Abstract
Previous studies have shown that males and females differ in personality and gender differences have also been reported in brain structure. However, effects of gender on this “personality–brain” relationship are yet unknown. We therefore investigated if the neural correlates of personality differ between males and females. Whole brain voxel-based morphometry was used to investigate the influence of gender on associations between NEO FFI personality traits and gray matter volume (GMV) in a matched sample of 182 males and 182 females. In order to assess associations independent of and dependent on gender, personality–GMV relationships were tested across the entire sample and separately for males and females. There were no significant correlations between any personality scale and GMV in the analyses across the entire sample. In contrast, significant associations with GMV were detected for neuroticism, extraversion, and conscientiousness only in males. Interestingly, GMV in left precuneus/parieto-occipital sulcus correlated with all 3 traits. Thus, our results indicate that brain structure–personality relationships are highly dependent on gender, which might be attributable to hormonal interplays or differences in brain organization between males and females. Our results thus provide possible neural substrates of personality–behavior relationships and underline the important role of gender in these associations.
Keywords: NEO-FFI, voxel brain morphometry, left precuneus/parieto-occipital sulcus, hormonal influence, extreme personality disorders
Introduction
Personality is what makes every human unique, as it denotes individual differences in behaviors, cognition, and emotion, which are stable over time and across situations (Mischel 2004). It has been shown that personality affects various domains in human life such as job performance (Rothmann and Coetzer 2003), social and political attitude (Riemann et al. 1993), quality and stability of social relationships (Asendorpf and Wilpers 1998), as well as risk for mental disorders (Costa and McCrae 1992a; Miller et al. 2001). One of the most widely recognized personality models is the NEO Five Factor Inventory (NEO FFI; Costa and McCrae 1992b), consisting of the dimensions neuroticism, extraversion, openness, agreeableness, and conscientiousness. Previous studies have indicated that there are gender differences in neuroticism and agreeableness, with women scoring higher on these 2 traits than men (Costa et al. 2001; Chapman et al. 2007; Weisberg et al. 2011).
Addressing the biological basis of personality, several voxel-based morphometry (VBM) studies have attempted to characterize the neural architecture of personality. For example, DeYoung et al. (2010) suggested different brain systems that might correlate with the traits of the NEO-FFI (DeYoung et al. 2010), but this view has been challenged by others (cf. Hu et al. 2011; Koelsch et al. 2013), illustrating the currently inconsistent and heterogeneous (with respect to both the associated regions and the direction of association) literature. Most importantly, the (generally rather low) sample size varies considerably between studies, and there is a substantial heterogeneity with regard to age and in particular gender distribution. For example, Barrós-Loscertales et al. (2006) only investigated males, Van Schuerbeck et al. (2011) only females, while others investigated unbalanced samples of males and females (i.e. Liu et al. 2013; Yamasue et al. 2008). It has, however, been shown that beyond mere brain size, males and females differ in brain structure. In particular, gender differences have been reported in gray matter volume (GMV) (Luders et al. 2009), cortical thickness (Im et al. 2006), and structural connectivity (Ingalhalikar et al. 2014). Given those gender differences in brain structure as well as the fact that males and females differ also in traits such as the NEO-FFI (Costa et al. 2001; Chapman et al. 2007; Weisberg et al. 2011), it is likely that gender also has an influence on the neural correlates of personality. However, effects of gender on personality/brain relationships have rarely been investigated to date. Rather, most studies investigating personality in association with brain structure treated gender only as covariate of no interest (Omura et al. 2005; Gardini et al. 2009; Cremers et al. 2011; Kapogiannis et al. 2013; Lu et al. 2014).
The aim of the current study was thus to investigate brain regions associated with personality across both genders as well as to assess a potential sexual dimorphism of the relationship between personality traits and local GMV. Importantly, since personality traits in their extreme forms are considered as vulnerability factors of personality and mood disorders (Costa and McCrae 1992a), which show important differences in prevalence for males and females (Afifi 2007), a better knowledge of the underlying neural correlates of personality and of potential gender differences of these should also contribute to a better understanding of those clinical conditions.
Materials and Methods
Subjects
Participants were selected from the data provided through the Human Connectome Project, WU-Minn Consortium, in the current “S500” release (HCP, http://www.humanconnectome.org, Van Essen et al. 2012, Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; and by the McDonnell Center for Systems Neuroscience at Washington University. Analyses of the HCP data were approved by the ethics committee of the Heinrich Heine University Düsseldorf.
The HCP sample is composed of monozygotic (MZ) and dizygotic (DZ) twins (at the moment of the selection of the subjects: 34 MZ males, 92 MZ females, 51 DZ males, 93 DZ females) and not-twins (132 males not-twins and 140 females not-twins). The category of the not-twins includes siblings of twins, just siblings, and only-children (including those that have a not yet scanned sibling but not twin).
Given this structure, we paid particular attention to select a well-matched sample from this data that is as large as possible while at the same time controlling for possible effects of heritability, age, and education. Evidently, we first selected all participants from the HCP sample for whom MRI images and personality data were available. Out of this sample, we then selected groups of males and females, respectively, which were closely matched with regard to their age and years of education. Importantly, we included only participants who met the following constraints to control for family structure and effects of premature birth (which is the norm in twins): only 1 subject per monozygotic twin pair was selected due to the high genetic similarity to the co-twin and the same amount of monozygotics was chosen for males and females, while for dizygotic pairs both twins were included since they are genetically equal to siblings. Although it would have been more straightforward to use only unrelated individuals, this would have extremely reduced the sample size and, consequently, the statistical power.
Based on these criteria, a sample of 182 males (age 22–36 years, mean 29.0 ± 3.4, education 14.7 ± 1.8) and 182 females (age 22–35 years, mean 29.2 ± 3.5, mean of years of education 14.7 ± 1.9) was selected. The percentage of twins and non-twins participants did not differ by gender (X21 = 2.2, n.s.). Moreover, no significant gender differences were detected for age (t362 = −0.47, ns.) and years of education (t362 = −0.25, ns.).
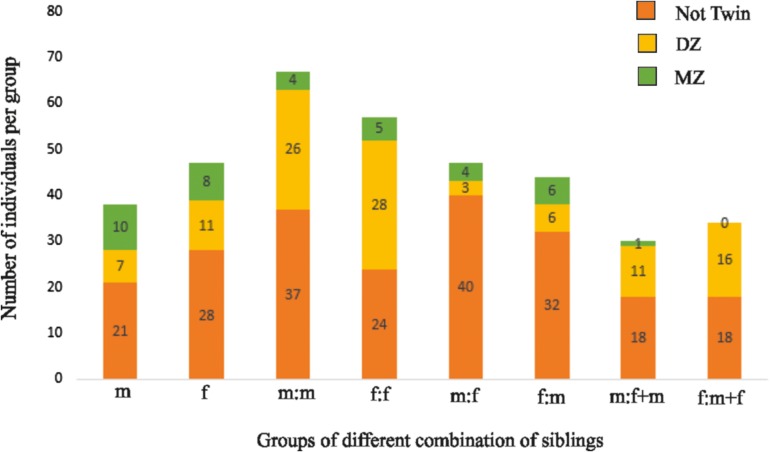
Figure 1 illustrates the distribution of siblings in the male and female sample. Specifically, 17 female–female dizygotic pairs and 27 females without the dizygotic twins were included in the female (for a total of 61 dizygotic females) and 15 pairs of male–male dizygotic twins and 17 dizygotic males without the dizygotic twin in the male sample (for a total of 47 dizygotic males). Furthermore, the sample consists of 19 monozygotic female individuals and 19 monozygotic male individuals as well as 102 non-twin females and 116 non-twin males. Therefore 80 individuals with a twin status and 102 individuals with a non-twin status formed the female group, while 66 individuals with a twin status and 116 individuals with a non-twin status formed the male group.
Figure 1.
Distribution of siblings in the male and female sample with their relative zygosity (Not Twin, Dizygotic, Monozygotic). Groups’ abbreviations: m (males with no siblings); f (females with no siblings); m:m (males who have at least another male sibling); f:f (females with at least another female sibling); m:f (males with at least a female sibling); f:m (females with at least a male sibling); m:f+m (males with at least a male and a female sibling); f:m+f (females with at least a male and a female sibling).
The 364 subjects belonged to a total of 200 different families, distributed as follows: 85 families were composed by just 1 individual, 75 families by 2 individuals, 31 families by 3 individuals, 8 families by 4 individuals, and 1 family by 5 individuals. Thus, 85 subjects were unrelated (38 males and 47 females) while 279 had at least one other subject in the sample that was related to him/her (144 males and 135 females). Thirty eight of the males and 47 of the females have no siblings; 67 of the males and 44 of the females have at least 1 male sibling, 47 of the males and 57 of the females have at least 1 female sibling; 30 of the males and 34 of the females have at least a male and a female sibling.
Questionnaire
Subjects completed the English version of the NEO Five Factor Inventory (NEO FFI, McCrae and Costa 2004). The NEO FFI consists of 60 items in the form of statements, 12 for each of the 5 factors (Neuroticism, Extraversion, Openness, Agreeableness, and Conscientiousness).
MR Imaging and Pre-processing
3D structural T1w MRI scans were acquired (Glasser et al. 2013) on a Siemens Skyra 3 T scanner using a 32-channel head coil and a 3D MPRAGE sequence (T1w MPR1, voxel size = 0.7 × 0.7 × 0.7 mm, FoV = 224 × 224 mm, matrix = 320, 256 sagittal slices in a single slab, TR = 2400 ms, echo time; TE = 2.14 ms, TI = 1000 ms, flip angle 8◦).
Data preprocessing was performed with SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK, http://www.fil.ion.ucl.ac.uk/spm/) and the VBM8 toolbox (http://www.neuro.uni-jena.de/vbm8), running under Matlab R2014a (Mathworks, Natick, MA). Structural images were normalized using the DARTEL algorithm (Ashburner 2007) to the ICBM-152 template using both affine and non-linear spatial normalization, bias-field corrected and segmented into gray matter, white matter, and cerebrospinal fluid tissues. The normalized gray matter segments were then linearly and non-linearly modulated. Finally, images were smoothed with an isotropic Gaussian kernel (full-width-half-maximum = 8 mm).
VBM Analyses: Relationships Between GMV and Personality
We performed multiple regression analysis in SPM8 using the voxel-wise GMV as dependent variable and the scores of the 5 factors of the NEO FFI as covariates of interest. Given the collinearity of the NEO FFI scores (Table 1), each factor was assessed by a separate GLM. For the analyses on the entire sample (364 participants) we included age, total brain volume (TBV), and gender as covariates of no interest. For the within-gender analyses only age and TBV were added. Inference was performed using threshold-free cluster enhancement (TFCE; Smith and Nichols 2009). The critical threshold to control the family-wise error at P < 0.05 was based on a non-parametric permutation framework (extend threshold of 50 voxels). In order to identify regions where GMV was correlated with more than one personality trait, conjunctions were performed using the minimum statistic (Nichols et al. 2005) and multiple linear regression analyses were conducted to examine how their predictive power on the correlation to the GMV was shared among them. All activations are reported in MNI space and were anatomically localized by using the SPM anatomy toolbox 2.1 (Eickhoff et al. 2005, 2007).
Table 1.
Intercorrelations (Pearson's r) among the 5 personality factors in males (n = 182), females (n = 182) and across the overall sample (n = 364)
| Neuroticism | Extraversion | Openness | Conscientiousness | Agreeableness | ||
|---|---|---|---|---|---|---|
| Neuroticism | Males | — | −0.5140a/ | 0.1001/ | 0.3996a/ | −0.3260a/ |
| Females | −0.3190a/ | −0.0204/ | −0.4287a/ | −0.3565a/ | ||
| Overall | −0.416a | 0.028 | −0.383a | −0.320a | ||
| Extraversion | Males | — | — | 0.0319/ | 0.4490a/ | 0.3398a/ |
| Females | 0.0741/ | 0.2624a/ | 0.2378a/ | |||
| Overall | 0.055 | 0.351a | 0.285a | |||
| Openness | Males | — | — | — | −0.1888a/ | 0.2009a/ |
| Females | −0.0557/ | 0.1299/ | ||||
| Overall | −0.136a | 0.157a | ||||
| Conscientiousness | Males | — | — | — | — | 0.2802a/ |
| Females | 0.1414/ | |||||
| Overall | 0.225a | |||||
| Agreeableness | — | — | — | — | — |
aMarks significance at P < 0.05.
We also calculated cortical thickness and cortical surface area in order to test whether the regions found in the VBM results were also detected in these other structural analyses. The description of method and results can be found in the supplement material.
Follow-up: Gender Differences in Volume–Personality Association
For regions where a significant correlation in either males or females was found, we further investigated if a significant difference in the correlation could be found between males and females. Therefore, Pearson correlations (r) between GMV and each personality score were calculated, separately for males and females, transformed into Fishers Z scores and compared between groups (Kenny 1987). For significant (P < 0.05) group differences we estimated the effect sizes by using Cohen's q measure (Cohen 1988) (q < 0.1: no effect, 0.1 < q < 0.3: small effect, 0.3 < q < 0.5: intermediate effect, q > 0.5: large effect).
Follow-up: Functional Decoding
All significant clusters were in a last step functionally characterized using the Behavioral Domain meta-data from the BrainMap database (http://www.brainmap.org; Fox and Lancaster 2002; Laird et al. 2009, 2011). In particular, we identified those meta-data labels (describing the task that was performed [paradigm class] as well as the computed contrast [behavioral domain]) that were significantly more likely than chance to result in activation of a given cluster (Henson 2005; Poldrack 2006). That is, functions were attributed to the identified morphological effects by quantitatively determining which types of experiments are associated with activation in the respective region.
Results
Gender Differences and Factors’ Correlations in NEO-FFI Scores
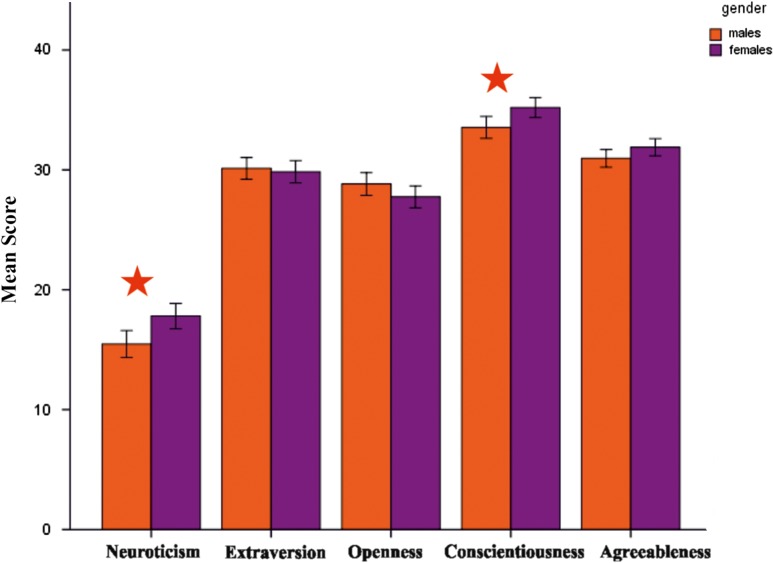
Comparison of the 5 personality scores between men and women (see Fig. 2) revealed a significant difference for neuroticism (t362 = −3.02; P < 0.05, d = 0.31) and conscientiousness (t362 = −2.7, P < 0.05, d = 0.29). For openness (t362 = 1.63, ns.), agreeableness (t362 = −1.79, ns) and extraversion (t362 = 0.43, ns) no significant gender differences were detected.
Figure 2.
Mean scores of the 5 NEO FFI personality scales (neuroticism, extraversion, openness, conscientiousness, and agreeableness) separately for males (orange) and females (violet); error bars represent standard errors. Significant differences between males and females, marked by a star, were found for neuroticism and conscientiousness.
Correlations between factors were calculated separately for males and females and across the whole sample using SPSS 20 (IBM Corp. Released, 2011). Most of them were significant at P < 0.05 (Bonferroni-corrected) for both males and females and across the entire sample; however, openness was found to be independent of most of the other factors, especially in the female sample (see Table 1). Furthermore, neuroticism was the only factor correlating negatively with most of the others: with agreeableness, conscientiousness, and extraversion in men and in the mixed sample, in women also with openness.
Association to GMV
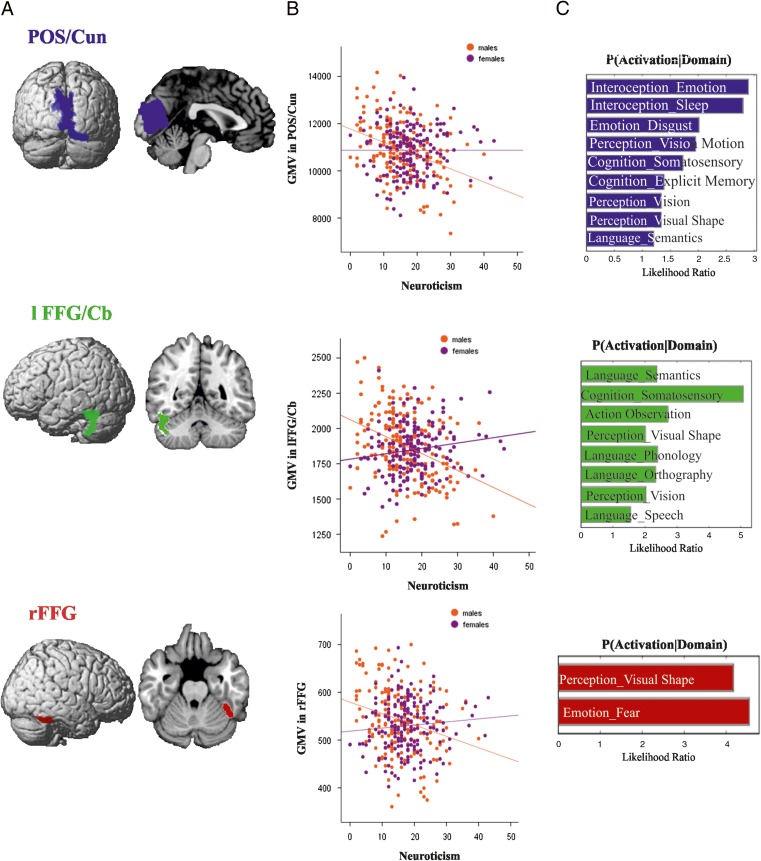
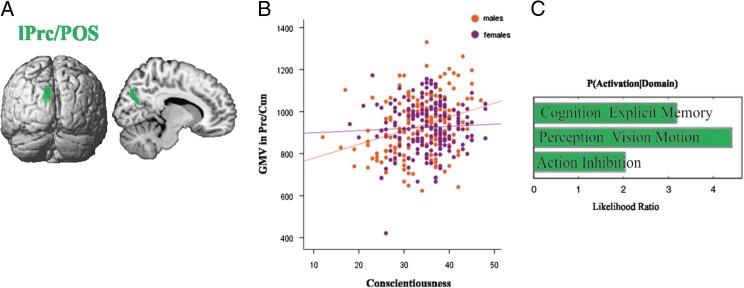
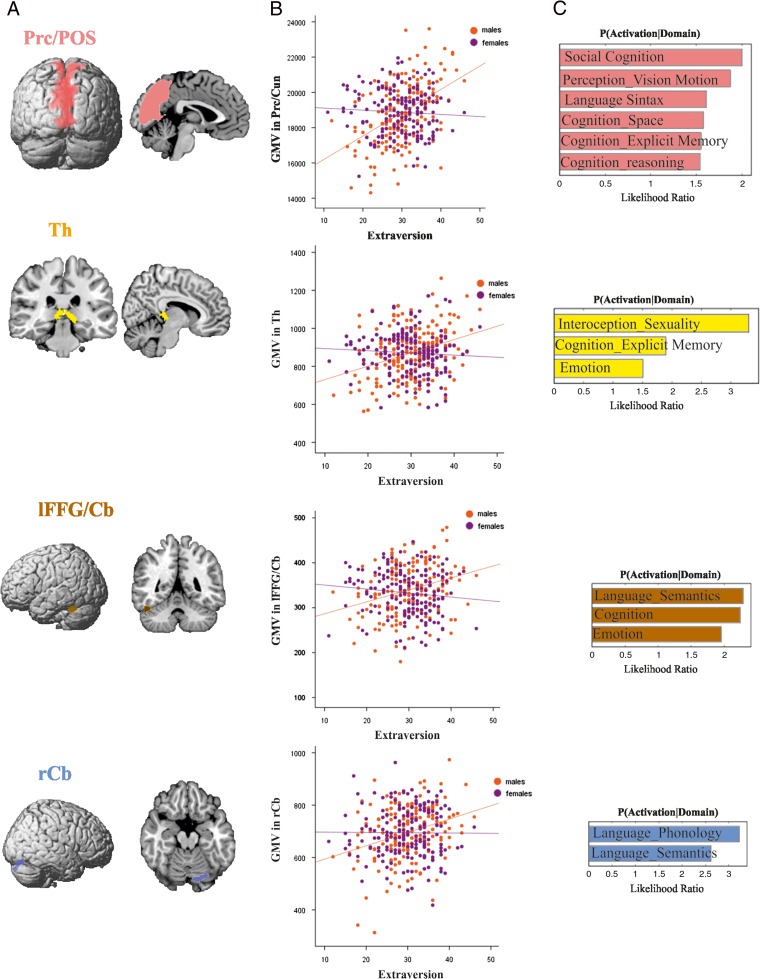
Across the entire sample, no significant correlation was found between any personality factor and GMV (controlling for age, TBV, and gender). Likewise, our analyses revealed no significant relationships between any of the 5 personality factors and GMV in females (controlling for age and TBV). However, we found significant (P < 0.05, FWE corrected) correlations in males. Negative correlations were found between neuroticism and GMV of bilateral parieto-occipital sulcus/cuneus (POS/Cun) extending into precuneus, left mid fusiform gyrus extending into cerebellum (lFFG/Cb), and right mid fusiform gyrus (rFFG). Positive correlations were found between extraversion and GMV of bilateral precuneus and parieto-occipital sulcus (Prc/POS), bilateral thalamus (Th), left mid FFG extending into the cerebellum (lFFG/Cb) and right cerebellum (rCb). Conscientiousness was positively correlated with GMV of left precuneus and parieto-occipital sulcus (Prc/POS) (Table 2and Fig. 3–5).
Table 2.
Regions where GMV was found to be correlated with neuroticism, extraversion, and conscientiousness in the male sample
| Regions (size in voxels) | X | Y | Z | Cytoarchitectonic assignments |
|---|---|---|---|---|
| Neuroticism | ||||
| Parieto-occipital sulcus/ Cuneus (6053 voxels) | 0 | −78 | 13 | left hOc1 (left hOc3d; right hOc1; left hOc1) |
| Left fusiform gyrus/ cerebellum (1027 voxels) | −46 | −49 | −28 | left lobule VIIa Crus I (left FG2; left lobule VIIa crus II; left lobule VI) |
| Right fusiform gyrus (297 voxels) | 42 | −48 | −25 | right FG2 (right lobule VI; right lobule VIIa crus I) |
| Extraversion | ||||
| Precuneus/parieto-occipital sulcus (11.001 voxels) | −1 | −69 | 33 | left hOc1 (right hOc1; right Area 5 L; left hOc3d) |
| Thalamus (621 voxels) | 9 | −31 | 0 | right Th-temporal (right Th-Parietal; left Th-Prefrontal; right Subiculum) |
| Left fusiform gyrus/Cerebellum (213 voxels) | −46 | −48 | −28 | left lobule VIIa crus I (left FG2; left lobule VI) |
| Right cerebellum (444 voxels) | 13 | −81 | −21 | right lobule VIIa crus I (right lobule VI; right hOc4v; right hOc3v) |
| Conscientiousness | ||||
| Left precuneus/parieto-occipital sulcus (491 voxels) | −9 | −73 | 30 | left hOc4d (left hOc3d; left Area 7 P; left Area 7 M) |
x, y and z coordinates denote the center of gravity in MNI space.
Reference for probabilistic cytoarchitectonic mapping of: hOc1 (Amunts et al. 2000); hOc3d, hOc4d (Kujovic et al. 2013); lobule VIIa crusI and lobule VI (Diedrichsen et al. 2009); FG2 (Caspers et al. 2013); Area 5 L, 7 P, 7 M (Scheperjans et al. 2008); hOc3v and hOc4v (Rottschy et al. 2007); Th-temporal, Th-parietal, Th-prefrontal, and Subiculum (Behrens et al. 2003) .
Figure 3.
Neural correlates of neuroticism in males. (A) Whole brain VBM results revealing negative relationships between neuroticism and GMV of POS/Cun, lFFG/Cb, and rFFG in males. (B) Correlations between neuroticism and GMV in POS/Cun, lFFG/Cb, and rFFG separately for males and females, with negative correlations in males but no correlation in females. (C) Functional decoding of the regions POS/Cun, lFFG/Cb, and rFFG; behavioral domains at P < 0.05 uncorrected for multiple comparison.
Figure 5.
Neural correlates of conscientiousness in males. (A) Whole brain VBM results revealing positive relationships between conscientiousness and GMV of Prc/POS in males. (B) Correlations between extraversion and GMV in Prc/POS separately for males and females, with positive correlations in males but no correlation in females. (C) Functional decoding of the regions Prc/POS; behavioral domains at P < 0.05 uncorrected for multiple comparison.
Given that in the male sample, we found a significant relationship of GMV in the region of the left Prc/POS with conscientiousness, neuroticism, and extraversion, we assessed a potential convergence between these effects by a minimum conjunction, confirming an association of a left Prc/POS with all 3 NEO FFI scores (Table 3, Fig. 6). The multiple regression model with all 3 predictors produced R² = 0.132, F(3, 178) = 9.0, P < 0 .001; specifically, conscientiousness was the only significant predictor of the model (t178 = 2.3, P < 0.05), while extraversion (t178 = 1.8, n.s.) and neuroticism (t178 = −1.4, n.s.) did not explain any additional variance.
Table 3.
Results of the three-way conjunction across the analyses of neuroticism, extraversion, and conscientiousness in the male sample
| Regions (size in voxels) | X | Y | Z | Cytoarchitectonic assignments |
|---|---|---|---|---|
| Neuroticism, Extraversion and Conscientiousness | ||||
| Left precuneus/parieto-occipital sulcus (477 voxels) | −9 | −73 | 30 | left hOc4d (left hOc3d) |
x, y and z coordinates denote the center of gravity in MNI space.
Reference for probabilistic cytoarchitectonic mapping of: hOc4d (Kujovic et al. 2013).
Figure 6.
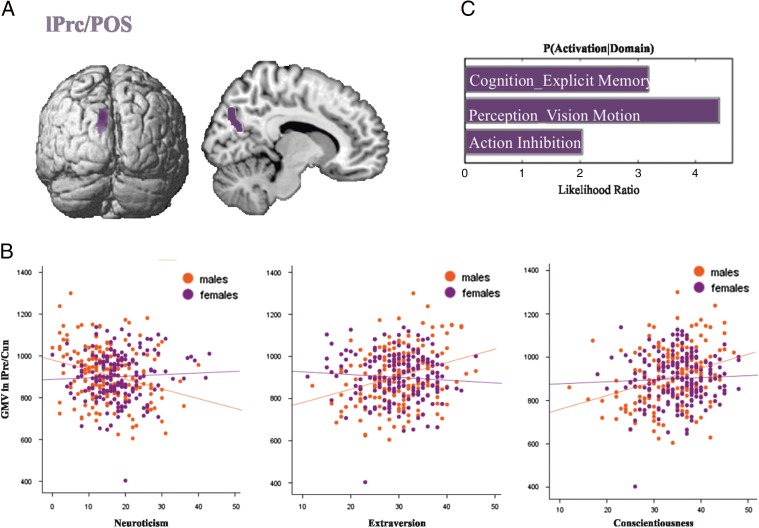
Three-way conjunction across the results of neuroticism, extraversion, and conscientiousness. (A) Results of the minimum conjunction analysis across the 3 traits revealing a cluster in lPrc/POS where GMV significantly correlated with all 3 personality scores in the male but not female sample. (B) Individual correlations between neuroticism, extraversion, and conscientiousness and GMV in lPrc/POS separately for males and females, with negative correlations in males but no correlation in females. (C) Behavioral characterization of lPrc/POS at P < 0.05, uncorrected for multiple comparison.
Another minimum conjunction was computed between the whole brain VBM results of neuroticism and extraversion revealing a further overlap in left FFG and right cerebellum (Table 4, Fig. 7).
Table 4.
Results of the two-way conjunction across the GMV results of Neuroticism and Extraversion
| Regions (size in voxels) | X | Y | Z | Cytoarchitectonic assignments |
|---|---|---|---|---|
| Neuroticism and extraversion | ||||
| Parieto-occipital sulcus/ Cuneus (4082 voxels) | −2 | −76 | 19 | left hOc1 (right hOc1, left hOc3d left hOc2) |
| Left fusiform gyrus/cerebellum (189 voxels) | −46 | −49 | −27 | left lobule VIIa crus I (left FG2, left lobule VI) |
| Right cerebellum (166 voxels) | 16 | −78 | −19 | right lobule VI (right hOc4v, right lobule VIIa crus I, in right hOc3v) |
x, y and z coordinates denote the center of gravity in MNI space.
Reference for probabilistic cytoarchitectonic mapping of: hOc1 and hOc2 (Amunts et al. 2000); hOc3d (Kujovic et al. 2013); lobule VIIa crusI and lobule VI (Diedrichsen et al. 2009); FG2 (Caspers et al. 2013); hOc3v and hOc4v (Rottschy et al. 2007).
Figure 7.
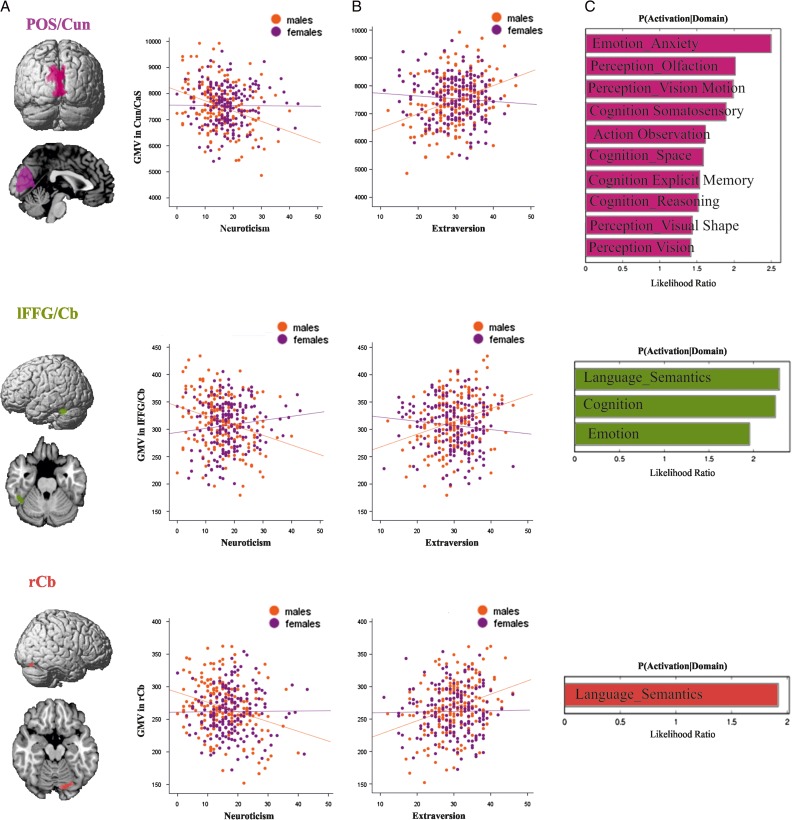
Two-way conjunction across the results of neuroticism and extraversion. (A) Results of the minimum conjunction analysis between these 2 traits revealing bilateral POS/Cun, lFFG, and rCb. (B) Individual correlations between neuroticism and extraversion and GMV in POS/Cun, lFFG/Cb, and rCb separately for males and females, with negative correlations in males but no correlation in females. (C) Behavioral domains significantly associated with POS/Cun, lFFG/Cb, and rCb at P < 0.05 uncorrected for multiple comparison.
Follow-up: Gender Differences in GMV–Personality Association
All clusters that showed a significant association with one of the NEO FFI scores in males (cf. Fig. 3B, 4B, 5B ) also showed a significant gender-difference in the correlation between GMV and personality scores (Table 5).
Figure 4.
Neural correlates of extraversion in males. (A) Whole brain VBM results revealing positive relationships between extraversion and GMV of Prc/POS, Th, lFFG/Cb, and rCb in males. (B) Correlations between extraversion and GMV in Prc/POS, Th, lFFG/Cb, and rCb separately for males and females, with positive correlations in males but no correlation in females. (C) Functional decoding of the regions Prc/POS, Th, lFFG/Cb, and rCb; behavioral domains at P < 0.05 uncorrected for multiple comparison.
Table 5.
Gender differences in GMV–Personality associations in regions individually correlating with neuroticism (POS/Cun, lFFG/Cb, rFFG), with extraversion (Prc/POS, Th, lFFG/Cb, rCb), and with conscientiousness (lPrc/POS)
| rmales | rfemales | Z value of gender comparison of r: (Zmales–Zfemales)/σ(Zmales–Zfemales) | Cohen's q of gender difference | |
|---|---|---|---|---|
| Neuroticism | ||||
| POS/Cun | −0.27a | 0.01 | −2.7a | 0.31 |
| lFFG/Cb | −0.36a | 0.15 | −4.8a | 0.53 |
| rFFG | −0.38a | 0.07 | −4.4a | 0.38 |
| Extraversion | ||||
| Prc/POS | 0.29a | −0.05 | 3.3a | 0.35 |
| Th | 0.32a | −0.07 | 3.8a | 0.4 |
| lFFG/Cb | 0.46a | −0.11 | 5.7a | 0.6 |
| rCb | 0.31a | −0.01 | 3.1a | 0.3 |
| Conscientiousness | ||||
| lPrc/POS | 0.3a | 0.05 | 2.9a | 0.3 |
aMarks a significant correlation coefficient or gender difference.
When comparing the GMV and NEO-FFI associations for the lPrc/POS, that is, the core region identified in the three-way conjunction (relationship to conscientiousness, neuroticism, and extraversion) between males and females, we also found a significant gender difference for all 3 scales (Table 6, Fig. 6B). Likewise, assessing the clusters found in two-way conjunctions across NEO FFI scores (POS/Cun, left FFG, and right cerebellum) also confirmed that the association between the GMV of these regions and the respective personality trait was significantly stronger in males (Table 6, Fig. 7B).
Table 6.
Gender differences in GMV–Personality associations of the regions found in the three-way conjunction region (lPrc/POS) and in the two-way conjunction (POS/Cun, lFFG/Cb, rCb)
| rmales | rfemales | Z value of gender comparison of r: (Zmales–Zfemales)/σ(Zmales–Zfemales) | Cohen's q of gender difference | |
|---|---|---|---|---|
| lPrc/POS | ||||
| Neuroticism | −0.27a | 0.04 | −2.24a | 0.31 |
| Extraversion | 0.29a | 0.07 | 2.16a | 0.20 |
| Conscientiousness | 0.30a | 0.04 | 2.54a | 0.27 |
| POS/Cun | ||||
| Neuroticism | −0.3a | 0.0 | −2.9a | 0.31 |
| Extraversion | 0.29a | −0.07 | 3.5a | 0.37 |
| lFFG/Cb | ||||
| Neuroticism | −0.33a | 0.11 | −4.7a | 0.45 |
| Extraversion | 0.33a | −0.1 | 4.2a | 0.35 |
| rCb | ||||
| Neuroticism | −0.28a | 0.0 | −2.7a | 0.28 |
| Extraversion | 0.31a | 0.02 | 3.2a | 0.3 |
aMarks a significant correlation coefficient or gender difference.
Follow-up: Functional Characterization
Functional decoding of the regions which correlated separately with neuroticism, extraversion, and conscientiousness is shown in Figures 3C, 4C and 5C, respectively. The functional decoding of the lPrc/POS found in the three-way conjunction (Fig. 6C) revealed that this region was significantly associated with explicit memory and perception of visual motion as well as action inhibition (P < 0.05). The functional characterization of the clusters from the conjunction between neuroticism and extraversion (Fig. 7C), that is, the only two-way conjunction yielding significant results outside the cluster already identified by the three-way conjunction, showed that the POS/Cun was associated with action observation, anxiety, olfactory, and visual perception, as well as multiple cognitive processes (P < 0.05). Finally, left FFG/Cb as well as right Cb were both associated with language processing, while the former was additionally related to emotion processing (P < 0.05).
Discussion
The current morphometric study investigated the neural correlates of personality traits assessed by the NEO-FFI and potential gender differences thereof. We found no significant correlations between any personality scales and GMV when investigating relationships across the entire sample. In contrast, when the sample was split by gender, significant associations were observed in males but not females. This sexual dimorphism was corroborated by the significant differences in GMV/personality correlations between males and females for the respective clusters. Together, these findings thus demonstrate that gender is a fundamental factor to consider when trying to understand the morphological underpinnings of inter-individual differences in personality traits.
Correlations Among Personality Traits
Correlations among the 5 personality traits (Table 1) revealed similar patterns as reported in the literature (Egan et al. 2000; McCrae and Costa 2004; van der Linden et al. 2010), with mostly negative correlations between neuroticism and the other factors, and positive ones among extraversion, agreeableness, and conscientiousness. Furthermore, also in line with previous reports (Egan et al. 2000; McCrae and Costa 2004; van der Linden et al. 2010) the lowest correlations were found between openness and the other factors, while the highest associations were observed for neuroticism with extraversion and neuroticism with consciousness (Egan et al. 2000; McCrae and Costa 2004; van der Linden et al. 2010). However, several correlation coefficients that were observed when performing separate analyses for males and females were somewhat higher than those observed in previous studies (e.g. extraversion and neuroticism in males: −0.514; conscientiousness and extraversion in males: 0.449; conscientiousness and neuroticism in females: −0.428). It may be speculated that this may relate to the fact that some of our subjects were related to each other. However, when testing correlations only in a subsample of unrelated subjects, higher than previously reported correlations persisted (Supplement Table 1). It is important to note that the meta-analytic intercorrelations reported in Van Der Linden et al. (2010) were based on different personality questionnaires and that previous studies investigating the NEO-FFI computed correlations in gender-mixed samples (Egan et al. 2000; McCrae and Costa 2004; van der Linden et al. 2010). Therefore, the higher values might be due to the fact that we did separate analyses for males and females, while correlations across the entire sample are comparable to previous reports. We would therefore argue that the discrepancy between our (gender-separated) correlations and those previously observed for gender-mixed samples (which we confirmed when analyzing males and females from our sample together) indicate that not only the mean NEO-scores but also their correlation structure shows a sexual dimorphism.
Gender Differences in Personality Traits
Previous studies investigating gender differences in NEO-FFI have shown that women score higher in neuroticism and agreeableness, while conscientiousness, extraversion, and openness did not show significant differences (Costa et al. 2001; Chapman et al. 2007; Weisberg et al. 2011). In line with these studies, we observed higher neuroticism-scores for women but no significant gender differences in openness and extraversion. However, in contrast to previous studies, we failed to find a significant gender difference in agreeableness, and found that women scored higher for conscientiousness than men. We would propose that these discrepancies may be attributable to the constitution of the cohort, most importantly the parenting experience. Indeed agreeableness has been associated with motherhood and nurturance in females but not in males (Jokela et al. 2011) and this trait shows a significant increase around the age of 30 (Soto et al. 2011), when more often decisions about starting a family are taken. Since the HCP data consists of a young sample, we hypothesize that some women in our sample might not have had kids yet, resulting in a similar men's mean score. When looking at mean agreeableness scores in older (32–35 years old) and younger females (22–27 years old) there is, indeed, an indication of an increasing score with age (younger: mean 30.8; older: mean 32.6) while in males the means are similar (younger: 30.5; older: 30.7). On the other hand, our female sample scored significantly higher than males in conscientiousness. This might reflect a potential societal shift favoring (work-related) conscientiousness in young female cohorts and confirm the study from Jokela and colleagues (Jokela 2012), already demonstrating a birth-cohort effect on conscientiousness and agreeableness scores.
Association of NEO-FFI Scores to GMV Across the Entire Sample
The absence of any significant relationships between personality traits and regional GMV across the entire sample contradicts the biological model of the NEO-FFI suggested by De Young (2010) and other previous studies that supported such association (Omura et al. 2005; Gardini et al. 2009; Cremers et al. 2011; Kapogiannis et al. 2013; Lu et al. 2014). However, it may be noted that GMV–personality relationships are highly inconsistent over these previous studies in terms of location and direction. Part of this heterogeneity may be attributable to methodological differences and analytic variability between studies, including differences in personality questionnaires, whole-brain vs. regional analysis, differences in data preprocessing, variable types of statistical thresholds and (no) correction for multiple comparisons, different combinations of nuisance covariates (NCs, specifically age, gender, and total brain size; cf. Hu et al. 2011). However, we would argue that probably the usually rather small sample size, leading to spurious associations, is the major culprit. In that context, it is interesting to note that our findings are in line with those by Liu and colleagues (Liu et al. 2013) who assessed a large sample (227 subjects) in a similar age range using a comparable approach and likewise found no significant associations between NEO-FFI personality items and GMV. However, it has to be noted that Liu and colleagues neither found any correlations when investigating relationships separately for males and females. However, considering that the male sample only consisted of 59 subjects, small and moderate effects, like those in our study, might thus have been missed.
Given that our study assessed a large and well-balanced sample, is by far the best powered to date, and capitalizes on the unprecedented data quality of the HCP project, the current negative result across both genders is particularly noteworthy given the backdrop of an inconsistent literature based on smaller samples. We would thus argue that the latter may have arisen from a combination of spurious associations in smaller samples (and/or liberal thresholding) and a publication bias towards positive findings (Wallentin 2009), a situation that may be a common problem in morphology/phenotype associations in basic and even more clinical neuroscience.
Association of NEO-FFI Scores to GMV in the Male Sample
Convergence of Neuroticism, Extraversion, and Conscientiousness
It has already been reported that extraversion and conscientiousness scores correlate positively with each other and negatively with neuroticism (McCrae and Costa 2004). In our male subsample, the neural correlates of all 3 traits overlap in the POS as well as in Prc (overlapping with cluster 1 of the connectivity-based parcellation of Bzdok et al. 2014), where their correlations with GMV resembled their correlation structure as higher extraversion and conscientious scores go along with higher GMV, whereas a lower amount of GMV is associated with higher neuroticism.
We furthermore showed that this region is activated by task-fMRI studies probing visual (motion) perception, memory, and action inhibition. It may thus be speculated that neuroticism, extraversion, and conscientiousness should, via the morphological substrate of the lPrc/POS, relate to inter-individual performance in these functions. It has, for example, been shown that higher conscientiousness is associated with better performance in tasks requiring cognitive control and action inhibition such as the Stroop (Bannon et al. 2002) and anti-saccade (Kelly et al. 2015) tasks. Similarly, higher extraversion goes along with an enhanced ability to ignore task-irrelevant information in a verbal Stroop task (Prabhakaran et al. 2011). Conversely, neuroticism is associated with a decreased ability to ignore irrelevant information (Prabhakaran et al. 2011). The latter has been related to a “hypervigilance of threats” (Mogg and Bradley 1998; Richards et al. 2014), that is, an adaptive behavior to perceive a potential risk faster, which comes at the cost of specificity and, consequently, less successful inhibition of irrelevant stimuli and response sets. While the association of personality traits to visual processing has received less attention, the positive relation between conscientiousness and (anti) saccade task performance corroborates the above picture, as does the role of extraversion as a positive predictor of attentional control in visual classification or change detection tasks (Stenberc 1994). There have also been several reports linking higher extraversion, as well as lower neuroticism, to better (long-term) memory performance (Nakamura et al. 1979; Ashby et al. 1999; Allen et al. 2011). Finally, conscientiousness was shown to correlate positively with subjective memory (Pearman 2009), which in turn might reflect performance in objective mnemonic tasks (Zimprich and Kurtz 2015).
In summary, we would thus argue that the observed convergence of morphometric substrates for neuroticism, extraversion, and conscientiousness in the lPrc/POS may provide the structural correlate of the association between these personality traits and inter-individual performance differences in the domains of action inhibition, visual perception, and memory.
Convergence of Neuroticism and Extraversion
For the male subsample, additional convergence in the morphometric substrate for extraversion and neuroticism was found in lFFG/Cb and rCb regions associated with language and in the case of lFFG/Cb, emotion, face, and reward processing. This suggests a link between these personality traits and inter-individual performance difference in language tasks, which is supported, for example, by previous work showing a positive association with extraversion and a negative one with neuroticism for verbal fluency tasks (Sutin et al. 2011). Regarding the specific effects in the fusiform face region and the relation to emotion, we would speculate that individuals with higher extraversion spend more time with others resulting in use-dependent plasticity in face-selective regions. Alternatively, however, already higher GMV in face selective regions might lead to a stronger tendency to spend more time with others and hence even predispose towards an extraverted personality.
In contrast, hypervigilance in high neuroticism might favor the detection of threats (Richards et al. 2014), and concurrently impair the processing of the neutral faces and other emotions (Andric et al. 2015). With regard to reward sensitivity, there is evidence of an opposite role of approach (associated with extraversion) and avoidance (associated with neuroticism) on the anticipatory role of reward: approach relies on a higher sensitivity to social (Wilkowski and Ferguson 2014) and monetary (Ostaszewski 1996) rewards, while avoidance is associated with reduced responsiveness to incentives (Bress et al. 2013).
Gender Differences in Brain Structure–Personality Relationships
Our analyses revealed not only several personality “hotspots” in males, but strikingly also failed to find any relationship in the female subgroup. This absence of localized morphology/personality relationships may well relate to observations that female brains are more decentralized (Zaidi 2010) and feature stronger interhemispheric structural connectivity (Ingalhalikar et al. 2014);that is, are potentially “hard-wired” towards multitasking (Zaidi 2010). In particular, such more distributed and integrated architecture may reduce the explanatory power of any local morphological effect.
Another factor that likely plays a major role in the observed dimorphism is the effect of sex hormones, given their influence on personality (Daendee et al. 2013) and brain structure (De Vries 2004). Both estrogens and progesterone, for example, influence neuroticism via antagonistic modulation of GABA receptors (Maggi and Perez 1986; Daendee et al. 2013), and have been hypothesized to play a crucial role in generating the higher neuroticism scores (Seeman 1997) that have been observed for females in several studies, including ours. These hormones also influence neuropsychological features related to personality obtained through the functional profile; for instance, estrogens positively modulate saccadic eye velocity (Wihlbäck et al. 2005), long-term memory (Barros et al. 2015), and self-regulation/inhibitory control (Hosseini-Kamkar and Morton 2014), while progesterone negatively modulates saccadic eye velocity (van Broekhoven et al. 2006), memory (Barros et al. 2015), and self-regulation (Hosseini-Kamkar and Morton 2014). Furthermore, their fluctuation over the menstrual cycle has been connected to neural changes, on both structural and functional level in different brain regions (Witte et al. 2010; Rasgon et al. 2014; Lisofsky et al. 2015).
The influence of sex hormones on personality features and neurobiology, combined with their massive changes over the menstrual cycle in women and relative stability in men, may well explain the lack of significant results in the female sample. In particular, since we could not control for menstrual cycle in our sample, we must assume that female participants were scanned randomly in all phases of a natural menstrual cycle or under contraceptive medication, that is, synthetic hormones. Given the ensuing variations in estrogens and progesterone levels, the female group should be substantially more heterogeneous than the male sample, which in turn should make it more difficult to detect associations between morphometric features and personality scores, if the increase in variance is not isomorphic between the phenotypical scores and (local) brain volume changes. In addition to the effects of female sex hormones, the higher levels of testosterone in males (Torjesen and Sandnes 2004) and its stable concentration across the life span (Liu et al. 2015) may also contribute to the differential findings. For example, testosterone is involved in regulating approach behavior and social status-seeking (Eisenegger et al. 2011) and therefore associated with extraversion (Smeets-janssen et al. 2015), but has also been shown to influence cortical thickness in cuneus and other visual areas (Bramen et al. 2012). Consequently, the above-discussed associations in males may reflect a common causal factor (i.e., testosterone) driving both morphometric features, personality and neuropsychological performance in various tasks.
Clinical Implications
Personality traits may become themselves clinically relevant in their extreme forms as personality disorders (Miller et al. 2001). Rather and more importantly, they also seem to predispose towards multiple Axis-I disorders. For example, high neuroticism and low extraversion are associated with social, agora- and specific phobias (Bienvenu et al. 2007), high neuroticism, low extraversion and conscientiousness with depression (Weiss et al. 2009), low extraversion and high agreeableness with eating disorders (Tasca et al. 2009), and high neuroticism and extraversion with substance abuse (Dubey et al. 2010). This strong link between personality traits and Axis-I disorders is corroborated by gender differences in prevalence. For example, mood, anxiety, social, and eating disorders are more frequently found in females (Mclean and Hofmann 2011; Viana and Andrade 2012; Seney and Sibille 2014) cf. Afifi (2007), while substance abuse is more common in males (Compton et al. 2007; Viana and Andrade 2012).
This convergence extends to the neurobiological level. For instance, depressed patients feature reduced GMV in the Prc/Cun (Grieve et al. 2013), which resonates well with its reduced volume in high neuroticism. Finally, in line with our result of decreased GMV in lPrc/POS going along with low extraversion, persistent GMV reduction of the precuneus has been demonstrated in anorexia (Joos et al. 2011). While more indirect evidence, it is also interesting to note that various Axis-I disorders also feature cognitive impairments in those domains that we found to be associated with the Prc/POS, that is, the convergent structural substrate for multiple personality dimensions. In particular, it has been shown that patients with social anxiety demonstrate a lack of attentional control and difficulties in focusing on task-relevant stimuli (Derakshan et al. 2009; Wieser et al. 2009) and that patients with depression show impairments involving visual attention, cognitive flexibility (Hoffstaedter et al. 2012; Doose-Grünefeld et al. 2015), and control (De Lissnyder et al. 2012) as well as memory (Roca et al. 2015).
In summary, our results in combination with previous findings suggest the Prc/POS as a key structure in the close relationship between personality traits, gender, major psychiatric disorders, and changes in brain structure as well as neuropsychological profiles. Moreover, they also highlight the importance of assessing potential sexual dimorphisms of these relationships.
Limitations
It has to be acknowledged that our sample partially consists of related subjects, which might have influenced the present findings. Therefore, in order to test if the association of personality with GMV in POS, lFFG, and rCB could be replicated in an unrelated (though substantially lower powered) sample, we reran our analysis again in a more restricted sample, consisting of 150 unrelated subjects, with men and women matched for their zygosity, age, and years of education. Also in this smaller group, we found a cluster located in the lPrc/POS, which GMV in males was positively associated with extraversion and negatively associated with neuroticism scores. However, correlations between lFFG and rCb with extraversion and neuroticism as well as between lPrc/POS with conscientiousness could not be reproduced. These effects may thus have arisen from the family structure, although we would strongly argue that their absence could very likely be related to the much lower power in the now substantially smaller sample. In conclusion, the correlation between lPrc/POS and extraversion and neuroticism can be considered as stable and independent from genetic influences, while the associations with lFFG/Cb and rCb should be interpreted with caution, and should be replicated in a larger unrelated sample.
Furthermore, the presented neuroanatomical changes associated with personality were found in males, who have generally larger brains than females (Ruigrok et al. 2014). It has previously been shown that volume of specific brain regions as well as inter- and intra-hemispheric connectivity differences between males and females may be related to brain size (Hänggi et al. 2014; Pintzka et al. 2015 but compare Im et al. 2006; Luders et al. 2009). Therefore the question arises whether the effects of the current study, which are only observed in men, can be fully attributed to gender, or are (partially) also driven by brain size. While we controlled for TBV by using it as a covariate of no interest in our statistical models, we would still refrain from claiming that our results are purely attributable to gender.
Lastly, we performed a surface-based analysis of cortical thickness and area in order to explore whether the correlations we found in VBM analysis could also be found in these more specialized measures of cortical morphometry. Results revealed a positive association in males between conscientiousness and cortical thickness of the lPOS at an uncorrected level (P < .001, uncorrected), while no correlations were observed with the traits of extraversion and neuroticism neither for the lPOS nor for the lFFG. Thus, based on our results, we would argue that, though on an uncorrected level (P < 0.001), the association between volume of lPOS and conscientiousness may be more related to changes in cortical thickness than surface area. However, given that the surface-based analyses yielded largely null-results it seems that GMV is more sensitive in detection brain structure–personality relationships than either of its 2 constituents, that is, cortical thickness or surface area. These results support the notion of GMV as a gross but robust anatomical index for morphometric changes, providing a mixed measure of regional gray matter properties including cortical surface area, thickness, and potentially folding. Consequently, the more specialized SBM measures might fail to detect changes driven by interactions of multiple surface-based features and, consequently, when used in isolation, significant associations with performances might not be revealed (Smolker et al. 2015).
Summary and Conclusion
Our study challenges existing notions on morphological substrates for personality traits, by yielding a negative result in a well-powered analysis of high-quality data in a balanced sample. Additionally, it demonstrates that relationships between personality traits and brain structure are highly dependent on gender. This observation is corroborated by converging neuropsychological and clinical evidence supporting a similar sexual dimorphism. We also identified the left precuneus as a convergent substrate for neuroticism, extraversion, and conscientiousness in males. This region was functionally implicated by our analysis in cognitive control, visual perception, and memory, that is, mental functions that show robust relationships to the aforementioned personality traits. Extraversion and neuroticism converged also in the left fusiform gyrus and right cerebellum, regions related to emotion processing and language skills that are likewise related to personality.
Taken together, our study provides a critical view on previous links between brain structure and personality traits, revealing the precuneus as a key region linking personality, gender, mental functions, and psychiatric disorders, and highlighting the need to account for sexual dimorphisms when trying to unravel the complex relationships between these aspects.
Financial Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This study was supported by the Deutsche Forschungsgemeinschaft (DFG, EI 816/4-1, LA 3071/3-1; EI 816/6-1.), the National Institute of Mental Health (R01-MH074457) and the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement no. 604102 (Human Brain Project).
Supplementary Material
References
- Afifi M. 2007. Gender Differences in Mental Health. Singapore Med J. 48:385–391. [PubMed] [Google Scholar]
- Allen PA, Kaut K, Baena E, Lien M-C, Ruthruff E.. 2011. Individual differences in positive affect moderate age-related declines in episodic long-term memory. J Cogn Psychol. 23:768–779. [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K.. 2000. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable. Neuroimage. 11:66–84. [DOI] [PubMed] [Google Scholar]
- Andric S, Maric NP, Knezevic G, Mihaljevic M, Mirjanic T, Velthorst E, Van Os J.. 2015. Brief Report Neuroticism and facial emotion recognition in healthy adults. Early Interv Psychiatry. 1–5. [DOI] [PubMed]
- Asendorpf JB, Wilpers S.. 1998. Personality effects on social relationships. J Pers Soc Psychol. 74:1531–1544 [Google Scholar]
- Ashburner J. 2007. A fast diffeomorphic image registration algorithm. Neuroimage. 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashby FG, Isen AM, Turken AU.. 1999. A neuropsychological theory of positive affect and its influence on cognition. Psychol Rev. 106:529–550. [DOI] [PubMed] [Google Scholar]
- Bannon S, Gonsalvez CJ, Croft RJ, Boyce PM.. 2002. Response inhibition deficits in obsessive-compulsive disorder. Psychiatry Res. 110:165–174. [DOI] [PubMed] [Google Scholar]
- Barros LA, Tufik S, Andersen ML.. 2015. The role of progesterone in memory: an overview of three decades. Neurosci Biobehav Rev. 49:193–204. [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Meseguer V, Sanjuán A, Belloch V, Parcet MA, Torrubia R, Ávila C.. 2006. Behavioral inhibition system activity is associated with increased amygdala and hippocampal gray matter volume: a voxel-based morphometry study. Neuroimage. 33:1011–1015. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, et al. . 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 6:750–757. [DOI] [PubMed] [Google Scholar]
- Bienvenu OJ, Hettema JM, Neale MC, Prescott CA, Kendler KS.. 2007. Low extraversion and high neuroticism as indices of genetic and environmental risk for social phobia, agoraphobia, and animal phobia. Am J Psychiatry. 164:1714–1721. [DOI] [PubMed] [Google Scholar]
- Bramen JE, Hranilovich JA, Dahl RE, Chen J, Rosso C, Forbes EE, Dinov ID, Worthman CM, Sowell ER.. 2012. Sex matters during adolescence: testosterone-related cortical thickness maturation differs between boys and girls. PLoS One. 7:e33850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Foti D, Kotov R, Klein DN, Hajcak G.. 2013. Blunted neural response to rewards prospectively predicts depression in adolescent girls. Psychophysiology. 50:74–81. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Heeger A, Langner R, Laird AR, Fox PT, Palomero-Gallagher N, Vogt BA, Zilles K, Eickhoff SB.. 2014. Subspecialization in the human posterior medial cortex. Neuroimage. 106:55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspers J, Zilles K, Eickhoff SB, Schleicher A, Mohlberg H, Amunts K.. 2013. Cytoarchitectonical analysis and probabilistic mapping of two extrastriate areas of the human posterior fusiform gyrus. Brain Struct Funct. 218:511–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman BP, Duberstein PR, Sörensen S, Lyness JM.. 2007. Gender differences in five factor model personality traits in an elderly cohort: extension of robust and surprising findings to an older generation. Pers Individ Dif. 43:1594–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. 1988. Statistical power analysis for the behavioral sciences. 2. New Jersey: Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF.. 2007. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States. Results from the National Epidemiologic Survey on alcohol and related conditions. Arch Gen Psychiatry. 64:566–576. [DOI] [PubMed] [Google Scholar]
- Costa P, McCrae R.. 1992. a. Normal personality assessment in clinical practice: the NEO personality inventory. Psychol Assess. 4:5–13. [Google Scholar]
- Costa PT, McCrae RR.. 1992. b. Professional manual: revised NEO personality inventory (NEO-PI-R) and NEO five-factor inventory (NEO-FFI). Odessa FL: Psychological Assessment Resources. [Google Scholar]
- Costa PT, Terracciano A, McCrae RR.. 2001. Gender differences in personality traits across cultures: robust and surprising findings. J Pers Soc Psychol. 81:322–331. [DOI] [PubMed] [Google Scholar]
- Cremers H, van Tol M-J, Roelofs K, Aleman A, Zitman FG, van Buchem MA, Veltman DJ, van der Wee NJA.. 2011. Extraversion is linked to volume of the orbitofrontal cortex and amygdala. PLoS One. 6:e28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daendee S, Thongsong B, Kalandakanond-Thongsong S.. 2013. Effects of time of estrogen deprivation on anxiety-like behavior and GABAA receptor plasticity in ovariectomized rats. Behav Brain Res. 246:86–93. [DOI] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EHW, Everaert J, Schacht R, Van den Abeele D, De Raedt R.. 2012. Internal cognitive control in clinical depression: General but no emotion-specific impairments. Psychiatry Res. 199:124–130. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. 2004. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology. 145:1063–1068. [DOI] [PubMed] [Google Scholar]
- De Young CG, Hirsh JB, Shane MS, Papademetris X. 2010. Testing predictions from personlaity neuroscience: brain structure and the big five. Psychol Sci 21:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW.. 2009. Anxiety, inhibition, efficiency, and effectiveness. Exp Psychol. 56:48–55. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR.. 2010. Testing predictions from personality neuroscience. Brain structure and the big five. Psychol Sci. 21:820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N.. 2009. A probabilistic MR atlas of the human cerebellum. Neuroimage. 46:39–46. [DOI] [PubMed] [Google Scholar]
- Doose-Grünefeld S, Eickhoff SB, Müller VI.. 2015. Audiovisual emotional processing and neurocognitive functioning in patients with depression. Front Integr Neurosci. 9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey C, Arora M, Gupta S, Kumar B.. 2010. Five factor correlates: a comparison of substance abusers and non-substance abusers. J Indian Aca. Appl Psychol. 36:107–114. [Google Scholar]
- Egan V, Deary I, Austin E.. 2000. The NEO-FFI : emerging British norms and an item-level analysis suggest N, A and C are more reliable than O and E. Pers Individ Dif. 29:907–920.
- Eickhoff SB, Paus T, Caspers S, Grosbras M-H, Evans AC, Zilles K, Amunts K.. 2007. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 36:511–521. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K.. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 25:1325–1335. [DOI] [PubMed] [Google Scholar]
- Eisenegger C, Haushofer J, Fehr E.. 2011. The role of testosterone in social interaction. Trends Cogn Sci. 15:263–271. [DOI] [PubMed] [Google Scholar]
- Fox PT, Lancaster JL.. 2002. Opinion: Mapping context and content: the BrainMap model. Nat Rev Neurosci. 3:319–321. [DOI] [PubMed] [Google Scholar]
- Gardini S, Cloninger CR, Venneri A.. 2009. Individual differences in personality traits reflect structural variance in specific brain regions. Brain Res Bull. 79:265–270. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, et al. . 2013. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage. 80:105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM.. 2013. Widespread reductions in gray matter volume in depression. NeuroImage Clin. 3:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hänggi J, Fövenyi L, Liem F, Meyer M, Jäncke L.. 2014. The hypothesis of neuronal interconnectivity as a function of brain size-a general organization principle of the human connectome. Front Hum Neurosci. 8:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson R. 2005. What can functional neuroimaging tell the experimental psychologist? Q J Exp Psychol A. 58:193–233. [DOI] [PubMed] [Google Scholar]
- Hoffstaedter F, Sarlon J, Grefkes C, Eickhoff SB.. 2012. Internally vs. externally triggered movements in patients with major depression. Behav Brain Res. 228:125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini-Kamkar N, Morton JB.. 2014. Sex differences in self-regulation: an evolutionary perspective. Front Neurosci. 8:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Erb M, Ackermann H, Martin JA, Grodd W, Reiterer SM.. 2011. Voxel-based morphometry studies of personality: Issue of statistical model specification—effect of nuisance covariates. Neuroimage. 54:1994–2005. [DOI] [PubMed] [Google Scholar]
- IBM Corp. Released. 2011. IBM SPSS Statistics for Windows, Version 20.0. 2011.
- Im K, Lee J, Lee J, Shin Y, Kim Y, Kwon JS, Kim SI.. 2006. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. NeuroImage. 31:31–38. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar M, Smith A, Parker D, Satterthwaite TD, Elliott MA, Ruparel K, Hakonarson H, Gur RE, Gur RC, Verma R.. 2014. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci U S A. 111:823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M. 2012. Birth-cohort effects in the association between personality and fertility. Psychol Sci. 23:835–841. [DOI] [PubMed] [Google Scholar]
- Jokela M, Alvergne A, Pollet T V, Lummaa V.. 2011. Reproductive behavior and personality traits of the five factor model. Eur J Pers. 25:487–500. [Google Scholar]
- Joos A, Hartmann A, Glauche V, Perlov E, Unterbrink T, Saum B, Tu O.. 2011. Grey matter deficit in long-term recovered anorexia nervosa patients. Eur Eat Disord Rev. 19:59–63. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Sutin A, Davatzikos C, Costa P, Resnick S.. 2013. The five factors of personality and regional cortical variability in the baltimore longitudinal study of aging. Hum Brain Mapp. 34:2829–2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CL, Sünram-lea SI, Crawford TJ.. 2015. The role of motivation, glucose and self- control in the antisaccade task. PLoS ONE. 10:e0122218. doi:10.1371/journal.pone.0122218. [DOI] [PMC free article] [PubMed]
- Kenny DA. 1987. Statistics for the social and behavioral sciences. Boston: Little, Brown. [Google Scholar]
- Koelsch S, Skouras S, Jentschke S.. 2013. Neural correlates of emotional personality : a structural and functional magnetic resonance imaging study. PLoS ONE. 8:e77196. doi:10.1371/journal.pone.0077196. [DOI] [PMC free article] [PubMed]
- Kujovic M, Zilles K, Malikovic A, Schleicher A, Mohlberg H, Rottschy C, Eickhoff SB, Amunts K.. 2013. Cytoarchitectonic mapping of the human dorsal extrastriate cortex. Brain Struct Funct. 218:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Fox PM, Uecker AM, Ray KL, Saenz JJ, McKay DR, Bzdok D, Laird RW, Robinson JL, et al. . 2011. The BrainMap strategy for standardization, sharing, and meta-analysis of neuroimaging data. BMC Res Notes. 4:349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT.. 2009. ALE meta-analysis workflows via the brainmap database: progress towards a probabilistic functional brain atlas. Front Neuroinform. 3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisofsky N, Mårtensson J, Eckert A, Lindenberger U, Gallinat J, Kühn S.. 2015. Hippocampal volume and functional connectivity changes during the female menstrual cycle. Neuroimage. 118:154–162. [DOI] [PubMed] [Google Scholar]
- Liu W-Y, Weber B, Reuter M, Markett S, Chu W-C, Montag C.. 2013. The Big Five of Personality and structural imaging revisited: a VBM—DARTEL study. Neuroreport. 24:375–380. [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu J, Shi X, Wang L, Yang Y, Tao M.. 2015. Dynamic alteration of serum testosterone with aging: a cross-sectional study from Shanghai, China. Reprod Biol Endocrinol. 13:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Huo Y, Li M, Chen H, Liu F, Wang Y, Long Z, Duan X, Zhang J, Zeng L, et al. . 2014. Relationship between personality and gray matter volume in healthy young adults : a voxel-based morphometric study. PLoS ONE. 9:e88763. doi:10.1371/journal.pone.0088763 [DOI] [PMC free article] [PubMed]
- Luders E, Gaser C, Narr KL, Toga AW.. 2009. Why sex matters: brain size independent differences in gray matter distributions between men and women. J Neurosci. 29:14265–14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi A, Perez J.. 1986. Estrogen-induced up-regulation of gamma-aminobutyric acid receptors in the CNS of rodents. J Neurochem. 47:1793–1797. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT.. 2004. A contemplated revision of the NEO Five-Factor Inventory. Pers Individ Dif. 36:587–596. [Google Scholar]
- Mclean CP, Hofmann SG.. 2011. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 45:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JD, Lynam DR, Widiger TA, Leukefeld C.. 2001. Personality disorders as extreme variants of common personality dimensions : can the five-factor model adequately represent psychopathy . J Pers. 69:253–276. [DOI] [PubMed] [Google Scholar]
- Mischel W. 2004. Toward an integrative science of the person. Annu Rev Psychol. 55:1–22. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP.. 1998. A cognitive-motivational analysis of anxiety. Behav Res Ther. 36:809–848. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Yoshihisa F, Iwao K, Nobukatsu K.. 1979. A comparison of the CNV in young and old subjects: its relation to memory and personality. Electromyogr Clin Neurophysiol. 46:337–344. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Brett M, Andersson J, Poline JB, Wager T.. 2005. Valid conjunction inference with the minimum statistic. Neuroimage. 25:653–660. [DOI] [PubMed] [Google Scholar]
- Omura K, Todd Constable R, Canli T.. 2005. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 16:1905–1908. [DOI] [PubMed] [Google Scholar]
- Ostaszewski P. 1996. The relation between temperament and rate of temporal discounting. Eur J Pers. 10:161–172. [Google Scholar]
- Pearman A. 2009. Predictors of Subjective Memory in Young Adults. J Adult Dev. 16:101–107.
- Pintzka CWS, Hansen TI, Evensmoen HR, Håberg AK.. 2015. Marked effects of intracranial volume correction methods on sex differences in neuroanatomical structures: A HUNT MRI study. Front Neurosci. 9:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA. 2006. Can cognitive processes be inferred from neuroimaging data. Trends Cogn Sci. 10:59–63. [DOI] [PubMed] [Google Scholar]
- Prabhakaran R, Kraemer DJM, Thompson-Schill SL.. 2011. Approach, avoidance, and inhibition: personality traits predict cognitive control abilities. Pers Individ Dif. 51:439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon NL, Geist CL, Kenna HA, Wroolie TE, Williams KE, Silverman DHS.. 2014. Prospective randomized trial to assess effects of continuing hormone therapy on cerebral function in postmenopausal women at risk for dementia. PLoS One. 9:e89095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards HJ, Benson V, Donnelly N, Hadwin JA.. 2014. Exploring the function of selective attention and hypervigilance for threat in anxiety. Clin Psychol Rev. 34:1–13. [DOI] [PubMed] [Google Scholar]
- Riemann R, Grubich C, Hempel S, Mergl S, Richter M.. 1993. Personality and attitudes towards current political topics. Pers Individ Dif. 15:313–321. [Google Scholar]
- Roca M, Vives M, López-navarro E, García-campayo J.. 2015. Cognitive impairments and depression : a critical review. Actas Esp Psiquiatr. 43:187–193. [PubMed] [Google Scholar]
- Rothmann S, Coetzer EP.. 2003. The big five personality dimensions and job performance. SA J Ind Psychol. 29:68–74. [Google Scholar]
- Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, Zilles K, Amunts K.. 2007. Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp. 28:1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruigrok AN V, Salimi-Khorshidi G, Lai M-C, Baron-Cohen S, Lombardo M V, Tait RJ, Suckling J.. 2014. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 39:34–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K.. 2008. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex. 18:846–867. [DOI] [PubMed] [Google Scholar]
- Seeman M V. 1997. Psychopathology in women and men: Focus on female hormones. Am J Psychiatry. 154:1641–1647. [DOI] [PubMed] [Google Scholar]
- Seney ML, Sibille E.. 2014. Sex differences in mood disorders : perspectives from humans and rodent models. Biol Sex Differ. 5:17. [DOI] [PMC free article] [PubMed]
- Smeets-Janssen MMJ, Roelofs K, Pelt V.. 2015. Salivary testosterone is consistently and positively associated with extraversion : results from The Netherlands study of depression and anxiety. Neuropsychobiology. 71:76–84. [DOI] [PubMed]
- Smith SM, Nichols TE.. 2009. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 44:83–98. [DOI] [PubMed] [Google Scholar]
- Smolker HR, Depue BE, Reineberg AE, Orr JM, Banich MT.. 2015. Individual differences in regional prefrontal gray matter morphometry and fractional anisotropy are associated with different constructs of executive function. Brain Struct Funct. 1291–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto CJ, John OP, Gosling SD, Potter J.. 2011. Age differences in personality traits from 10 to 65 : big five domains and facets in a large cross-sectional sample. J Pers Soc Psychol. 100:330–348. [DOI] [PubMed] [Google Scholar]
- Stenberc G. 1994. Extraversion and the P300 in a visual classification task. Pers Individ Dif. 16:543–560.
- Sutin AR, Terracciano A, Kitner-Triolo MH, Uda M, Schlessinger D, Zonderman AB.. 2011. Personality traits prospectively predict verbal fluency in a lifespan sample. Psychol Aging. 26:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasca GA, Demidenko N, Krysanski V, Bissada H, Illing V, Gick M, Weekes K, Balfour L.. 2009. Personality Dimensions among Women with an Eating Disorder : Towards Reconceptualizing DSM Eur Eat Disord Rev. 17:281–289. [DOI] [PubMed] [Google Scholar]
- Torjesen PA, Sandnes L.. 2004. Serum testosterone in women as measured by an automated immunoassay and a RIA To. Clin Chem. 50:678–679. [DOI] [PubMed] [Google Scholar]
- van Broekhoven F, Bäckström T, Verkes RJ.. 2006. Oral progesterone decreases saccadic eye velocity and increases sedation in women. Psychoneuroendocrinology. 31:1190–1199. [DOI] [PubMed] [Google Scholar]
- van der Linden D, te Nijenhuis J, Bakker AB.. 2010. The general factor of personality: a meta-analysis of big five intercorrelations and a criterion-related validity study. J Res Pers. 44:315–327. [Google Scholar]
- Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, et al. . 2012. The Human Connectome Project: a data acquisition perspective. Neuroimage. 62:2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schuerbeek P, Baeken C, De Raedt R, De Mey J, Luypaert R.. 2011. Individual differences in local gray and white matter volumes reflect differences in temperament and character: A voxel-based morphometry study in healthy young females. Brain Res. 1371:32–42. [DOI] [PubMed] [Google Scholar]
- Viana MC, Andrade LH.. 2012. Lifetime prevalence, age and gender distribution and age-of-onset of psychiatric disorders in the São Paulo Metropolitan Area, Brazil: results from the São Paulo Megacity Mental Health Survey. Rev Bras Psiquiatr. 34:249–260. [DOI] [PubMed] [Google Scholar]
- Wallentin M. 2009. Putative sex differences in verbal abilities and language cortex: a critical review. Brain Lang. 108:175–183. [DOI] [PubMed] [Google Scholar]
- Weisberg YJ, DeYoung CG, Hirsh JB.. 2011. Gender differences in personality across the ten aspects of the big five. Front Psychol. 2:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A, Sutin AR, Duberstein PR, Friedman B, Bagby RM, Costa PT.. 2009. The personality domains and styles of the five-factor model are related to incident depression in Medicare recipients aged 65 to 100. Am J Geriatr Psychiatry. 17:591–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Mühlberger A.. 2009. Probing the attentional control theory in social anxiety: an emotional saccade task. Cogn Affect Behav Neurosci. 9:314–322. [DOI] [PubMed] [Google Scholar]
- Wihlbäck A-C, Nyberg S, Bäckström T, Bixo M, Sundström-Poromaa I.. 2005. Estradiol and the addition of progesterone increase the sensitivity to a neurosteroid in postmenopausal women. Psychoneuroendocrinology. 30:38–50. [DOI] [PubMed] [Google Scholar]
- Wilkowski BM, Ferguson EL.. 2014. Just loving these people: Extraverts implicitly associate people with reward. J Res Pers. 53:93–102. [Google Scholar]
- Witte AV, Savli M, Holik A, Kasper S, Lanzenberger R.. 2010. Regional sex differences in grey matter volume are associated with sex hormones in the young adult human brain. Neuroimage. 49:1205–1212. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Abe O, Suga M, Yamada H, Inoue H, Tochigi M, Rogers M, Aoki S, Kato N, Kasai K.. 2008. Gender-common and -specific neuroanatomical basis of human anxiety-related personality traits. Cereb Cortex. 18:46–52. [DOI] [PubMed] [Google Scholar]
- Zaidi ZF. 2010. Gender Differences in Human Brain: A Review. Open Anat J. 2:37–55. [Google Scholar]
- Zimprich D, Kurtz T.. 2015. Subjective and Objective Memory Changes in Old Age across Five Years. Gerontology. 61:223–231. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.