickness from these topographically determined post hoc visual regions was compared across groups in a 3 × 3 ANOVA. Consistent with known neuroanatomical differences, there was a large main effect of region [F(2,90) = 783.80, P< 0.001, partial η2 = 0.95]. V1 was thinner than V2, which was thinner than V3, across all three groups (Fig. 3). Only a trend-level main effect of group appeared in this analysis [F(2,91) = 2.34, P= 0.10, partial η2 = 0.05]. There was no interaction between group and region for these three post hoc areas [F(4,180) = 0.59, P= 0.67, partial η2 = 0.01].
Figure 3.
Comparison of V1, V2, and V3 across groups. There was no significant difference in thickness between the three groups, although there was a significant main effect of region.
Follow-up Analyses: Cortical Thickness Variability and Image Quality
To evaluate the possibility that the groups had different cortical thickness estimate variability, we compared subject-wise standard deviations of the thickness estimates for the two visual ROIs across groups. While there was a significant difference in standard deviation of cortical thickness estimates across ROIs [F(1,91) = 65.27, P <0.01, partial η2 = 0.42], likely because of the larger size of retinotopic cortex than LOC, there was no significant difference in standard deviation of the thicknesses across groups [F(2,91) = 0.14, P= 0.87, partial η2 = 0.003], and no significant interaction between ROI and group [F(2,91) = 0.30, P= 0.74, partial η2 = 0.01].
To evaluate the possibility of group differences in MRI image quality that could influence estimates of cortical thickness, we compared the image intensity and standard deviation of intensity for cortical voxels in the raw structural scans across groups in two one-way ANOVAs. Image statistics of cortical voxels in the raw structural scans did not differ across groups, either in terms of image intensity [schizophrenia = 103, bipolar = 101, healthy control = 104 on an 8-bit scale; F(2,91) = 0.44, P= 0.64] or standard deviation of image intensity [schizophrenia = 19.0, bipolar = 18.2, healthy control = 19.4; F(2,91) = 1.91, P= 0.15].
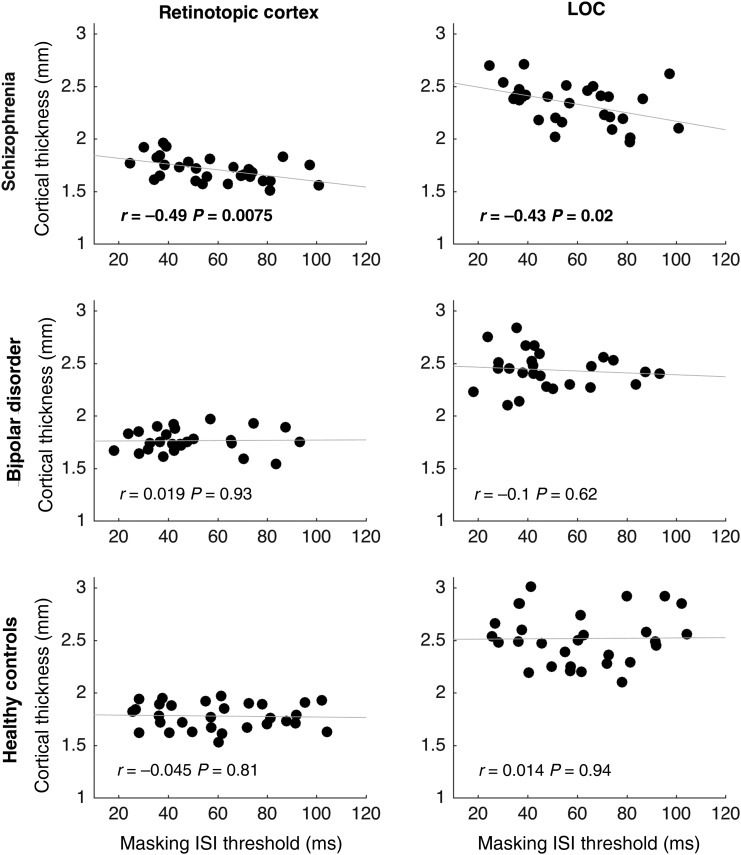
Follow-up Analysis: Masking Threshold Correlations
To investigate whether group differences in cortical thickness are related to impairments in perception, we performed exploratory correlations between cortical thickness of the visual ROIs and visual object masking performance within the three groups. There was no significant difference in object masking thresholds among the three groups [F(2,82) = 2.16, P= 0.12]. However, in the schizophrenia group, thicker cortex in both ROIs predicted better performance (i.e., lower detection thresholds) on the masking task. There was no significant relationship between the cortical thickness of either ROI and performance on the masking task in bipolar participants or healthy controls. Scatterplots and correlation statistics for all comparisons are shown in Figure 4.
Figure 4.
Correlation between cortical thickness and masking task performance, by group and region. Cortical thickness of both visual ROIs significantly predicted masking thresholds for the schizophrenia group, but there were no significant relationships in either the bipolar or control groups.
Follow-up Analyses: Demographic Differences, Symptoms, and Medication
We performed follow-up analyses to explore the relationships between cortical thickness and the two demographic variables that differed significantly between the groups: gender and personal education. The effects of gender were evaluated by adding it as a factor to the ANOVA used to compare cortical thickness across ROIs and groups. This 2 × 2 × 3 ANOVA showed no significant main effect of gender [F(1,88) = 0.56, P= 0.46], gender did not interact significantly with any other factor, and it did not change the significance of the main effects of ROI [F(1,88) = 849.95, P< 0.001] or group [F(2,88) = 4.50, P= 0.01]. Neither retinotopic cortex thickness nor LOC thickness was significantly associated with participant education [retinotopic cortex: r(94) = 0.02, P= 0.82; LOC: r(94) = −0.12, P= 0.25]. Additionally, we performed a correlation between BPRS score and cortical thickness in the two patient groups, since the two groups' BPRS scores differed significantly. There was no significant correlation within either the schizophrenia or bipolar groups between BPRS total score and the cortical thickness of either area [schizophrenia retinotopic cortex: r(33) = 0.21, P= 0.25 and LOC: r(33) = −0.01, P= 0.97; bipolar retinotopic cortex: r(30) = 0.20, P= 0.29 and LOC: r(30) = 0.11, P= 0.55].
We also correlated antipsychotic medication with cortical thickness within each patient group. Dosages were converted to chlorpromazine-equivalent units (Andreasen et al. 2010). Some participants were taking antipsychotic medications without a published conversion factor (iloperidone, loxapine, lurasidone, or asenapine) and they were not included in this analysis (9 schizophrenia and 5 bipolar disorder). There were no significant relationships between antipsychotic dosage and the cortical thickness of any ROI in either patient group. Cortical thickness was not correlated with antipsychotic dosage [retinotopic cortex: r(20) = 0.22, P= 0.36; LOC: r(20) = 0.31, P= 0.19]. The same held true for the BD group: neither thickness was correlated with antipsychotic dosage [retinotopic cortex: r(15) = 0.34, P= 0.22; LOC: r(15) = 0.19, P= 0.50].
Follow-up Analysis: Motor Cortex
Because we found group differences in the cortical thickness of both early retinotopic cortex and LOC, we conducted a follow-up analysis of a nonvisual region—motor cortex—to determine whether the similar pattern of cortical thickness differences in the two visual ROIs was a general pattern of differences that held true across all cortical areas. There was no difference in the thickness of motor cortex across groups [F(2,91) = 1.14, P= 0.33, partial η2 = 0.02; schizophrenia patients M = 2.46 mm, standard error = 0.03; bipolar M = 2.50 mm, standard error = 0.02; controls M = 2.50 mm, standard error = 0.02].
Discussion
In a targeted, hypothesis-driven analysis of two functionally defined visual brain areas in patients with schizophrenia and bipolar disorder, as well as healthy controls, we found consistent group differences in cortical thickness. In both early retinotopic cortex and LOC, patients with schizophrenia had thinner cortex than healthy controls, and bipolar patients had intermediate cortical thickness. These group differences were not explained by other demographic or clinical differences between the groups. An exploratory analysis showed a significant correlation between the thickness of the two visual ROIs and performance on a visual masking task in schizophrenia patients, but not bipolar patients or healthy controls. Follow-up analyses of early retinotopic areas V1, V2, and V3 using a topography-based localization technique showed no significant pattern of group thickness differences in those areas. The thickness of motor cortex, a control region that was localized based on anatomical features, also did not differ significantly across groups. These results suggest that the neuroanatomical properties of visual cortex are abnormal in schizophrenia and bipolar disorder, and that those abnormalities are linked to a functional visual impairment in schizophrenia.
There are two likely reasons why we found group differences in visual cortical thickness that were often missed in earlier whole-brain analyses of cortical thickness. First, while whole-brain analyses of cortical thickness are useful for exploratory studies, they suffer from poor statistical power because they require substantial corrections for multiple comparisons. Our novel ROI-based approach maximizes statistical power by constraining tests to particular regions relevant to study hypotheses. Second, earlier whole-brain analyses compared cortical thickness using purely anatomical techniques to define comparison-points on the cortical surface across subjects. That approach fails to account for known heterogeneity in the anatomical location of functionally specialized brain regions. In this study, we created individualized ROIs based on functional localizer scans so that cross-subject comparisons of thickness included the same functional areas in each participant. To our knowledge, this is the first study to use such targeted analytical techniques in these clinical populations. However, our results suggest that this targeted approach could be a fruitful source of new insights about neuroanatomical differences in other patient populations and other functional brain areas.
One intriguing result of the present study is that both LOC and early retinotopic cortex were thinner in patients with schizophrenia—and, to a lesser extent, patients with bipolar disorder—than in controls. While it is not possible to make strong claims about the regional specificity of this difference without additional functional ROIs for comparison, the finding of group differences in both LOC and early visual cortex is consistent with the idea that structural differences of visual cortex in schizophrenia may be distributed across early-, mid-, and high-level processing areas. Previous studies using functional imaging have tended to implicate LOC as dysfunctional in schizophrenia more frequently than early visual cortex (Green, Lee, et al. 2009; Green et al. 2011; Harvey et al. 2011; Silverstein et al. 2015). It is possible that early retinotopic cortex is indeed dysfunctional in severe mental illnesses such as schizophrenia, but that prior fMRI studies have used tasks that fail to elicit low-level visual dysfunction reliably. Mounting behavioral evidence for low-level visual dysfunction in patients (e.g., Must et al. 2004; Dakin et al. 2005; Yoon et al. 2009, 2010; Yang et al. 2012a, 2012b; Schallmo et al. 2013, 2015) also points to this conclusion.
The intermediate thickness of LOC and early retinotopic cortex in bipolar disorder, relative to schizophrenia patients and healthy controls, is consistent with the possibility that bipolar disorder can manifest with visual system pathology similar to schizophrenia, albeit less frequently or to a lesser degree. One intriguing question for future research is whether bipolar patients with a history of psychosis have neuroanatomical characteristics more similar to schizophrenia patients than bipolar patients without a history of psychosis. The present study had only 9 bipolar patients with a history of psychosis, so it was not possible to address this question with adequate power in the present sample.
Another intriguing issue for future research is to determine whether cortical thickness might be influenced by training or other experiential factors. There is currently substantial disagreement over the extent to which cortical thickness can be influenced by training (Thomas and Baker 2013 but see Lövdén et al. 2013). If it can, then group differences in thickness could be related to differences in perceptual experience across the lifespan among those groups.
Future research might also address several important limitations of the present study. First, patients in the study were medicated and in most cases had been on antipsychotic medication, mood stabilizers, or both, for many years. Although current antipsychotic dosages were not associated with cortical thickness in our sample, it is not possible to rule out the possibility that cortical thickness differences might be influenced by long-term effects of medication. Future studies of unmedicated first-episode or prodromal populations could address this issue. The present sample was also disproportionately male and contained too few bipolar disorder patients to permit separate analyses of different bipolar disorder subtypes (e.g., those with and without a history of psychosis). Also, the lack of a planned, functionally defined nonvisual control region in this study limits the extent to which the specificity of the observed cortical thickness differences in visual cortex can be established.
Nevertheless, the present study identifies a likely anatomical substrate for the types of functional abnormalities in visual processing that have been observed in measures of neural activity and behavior in schizophrenia. Indeed, we found that the thickness of retinotopic cortex and LOC predicted schizophrenia patients' performance on a visual object masking task. Although visual masking deficits have long been known to exist in schizophrenia, this is the first study to identify a link between neuroanatomical abnormalities and visual masking abnormalities in schizophrenia. We found no similar correlation between cortical thickness and perception in the bipolar or healthy control groups, which could suggest that individual differences in gross neuroanatomy are not a good predictor of masking performance except when they are related to particular pathophysiological abnormalities.
The specific microstructural causes of thickness abnormalities in patient visual cortex are unclear. Early studies of postmortem tissue found reduced cortical thickness but an abnormally high density of small neurons in primary visual cortex in schizophrenia, implicating reduction in neuropil (axonal and dendritic tissue) as a likely cause of reduced cortical thickness (Selemon et al. 1995; Rajkowska et al. 1998; Selemon and Goldman-Rakic 1999). Such neuropil reductions could be related to decreases in synaptic density, which have long been thought to contribute to the pathophysiology of schizophrenia (Feinberg 1982; Sekar et al. 2016). However, some studies have found no differences in cortical thickness or neural density in schizophrenia patients' postmortem primary visual cortex (Dorph-Petersen et al. 2007). It remains unclear what types of microstructural abnormalities exist in visual cortex in schizophrenia, and it is even less clear in the case of bipolar disorder.
This could present an important opportunity for the application of new methods such as high-field structural imaging capable of estimating the thickness of different cortical lamina in vivo, or recent techniques for fine-scale connectomics. The use of such advanced methods might help to shed light on specific microstructural abnormalities in visual cortex in schizophrenia and bipolar disorder. For example, if thickness differences in schizophrenia patients' visual cortex can be attributed to structural abnormalities in particular cortical layers, it will provide more precise clues about the nature of neural circuit abnormalities in the disorder. The same could be true for bipolar disorder and other serious mental illnesses. Thus, the visual system could prove a useful model system for psychopathology research as it has been in other areas of neuroscience (Albright et al. 2000; Koch and Reid 2012). Our results emphasize the likely utility of focused investigation into visual cortical structure in schizophrenia, bipolar disorder, and other mental illnesses.
Funding
This work was supported by the National Institutes of Health: R01 MH095878, to M.F.G., “Visual Tuning in Psychosis.”
Notes
The authors wish to thank Ana Ceci Meyers for help with data collection and two anonymous reviewers for helpful feedback. Some of these data were presented as a poster at the Society for Research in Psychopathology meeting in 2015.
References
- Albright TD, Jessell TM, Kandel ER, Posner MI. 2000. Neural science: a century of progress and the mysteries that remain. Neuron. 25:S1–S55. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. 2010. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson NC, Butt OH, Brainard DH, Aguirre GK. 2014. Correction of distortion in flattened representations of the cortical surface allows prediction of V1–V3 functional organization from anatomy. PLoS Comput Biol. 10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson NC, Butt OH, Datta R, Radoeva PD, Brainard DH, Aguirre GK. 2012. The retinotopic organization of striate cortex is well predicted by surface topology. Curr Biol. 22:2081–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Schechter I, Zemon V, Schwartz SG, Greenstein VC, Gordon J, Schroeder CE, Javitt DC. 2001. Dysfunction of early-stage visual processing in schizophrenia. Am J Psychiatry. 158(7):1126–1133. [DOI] [PubMed] [Google Scholar]
- Butler PD, Silverstein SM, Dakin SC. 2008. Visual perception and its impairment in schizophrenia. Biol Psychiatry. 64:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler PD, Zemon V, Schechter I, Saperstein AM, Hoptman MJ, Lim KO, Revheim N, Silipo G, Javitt DC. 2005. Early-Stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 62(5):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Bidwell LC, Holzman PS. 2005. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr Res. 74:271–281. [DOI] [PubMed] [Google Scholar]
- Chkonia E, Roinishvili M, Reichard L, Wurch W, Puhlmann H, Grimsen C, Herzog MH, Brand A. 2012. Patients with functional psychoses show similar visual backward masking deficits. Psychiatry Res. 198:235–240. [DOI] [PubMed] [Google Scholar]
- Dakin S, Carlin P, Hemsley D. 2005. Weak suppression of visual context in chronic schizophrenia. Curr Bio. 15(20): R822–R824. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno M. 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 194:179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno M. 1993. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 5:162–176. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT et al. . 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- DeYoe E, Carman G, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. 1996. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A. 93:2382–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Fenstermacher E, Salat D, Wolk D, Maguire RP, Desikan RS, Pacheco J, Quinn BT, van der Kouwe A, Greve D et al. . 2008. Detection of cortical thickness correlates of cognitive performance: reliability across MRI scan sessions, scanners, and field strengths. Neuroimage. 39:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. 2007. Primary visual cortex volume and total neuron number are reduced in schizophrenia. J Comp Neurol. 501:290–301. [DOI] [PubMed] [Google Scholar]
- Engel SA, Glover G, Wandell B. 1997. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 7:181–192. [DOI] [PubMed] [Google Scholar]
- Feinberg I. 1982. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence? J Psychiatr Res. 17:319–334. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. 1991. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1:1–47. [DOI] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J, Benjamin L. 1996. Structured clinical interview for DSM-IV axis II personality disorders. New York: State Psychiatric Institute. [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. 1997. Structured clinical interview for DSM-IV axis I disorders—Patient Edition. New York: Biometrics Research Department, New York State Psychiatric Institute. [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Van Der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D et al. . 2004. Automatically parcellating the human cerebral cortex. Cereb Cortex. 14:11–22. [DOI] [PubMed] [Google Scholar]
- Frank SM, Reavis EA, Greenlee MW, Tse PU. 2016. Pretraining cortical thickness predicts subsequent perceptual learning rate in a visual search task. Cereb Cortex. 26(3):1211–1220. [DOI] [PubMed] [Google Scholar]
- Green MF, Butler PD, Chen Y, Geyer MA, Silverstein S, Wynn JK, Yoon JH, Zemon V. 2009. Perception measurement in clinical trials of schizophrenia: promising paradigms from CNTRICS. Schizophr Bull. 35:163–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Hellemann G, Horan WP, Lee J, Wynn JK. 2012. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch Gen Psychiatry. 69:1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Lee J, Cohen MS, Engel SA, Korb AS, Nuechterlein KH, Wynn JK, Glahn DC. 2009. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch Gen Psychiatry. 66:1295–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Lee J, Wynn JK, Mathis KI. 2011. Visual masking in schizophrenia: overview and theoretical implications. Schizophr Bull. 37:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. 1960. A rating scale for depression. J Neurol Neurosurg Psychiatry. 23:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey PO, Lee J, Cohen MS, Engel SA, Glahn DC, Nuechterlein KH, Wynn JK, Green MF. 2011. Altered dynamic coupling of lateral occipital complex during visual perception in schizophrenia. Neuroimage. 55:1219–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahshan C, Wynn JK, McCleery A, Glahn DC, Altshuler LL, Green MF. 2014. Cross-diagnostic comparison of visual processing in bipolar disorder and schizophrenia. J Psychiatr Res. 51:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen J, Alemán-Gómez Y, Schnack H, Balaban E, Pina-Camacho L, Alfaro-Almagro F, Castro-Fornieles J, Otero S, Baeza I, Moreno D et al. . 2014. Cortical morphology of adolescents with bipolar disorder and with schizophrenia. Schizophr Res. 158:91–99. [DOI] [PubMed] [Google Scholar]
- Javitt DC. 2009. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 5:249–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Freedman R. 2015. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz JT, Butler PD, Schecter I, Silipo G, Javitt DC. 2009. Seeing the world dimly: the impact of early visual deficits on visual experience in schizophrenia. Schizophr Bull. 35:1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch C, Reid RC. 2012. Neuroscience: observatories of the mind. Nature. 483(7390):397–398. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW et al. . 2003. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 60:878–888. [DOI] [PubMed] [Google Scholar]
- Larsson J, Heeger DJ. 2006. Two retinotopic visual areas in human lateral occipital cortex. J Neurosci. 26:13128–13142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Altshuler L, Glahn DC, Miklowitz DJ, Ochsner K, Green MF. 2013. Social and nonsocial cognition in bipolar disorder and schizophrenia: relative levels of impairment. Am J Psychiatry. 170(3):334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Wenger E, Mårtensson J, Lindenberger U, Bäckman L. 2013. Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev. 37(9 Pt B):2296–2310. [DOI] [PubMed] [Google Scholar]
- Lyoo IK, Sung YH, Dager SR, Friedman SD, Lee JY, Kim SJ, Kim N, Dunner DL, Renshaw PF. 2006. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 8:65–74. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, Benson RR, Kwong KK, Jiang H, Kennedy WA, Ledden PJ, Brady TJ, Rosen BR, Tootell RB. 1995. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proc Natl Acad Sci USA. 92:8135–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Must A, Janka Z, Benedek G, Kéri S. 2004. Reduced facilitation effect of collinear flankers on contrast detection reveals impaired lateral connectivity in the visual cortex of schizophrenia patients. Neurosci Lett. 357(2):131–134. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP et al. . 2005. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 15:708–719. [DOI] [PubMed] [Google Scholar]
- Padmanabhan JL, Tandon N, Haller CS, Mathew IT, Eack SM, Clementz BA, Pearlson GD, Sweeney JA, Tamminga CA, Keshavan MS. 2014. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders. Schizophr Bull. 41:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pol HEH, van Baal GCM, Schnack HG, Brans RGH, van der Schot AC, Brouwer RM, van Haren NEM, Lepage C, Collins DL, Evans AC et al. . 2012. Overlapping and segregating structural brain abnormalities in twins with schizophrenia or bipolar disorder. Arch Gen Psychiatry. 69:349–359. [DOI] [PubMed] [Google Scholar]
- Prins N, Kingdom F. 2009. Palamedes: Matlab routines for analyzing psychophysical data. Available from: URL http://www.palamedestoolbox.org.
- Rajkowska G, Selemon LD, Goldman-Rakic PS. 1998. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 55(3):215–224. [DOI] [PubMed] [Google Scholar]
- Reuter M, Tisdall MD, Qureshi A, Buckner RL, van der Kouwe AJW, Fischl B. 2015. Head motion during MRI acquisition reduces gray matter volume and thickness estimates. Neuroimage. 107:107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH et al. . 2010. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 68:41–50. [DOI] [PubMed] [Google Scholar]
- Rimol LM, Nesvåg R, Hagler DJ, Bergmann Ø, Fennema-Notestine C, Hartberg CB, Haukvik UK, Lange E, Pung CJ, Server A et al. . 2012. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 71:552–560. [DOI] [PubMed] [Google Scholar]
- Schallmo MP, Sponheim SR, Olman CA. 2013. Abnormal contextual modulation of visual contour detection in patients with schizophrenia. PLoS ONE 8(6):e68090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallmo MP, Sponheim SR, Olman CA. 2015. Reduced contextual effects on visual contrast perception in schizophrenia and bipolar affective disorder. Psychol Med. 45(16):3527–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz CC, Koch K, Wagner G, Roebel M, Schachtzabel C, Gaser C, Nenadic I, Reichenbach JR, Sauer H, Schlösser RGM. 2010. Reduced cortical thickness in first episode schizophrenia. Schizophr Res. 116:204–209. [DOI] [PubMed] [Google Scholar]
- Sekar A, Bialas AR, de Rivera H, Davis A, Hammond TR, Kamitaki N, Tooley K, Presumey J, Baum M, Van Doren V et al. . 2016. Schizophrenia risk from complex variation of complement component 4. Nature. 530(7589):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. 1999. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 45:17–25. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. 1995. Abnormally high neuronal density in the schizophrenic cortex: a morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 52:805–818. [DOI] [PubMed] [Google Scholar]
- Silverstein SM, Harms MP, Carter CS, Gold JM, Keane BP, MacDonald A, Daniel Ragland J, Barch DM. 2015. Cortical contributions to impaired contour integration in schizophrenia. Neuropsychologia. 75:469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprooten E, Papmeyer M, Smyth AM, Vincenz D, Honold S, Conlon GA, Moorhead TWJ, Job D, Whalley HC, Hall J et al. . 2013. Cortical thickness in first-episode schizophrenia patients and individuals at high familial risk: a cross-sectional comparison. Schizophr Res. 151:259–264. [DOI] [PubMed] [Google Scholar]
- Thomas C, Baker CI. 2013. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage. 73:225–236. [DOI] [PubMed] [Google Scholar]
- Ventura J, Green MF, Shaner A, Liberman RP. 1993. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters.” Int J Methods Psychiatr Res. 3:221–224. [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. 1998. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P). Psychiatry Res. 79:163–173. [DOI] [PubMed] [Google Scholar]
- Wagstyl K, Ronan L, Goodyer IM, Fletcher PC. 2015. Cortical thickness gradients in structural hierarchies. Neuroimage. 111:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Tadin D, Glasser DM, Hong SW, Blake R, Park S. 2012. a. Visual context processing in bipolar disorder: a comparison with schizophrenia. Front Psychol. 4(569):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Tadin D, Glasser DM, Hong SW, Blake R, Park S. 2012. b. Visual context processing in schizophrenia. Clin Psychol Sci. 1(1):5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Maddock RJ, Rokem A, Silver MA, Minzenberg MJ, Ragland JD, Carter CS. 2010. GABA concentration is reduced in visual cortex in schizophrenia and correlates with orientation-specific surround suppression. J Neurosci. 30(10):3777–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Rokem AS, Silver MA, Minzenberg MJ, Ursu S, Ragland JD, Carter CS. 2009. Diminished orientation-specific surround suppression of visual processing in schizophrenia. Schizophr Bull. 35(6):1078–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. 1978. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 133:429–435. [DOI] [PubMed] [Google Scholar]